Abstract
Recent work has highlighted the importance of cobalamin deficiency in cats with a range of alimentary tract diseases. The primary aim of our study was to determine the incidence of subnormal cobalamin concentrations in sick cats with and without alimentary system disorders. Firstly, serum cobalamin concentrations were measured in a population of cats, with and without gastrointestinal (GI) disease, evaluated at a referral hospital. In the second part of the study, the incidence of cobalamin deficiency was assessed in samples submitted to a commercial laboratory specifically for cobalamin measurement. For both studies, a validated radioimmunoassay was used to measure serum cobalamin concentrations (reference range: >150 pg/ml). In the first part of the study, 132 cats were included and none of these cats had subnormal cobalamin concentrations (median=1172; range: 278 to >2000). There were no differences in cobalamin concentrations between cats with alimentary system disorders, and those with diseases of other organs. In the second part, 682 samples were submitted for cobalamin assay over a period of 3 years, and only one cat had a result below the reference range (median=794; range: 147 to >2000). Cobalamin deficiency was rare in the population tested and this may suggest that the incidence of this biochemical abnormality is less common than reported in the USA.
Cobalamin (vitamin B12) is a water-soluble cobalt-containing vitamin, which acts as a cofactor for a number of enzymes, including methylmalonic-CoA mutase and methionine synthase (Allen et al 1993). Both are essential enzymes for normal cellular metabolism. Decreased activity of these two enzymes can lead to abnormal function in all body tissues, especially in those with rapidly dividing cells such as haemopoietic tissue, intestinal epithelium, fetal and testicular tissue. Neuronal tissue can also be affected. In humans, cobalamin deficiency can be associated with demyelinating neuropathies, dementia and pernicious anaemia. Signs of cobalamin deficiency in companion animals more commonly include inappetence, failure to thrive and haematological changes such as anaemia and leukopenia (Fyfe et al 1989, 1991, Vaden et al 1992). Neurological signs have been recently documented in cats with cobalamin deficiency (Salvadori et al 2003).
Cobalamin deficiency has most commonly been associated with disorders of the alimentary system, and this not surprising given its importance in cobalamin metabolism. In this regard, ingested cobalamin is initially bound to dietary protein. After protein is digested in the stomach and proximal duodenum, the released cobalamin immediately binds to haptocorrin, which is found in saliva and gastric secretions. In the small intestine, haptocorrin is digested by pancreatic enzymes and cobalamin then binds to intrinsic factor (IF), which in cats is mainly (99%) secreted by the pancreas (Fyfe 1993). Cobalamin bound to IF is then absorbed by a specific carrier mechanism in the ileum travelling via the portal blood stream to the liver where it gets absorbed by hepatocytes. A problem at any level in this multi-step process can lead to cobalamin deficiency (Fyfe 2000).
In dogs, subnormal serum cobalamin concentrations have been documented secondary to exocrine pancreatic insufficiency (EPI), intestinal diseases causing malabsorption and small intestinal bacterial overgrowth (SIBO) (Batt and Morgan 1982). Further, Giant Schnauzers can suffer primary cobalamin deficiency caused by defective localisation of the ileal cobalamin–IF receptor leading to malabsorption of cobalamin (Fyfe 1991). There are sporadic reports in the literature of cats with subnormal cobalamin concentrations and signs of cobalamin deficiency (Frank and Feinstein 1991, Perry et al 1991, Vaden et al 1992). Recent work has also documented the metabolic consequences of severe cobalamin deficiency (cobalamin concentrations<100 pg/ml, n=40) (Ruaux et al 2001). Most notably, markedly increased serum and urinary concentrations of methylmalonic acid (MMA) were present. This study confirmed that cats with low serum cobalamin concentrations had biochemical abnormalities due to cobalamin depletion in tissues.
Despite such reports demonstrating the importance of severe cobalamin deficiency in cats, there are limited data reviewing the incidence of subnormal cobalamin concentrations in cats. One recent study documented hypocobalaminaemia in 49/80 (61%) of cats with confirmed GI disease, when compared with a control population of healthy cats (Simpson et al 2001). In contrast, a similar study failed to document subnormal serum cobalamin concentrations in 19 cats with chronic GI disease (Johnston et al 2001). Furthermore, given that both studies compared cats with gastrointestinal disorders with healthy cats, it was also not clear whether hypocobalaminaemia is always the result of diseases of the alimentary system, or whether it could be a non-specific marker of illness in cats. Although no published evidence exists for the latter, parenteral cobalamin is commonly given in first opinion practice to cats with vague signs of illness.
In view of the current controversy, the current study sought to assess both the overall incidence of subnormal cobalamin concentrations in sick cats, and its incidence in cats with diseases of the alimentary system.
Materials and methods
Study animals
The aim of the first part of the study was to assess the overall incidence of hypocobalaminaemia in the population of cats evaluated at a referral hospital (University of Liverpool, Small Animal Teaching Hospital; UOL). The hospital computer database was searched to identify all the cats referred over a period of 18 months (from January 2002 to June 2003). The main inclusion criteria were: (1) availability of sufficient serum for analysis, (2) no history of recent cobalamin administration either at the referring veterinary surgeons or at UOL prior to blood sampling, and (3) a definitive diagnosis had been achieved. To exclude the possibility of prior cobalamin supplementation, referring veterinary surgeons provided complete medical records for every cat, and all previous therapies were reviewed. After referral, all cases were investigated as completely as possible, and the diagnostic investigations performed were appropriate to the clinical presentation. For cases that presented more than once during the study period, only the samples taken on the first visit (invariably within 24 h of admission) were included for analysis.
The aim of the second part of the study was to assess the incidence of hypocobalaminaemia in feline serum samples submitted to a commercial laboratory (Cambridge Specialist Laboratory Services; CSLS, Cambridge, UK) specifically for cobalamin measurement. The computer database was reviewed to identify all submissions for cobalamin assessment between November 1998 and December 2004 inclusive. Signalment data were available for all submissions, although incomplete in some cases. Detailed historical data was limited but based on the history provided by the veterinary surgeon in the submission form, the reason for sampling in most cases was suspected GI disease.
Sample collection and processing from referred cases
Serum samples used in the first part of the study represented surplus material after routine biochemical analyses had been performed. All samples were coded, batched, transported on ice for analysis at CSLS, and subsequently assayed blind, in a single batch.
Cobalamin measurement
Cobalamin was measured by a dual isotope standard radioimmunoassay (RIA) method without a boil phase (SimulTRAC-SNB, MP Biomedicals). This assay is a competitive RIA which has been widely used in the human field and shown to be accurate for detecting low serum concentrations of cobalamin (Arnaud et al 1994). This assay was validated for use in the feline species, and the lower limit of the laboratory reference range was 150 pg/ml (H. Evans, unpublished data). This range was established using a population of cats (representing a range of ages, genders and breeds) without GI disease.
Statistical analysis
A computer software package (Minitab for Windows release 14.1; Minitab Inc., State College, USA) was employed for all statistical analyses. Given that many results were reported as >2000 pg/ml, the data were not normally distributed and non-parametric tests were performed throughout including Kruskal–Wallis analysis of variance (ANOVA) and Mann–Whitney U tests. For part 1 of the study, baseline data (eg, age, gender, breed) were first analysed to eliminate any possible confounding effects, before differences amongst groups with different final diagnoses were examined. Age, breed and gender effects were examined in more detail in part 2 of the study. For this, only the submissions with complete signalment data were included. For assessment of age effects, the data were assigned to five groups: <1 year, 2–5 years, 6–10 years, 11–15 years and >15 years. Group differences were initially assessed by Kruskal–Wallis ANOVA, with the Mann–Whitney U test used for post hoc analysis. Gender effects were first compared as four separate groups (male, female, male neuter, and female neuter) by Kruskal–Wallis ANOVA, and then as two groups (male [including neuter and entire] and female [including neuter and entire]) by Mann–Whitney U test. To assess the effects of breed, data for domestic shorthair and domestic longhair were combined, and compared against other breeds (by Mann–Whitney U test). Only breeds with >10 individuals were assessed. To account for any possible confounding effect of age, breed comparisons were made both prior to and after regression analysis (based on age). For all statistical tests, the level of significance was set at P<0.05.
Results
Part 1
For the first part of the study, 426 cats were seen between January 2002 and June 2003. Of these cases, 294 cats were excluded for not meeting any of the three inclusion criteria. Most notably, three cats had evidence of prior cobalamin supplementation at the referring veterinary surgeon. Therefore, 132 cats fulfilled the inclusion criteria and full details are reported in Table 1. Of these, 21/132 (16%) had alimentary system disorders, including 12 cats with gastrointestinal, three with hepatic and six with pancreatic disorders. These included four cats with alimentary lymphoma, a disorder reportedly associated with marked decreases in serum cobalamin concentrations (Simpson et al 2001). The range of cobalamin concentrations in these four cats was from 553 to 1748 pg/ml. Based on the established reference interval, none of the 132 cats had subnormal cobalamin concentrations. The overall range of concentrations was from 278 pg/ml to >2000 pg/ml (upper limit of the reference range), with a median value of 1172 pg/ml (Table 1). None of the baseline parameters had a significant confounding effect on these results. Furthermore, no differences were found amongst all groups with differing final diagnoses (P>0.05 for all). More specifically, there was no difference between cats with alimentary system disease and those with diseases in other organs (P>0.05). The two cats with the lowest cobalamin concentrations (278 pg/ml and 284 pg/ml) were finally diagnosed with hypertrophic cardiomyopathy and inflammatory bowel disease, respectively.
Table 1.
Clinical details for cases in part 1 of the study
| Alimentary tract disease | Other organ disease | |||
|---|---|---|---|---|
| GI | Liver | Pancreatic | ||
| Number | 12 | 3 | 6 | 111 |
| Gender | 11 NM, 1 NF | 1 NM, 2 NF | 3 NM, 3 NF | 67 NM, 44 NF |
| Age (years) | 8 (4–15) | 9 (2–20) | 11 (2–14) | 7 (1–18) |
| Breed | 74 DSH, 7 DLH | |||
| 9 DSH | 3 BSH, 5 Oriental | |||
| 1 Persian | 4 DSH | 2 Maine Coon | ||
| 1 Siamese | 3 DSH | 1 Oriental | 5 Siamese, 2 Birman | |
| 1 Egyptian Mau | 1 Birman | 3 Burmese, 8 Persian | ||
| 1 Bengal, 1 Ragdoll | ||||
| Final diagnosis | lymphoma (4) | Renal (11), lower urinary tract (11), respiratory (12), infectious (11), neoplasia (28), skin disease (11), neurological (4), haematological (4), cardiac (10), endocrine (5) | ||
| IBD (5) | Hepatic adenocarcinoma | |||
| Megacolon (1) | Cholangiohepatitis | Pancreatitis (5) | ||
| Pyloric stenosis (1) | Portosystemic shunt | Neoplasia (1) | ||
| Adverse food reaction (1) | ||||
| Miscellaneous (4) | ||||
| Serum cobalamin (pg/ml) | 1111 (284 to >2000) | 1872 (345 to >2000) | 676 (488 to 1530) | 1178 (278 to >2000) |
GI=gastrointestinal disease; NM=neutered male; NF=neutered female; DSH=domestic shorthair; DLH=domestic longhair; BSH=British shorthair; IBD=inflammatory bowel disease. Both age and serum cobalamin concentrations are reported as: median (range).
Part 2
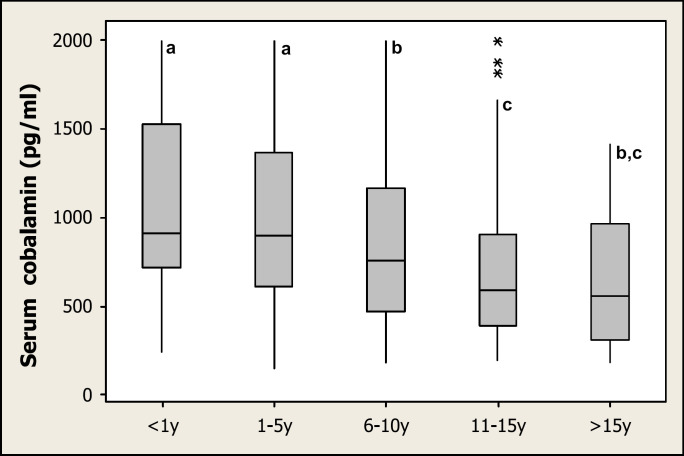
The second part of the study included 679 cats (Table 2). No gender effects were evident, but a significant age effect was noted (P<0.0001), with younger cats having significantly higher serum cobalamin concentrations than older cats (Fig 1). The age range for most breeds was significantly younger than for the ‘domestic’ cat group (Table 2). Significant breed effects were noted for Birmans, and Maine Coons, both of which had higher serum cobalamin concentrations than cats in the domestic group (DSH and DLH) (P=0.011 and P=0.0003, respectively). These effects remained after the possible compounding effect of age was excluded (P=0.0098 and P=0.004, respectively). Although a significant breed effect was also noted for Bengals on preliminary analysis (P=0.011), this effect no longer existed when the compounding effect of age was excluded (P=0.49). No other breed effects were noted.
Table 2.
Clinical details for cases in part 2 of the study
| Breed group | Number | Gender | Age (years) | Serum cobalamin concentrations (pg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | NM | F | NF | U | Median | Range | Median | Range | Number<150 | Number<290 | ||
| All cats | 682 | 99 | 226 | 89 | 160 | 108 | 6 | 0–18 | 794 | 147–2000 | 1 | 41 |
| Domestic | 336 | 43 | 115 | 48 | 96 | 34 | 9 | 0–17 | 733 | 185–2000 | 0 | 26 |
| Abyssinian | 7 | 2 | 3 | 0 | 2 | 0 | 10 | 0–13 | 860 | 589–1172 | 0 | 0 |
| Balinese | 3 | 0 | 2 | 1 | 0 | 0 | 7 | 4–10 | 866 | 488–1231 | 0 | 0 |
| Bengal | 12 | 7 | 2 | 2 | 1 | 0 | 0a | 0–14 | 1568 | 152.0–2000 | 0 | 1 |
| Birman | 14 | 3 | 5 | 1 | 5 | 0 | 4 | 1–11 | 1146b | 329–2000 | 0 | 0 |
| British Short Hair | 19 | 3 | 10 | 4 | 1 | 1 | 4c | 0–12 | 678 | 306–2000 | 0 | 0 |
| Burmese | 20 | 5 | 10 | 1 | 4 | 0 | 4b | 0–13 | 822 | 356–2000 | 0 | 0 |
| Burmilla | 1 | 0 | 1 | 0 | 0 | 0 | 4 | * | 1836 | 0 | 0 | |
| Cameo | 1 | 0 | 0 | 0 | 1 | 0 | 11 | * | 1039 | 0 | 0 | |
| Chinchilla | 3 | 2 | 1 | 0 | 0 | 0 | 10 | 3–12 | 1027 | 675–1314 | 0 | 0 |
| Devon Rex | 10 | 2 | 1 | 2 | 3 | 2 | 5c | 0–8 | 693 | 377–1624 | 0 | 0 |
| Egyptian Mau | 1 | 1 | 0 | 0 | 0 | 0 | 5 | * | 875 | 0 | 0 | |
| Maine Coon | 24 | 5 | 11 | 3 | 4 | 1 | 1.5a | 0–14 | 1069b | 355–2000 | 0 | 0 |
| Norwegian Forest | 2 | 1 | 0 | 0 | 1 | 0 | 1.5 | 0, 3 | 1344 | 880, 1808 | 0 | 0 |
| Oriental | 6 | 0 | 4 | 0 | 0 | 2 | 1 | 0–7 | 756 | 670.0–1819 | 0 | 0 |
| Persian | 53 | 9 | 15 | 7 | 14 | 8 | 2a | 0–14 | 773 | 190–2000 | 0 | 4 |
| Ragdoll | 8 | 4 | 2 | 0 | 2 | 0 | 2 | 0–13 | 1541 | 516–2000 | 0 | 0 |
| Russian Blue | 5 | 1 | 1 | 1 | 2 | 0 | 7 | 7–11 | 1562 | 355–1877 | 0 | 0 |
| Siamese | 45 | 1 | 25 | 6 | 8 | 5 | 3a | 0–14 | 788 | 209–2000 | 0 | 3 |
| Siberian Tiger | 1 | 1 | 0 | 0 | 0 | 0 | 0 | * | 358 | 0 | 0 | |
| Somali | 1 | 0 | 0 | 0 | 1 | 0 | 5 | * | 1945 | 0 | 0 | |
| Sphinx | 2 | 0 | 0 | 1 | 0 | 1 | 11 | 8,14 | 558 | 318.0, 797 | 0 | 0 |
| Tonkinese | 1 | 0 | 0 | 1 | 0 | 0 | 0 | * | 1342 | 0 | 0 | |
| Turkish | 1 | 0 | 1 | 0 | 0 | 0 | 1 | * | 1513 | 0 | 0 | |
| Unknown | 106 | 9 | 17 | 11 | 15 | 54 | 6 | 0–18 | 680 | 147.2000 | 1 | 7 |
Statistical analyses were only performed on breed groups with >10 cats. For age and cobalamin data, median results with letter subscripts are significantly different from cats in the ‘domestic’ group at P<0.001, P<0.01 and P<0.05 for subscripts a, b and c, respectively. Range is quoted for three or more individuals only; otherwise, absolute values are included.
‘Domestic’ breed group includes domestic shorthair and domestic longhair combined. M=male; NM=neutered male; F=female; NF=neutered female; U=unknown.
Fig 1.
Box and whisker plot demonstrating the effect of age on serum cobalamin concentrations in part 2 of the study. Serum cobalamin concentrations, from samples submitted to a commercial laboratory, were assigned to five age groups: <1 year=cats under 1 year of age; 1–5 years=cats between 1 and 5 years of age; 6–10 years=cats between 6 and 10 years of age; 11–15 years=cats between 11 and 15 years of age; and >15 years=cats older than 15 years of age. Horizontal bars denote median values; boxes contain all results between the first and third quartiles; the upper whisker extends to Q3+1.5[Q3−Q1]); the lower whisker extends to Q1−1.5(Q3−Q1); asterisks represent outliers. Groups with different letters are significantly different from one another (at P<0.05).
Only one cat had cobalamin concentrations (147 pg/ml) below the reference range (>150 pg/ml). None of the other cats had subnormal cobalamin concentrations (median=794 pg/ml; range: 147 to >2000 pg/ml).
Discussion
The current study had two main objectives: to assess the incidence of hypocobalaminaemia in sick cats in the United Kingdom, and to assess more specifically the incidence of subnormal cobalamin concentrations in alimentary system disorders. Two populations were used for this study, a population of cats from a referral hospital, and a population of cats from which serum had been submitted for cobalamin measurement at a commercial laboratory. The investigations were undertaken in the light of previous conflicting data on the incidence of hypocobalaminaemia, and in the light of the fact that only cats with GI disease or control cats had previously been studied in detail.
In contrast to the previous data reported from another referral population where 49/80 (61%) of cats were hypocobalaminaemic (Simpson et al 2001), not a single cat from our study population had subnormal cobalamin concentrations. There are a number of possible reasons for such a discrepancy. First, the assay used in the studies varied; the previous study used a radioassay performed with a kit that simultaneously measures serum cobalamin and folate concentrations (Dualcount solid-phase boil assay, Diagnostics Products Corporation, Los Angeles, CA). In contrast, the current work employed an RIA that had already been used in the commercial field. Second, the reference interval differed between studies, with a lower limit adopted in this work (150 pg/ml) compared with the previous study (900 pg/ml). If a reference limit of similar magnitude were used with our population, 39/132 cats (30%) would have been classified as hypocobalaminaemic. The higher magnitude reference range adopted in the Simpson et al (2001) study might reflect the reference population used, that included a significant proportion of young male cats from a single colony.
One limitation of this work is that a contemporaneous control group was not included. However, a reference range previously validated by the laboratory was used in this study (H. Evans, unpublished data). Furthermore, the range adopted in this study was broadly similar to that adopted by a commercial laboratory in the USA (290–1500 pg/ml; GI Lab, Texas A&M University). If the lower limit adopted by this laboratory were applied to our results, only two cats (278 pg/ml and 284 pg/ml) would have been marginally hypocobalaminaemic. Furthermore, previous studies have shown that tissue-level cobalamin deficiency and elevation of serum MMA only occurs in cats with severe hypocobalaminaemia (<100 pg/ml) (Williams et al 2001). Only hypocobalaminaemia of this magnitude is, therefore, likely to be associated with metabolic changes and be clinically significant. Cases with cobalamin concentrations of such magnitude were not present in either part of the current study.
Other possible explanations for the discrepancy between UK and USA cats include differences in diet, genetic make up and prior cobalamin supplementation. Unfortunately, limited specific information, on diet and genetic factors, was available for review, and thus possible effects are difficult to assess. With regard to prior cobalamin supplementation, none of the cats in part 1 of the study received such therapy. Unfortunately, for part 2 of the study, less information was available regarding prior treatment with cobalamin; however, it is unlikely that a significant number of cats received supplementation with no mention in the clinical history. Furthermore, it would be surprising for veterinary surgeons first to supplement cobalamin and then subsequently measure serum cobalamin concentrations, unless assessing response to therapy. However, no repeat samples were included in the population studied in part 2.
A final explanation for the difference between studies may be the study populations examined. In the previous study, cats with GI disease were compared with a population of healthy controls (Simpson et al 2001). Therefore, it was not clear whether the previously documented hypocobalaminaemia was unique to diseases of the alimentary system, or could instead have been a general marker of systemic illness. In contrast, the current study recruited cats without reference to the affected organ system. Therefore, the reduced incidence may partly be explained by the fact that fewer individuals with alimentary tract disorders were examined (21 cats). Nevertheless, the diseases represented in this population were broadly similar to the previous study, and four cats had alimentary tract lymphoma, previously associated with some of the lowest serum cobalamin concentrations. Therefore, we would have expected that at least some of these cats would have had low cobalamin concentrations if this biochemical abnormality were common in cats with GI disease.
Given that the first part of the study was limited by the number of cats with GI diseases, a larger population was examined in the second part which used recent serum sample submissions to a commercial laboratory for cobalamin measurement. Although clinical details were limited from this population, and the final diagnosis was not available, most samples came from cats with suspected GI disease. Furthermore, the major advantage to this approach is that it enables a larger population to be examined. The results again suggested that hypocobalaminaemia is an uncommon finding, even in cats with suspected GI disease.
Interestingly, in part 2 of the study significant age and breed effects were noted for serum cobalamin concentrations in cats. Age had a significant effect on cobalamin concentrations, with younger cats having significantly greater concentrations than older cats. This is an important finding and it may explain the wide discrepancy among the reference range for cobalamin in different laboratories, where populations of young cats are used to establish the reference range. Furthermore, Birman and Maine Coon cats had significantly higher cobalamin concentrations than the cats in the domestic group. There could be several reasons for these findings. First, it is possible that there are genuine breed and age variations in serum cobalamin concentrations in cats. However, given the lack of a contemporaneous control population in part 2 of the study, the differences might instead reflect the spectrum of GI disease in each breed. In this regard, Birman and Maine Coon cats may be less prone to suffering vitamin malabsorption concurrent with diseases of the alimentary system. Given that the Maine Coon population examined was significantly younger than the domestic group, the difference noted may simply reflect differences in age distribution. However, such an explanation could not account for the difference seen with Birman cats, because the age range for this breed was not significantly different from the domestic group. Furthermore, it is of note that other breeds also had younger populations, yet cobalamin concentrations were equivalent to those of the domestic group. To the authors' knowledge, this is the first study to document age and breed differences for serum cobalamin concentrations in cats and, whatever the reasons behind these trends, different reference ranges may be needed for different populations in the future. Further studies in this area are necessary to determine ‘normal’ serum cobalamin ranges and the use of a random population of healthy, young cats may not be appropriate for the validation of an assay.
Despite the findings of this study, cobalamin deficiency remains an important potential complication of GI disease in cats. Previous studies have documented the metabolic consequences of serum cobalamin deficiency (Ruaux et al 2001), leading to complicating clinical signs in cases with GI disease. Furthermore, there is some evidence that the cobalamin deficiency can be a complicating factor in therapy, in that cats may respond suboptimally if the deficiency is not corrected (Williams et al 2001). Nevertheless, whilst cobalamin supplementation is relatively safe and inexpensive, this study does not support the approach of parenteral supplementation in all cats with alimentary system disorders. Furthermore, unless supranormal cobalamin concentrations have specific therapeutic benefits, the findings of the current study would not support the use of parenteral cobalamin in all sick cats. Instead, this study would support an approach based on measurement of serum cobalamin concentrations and supplementation when hypocobalaminaemia is documented.
In conclusion, this study has shown that hypocobalaminaemia is an uncommon abnormality in both the populations examined, namely sick cats from a referral hospital, and cats with signs of GI disease. Further work is required to assess the incidence of serum cobalamin concentrations in populations from other countries.
Acknowledgements
The authors would like to acknowledge the staff at Cambridge Specialist Laboratory Services for their technical help with the running of the samples. Thanks are also due to Peter Taylor for excellent technical support storing surplus serum samples from our clinical cases. We are also grateful to the clinicians and support staff of the Small Animal Hospital, University of Liverpool for managing the clinical cases. We acknowledge the referring veterinary surgeons for referring all cats who participated in this study. AG lectureship is currently funded by Royal Canin.
References
- Allen R.H., Stabler S.P., Savage D.G., Lindenbaum J. Metabolic abnormalities in cobalamin and folate deficiency, FASEB Journal 7, 1993, 1344–1353. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Cotisson A., Meffre G., Bourgeay-Causse M., Augert C., Favier A., Vuillez J.P., Ville G. Comparison of three commercial kits and a microbiological assay for the determination of vitamin B12 in serum, Scandinavian Journal of Clinical Laboratory Investigation 54, 1994, 235–240. [DOI] [PubMed] [Google Scholar]
- Batt R.M., Morgan J.O. Role of serum folate and vitamin B12 concentrations in the differentiation of small intestinal abnormalities in the dog, Research in Veterinary Science 32, 1982, 17–22. [PubMed] [Google Scholar]
- Frank A., Feinstein R.E. Hepatic lipidosis associated with severe vitamin B12 deficiency recognized by liver cobalt status in three cats, Feline Practice 19, 1991, 17–20. [Google Scholar]
- Fyfe J.C., Jezyk P.F., Giger U., Patterson D.F. Inherited selective malabsorption of vitamin B12 in Giant Schnauzers, Journal of the American Animal Hospital Association 25, 1989, 533–539. [Google Scholar]
- Fyfe J.C., Giger U., Hall C.A., Jezyk P.F., Klumpp S.A., Levine J.S., Patterson D.F. Inherited selective cobalamin malabsorption and cobalamin deficiency in dogs, Pediatric Research 29, 1991, 24–31. [DOI] [PubMed] [Google Scholar]
- Fyfe J.C. Feline intrinsic factor (IF) is pancreatic in origin and mediates ileal cobalamin (CBL) absorption, Journal of Veterinary Internal Medicine 7, 1993, 133. [Google Scholar]
- Fyfe J.C. Haematology of selective intestinal cobalamin malabsorption. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Haematology, 5th edn, 2000, Lippincott Williams and Wilkins: Philadelphia, 965–970. [Google Scholar]
- Johnston K.L., Swift N.C., Forster-van Hijfte M., Rutgers H.C., Lamport A., Ballevre O., Batt R.M. Comparison of the bacteria flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease, Journal of the American Veterinary Medical Association 218, 2001, 48–51. [DOI] [PubMed] [Google Scholar]
- Perry L.A., Williams D.A., Pidgeon G.L., Boosinger T.R. Exocrine pancreatic insufficiency with associated coagulopathy in a cat, Journal of the American Animal Hospital Association 27, 1991, 109–114. [Google Scholar]
- Ruaux CG, Steiner JM, Wiliams DA. (2001) Biochemical markers of cobalamin deficiency accompanying severe hypocobalaminaemia in the cat (Abstract). Proceedings 19th ACVIM Forum, Denver, CO, USA, p. 837.
- Salvadori C., Cantile C., De Ambrogi G., Arispici M. Degenerative nyelopathy associated with cobalamin deficiency in a cat, Journal of Veterinary Medicine 50, 2003, 292. [DOI] [PubMed] [Google Scholar]
- Simpson K.W., Fyfe J., Cornetta A., Sachs A., Strauss-Ayali D., Lamb S.V., Reimers T.J. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease, Journal of Veterinary Internal Medicine 15, 2001, 26–32. [DOI] [PubMed] [Google Scholar]
- Vaden S.L., Wood P.A., Ledley F.D., Cornwell P.E., Miller R.T., Page R. Cobalamin deficiency associated with methylmalonic acidemia in a cat, Journal of the American Veterinary Medical Association 200, 1992, 1101–1103. [PubMed] [Google Scholar]
- Williams DA, Ruaux CG, Steiner JM. (2001) Biochemical and clinical responses to cobalamin supplementation in cats with gastrointestinal disease and severe hypocobalaminaemia (Abstract). Proceedings of 11th ESVIM Congress, Dublin, Ireland, pp. 52–53. [DOI] [PubMed]