Abstract
Practical relevance Accurate diagnosis of the distinct subtypes of alimentary lymphoma (AL) that occur in cats is important as there are major differences between them in clinical presentation, treatment and prognosis. Unlike intermediate- and high-grade alimentary lymphoma (I/HGAL) and large granular lymphocyte lymphoma (LGLL), which can often be diagnosed by aspiration cytology, full-thickness intestinal biopsies are usually required for the diagnosis of low-grade alimentary lymphoma (LGAL).
Clinical challenges LGAL is an increasingly recognised clinical problem and it can be challenging to differentiate from inflammatory disease. Where there is ambiguity on histology, further diagnostics (immunophenotyping and clonality analysis) may be required. The diagnosis of LGLL requires an index of suspicion as it may be missed with routine diagnostics. While cats with LGAL typically achieve durable remissions with oral prednisolone and chlorambucil, I/HGAL runs a more aggressive clinical course and requires multi-agent chemotherapeutic protocols. Information on the treatment of LGLL is limited and this form of AL has the poorest prognosis. Preliminary studies suggest that abdominal irradiation may potentially be of benefit in cats with AL and further investigations are warranted.
Evidence base The evidence supporting this review is derived from grade II, III and IV prospective studies, retrospective case series, reviews, extrapolation from other species, pathophysiological justification and the combined clinical experience of those working in the field.

Alimentary lymphoma – and its particular diagnostic challenges
As discussed in part 1, alimentary lymphoma (AL) in cats can be considered clinically as low-grade (LGAL), intermediate- or high-grade (I/HGAL) or, the less commonly identified, large granular lymphocyte lymphoma (LGLL). These diseases differ in clinical presentation, techniques required for diagnosis, response to treatment and prognosis. Here, in part 2, the benefits and limitations of different biopsy techniques are discussed. Special emphasis is given to LGAL, an increasingly recognised clinical problem, and the challenges faced when differentiating this entity from inflammatory disease. The clinician should be aware of the criteria required to diagnose LGAL, both to facilitate communication with pathologists and to optimise the environment for diagnosis of this common disease. Treatment options and prognosis for the different subtypes of AL are also reviewed.
Ultrasound-guided, fine-needle aspiration cytology
Aspiration cytology of diffusely thickened intestinal walls, as are typical of LGAL, can be technically difficult and is usually non-diagnostic. Similarly, cytology of enlarged mesenteric lymph nodes is not helpful in establishing a diagnosis of LGAL as it is not possible to distinguish well-differentiated neoplastic lymphocytes characteristic of low-grade disease from benign lymphoid hyperplasia (Figure 1). 1 Tissue biopsy is required for histological demonstration of disruption of normal lymph node architecture by the neoplastic infiltrate.
Figure 1.
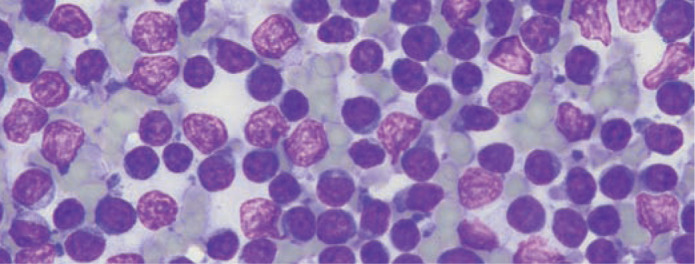
Modified Wright-Giemsa stained smear of an ultrasound-guided fine-needle aspirate from the mesenteric lymph node of a cat with low-grade alimentary lymphoma (LGAL). While there is a preponderance of small lymphocytes, it is not possible to distinguish between LGAL and benign lymphoid hyperplasia on cytology. Courtesy of Dr Patricia Martin, Veterinary Pathology Diagnostic Services, University of Sydney
This contrasts with the diagnosis of I/HGAL and LGLL, which can often be made on the basis of aspiration cytology of focal intestinal wall masses, enlarged mesenteric lymph nodes or extraintestinal mass lesions (Figure 2).2–6 This is because of the characteristic morphology of the neoplastic infiltrate (large lymphoblastic cells or large granular lymphocytes [LGLs]), which facilitates differentiation from the background population of lymphocytes. Also, modified Wright-Giemsa stains (eg, Diff Quik; Dade Shearing) used for cytological specimens are more sensitive for detecting LGLs than haematoxylin and eosin (HE) stains used for histological specimens. On cytology, LGLs are identified as large mononuclear cells with moderate amounts of deeply basophilic cytoplasm containing multiple blue or purple granules (Figure 3).5,7 On HE sections, LGLL may be erroneously reported as I/HGAL. Reliable identification of cytoplasmic granules in fixed tissues requires evaluation of Giemsa-stained, plastic-embedded tissue or immunohistochemistry to detect the cytotoxic granule protein, granzyme B.8,9
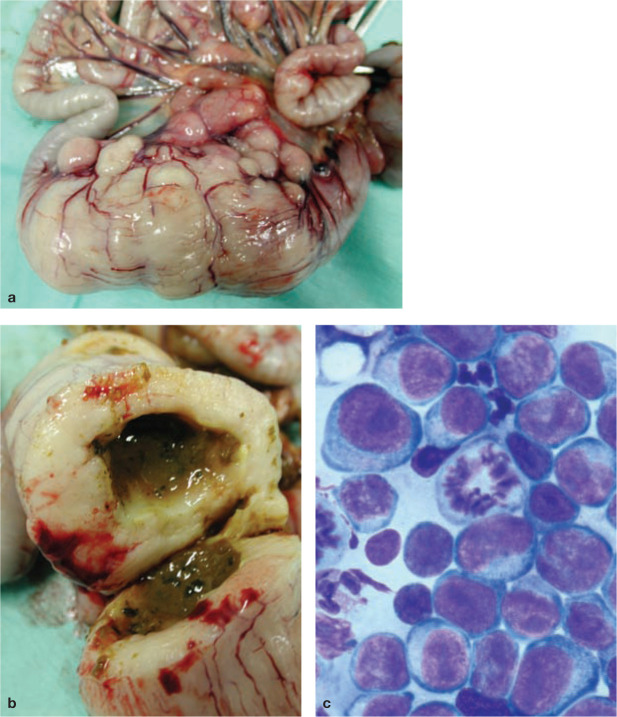
Figure 2.
(a) Ileocaecocolic high-grade alimentary lymphoma (HGAL) and (b) a cut surface from the same specimen. Diagnosis of HGAL can often be based on cytology of ultrasound-guided fine-needle aspirates from a focal intestinal mass or an enlarged mesenteric lymph node. (c) Diff Quik cytology of a concurrent abdominal effusion, showing neoplastic round cells and a mitotic figure. Image (c) courtesy of Dr Patricia Martin, Veterinary Pathology Diagnostic Services, University of Sydney
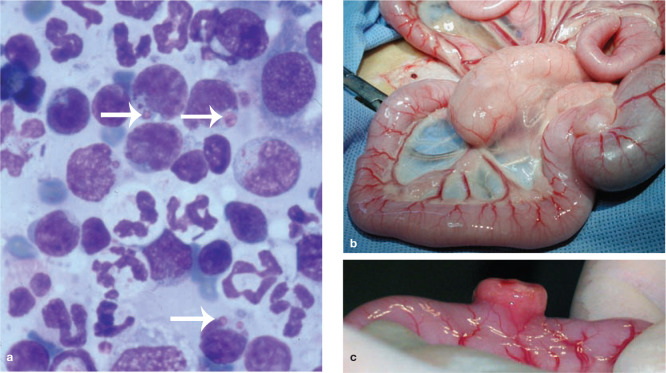
Figure 3.
(a) Diff Quik stained smear of a fine-needle aspirate biopsy from an enlarged mesenteric lymph node (b) and intestinal mass (c) in a cat with large granular lymphocyte lymphoma (LGLL). In (a) neoplastic round cells have a basophilic cytoplasm and contain large purple intracytoplasmic granules (arrows). Image (a) courtesy of Dr Patricia Martin, Veterinary Pathology Diagnostic Services, University of Sydney
Diagnostic considerations for LGAL
Intestinal biopsy procurement
Histological evaluation of intestinal biopsies is required for diagnosis of LGAL and for other forms of AL where cytological evaluation is not definitive (Figures 4 and 5). LGAL is typically a diffuse or multifocal disease affecting more than one region of the gastrointestinal tract. There is jejunal and ileal involvement in over 90% of cases, duodenal involvement in over 70% and gastric involvement in 7–40% of cases.1,10,11 The distribution of gastrointestinal involvement in lymphoplasmacytic enteritis (LPE) is similar to LGAL except that gastric involvement is more common in LPE. 11
Figure 4.
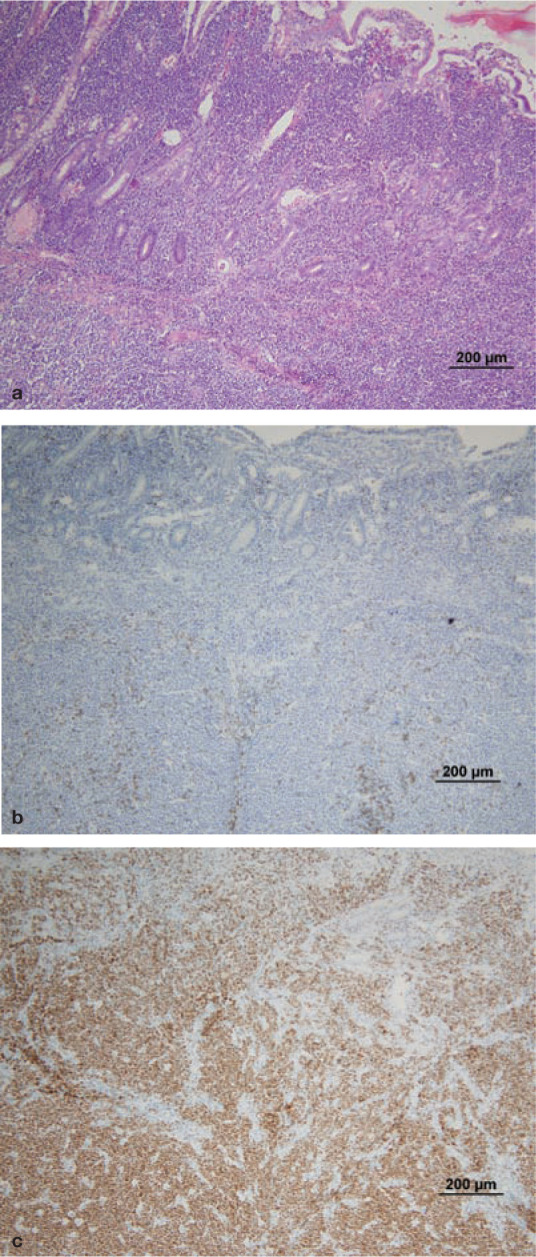
Histology and immunohistochemistry of intestinal wall biopsies from a cat with B cell HGAL. Heavy infiltration of the lamina propria and submucosa can be seen, destroying the normal architecture of the intestine. Villous and crypt architecture is also severely altered. (a) HE stain; (b) CD3 (T cell marker) stain; (c) CD79a (B cell marker) stain. Note the heavy staining of the neoplastic B cell infiltrate. Courtesy of Associate Professor Mark Krockenberger (image a) and Dr Katherine Briscoe (images b and c), University of Sydney
Figure 5.
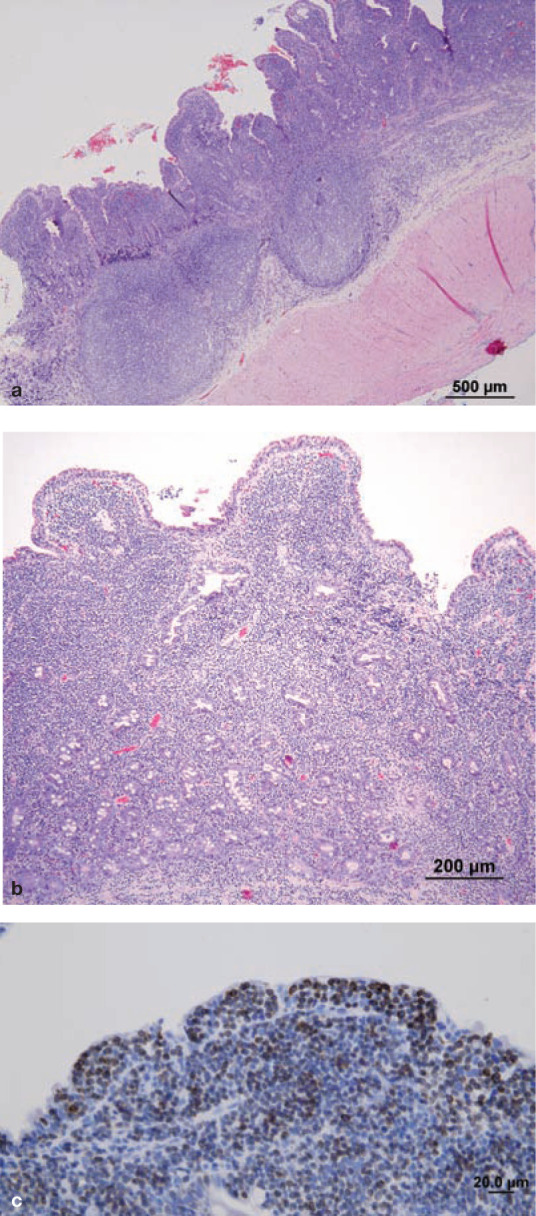
T cell LGAL. (a,b) HE-stained section of full-thickness intestinal biopsy. There is severe villous blunting and distension. The lamina propria and submucosa are diffusely infiltrated with monomorphous sheets of small lymphocytes. Intraepithelial lymphocytes (IELs) can be difficult to identify in HE-stained sections, but are easily identified in CD3-stained sections (c). This pattern of epitheliotropism with aggregates of neoplastic IELs is common in LGAL and facilitates differentiation from LPE. Courtesy of Associate Professor Mark Krockenberger (image a) and Dr Katherine Briscoe (images b and c), University of Sydney
Given the propensity of LGAL to invade the distal small intestine, if only the stomach and duodenum are biopsied using gastroduodenoscopy, a diagnosis of LGAL could be missed. The current recommendation for endoscopic investigation of enteropathies is to sample both the duodenum and ileum, necessitating both gastroduodenoscopy and ileocolonoscopy. 12 Endoscopic biopsy (EB) of the jejunum is not possible using conventional push endoscopy in cats. Adaptation of double-balloon enteroscopic techniques used in humans, as reported in dogs, would facilitate this procedure. 13 At laparotomy, the standard intestinal regions sampled are duodenum descendens, mid-jejunum and ileum. 14 Laparoscopic intestinal biopsies are usually single site, from the jejunum (Table 1). 15 The utility of this biopsy technique for the diagnosis of LGAL in cats has not been evaluated. Intestinal biopsies obtained at exploratory laparotomy or laparoscopically are full thickness, while optimal EBs include intestinal mucosa and submucosa.
Table 1.
Comparison of full-thickness with partial-thickness intestinal biopsies for diagnosis of LGAL
| Full-thickness biopsy | Partial-thickness biopsy | ||
|---|---|---|---|
| (laparotomy) | (laparoscopy) | (endoscopy) | |
| Intestinal wall layers sampled | Mucosa, submucosa, muscularis, serosa | Mucosa, submucosa, muscularis, serosa | Mucosa and submucosa only |
| Assessment of other organs | Visual inspection of serosal surface of gastrointestinal tract (GIT) and inspection/biopsy of other abdominal organs | Visual inspection of serosal surface of GIT and inspection/biopsy of other abdominal organs | Visual assessment of mucosal surface of GIT only |
| Degree of invasiveness | Highest; longer hospital stays | Intermediate | Lowest; shorter hospital stays |
| Gastrointestinal regions accessed | All | All regions, but only jejunum biopsied routinely | Stomach, duodenum (gastroduodenoscopy) Colon, ileum (colonoscopy) Jejunum is not accessible |
| Requirements for timing of chemotherapy | Delay (7 days) required because of risk of intestinal dehiscence during postoperative wound healing | No delay required | |
| Operator skill | Advanced training not required | Advanced training required | Advanced training required to reliably access duodenum and ileum to obtain good quality biopsies. Diagnosis may not be possible if biopsies are suboptimal |
| Pathologist skill and interpretation | Full-thickness biopsies are more likely to be oriented in the correct plane than endoscopic biopsies, aiding interpretation. Less subject to artefact | Poor quality biopsies hamper interpretation. Greater level of pathologist expertise required for correct interpretation of poor quality endoscopic biopsies | |
While EBs are minimally invasive, diagnosis of LGAL by EB requires significant expertise on the part of both endoscopist and pathologist, as well as appropriate laboratory tissue processing (Table 1). Full-thickness biopsy should be considered when these factors are not optimal. In one small series, histological evaluation of full-thickness biopsies of the gastrointestinal tract was found to be more sensitive than EBs procured by gastroduodenoscopy for the diagnosis of LGAL. 10 However, in that study, technical difficulties may have hampered the quality of EB specimens, as some cats underwent only ‘partial’ duodenal assessment or blind duodenal biopsy. The quality of EB samples has a profound effect on their sensitivity for identifying certain lesions. 16 Substantially fewer biopsy samples are needed to establish a diagnosis as the quality of the tissue increases. It has been shown that if six ‘marginal’ or ‘adequate’ quality duodenal or gastric EBs are taken, as defined by the presence of at least one villus and subvillus lamina propria, correct histological diagnosis is very likely to be achieved. 16 Optimal histological processing, including biopsy orientation, positioning and staining, is also essential for correct interpretation. 17
Further studies are required to compare the results of ileal EB and full-thickness biopsy specimens in the diagnosis of LGAL in cats.
Histological features
In contrast to higher grades of AL, the histological diagnosis of LGAL is not straightforward as neoplastic infiltrates of small lymphocytes are often morphologically indistinguishable from those present in the gastrointestinal tract of healthy cats or, in particular, cats with LPE. In a histopathological review of LPE and LGAL, there was disparity in diagnosis between two pathologists for 8/12 cases of moderate to marked LPE, requiring assessment by a third pathologist to reach a consensus diagnosis. 11 In another study, the use of adjunctive immunophenotyping and analysis of clonality of lymphoid infiltrates by polymerase chain reaction (PCR) resulted in 10/19 cases diagnosed as inflammatory on histological evaluation of HE sections being reclassified as T cell lymphoma, while three cases of T cell lymphoma were reclassified as inflammatory. 18
Histological criteria distinguishing LGAL from LPE include, in the former, the relative absence of mixed lymphoid and granulocytic cells and their replacement with monomorphous sheets of neoplastic lymphoid cells involving the lamina propria. In early disease, neoplastic cells form lamina propria ‘patches’, which are discrete regions of lymphocytic infiltration within some villi but not others.8,19–23 As disease progresses, lamina propria ‘bands’ of lymphocytes spanning the crypt–villous junction are seen, followed by ‘villous laminal propria obliteration’ by dense, monomorphic, lymphocyte infiltrates. In the most severe lesions ‘villous and crypt laminal propria obliteration’ occurs due to complete lymphocytic infiltration and formation of a band of lymphocytes beneath the crypt epithelium but above the muscularis mucosae. 8
Epitheliotropism, characterised by increased numbers of IELs, is a feature of both LGAL and LPE.11,12,18,24–26 However, particular patterns of epitheliotropism, including the formation of ‘nests’ (≥5 clustered IELs) or ‘plaques’ (≥5 adjacent epithelial cells overrun by IELs) in the villous or crypt epithelium, are highly specific for LGAL.8,11,18,20,23,27,28 Other histological features of LGAL that help to differentiate this disease from LPE include extension of the lymphocytic infiltrate into layers deep to the mucosa,11,18 more severe disruption to villous and crypt architecture (Figure 5), 11 intravascular lymphocytic infiltrates 18 and the presence of neoplastic cellular infiltrates in mesenteric lymph nodes.19,22,29

Immunophenotype
Immunophenotyping assists in the differentiation of LGAL from LPE. A monomorphic lymphocytic population supports a diagnosis of lymphoma, while a mixed lymphocytic population supports a diagnosis of inflammation. Of 32 cats diagnosed with lymphoma based on HE-stained sections, immunohistochemical stains revealed that, in five cats, the ‘neoplastic’ infiltrate was composed of a mixed population of small B and T lymphocytes and plasma cells. On this basis, all five cases were reclassified as having inflammatory enteropathies. 26 Importantly, LGAL cannot be diagnosed on the basis of T cell phenotype alone, since expansion of T cell populations in intestinal mucosal-associated lymphoid tissue can occur in inflammatory intestinal disease in cats.11,18,30 An additional feature of CD3-stained intestinal sections is that IELs can be more clearly visualised than in HE-stained sections.
Clonality testing
Determination of clonality of T cell populations in lymphocytic intestinal infiltrates is a useful diagnostic tool when the distinction between LGAL and LPE remains ambiguous after histological evaluation and immunophenotyping.8,18,30,31 The clonality of infiltrates of T lymphocytes in intestinal sections can be determined by assessment of T cell receptor gamma (TCRG) V–J junctional diversity.18,30–32 Similarly, B cell clonality can be determined by PCR of immunoglobulin heavy chain (IgH) variable regions.32–34
During development in the thymus, T cells rearrange their antigen receptor genes TCRA, TCRB, TCRG and TCRD to form two lineages, αβ and δγ T cells. Most αβ T cells rearrange TCRG before rearrangement of TCRA and TCRB. The TCRD gene is deleted from the genome during rearrangement of the TCRA gene. Thus, TCRG gene rearrangements occur in both αβ and δγ T lymphocytes. During this process the V-domains are somatically rearranged in a process called V–J recombination, where the V-region is randomly and imprecisely joined to the J-region. Random nucleotides are added at the joining sites, further enhancing diversity and length polymorphism. This length polymorphism can be visualised by conventional PCR of the resultant hypervariable region of the V-domain, known as the complementarity determining region 3 (CDR3), using primers directed against relatively conserved framework regions. Amplified products are analysed using heteroduplex gel electrophoresis and clonality is determined by the number and size of the bands in duplicate samples run side by side.
Clonal lymphocyte populations produce one or two sharp bands that are consistent in duplicate samples; oligoclonal populations produce three bands; while polyclonal populations produce a broad band, smear or ladder of bands. Pseudoclonal populations contain one or two bands that are of different sizes or are non-reproducible in duplicate analyses. Neoplastic populations of T lymphocytes are clonal or oligoclonal, while inflammatory populations are polyclonal. 30
In three studies, determination of lymphocyte clonality by PCR was 78–90% sensitive in the detection of intestinal T cell lymphoma, where clonal or oligoclonal T cell populations were considered to be neoplastic.8,30,31 In one of these studies, 22/28 cats were found to have clonal rearrangements of the TCRG gene, while three had oligoclonal rearrangements. In comparison, polyclonal rearrangements were detected in 3/3 cats with normal intestinal histology and in 9/9 cats with LPE. 30
Performing concurrent T and B cell clonality analysis can increase the sensitivity of detection of T cell lymphomas due to cross-lineage IgH gene rearrangements.18,32 As clonality is not always specific for malignancy, this technique cannot be used as a stand-alone diagnostic test for LGAL. A diagnostic approach that combines the assessment of histological features, immunophenotype and clonality analysis is optimal to distinguish LGAL from LPE, especially where EBs are submitted. 18 Lymphocyte lineage should be based on immunophenotypic assessment rather than clonality determination by PCR if the results of these two techniques are divergent. 30
Chemotherapy protocols and response to therapy
LGAL
Most cats with LGAL show an excellent response to treatment with oral, slow-alkylating agents and prednisolone.1,27,43,44 Combinations of oral prednisolone and chlorambucil used for induction, and median ST or remissions achieved, are listed in Table 2.1,18,27,43,44 Complete remission (CR), defined as complete resolution of clinical signs for ≥30 days, occurred in 70% of cases of LGAL in two studies.1,27 For cats achieving CR, median durations of remission of 26 and 29 months are reported.43,44 In these studies, since the assignation of anything less than a CR was considered arbitrary due to insufficient data, any cats with a partial response (PR) were included in the ‘no response’ category. In contrast, another report found a lower CR rate of 56%. 43 A key difference in this study was that a third (partial) response category was included – defined as >50% but less than 100% response. However, 95% of the 41 cats achieved either CR or PR with median remission durations of 29 and 14 months, respectively. This would be considered a good outcome, even for cats with PR.
Table 2.
Remission and survival data, and induction chemotherapy protocols for cats with LGAL
| Study | n | % CR | MST (months) | MST* or MDR† for cats in CR (months) | Prednisolone | Chlorambucil |
|---|---|---|---|---|---|---|
| Fondacaro et al (1999) 27 | 29 | 69 | 17 | 23* | 10 mg/cat PO q24h | 15 mg/m2 PO q24h for 4 d q3wk |
| Kiselow et al (2008) 43 | 25 | 36 | 25 | 29† | 5 mg/cat PO q12–24h | 2 mg/cat PO q48h |
| Lingard et al (2009) 1 | 17 | 76 | 15 | 19* | 3 mg/kg PO q24h | 15 mg/m2 PO q24h for 4 d q3wk |
| Stein et al (2010) 44 | 28 | 96 | NS | 26† | 2 mg/kg PO q24h | 20 mg/m2 PO q2wk |
| Kiupel et al (2011) 18 | 12 | NS | 25 | NS | NS | NS |
n = number of cats, CR = complete remission, NS = not specified, MST = median survival time, MDR = median duration of remission, d = day, wk = week
Reported doses of prednisolone to maintain remission are 5 mg q24h PO or 1–3 mg/kg q48h PO.1,27,43 Cyclophosphamide (10 mg/kg PO every 3–4 weeks, 225 mg/m 2 PO every 3 weeks or 200–250 mg/m 2 PO given on days 1 and 3, every 2 weeks) in combination with prednisolone at induction or maintenance doses has been used as a rescue protocol for cats that come out of clinical remission.27,44,48 The median ST of 12 cats in which remission was re-induced using cyclophosphamide was 29 months, while for four other cats the median duration of second remission was 9 months.27,44
I/HGAL
There is little precise information regarding remission rates and treatment responses for I/HGAL since, in many studies, all anatomical forms (mediastinal, multicentric, alimentary and extranodal) are considered as a single group for analysis. A median ST of 7–10 months is expected with chemotherapy protocols that include doxorubicin.3,37,38,40,42,45 In a series of 28 cats with AL treated with a COP protocol, of which 25 cases were HGAL, the median ST was 50 days. 4 For cats achieving CR (32%), the median remission time was 7 months. In another report of 21 cats with AL, where cytological and ultrasonographic findings were consistent with HGAL in most cases, treatment with a CHOP-based protocol (COP plus doxorubicin [hydroxydaunorubicin]) gave a median ST of 9 months and 38% of cats achieved CR. 3 Surgical resection of an intestinal mass prior to chemotherapy has not been demonstrated to improve survival compared with chemotherapy alone.4,49
Traditionally, CHOP-based chemotherapy protocols have involved 1 or more years of chemotherapy.42,45,50 In one study, COP and doxorubicin for a total of 6 months gave median remission times of 9.5 months compared with 3 months for cats treated with COP alone. 38 It was postulated that shorter CHOP-based protocols (6 months) are adequate for the treatment of feline lymphoma. 38 An example of a currently recommended 25-week CHOP-based protocol is given in Table 3. 51 In COP-treated cats, doxorubicin was shown not to be an effective rescue protocol for I/HGAL. 52 Other rescue protocols (eg, CCNU, MOPP) are generally associated with a 40–50% response rate and a short median duration of second remission of 1.5–2 months. 51
Table 3.
CHOP-based chemotherapy protocol for cats with lymphoma 52
| Week | Drug, dosage and route of administration* |
|---|---|
| 1 | Vincristine 0.5–0.7 mg/m2 IVM L-asparaginase 400 IU SC Prednisolone 2 mg/kg PO |
| 2 | Cyclophosphamide 200 mg/m2 IV Prednisolone 2 mg/kg PO |
| 3 | Vincristine 0.5–0.7 mg/m2 IV Prednisolone 1 mg/kg PO |
| 4 | Doxorubicin 25 mg/m2 IV Prednisolone 1 mg/kg PO† |
| 6 | Vincristine 0.5–0.7 mg/m2 IV |
| 7 | Cyclophosphamide 200 mg/m2 IV |
| 8 | Vincristine 0.5–0.7 mg/m2 IV |
| 9 | Doxorubicin 25 mg/m2 IV |
| 11 | Vincristine 0.5–0.7 mg/m2 IV |
| 13 | Cyclophosphamide 200 mg/m2 IV |
| 15 | Vincristine 0.5–0.7 mg/m2 IV |
| 17 | Doxorubicin 25 mg/m2 IV |
| 19 | Vincristine 0.5–0.7 mg/m2 IV |
| 21 | Cyclophosphamide 200 mg/m2 IV |
| 23 | Vincristine 0.5–0.7 mg/m2 IV |
| 25 | Doxorubicin 25 mg/m2 IV |
A complete blood count (CBC) should be performed before each chemotherapy treatment. If the neutrophil count is <1500 cells/µl, delay chemotherapy for 5–7 days, repeat a CBC and, if the neutrophil count has risen above 1500 cells/µl, administer the drug
Prednisolone is continued at 1 mg/kg q48h PO from this point forward
LGLL
LGLL has the poorest prognosis of all forms of AL and, from limited reports, appears to be minimally responsive to standard chemotherapy protocols. Of 24 cats receiving combination chemotherapy (COP [n = 20], CHOP [n = 4]), the overall response rate was 30% (one CR, six partial remission) with a median ST of only 45 days. 6
Radiation protocols and response to therapy
Targeted abdominal irradiation has recently been evaluated for the treatment of feline AL.53,54 In a pilot study, eight cats with I/HGAL (n = 5) or multicentric lymphoma confined to the abdomen (n = 3), which were assessed to be in remission following treatment with a 6-week CHOP-based chemotherapy protocol, received whole-abdomen radiation therapy in 10 daily fractions of 1.5 Gy. 53 Two cats relapsed following treatment, one of which was subsequently diagnosed with LGLL, and another was euthanased for unrelated reasons. The other five cats were all in remission at the time of publication, with durations ranging from >266 to >1332 days. In a retrospective study, 10/11 cats with AL (six LGAL, four I/HGAL, one LGLL) responded to whole-abdomen radiation (8 Gy over 2 days) as a rescue therapy. Median ST overall was 355 days and median ST post-radiation therapy was 214 days. Radiation therapy was well tolerated in both studies.
Further investigations of the use of abdominal radiation for the treatment of feline AL are warranted.
Supportive treatment
For stabilisation of cats presenting acutely with AL, intravenous fluid therapy, blood products, antimicrobials and antiemetics may be required. Many cats with AL have a low body condition score and are inappetent or anorexic at presentation, necessitating provision of enteral or parenteral nutrition. Consideration should be given to placement of oesophagostomy or gastrostomy tubes during general anaesthesia for intestinal biopsies. In the postoperative period, appetite stimulants such as mirtazapine or cyproheptadine may be beneficial until glucocorticoid therapy can be initiated. When gastrointestinal ulceration is identified or suspected, treatment with proton-pump inhibitors (eg, omeprazole) or H2-antagonists (eg, ranitidine or famotidine) and mucosal protectants (eg, sucralfate) is indicated.
A further consideration is dietary modification for treatment of concurrent LPE or dietary intolerance. The diet should be gluten-free and contain a novel protein component that is single source or hydrolysed. 55 The carbohydrate component should also be single source and highly digestible (eg, cooked rice). Although evidence of efficacy is currently lacking, use of prebiotics and probiotics may be considered. Folate deficiency should be treated with oral folic acid supplementation. Parenteral cobalamin supplementation is indicated if the serum cobalamin concentration is subnormal (see Case Notes). Because cobalamin deficiency itself can result in inflammatory infiltration of the gastrointestinal mucosa and villous atrophy, the response to chemotherapy may be suboptimal in cats with untreated hypocobalaminaemia. 56 In cats showing relapse of clinical signs, serum cobalamin concentration should also be measured routinely.
Key points
While I/HGAL can often be diagnosed by aspirate cytology, the diagnosis of LGAL requires histological evaluation of intestinal biopsies. Immunophenotyping is a useful adjunctive technique for diagnosis.
In cases where the distinction between LGAL and LPE remains ambiguous, PCR assays for lymphocyte receptor gene rearrangements can provide information on the clonality of the infiltrate.
I/HGAL runs an aggressive clinical course and multi-agent chemotherapy is recommended. Cats with LGAL typically present with chronic signs and achieve durable remissions with oral prednisolone and chlorambucil.
Less is known about LGLL although it seems to carry a poor prognosis. It is important to maintain an index of suspicion for LGLL as it may not be identified on routine diagnostics.
Case notes

Sooty, an 11-year-old male neutered domestic longhair cat, was presented with a history of weight loss and chronic vomiting of 10 months duration.
History In the preceding 2 months, the frequency of vomiting had increased from once a fortnight to twice weekly. There had been no response to a diet change from commercial tinned food to a hypoallergenic, hydrolysed protein diet (Hill’s z/d). Sooty’s bodyweight had decreased to 4.3 kg from 4.96 kg 6 months prior. Appetite had been normal until a week before presentation when Sooty became inappetent. Stool composition and frequency were normal. Oral milbemycin had been administered every 3 months for routine treatment of gastrointestinal parasites.
Physical examination Abnormalities noted on physical examination were tacky mucous membranes, reduced skin turgor, a low body condition score (2/5), mild enlargement of the thyroid glands bilaterally and thickened small intestinal loops. Heart rate (184 bpm), respiratory rate (32/min) and rectal temperature (38.2°C) were normal.
Clinical investigations A complete blood count and serum biochemistry profile identified mild prerenal azotaemia (urea 12.8 mmol/l, reference interval [RI] 7.2–10.7 mmol/l; creatinine 169 µmol/l, RI 90–190 µmol/l; USG >1.050) and mild hyperproteinaemia (total plasma protein 80 g/l, RI 54–78 g/l), which was attributed to dehydration. Total serum T4 was normal (26 nmol/l, RI 5–52 nmol/l). Serum cobalamin was decreased (197 ng/l, RI 290–1500 ng/l) and folate was normal (14 µg/l, RI 10–22 µg/l). On abdominal ultrasonography mesenteric lymph nodes were rounded, hypoechoic and mildly enlarged (Figure 6), while jejunal and ileal wall thicknesses were increased, ranging from 3.6–4.2 mm (Figure 7). Intestinal wall layering was normal. Thoracic radiographs were unremarkable.
Figure 6.
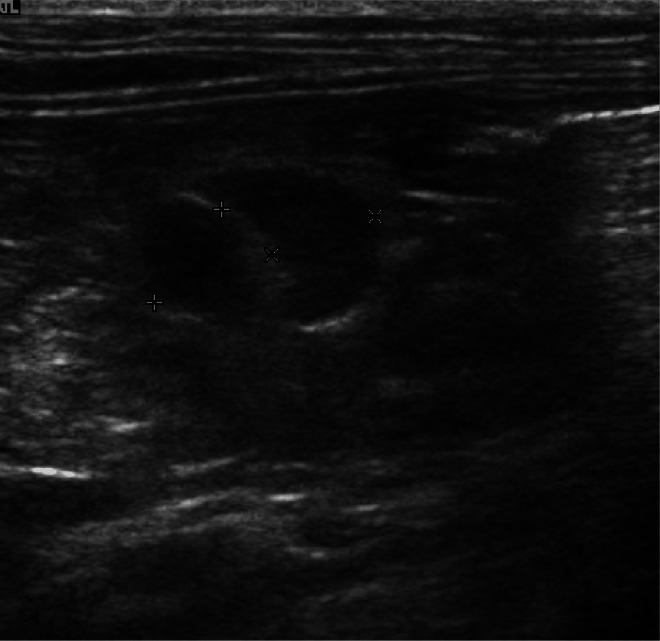
Rounded, hypoechoic and mildly enlarged (6.3 and 6.5 mm diameter) mesenteric lymph nodes
Figure 7.
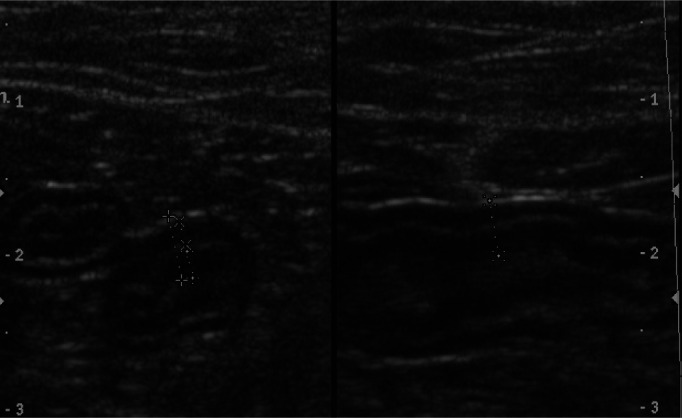
Thickened jejunal and ileal walls
Cytology of an ultrasound-guided mesenteric lymph node aspirate revealed 92% small lymphocytes, 5% medium lymphoid cells, 2% neutrophils, 1% plasma cells and occasional large lymphoid cells. At laparotomy, the ileum and distal jejunum were diffusely thickened and biopsies were obtained from the stomach, duodenum, jejunum, ileum, liver, mesenteric lymph nodes and pancreas. An oesophagostomy tube was placed for enteral nutritional support during the postoperative period.
On histology of the jejunum and ileum, abnormalities in villous architecture, including widening of the base, shortening and fusion, were noted. The lamina propria of the villi and crypts was distended by an infiltrate of large numbers of small lymphocytes arranged in sheets. The infiltrate showed positive staining with CD3 and no staining with CD79a. Clusters of IELs were identified in the villous epithelium on CD3-stained sections. In the lymph node, there was an expanded paracortical zone comprising small lymphocytes that stained positively for CD3. The liver and pancreas were normal.
• WHAT IS YOUR ASSESSMENT?
1 What does the cytology of the mesenteric lymph node suggest?
(a) LGAL with metastasis to the lymph node.
(b) Benign lymphoid hyperplasia.
(c) HGAL (given the presence of occasional large lymphoid cells).
(d) Answers (a), (b) or (c) are equally likely.
(e) It could be (a) or (b).
2 In cases where differentiation between LPE and LGAL cannot be made on routine HE-stained intestinal sections, what further diagnostic approach would you recommend?
(a) Cytology of a repeat ultrasound-guided fine-needle aspirate of the enlarged mesenteric lymph node.
(b) Immunohistochemistry of intestinal biopsy sections using markers for CD3 and CD79a.
(c) PCR of intestinal biopsies for detection of T cell and B cell clonality.
(d) No further diagnostic tests are necessary – a therapeutic trial with prednisolone is indicated as LPE and LGAL are treated the same way.
(e) Approach (b) interpreted together with the HE-stained intestinal biopsy sections. If still not diagnostic, proceed to (c).
Sooty was diagnosed with LGAL (intestinal T cell lymphoma).
Treatment protocol The following treatment was initiated:
Cobalamin supplementation was commenced (250 µg subcutaneously once weekly for 6 weeks, then fortnightly for 6 weeks, then monthly).
Seven days postoperatively, treatment was commenced with prednisolone (7.5 mg q12h PO) and chlorambucil (15 mg/m2 q24h for 4 consecutive days every 3 weeks PO).
Response and outcome At a recheck examination 3 weeks after discharge, Sooty had gained 200 g in body weight, had an improved appetite and no further episodes of vomiting were recorded. A complete blood count was unremarkable. Over the next 6 months Sooty’s weight increased to 5.2 kg. The prednisolone dose was gradually tapered to 7.5 mg q24h and the frequency of chlorambucil administration was decreased to 15 mg/m 2 q24h for 4 consecutive days every month.
Sooty remained in remission for 19 months before vomiting and weight loss recurred. The results of clinical investigations were consistent with relapse of LGAL. A rescue chemotherapy protocol was commenced comprising 15 mg prednisolone q24h PO and 50 mg cyclophosphamide once every 3 weeks. Sooty achieved clinical remission for a further 11 months before relapsing, at which point euthanasia was performed.
Answers 1 (e), 2 (e)
Funding
The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this review article.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Lingard AE, Briscoe K, Beatty JA, Moore AS, Crowley AM, Krockenberger M, et al. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg 2009; 11: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wellman M, Hammer AS, DiBartola SP, Carothers MA, Kociba GJ, Rojko JL. Lymphoma involving large granular lymphocytes in cats: 11 cases (1982–1991). J Am Vet Med Assoc 1992; 201: 1265–1269. [PubMed] [Google Scholar]
- 3. Zwahlen CH, Lucroy MD, Kraegel SA, Madewell BR. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). J Am Vet Med Assoc 1998; 213: 1144–1149. [PubMed] [Google Scholar]
- 4. Mahony OM, Moore AS, Cotter SM, Engler SJ, Brown D, Penninck DG. Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Assoc 1995; 207: 1593–1597. [PubMed] [Google Scholar]
- 5. Franks PT, Harvey JW, Mays MC, Senior DF, Bowen DJ, Hall BJ. Feline large granular lymphoma. Vet Pathol 1986; 23: 200–202. [DOI] [PubMed] [Google Scholar]
- 6. Krick EL, Little L, Patel R, Shofer FS, Sorenmo K, Clifford CA, et al. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996–2004). Vet Comp Oncol 2008; 6: 102–110. [DOI] [PubMed] [Google Scholar]
- 7. Roccabianca P, Vernau W, Caniatti M, Moore PF. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8 alpha alpha phenotype. Vet Pathol 2006; 43: 15–28. [DOI] [PubMed] [Google Scholar]
- 8. Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype and molecular clonality. Vet Pathol. Epub ahead of print 19 April 2011. PMID: 21505197. [DOI] [PubMed] [Google Scholar]
- 9. Darbes J, Majzoub M, Breuer W, Hermanns W. Large granular lymphocyte leukemia/lymphoma in six cats. Vet Pathol 1998; 35: 370–379. [DOI] [PubMed] [Google Scholar]
- 10. Evans SE, Bonczynski JJ, Broussard JD, Han E, Baer KE. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 11. Briscoe KA, Krockenberger M, Beatty JA, Crowley A, Dennis MM, Canfield PJ, et al. Histopathological and immunohistochemical evaluation of 53 cases of feline lymphoplasmacytic enteritis and low-grade alimentary lymphoma. J Comp Pathol 2011; 145: 187–198. [DOI] [PubMed] [Google Scholar]
- 12. The WSAVA International Gastrointestinal Standardization Group, Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010; 24: 10–26. [DOI] [PubMed] [Google Scholar]
- 13. Lopez Albors O, Rojo D, Sarria R, Soria F, Perez Cuadrado E, Latorre R. Morphometry of the canine intestine with reference to the use of double balloon endoscopy. Vet J 2011; 190: 113–118. [DOI] [PubMed] [Google Scholar]
- 14. Kleinschmidt S, Harder J, Nolte I, Marsilio S, Hewicker-Trautwein M. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J Feline Med Surg 2010; 12: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Washabau RJ, Day MJ, Willard MD. Response letter to Dr JA Impellizeri [letter]. J Vet Intern Med 2010; 24: 456.20565567 [Google Scholar]
- 16. Willard MD, Mansell J, Fosgate GT, Gualtieri M, Olivero D, Lecoindre P, et al. Effect of sample quality on the sensitivity of endoscopic biopsy for detecting gastric and duodenal lesions in dogs and cats. J Vet Intern Med 2008; 22: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 17. Willard MD, Moore GE, Denton BD, Day MJ, Mansell J, Bilzer T, et al. Effect of tissue processing on assessment of endoscopic intestinal biopsies in dogs and cats. J Vet Intern Med 2010; 24: 84–89. [DOI] [PubMed] [Google Scholar]
- 18. Kiupel M, Smedley RC, Pfent C, Xie Y, Xue Y, Wise AG, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline intestinal biopsy specimens. Vet Pathol 2011; 48: 212–222. [DOI] [PubMed] [Google Scholar]
- 19. Brown CC. Alimentary system. In: Maxie MG. (ed). Jubb, Kennedy, and Palmer’s pathology of domestic animals. 5th ed. Oxford; Saunders Elsevier, 2007, pp 124–125. [Google Scholar]
- 20. Valli VE, Jacobs RM, Parodi AL, Vernau W, Moore PF. Tumors of the lymphoid system. 2nd ed. Washington, DC: Armed Forces Institute of Pathology, Washington DC, 2002. [Google Scholar]
- 21. Valli VE. Enteropathy-type T-cell lymphoma. In: Valli VE. (ed). Veterinary comparative hematopathology. Ames: Blackwell Publishing, 2007, pp 318–327. [Google Scholar]
- 22. Valli VE, Jacobs RM, Norris A, Couto CG, Morrison WB, McCaw D, et al. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest 2000; 12: 295–306. [DOI] [PubMed] [Google Scholar]
- 23. Richter KP. Feline gastrointestinal lymphoma. Vet Clin North Am Small Anim Pract 2003; 33: 1083–1098. [DOI] [PubMed] [Google Scholar]
- 24. Hart JR, Shaker E, Patnaik E, Garvey MS. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988–1990). J Am Anim Hosp Assoc 1994; 30: 505–514. [Google Scholar]
- 25. Day MJ, Bilzer T, Mansell J, Wilcock B, Hall EJ, Jergens A, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008; 138 Suppl 1: S1–S43. [DOI] [PubMed] [Google Scholar]
- 26. Waly NE, Gruffydd-Jones TJ, Stokes CR, Day MJ. Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. J Comp Pathol 2005; 133: 253–260. [DOI] [PubMed] [Google Scholar]
- 27. Fondacaro JV, Richter KP, Carpenter JL, Hart JR, Hill SL, Fettman MJ. Feline gastrointestinal lymphoma: 67 cases (1988–1996). Eur J Comp Gastroenterol 1999; 4: 5–11. [Google Scholar]
- 28. Carreras JK, Goldschmidt M, Lamb M, McLear RC, Drobatz KJ, Sorenmo KU. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000). J Vet Intern Med 2003; 17: 326–331. [DOI] [PubMed] [Google Scholar]
- 29. Ben-Ezra J. Small lymphocytic lymphoma. In: Knowles D, Danile M. (eds). Neoplastic hematopathology. Philadelphia: Lippincott Williams & Wilkins, 2001, pp 773–787. [Google Scholar]
- 30. Moore PF, Woo JC, Vernau W, Kosten S, Graham PS. Characterization of feline T-cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T-cell lymphoma. Vet Immunol Immunopathol 2005; 106: 167–178. [DOI] [PubMed] [Google Scholar]
- 31. Weiss AT, Klopfeisch R, Gruber AD. T-cell receptor gamma chain variable and joining region genes of subgroup 1 are clonally rearranged in feline B- and T-cell lymphoma. J Comp Pathol 2011; 144: 123–134. [DOI] [PubMed] [Google Scholar]
- 32. Sato H, Fujino Y, Uchida K, Ohno K, Nakayama H, Tsujimoto H. Comparison between immunohistochemistry and genetic clonality analysis for cellular lineage determination in feline lymphomas. J Vet Med Sci 2011; 73: 945–947. [DOI] [PubMed] [Google Scholar]
- 33. Werner JA, Woo JC, Vernau W, Graham PS, Grahn RA, Lyons LA, et al. Characterization of feline immunoglobulin heavy chain variable region genes for the molecular diagnosis of B-cell neoplasia. Vet Pathol 2005; 42: 596–607. [DOI] [PubMed] [Google Scholar]
- 34. Henrich M, Hecht W, Weiss AT, Reinacher M. A new subgroup of immunoglobulin heavy chain variable region genes for the assessment of clonality in feline B-cell lymphomas. Vet Immunol Immunopathol 2009; 130: 59–69. [DOI] [PubMed] [Google Scholar]
- 35. Moore AS, Ogilvie GK. Lymphoma. In: Ogilvie GK, Moore AS. (eds). Feline oncology. Trenton, NJ: Veterinary Learning Systems, 2001, pp 191–219. [Google Scholar]
- 36. Mooney SC, Hayes AA, Macewen EG, Matus RE, Geary A, Shurgot BA. Treatment and prognostic factors in lymphoma in cats – 103 cases (1977–1981). J Am Vet Med Assoc 1989; 194: 696–699. [PubMed] [Google Scholar]
- 37. Vail DM, Moore AS, Ogilvie GK, Volk LM. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 38. Moore AS, Cotter SM, Frimberger AE, Wood CA, Rand WM, Lheureux DA. A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. J Vet Intern Med 1996; 10: 372–375. [DOI] [PubMed] [Google Scholar]
- 39. Brenn SH, Couto SS, Craft DM, Leung C, Bergman PJ. Evaluation of p-glycoprotein expression in feline lymphoma and correlation with clinical outcome. Vet Comp Oncol 2008; 6: 201–211. [DOI] [PubMed] [Google Scholar]
- 40. Rassnick KM, Mauldin GN, Moroff SD, Mauldin GE, McEntee MC, Mooney SC. Prognostic value of argyrophilic nucleolar organizer region (AgNOR) staining in feline intestinal lymphoma. J Vet Intern Med 1999; 13: 187–190. [DOI] [PubMed] [Google Scholar]
- 41. Hadden AG, Cotter SM, Rand W, Moore AS, Davis RM, Morrissey P. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J Vet Intern Med 2008; 22: 153–157. [DOI] [PubMed] [Google Scholar]
- 42. Malik R, Gabor LJ, Foster SF, McCorkell BE, Canfield PJ. Therapy for Australian cats with lymphosarcoma. Aust Vet J 2001; 79: 808–817. [DOI] [PubMed] [Google Scholar]
- 43. Kiselow MA, Rassnick KM, McDonough SP, Goldstein RE, Simpson KW, Weinkle T, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). J Am Vet Med Assoc 2008; 232: 405–410. [DOI] [PubMed] [Google Scholar]
- 44. Stein TJ, Pellin M, Steinberg H, Chun R. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc 2010; 46: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Milner RJ, Peyton J, Cooke K, Fox LE, Gallagher A, Gordon P, et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin–Madison chemotherapy protocol: 38 cases (1996–2003). J Am Vet Med Assoc 2005; 227: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 46. Patterson-Kane JC, Kugler BP, Francis K. The possible prognostic significance of immunophenotype in feline alimentary lymphoma: a pilot study. J Comp Pathol 2004; 130: 220–222. [DOI] [PubMed] [Google Scholar]
- 47. Kristal O, Lana SE, Ogilvie GK, Rand WM, Cotter SM, Moore AS. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994–1997). J Vet Intern Med 2001; 15: 125–130. [DOI] [PubMed] [Google Scholar]
- 48. Barrs VR, Beatty JA. Diagnosis and treatment of low-grade alimentary lymphoma. In: August JR. (ed). Consultations in feline internal medicine. Oxford; Saunders Elsevier, 2010, pp 187–199. [Google Scholar]
- 49. Slawienski MJ, Mauldin GE, Mauldin GN, Patnaik AK. Malignant colonic neoplasia in cats: 46 cases (1990–1996). J Am Vet Med Assoc 1997; 211: 878–881. [PubMed] [Google Scholar]
- 50. Simon D, Eberle N, Laacke-Singer L, Nolte I. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 51. Vail DM. Feline lymphoma and leukaemia, hematopoietic tumours. In: Withrow SJ, Vail DM. (eds). Withrow & MacEwen’s small animal clinical oncology. 4th ed. Oxford: Saunders Elsevier, 2007, p 24. [Google Scholar]
- 52. Oberthaler KT, Mauldin E, McManus PM, Shofer FS, Sorenmo KU. Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. J Feline Med Surg 2009; 11: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams LE, Pruitt AF, Thrall DE. Chemotherapy followed by abdominal cavity irradiation for feline lymphoblastic lymphoma. Vet Radiol Ultrasound 2010; 51: 681–687. [DOI] [PubMed] [Google Scholar]
- 54. Parshley DL, LaRue SM, Kitchell B, Heller D, Dhaliwal RS. Abdominal irradiation as a rescue therapy for feline gastrointestinal lymphoma: a retrospective study of 11 cats (2001–2008). J Feline Med Surg 2011; 13: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guilford WG, Jones BR, Markwell PJ, Arthur DG, Collett MG, Harte JG. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med 2001; 15: 7–13. [DOI] [PubMed] [Google Scholar]
- 56. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 2005; 19: 155–160. [DOI] [PubMed] [Google Scholar]