Abstract
Thyroid gland palpation is an important aid for diagnosing feline hyperthyroidism in an early stage to prevent development of deleterious complications. Our objectives were to assess within- and between-examiner agreement for two thyroid gland palpation techniques in cats and to correlate palpation results with ultrasonographic thyroid measurements. Nine client-owned hyperthyroid (12.6±2.4 years) and 10 healthy control cats (7.4±5.4 years) entered this prospective study. Both thyroid glands of all cats were palpated twice by three blindfolded clinicians with the classic palpation technique [technique 1 (T1)] and the technique described by Norsworthy GD, Adams VJ, McElhaney MR, Milios JA [(2002a) Relationship between semi-quantitative thyroid palpation and total thyroxine concentration in cats with and without hyperthyroidism. Journal of Feline Medicine and Surgery 4, 139–143] [technique 2 (T2)]. A semi-quantitative score from 1 to 6 was assigned to the gland size. After clipping of the ventral cervical region, another palpation session followed by ultrasonography of the thyroid glands was performed. Average weighted κ-values within- and between-examiners were 0.864 and 0.644 for T1 and 0.732 and 0.532 for T2. T1 did lead to significantly smaller within- (P=0.007) and between-examiner (P=0.048) differences than T2. Significant correlation coefficients (P<0.001) between the palpation scores of both techniques and ultrasonographic thyroid lobe length (T1: 0.43; T2: 0.38) were observed. No significant difference before and after clipping was found (T1: P=0.503; T2: P=0.607). The first time that all cats were palpated by either technique, significant score differences between control and hyperthyroid cats were observed both for T1 (P=0.002) and T2 (P=0.003). Both feline thyroid gland palpation techniques have good within- and between-examiner agreements. Based on this study, the classic palpation technique is preferred.
Feline hyperthyroidism is the most common endocrine disorder of older cats (Mooney 2005). Due to the progressive nature, an early diagnosis and subsequent treatment prevents development of deleterious complications. Thyroid gland palpation is an important aid for diagnosing thyrotoxicosis in an early stage. This is particularly true in hyperthyroid cats with a serum total thyroxine concentration (TT4) within reference ranges due to fluctuating thyroid hormone levels or due to suppression of thyroid hormone production by concurrent disorders (Feldman and Nelson 2004). As thyroid gland palpation should be a standard part of the physical examination of all, especially older, cats, the reliability of palpation results should be known. Currently, there are two thyroid gland palpation techniques described in cats, namely the classic technique with the cat in sitting position and the neck extended (Feldman and Nelson 2004, Mooney 2005) and the technique described by Norsworthy et al (2002a) with the cat in standing position and the head elevated and turned to one side. To date, the diagnostic value of both techniques has not been compared. Recent studies show that almost all hyperthyroid and a significant number of euthyroid cats have a palpable thyroid gland. The presence of a palpable goitre is, therefore, a poor indicator of hyperthyroidism, although the size of the enlarged thyroid gland can be valuable in distinguishing euthyroid and hyperthyroid cats (Norsworthy et al 2002a, Boretti et al 2005). However, evaluation of the size of the thyroid gland by palpation is subjective and, therefore, subject to within- and between-examiner variability (Polzin et al 2000, Dohoo et al 2003). In human medicine, the intra- and interobserver variation on thyroid gland palpation is variable and large (Jarløv et al 1991, Brander et al 1992). Based on low positive (27%) and high negative (95%) predictive values, thyroid gland palpation has been shown to be a good screening test ruling out goitres in humans but is less valuable at assessing thyroid gland size (Lisbôa et al 1996). To the authors' knowledge, the reproducibility of thyroid gland palpation in cats has not yet been determined.
Because of the poor reliability for determining thyroid gland size and for detection of thyroid gland nodules or small goitres by palpation alone, thyroid gland ultrasonography is an important diagnostic tool in human medicine (Jarløv et al 1991, Brander et al 1992, Lisbôa et al 1996). Ultrasonography can accurately estimate thyroid gland volume and evaluate thyroid gland morphology (Hopkins and Reading 1995, Barraclough and Barraclough 2000, Hegedüs 2001). However, the correlation between palpation and ultrasonographic findings is poor (Brander et al 1992, Vitti et al 1994, Lisbôa et al 1996). The value of thyroid ultrasonography in diagnosing feline hyperthyroidism is limited because most hyperthyroid cats are easily identified based on clinical signs, thyroid palpation and TT4 measurements (Feldman and Nelson 2004). However, the correlation between thyroid palpation and ultrasonography has not yet been investigated in cats.
The primary objective of the present study was to define within- and between-examiner agreement for thyroid palpation by the two palpation techniques, described above, commonly used in clinical practice. Further objectives were to correlate the results of these palpation techniques with ultrasonographic thyroid length measurements and to compare palpation scores before and after clipping.
Material and methods
Animals
Ten hyperthyroid and 10 healthy control cats were included in the study.
Hyperthyroid client-owned cats, presented at the Faculty of Veterinary Medicine, Department of Small Animal Medicine and Clinical Biology of the Ghent University between December 2005 and February 2006 were considered for inclusion. The diagnosis of hyperthyroidism was confirmed by measurement of serum TT4. Exclusion criteria were previous treatment with radioactive iodine (131I) or bilateral thyroidectomy and the presence of severe concurrent disease determined on physical examination, complete blood count (CBC) or routine serum biochemistry profile. In addition, cats that needed sedation or anaesthesia to undergo the study protocol were also excluded.
The cats in the control group were owned by veterinarians, veterinary students, personnel and friends. Inclusion criteria were a history without medical problems and the absence of clinically relevant abnormalities on physical examination, CBC, routine serum biochemistry profile and TT4; and sufficient cooperativeness. All owners signed a consent form before enrolling their cats in the study.
Study design
The study was organised on days that hyperthyroid cats were scheduled for treatment with radioactive iodine (four Mondays between December 2005 and February 2006). The clinicians who performed the thyroid gland palpations were blinded to the number of hyperthyroid cats scheduled on each study day. In addition to these hyperthyroid cats, control cats were added to the groups so that each group consisted of four or five animals. There were one group of four cats and three groups of five cats. One group consisted of one hyperthyroid and four healthy cats. In the other groups, at least two hyperthyroid cats were present. The cats in each group were randomly presented by assistants to three blindfolded clinicians (clinician 1: SD, clinician 2: DP, clinician 3: IvH) who performed four palpation sessions on all cats: technique 1 (T1), technique 2 (T2), T1 and finally again T2. After these four palpation sessions, a rectangular zone of the ventral cervical region from the larynx to the thoracic inlet was clipped. Afterwards, T1 and T2 were again performed on all cats. All cats of a group were presented to and examined by all clinicians on the same day. To achieve randomisation, prior to each study day, one of the authors (PS) made a scheme by the hat method to determine the order of presentation of the cats (numbers 1–4 or 5) for each palpation technique for each clinician.
Prior of the study, all clinicians had substantial experience in thyroid palpation in cats. For each palpation session (six sessions/day), the cats were presented in a different order (determined by the hat method) to prevent the clinicians remembering the previously given palpation scores. The clinicians knew that each group consisted of a maximum of five cats, but did not know the subdivision of hyperthyroid and control cats within the group. In addition, all studied cats were presented at our clinic for the first time on the study day. Therefore, none of the clinicians examined the cats prior to inclusion in this study.
Thyroid gland palpation
T1 was the classic technique, in which the cat is restrained in sitting position and the front legs held still. The neck of the cat is extended, and the clinicians' thumb and forefinger are placed on each side of the trachea and swept downwards from the larynx to the sternal manubrium. Palpation of a mobile subcutaneous nodule or a ‘blip’ that slips under the fingertips determines the presence of a goitre (Mooney 2005).
T2 was the recently described palpation technique of Norsworthy et al (2002a). The clinician is positioned directly behind the cat which is placed in standing position or sternal recumbency. The head of the cat is elevated and turned (45°) alternatively to the right or left, depending on which side is assessed. The clinician's index finger is placed in the groove formed by the trachea and sternothyroid muscle just below the larynx and then moved downwards in the groove to the thoracic inlet. If the thyroid lobe is enlarged, a characteristic ‘blip’ is felt as the index finger passes the goitre (Norsworthy et al 2002a).
In our clinic, the classic palpation technique is routinely performed, whereas the technique of Norsworthy et al (2002a) was newly introduced to perform this study.
For each palpation a semi-quantitative score from 1 to 6, following the scoring system proposed by Boretti et al (2005) was given to the length of the left and right thyroid glands (score 0=non-palpable, score 1=1 to <3 mm, score 2=3 to <5 mm, score 3=5 to <8 mm, score 4=8 to <12 mm, score 5=12 to <25 mm, and score 6=≥25 mm). To improve objectiveness, all clinicians received a paper with drawings of thyroid glands of the six scores to compare the palpated size with the size of the drawings.
Thyroid gland ultrasonography
Following all palpation sessions, ultrasonography of the thyroid glands of all cats was performed. This was undertaken by an ECVDI-diplomate (JS), with a linear transducer of 7–14 MHz (Logic 7, General Electric Medical Systems, Milwaukee, Wisconsin, USA).
The cats were restrained, without sedation, in a sitting position with the neck extended. The thyroid gland was identified by a method described by Wisner and Nyland (1998) with the common carotid artery as major internal landmark. On the longitudinal section, the length of each thyroid lobe was measured. Afterwards, the transducer was turned 90° to view the transverse section of the thyroid gland. If possible, the width of the thyroid gland lobe was measured.
Statistical analysis
A two-sided t-test was used to determine if the age of the healthy and hyperthyroid cats was significantly different.
The evaluation of the within- and between-examiner agreement was based on the absolute difference in score between the two assessments of the same observer (within-examiner agreement) and the absolute average difference in score between the three examiners (between-examiner agreement) on the same thyroid gland lobe (left or right) using the same technique and clipping mode (yes or no). The two techniques and the effect of clipping were compared for absolute difference in score using the Wilcoxon signed rank test. The within- and between-examiner agreement was further quantified by weighted κ-values. The κ-values were interpreted as described by Dohoo et al (2003) in which a κ-value<0.2 corresponds with slight agreement, between 0.2 and 0.4 with fair agreement, between 0.4 and 0.6 with moderate agreement, between 0.6 and 0.8 with substantial agreement and >0.8 with almost perfect agreement. Bland–Altman plots were used to visualise the agreement of thyroid palpation with T1 and T2. Because the Bland–Altman plots are based on scores (and not on continuous data), some points occur frequently. Therefore, the points are represented by their occurring frequency.
The scores of healthy and hyperthyroid cats were compared by the Wilcoxon rank sum test.
The Spearman rank correlation coefficient between palpation scores and echographic measurements was derived to investigate the relationship between palpation and ultrasonography.
Results were considered significantly different when a P-value was smaller than or equal to 0.05.
Results
Animals
Ten hyperthyroid cats were recruited to the study. However, one was excluded due to aggressive behaviour. Therefore, the study group comprised nine hyperthyroid cats. These cats were aged between 8 and 15 years [mean 12.6; standard deviation (SD) 2.4]. All cats were domestic shorthairs; four cats were spayed females and five were castrated males. One cat had previously undergone unilateral thyroidectomy at the right side.
Ten healthy cats, aged between 1.5 and 19 years (mean 7.4; SD 5.4) were included. This group consisted of eight domestic shorthair cats and two Persian cats. There were five males (one intact) and five females (three intact). The age of the control and hyperthyroid cats was not significantly different (P=0.07).
Thyroid gland palpation
T2 did lead to a significantly larger difference between the two measurements of the same thyroid gland lobe performed by the same clinician (P=0.007). T2 demonstrated an average of 0.19 units more variation between clinicians than T1. T2 did also lead to a significantly larger difference between the examiners for the palpation of the same thyroid gland lobe (P=0.048).
The average weighted κ-values within- and between-examiners were 0.864 and 0.644 for T1 and 0.732 and 0.532 for T2. The individual κ-coefficients for the three clinicians and between the three clinicians are shown in Table 1. A summary of the absolute difference in score is given in Table 2.
Table 1.
κ-Values for within- and between-examiners agreement for two techniques of thyroid palpation
| T1 | T2 | |
|---|---|---|
| Within-examiner agreement | ||
| Clinician 1 | 0.968 | 0.906 |
| Clinician 2 | 0.871 | 0.672 |
| Clinician 3 | 0.752 | 0.619 |
| Between-examiners agreement | ||
| Clinician 1–2 | 0.632 | 0.568 |
| Clinician 1–3 | 0.705 | 0.546 |
| Clinician 2–3 | 0.596 | 0.456 |
Table 2.
The absolute differences in palpation score between two assessments of the same thyroid gland lobe by the same clinician and the same technique
| Clinician | Number of palpations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference in score with T1 | Difference in score with T2 | ||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 4 | |
| Clinician 1 | 36 | 2 | 0 | 0 | 35 | 2 | 0 | 1 | 0 |
| Clinician 2 | 31 | 6 | 0 | 1 | 25 | 8 | 2 | 3 | 0 |
| Clinician 3 | 28 | 6 | 3 | 1 | 23 | 11 | 2 | 1 | 1 |
Each clinician assessed 38 thyroid gland lobes twice with both techniques, because 19 cats with two thyroid gland lobes were palpated.
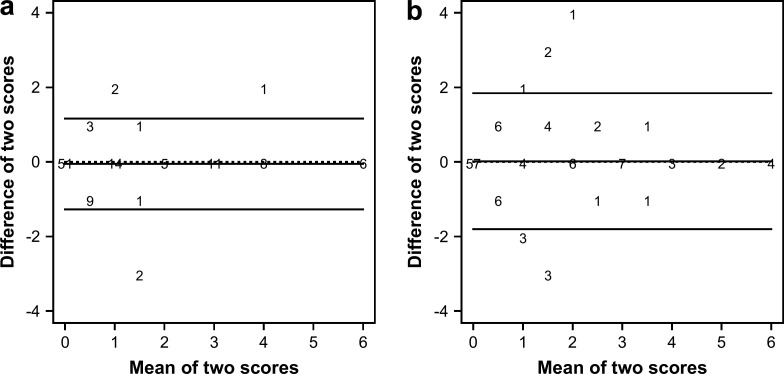
The scores of T1 were significantly higher (P=0.035) than the scores of T2. For both techniques, the scores given by the same clinician to the same thyroid gland lobe differed less for higher scoring categories (score≥3) compared to lower scoring categories (score<3) (Fig. 1). Furthermore, more extreme differences between the two assessments of the same examiner were observed for T2 (Fig. 1b) compared with T1 (Fig 1a).
Fig 1.
Bland–Altman plots for the two repeated measurements of the same clinician with T1 (a) and T2 (b). The X-axis shows the mean of the two repeated scores, whereas the Y-axis shows the difference between the two repeated scores. The numbers on the figure represent how many times a specific combination occurred. The horizontal lines on the figure correspond with the mean difference in score (middle horizontal line) and the 95% CI (upper and lower horizontal line). The dotted horizontal line corresponds with a zero mean difference in score.
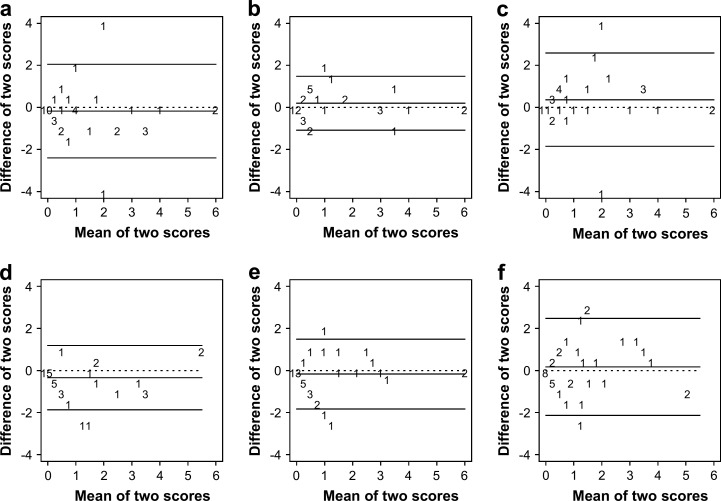
For both techniques, the scores given by different examiners to the same thyroid gland lobe differed less for higher scoring categories compared to lower scoring categories (Fig. 2).
Fig 2.
Bland–Altman plots for the palpation scores of the same thyroid gland lobe between clinicians. (a–c) represent T1 and (d–f) represent T2. In (a) and (d) the difference between clinicians 1 and 2 is depicted. Similarly, (b) and (e) refer to the difference between clinicians 1 and 3; and (c) and (f) refer to the difference between clinicians 2 and 3. The X-axis shows the mean of the two scores, whereas the Y-axis shows the difference between the two scores. The numbers on the figure represent how many times a specific combination occurred. The horizontal lines on the figure correspond with the mean difference in score (middle horizontal line) and the 95% CI (upper and lower horizontal line). The dotted horizontal line corresponds with a zero mean difference in score.
The scores before and after clipping did not differ significantly (P=0.503 for T1 and P=0.607 for T2).
Healthy cats had a much lower and significantly different score than hyperthyroid cats both for T1 (P=0.002) and for T2 (P=0.003).
The first time that the three clinicians performed T1 on all cats, a goitre was palpated in 96.3% (26/27) of the assessments of hyperthyroid cats and in 36.7% (11/30) of the assessments of control cats. When T2 was first performed, a goitre was palpated in 88.9% (24/27) of the palpations of hyperthyroid cats and in 33.3% (10/30) of the palpations of control cats. If a goitre was felt, the palpation score attributed to the largest thyroid gland lobe varied in hyperthyroid cats between ‘2’ and ‘6’ for T1 and between ‘1’ and ‘6’ for T2 and in control cats between ‘1’ and ‘2’ for T1 and between ‘1’ and ‘3’ for T2 (Table 3). Table 3 also indicates that a score of ‘3’ or more was attributed to none of the healthy cats with T1 and to only two of the healthy cats with T2. In contrast, in hyperthyroid cats 85.2% (23/27) of T1 palpations and 66.7% (18/27) of T2 palpations received a score of ‘3’ or more. Therefore, a score of ‘3’ or more had sensitivities of 85% and 67% and specificities of 100% and 93%, respectively for T1 and T2, to predict hyperthyroidism.
Table 3.
The palpation scores for the largest thyroid gland lobe of each cat given by the three clinicians the first time that all cats were palpated with T1 and T2
| Palpation score | Control cats | Hyperthyroid cats | ||
|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |
| 0 | 19 | 20 | 1 | 3 |
| 1 | 10 | 6 | 0 | 1 |
| 2 | 1 | 2 | 3 | 5 |
| 3 | 0 | 2 | 11 | 10 |
| 4 | 0 | 0 | 8 | 5 |
| 5 | 0 | 0 | 1 | 1 |
| 6 | 0 | 0 | 3 | 2 |
In total there are 30 assessments for the control cats and 27 for the hyperthyroid cats for each technique.
The cat that previously did undergo unilateral thyroidectomy at the right side now had a palpable goitre at the left side. Palpation scores for this cat varied between ‘3’ and ‘4’ for the left side and between ‘0’ and ‘1’ for the right side. None of the clinicians palpated a scar at the side of the thyroidectomy.
Thyroid gland ultrasonography
Because thyroid length on longitudinal section was the only parameter measured on all thyroid gland lobes during ultrasonography only the correlation between palpation scores and echographic thyroid lobe length was calculated. The Spearman rank correlation coefficients between palpation scores and length of the thyroid gland lobes were 0.43 (95% confidence interval (CI): [0.31; 0.55]) for T1 and 0.38 for T2 (95% CI: [0.25; 0.51]), which are both low values and similar to each other.
Discussion
The κ-values for the variability of determining the size of the thyroid gland in this study are good for both techniques. According to Dohoo et al (2003), the calculated κ-values represent substantial to almost perfect agreement within observers and moderate to substantial agreement between observers. This is in contrast to the findings in human medicine. In one study in humans, κ-values for within- and between-examiner agreement to detect a goitre were 0.52–0.83 and 0.37–0.61 (Jarløv et al 1991). In another study, κ-values between observers for estimating the thyroid gland size were 0.620 for the right and 0.322 for the left thyroid lobe (Brander et al 1992). Possible explanations for the better results of our study compared to human studies are differences in conformation, different causes for goitres and different prevalence of the various thyroid disorders between cats and humans. In general, cats are less cooperative than human patients which leads to the suggestion that thyroid gland palpation is subject to more errors in cats than in humans. On the other hand, feline goitres are usually small and almost always fit between two fingers, whereas human goitres can be large and require the examiner to estimate a mass on the order of centimetres instead of millimetres. This can be another explanation for the lower agreements for human thyroid gland palpation.
Several efforts were made to minimise the possibility of memory influencing assessments. Each day of the study, every clinician had to evaluate the size of 48 (group of four cats) or 60 (groups of five cats) thyroid gland lobes. In addition, the clinicians were blindfolded and the cats were presented to the clinicians in a random order. A limitation of the study design was that the clinicians knew that the study was organised on days that hyperthyroid cats were scheduled for radioactive iodine treatment. Therefore, they knew that at least one hyperthyroid cat was present in each group. Because they were blinded to the number of scheduled hyperthyroid cats, they did not know the exact number of hyperthyroid cats in each group. Therefore, it is assumed that this limitation did not or only minimally influences the study results.
We demonstrated that T1 is more repeatable than T2. This could be due to bias towards T1 because all clinicians performing the palpations were more familiar with T1 than with T2, because the latter technique is a recently developed technique. Prior to the study, all clinicians routinely performed feline thyroid palpation with T1. It is possible that this bias would have been reduced if we allowed more time for each clinician to become more familiar with T2. The individual within-examiner agreement may also reflect the influence of experience (Table 1). The κ-values of clinician 1 (SD), who was the most experienced clinician, were higher than for clinicians 2 and 3 (DP, IvH). Furthermore, the difference in score between two assessments of the same thyroid gland lobe for clinician 1 was smaller than for the other clinicians for either techniques (Table 2). However, even without training in T2, substantial to almost perfect agreement was obtained which suggests that thyroid palpation in untrained observers may have acceptable results. Although T1 seems to be preferred, all clinicians subjectively found T2 more appropriate to assess which side was involved, especially in hyperthyroid cats with a large goitre crossing the midline which could be felt on both sides of the trachea.
There was no significant difference observed in palpation results before and after clipping the ventral cervical region. However, the majority of the cats studied were shorthaired and, therefore, the impact of clipping on longhaired cats was not investigated.
There was an obvious and very significant difference (P<0.001) in palpation scores between control and hyperthyroid cats (Table 3). Almost all hyperthyroid and approximately one third of the control cats had a palpable goitre. In two previous studies 96% and 100% of hyperthyroid cats and 59% and 69% of euthyroid cats had an enlarged thyroid gland (Norsworthy et al 2002a, Boretti et al 2005). Possible explanations for the palpation of goitre in euthyroid cats may be the presence of pathological changes in thyroid glands, parathyroid glands or other cervical structures or the fact that experienced clinicians are able to palpate healthy thyroid glands in cats. The lower proportion of healthy cats with a palpable goitre in our study may be due to the younger median age in comparison with the study by Norsworthy et al (2002a). Observed histopathological abnormalities after thyroidectomy in euthyroid cats with palpable nodules revealed mainly adenomatous hyperplasia and less frequently adenomas or non-proliferative thyroid gland changes, thyroiditis and parathyroid abnormalities (Norsworthy et al 2002b, Ferguson and Freedman 2006), which are all more likely to occur in older cats. In the study by Norsworthy et al (2002a) no euthyroid but most hyperthyroid cats (18/23) received a palpation score of more than ‘3’. Also Boretti et al (2005) found significantly different palpation scores for hyperthyroid and euthyroid cats of 3.5 and 1, respectively. In comparison, in our study, a score of ‘3’ or more had moderate sensitivities (T1: 85%; T2: 67%) and high specificities (T1: 100%; T2: 93%) to predict hyperthyroidism. However, these values need further investigation in the general cat population and, therefore, must be interpreted carefully. Although our and previous studies indicate that a cat with a palpation score ‘≥3’ is very likely to be hyperthyroid (Norsworthy et al 2002a, Boretti et al 2005), thyroid gland palpation cannot be used as a single test to diagnose hyperthyroidism because many healthy cats have a palpable goitre. Furthermore, the comparison between these studies should be interpreted with caution as different scoring systems were used. Norsworthy et al (2002a) used a semi-quantitatively scoring system on an arbitrary scale from 0 to 6. Non-palpable lobes were scored as ‘0’, barely palpable lobes as ‘1’ and lobes approximately 2.5 cm or greater in length as ‘6’. The other values were assigned proportionally to measurements between ‘1’ and ‘6’. In our study, the semi-quantitative scoring system proposed by Boretti et al (2005) was used. Hereby, the difference between lower score categories is very small (score 1=1 to <3 mm versus score 2=3 to <5 mm). The clinicians of our study had more difficulties to discriminate lower scoring categories than higher scoring categories (Fig. 1). Because most hyperthyroid cats have larger thyroid gland scores, and because it is very subjective to discriminate millimetres by palpation, the difference in these lower score categories is probably not clinically significant. The development of an international acknowledged and clinically relevant scoring system for evaluating thyroid enlargement in cats, comparable to the WHO-criteria for goitre in humans (World Health Organization 2001), may be indicated.
As in human medicine (Brander et al 1992, Vitti et al 1994, Lisbôa et al 1996), the correlation between thyroid gland palpation scores and echographic measurements was poor. Caution must be employed in interpreting these results because the primary objective of the study design was determination of reproducibility for thyroid gland palpation. A limitation of this study is the lack of measurement of the thyroid gland volume. Abnormal thyroid lobes can have an abnormal volume despite a normal length (Wisner et al 1994). Further investigation of the correlation of thyroid gland palpation scores and ultrasonographic thyroid gland volume to define the diagnostic yield of ultrasonography in hyperthyroid cats is warranted.
To conclude, our study indicates that both techniques for thyroid gland palpation in cats have a very good within- and good between-examiner agreement. Secondly, palpation scores between control and hyperthyroid cats differed significantly. Therefore, thyroid gland palpation is an important uninvasive and inexpensive diagnostic tool to detect hyperthyroid cats, also if TT4 concentrations are within the reference range. As in humans, correlation of palpation scores and ultrasound results seems to be poor, although this needs further research. Based on our results, T1 is preferred because it leads to more repeatable scores both within- and between-examiners. We also observed higher sensitivity and specificity and better correlation with ultrasound measurements for T1, but these differences were not significant.
References
- Barraclough B.M., Barraclough B.H. Ultrasound of the thyroid and parathyroid glands, World Journal of Surgery 24, 2000, 158–165. [DOI] [PubMed] [Google Scholar]
- Boretti F.S., Sieber-Ruckstuhl N.S., Lahula P., Reusch C.E. Thyroid enlargement and its relationship to serum T4 status in clinically suspected hyperthyroid cats. 15th ECVIM-CA Congress Proceedings, abstract 22, Journal of Veterinary Internal Medicine 19, 2005, 928–950. [Google Scholar]
- Brander A., Viikinkoski P., Tuuhea J., Voutilainen L., Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice, Journal of Clinical Ultrasound 20, 1992, 37–42. [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. Screening and diagnostic tests. McPike S.M. Veterinary Epidemiologic Research, 1st edn, 2003, AVC Inc.: Charlottetown, 85–120. [Google Scholar]
- Feldman E.C., Nelson R.W. Feline hyperthyroidism (thyrotoxicosis). Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, 3rd edn, 2004, Elsevier Saunders: St. Louis, 152–218. [Google Scholar]
- Ferguson D.C., Freedman R. Goiter in apparently euthyroid cats. August J.R. Consultations in Feline Internal Medicine vol. 5, 2006, Elsevier Saunders: St. Louis, 207–215. [Google Scholar]
- Hegedüs L. Thyroid ultrasound, Endocrinology and Metabolism Clinics of North America 30, 2001, 339–360. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Reading C.C. Thyroid and parathyroid imaging, Seminars in Ultrasound, CT and MRI 16, 1995, 279–295. [DOI] [PubMed] [Google Scholar]
- Jarløv A.E., Hegedüs L., Gjørup T., Hansen J.M. Observer variation in the clinical assessment of the thyroid gland, Journal of Internal Medicine 229, 1991, 159–161. [DOI] [PubMed] [Google Scholar]
- Lisbôa H.R.K., Gross J.L., Orsolin A., Fuchs S. Clinical examination is not an accurate method of defining the presence of goitre in schoolchildren, Clinical Endocrinology 45, 1996, 471–475. [DOI] [PubMed] [Google Scholar]
- Mooney C.T. Hyperthyroidism. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 6th edn, 2005, Elsevier Saunders: St. Louis, 1544–1560. [Google Scholar]
- Norsworthy G.D., Adams V.J., McElhaney M.R., Milios J.A. Relationship between semi-quantitative thyroid palpation and total thyroxine concentration in cats with and without hyperthyroidism, Journal of Feline Medicine and Surgery 4, 2002a, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsworthy G.D., Adams V.J., McElhaney M.R., Milios J.A. Palpable thyroid and parathyroid nodules in asymptomatic cats, Journal of Feline Medicine and Surgery 4, 2002b, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin D.J., Lund E., Walter P., Klausner J. From journal to patient: evidence-based medicine. Bonagura Kirk's Current Veterinary Therapy, 13th edn, 2000, WB Saunders: Philadelphia, 2–8. [Google Scholar]
- Vitti P., Martino E., Aghini-Lombardi F., Rago T., Antonangeli L., Maccherini D., Nanni P., Loviselli A., Balestrieri A., Araneo G., Pinchera A. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency, Journal of Clinical Endocrinology and Metabolism 79, 1994, 600–603. [DOI] [PubMed] [Google Scholar]
- Wisner E.R., Nyland T.G. Ultrasonography of the thyroid and parathyroid glands, Veterinary Clinics of North America Small Animal Practice 28, 1998, 973–991. [DOI] [PubMed] [Google Scholar]
- Wisner E.R., Théon A.P., Nyland T.G., Hornof W.J. Ultrasonographic examination of the thyroid gland of hyperthyroid cats: comparison of 99mTcO4− scintigraphy, Veterinary Radiology and Ultrasound 35, 1994, 53–58. [Google Scholar]
- World Health Organization. United Nation Children's Fund. International Council for Control of iodine Deficiency Disorders. Assessment of the Iodine Deficiency Disorders and Monitoring their Elimination, 2001, World Health Organization, WHO document WHO/NHD/01.1 [Google Scholar]