Abstract
An understanding of the anatomy of the feline vestibular system is essential for interpretation of the clinical signs associated with vestibular dysfunction, for precise lesion localisation, and for accurate interpretation of results of diagnostic imaging. Appropriate recognition and interpretation of the clinical signs of vestibular disease is also an essential aspect of the precise diagnosis of the cause of vestibular dysfunction in cats. The objectives of this review are to provide an overview of the anatomy of the feline vestibular system, and to review the clinical signs of peripheral and central vestibular dysfunction of cats.
The vestibular system is the primary sensory system that maintains proper balance and posture, and normal orientation of the body relative to gravity. This is accomplished by a continuous contribution of information to the central nervous system (CNS) regarding the position and motion of all parts of the body, including the head and eyes (deLahunta 1983, Kandel et al 1991).
There are two main functions of the vestibular system (Kandel et al 1991). The first is to stabilise the position of the head in space by means of vestibulospinal reflexes, thus ensuring that the position of the body remains stable. The second is to maintain the visual image by means of vestibulo-ocular reflexes, thus stabilising the eyes in space during head movements. The results of these interactions is that the position of the eyes, trunk, and limbs are maintained in an appropriate relationship with the position of the head, during movement and at rest.
Disorders of the vestibular system of cats occur frequently. A basic understanding of the functional anatomy and physiology of the vestibular system is essential for interpretation of the clinical signs associated with vestibular dysfunction, and for precise lesion localisation. Appropriate management of a cat with a vestibular disorder requires recognition and interpretation of the clinical signs of vestibular disease, determination of an appropriate list of differential diagnoses, completion of a diagnostic plan, interpretation of appropriate diagnostic test results, and formulation of a treatment plan.
Anatomy and physiology
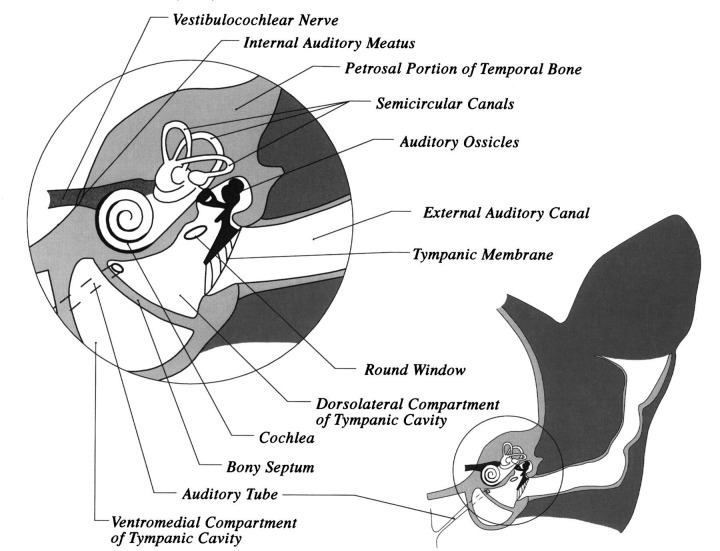
The ear consists of three parts: (1) the external ear consisting of the pinna (cartilaginous portion outside the head) and the vertical and horizontal portions of the external auditory canal; (2) the middle ear, consisting of the tympanic membrane, the middle ear cavity (tympanic cavity), auditory ossicles, and associated muscles; and (3) the inner ear, consisting of the cavity within the petrosal portion of the temporal bone and the membranous labyrinth of sacs and ducts within this cavity (Fig 1) (Gilbert 1968). Vestibular dysfunction most frequently results from disorders affecting the middle and inner ear.
Fig 1.
Schematic line drawing of a transverse section of the right side of the head of a cat at the level of the external auditory canal.
The pinna consists of a thin auricular cartilage that is covered by skin. The various parts of the auricular cartilage are connected by six muscles. The auricular cartilage continues in a medial direction as the external auditory canal. The external auditory canal ends medially at the external auditory meatus of the temporal bone, which is covered by the tympanic membrane (Gilbert 1968).
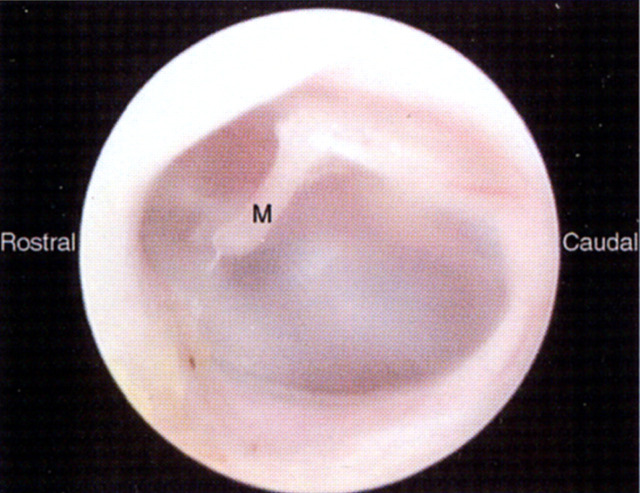
The tympanic membrane is oval in shape, and slightly concave from a lateral view (Fig 2). It is set into the external auditory meatus, and is oblique to the midline, angled in a rostral and medial direction (Holzworth 1987). The membrane is a shiny, pale grey, translucent sheet of fibrous tissue covered by a thin layer of stratified squamous epithelium. Through its dorsal part may be seen the manubrium (handle) of the malleus, the auditory ossicle attached to the medial surface of the membrane. Blood vessels are visible only in the region of the manubrium in a normal tympanic membrane.
Fig 2.
Left tympanic membrane of a normal cat viewed by means of a video otoscope. Note the manubrium of the malleus (M) visible through the shiny, pale grey and translucent membrane.
The middle ear consists of an air-filled tympanic cavity that is lined by ciliated epithelium. It is connected to the nasopharynx by the auditory (eustachian) tube, and is separated from the external environment by the tympanic membrane (Fig 1). In cats, unlike dogs, the tympanic cavity is divided into a larger ventromedial and a smaller dorsolateral compartment by a thin bony septum, which arises along a line on the bulla wall running from mid-rostral to mid-lateral (Fig 3) (Little & Lane 1986). The ventromedial and dorsolateral compartments communicate through a narrow fissure on the caudomedial aspect of the dorsolateral compartment, where the septum is incomplete (Fig 4). The smaller dorsolateral compartment is arbitrarily divided into the epitympanum dorsally and the mesotympanum.
Fig 3.
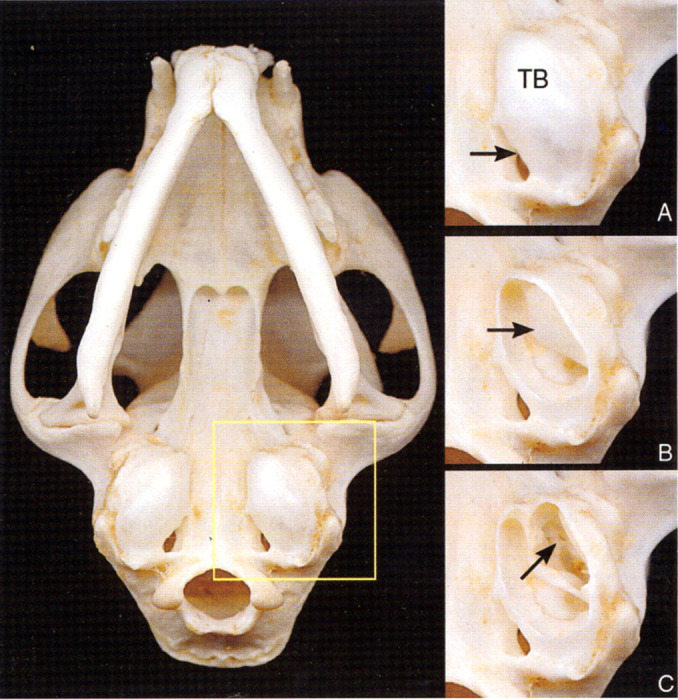
Ventral view of the skull of a cat. (A) Enlargement of the area of the left side of the skull outlined by the yellow square. Note the tympanic bulla (TB) and the jugular foramen (arrow). (B) A portion of the ventral tympanic bulla seen in (A) has been removed to expose the ventromedial compartment of the tympanic cavity, and the bony septum (arrow). (C) The bony septum seen in (B) has been partially removed to expose the dorsolateral compartment of the tympanic cavity, containing the auditory ossicles (arrow).
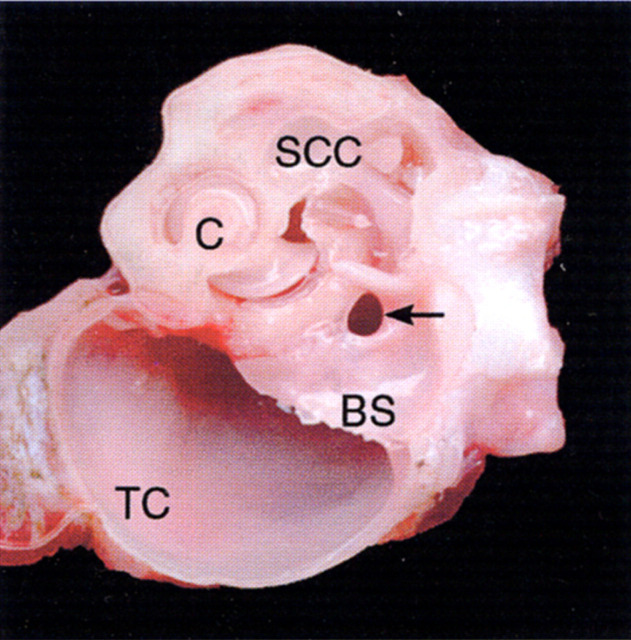
Fig 4.
Transverse view of an anatomical dissection of the left middle ear and inner ear of a cat, at a level similar to that in Fig 1. Note the large caudal extent of the ventromedial compartment of the tympanic cavity (TC), the cochlea (C), and the semicircular canals (SCC). Within the most caudal extent of the bony septum (BS) the communicating fissure (arrow) between the ventromedial and dorsolateral compartments of the tympanic cavity may be seen.
Vibrations are conveyed from the tympanic membrane to the inner ear by an articulated chain of three small bones called auditory ossicles. The three auditory ossicles (malleus, incus and stapes), and their associated muscles, occupy the epitympanum and the lateral part of the mesotympanum, immediately medial to the tympanic membrane (Fig 1). The largest of these ossicles is the malleus, which has its manubrium attached to the tympanic membrane. At the base of the manubrium near the neck of the malleus is a muscular process to which the tensor tympani muscle attaches (Fig 5). Immediately ventral to this process the chorda tympani crosses the medial aspect of the malleus on its way to join the lingual nerve. The incus lies between the malleus and the stapes (Fig 1). The base of the stapes faces medially and lies within the oval window of the labyrinth to articulate by a syndesmosis with the thin cartilage covering the edge of the oval window (Fig 1). The caudal crus of the stapes has a muscular projection to which attaches the tendon of the stapedius muscle. The stapedius muscle and tensor tympani muscle function as safety mechanisms by regulating the amount of tension exerted on the tympanic membrane and the oval window by the ossicles (Girgis & Maurice 1982). A conical cartilaginous structure named the ‘T-ossicle’ (Girgis & Maurice 1982) or the conical cartilage (Khalil & Spector 1985) has been identified in the dorsolateral compartment of the tympanic cavity of cats. The function of this ‘fourth’ ossicle remains undetermined, however, it is likely to be a vestigial remnant of the second arch bar (Reichert's cartilage) (Khalil & Spector 1985). Ventral and caudal to the oval window is the round window of the cochlea, which is covered by a delicate membrane (Figs 1, 5). The round window faces laterally. The rounded promontory extends medially from the round window on the dorsal wall of the tympanic cavity (Fig 6) (Little & Lane 1986).
Fig 5.
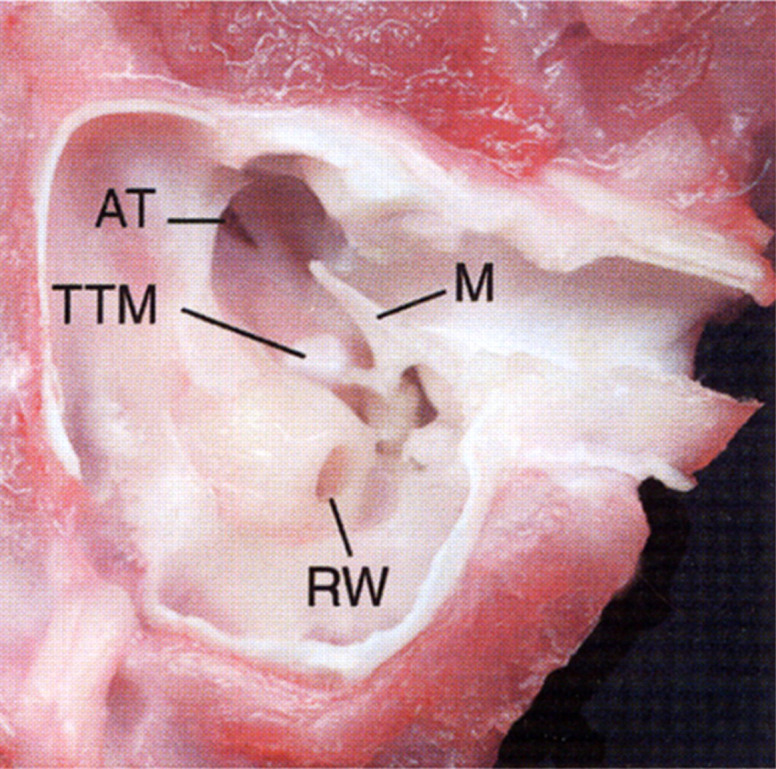
Ventral view of an anatomical dissection of the left tympanic cavity of a cat. The tympanic bulla and bony septum have been removed to expose the epitympanic recess. Note the round window (RW), malleus (M), tensor tympani muscle (TTM), and opening of the auditory tube (AT).
Fig 6.
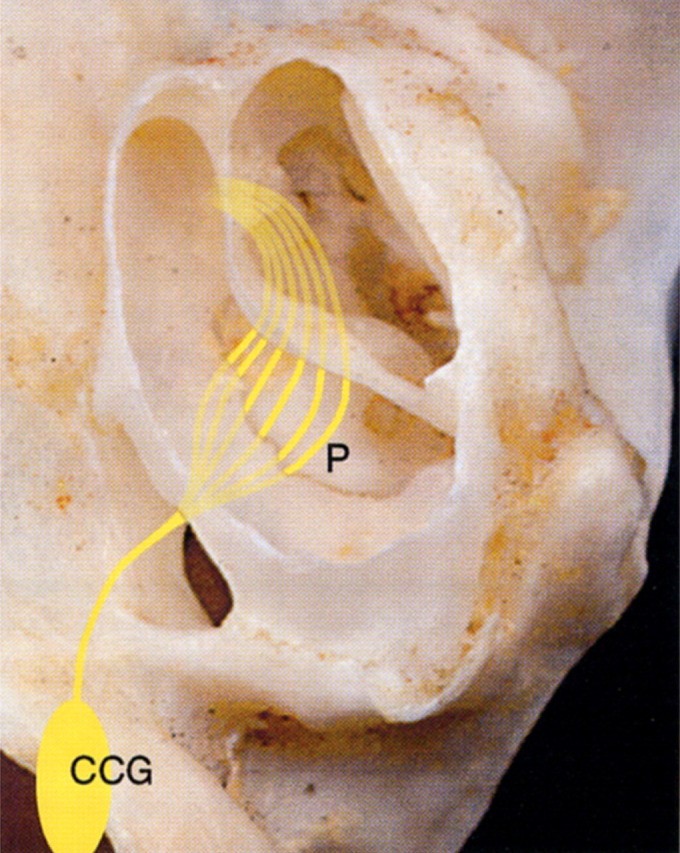
View of the ventral surface of the left petrous portion of the temporal bone with the left tympanic bulla and bony septum partially removed. The course of the sympathetic nerves that negotiate the middle ear of the cat on their course from the cranial cervical ganglion (CCG) to the eye are represented diagrammatically. The lighter colored lines represent nerve fibres that pass under the endotympanic plate, and are, thus, protected from surgical trauma. The darker yellow lines represent the fibres of the tympanic plexus that extend rostrally across the promontory (P), where they are highly vulnerable to surgical trauma. Interruption of the sympathetic nerve fibres at this location will result in Horner's syndrome. [Adapted with permission from Barlow & Root (1949) Journal of Comparative Neurology 91, 195–207.]
The auditory (eustachian) tube is a 5–8 mm long, partially cartilaginous tube that extends between the nasopharynx and a permanent opening in bone of the dorsomedial and rostral extremity of the dorsolateral compartment of the tympanic cavity (Figs 1, 5) (Rose 1978, Holzworth 1987, Little & Lane 1986). It is lined by pseudostratified columnar respiratory epithelium (Holzworth 1987). The auditory tube emerges by a 4 mm slit-like opening on the lateral wall of the nasopharynx, dorsal to the soft palate, where it is hidden from view unless the soft palate is pulled rostrally (Rose 1978). The auditory tube is closed in its resting state, and opens during sneezing, swallowing or yawning to equalise pressure in the middle ear.
Post-ganglionic sympathetic nerve fibres traverse the tympanic cavity of the cat (Fig 6) (Barlow & Root 1949, Little & Lane 1986). Interruption of this pathway will result in ipsilateral Horner's syndrome (miosis, ptosis, enophthalmos and protrusion of the nictitating membrane) (Fig 7). The sympathetic fibres arise from the cranial cervical ganglion which is located immediately caudal and medial to the tympanic bulla (Fig 6). After exiting the ganglion, the sympathetic fibres are associated with the degenerate internal carotid artery (Crouch 1969). In fetal cats the sympathetic nerve fibres traverse the middle ear in close proximity to the internal carotid artery, however, the internal carotid artery is degenerate in cats, and is occluded soon after birth (King 1987). The sympathetic nerve fibres enter the tympano-occipital fissure caudal to the tympanic bulla, and then pass between the tympanic bulla and the petrosal part of the temporal bone (Fig 6). The nerve fibres enter the tympanic cavity at the caudal extremity of the promontory, where they branch to form the tympanic plexus. The tympanic plexus is superficially located on the surface of the promontory, and is highly vulnerable to surgical trauma at this location (Little & Lane 1986). Careless removal of the bony septum, or overzealous curettage of the promontory, during bulla osteotomy, will result in a Horner's syndrome that may be permanent.
Fig 7.
Cat with Horner's syndrome in the right eye following a bulla osteotomy. Note the protrusion of the third eyelid, miosis, ptosis, and enophthalmos of the right eye.
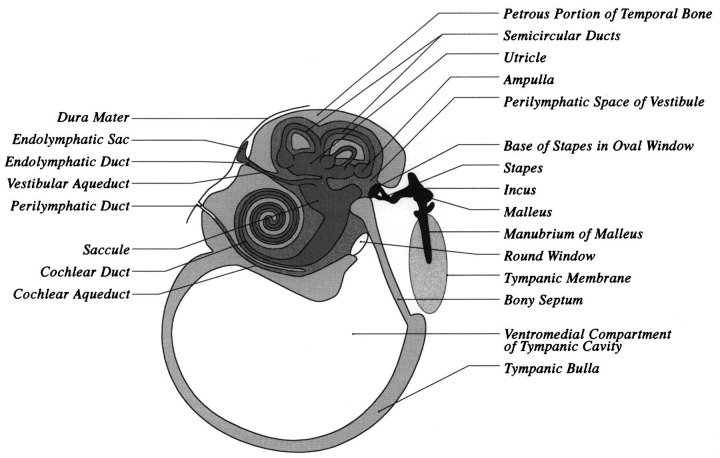
The inner ear consists of the bony labyrinth, which is a cavity within the petrosal portion of the temporal bone, and the membranous labyrinth, which is a delicate system of ducts and sacs within the bony labyrinth (Fig 8) (Gilbert 1968). The membranous labyrinth lies within the bony labyrinth, and closely resembles it in shape. The space between the membranous labyrinth and bony labyrinth is filled with a clear fluid (perilymph) and the membranous labyrinth is filled with fluid called endolymph. The ramifications of the vestibulocochlear nerve (VIIIth cranial nerve) terminate in sensory areas in the membranous labyrinth.
Fig 8.
Schematic line drawing of a transverse section of the middle ear (tympanic cavity) and inner ear of the cat at the level immediately caudal to the external auditory meatus. The perilymphatic duct, within the cochlear aqueduct, is shown as being continuous with the subarachnoid space of the brain.
Endolymph and perilymph are chemically different (Durrant & Lovrinic 1984, Jenkins 1978, Kandel et al 1991). The outstanding difference is that perilymph chemically is similar to the extracellular or cerebrospinal fluid (CSF), both having a high Na+ content and a low K+ content, whereas endolymph is similar to intracellular fluid, having a high K+ content and a low Na+ content. Perilymph is thought to be secreted by arterioles in the periosteum surrounding the labyrinth. A tube called the cochlear aqueduct connects the scala tympani of the cochlea with the subarachnoid space of the brain (Fig 8). Within this tube is the perilymphatic duct, which permits exchange between perilymph and CSF within the subarachnoid space (Durrant & Lovrinic 1984). The vestibular aqueduct contains the endolymphatic duct, which is formed by the utricular and saccular ducts, and extends to the extradural space, where it terminates in a dilated endolymphatic sac adjacent to the dura mater (Fig 8). Endolymph is probably produced by secretory cells in the transitional epithelium surrounding the sensory epithelia, and by the stria vascularis, the epithelium lining the upper part of the cochlear duct. Endolymph drains into the venous sinuses of the dura mater through the endolymphatic duct and sac.
The inner ear houses the structures that regulate equilibrium (semicircular canals, utricle and saccule), and the receptors for hearing (cochlea, which encases the organ of Corti) (Fig 8). The vestibule is the middle portion of the inner ear, and contains the saccule and utricle. There are two membrane covered openings (round and oval windows) into the bony labyrinth from the tympanic cavity. The membranous round (cochlear) window separates the air in the tympanic cavity from the scala tympani of the labyrinth, and compensates for pressure fluctuations caused by fluid waves through the perilymph. The oval (vestibular) window is located in the lateral wall of the vestibule, and contains the stapes, which transmits impulses to the perilymph circulating around the semicircular canals and cochlea.
The vestibular organ consists of interconnecting canals in the bone (bony labyrinth), and a single large cavity to which all canals connect, called the vestibule (Figs 1, 8). The vestibular portion of the membranous labyrinth consists of three delicate curved tubes (lateral, caudal and rostral semicircular canals) and two membranous sacs (the saccule and the utricle). The three semicircular canals each are in a different plane, and each contains a dilatation (ampulla), that in turn contains a crista. Receptor cells (hair cells) are located within each crista. Receptor cells are also located within the macula of the two sac-like structures within the bony vestibule—the saccule and the utricle. The macula in the sacculus is orientated in a vertical direction, whereas the macula in the utricle is orientated in a horizontal plane.
The receptors located in the semicircular canals are responsive to angular (rotational) acceleration or deceleration, while the receptors located in the utricle and saccule are responsive to linear acceleration (or deceleration) and gravity (Kandel et al 1991). The receptor cells show a tonic discharge at rest. This tonic rate of discharge is either accelerated or decelerated when the receptor cells are excited by movement. The receptor cells generate a potential in the primary afferent fibres in the vestibular portion of the vestibulocochlear nerve.
Primary afferent fibres from the vestibular receptors within the vestibulocochlear nerve pass through the skull via the internal acoustic meatus (along with the facial nerve), and enter the brain stem at the level of the rostral medulla. Many of the nerve fibres synapse in one of the four vestibular nuclei within the medulla, however, some bypass the vestibular nuclei, and enter the cerebellum via the caudal cerebellar peduncle.
There are numerous vestibular connections within the CNS. Connections descend the spinal cord via the lateral vestibulospinal tract to lower motor neurons of muscles of the neck, and thoracic and pelvic limbs. This is the largest (and thus the fastest) of all the descending pathways and is important for posture and locomotion. Interruption of input to this pathway from one side results in ipsilateral inhibition of extensor muscles and facilitation of flexor muscles, and contralateral facilitation of extensor muscles. The medial vestibulospinal tract descends in the medial longitudinal fasciculus to terminate in lower motor neurons of extensor muscles of the neck and back. Interruption of this pathway may result in flexion of the neck and trunk laterally, with the concavity directed toward the side of a vestibular lesion. Nerve fibres from all of the vestibular nuclei synapse with the lower motor neurons of the IIIrd, IVth and VIth cranial nerves, that innervate the extraocular muscles. These fibres are involved in the oculocephalic reflexes (eg, rolling of the eyes when the head is moved). There are numerous projections from vestibular nuclei into the reticular formation. Some of these are involved in the vomiting and cardiovascular reactions that may occur in vestibular disturbances.
Clinical signs of vestibular dysfunction
Disorders of the vestibular system produce varying degrees of loss of equilibrium resulting in loss of balance, ataxia and nystagmus (de Lahunta 1983). Vestibular disease is usually unilateral or asymmetrical, however, bilateral vestibular dysfunction may occur. Since strength is not affected by vestibular dysfunction, paresis is not observed. Animals with vestibular dysfunction may have one or more of the following clinical abnormalities: abnormal posture (eg, head tilt), asymmetrical ataxia, nystagmus or strabismus.
From a clinical standpoint, all these clinical signs should not be expected to occur in any one case. Jenkins (1978) states that the following points are important to consider: (1) many of the clinical signs of vestibular dysfunction are transient, and, therefore, may have disappeared, or have been compensated for, by the time of clinical examination; (2) many of the isolated clinical signs result from lesions other than those of the labyrinth (eg cerebellar lesions look similar to vestibular lesions); and, (3) nystagmus is not pathognomonic for any syndrome. Carpenter et al (1959) also address the alteration in signs of vestibular dysfunction that may be seen over time, making the following observations: (1) unilateral labyrinthectomy results in a horizontal nystagmus (rapid phase towards the contralateral side), however, this condition disappears after the 3rd or 4th post-operative day; (2) at first the ataxia seems more pronounced in the thoracic limbs, but later becomes greater in the pelvic limbs; and (3) after 3 weeks the severity of the neurologic deficits lessens tremendously (eg, only when an animal runs or jumps does it show evidence of disequilibrium).
Unilateral vestibular dysfunction
Loss of coordination and balance is reflected in a head tilt, with the more ventral ear usually directed towards the side of a vestibular lesion (Fig 9). Affected animals may also lean, fall, roll, or circle tightly, usually toward the side of a lesion. Animals roll towards the side of the lesion, due to reduced extensor tone on that side and increased extensor tone on the side opposite the lesion (Jenkins 1978). In time, visual compensation may assist a cat to compensate for a vestibular system deficit. Blindfolding an animal with subtle signs of vestibular disease may exacerbate signs of vestibular dysfunction, particularly a head tilt or circling. Cats with vestibular dysfunction assume a wide-based stance, and may lean or drift, usually toward the side of a lesion. Animals with acute vestibular dysfunction may also vomit, and /or ‘appear to be nauseous’.
Fig 9.
Deaf, white kitten with blue eye colour, and a head tilt toward the right side. The kitten had a congenital deafness and an acquired right-sided peripheral vestibular syndrome associated with head trauma.
Nystagmus is defined as involuntary rhythmic oscillations of the eye (Jenkins 1978). It may be a clinical sign of dysfunction of the central nervous system (cerebellum or brain stem), the eye, labyrinth (inner ear), or it may be induced in normal cats, where it is termed physiological nystagmus (eg, when moving the head from side to side, or following rotation of the head, or by irrigation of the external ear canals with either warm or cold water). The oscillations of the eye may occur either with equal movements in each direction (pendular nystagmus), or with a fast and slow phase (jerk nystagmus). Pendular nystagmus is usually associated with visual pathway deficits in (Siamese) cats (Guillery et al 1974). Should nystagmus occur when the head is held still, it is termed abnormal or spontaneous nystagmus.
Abnormal nystagmus is an involuntary, rhythmic oscillation of the eyeball due to disturbed vestibular inputs to the neurons innervating the extraocular muscles. Nystagmus probably occurs at some time during all types of vestibular disease. Typically nystagmus consists of a slow phase in one direction and a fast phase in the opposite direction. The direction of the nystagmoid movement is said to be that of the more easily observed fast phase (cerebellum or brain stem in origin), even though this is the compensatory direction (Jenkins 1978). The slow phase of rhythmic nystagmus is the active physiological component, and in most cases the slow phase is directed towards the side of a lesion. It should be noted that a cat may have a nystagmus in one direction early in a disease, and later may develop a nystagmus in the opposite direction. This change of direction is explained by the probability of an early disease process acting as an irritating lesion to produce ipsilateral nystagmus, and the progression of the lesion later resulting in a destructive process producing contralateral nystagmus (Jenkins 1978).
Nystagmus is described in terms of: (1) its direction (horizontal, rotary, or vertical); (2) whether it is non-positional (constant direction in all head positions) or positional (nystagmus that occurs only when the head is placed in an unusual position (eg, laterally or dorsally), or changes direction with altered head position; and (3) whether it is conjugate (same direction in each eye) or dysconjugate (different direction in each eye). In some cats with severe acute vestibular dysfunction, there may be a head oscillation or eyelid contraction that corresponds to the rate of nystagmus (Carpenter et al 1959).
Strabismus is a deviation of the eyeball from its normal position in the orbit. Strabismus often occurs with unilateral vestibular disturbances, resulting in a ventrally (or occasionally ventrolaterally) deviated eyeball. Strabismus usually occurs in the eye on the side of a unilateral vestibular lesion.
Bilateral vestibular dysfunction
Cats with bilateral peripheral vestibular disease, with complete loss of vestibular function, do not have postural asymmetry (eg, head tilt) nor do they exhibit nystagmus (Carpenter et al 1959). Normal oculocephalic reflexes are absent (ie, the eyes do not move when the head is moved), and affected cats usually have a symmetrical ataxia, may fall to either side, and may display characteristic ‘side to side’ excursions of the head.
Peripheral vs central vestibular disease
Once vestibular dysfunction has been recognised in an animal, it is then necessary to classify the vestibular dysfunction as peripheral or central (Table 1).
Table 1.
Clinical signs of peripheral and central vestibular disease
| Neurological sign | Peripheral | Central |
|---|---|---|
|
| ||
| Head tilt | yes | yes |
| Nystagmus | yes | yes |
| horizontal | yes | yes |
| rotary | yes | yes |
| vertical | no | yes |
| positional | no | yes |
| non-positional | yes | yes |
| conjugate | yes | yes |
| dysconjugate | no | yes |
| Strabismus | yes | yes |
| Asymmetrical ataxia | yes | yes |
| Cranial nerve deficits | VII possible | V, VI, VII possible |
| Horner's syndrome | yes | no |
| Cerebellar signs | no | possible |
| Mentation | normal | possibly reduced |
| Hemiparesis | no | possible |
| Proprioceptive deficits | no | possible |
Clinical signs of peripheral vestibular dysfunction
Clinical signs of peripheral vestibular dysfunction result from disorders of the middle and inner ear, that involve the receptors in the labyrinth and the vestibular nerve. Vestibular dysfunction does not occur with disease of the middle ear alone, however, many middle ear diseases extend to involve the inner ear, and result in signs of peripheral vestibular disease. Horner's syndrome (miosis, ptosis, enophthalmos and protrusion of the third eyelid) of the ipsilateral eye may be present with either middle or inner ear disease because the sympathetic trunk passes through the middle ear in close proximity to the promontory of the temporal bone (Figs 6, 7). The facial nerve also courses through this bone in contact with the vestibulocochlear nerve, and may be affected by middle and inner ear disorders.
Head tilt and ataxia, resulting from peripheral vestibular disease, are directed toward the side of the lesion. Nystagmus is horizontal or rotary, (with the fast phase directed away from the side of the lesion), non-positional, and conjugate. Strabismus is usually ventrolateral affecting the eye on the same side as a lesion. Mental status is normal, and paresis or deficits of proprioceptive positioning should not be present.
Clinical signs of central vestibular dysfunction
Clinical signs of central vestibular dysfunction result from disorders affecting the brain stem and associated structures. Any sign of brain stem dysfunction seen in association with vestibular signs indicates that central vestibular involvement is present. A common differentiating feature between central and peripheral vestibular disease is a deficit in postural reactions, as central vestibular lesions most often result in paresis or loss of conscious proprioception on the side of a unilateral lesion. Nystagmus is usually present and may also be important in differentiating central from peripheral disease. Nystagmus may vary in direction with change in head position (positional nystagmus) and may also be dysconjugate. Vertical nystagmus, in any head position, almost always is indicative of central vestibular disease. Affected cats usually have a head tilt, ventrolateral strabismus, and asymmetrical ataxia. Other clinical signs of brain stem disease (eg, ipsilateral proprioceptive positioning deficits, hemiparesis, reduced mental status, and other cranial nerve deficits [particularly cranial nerve V], or signs of cerebellar disease (eg, dysmetria and intention tremors), may be present. Clinical signs (eg, seizures) reflecting involvement of other areas of the CNS, seen in association with clinical signs of central vestibular dysfunction, may be apparent in animals with multifocal disease.
Clinical signs of paradoxical central vestibular disease
Unilateral lesions affecting the peripheral vestibular system produce clinical signs of vestibular dysfunction on the same side as the vestibular system lesion. The same is most often true of unilateral lesions of the central vestibular system. Exceptions, therefore, are termed paradoxical. Lesions of the central vestibular system that affect the rostral and medial vestibular nuclei, caudal cerebellar peduncle, and flocculonodular lobe of the cerebellum, produce clinical signs of vestibular system dysfunction that occur on the side opposite the lesion (de Lahunta 1983). Such lesions are usually space-occupying lesions of the cerebellomedullary angle. In the presence of clinical signs of vestibular dysfunction, the observation of postural reaction deficits, or additional cranial nerve abnormalities (eg, facial or trigeminal nerve deficits) is the most reliable indicator of the side on which a lesion is located.
Acknowledgement
The authors thank John Doval for preparation of illustrations and figures.
References
- Barlow CM, Root WS. (1949) The ocular sympathetic path between the superior cervical ganglion and the orbit in the cat. Journal of Comparative Neurology 91, 195–207. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Fabrega H, Glinsmann W. (1959) Physiological deficits occurring with lesions of the labyrinth and fastigeal nuclei. Journal of Neurophysiology 22, 222–234. [DOI] [PubMed] [Google Scholar]
- Crouch JE. (1969) Text-Atlas of Cat Anatomy. Philadelphia: Lea & Febiger. pp 212–213. [Google Scholar]
- de Lahunta A. (1983) Veterinary Neuroanatomy and Clinical Neurology. 2nd edn. Philadelphia: WB Saunders. pp 238–254. [Google Scholar]
- Durrant JD, Lovrinic JH. (1984) Bases of Hearing Science. 2nd edn. London: Williams & Wilkins. pp 85–113. [Google Scholar]
- Gilbert SG. (1968) Pictorial Anatomy of the Cat. Seattle: University of Washington Press. pp 106–108. [Google Scholar]
- Girgis IH, Maurice M. (1982) The ossicular system of cats. Journal of Laryngology and Otolaryngology 96, 195–203. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Casagrande VA, Obendorer MD. (1974) Congenitally abnormal vision in Siamese cats. Nature 252, 195–199. [DOI] [PubMed] [Google Scholar]
- Holzworth J. (1987) Diseases of the Cat. Philadelphia: WB Saunders. pp 724–738. [Google Scholar]
- Jenkins TW. (1978) Functional Mammalian Neuroanatomy. 2nd edn. Philadelphia: Lea & Febiger. pp 337–356. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. (1991) Principles of Neural Science. 3rd edn. Connecticut: Appleton & Lange. pp 500–510. [Google Scholar]
- Khalil MA, Spector M. (1985) The conical cartilage of the cat's middle ear. Journal of Laryngology and Otolaryngology 99, 831–838. [DOI] [PubMed] [Google Scholar]
- King AS. (1987) Physiological and Clinical Anatomy of the Domestic Mammals. Volume 1. Central Nervous System. New York: Oxford University Press, pp 2–4. [Google Scholar]
- Little CJL, Lane JG. (1986) The surgical anatomy of the feline bulla tympanica. Journal of Small Animal Practice 27, 371–378. [Google Scholar]
- Rose WR. (1978) The eustachian tube–1: General considerations. Veterinary Medicine Small Animal Clinician 73, 882–887. [PubMed] [Google Scholar]