Adverse reactions to phenobarbital administration have been reported in humans and dogs. This case history describes a young domestic shorthair cat that presented with clinical signs compatible with an adverse drug reaction to phenobarbital. Clinical signs included depression, anorexia, cutaneous eruptions, and a severe, generalised lymphadenopathy. These signs began approximately 21 days after beginning phenobarbital administration. Similarities between this reaction and the anticonvulsant hypersensitivity syndrome are demonstrated and possible aetiologies are discussed.
Case Report
An 18-month-old castrated male domestic short-hair cat was presented to the Veterinary Teaching Hospital at Texas A&M University for a recent onset of generalised seizures. Previous medical history included a head injury approximately 1 year prior to presentation. Neurological examination localised the lesion to the right forebrain. Complete blood count (CBC) and serum chemistry profile results were normal. Feline leukaemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody assays were negative. Cerebrospinal fluid analysis results were within normal limits. Computed tomography (CT) of the brain revealed a triangular-shaped area of a decreased (fluid) opacity in the peripheral right frontal and parietal lobes of the cerebrum. This area did not enhance with intravenous contrast medium. Based on the CT results, the seizures were suspected to be posttraumatic or vascular in origin. Treatment was started with phenobarbital 4 mg/kg every 12 h. Seizure frequency decreased over a 1-week period, and the cat was discharged from the hospital.
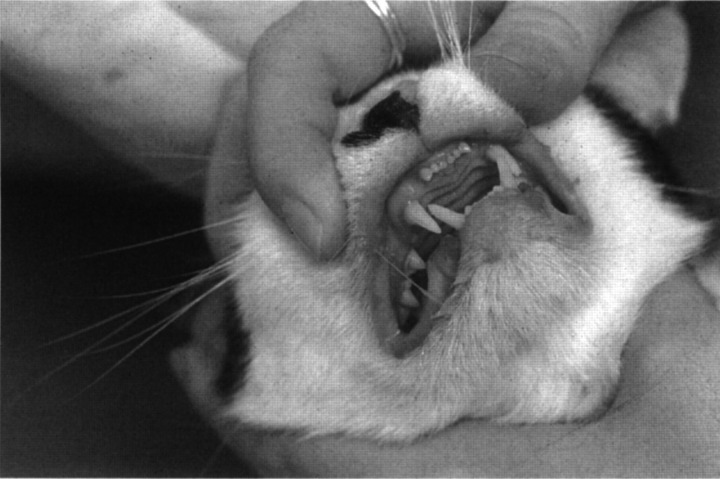
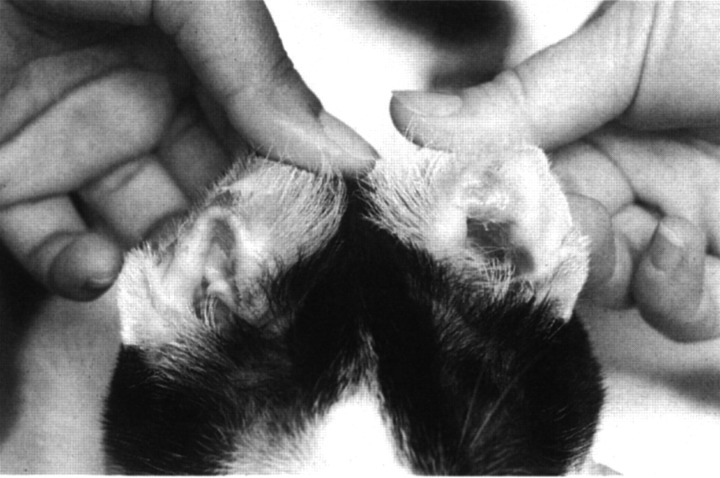
After 4 weeks of phenobarbital treatment, the cat presented with a 1-week history of lethargy, decreased appetite and difficulty with walking. He was depressed and seemed to be in pain. His body temperature was 38°C, pulse was 144 beats per minute, and respiratory rate was 32 breaths per minute. On physical examination, he was approximately 5% dehydrated. Heart and lung sounds were within normal limits. The submandibular, retropharyngeal, prescapular and popliteal lymph nodes were enlarged. His hair-coat was unkempt. There was swelling and erythema of all four paws, most severe at the nail beds. Numerous ulcerated, exudative lesions were present in the mouth, located mostly at the mucocutaneous junctions (Fig 1). Halitosis and ptyalism were noted. There were bilaterally symmetric, ulcerated, encrusted lesions on the inside of the pinna of both ears (Fig 2). Results of neurological examinations were unchanged from 4 weeks previously. He was reluctant to walk and appeared to be in pain when he put any pressure on his paws. The cat was re-admitted to the hospital for further evaluation and treatment.
Fig 1.
Oral lesions seen in a cat with phenobarbital hypersensitivity. Note the multiple erosions and erythematous lesions at the mucocutaneous junction.
Fig 2.
Bilaterally symmetric vesicular lesions on the inside of the pinna of both ears in a cat with hypersensitivity to phenobarbital. Lesions are ulcerated and encrusted. Similar lesions were present surrounding the pads and nailbeds of all four paws.
A CBC detected a mild monocytosis (950 cells/μl), increased haematocrit (47%), and increased total protein concentration (8.6 g/dl). Serum alanine transferase, alkaline phosphatase, albumin, blood urea nitrogen, creatinine, and electrolytes were within normal limits. The serum phenobarbital concentration was 39.1 μg/ml (target range 15.0–45.0 μg/ml). Based on the history and clinical signs, an adverse drug reaction was suspected. Histopathology of skin lesions was not performed.
Phenobarbital was discontinued, and the cat was administered a loading dose of potassium bromide to treat seizure (400 mg/kg divided over 5 days) (Boothe 1998). His mouth was flushed with sterile 0.9% NaCl and chlorhexidine gel (CIIX-guard LA, VRX Products) was applied three times a day. The patient was initially anorexic. On day 3 of hospitalisation, the oral lesions were mildly improved, but he remained uninterested in food. By day 6, the cat's oral erosions were almost resolved; he began to eat canned tuna and moist cat food (a/d, Hill's Pet Products). The generalised lymphadenopathy was still present, but the lymph nodes were reduced in size since presentation. The swelling and erythema of the paws and nail beds had resolved. On day 7, the serum phenobarbital level was 9.8 μg/ml and the scrum bromide level was 0.45 mg/ml (therapeutic range 1.0–3.0 mg/ml). The cat was discharged from the hospital with potassium bromide at a maintenance dose of 30 mg/kg/day Two weeks later the patient was re-evaluated. Seizures had not recurred. According to the owner, his appetite was good and he appeared well groomed. No cutaneous lesions were found, although a mild retropharyngeal lymphadenopathy remained on the right side of his neck.
Discussion
Adverse reactions to drugs generally fall into one of two groups: (1) acute toxicity related to dosage, or (2) delayed hypersensitivity that is idiosyncratic and much more difficult to diagnose (Boothe 1998). An example of the second type would include a drug-induced Type II hypersensitivity reaction. Adverse reactions to phenobarbital have been reported in dogs (Bunch 1982, 1984, Jacobs 1998, Inzana 1998). These dogs had either hepatic dysfunction, which was believed to be related to the dose of phenobarbital administered, or varying degrees of blood dyscrasias, which were manifested as neutropenia, thrombocytopenia, or anaemia. The latter reactions were suspected to be immune mediated (Type II hypersensitivity reactions), possibly due to hapten formation between the drug and blood cell proteins, which induced a cytotoxic immune response (Jacobs 1998).
In humans, a typical group of clinical and laboratory findings are associated with a syndrome of delayed hypersensitivity to phenobarbital named anti-convulsant hypersensitivity syndrome (AHS) (Shear & Spielberg 1988). Clinical signs usually begin 1–4 weeks after starting phenobarbital therapy, but can occur as late as 2–3 months into the course of treatment. Patients usually present with a fever and generalised lymphadenopathy. Dermatitis is common, and may evolve into toxic epidermal necrolysis. The syndrome may gradually progress to involve several body systems; hepatitis, haematologic abnormalities, and renal dysfunction may ensure. All or just a few of these clinical signs may occur in a single patient. The majority of patients recover after discontinuation of phenobarbital treatment, but clinical signs may persist for weeks or months (Morkunas & Miller 1997).
Cross-reactivity to other anti-convulsants is a disturbing problem and has been seen with phenytoin and carbamazepine (Handfield-Jones 1993). In vitro studies with lymphocytes from hypersensitive patients have suggested the aetiology of this syndrome (Shear & Spielberg 1988). Phenobarbital, phenytoin, and carbemazepine have in common an aromatic benzene ring, which is metabolised by cytochrome P450 to an arene oxide. Arene oxide metabolites are unstable, and are normally detoxified by the enzyme epoxide hydrolase. When there are insufficient quantities of epoxide hydrolase, arene oxide metabolites will covalently bond to macromolecules, and cause cytotoxicity or form co-antigens that may stimulate an immune response (Anonymous 1994, Brown 1997). It is hypothesised that this enzymatic deficiency leads to a build-up of toxic arene oxide metabolites and, subsequently, delayed hypersensitivity. Furthermore, a genetic basis for epoxide hydrolase deficiency is suspected (Shear & Spielberg 1988), and may lead to the familial occurrence of AHS in patients. An effective screening test for AHS is not currently available. The previously mentioned in vitro studies have not been reported in dogs or cats.
The cat in this report had severe cutaneous eruptions and marked lymphadenopathy. He was afebrile while hospitalised, although he had a 1-week history of illness prior to hospitalisation, during which time his body temperature was not evaluated. The only haematologic abnormalities noted were those secondary to mild dehydration (elevated haematocrit and plasma protein concentration) and a monocytosis. There was no laboratory or clinical evidence of hepatic dysfunction.
The clinical signs in this patient resolved within 7–14 days after discontinuation of phenobarbital. No other definitive therapy was given and the clinical signs have not recurred. This presumably represented an adverse reaction to phenobarbital in this cat. An immune mediated drug reaction, or hypersensitivity, would be most likely. It appears this hypersensitivity may be analogous to AHS in humans, although this cannot be proven. Significant haematologic changes and hepatitis were not seen in this patient, but it is possible that these clinical findings might be a part of the progression of this syndrome when left untreated. Only a repeat challenge with phenobarbital or the development of an in vitro assay for detection of epoxide hydrolase levels in cats would allow a more definitive diagnosis.
The choices for an anti-convulsant drug to use in cats are limited. Diazepam and phenobarbital are currently the most commonly used drugs (Quesnal 1997, Cochrane 1990). Recently, several cats were reported with acute hepatic necrosis and death secondary to long-term oral diazepam treatment (Hughes 1996). Phenobarbital, therefore, is now the logical drug to use in cats with seizures. However, clinicians should be cautious and should monitor patients for possible signs of an adverse drug reaction. Potassium bromide is an alternative possibility for anti-convulsant therapy in cats (George 1996). This case illustrates the need for well-designed clinical trials of new anti-convulsant drugs for use in cats.
References
- Anonymous (1994) Anticonvulsant hypersensitivity. The Medical Journal of Australia 160, 650–651. [PubMed] [Google Scholar]
- Boothe DM. (1998) Adverse drug reactions in cats. Proceedings of the North American Veterinary Conference 29–42.
- Brown CE, Smith GD, Coniglione T. (1997) Anticonvulsant hypersensitivity: An unfortunate case of triple exposure to phenytoin. The Journal of Family Practice 45, 434–437. [PubMed] [Google Scholar]
- Bunch SE, Castleman WL, Hornbuckle WE, Tennant BC. (1982) Hepatic cirrhosis associated with long-term anticonvulsant drug therapy in dogs. Journal of the American Veterinary Medical Association 181, 357–362. [PubMed] [Google Scholar]
- Bunch SE, Baldwin BH, Hornbuckle WE, Tennant BC. (1984) Compromised hepatic function in dogs treated with anticonvulsant drugs. Journal of the American Veterinary Medical Association 184, 444–448. [PubMed] [Google Scholar]
- Cochrane SM, Black WD, Parent JM, Allen DG, Lumsden JH. (1990) Pharmacokinetics of phenobarbital in the cat following intravenous and oral administration. Canadian Journal of Veterinary Research 54, 132–138. [PMC free article] [PubMed] [Google Scholar]
- Cochrane SM, Black WD, Parent JM, Allen DG, Lumsden JH. (1990) Pharmacokinetics of phenobarbital in the cat following multiple oral administration. Canadian Journal of Veterinary Research 54, 309–312. [PMC free article] [PubMed] [Google Scholar]
- George K, Boothe DM. (1996) Disposition of bromide in cats following oral administration of the potassium salt (abstract). Proceedings of the 14th ACVIM Forum, San Antonio, Texas, 757.
- Handfield-Jones SE, Jenkins RE, Whittaker SJ, Besse CP, McGibbon DH. (1993) The anticonvulsant hypersensitivity syndrome. British Journal of Dermatology 129, 175–177. [DOI] [PubMed] [Google Scholar]
- Hughes D, Moreau RE, Overall KL, Van Winkle TJ. (1996) Acute hepatic necrosis and liver failure associated with benzodiazepine therapy in six cats, 1986–1995. The Journal of Veterinary Emergency and Critical Care 6, 13–20. [Google Scholar]
- Inzana KD, Allyn M, Davies C. (1998) Pancytopenia in three dogs associated with phenobarbital therapy. Proceedings of the 16th ACVIM Forum, San Diego, California, 725.
- Jacobs G, Calvert C, Kaufman A. (1998) Neutropenia and thrombocytopenia in three dogs treated with anticonvulsants. Journal of the American Veterinary Medical Association 212, 681–684. [PubMed] [Google Scholar]
- Morkunas AR, Miller MB. (1997) Anticonvulsant hypersensitivity syndrome. Critical Care Clinics 13, 727–739. [DOI] [PubMed] [Google Scholar]
- Quesnal AD, Parent JM, McDonell W. (1997) Clinical management and outcome of cats with seizure disorders: 30 cases (1991–1993). Journal of the American Veterinary Medical Association 210, 72–77. [PubMed] [Google Scholar]
- Shear NH, Spielberg SP. (1988) Anticonvulsant hypersensitivity syndrome in vitro assessment of risk. Journal of Clinical Investigation 82, 1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]