Abstract
Estimates of in vivo insulin sensitivity (SI) can be derived from minimal model analysis of a frequently sampled intravenous glucose tolerance test (FSIVGTT). Modification of the FSIVGTT by the injection of insulin allows insulin sensitivity to be measured in diabetics. To establish and compare reference values for insulin sensitivity in clinically normal and diabetic cats, we subjected 10 clinically normal cats and five diabetic cats to the insulin-modified FSIVGTT with minimal model analysis. Diabetic cats had a significantly lower insulin sensitivity than clinically normal cats (P <0.05). Mean insulin sensitivity in clinically normal cats was 3.22 × 10−4/min/μU/ml (range 1.71–5.23 × 10−4/min/μU/ml). In contrast, the mean insulin sensitivity in diabetic cats was 0.58 × 10−4/min/μU/ml (range 0.136–0.88 × 10−4/min/μU/ml), or approximately six times less insulin sensitive than clinically normal cats. Mean glucose effectiveness in clinically normal cats was 0.030/min (range 0.021–0.045/min). Mean glucose effectiveness in diabetic cats was 0.014/min (range 0.008–0.021/min). Our data demonstrate that insulin resistance is a feature of feline diabetes mellitus and that diabetic cats have a similar relative decrease in insulin sensitivity to humans with type 2 diabetes.
Diabetes mellitus is one of the most frequent endocrinopathies in cats, with an estimated incidence of between one in 100 and one in 400 (Panciera et al 1990, Rand 1997). Feline diabetes has been classified as type 1, type 2 or secondary (Lutz & Rand 1995). Histological, clinical and laboratory data indicate that the most frequent form of diabetes in cats is analogous to human type 2 diabetes (Lutz & Rand 1995). Although insulin dependence and ketosis are more frequent (Nelson et al 1993), feline diabetes has many of the characteristics of the human disease. In humans it is recognised that type 2 diabetes mellitus is a combined abnormality of impaired beta cell function (reduced insulin secretion) and insulin resistance, although the severity of these two defects may vary in different subgroups of type 2 diabetic patients (Eriksson et al 1989, Leahy 1990, Porte 1991, Ostenson et al 1992, Taylor et al 1994). In cats, variable degrees of beta cell function have also been reported (O'Brien et al 1985, Lutz & Rand 1996).
Insulin resistance is a pathological condition in which the magnitude of the biological response to insulin is decreased (Kahn 1978). When insulin and glucose concentrations are compared between normal and diabetic cats, it suggests that diabetic cats are also insulin resistant (O'Brien et al 1985). However, insulin resistance has not yet been documented in diabetic cats using accepted measures of insulin sensitivity.
Insulin resistance is postulated to contribute to beta cell failure and decreased insulin secretion through a mechanism of cellular exhaustion (Porte 1991). Hepatic and peripheral resistance to insulin results in increased hepatic glucose production and decreased peripheral glucose utilisation, leading to hyperglycaemia (Porte 1991). Hyperinsulinaemia then develops as a compensatory mechanism. When sufficient islet function is present, insulin levels continue to rise until the effects of insulin resistance are overcome (Porte 1991). As islet function declines due to beta cell ‘exhaustion’, a greater degree of hyperglycaemia is necessary to stimulate the beta cells to produce sufficient insulin to compensate for the insulin resistance. Eventually, however, the beta cells are no longer able to compensate and absolute hypoinsulinaemia occurs (Porte 1991).
A number of different methods have been developed to estimate insulin sensitivity. While the euglycaemic insulin clamp has been considered to be the ‘gold standard’, the minimal model method is a simpler protocol which provides estimates of insulin sensitivity that correlate significantly with those from the glucose clamp (Bergman et al 1986, 1987, Groop et al 1993). A tolbutamide modified protocol improves the correlation between minimal model and glucose clamp estimates of insulin sensitivity in humans (Beard et al 1986). However, this protocol is unable to calculate insulin sensitivity in diabetic patients, as it requires the endogenous secretion of insulin (Beard et al 1986). An insulin-modified protocol was developed, which allows insulin sensitivity to be calculated across the spectrum of glucose tolerance (Welch et al 1990, Saad et al 1994). Petrus et al (1996) showed that values for insulin sensitivity in normal cats obtained by the euglycaemic clamp method were highly correlated (r = 0.93) with estimates using the minimal model. In their study, the standard minimal model protocol was used, and only values for normal cats were reported.
The aim of the present study was to establish reference values for insulin sensitivity using the insulin-modified minimal model method in clinically normal adult cats with normal glucose tolerance and to compare these with values from diabetic cats.
Research design and methods
Animals
This study was approved by the Animal Experimentation Ethics Committee of the University of Queensland. To measure insulin sensitivity in normal cats, 10 mixed-bred cats (six castrate males and four castrate females) were obtained from the University of Queensland veterinary research colony. The cats were adults, but exact age was unknown. Mean body weight of the cats was 4.6 kg (range 3.7–5.8 kg). Normal body condition was determined using a five-point body condition scoring system (Donoghue & Kronfeld 1993). Non-obese cats with a score of 2–4 were used in the study. For inclusion in the study, normal cats had to have fasting normoglycaemia and normal intravenous glucose tolerance test (IVGTT) results (T1/2<86 min) (Link et al 1997). Clinical characteristics of the normal cats are shown in Table 1.
Table 1.
Clinical characteristics of normal cats
| Cat | Breed | Sex | Age (years) | Body weight (kg) | Body condition score (1–5) |
|---|---|---|---|---|---|
|
| |||||
| 1 | DSH | Mc | 1–2 | 4.2 | 3 |
| 2 | DSH | Mc | 1–2 | 5.2 | 3 |
| 3 | DSH | Mc | 1–2 | 4.6 | 3 |
| 4 | DSH | Mc | 1–2 | 4.7 | 3 |
| 5 | DLH | Fs | 1–2 | 3.9 | 3 |
| 6 | DSH | Mc | 1–2 | 4.5 | 3 |
| 7 | DSH | Fs | 1–2 | 3.8 | 2 |
| 8 | DSH | Fs | 1–2 | 5.7 | 4 |
| 9 | DSH | Mc | 1–2 | 3.7 | 2 |
| 10 | DSH | Fs | 1–2 | 5.8 | 4 |
DSH—domestic short hair, DLH—domestic long hair, Mc—male castrate, Fs—female spayed.
Five newly diagnosed (insulin treatment <5 days) diabetic cats were recruited from the University of Queensland Small Animal Clinic after owner consent was obtained. Diabetes was defined as a persistent fasting blood glucose concentration greater or equal to 12.6 mmol/l (227 mg/dl) in cats with clinical signs of diabetes (Rand 1997). Signs considered consistent with feline diabetes were polyuria/polydipsia, glycosuria, and weight loss (Crenshaw & Peterson 1996). Only cats judged to be in good health, as determined by clinical examination and routine haematological and biochemical analyses, were used in the study. The mean morning blood glucose before insulin administration was 20.7 mmol/l (374 mg/dl) (range 17.8–23.7 mmol/l; 320–427 mg/dl). Serum triglycerides were increased in the three cats in which it was measured (mean 7.9 mmol/l, range 1.5–18.7, normal <1.1 mmol/l). All cats were negative for urine ketones. All cats were non-obese with body condition scores of 2–4. Clinical characteristics and morning glucose concentration of the diabetic cat are shown in Table 2. Diabetic cats were treated with porcine lente insulin 0.25–0.5 U/kg twice daily. Insulin therapy was withheld for at least 24 h before the insulin sensitivity test.
Table 2.
Clinical characteristics of diabetic cats
| Cat | Breed | Sex | Age (years) | Body weight (kg) | Body condition score (1–5) | Fasting glucose prior to morning insulin (mmol/l) |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | DSH | Mc | 12 | 5.5 | 3 | 17.8 |
| 2 | DSH | Fs | 8.5 | 4.8 | 3 | 19.3 |
| 3 | DSH | Mc | 10 | 6.8 | 4 | 23.7 |
| 4 | DSH | Mc | 5 | 6.5 | 3 | 21.9 |
| 5 | Bur | Mc | 13 | 4.7 | 2 | 21.0 |
DSH—domestic short hair, Bur—Burmese.
Experimental protocol
In the clinically normal cats, a jugular catheter was placed, followed 2 days later by an intravenous glucose tolerance test. The minimal model experiment was performed the day after the glucose tolerance test. Diabetic cats were tested with the minimal model method 2 days after jugular catheter placement.
Jugular catheter placement
A jugular catheter (18 gauge × 8 cm, Cook Australia) was placed into the left jugular vein under general anaesthesia (Propofol 5 mg/kg iv; ICI) for blood sampling and drug administration (Martin & Rand 1999). Catheter patency was maintained by flushing with 2 ml of heparinised saline (20 IU heparin [Multiparin; Fisons Pharmaceutical Pty] per ml of saline [0.9% sodium chloride intravenous infusion BP, Baxter Healthcare Pty]) daily. Tests were performed at least 48 h after catheter placement.
Intravenous glucose tolerance test
The test was performed at 0800 hours after a 12-h fast. Glucose (0.5 g/kg, Glucose Injection B.P.; Astra Pharmaceuticals) was injected through the jugular catheter over 30–45 s, followed immediately by a 1 ml flush with saline (0.9% sodium chloride intravenous infusion BP). Blood samples of 0.5 ml each were collected at 0, 2, 5, 10, 15, 30, 45, 60, 90 and 120 min. Prior to each blood collection, 0.8 ml of blood was removed from the jugular catheter using a sterile syringe and re-injected into the catheter immediately after the sample collection. The catheter was then flushed with 1 ml of saline (0.9% sodium chloride intravenous infusion BP). Blood samples were placed in EDTA tubes in which 0.05 ml of 10 000 KIU/ml aprotinin (Trasolyl; Bayer Pharmaceuticals) had been added, and immediately centrifuged at 1500 ×g for 10 min. The plasma obtained was pipetted into 0.6 ml microcentrifuge tubes (Quality Scientific Plastics) and immediately frozen for subsequent glucose determination. Results were compared with reference values for our laboratory (Link & Rand 1998).
The insulin-modified FSIVGTT with minimal model analysis
The minimal model was performed 1 day after the glucose tolerance test. Testing began at 0800 h after a 12-h fast. Four baseline blood samples were collected over 15 min (−15, −10, −5 and −1 min) before 0.3 g/kg of glucose (Glucose Injection B.P.) was administered as an intravenous bolus. Twenty-seven blood samples were then collected over the next 3 h at times 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160 and 180 min for glucose and insulin determination. Twenty minutes after the glucose injection, insulin was injected intravenously (human regular insulin [Actrapid], 0.05 U/kg in non diabetics; 0.3 U/kg in diabetics). These insulin doses have been shown to give optimal results in humans and, in our experiment, peak insulin levels after the injection of insulin were not significantly different in normal and diabetic cats. Blood samples were collected and handled as for the intravenous glucose tolerance test. After the plasma was collected, the red blood cells were resuspended in 1 ml of normal saline. The tubes were spun at 1500 ×g for 5 min and the supernatant pipetted off and discarded to remove any aprotinin (Trasolyl; Bayer Pharmaceuticals) and EDTA from the red blood cells. The red blood cells were resuspended in 0.5 ml of normal saline and reinjected into the cat through the jugular catheter at the end of the experiment.
Glucose and insulin measurements
Glucose was measured using a YSI glucose analyser (Yellow Springs Instruments, Yellow Springs, OH, USA). Insulin was measured using the Pharmacia insulin radioimmunoassay kit which detects both human and feline insulin (Lutz & Rand 1993).
Minimal model analysis
Insulin sensitivity was determined using the MINMOD computer program (Bergman et al 1979), kindly donated by R.N. Bergman.
Statistical analysis
Statistical comparisons between the insulin sensitivity of clinically normal and diabetic cats were made using the ANOVA test (Dunn's method). Statistical significance was accepted at P <0.05.
Results
Intravenous glucose tolerance test
The glucose half-life (T1/2) for the 10 clinically normal cats ranged from 49.84 to 65.27 min (mean T1/2 55.75±6.16 min) and was within the reference range for our laboratory (<86 min; Link et al 1997).
Insulin-modified FSIVGTT with minimal model analysis
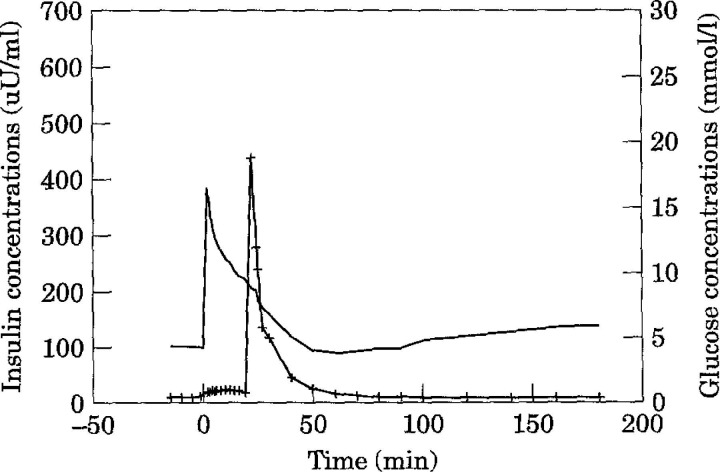
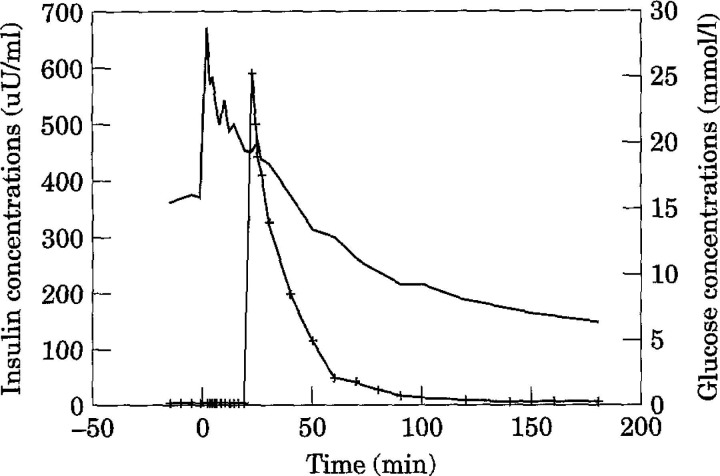
Based on the insulin-modified FSIVGTT with minimal model analysis, mean insulin sensitivity (SI) in clinically normal cats was 3.22 × 10−4min/μU/ml (range 1.71–5.23 × 10−4min/μU/ml) (Table 3). The calculated 95% confidence interval for the population was 0.94–5.50 ×10−4/min/μU/ml. In contrast, the mean SI in diabetic cats was 0.58×10−4/min/μU/ml (range 0.136–0.88×10−4/min/μU/ml) (Table 4). Diabetic cats had a significantly lower SI than clinically normal cats (P <0.05) and were on average six times less sensitive to insulin than normal cats. One diabetic cat displayed profound insulin resistance (SI = 1.84 ×10−10/min/μU/ml) (Table 4). However, the peak insulin concentration was three times higher than the other diabetic cats, even though basal insulin levels were similar. The reason for this is unknown. As it may have been due to an error in the amount of insulin administered, statistical values were calculated omitting this result. Mean insulin and glucose concentrations during the insulin-modified FSIVGTT are shown for normal (Fig 1) and diabetic (Fig 2) cats. Areas under the insulin and glucose curves were significantly different between normal and diabetic cats (P <0.05).
Table 3.
Insulin-modified FSIVGTT in normal cats
| Cat | p2×10−2 (per min) | p3×10−5 (per min) | G (0) | SG (per min) | SI×10−4 (per min/μU/ml) | |
|---|---|---|---|---|---|---|
|
| ||||||
| (mg/dl) | (mmol/l) | |||||
|
| ||||||
| 1 | 3.07 | 1.21E+00 | 235 | (13.1) | 0.021 | 3.94 |
| 2 | 3.25 | 6.88E–01 | 276 | (15.3) | 0.022 | 2.12 |
| 3 | 6.40 | 2.28E+00 | 300 | (16.7) | 0.034 | 3.56 |
| 4 | 11.35 | 3.05E+00 | 477 | (26.5) | 0.045 | 2.68 |
| 5 | 10.92 | 2.59E+00 | 520 | (28.9) | 0.036 | 2.37 |
| 6 | 3.57 | 8.62E–01 | 269 | (14.9) | 0.024 | 2.41 |
| 7 | 3.94 | 2.06E+00 | 214 | (11.9) | 0.022 | 5.23 |
| 8 | 3.54 | 1.68E+00 | 314 | (17.4) | 0.034 | 4.74 |
| 9 | 9.21 | 1.58E+00 | 391 | (21.7) | 0.026 | 1.71 |
| 10 | 6.19 | 2.12E+00 | 294 | (16.3) | 0.038 | 3.43 |
| Mean | 6.14 | 1.81E+00 | 329 | (18.3) | 0.030 | 3.22 |
| SD | 3.26 | 0.75E+00 | 102 | (5.7) | 0.008 | 1.16 |
| 95% CI of the mean | 0.72 | |||||
| 95% CI of the population | 2.28 | |||||
SI—insulin sensitivity index, SG—glucose effectiveness, G (0)—theoretical plasma glucose at time 0, as extrapolated from the minimal model.
Parameter p2 is the disappearance rate constant of insulin from the remote (interstitium) compartment.
Parameter p3 characterises the ability of insulin to cross the capillary endothelium and its subsequent effects to both increase peripheral glucose disposal and inhibit net hepatic glucose production.
SD—standard deviation, CI—confidence interval.
Table 4.
Insulin-modified FSIVGTT in diabetic cats
| Cat | p2×10−2 (min) | p3×10−5 (min) | G (0) | SG (min) | SI×10−4 (min/μU/ml) | |
|---|---|---|---|---|---|---|
|
| ||||||
| (mg/dl) | (mmol/l) | |||||
|
| ||||||
| 1 | 1.79 | 1.23E–01 | 367 | (20.4) | 0.008 | 0.69 |
| 2 | 2.05 | 1.28E–01 | 437 | (24.3) | 0.011 | 0.62 |
| 3 | 1.24 | 1.68E–02 | 692 | (38.4) | 0.016 | 0.14 |
| 4 | 1.29 | 1.14E–01 | 446 | (24.8) | 0.021 | 0.88 |
| 5 | 189.5 | 3.47E–07 | 546 | (30.0) | 0.036 | 1.83×10−6* |
| Mean | 1.59 | 9.55E–02 | 486 | (27.0) | 0.014 | 0.58 † |
| SD | 0.40 | 5.28E–02 | 142 | (7.9) | 0.006 | 0.31 † |
| 95% CI of the mean | 0.31 † | |||||
| 95% CI of the population | 0.62 † | |||||
Peak insulin concentration was three times higher than the other diabetic cats. The reason for this is unknown. It may have been due to an error in the amount of insulin administered.
Statistical values were calculated omitting the result for cat 5.
Fig 1.
Mean insulin and glucose concentrations in normal cats (n = 10) during an insulin-modified (0.05 U/kg insulin, —+—) frequently sampled intravenous glucose tolerance test (0.3 g/kg glucose, ——).
Fig 2.
Mean insulin and glucose concentrations in diabetic cats (n = 4) during an insulin-modified (0.3 U/kg insulin, —+—) frequently sampled intravenous glucose tolerance test (0.3 g/kg glucose, ——).
Mean glucose effectiveness (SG) in clinically normal cats was 0·030/min (range 0.021–0.045/min) and in diabetic cats was 0.014/min (range 0.008–0.021/min). It is reported that because of an artifact in the minimal model, comparison of values calculated from the minimal model for glucose effectiveness between groups with markedly different insulin secretory capacity, and therefore no statistical comparison was performed between normal and diabetic cats (Finegood & Tzur, 1996).
Discussion
Our data demonstrate that diabetic cats have a significantly lower insulin sensitivity than clinically normal cats. Thus, insulin resistance, a hallmark feature of type 2 diabetes in humans, is also a feature of the feline disease.
Only one other study has measured insulin sensitivity in clinically normal cats using the minimal model method (Petrus et al 1996). The mean insulin sensitivity for this study was 12.41 × 10−4/min/μU/ml, which is significantly higher than our mean value for clinically normal cats (3.22 × 10−4/min/μU/ml). However, the study by Petrus et al (1996) used the standard minimal model method, rather than the insulin-modified protocol. Studies in clinically normal humans comparing the standard and tolbutamide-modified protocols for the minimal model method found no difference in mean insulin sensitivity (Beard et al 1986). However, studies comparing the tolbutamide and insulin-modified protocols have shown differences in insulin sensitivity estimates, with the tolbutamide modified protocol yielding substantially higher values (Welch et al 1990, Saad et al 1997). Thus, comparison of the standard and insulin-modified protocols is predicted to show a decrease in mean insulin sensitivity with the injection of insulin. Quon et al (1994) compared the standard and insulin-modified protocols and showed a 10% decrease in mean insulin sensitivity with the insulin-modified protocol. Thus, while these three protocols give insulin sensitivity measures that correlate strongly with each other, the data are not directly comparable and the same protocol must be used in any single cross-sectional or longitudinal study (Welch et al 1990, Quon et al 1994, Saad et al 1997). In our study, the insulin-modified protocol was used so that values from clinically normal cats could be compared with diabetic cats.
Five different studies of clinically normal humans using the identical insulin-modified minimal model method yielded mean insulin sensitivities ranging from 2.00 to 5.11 × 10−4/min/μU/ml and the mean of the five studies was 2.93 × 10−4/min/μU/ml (Finegood et al 1990, Quon et al 1994, Saad et al 1994, Osei & Schuster 1995, Saad et al 1997). These values are similar to our results for healthy young cats (range 1.71 to 5.23 × 10−4/min/μU/ml, mean 3.22 × 10−4/min/μU/ml). The identical insulin-modified minimal model method in three different studies of type 2 diabetic human patients gave insulin sensitivities ranging from 0.106 to 0.67 × 10−4/min/μU/ml (Welch et al 1990, Saad et al 1994, Coates et al 1995). The mean of the three studies is 0.462 × 10−4/min/μU/ml, which is slightly lower than our mean value of 0.58 × 10−4/min/μU/ml for diabetic cats.
However, when comparing results between species, it may be more valid to compare the relative change in insulin sensitivity between normal and diabetics of the same species, rather than compare absolute values between species. This is because differences in insulin assays, lean body mass, and the volume of distribution affect the absolute value. When the change in insulin sensitivity is compared, diabetic cats had a similar decrease in insulin sensitivity to human type 2 diabetics, relative to normal values for their species.
Our normal cats were significantly younger than our diabetic cats. However, in healthy, non-obese humans, the decline in insulin sensitivity with age is small (Reaven et al 1989). In healthy, adult humans, insulin sensitivity and glucose tolerance are affected primarily by regional body fat distribution and not age (Coon et al 1992). Whether an age-related change occurs in cats, independent of weight gain and physical inactivity, needs to be determined. None of our diabetic cats was obese at the time of testing. As obesity increases insulin resistance, insulin sensitivity would be expected to decrease in older cats if they had more body fat than young cats (Nelson et al 1990, Walker 1995).
In our study of diabetic cats and in the human studies using the same insulin-modified protocol, the patients had not been previously treated with insulin or had received insulin therapy for less than 5 days. It would be expected that with insulin treatment and the resultant reduction in hyperglycaemia and hyperlipaemia, insulin sensitivity would improve (Yki-Järvinen 1992). Both persistent hyperglycaemia and hyperlipidaemia have similar detrimental effects on peripheral insulin action and beta-cell function, referred to as glucose and lipid toxicity (Abbott et al 1987, Yki-Järvinen 1992, Schermerhorn 1998). Once persistently elevated, triglyceride and glucose concentrations are strongly correlated with the degree of whole-body insulin resistance and add to the underlying insulin resistance in humans with type 2 diabetes (Abbott et al 1987, Yki-Järvinen 1992, Reaven 1995). Therefore, part of the insulin resistance seen in our diabetic cats is probably due to glucose and lipid toxicity and may be reversible with appropriate therapy. Due to the implications for appropriate insulin dosage, further studies are needed to determine if well-controlled diabetic cats have substantially improved insulin sensitivity compared to diabetic cats at initial diagnosis.
Various insulin doses have been used for humans and dogs in the insulin-modified minimal model when normal and diabetic patients are compared (Welch et al 1990, Saad et al 1994, 1997, Finegood & Tzur 1996). It has been found that, when using the minimal model to compare groups with different levels of insulin sensitivity, it is better to use insulin doses which produce plasma insulin concentrations that inversely reflect the levels of insulin action rather than to match plasma insulin concentrations between groups. We used a dose of 0.05 U/kg in normal cats and 0.3 U/kg in diabetics, which has been used in humans to compare non-insulin-dependent diabetics with normal patients (Welch et al 1990, Saad et al 1994, Coates et al 1995). Although peak insulin concentrations were not significantly different between normal and diabetic cats, the area under the insulin curve was higher in diabetic cats (P <0.05) (Figs 1 and 2). Further work may be required in cats to determine the optimal dose of insulin for the insulin-modified minimal model method.
Skeletal muscle is a major site of insulin resistance, as it is responsible for the great majority of insulin-stimulated glucose metabolism (Olefsky 1995). Although other tissues are insulin-resistant in type 2 diabetes, they do not account for a significant proportion of overall glucose uptake (Olefsky 1995). Thus, all measures of in vivo insulin action on glucose disposal, such as the minimal model method, largely assess the resistance of skeletal muscle to take up glucose under the influence of insulin (Olefsky 1995). The other major site of insulin resistance is the liver (Porte 1991). Hepatic insulin resistance results in increased hepatic glucose production, a major contributor to hyperglycaemia in diabetics (Porte 1991). However, the minimal model allows calculation of only glucose disposal and not of hepatic glucose production and its sensitivity to suppression by insulin (Groop et al 1993). In the euglycaemic insulin clamp, insulin sensitivity is calculated from the amount of glucose required to maintain normoglycaemia during an infusion of insulin. When this method of measuring insulin sensitivity is combined with the infusion of labelled glucose, it allows measurement of hepatic glucose production and its sensitivity to insulin (Groop et al 1993). Further studies are needed to determine the relative importance of hepatic and peripheral insulin resistance in diabetic cats.
Glucose effectiveness (SG) has been defined as the effect of glucose to enhance its own uptake and suppress its production at basal insulin levels (Finegood & Tzur 1996). A study by Ader et al (1985) has shown that glucose effectiveness is a major determinant of glucose tolerance status and is at least as important as the dynamic insulin response for normalisation of glucose. Mean glucose effectiveness from four different studies in normal humans using the insulin-modified minimal model method was 0.024/min (range 0.020–0.028/min) (Finegood et al 1990, Saad et al 1994, 1997, Osei & Schuster 1995). This is similar to our mean value of 0.030/min for normal cats. Two different studies in type 2 diabetic humans yielded mean glucose effectiveness values of 0.0137 and 0.0146/min (Welch et al 1990, Coates et al 1993). The mean of these two values (0.0142/min), is nearly identical to our mean value of 0.0141/min for diabetic cats. While comparisons can be made between subjects of similar insulin secretory capacity, the minimal model estimates of glucose effectiveness should not be compared between subject groups with substantially different levels of insulin secretory function (Finegood & Tzur 1996). This is because values for glucose effectiveness calculated using the minimal model vary artefactually with differences in insulin secretory capacity and are overestimated in normal subjects (Finegood & Tzur 1996). Caution should also be used in comparing absolute values for glucose effectiveness between species until further studies demonstrate its validity.
In conclusion, diabetic cats have a significantly lower insulin sensitivity than clinically normal cats. The magnitude of the decrease was similar to that reported for human diabetics using the same insulin-modified minimal model method. Thus, insulin resistance is a feature of feline diabetes mellitus. As our sample size for diabetic cats was only small (n = 4), further work needs to be done in this area in order to establish the range of insulin sensitivity that occurs in diabetic cats. Direct comparison of absolute levels for insulin sensitivity between species may not be valid unless identical protocols and assays are used and corrections are made for lean body mass and volume of distribution.
Acknowledgements
Funding for this study was provided by the Australian Companion Animal Health Foundation, Intervet International Ltd, and the University of Queensland. Juanita Feldhahn was supported by an Australian Postgraduate Award and a University of Queensland Postgraduate Scholarship. The authors thank R.N. Bergman for the kind donation of the MINMOD computer program.
References
- Abbott WGH, Lillioja S, Young AA, Zawadzki JK, Yki-Jarvinen H, Christin L, Howard BV. (1987) Relationships between plasma lipoprotein concentrations and insulin action in an obese hyperinsulinaemic population. Diabetes 36, 897–904. [DOI] [PubMed] [Google Scholar]
- Ader M, Pacini G, Yang YJ, Bergman RN. (1985) Importance of glucose per se to intravenous glucose tolerance. Diabetes 34, 1092–1103. [DOI] [PubMed] [Google Scholar]
- Beard JC, Bergman RN, Ward WK, Porte D. (1986) The insulin sensitivity index in non diabetic man: Correlation between clamp-derived and IVGTT-derived values. Diabetes 35, 362–369. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C. (1979) Quantitative estimation of insulin sensitivity. American Journal of Physiology 236, E667–E677. [DOI] [PubMed] [Google Scholar]
- Bergman R, Volund A, Yang Y, Youn J, Ader M. (1986) Simplified intravenous injection protocol in man yields insulin sensitivity index equal to that from eugylcaemic clamps. Diabetes 35, 14A. [Google Scholar]
- Bergman RN, Prager R, Volund A, Olefsky JM. (1987) Equivalence of the insulin sensitivity index in man derived by the minimal model method on the euglycemic glucose clamp. Journal of Clinical Investigation 79, 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand Miller JC, Colagiuri S. (1994) The carnivore connection: Dietary carbohydrate in the evolution of NIDDM. Diabetologia 37, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Coates PA, Ollerton RL, Luzio SD, Ismail IS, Owens DR. (1993) Reduced sampling protocols in estimation of insulin sensitivity and glucose effectiveness using the minimal model in NIDDM. Diabetes 42, 1635–1641. [DOI] [PubMed] [Google Scholar]
- Coates PA, Luzio SD, Brunei P, Owens DR. (1995) Comparison of estimates of insulin sensitivity from minimal model analysis of the insulin-modified frequently sampled intravenous glucose tolerance test and the isoglycemic hyperinsulinemic clamp in subjects with NIDDM. Diabetes 44, 631–635. [DOI] [PubMed] [Google Scholar]
- Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. (1992) Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. Journal of Clinical Endocrinology and Metabolism 75, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Crenshaw KL, Peterson ME. (1996) Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992–1994). Journal of the American Veterinary Medical Association 209, 943–949. [PubMed] [Google Scholar]
- Donoghue S, Kronfeld DS. (1993) Feeding the hospitalised cat. In: Wills J, Wolf A. (eds) Handbook of Feline Medicine. UK: Pergamon Press, pp. 35–47. [Google Scholar]
- Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, Groop L. (1989) Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. New England Journal of Medicine 321, 337–343. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Tzur D. (1996) Reduced glucose effectiveness associated with reduced insulin release: An artifact of the minimal-model method. American Journal of Physiology 271, E485–E495. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Hramiak IM, Dupre J. (1990) A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. Journal of Clinical Endocrinology and Metabolism 70, 1538–1549. [DOI] [PubMed] [Google Scholar]
- Groop LC, Widen E, Ferrannini E. (1993) Insulin resistance and insulin deficiency in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus: Errors of metabolism or of methods? Diabetologia 36, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Kahn CR. (1978) Insulin resistance, insulin insensitivity, and insulin unresponsiveness: A necessary distinction. Metabolism—Clinical and Experimental 27 (Suppl. 2), 1893–1902. [DOI] [PubMed] [Google Scholar]
- Leahy JL. (1990) Natural history of beta-cell dysfunction in NIDDM. Diabetes Care 13, 992–1010. [DOI] [PubMed] [Google Scholar]
- Link KR, Rand JS. (1996) Arginine and phentolamine response tests in cats. Journal of Veterinary Internal Medicine 10, 185. [Google Scholar]
- Link KR, Rand JS. (1998) Reference values for glucose tolerance and glucose tolerance status in cats. Journal of the American Veterinary Medical Association 213, 492–496. [PubMed] [Google Scholar]
- Link KR, Rand JS, Hendrikz JK. (1997) Evaluation of a simplified intravenous glucose tolerance test and a reflectance glucose meter for use in cats. Veterinary Record 140, 253–256. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1993) Comparison of five commercial radioimmunoassay kits for the measurement of feline insulin. Research in Veterinary Science 55, 64–69. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1995) Pathogenesis of feline diabetes mellitus. Veterinary Clinics of North America, Small Animal Practice 25, 527–552. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1996) Plasma amylin and insulin concentrations in normoglycaemic and hyperglycaemic cats. Canadian Veterinary Journal 37, 27–34. [PMC free article] [PubMed] [Google Scholar]
- Martin G, Rand JS. (1999) Evaluation of polyurethane jugular catheter in cats placed using a modified Seldinger technique. Australian Veterinary Journal 77, 250–254. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Himsel CA, Feldman EC, Bottoms GD. (1990) Glucose tolerance and insulin response in normal-weight and obese cats. American Journal of Veterinary Research 51, 1357–1362. [PubMed] [Google Scholar]
- Nelson RW, Feldman EC, Ford SL, Roemer OP. (1993) Effect of an orally administered sulfonylurea, glipizide, for treatment of diabetes mellitus in cats. Journal of the American Veterinary Medical Association 203, 821–827. [PubMed] [Google Scholar]
- O'Brien TD, Hayden DW, Johnson KH, Stevens JB. (1985) High dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: Relationships to insular amyloidosis. Veterinary Pathology 22, 250–261. [DOI] [PubMed] [Google Scholar]
- Osei K, Schuster DP. (1995) Metabolic characteristics of African descendants: A comparative study of African-Americans and Ghanian immigrants using minimal model analysis. Diabetologia 38, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Ostenson C-G, Khan A, Efendic S. (1992) Impaired glucose-induced insulin secretion: Studies in animal models with spontaneous NIDDM. New Concepts in the Pathogenesis of NIDDM. Advances in Experimental Medicine and Biology 334, 1–11. [DOI] [PubMed] [Google Scholar]
- Panciera DL, Thomas CB, Eicker SW, Atkins CE. (1990) Epizootiologic patterns of diabetes mellitus in cats: 333 cats (1980–1986). Journal of the American Veterinary Medical Association 197, 1504–1508. [PubMed] [Google Scholar]
- Petrus DJ, Jackson MW, Kemnitz JW, Finegood DT, Panciera DL. (1996) Assessment of insulin sensitivity in the cat. Proceedings of 14th American College of Veterinary Internal Medicine Forum, p. 765.
- Porte D. (1991) Cells in type II diabetes mellitus. Diabetes 40, 166–180. [DOI] [PubMed] [Google Scholar]
- Quon MJ, Cochran C, Taylor SI, Eastman RC. (1994) Direct comparison of standard and insulin-modified protocols for minimal model estimation of insulin sensitivity in normal subjects. Diabetes Research 25, 139–149. [PubMed] [Google Scholar]
- Rand JS. (1997) Understanding feline diabetes. Australian Veterinary Practitioner 27, 17–26. [Google Scholar]
- Reaven GM, Chen N, Hollenbeck C, Chen YDI. (1989) Effect of age on glucose tolerance and glucose uptake in healthy individuals. Journal of the American Geriatrics Society 37, 735–740. [DOI] [PubMed] [Google Scholar]
- Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Ida Chen YD, Evan Sands R, Pie D, Savage PJ, Bergman RN. (1994) A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Diabetes 43, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Saad MF, Steil GM, Kades WW, Ayad MF, Elsewafy WA, Boyadjian R, Jinagouda SD, Bergman RN. (1997) Differences between the tolbutamide-boosted and the insulin-modified minimal model protocols. Diabetes 46, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Schermerhorn T. (1998) Beta cell dysfunction in diabetes mellitus: recent developments. Proceedings of the 16th American College of Veterinary Internal Medicine Forum, pp. 498–500.
- Taylor SI, Accili D, Imai Y. (1994) Perspectives in diabetes. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 43, 735–740. [DOI] [PubMed] [Google Scholar]
- Walker M. (1995) Obesity, insulin resistance, and its link to non-insulin-dependent diabetes mellitus. Metabolism — Clinical and Experimental 44 (Suppl. 3), 18–20. [DOI] [PubMed] [Google Scholar]
- Welch S, Gebhart SSP, Bergman RN, Phillips LS. (1990) Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. Journal of Clinical Endocrinology and Metabolism 71, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. (1992) Glucose toxicity. Endocrine Reviews 13, 415–430. [DOI] [PubMed] [Google Scholar]