Abstract
In in vitro studies, the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine (PMPDAP) inhibited the replication of feline immunodeficiency virus (FIV). No information about its clinical efficacy is available so far. The aim of this prospective placebo-controlled, double-blinded study was to evaluate the antiviral efficacy of PMPDAP in cats naturally infected with FIV. Twenty cats were randomly assigned to two treatment groups receiving either PMPDAP (25 mg/kg) or placebo twice per week subcutaneously for 6 weeks. The general health status (Karnofsky’s score), clinical signs, laboratory, immunological, and surrogate parameters were evaluated. No significant differences were found between PMPDAP- and placebo-treated cats, although cats treated with PMPDAP showed a tendency for improvement in their Karnofsky’s score and clinical signs. Haematological side effects were noted in the PMPDAP-treated cats. Thus, PMPDAP may be an option in treating cats if it becomes available for veterinarians, but side effects have been monitored.
Introduction
Natural infections with feline immunodeficiency virus (FIV) are found worldwide. Recent studies report a prevalence of 3.1–14.0% in Europe,1–4 2.5–6.4% in North America,5–7 9.8–23.2% in Asia,8–10 and 0–25% in Australia. 11 Infections are persistent and clinical signs are characterised by their chronicity and their progressive nature.
The similarities between FIV and human immunodeficiency virus (HIV) reverse transcriptase (RT) lead to a similar susceptibility of the virus to several antiviral compounds. 12 Different drugs have been tested in the treatment of FIV infections, of which inhibitors of the viral RT were the most efficient. The replication of the virus is discontinued in the phase of transcription by the integration of a modified nucleoside. The nucleoside analogues 3’-azido-2’,3’-dideoxythymidine (zidovudine, AZT), 9-(2-phosphonylmethoxyethyl) adenine (PMEA), and (S)-9-(3-fluoro-2-phosphonylmethoxypropyl) adenine (FPMPA) have been shown to improve the severity of clinical signs, such as stomatitis, and the immunological status shown by an increase of the CD4/CD8 ratio. However, AZT and PMEA show toxic effects on the bone marrow, leading to anaemia which limits their long-term use in cats.13,14 FPMPA is less toxic, but also less effective than PMEA. 15
The nucleoside analogue (R)-9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine (PMPDAP) was shown to be a potent inhibitor of FIV replication in cell culture using different cell lines.16,17 In a preliminary study, four cats were experimentally infected with FIV and treated with 20 mg/kg PMPDAP subcutaneously three times per week for 6 weeks. The viral RNA load was reduced in three out of the four cats during treatment. No changes in the red blood cell counts or haemoglobin values were observed. 16 PMPDAP showed an excellent inhibition of viral replication in vitro 16 and was shown to have fewer side effects when used long term.16,18 The present study was designed to evaluate its clinical efficacy in a population of cats naturally infected with FIV.
Materials and methods
Cat population
Twenty cats were included in the study that had been presented at the Clinic of Small Animal Medicine of the Ludwig Maximilian University of Munich in Germany. All cats tested positive for the presence of antibodies against FIV p24 antigen by enzyme-linked immunosorbent assay (ELISA) (PetCheck Anti-FIV; Idexx, Ludwigsburg, Germany). Each positive result was confirmed by Western blot. The Western blot was considered to be positive if antibodies against p15 and p24 proteins could be demonstrated. 19 Only cats were included in the study for which the owners gave their consent for participation. For all cats, an informed consent of participation was signed by the owners. This study fulfilled the general German guidelines for prospective studies requiring owner consent. The cases were consecutive cases with FIV infection that had owners willing to participate in the study. Cats in a moribund condition or pregnant cats were excluded from the study.
Of the 20 cats included in the study, represented breeds were Maine Coon (one cat), domestic longhair (one cat), and domestic shorthair (18 cats). Fifteen cats were male (10/15 neutered) and five cats were female (3/5 neutered). The average age was 6.7 ± 3.7 years. Seventeen cats were free roaming; the others were house indoors only.
Design of the study
The study was designed as a prospective, placebo- controlled, double-blinded study. Cats were randomly assigned to two treatment groups receiving either PMPDAP (25 mg/kg) (group T) or phosphate buffered saline (PBS) as a placebo (group P). Both substances were given subcutaneously twice per week. The treatment period was 6 weeks. No other medications were administered to the cats.
Examination and sample collection
Clinical examinations were performed at the beginning, once per week thereafter, and at the end of the study (day 0, 7, 14, 21, 28, 35, 42). The severity of stomatitis and conjunctivitis, as the two most common clinical signs in the study population, were evaluated in a score ranging from 0 (no clinical signs) to 10 (severe clinical signs).
General health status and the quality of life were determined using the modified Karnofsky’s score. 20 This scoring system uses behaviour patterns such as eating, playing, sleeping, grooming, and outdoor activities to evaluate the degree of quality of life expressed in percentage ranging from 100% (cat with normal behaviour) to 0% (death of the cat).
Blood samples were taken after each clinical examination. On day 0, 21, and 42, a urine sample was obtained in addition. On these days, complete blood cell counts (CBC) and serum biochemistry profiles were evaluated. Flow cytometry was used to differentiate CD4+ and CD8+ lymphocytes. Serum and urine samples taken for the analysis of the pterins (biopterin, 7-xanthopterin) were covered with aluminium foil immediately after sampling and stored at -20°C until processed further. Pterins were measured by high performance liquid chromatography (HPLC).
Flow cytometry for the measurement of CD4+ and CD8+ lymphocytes
For the detection of lymphocyte subpopulations a method described previously was used. 15
HPLC for the detection of biopterin and 7-xanthopterin
Preparation of the samples were performed as described previously. 21 The samples and a mobile phase were pumped through a column containing a stationary phase. The analyte retention time was depicted by a fluorescence detector. Measurements of pyterins were performed at an excitation of 350 and an emission of 450 nm. By comparing the analyte retention time to standards, the compounds and their quantity were identified. The exact performance of the HPLC and analysis are described elsewhere. 21
Statistics
The commercially available software package SPSS 13.0.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. The Mann Whitney U test was used to detect differences in clinical parameters between both groups on day 0 and 42 and between day 0 and day 42 in each group. The Student’s t-test was used for laboratory parameter to detect differences between groups at the beginning and the end of the treatment period. A P-value of ≤ 0.05 was considered statistically significant.
Results
Clinical parameters
Mean values of clinical parameters are summarised in Table 1. No statistically significant differences were found between both groups and within each group. However, the Karnofsky’s score increased continuously throughout treatment in cats treated with PMPDAP. In cats receiving placebo, however, the score remained at the same level throughout the study, finishing slightly lower compared to day 0 (Figure 1). Stomatitis (Figure 2) and conjunctivitis (Figure 3) improved during treatment in both groups, but no significant difference was detected. Other clinical signs, such as rhinitis (three cats in group P), bronchitis (six cats in group T and four cats in group P), enteritis (two cats in group P), and skin problems (three cats in group P, one cat in group P), were present. However, these clinical signs were not common enough to allow statistical evaluation.
Table 1.
Clinical, laboratory, immunological, and surrogate parameter (mean ± standard deviation) determined at the beginning and the end of the treatment period with PMPDAP. Changes on day 42 between both groups were compared by Mann Whitney U-test for clinical parameter and by Student’s t-test for laboratory parameter (P ≤ 0.05)
| PMPDAP | Placebo | P | |||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 42 | P | Day 0 | Day 42 | P | PMPDAP vs placebo (day 42) | |
| Clinical parameter | |||||||
| Karnofsky’s score (%) (score 0–100) | 72.0 ± 14.0 | 83.0 ± 12.5 | 0.084 | 83.0 ± 17.0 | 82.0 ± 19.3 | 0.814 | 0.938 |
| Conjunctivitis (score 0–10) | 2.0 ± 0.9 | 1.2 ± 1.3 | 0.129 | 1.9 ± 2.1 | 1.7 ± 2.0 | 0.938 | 0.455 |
| Stomatitis (score 0–10) | 4.0 ± 3.1 | 3.0 ± 2.4 | 0.400 | 1.2 ± 1.2 | 1.1 ± 1.2 | 0.874 | 0.074 |
| Body weight (kg) | 4.8 ± 1.4 | 4.5 ± 1.2 | 0.626 | 4.5 ± 1.0 | 4.3 ± 0.8 | 0.690 | 0.376 |
| Laboratory parameter | |||||||
| Packed cell volume (%) (29.3–49.8 %) | 36.8 ± 5.9 | 31.7 ± 6.8 | 0.089 | 37.3 ± 5.5 | 36.4 ± 3.7 | 0.672 | 0.070 |
| Haemoglobin (g/dl) (9.0–15.6 g/dl) | 11.7 ± 2.3 | 10.5 ± 2.5 | 0.254 | 12.6 ± 2.1 | 12.2 ± 1.6 | 0.621 | 0.090 |
| White blood cells (x 103/µl) (4.87–20.10 × 103/µl) | 10.46 ± 4.48 | 9.83 ± 6.03 | 0.794 | 11.05 ± 4.46 | 12.82 ± 8.41 | 0.562 | 0.372 |
| Lymphocytes (× 103/µl) (1.5–7.0 x 103/µl) | 2.75 ± 1.42 | 3.17 ± 2.30 | 0.628 | 3.27 ± 1.31 | 3.79 ± 2.05 | 0.502 | 0.528 |
| Immunologic parameter | |||||||
| CD4+ (× 103/µl) (0–2.87 × 103/µl) | 0.51 ± 0.32 | 0.64 ± 0.53 | 0.547 | 0.63 ± 0.36 | 0.70 ± 0.39 | 0.705 | 0.796 |
| CD4+ (%) (14.7–50.3%) | 20.1 ± 9.5 | 22.1 ± 7.3 | 0.622 | 21.1 ± 8.0 | 20.4 ± 7.5 | 0.857 | 0.640 |
| CD8+ (x 103/µl) (0–1.37 × 103/µl) | 0.79 ± 0.51 | 0.83 ± 0.70 | 0.892 | 0.69 ± 0.41 | 0.81 ± 0.45 | 0.547 | 0.951 |
| CD8+ (%) (7.0–25.8%) | 26.8 ± 10.6 | 23.1 ± 8.7 | 0.403 | 21.1 ± 9.3 | 23.9 ± 10.3 | 0.193 | 0.857 |
| CD4/CD8 (0.4–4.0) | 0.91 ± 0.61 | 1.20 ± 0.83 | 0.429 | 1.18 ± 0.62 | 1.08 ± 0.72 | 0.753 | 0.752 |
| Surrogate parameter | |||||||
| Biopterin serum (nmol/l) (7.2–7.1 nmol/l) | 18.18 ± 7.6 | 17.70 ± 7.17 | 0.888 | 21.53 ± 6.66 | 19.16 ± 11.75 | 0.587 | 0.741 |
| Biopterin urine (nmol/mmol urine creatine) (0–593.2 nmol/mmol urine creatine) | 225.4 ± 126.9 | 219.4 ± 102.9 | 0.667 | 270.4 ± 98.9 | 219.4 ± 117.4 | 0.363 | 0.713 |
| 7-xanthopterin serum (nmol/l) (3.4–18.6 nmol/l) | 16.36 ± 3.25 | 17.03 ± 5.25 | 0.813 | 19.30 ± 6.91 | 17.71 ± 6.47 | 0.601 | 0.590 |
| 7-xanthopterin urine (nmol/mmol urine creatine) (0–1043.5 nmol/mmol urine creatine) | 697.9 ± 277.1 | 691.7 ± 370.1 | 0.971 | 619.9 ± 129.4 | 595.0 ± 217.0 | 0.791 | 0.491 |
Figure 1.
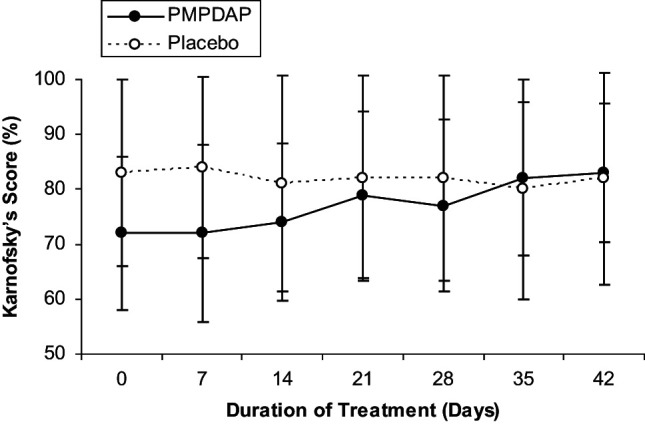
Changes in the Karnofsky’s score during the 6 weeks of treatment with PMPDAP = 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine or placebo
Figure 2.
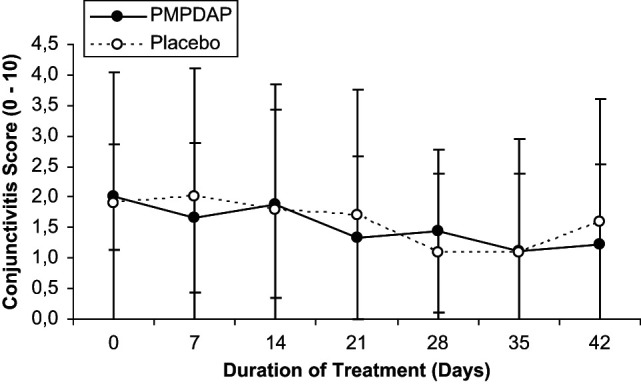
Changes in the stomatitis score during the 6 weeks of treatment with PMPDAP = 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine or placebo
Figure 3.
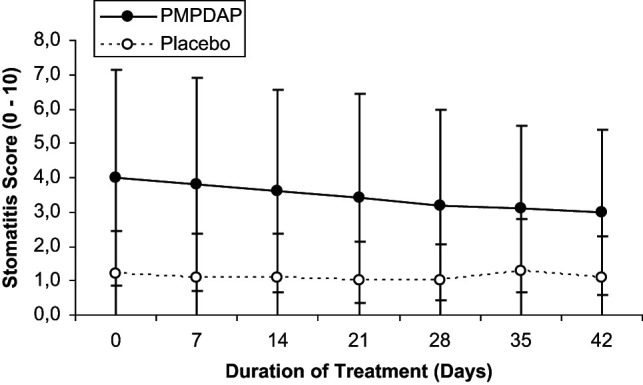
Changes in the conjunctivitis score during the 6 weeks of treatment with PMPDAP = 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine or placebo
Laboratory parameters
Packed cell volume (PCV) and the concentration of haemoglobin decreased in the cats receiving PMPDAP during treatment (Table 1). White blood cells (WBC) and lymphocyte counts varied between groups and days (Table 1). Biochemistry, including electrolytes, showed no changes during the observation period.
Immunological and surrogate parameters
In Table 1, the values of immunological and surrogate parameters are summarised for each group at the beginning and the end of the study. There were no statistically significant differences between both groups and within each group.
Discussion
This study tried to evaluate the clinical efficacy of PMPDAP in the treatment of cats naturally infected with FIV. There were, however, no significant differences found, although there was a tendency that the Karnofsky’s score and the clinical signs stomatitis and conjunctivitis improved in cats treated with PMPDAP. There are possible explanations why no statistically significant differences could be found. A limitation of the study is the group size — it is always very difficult to achieve a statistically significant difference with a small number of patients in each group. Therefore, the changes observed could become significant using a higher number of patients in each group. The number of cats in this study was based on a study from Vahlenkamp et al, 16 in which a significant difference was observed using only four, experimentally-infected cats. However, it may be that differences are easier to assess after experimental infection, but it seems that PMPDAP is less effective in vivo compared with the promising results of the in vitro studies. One reason could be that the dosage and administration intervals based on the preliminary studies may not have been sufficient. 16 A prolonged treatment period or decreased treatment intervals could lead to better results. Another reason could be that nucleoside analogues are generally not as effective in vivo in the treatment of FIV in cats compared with their efficacy against HIV in human beings. Co-infections could also have influenced the results. FIV-positive cats co-infected with either feline herpesvirus or feline calicivirus develop more severe clinical signs that are more difficult to treat.22–24 Results of other studies showing a better clinical efficacy in FIV-infected cats may be influenced by their effects against co-infections.13,14 ANPs other than PMPDAP were shown to be potent inhibitors of herpesvirus infections, e.g., PMEA, while PMPDAP and FPMPA are ineffective. 25
In this study, PMPDAP showed a tendency to improve clinical signs. A continuous improvement throughout the weeks of treatment was observed. As with the general health status, an improvement in the stomatitis and conjunctivitis score was observed with PMPDAP which was not significant; however, the results are in line with other studies.13,15,26 Nevertheless, PMEA seems to be a more potent compound in respect to its clinical efficacy than PMPDAP, as it was possible to demonstrate a statistically significant difference with respect to its effects on general health status, stomatitis, and conjunctivitis using PMEA in a similar study design over a period of 6 weeks. 15
Although, again, no significant changes were observed, CD4+ lymphocyte counts and the CD4/CD8 ratio improved during treatment. Relative CD8+ lymphocytes decreased, contributing to an increase of the CD4/CD8 ratio. Similar results were obtained in a study evaluating PMEA and FPMPA. The results of PMPDAP are comparable to FPMPA, whereas PMEA led to an even better response. 15
Pterines are surrogate markers for HIV-infected patients that are used to predict the progression of the disease and as a control during treatment.27,28 Biopterin and 7-xanthopterin vary naturally between individuals and within repetitive controls in single individuals. The makers have been evaluated in cats infected with FIV and healthy non-infected control cats. 29 Serum biopterin values determined at the beginning of the study were within the normal range for healthy cats. Average values during, and at the end of, the study were also within the physiological range of healthy cats. Serum and urine 7-xanthopterin values showed marked fluctuations within the groups and compared with each other. Therefore, these markers do not seem to be stable or reliable, and are subject to marked deviation and individual differences. Thus, in cats infected with FIV, both substances seem to be neither suitable for predicting the progression of disease nor useful as parameters to control treatment.
Toxic side effects of nucleoside analogues on the haematopoietic system are frequently reported.13,14,30 PMPDAP seems to be slightly less toxic compared with other ANPs. A relatively mild decline in the packed cell volume and haemoglobin values were noted. Average haemoglobin values remained within the physiological range throughout the treatment period. Therefore, haematopoetic side effects are considered to be mild, if PMPDAP is used in this concentration. In vitro, PMPDAP also showed a high selectivity index and, depending on the cell type, a higher 50% cytotoxic dosage (CD50) compared with FPMPA.16,31
A limitation of the study is the small number of patients — the study was intended to be a pilot study. The number of patients was chosen on the basis of other studies evaluating ANPs in naturally FIV-infected positive cats, 15 as well as those in one study using PMPDAP in experimentally-infected cats. 16 In this study, no quantitative evaluation of the provirus and virus load was performed. Changes in the provirus load can be seen in patients on extended treatment regimes; however, during a relative short treatment period of 6 weeks, no such changes are expected. 18 Virus load would be a better option to detect changes of virus replication; however, virus load detection can be very difficult in naturally infected cats. In this study, the RNA polymerase chain reaction quantitative measurement was only possible in very few cats; therefore, this parameter could not be evaluated. In this study, the exact aetiology of the clinical signs was not determined. We assumed that the clinical signs were caused by FIV-related immunosuppression. However, we cannot exclude that the cats were immunocompetent asymptomatic FIV-infected cats suffering from conjunctivitis or stomatitis not related to immunosuppression. This is another limitation of the study.
PMPDAP showed a tendency for improvement of clinical parameters but although hematologic side effects, that however, were less severe than in most other ANPs so far used in cats with FIV infections. Therefore, further investigations with PMPDAP with respect to its effect on retroviral infections are useful, e.g., using more cats to reach statistical significance, increasing dosage or decreasing treatment intervals or increasing duration of treatment.
Acknowledgments
The authors thank PD Dr M Goldberg (Institut für Physiologie, Ludwig-Maximilians-Universität, München) for the HPLC analysis and Prof Dr T Vahlenkamp (Institut für Virologie, Veterinärmedizinische Fakultät, Universität Leipzig, Leipzig) for the Western blot analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Arjona A, Escolar E, Soto I, et al. Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. J Clin Microbiol 2000; 38: 3448–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopes AP, Cardoso L, Rodrigues M. Serological survey of Toxoplasma gondii infection in domestic cats from northeastern Portugal. Vet Parasitol 2008; 155: 184–9. [DOI] [PubMed] [Google Scholar]
- 3. Muirden A. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus and feline coronavirus in stray cats sent to an RSPCA hospital. Vet Rec 2002; 150: 621–5. [DOI] [PubMed] [Google Scholar]
- 4. Murray JK, Roberts MA, Skillings E, et al. Risk factors for feline immunodeficiency virus antibody test status in Cats Protection adoption centres (2004). J Feline Med Surg 2009; 11: 467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy JK, Scott HM, Lachtara JL, Crawford PC. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 2006; 228: 371–6. [DOI] [PubMed] [Google Scholar]
- 6. Little S, Sears W, Lachtara J, Bienzle D. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can Vet J 2009; 50: 644-8. [PMC free article] [PubMed] [Google Scholar]
- 7. Luria BJ, Levy JK, Lappin MR, et al. Prevalence of infectious diseases in feral cats in Northern Florida. J Feline Med Surg 2004; 6: 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maruyama S, Kabeya H, Nakao R, et al. Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiol Immunol 2003; 47: 147–53. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Miyazawa T, Ikeda Y, et al. Contrastive prevalence of feline retrovirus infections between northern and southern Vietnam. J Vet Med Sci 2000; 62: 921–3. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura Y, Ura A, Hirata M, et al. An updated nation-wide epidemiological survey of feline immunodeficiency virus (FIV) infection in Japan. J Vet Med Sci 2010; 72: 1051–6. [DOI] [PubMed] [Google Scholar]
- 11. Norris JM, Bell ET, Hales L, et al. Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. J Feline Med Surg 2007; 9: 300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. North TW, North GL, Pedersen NC. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother 1989; 33: 915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann K, Donath A, Beer B, et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol 1992; 35: 167–75. [DOI] [PubMed] [Google Scholar]
- 14. Kuffer M, Balzarini J, Rolinski B, et al. Comparative investigation of the efficacy of two nucleocapsid analogs in FIV infected cats. Tierarztl Prax 1997; 25: 671–7. [PubMed] [Google Scholar]
- 15. Hartmann K, Kuffer M, Balzarini J, et al. Efficacy of the acyclic nucleoside phosphonates (S)-9-(3-fluoro-2-phosphonylmethoxypropyl)adenine (FPMPA) and 9-(2-phosphonylmethoxyethyl)adenine (PMEA) against feline immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17: 120–8. [DOI] [PubMed] [Google Scholar]
- 16. Vahlenkamp TW, De Ronde A, Balzarini J, et al. (R)-9- (2-phosphonylmethoxypropyl)-2,6-diaminopurine is a potent inhibitor of feline immunodeficiency virus infection. Antimicrob Agents Chemother 1995; 39: 746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van der Meer FJ, Schuurman NM, Balzarini J, Egberink HF. Comparative evaluation of the activity of antivirals towards feline immunodeficiency virus in different cell culture systems. Antiviral Res 2007; 76: 198–201. [DOI] [PubMed] [Google Scholar]
- 18. Vahlenkamp TW, De Ronde A, Horzinek MC, HF. Quantification of feline immunodeficiency virus (FIV) RNA in the plasma of infected cats. Berl Munch Tierarztl Wochenschr 1996; 109: 265–9. [PubMed] [Google Scholar]
- 19. Egberink HF, Lutz H, Horzinek MC. Use of Western blot and radioimmunoprecipitation for diagnosis of feline leukemia and feline immunodeficiency virus infections. J Am Vet Med Assoc 1991; 199: 1339–42. [PubMed] [Google Scholar]
- 20. Hartmann K, Kuffer M. Karnofsky’s score modified for cats. Eur J Med Res 1998; 3: 95–8. [PubMed] [Google Scholar]
- 21. Goldberg M, Gassner F, Singer L, Merkenschlager M. Pteridinkonzentrationen in Harn und Organgeweben von Hund und Katze bei verschiedenen Neoplasien und Virusinfektionen. Tierarztl Prax 1989; 5 (Suppl.): 37–41. [Google Scholar]
- 22. Tenorio AP, Franti CE, Madewell BR, Pedersen NC. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet Immunol Immunopathol 1991; 29: 1–14. [DOI] [PubMed] [Google Scholar]
- 23. Reubel GH, George JW, Higgins J, Pedersen NC. Effect of chronic feline immunodeficiency virus infection on experimental feline calicivirus-induced disease. Vet Microbiol 1994; 39: 335–51. [DOI] [PubMed] [Google Scholar]
- 24. Reubel GH, George JW, Barlough JE, et al. Interaction of acute feline herpesvirus-1 and chronic feline immunodeficiency virus infections in experimentally infected specific pathogen-free cats. Vet Immunol Immunopathol 1992; 35: 95–119. [DOI] [PubMed] [Google Scholar]
- 25. Reymen D, Naesens L, Balzarini J, et al. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antiviral Res 1995; 28: 343–57. [DOI] [PubMed] [Google Scholar]
- 26. Egberink H, Borst M, Niphuis H, et al. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc Natl Acad Sci USA 1990; 87: 3087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med 1990; 322: 166–72. [DOI] [PubMed] [Google Scholar]
- 28. Reddy MM, McKinley GF, Grieco MH. Evaluation of HIV P24 antigen, beta 2-microglobulin, neopterin, soluble CD4, soluble CD8, and soluble interleukin-2 receptor levels in patients with AIDS or AIDS-related complex treated with 2’,3’-dideoxyinosine (ddI). J Clin Lab Anal 1991; 5: 39–8. [DOI] [PubMed] [Google Scholar]
- 29. Hartmann K, Goldberg M, Wilhelm N, et al. Pteridine - prognostische Marker bei Retrovirusinfektionen der Katze? Proceedings of the 5th Annual Conference of the German Society of Internal Medicine and Clinical Pathology of the German Veterinary Association (DVG); Munich, Germany, 1995: 76. [Google Scholar]
- 30. Hoover EA, Ebner JP, Zeidner NS, Mullins JI. Early therapy of feline leukemia virus infection (FeLV-FAIDS) with 9-(2-phosphonylmethoxyethyl)adenine (PMEA). Antiviral Res 1991; 16: 77–92. [DOI] [PubMed] [Google Scholar]
- 31. Balzarini J, De Clercq E. Acyclic purine nucleoside phosphonates as retrovirus inhibitors. In: Jeffries D, De Clercq E, eds. Antiviral chemotherapy. Hoboken, NJ: John Wiley & Sons, 1995: 41–63. [Google Scholar]


