Abstract
The objective of this study was to evaluate three commercially-available human assays for the determination of adiponectin, ghrelin and insulin-like growth factor 1 (IGF-1) concentrations in feline serum samples. Intra- and interassay coefficients of variation were lower than 20%, 15% and 6% for adiponectin, ghrelin and IGF-1 assays, respectively. Dilutions of feline serum pools resulted in linear regression equations in all kits. Mean recovery of adiponectin, ghrelin and IGF-1 assays were 107%, 102% and 105%, respectively. Significant differences were detected in adiponectin and ghrelin concentrations between lean and obese cats (P <0.05 in both cases), but there was no difference in IGF-1 concentrations (P = 0.12).
Introduction
Obesity is an important disease in cats 1 and research interest in this topic is growing, driving a demand for more investigations in this field. Numerous studies have reported changes in different analytes that could proceed to obesity-related diseases such as diabetes mellitus and skin and oral pathologies, among others.2,3 Thus, accessible, fast, precise and accurate methods for evaluating the physiopathological changes associated with obesity and its related diseases are necessary in this species. 4
Adiponectin, ghrelin and insulin-like growth factor 1 (IGF-1) are serum proteins associated with obesity and different metabolic diseases.5–7 In cats, adiponectin concentrations decrease in obesity and return to their initial values after weight loss. 8 Additionally, low concentrations of adiponectin are associated with decreased insulin sensitivity and increased susceptibility to inflammation. 9 Ghrelin is gaining importance in human medicine owing to its orexigenic and gastric motility stimulating effects, 10 and also as a clinical pharmacological substrate in the treatment of obesity and anorexia. 11 IGF-1 is involved in the regulation of growth and metabolism, and also mediates many of the anabolic effects of growth hormone in different tissues. 6
Currently, measurement of these analytes in cats is limited. Ghrelin and IGF-1 assays have usually been performed with radioimmunoassay (RIA) technology which requires special equipment and facilities12,13 and there is a paucity of commercially-available validated assays for adiponectin measurements in feline serum samples.8,14 Therefore, the objective of this study was to perform an analytical evaluation of commercially-available enzyme-linked immunosorbent assays (ELISAs) for adiponectin, ghrelin and IGF-1 on cat serum samples. Effects of lipaemia, haemolysis and bilirubinaemia on the determination of the three analytes were also investigated. In addition, overlap performance of the assays was studied in order to assess the ability of the assays to detect different concentrations of adiponectin, ghrelin and IGF-1 concentrations in obese and lean cats.
Materials and methods
Adiponectin, ghrelin and IGF-1 analysis
A human adiponectin ELISA (High Sensitivity Kit; BioVendor-Labaratorni Medicina, Modrice, Czech Republic) was used for measurement of serum adiponectin. This assay employs microtitre wells coated with polyclonal antibody, horseradish peroxidase, hydrogen peroxide/3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution and acidic solution to stop the reaction. Fifteen minutes after substrate is added, the reaction is stopped by the addition of the acidic solution. The enzyme activity is measured spectrophotometrically at 450 nm in a microtitre plate reader (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA). A standard curve was constructed from the measurement of calibrators by plotting the absorbance values versus adiponectin concentrations of calibrators. Concentrations of unknown samples were then determined using this standard curve. All samples were diluted 1:300 with the dilution buffer provided in the kit prior to analysis, as recommended by the manufacturers. A dilution of 1:200 was also tested but gave poorer results in the linearity tests (data not shown).
A human ghrelin double-antibody sandwich ELISA (BioVendor-Labaratorni Medicina) was used for measurement of circulating ghrelin concentration. It employs microtitre wells coated with monoclonal antibodies specific to the C-terminal of ghrelin, acetylcholinesterase-Fab’ conjugate, and Ellman’s reagent. Absorbance is measured spectrophotometrically at 450 nm, 30–60 min after adding the substrate solution in a microtitre plate reader (PowerWave XS; Bio-Tek Instruments). A standard curve was then constructed from measurement of calibrators by plotting absorbance values versus ghrelin concentrations of calibrators; concentrations of unknown samples were then determined using this standard curve.
In order to verify any changes that could occur with different final incubation times, absorbance readings were initially preformed at 30, 45 and 60 min after the substrate was added. As no significant differences were noted amongst the three time points (data not shown), all measurements were performed at 30 min after adding the substrate solution. Although the manufacturers of the ghrelin assay recommend diluting samples 1:10, preliminary analyses demonstrated that a 1:2 dilution gave more repeatable and accurate results for feline serum ghrelin (data not shown).
For IGF-1, the Immulite 1000 IGF-1 assay (Immulite 1000; Diagnostic Products, Los Angeles, USA), an automated solid-phase, enzyme-labelled chemiluminescent immunometric assay was used. The kit was configured and calibrated on an instrument in our laboratory according to the manufacturer’s instructions. It employs test units containing one bead coated with monoclonal murine anti-IGF-1, alkaline phosphatase (bovine calf intestine) conjugated to polyclonal rabbit anti-IGF-1 in buffer, chemiluminescent substrate [phosphate ester of adamantyl dioxetane in a 2-amino-2-methyl-1-propanol (AMP) buffer with enhancer]. A dilution of 1:5 was used, as recommended by the manufacturers.
Analytical performance
Feline serum samples used for the analytical performance study were obtained from six lean [body condition score (BCS), 4–5 based on a 9-point scale] 15 and six obese (BCS, 7–9/9) cats of different sexes, breeds and ages presented to the Veterinary Teaching Hospital of Murcia University for routine evaluation. Cats had no abnormalities on physical examination (apart from obesity in six cats). Routine haematology (Advia 120; Siemens Healthcare Diagnostics, Bad Nauheim, Germany) and serum biochemistry (Olympus AU2700; Olympus Diagnostica, Melville, NY, USA) were within the reference intervals established for the cats.
Blood samples were collected by jugular or saphenous venepuncture in the morning, after an overnight fast of at least 12 h, and placed in tubes containing clotting accelerator (TapVal; Aquisel, Barcelona, Spain). The samples were then centrifuged at 2000 × g for 10 min at room temperature to obtain serum and were then stored in aliquots in plastic vials at −20°C until analysis. On the day of analysis, samples were brought to room temperature prior to measuring adiponectin, ghrelin and IGF-1 concentrations.
For analytical performance of the three methods, assay precision, accuracy and limit of detection were calculated. All samples used for repetitive analysis were frozen in aliquots and only vials needed for each assay run were used, to avoid possible changes caused by repetitive thawing and freezing.
For assay precision, three pools of sera with different concentrations of analytes that corresponded to low, mid-point and high ranges of the assays were prepared from serum samples obtained from obese and lean cats and used in each assay. For interassay precision, the pools were divided into aliquots and stored in plastic vials at −20 °C until analysis. Intra-assay coefficient of variation (CV) was calculated, after analysis of the three serum pools, five times in a single assay run. Inter-assay CV was determined by analysing the same samples in six separate runs carried out on different days.
The accuracy of the assays was evaluated indirectly by linearity under dilution and recovery studies. 16 For linearity under dilution, the two feline serum pools were serially diluted with diluent provided with the kit (adiponectin, ghrelin, IGF-1) and analysed using the procedures previously described. The pools had the concentration of 7121.27 ng/ml and 4022.68 ng/ml of adiponectin, 233.62 ng/ml and 120.18 ng/ml of ghrelin, and 315.00 ng/ml and 114.00 ng/ml of IGF-1. As certified species-specific reference material was not available, to perform a spiking recovery test, two samples with different amount of analytes were mixed in different ratios.16–18 Thus, samples with high adiponectin, ghrelin and IGF-1 concentrations (5553.40 ng/ml, 109.07 ng/ml and 1562.00 ng/ml, respectively) were mixed at different ratios with the serum samples with low adiponectin, ghrelin and IGF-1 concentrations (1788.79 ng/ml, 44.19 ng/ml and 205.00 ng/ml, respectively) to reach % dilutions of 100, 75, 62, 50, 37.5, 25 and 0%. Test recovery, expressed as a percentage, was calculated for each dilution for comparison of expected versus measured concentrations. Limit of detection was calculated on the basis of data from 20 replicate determinations of the zero standard (buffer of assays), as mean value plus three standard deviations.
To investigate the effects of haemolysis, lipaemia and bilirubinaemia, two feline serum pools were mixed with different concentrations of haemoglobin, lipid or bilirubin solution following previously-described procedures. 19
Effects of haemolysis and lipaemia
To investigate the effects of lipids, a commercial fat emulsion (Lipofundina 20%; Braun Medical, Barcelona, Spain) with a triglyceride concentration of 200 g/l was serially diluted with sample diluent buffer; 10 µl of each dilution was added to two samples of 90 µl of feline serum. Sample homogeneity was then achieved by vortexing. The final triglyceride concentrations were 5, 2.50, 1.25, 0.625 and 0.3125 g/l. These triglyceride concentrations would correspond to slight lipaemia (0.3125 and 0.625 g/l), moderate lipaemia (1.25 and 2.5 g/l) and marked lipaemia (5 g/l).
To investigate the effect of haemolysis, a fresh haemolysate was prepared by addition of distilled water to packed, saline-washed, feline red cells from a healthy cat. The haemoglobin concentration in the haemolysate was determined by using a Veterinary Animal Blood Counter (Vet ABC; ABX Diagnostics, Montpellier, France) and adjusted to 200 g/l by adding assay buffer to produce a stock solution. This stock solution was serially diluted with sample buffer and 10 µl of each dilution was added to the samples of 90 µl of feline serum. The final haemoglobin concentrations were 8, 4, 2, 1, 0.5 and 0.0 g/l (10 µl of sample diluent buffer were added to the samples to give 0.0 g/l concentration). These haemoglobin concentrations would correspond to slight haemolysis (0.5 g/l), moderate haemolysis (1 and 2 g/l) and marked haemolysis (4 and 8 g/l).
To study the effects of hyperbilirubinaemia, 6 mg of bilirubin (Sigma Chemical, St Louis, MO, USA) were suspended in 1 ml of the sample diluent provided in the corresponding commercial kit for adiponectin, ghrelin and IGF-1 assays. This stock solution was serially diluted with sample buffer and 10 µl of each dilution was added to the samples of 90 µl of feline serum. The final bilirubin concentrations were 0.15, 0.075, 0.037, 0.018, 0.009 and 0.0 g/l (10 µl of sample diluent buffer were added to the samples to give 0.0 g/l concentration). These bilirubin concentrations would correspond to slight bilirubinaemia (0.009 g/l), moderate bilirubinaemia (0.018 and 0.037 g/l) and marked bilirubinaemia (0.075 and 0.15 g/l).
Overlap performance
In order to evaluate whether the assays were able to detect differences in analyte concentrations between lean and obese individuals, samples from 20 client-owned cats were tested. Two groups were studied: group C1 comprised 10 overweight/obese neutered cats (BCS 7–9/9), all of which had been referred to the Royal Canin Weight Management Clinic, University of Liverpool (female/male 2/8; body weight range 5.8–10.7 kg; age range 5–10 years; mixed breeds); group C2 comprised 10 healthy normal weight entire cats (BCS 4–5/9), presented to the Veterinary Hospital, University of Murcia, Spain for routine check-ups or castration (female/male 2/8; body weight range 3.1–4.5 kg; age range 4.2–8.0 years; mixed breeds). Blood samples were collected by jugular or saphenous venepuncture in the morning after an overnight fast of at least 12 h. All cats were clinically normal (apart from obesity in 10 cats) and had normal haematology (Baker 9010 analyzer; Serono-Baker: Advia 120; Siemens Healthcare Diagnostics) and serum biochemistry (Kone Specific Supra biochemistry analyser; Thermo Fisher Scientific: Olympus AU2700; Olympus Diagnostica) results.
Statistical methods
Arithmetic means, medians, intra- and interassay CVs were calculated using routine descriptive statistical procedures and software (Excel, Microsoft, Redmond, WA, USA; GraphPad Prism, GraphPad Software, La Jolla, CA, USA). Linearity under dilution was accomplished by ordinary linear regression analysis comparing the measured concentrations of analyte with the expected levels. Interferograms were prepared to show the differences in analyte concentrations when triglycerides, haemoglobin or bilirubin were added, as previously described. 19 The influence of haemoglobin, triglycerides or bilirubin was investigated using one-way repeated measures analysis of variance (ANOVA) and Tukey’s multiple comparison post-test. Student’s t-test for similar variances was used to evaluate the difference of adiponectin and IGF-1 as an F test revealed that the variances did not differ significantly, while Student’s t-test for different variances was used to evaluate the difference of ghrelin between healthy and obese cats as an F test revealed that the variances differed significantly. The significance level used in each case was P <0.05.
Results
Assay characteristics
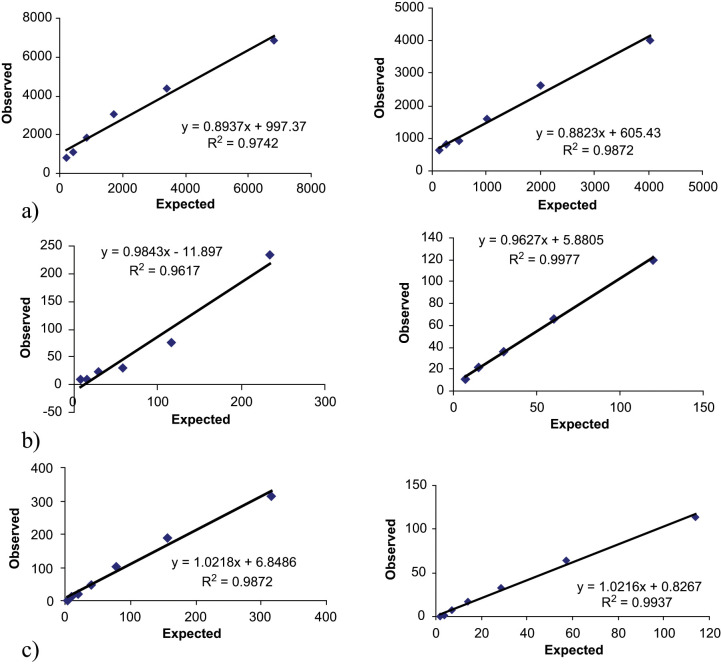
Intra- and interassay CVs of the three pools were below 7–16%, respectively (Table 1). The linearity under dilution of two feline serum pools is shown in Figure 1. Dilution of feline serum samples with different adiponectin concentrations resulted in linear regression equations with correlation coefficient close to 1.0 in the mean range from 90–7000 ng/ml. The recovery between observed and expected adiponectin concentrations ranged from 100–112%, with a mean of 107% (Table 2). The detection limit was 2.0 ng/ml [mean 0.2 ng/ml, standard deviation (SD) 0.6 ng/ml].
Table 1.
Intra- and interassay coefficients of variation (CV) of adiponectin, ghrelin and IGF-1 assays in feline serum pools
| Assay | Comparison | Pool | Mean | SD | CV(%) |
|---|---|---|---|---|---|
| Adiponectin ng/ml | Intra | Pool 1 | 249.8 | 16.2 | 6.5 |
| Pool 2 | 2070.6 | 102.6 | 5.0 | ||
| Pool 3 | 5969.7 | 89.3 | 1.5 | ||
| Inter | Pool 1 | 235.6 | 37.4 | 15.9 | |
| Pool 2 | 1990.0 | 309.6 | 15.6 | ||
| Pool 3 | 6998.0 | 712.7 | 10.2 | ||
| Ghrelin pg/ml | Intra | Pool 1 | 43.59 | 4.0 | 9.2 |
| Pool 2 | 76.5 | 5.6 | 7.3 | ||
| Pool 3 | 115.17 | 3.3 | 2.9 | ||
| Inter | Pool 1 | 41.68 | 5.5 | 13.2 | |
| Pool 2 | 79.3 | 8.0 | 10.1 | ||
| Pool 3 | 123.98 | 10.0 | 8.1 | ||
| IGF-1 ng/ml | Intra | Pool 1 | 58.0 | 0.5 | 0.9 |
| Pool 2 | 374.6 | 8.3 | 2.2 | ||
| Pool 3 | 1005.8 | 31.2 | 3.1 | ||
| Inter | Pool 1 | 58.6 | 2.7 | 4.6 | |
| Pool 2 | 353.4 | 18.8 | 5.3 | ||
| Pool 3 | 1003.5 | 55.2 | 5.5 |
Figure 1.
Linearity under dilution of two feline serum pools with different adiponectin (A), ghrelin (B) and IGF-1 (C) concentrations
Table 2.
Spiking recovery. Two samples with different amounts of analytes were mixed in different ratios and recovery, expressed as a percentage, was calculated for each dilution for comparison of expected versus measured concentrations
| Volume, % | |||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Expected | Observed | % | |
| Adiponectin | 100 | 0 | 5553.40 | 5553.40 | 100 |
| 75 | 25 | 4612.25 | 5134.75 | 111.3 | |
| 62.5 | 37.5 | 4141.67 | 4591.60 | 110.9 | |
| 50 | 50 | 3671.09 | 4095.93 | 111.6 | |
| 37.5 | 62.5 | 3200.52 | 3525.41 | 110.2 | |
| 25 | 75 | 2729.94 | 2951.53 | 108.1 | |
| 0 | 100 | 1788.79 | 1788.79 | 100 | |
| Ghrelin | 100 | 0 | 109.07 | 109.07 | 100 |
| 75 | 25 | 92.85 | 104.14 | 112.2 | |
| 62.5 | 37.5 | 84.74 | 76.92 | 90.8 | |
| 50 | 50 | 76.63 | 79.84 | 104.2 | |
| 37.5 | 62.5 | 68.52 | 73.16 | 106.8 | |
| 25 | 75 | 60.41 | 59.90 | 99.1 | |
| 0 | 100 | 44.19 | 44.19 | 100 | |
| IGF-1 | 100 | 0 | 1562.00 | 1562.00 | 100 |
| 75 | 25 | 1222.75 | 1288.00 | 105.3 | |
| 62.5 | 37.5 | 1053.13 | 1155.00 | 109.7 | |
| 50 | 50 | 883.50 | 992.00 | 112.3 | |
| 37.5 | 62.5 | 713.88 | 747.00 | 104.6 | |
| 25 | 75 | 544.25 | 551.00 | 101.2 | |
| 0 | 100 | 205.00 | 205.00 | 100 | |
For ghrelin, intra- and interassay CVs of the three pools were below 15% in both cases (Table 1). The linearity under dilution of two feline serum pools is shown in Figure 1. Dilution of feline serum samples with different adiponectin concentrations resulted in linear regression equations with correlation coefficient close to 1.0 in the mean range from 30–130 pg/ml. The recovery between observed and expected ghrelin concentrations ranged from 91–112%, with a mean of 102% (Table 2). The detection limit was 9.6 pg/ml (mean 1.8 pg/ml, SD 2.6 pg/ml).
For IGF-1, intra- and interassay CVs of the three pools were below 3% and 6%, respectively (Table 1). The linearity under dilution of two feline serum pools is shown in Figure 1. Dilution of feline serum samples with different adiponectin concentrations resulted in linear regression equations with correlation coefficient close to 1.0 in the mean range from 10–1600 ng/ml. The recovery between observed and expected IGF-1 concentrations ranged from 100–112%, with a mean of 105% (Table 2). The detection limit was lower than 0.01 ng/ml.
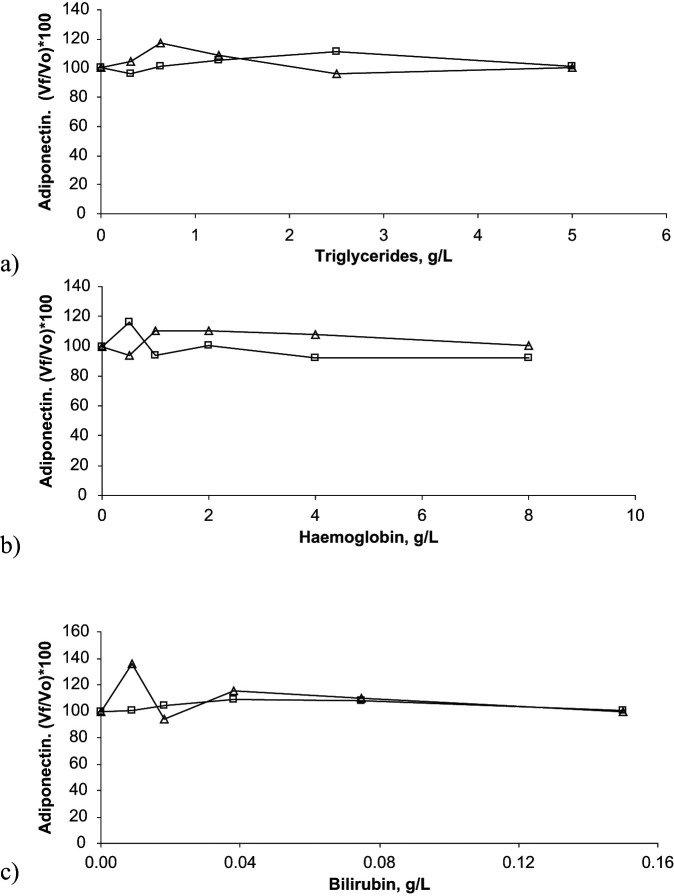
Interferograms showing effects of lipaemia, haemolysis and bilirubinaemia on adiponectin, ghrelin and IGF-1 concentrations determination are presented in Figures 2–4.
Figure 2.
Interferograms corresponding to the effect of triglycerides (A), haemoglobin (B) and bilirubin (C) concentrations on adiponectin determination in two feline serum pools. In these graphs, results obtained with the two pools are presented with open boxes and with open triangles, respectively. X-axes show increasing concentrations of haemoglobin, triglycerides or bilirubin, whilst Y-axes show percentage of change in adiponectin [(Vƒ/V0)×100]. Vƒ = final value; V0 = original value
Figure 4.
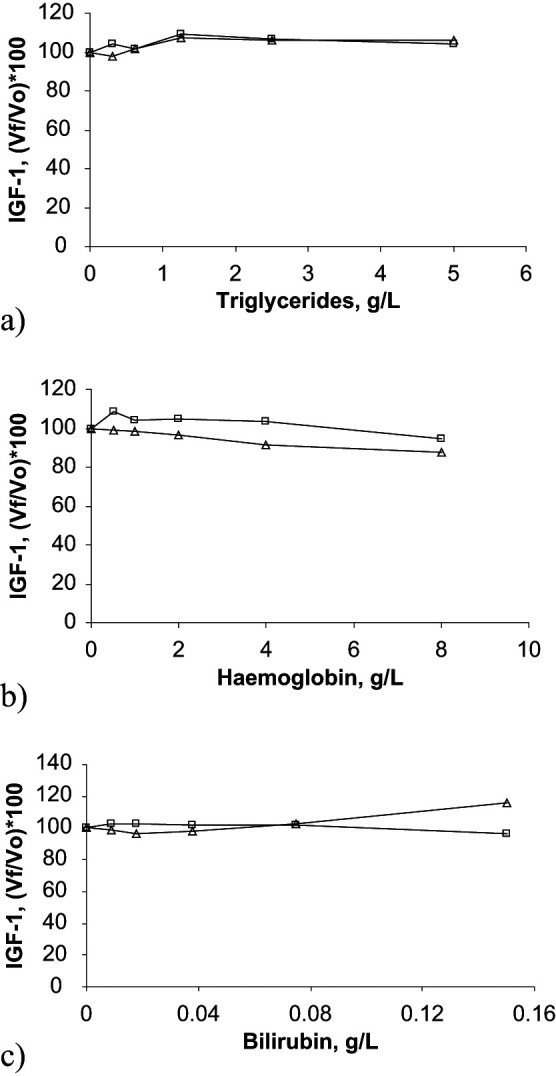
Interferograms corresponding to the effect of triglycerides (A), haemoglobin (B), and bilirubin (C) concentrations on IGF-1 determination in two feline serum pools. In these graphs, results obtained with the two pools are presented with open boxes and with open triangles, respectively. X-axes show increasing concentrations of haemoglobin, triglycerides, or bilirubin, whilst Y-axes show percentage of change in IGF-1 [(Vƒ/ V0)×100]. Vƒ = final value; V0 = original value
Figure 3.
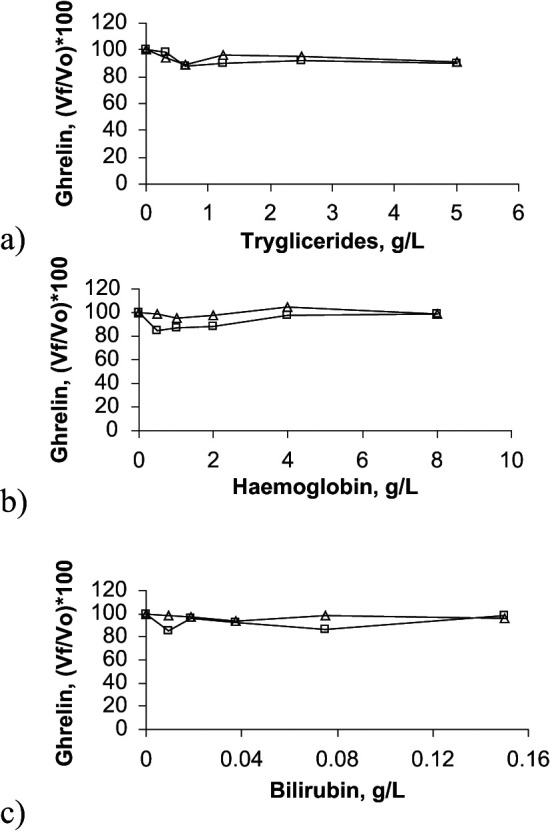
Interferograms corresponding to the effect of triglycerides (A), haemoglobin (B) and bilirubin (C) concentrations on ghrelin determination in two feline serum pools. In these graphs, results obtained with the two pools are presented with open boxes and with open triangles, respectively. X-axes show increasing concentrations of haemoglobin, triglycerides or bilirubin, whilst Y-axes show percentage of change in ghrelin [(Vƒ/ V0)×100]. V = final value; V0 = original value
The different degrees of lipaemia, haemolysis and bilirubinaemia tested in this study did not affect the measured concentrations of adiponectin or ghrelin in the feline serum samples. Lipaemia with a lipid concentration up to 5 g/l and bilirubinaemia with a bilirubin concentration of up to 0.15 g/l did not affect IGF-1 determination in feline serum. However, one-way ANOVA showed a significant decrease (P <0.05) in IGF-1 concentrations in the presence of severe haemolysis with a haemoglobin concentration of 8 g/l.
Overlap performance
Serum adiponectin (P = 0.01) and ghrelin (P = 0.03) concentrations were lower in obese cats (group C1) compared with normal weight cats (group C2), but there was no difference in IGF-1 concentrations between groups (P = 0.12) (Table 3).
Table 3.
Serum adiponectin, ghrelin and IGF-1 in obese (C1) and normal weight (C2) cats
| Obese cats (group C1) | Lean cats (group C2) | ||||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | P | |
| Adiponectin pg/ml | 875.85 | (182.9–2822.3) | 3567.58 | (169.5–6578.9) | 0.01 |
| Ghrelin pg/ml | 220.46 | (130.21–306.6) | 287.40 | (196.0–332.9) | 0.03 |
| IGF-1 ng/ml | 426.22 | (293–570) | 498.33 | (291–998) | 0.18 |
Discussion
A primary function of any methodology is to minimise the amount of error so that test interpretation, patient care and consumer safety are not compromised. 16 Therefore, a common task in clinical laboratories is to validate new tests, assuring that test results reflect the status of the animals more than they reflect variation caused by the laboratory itself. Furthermore, validation studies must ensure that analytical methods can detect the corresponding analyte and provide repeatedly accurate results. 20 The three assays evaluated in the present study showed adequate precision for adiponectin, ghrelin and IGF-1 measurements in feline serum samples with intra- and interassay CVs lower than 20%, the limit of the objective analytic performance standard for precision. 21 However, interassay CVs for low and medium adiponectin concentrations were greater than 15%, which is considered to be poor by some authors. Therefore, when adiponectin is measured, analysing all samples in a single batch, if possible, would be recommended in order to minimise the effect of problems with interassay precision. Further, given such variability, it would not be advisable to use such assays for the purpose of clinical diagnosis, where assays with better precisions are preferred. The IGF-1 assay gave results with the best precision, possibly because it was automated. Automated assays are usually more robust and have the additional advantage of more rapid turnaround time. Similar results (CV <20%) were obtained when these three assays were validated in the authors’ laboratory for use with canine serum samples.22–24
Given that neither certified species-specific reference material nor a ‘reference’ method were available, accuracy was indirectly evaluated by linearity under dilution and spiking recovery, 25 performed by mixing different samples at different proportions. Ideally, the regression analysis of the relationship of the obtained and expected analyte values should result in a regression equation approximating R2 to 1.0. For spiking recovery, percentages between 80% and 120%, should be recorded. 16 In the present study, regression analysis revealed that the three assays were linear when measuring adiponectin, ghrelin and IGF-1, respectively, in different dilutions of feline serum samples with the regression equation R2 close to 1.0. Additionally, given the lack of defined species-specific standards, the methods performed satisfactorily with the materials available for the recovery procedures, the recovery study showed that the three assays could accurately measure the different concentrations of adiponectin, ghrelin and IGF-1 when two samples were mixed at different ratios with a mean recovery close to 100% (107%, 102% and 105%, respectively). These findings confirm all three assays to be accurate when quantifying their respective analytes in feline serum samples.
The lower linearity ranges observed in evaluated tests were acceptable for measurement of adiponectin, ghrelin and IGF-1 in all studied cats. In some cases, serum from lean cats had analyte concentrations exceeding the linearity ranges of the assays. In such cases, the authors would recommend diluting the sample further and repeating the analysis.
In the present study, the three methods were performed with the human standards provided by the respective manufacturers because of a lack of feline reference material with known amounts of adiponectin, ghrelin and IGF-1, respectively. However, for the future, it would be desirable to use species-specific standards in order to achieve similar affinity of antiserum against standards and samples. 26
Adiponectin and ghrelin concentrations were significantly less in obese cats compared with normal weight cats, which is similar to findings from previous reports.8,12,14 Mean concentrations of adiponectin found in our study in obese and normal weight cats are in an equivalent range to those found by Hoenig et al 8 who reported concentrations of around 3 ng/ml (3000 pg/ml) in lean and 1 ng/ml (1000 pg/ml) in obese cats; however, they are less than those reported by Ishioka et al 14 who found concentrations of 18000 ng/ml in lean and 7200 ng/ml in obese cats. Also, our concentrations for ghrelin are less than those reported by Backus et al 12 in cats, with values of around 2000 pg/ml. Differences in the standard used in the assays, as well as affinity in the antibodies, could be a factor that contributed to such diverse results. No difference in serum IGF-1 concentration has been observed between lean and obese cats in contrast to the findings in obese humans.27,28 This could be because of a high interindividual variability of the serum IGF-1 in cats 13 or because of the study was performed comparing neutered and intact cats, as castration can affect IGF-1 concentrations. 29 It may be that differences in circulating IGF-1 levels would be evident between lean and obese cats either if larger groups were compared, or if IGF-1 was measured in the same group of cats before and after weight loss. However, mean concentrations found for IGF-1 in our study for lean cats were similar to the values previously reported by Alt et al. 13 which reported mean IGF-1 concentrations of 452 ng/ml.
In conclusion, we have made a preliminary assessment of commercially-available assays for adiponectin, ghrelin and IGF-1 for use with feline serum samples. All methods exhibited acceptable analytical characteristics, allowing their use in the laboratory with an adequate precision, linearity under dilution and recovery. Further, each assay can detect different concentrations of its respective analyte in lean and obese cats, respectively. Use of these assays in future research studies may help to improve understanding of the pathogenesis of feline obesity and obesity-related diseases.
Acknowledgments
The authors wish to thank Isabel M Rodrigez-Muñoz, Josefa Hernandez-Ruiz, and Susana Ros-Lara from University of Murcia, for their technical support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. German AJ. The growing problem of obesity in dogs and cats. J Nutr 2006; 136: 1940S–6S. [DOI] [PubMed] [Google Scholar]
- 2. German AJ, Ryan VH, German AC, et al. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 2010; 185: 4–9. [DOI] [PubMed] [Google Scholar]
- 3. Zoran DL. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 2010; 40: 221–39. [DOI] [PubMed] [Google Scholar]
- 4. Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: A comparative review. Vet J 2011. doi:10.1016/j.tvjl.2011.04.009 10.1016/j.tvjl.2011.04.009 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Tschop M, Smiley DL, Heiman HL. Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–13. [DOI] [PubMed] [Google Scholar]
- 6. Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res 2003; 13: 113–70. [DOI] [PubMed] [Google Scholar]
- 7. Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des 2010; 16: 1896–901. [DOI] [PubMed] [Google Scholar]
- 8. Hoenig M, Thomaseth K, Waldron M, et al. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol 2007; 292: R227–34. [DOI] [PubMed] [Google Scholar]
- 9. Jaso-Friedmann L, Leary JH, III, Praveen K, et al. The effects of obesity and fatty acids on the feline immune system. Vet Immunol Immunopath 2008; 122: 146–152. [DOI] [PubMed] [Google Scholar]
- 10. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 2005; 85: 495–522. [DOI] [PubMed] [Google Scholar]
- 11. Yokoyama M, Nakahara K, Kojima M, et al. Influencing the between-feeding and endocrine responses of plasma ghrelin in healthy dogs. Eur J Endocrinol 2005; 152: 155–60. [DOI] [PubMed] [Google Scholar]
- 12. Backus RC, Cave NJ, Keisler DH. Gonadectomy and high dietary fat but not high dietary carbohydrate induce gains in body weight and fat of domestic cats. Br J Nutr 2007; 98: 641–50. [DOI] [PubMed] [Google Scholar]
- 13. Alt N, Kley S, Tschuor F, et al. Evaluation of IGF-1 levels in cats with transient and permanent diabetes mellitus. Res Vet Sci 2007; 83: 331–5. [DOI] [PubMed] [Google Scholar]
- 14. Ishioka K, Omachi A, Sasaki N, et al. Feline adiponectin: molecular structures and plasma concentrations in obese cats. J Vet Med Sci 2009; 71: 189–94. [DOI] [PubMed] [Google Scholar]
- 15. Laflamme DP. Development and validation of a body condition score for cats: a clinical tool. Feline Pract 1997; 25: 13–8. [Google Scholar]
- 16. Jensen AL, Kjelgaard-Hansen M. Diagnostic test validation. In: Weiss DJ, Wardrop KJ. (eds). Schalm’s veterinary haematology. 6th edn. Iowa: Blackwell Publishing, 2010, pp.1027–33. [Google Scholar]
- 17. Furlanello T, Simonato G, Caldin M, et al. Validation of an automated spectrophotometric assay for the determination of cholinesterase activity in canine serum. Vet Res Comm 2006; 30: 723–3. [DOI] [PubMed] [Google Scholar]
- 18. Schellenberg S, Grenacher B, Kaufmann K, et al. Analytical validation of commercial immunoassays for the measurement of cardiovascular peptides in the dog. Vet J 2008; 178: 85–90. [DOI] [PubMed] [Google Scholar]
- 19. Martínez-Subiela S, Ceron JJ. Effects of hemolysis, lipemia, hyperbilirubinemia, and anticoagulants in canine C-reactive protein, serum amyloid A, and ceruloplasmin assays. Can Vet J 2005; 46: 625–9. [PMC free article] [PubMed] [Google Scholar]
- 20. Tecles F, Fuentes P, Martinez-Subiela S, et al. Analytical validation of commercially available methods for acute phase proteins quantification in pigs. Res Vet Sci 2007; 83: 133–9. [DOI] [PubMed] [Google Scholar]
- 21. Guidance for Industry: Bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. (2001, accessed 24 November 2011).
- 22. Tvarijonaviciute A, Martínez-Subiela S, Ceron JJ. Validation of 2 commercially available enzyme-linked immunosorbent assays for adiponectin determination in canine serum samples. Can J Vet Res 2010; 74: 279–85. [PMC free article] [PubMed] [Google Scholar]
- 23. Tvarijonaviciute A, Martínez-Subiela S, Ceron JJ. Validation of two ELISA assays for total ghrelin measurement in dogs. J Anim Physiol Anim Nutr (Berl) 2010. doi: 10.1111/j.1439-0396.2010.01112.x 10.1111/j.1439-0396.2010.01112.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24. Tvarijonaviciute A, Tecles F, Carillo JM, Rubio M, Ceron JJ. Serum IGF-1 measurements in dogs: performance characteristics of an automated assay and study of some sources of variation. Can Vet J 2010. 2011; 75: 312–316. [PMC free article] [PubMed] [Google Scholar]
- 25. Jensen AS. Validation of diagnostic tests in hematology laboratories. In: Feldman BF, Zinkl JG, Jain NC. (eds). Schalm’s veterinary hematology. 5th edn. Iowa: Blackwell Publishing, 2006, pp. 20–28. [Google Scholar]
- 26. Heddle RJ, Rowley D. Dog immunoglobulins. I. Immunochemical characterization of dog serum, parotid saliva, colostrum, milk and small bowel fluid. Immunol 1975; 29: 185–95. [PMC free article] [PubMed] [Google Scholar]
- 27. Pardina E, Ferrer R, Baena-Fustegueras JA, et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg 2010; 20: 623–32. [DOI] [PubMed] [Google Scholar]
- 28. Belobrajdic DP, Frystyk J, Jeyaratnaganthan N, et al. Moderate energy restriction-induced weight loss affects circulating IGF levels independent of dietary composition. Eur J Endocrinol 2010; 162: 1075–82. [DOI] [PubMed] [Google Scholar]
- 29. Martin LJM, Siliart B, Dumon HJW, Nguyen P. Spontaneus hormonal variations in male cats following gonadectomy. J Feline Med Surg 2006; 8: 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]