Abstract
Clarithromycin (CLM) has been known to increase the cyclosporine (CsA) trough levels in human transplant patients. However, the interaction of CLM with CsA has not been reported in cats. In this study, the effects of oral dosing of CLM on the pharmacokinetics and dosing of CsA in cats were investigated. Co-administration of CLM with CsA resulted in significant increases of oral bioavailability of CsA. In addition, CLM reduced the CsA dosage required to maintain the therapeutic CsA trough levels to almost 35% of the initial CsA therapy and the dose frequency was successfully replaced from a twice a day schedule to once a day in a feline kidney transplant patient. The addition of CLM to the regular CsA-based immunosuppression could be used as an effective alternative to classical ketoconazole treatment in feline kidney transplant patients and may result in substantial cost saving and convenience for the cat owners.
Introduction
Kidney transplantation has been developed as one of the therapeutic options for end-stage kidney failure in cats since the 1990s. Cyclosporine (CsA) is currently the core immunosuppressive drug for the prevention of allograft rejection after renal transplantation in cats. 1 CsA is a lipophilic, cyclic polypeptide isolated from the fungus Tolypocladium inflatum which exerts powerful immunosuppressive properties. CsA specifically inhibits synthesis of interleukin (IL)-2, which induces activation and proliferation of T cells and results in suppression of secondary synthesis of various cytokines, such as IL-4, interferon-γ and granulocyte–macrophage colony stimulating factor. CsA has been reported to be effective in the treatment of canine atopic dermatitis, perianal fistula and keratoconjunctivitis sicca.2–4 CsA is widely known as a substrate of cytochrome P450 (CYP) 3A and P-glycoprotein (P-gp) in humans.5–7 In feline kidney transplantation, CsA typically needs to be administered twice a day throughout the lifetime of the patients. Although kidney transplantation has become a therapeutic option for end-stage kidney failure, the cost of CsA-based immunosuppressive therapy has been a significant obstacle. To circumvent this problem, ketoconazole (Kcz) has been used to reduce the dosage of CsA, 8 which suggests that CsA metabolism is mediated by inhibition of CYP3A and/or P-gp in cats, as in humans. It was reported that Kcz administration combined with a twice-daily CsA dosage regimen reduced approximately 60% of the total amount of CsA required to maintain the therapeutic CsA blood levels compared with the treatment without Kcz 8 . However, the use of Kcz leads to other problems associated with side effects of Kcz, the main problem being its hepatotoxicity. If hepatotoxicity develops, Kcz needs to be removed from the immunosuppressive regimen, which may result in acute allograft rejection.
It is known that clarithromycin (CLM) can also inhibit CYP3A and P-gp,9–12 and it appears to be less toxic than Kcz. It was reported that CLM significantly increased bioavailability of CsA and lowered the daily drug cost in human lung transplant patients treated with CsA-based immunosuppressive therapy. 13 Based on these findings, CLM may become an excellent alternative for Kcz for feline kidney transplant patients. In cats, however, it has not been reported whether CLM affects the oral bioavailability of CsA. In this study, we hypothesized that CLM increases the blood levels of CsA and, as a result, it decreases the required CsA dosage in feline kidney transplantation.
The aim of this study was to evaluate the effect of CLM on the pharmacokinetic parameters of CsA in cats. In addition, we investigated whether CLM decreases the CsA dosage while maintaining a therapeutic CsA trough level in a feline kidney transplant patient. The aim was to develop a new immunosuppressive therapy that was cost effective and clinically acceptable for cats receiving kidney transplantation.
Materials and methods
This study consisted of two components (pharmacokinetic and clinical studies). Both studies were approved by the Iwate University Animal Care and Use Committee.
Pharmacokinetic study in healthy cats
Four healthy male cats were used for this study. Their body weights ranged from 4.1–6.5 kg and their ages ranged from 2–4 years old. Prior to this study, all cats were confirmed to be healthy based on physical examination, complete blood count, biochemical profile and urinalysis. The cats were allocated to two treatment groups and the study was conducted by a crossover design. In a clinical setting, CsA is administered at a dosage of 3–5 mg/kg q12h for renal transplantation in cats. 14 Therefore, the dose of CsA (Ciclosporin fine granule 17%; Mylan, Tokyo, Japan) was adjusted to 5 mg/kg. In treatment group A, the cats received oral CsA alone. In treatment group B, the cats were premedicated with approximately 10 mg/kg (therapeutic dose level) of CLM (Clarithromycin Capsule 50 mg; Chouseido, Tokushima, Japan: range 7.96–11.11 mg/kg, mean dose 9.82 mg/kg) 2 h before CsA administration. The washout times of each treatment were more than 1 month. The cats were fasted 12 h prior to each treatment and they had access to water ad libitum throughout the study.
Whole blood samples were drawn through the jugular vein at 0.5, 1, 2, 4, 6, 10, 12 and 24 h after CsA administration and were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). Blood samples were stored overnight at 0°C until measurements were taken. Measurement of the whole blood CsA concentration was performed using a radioimmunoassay (RIA) by the commercial laboratory (SRL, Tokyo, Japan).
The maximum blood concentration (Cmax) and its corresponding time (tmax) were determined for each cat by observation of the blood CsA concentration versus time profile. The area under the curve from 0–24 h (AUC0–24) after a CsA administration was calculated by the linear trapezoidal method. The terminal elimination rate constant (k) was calculated by linear least squares regression analysis using the last three measurement points in the log-linear terminal phase. The t1/2 was estimated as 0.693/k. The area under the first moment curve from 0–24 h (AUMC0–24) after a CsA administration was also calculated by the linear trapezoidal method. The mean residence time (MRT) was calculated as AUMC0–24/AUC0–24.
Differences in the pharmacokinetic parameters between each treatment were analyzed using the paired t-test and were regarded to be statistically significant at P <0.05. Each value is shown as the mean ± standard error (SE).
Clinical case
An 8-year-old male domestic shorthair cat was transplanted an allograft kidney at the Iwate University Veterinary Teaching Hospital (IUVTH) because of end-stage chronic kidney failure. The cat was managed with a classical immunosuppressive drug regimen consisting of CsA (Ciclosporin fine granule 17%; Mylan, Tokyo, Japan) and prednisolone (Pre) to prevent acute allograft rejection. In this cat, buprenorphine (0.2 mg/head) was administered intravenously to control pain after the surgery. CsA was started 2 days prior to transplantation. Pre was started at 1 mg/kg/day on the day of transplantation and tapered to 0.25 mg/kg/day over a 5-month period after surgery. The cat was administered with two treatment protocols with or without CLM. Initially, the cat was orally administered CsA which was adjusted to 5 mg/kg q12h. CsA trough levels were monitored as required and the dosage of CsA was adjusted to maintain the CsA trough levels within the therapeutic range of 400–600 ng/ml. Once CsA blood levels were stabilized, oral CLM was co-administered with CsA at the dosage of approximately 10 mg/kg q24h. Simultaneously, the dose frequency of CsA was changed from q12h to q24h. The dosage of CsA was re-adjusted to maintain the trough levels within the therapeutic range. Measurements of whole blood CsA concentration was performed using RIA as in the pharmacokinetic study.
Results
Pharmacokinetic study in healthy cats
The CsA blood concentration-time curves after the two treatments are shown in Figure 1. The AUC0–24 of cats with CLM treatment (treatment B) was significantly higher than that of cats treated with CsA alone (treatment A) (P < 0.02). The Cmax of cats with treatment B was significantly higher than that with treatment A (P < 0.03). The average CsA blood concentrations of cats with treatment B were almost two times higher than those of cats with treatment A at 12 and 24 h (P < 0.02 and P < 0.04, respectively). Pre-administration of a single dose of CLM did not significantly affect tmax, t1/2 and MRT. The pharmacokinetic parameters of CsA with or without CLM are listed in Table 1.
Figure 1.
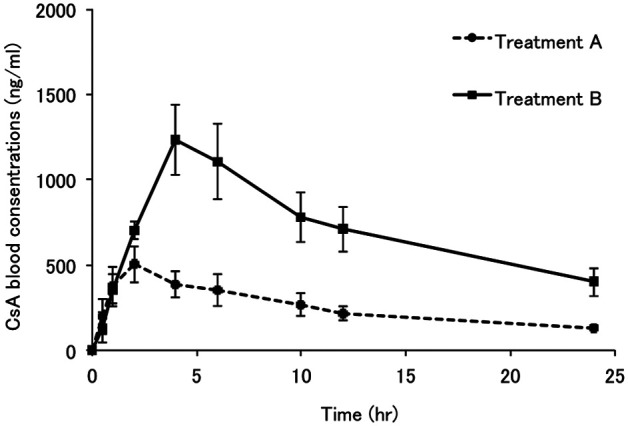
Mean cyclosporine (CsA) blood concentration-time curves of the four healthy cats following treatment A (CsA alone) and treatment B (CsA + single therapeutic dose of clarithromycin (CLM)). In this study, the dosages of CsA and CLM were adjusted to 5 mg/kg and approximately 10 mg/kg (range; 7.96–11.11 mg/kg according to the capsule strength and cat’s weight) respectively
Table 1.
Effects of a single therapeutic dose of oral clarithromycin (CLM) on the pharmacokinetic parameters of cyclosporine (CsA) in four healthy cats
| Parameter | Treatment A (CsA alone) | Treatment B (CsA + CLM) |
|---|---|---|
| AUC0–24 (ng•h/ml) | 6102 ± 1050 | 16879 ± 2867 a |
| Cmax (ng/ml) | 553 ± 99 | 1258 ± 201 b |
| tmax (h) | 2.38 ± 1.25 | 4.00 ± 0 |
| t1/2 (h) | 19.24 ± 4.82 | 14.31 ± 0.65 |
| C12 (ng/ml) | 218 ± 42 | 710 ± 132 c |
| C24 (ng/ml) | 133 ± 14 | 400 ± 84 d |
| AUMC0–24 (ng•h2/ml) | 56749 ± 9932 | 172833 ± 32848 e |
| MRT0–24 (h) | 9.36 ± 0.45 | 10.16 ± 0.25 |
In this study, the dosage of CsA and CLM were adjusted to 5 mg/kg and approximately 10 mg/kg (range; 7.96–11.11 mg/kg according to the capsule strength and cat’s weight), respectively. Values are presented as means ± SE
C12 and C24 = CsA blood concentrations at 12 h and 24 h after CsA intake, respectively
Statistically different from treatment group A (P < 0.02, P < 0.03, P < 0.02, P < 0.04 and P < 0.02, respectively).
Clinical case
The initial CsA dosage required to achieve whole blood trough levels between 400 and 600 ng/ml was 2.64 mg/kg/day, divided twice daily. The CsA blood concentrations were stabilized at 580 ng/ml 134 days after the initiation of the CsA administration. The next day, CLM was co-administered with CsA and the dose frequency was changed from q12h to q24h. CLM immediately affected the CsA blood levels, which increased up to 1000 ng/ml within the first 2 days. On day 37 after CLM, it was difficult to stabilize the CsA blood levels within the therapeutic range by continuous administration of the same dosage of CsA. Therefore, CsA dosage was adjusted irregularly as follows: 0.84–0.96 mg/kg on Tuesday, Thursday, Saturday and Sunday; and, 1.11–1.28 mg/kg on Monday, Wednesday and Friday, based on the cat’s weight. On day 69 after CLM, CsA blood levels were stabilized at 510 ng/ml and the average CsA dosage of 1 week treatment decreased to 1.08 mg/kg/day. Thereafter, CsA dosage continued to decrease based on cat’s weight. On day 210 after CLM, CsA blood level was stabilized at 600 ng/ml and the average CsA dosage of 1 week treatment finally decreased to 0.91 mg/kg/day. The CsA requirements decreased significantly by 34.5% compared with initial CsA doses. The time courses of CsA blood trough levels and CsA dosage after CLM are shown in Figure 2.
Figure 2.
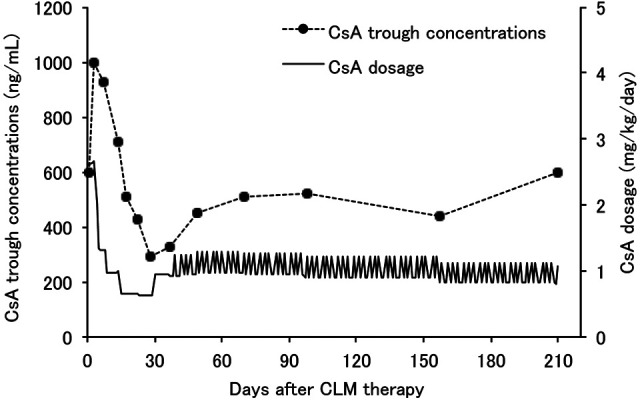
Time courses of cyclosporine (CsA) trough concentrations and dosage after clarithromycin (CLM) treatment in a feline kidney transplant patient
After CLM, gastrointestinal signs such as vomiting and diarrhoea associated with the adverse effects of macrolides, hepatotoxicity and acute allograft rejection have not been observed so far. At the time of writing, the cat was scored as stage II [blood urea nitrogen 39.8 mg/dl, creatinine 2.24 mg/dl, inorganic phosphorus 4.1 mg/dl, calcium (albumin corrected value) 11.7 mg/dl, packed cell volume 31.0%] defined by the chronic kidney disease staging chart of the International Renal Interest Society; 15 progression of clinical signs and staging has not been recognized.
Discussion
CsA is widely known to be the substrate of CYP3A and P-gp. When CsA is combined with the inhibitor of these proteins, CsA metabolism will be more or less affected. In human lung, liver and heart transplant patients, CLM, one of the 14-membered ring macrolides, significantly increased the CsA blood concentrations and resulted in the reduction of CsA dosage.13,16 CLM has been known to inhibit both CYP3A and P-gp activities.9–12 Macrolides have been recognized as having the ability of metabolite-intermediate (MI) complex formation resulting in inhibition of the CYP3A mediated catalytic activity.17,18 This mechanism accounts for most of the drug interactions produced by macrolides. On one hand, Pinto et al 11 reported that CLM caused inhibition of intestinal CYP3A activity, most likely as a result of formation of MI complex in humans. On the other hand, CLM could form MI complex to a lesser extent in rats, 19 which appeared to have a weak ability to inhibit CYP3A. In addition to CYP3A, it has emerged recently that P-gp is involved in the pharmacokinetic behaviour of CsA. P-gp belongs to the subfamily of ATP-binding cassette transporters and is also associated with excretion of CsA, acting as a drug efflux pump that actively transports CsA back into the intestinal lumen. 5 Wakasugi et al 9 reported that CLM inhibited the P-gp mediated tubular excretion of digoxin in a concentration-dependent manner. In addition, Hughes et al 12 also demonstrated that CLM had strong P-gp inhibitory action against digoxin and its metabolites using Caco-2 sub clone cells highly expressing P-gp. These findings suggest that P-gp, rather than CYP3A, may play a central role in the CLM inhibitory mechanism of CsA metabolism. However, the details of the effects of CLM on CYP3A and P-gp have not yet been investigated in cats.
Hepatic metabolism is generally considered to be primarily important for drug bioavailability, with other areas of metabolism playing a comparatively minor role. However, intestinal metabolism may play a much greater role in the pharmacokinetics of orally-administered drugs than previously thought. Although CsA is metabolized both in the liver and small intestine in humans,5,6 it was reported that as much as 50% of oral CsA metabolism may be attributed to intestinal metabolism in humans.5,7 Based on the results of our pharmacokinetic study, administration of CLM could significantly increase the blood concentration and AUC0–24 of CsA compared with CsA alone. In addition, regarding t1/2 and MRT, there were no statistical differences between CsA alone and CsA with CLM. This suggests that administration of a single dose of CLM may increase oral bioavailability of CsA in cats, mainly by decreasing the first-pass effect owing to CYP3A and/or P-gp inhibition.
In this study, the mean t1/2 value of a single oral CsA treatment was 19.24 h compared with 12.15 h in our previous pharmacokinetic study in cats. 20 The blood samplings were scheduled similarly in both studies. The difference between these t1/2 values may be partially owing to individual differences among cats and/or CsA drug formulation.
In addition to the effects of CLM therapy on CsA blood levels, macrolides are well known to have direct or indirect effects on the immune system. 21 Morikawa et al 22 reported that CLM inhibited the production of IL-2 by mitogen-stimulated human T lymphocytes in a dose-dependent manner, which may indicate that the immunosuppressive effects of CLM may be based, in part, on the ability to inhibit the production of IL-2 by T lymphocytes. Combined treatment with CLM and CsA may enhance the immunosuppressive effects on T lymphocyte proliferation, leading to acute allograft rejection. Perhaps CLM may be beneficial to feline kidney transplant patients as a result of its immunosuppressive effects, thereby minimizing potential insults to the allograft. CLM has been used primarily to treat respiratory tract infections in humans and is considered to be one of the safe drugs with fewer side effects.23,24 In cats, although its use is limited, its use in the treatment of infections of Mycobacterium and Helicobacter species has been reported.23–25 In feline kidney transplant patients, the occurrence of bacterial infections, such as upper respiratory tract infections and pneumonia as a postoperative complication owing to long-term immunosuppression, has been reported.26,27 Schmiedt et al 28 reported that 37% of feline kidney transplant patients showed infection at some point after transplantation — with bacteria being the most common cause (62% of all infections). Thus, prophylactic use of CLM may be effective for the prevention of respiratory bacterial infections in feline kidney transplantation. In cats, it was reported that the occurrence of side effects had not been observed after more than 3 months of use of CLM (50.0–62.5 mg/head, q12h, PO).23,25 In our case, side effects associated with CLM have not been observed so far, even when used at 50 mg/head/day for 10 months. This suggests that CLM may be a relatively safe drug for cats, as it is for humans. In our clinical transplant patient, co-administration of CLM with CsA resulted in a 65.5% reduction in CsA requirement, leading to an approximately 30% reduction in total medical costs. From the above viewpoint, the application of triple drug immunosuppressive therapy including CLM may be clinically beneficial for the management of feline kidney transplant patients. However, long-term use of CLM may induce the development of antibiotic-resistant bacteria. Therefore, further research is required to investigate the effectiveness and safety of long-term CLM therapy after kidney transplantation in cats.
In this study, RIA, which is widely known to cross- react with both the parent CsA and its metabolites, was used as an alternative to high performance liquid chromatography for CsA measurement. Therefore, the CsA blood concentrations measured in this study by RIA may have been overestimated to some degree. In addition, we did not monitor the blood CLM concentration. We would expect that the blood concentrations of CLM might reach a plateau by continuous administration of therapeutic doses of CLM in cats. When CsA is co-administered with CLM, the increase in blood CsA concentration may partially depend upon the blood CLM concentration. However, the optimal concentration of CLM to up-regulate the CsA blood level in cats is not clear.
In conclusion, the results of our study showed that CLM significantly increased the oral bioavailability of CsA in healthy cats and co-administration of CsA with CLM decreased the CsA dosage required for the prevention of acute allograft rejection. In addition, CLM replaced the CsA dose frequency from a twice a day to a once a day schedule in a clinical feline kidney transplant patient. These effects of CLM provide economic benefits to the pet owners. However, a larger scale, prospective study is warranted to explore more extensively the impact of CLM on the CsA dosage in kidney transplanted cats because of a small number of animals used in this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1. Katayama M, McAnulty J. Renal transplantation in cats: patient selection and preoperative management. Compend Cont Edu Pract Vet 2002; 24: 868–73, 874-882. [Google Scholar]
- 2. Guaguere E, Steffan J, Olivry T. Cyclosporin A: a new drug in the field of canine dermatology. Vet Dermatol 2004; 15: 61–74. [DOI] [PubMed] [Google Scholar]
- 3. Mathews KA, Ayres SA, Tano CA, et al. Cyclosporin treatment of perianal fistulas in dogs. Can Vet J 1997; 38: 39–41. [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan RV, Abrams KL. Topical administration of cyclosporine for treatment of keratoconjunctivitis sicca in dogs. J Am Vet Med Assoc 1991; 199: 1043–1046. [PubMed] [Google Scholar]
- 5. Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev 1997; 27: 201–214. [DOI] [PubMed] [Google Scholar]
- 6. Whalen RD, Tata PN, Burckart GJ, et al. Species differences in the hepatic and intestinal metabolism of cyclosporine. Xenobiotica 1999; 29: 3–9. [DOI] [PubMed] [Google Scholar]
- 7. Kolars JC, Awni WM, Merion RM, et al. First-pass metabolism of cyclosporin by the gut. Lancet 1991; 338: 1488–1490. [DOI] [PubMed] [Google Scholar]
- 8. McAnulty JF, Lensmeyer GL. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats. Vet Surg 1999; 28: 448–455. [DOI] [PubMed] [Google Scholar]
- 9. Wakasugi H, Yano I, Ito T, et al. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin Pharmacol Ther 1998; 64: 123–128. [DOI] [PubMed] [Google Scholar]
- 10. Spicer ST, Liddle C, Chapman JR, et al. The mechanism of cyclosporine toxicity induced by clarithromycin. Br J Clin Pharmacol 1997; 43: 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinto AG, Wang YH, Chalasani N, et al. Inhibition of human intestinal wall metabolism by macrolide antibiotics: effect of clarithromycin on cytochrome P450 3A4/5 activity and expression. Clin Pharmacol Ther 2005; 77: 178–188. [DOI] [PubMed] [Google Scholar]
- 12. Hughes J, Crowe A. Inhibition of P-glycoprotein-mediated efflux of digoxin and its metabolites by macrolide antibiotics. J Pharmacol Sci 2010; 113: 315–324. [DOI] [PubMed] [Google Scholar]
- 13. Knower MT, Labella-Walker K, McFadden PM, et al. Clarithromycin for safe and cost-effective reduction of cyclosporine doses in lung allograft recipients. South Med J 2000; 93: 108792. [PubMed] [Google Scholar]
- 14. Gregory C, Bernsteen L. Organ transplantation in clinical veterinary practice. In: Slatter D. (ed). Textbook of small animal surgery. 3rd ed. Philadelphia, PA: Saunders, 2003, pp 112–136. [Google Scholar]
- 15. Roudebush P, Polzin DJ, Ross SJ, et al. Therapies for feline chronic kidney disease. What is the evidence? J Feline Med Surg 2009; 11: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadaba B, Lopez de, Ocariz A, Azanza JR, et al. Concurrent clarithromycin and cyclosporin A treatment. J Antimicrob Chemother 1998; 42: 393–395. [DOI] [PubMed] [Google Scholar]
- 17. Murray M. Drug-mediated inactivation of cytochrome P450. Clin Exp Pharmacol Physiol 1997; 24: 465–470. [DOI] [PubMed] [Google Scholar]
- 18. Pessayre D, Larrey D, Vitaux J, et al. Formation of an inactive cytochrome P-450 Fe(II)-metabolite complex after administration of troleandomycin in humans. Biochem Pharmacol 1982; 31: 1699–1704. [DOI] [PubMed] [Google Scholar]
- 19. Ohmori S, Ishii I, Kuriya S, et al. Effects of clarithromycin and its metabolites on the mixed function oxidase system in hepatic microsomes of rats. Drug Metab Dispos 1993; 21: 358–363. [PubMed] [Google Scholar]
- 20. Katayama M, Katayama R, Kamishina H. Effects of multiple oral dosing of itraconazole on the pharmacokinetics of cyclosporine in cats. J Feline Med Surg 2010; 12: 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Culic O, Erakovic V, Parnham MJ. Anti-inflammatory effects of macrolide antibiotics. Eur J Pharmacol 2001; 429: 209–229. [DOI] [PubMed] [Google Scholar]
- 22. Morikawa K, Oseko F, Morikawa S, et al. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother 1994; 38: 2643–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elsner L, Wayne J, O’Brien CR, et al. Localised Mycobacterium ulcerans infection in a cat in Australia. J Feline Med Surg 2008; 10: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khoshnegah J, Jamshidi S, Mohammadi M, et al. The efficacy and safety of long-term Helicobacter species quadruple therapy in asymptomatic cats with naturally acquired infection. J Feline Med Surg 2011; 13: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Courtin F, Huerre M, Fyfe J, et al. A case of feline leprosy caused by Mycobacterium lepraemurium originating from the island of Kythira (Greece): diagnosis and treatment. J Feline Med Surg 2007; 9: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kadar E, Sykes JE, Kass PH, et al. Evaluation of the prevalence of infections in cats after renal transplantation: 169 cases (1987–2003). J Am Vet Med Assoc 2005; 227: 948–953. [DOI] [PubMed] [Google Scholar]
- 27. Mishina M, Watanabe T, Maeda H, et al. Renal transplantation in cats with chronic renal failure. J Vet Med Sci 1996; 58: 655–658. [DOI] [PubMed] [Google Scholar]
- 28. Schmiedt CW, Holzman G, Schwarz T, et al. Survival, complications, and analysis of risk factors after renal transplantation in cats. Vet Surg 2008; 37: 683–695. [DOI] [PubMed] [Google Scholar]


