Abstract
Twenty-one cats presented with a history of slowly progressive neurological signs characterised by a stiff extended tail, behavioural changes, and spastic and ataxic gait. All cats had outdoor access and lived in the same geographical rural area in north-east Scotland. Histological findings were consistent with lymphohistiocytic meningoencephalomyelitis. Immunohistochemistry ruled out 15 pathogens and showed a significant expression of the interferon-inducible Mx protein, suggesting an as yet unidentified infective or environmental immunogenic trigger as the possible causative agent. The late age at onset (mean 9 years), the very slow progression of clinical signs (mean 11 months) and the peculiar clinical presentation (particularly the posture of the tail) have not been reported previously in cats with lymphohistiocytic meningoencephalomyelitis.
Introduction
Inflammatory diseases have been reported to account for 32–44% of histologically confirmed central nervous system (CNS) diseases in cats.1,2 The most common cause of meningoencephalomyelitis in cats is feline coronavirus (FCoV), the aetiological agent of feline infectious peritonitis (FIP). Its frequency has been reported as 44–51%1,2 in cats with histologically-confirmed CNS inflammation. The incidence of FIP is highest in cats 6 months to 2 years of age. 3 Neurological signs are consistent with multifocal CNS involvement; however, signs most commonly reflect caudal cranial fossa (cerebellum, pons and medulla oblongata) involvement. 4 Progression can be acute to chronic. Pathological findings typical of CNS FIP include a pyogranulomatous inflammatory cell infiltration of leptomeninges, choroid plexus, ependyma and brain parenchyma with the most severe changes at the inner and outer surfaces of the CNS. Hydrocephalus, vasculitis and extraneural changes are commonly present. 5
Other known aetiologies of feline meningoencephalomyelitis are uncommon and include viruses such as feline immunodeficiency virus (FIV), feline leukaemia virus (FeLV), feline parvovirus (FPV), pseudorabies virus/porcine herpesvirus 1 (PHV-1), rabies virus, Borna disease virus (BDV), West Nile virus (WNV), encephalomyocarditis virus (EMCV), and protozoal, bacterial, rickettsial, fungal and parasitic agents.6,7 A large number of cats with inflammatory CNS disease other than FIP (35–40%) are found to have histopathological changes consistent with lymphohistiocytic (non-suppurative) meningoencephalomyelitis. 6 This is characterised by perivascular and parenchymal infiltration with lymphocytes, monocytes and plasma cells, usually associated with meningitis and, occasionally, with inflammation of the choroid plexus and the ependyma. 5 Lymphohistiocytic meningoencephalomyelitis is usually suggestive of viral infection; however, the causative agent is often not identified. In a study on 33 cats with lymphohistiocytic meningoencephalitis, immunohistochemistry with antibodies for 17 different infectious agents failed to reveal the aetiology in 61% of cats. 7
The onset of neurological signs in cats with lymphohistiocytic meningoencephalomyelitis is usually at a young age (2 years or less) and progression is generally no longer than a couple of weeks.2,6
The purpose of this study is to describe the clinical, diagnostic, histological and immunohistochemical findings in a group of cats with an adult-onset, slowly-progressive lymphohistiocytic meningoencephalomyelitis resulting in a peculiar and distinctive clinical presentation.
Materials and methods
Cats that presented with behavioural changes, a stiff extended tail and a spastic and ataxic gait to the Strathbogie Veterinary Centre, Huntly, Scotland and to Morven Veterinary practice, Alford, Scotland, between January 2001 and May 2010 were included. Clinical follow-up information had to be available until death/euthanasia or May 2010. The medical records, including results of laboratory investigations, clinical follow-up, post-mortem examination (when available) and videos of the neurological examinations, were reviewed by the first author. A general pathological examination, including necropsy and histological examination, was carried out routinely on all cats that underwent post-mortem examination.
The CNS of all cats that underwent post-mortem examination was examined at the Neuropathology Laboratories of the Animal Health Trust (AHT) and Ludwig Maximilians University of Munich. The brain was trimmed in transverse sections from the olfactory bulb to the oculomotor nerve emergence. Further oblique vertical sections were performed on the occipital cortex, midbrain, rostral pons and the rostral lobe of the cerebellum. The brain was subjected to transverse sectioning from the middle cerebellar peduncles to the obex. Histological slides were obtained from eight different levels [at genu of callosal body, interthalamic adhesion, caudal commissure, interpeduncular nucleus, trapezoid body, lateral foramina (Luschkae), obex, and first cervical segment] after paraffin embedding. Sections of the lower cervical, thoracic, upper lumbar and lumbosacral spinal cord were obtained.
Histological sections were stained routinely with haematoxylin-eosin, luxol fast blue and periodic acid-Schiff. The investigation was carried out on a Zeiss Axiophot and an Olympus BH2 microscope at magnification ×20 to ×1000. The assessment and interpretation of histological abnormalities was based on established neuropathological algorithms. 5
Routine immunohistochemistry on paraffin embedded CNS tissue was performed after antigen retrieval as described elsewhere. 7 Target antigens were: FCoV, FIV, FeLV, FPV, PHV-1, rabies virus, classical and avian BDV, WNV, EMCV, canine distemper virus (CDV), hantavirus, avian paramyxovirus I/Newcastle disease virus (NDV), tick-borne encephalitis virus (TBEV), Toxoplasma gondii and Neospora caninum. The staining employed an avidin-biotin system and horseradish peroxidase-diaminobenzidine detection kit. A screen for FPV, NDV and EMCV nucleic acid was also carried out via reverse transcriptase-polymerase chain reaction (RT-PCR). 8 Additional immunohistochemical investigations were carried out for immune cell markers CD3, CD20, CD79a, MAC387 and lysozyme, as well as for the dynamin-like GTPase Mx protein. 9
Results
Twenty-one cats were identified. One of these 21 cats, after examination at the Strathbogie Veterinary Centre in Huntly, was referred to the Neurology/Neurosurgery Service at the AHT for further clinical and diagnostic investigations. Videos of the neurological examination of the other cats (performed by the second author), were reviewed by the first author. All cats had outdoor access, were active hunters (predominantly of rodents and also of birds) before developing the condition and lived in the same geographical rural area in north-east Scotland between Inverness and Aberdeen, predominately near Huntly and Alford. Mean age at onset of signs was 9 years (range: 4.5–14 years) and mean progression of signs was 11 months (range: 6–24 months). Disease occurrence appeared sporadic with no seasonality, environmental factor or exposure identified. Nineteen cats were domestic shorthair and two were Persian-cross. Seven were male, four were male neutered, six were female, two were female spayed, and in two cats the sex was not recorded. General physical examination revealed an unkempt hair coat over the lumbar region which was most likely the result of decreased ability or inability to groom this region. Neurological examination revealed disorientation, obtunded mental status, a stiff extended tail posture, spastic and ataxic gait (see supplementary video 1, online), increased muscle tone, decreased-to-absent postural reactions in all four limbs and decreased-to-absent menace response bilaterally. The degree of neurological dysfunction varied depending on the duration of the disease, becoming worse over time in all cats. Mild behavioural changes and the rigid extension of the tail were the most consistent early signs of the disease.
Haematology and serum biochemistry were performed in six cats and did not reveal any specific abnormalities. Bile acids were normal in the only tested cat and T4 was normal in four cats. Serology for FIV and FeLV was performed in 13 cats and was negative in all but one cat that was FIV-positive. Serology for BDV was performed in one cat and results were negative. Magnetic resonance imaging was performed in the cat referred to the AHT and revealed subjective atrophy of the cerebral cortex with expanded subarachnoid spaces and a normal spinal cord. Cisternal cerebrospinal fluid (CSF) analysis and CSF PCRs for FIV, FeLV, FCoV, FPV, BDV and T gondii were performed in this cat and did not reveal any abnormalities.
Treatment with antibiotics (including amoxicillin and clavulanic acid and enrofloxacin) (in one cat), prednisolone (in four cats), and vitamin B1 and B12 (in three cats) did not result in any improvement. The disease was slowly progressive to the stage where euthanasia had to be performed because of poor quality of life in all but two cats that were still alive at the time of the study. All cats were still able to walk in the late stage of the disease.
A complete post-mortem examination was conducted in 10 cats, none of which showed significant macroscopic or histological changes in the visceral organs. Peripheral nerves were not available for histological assessment. On external inspection, the volume relationship between forebrain, brainstem and cerebellum were within normal anatomic limits. At subgross magnification, a moderate atrophy of the cerebral cortex with expanded subarachnoid spaces was recognised in all cats. The cortical patterning, nuclear histoarchitecture and the organisation of the central white matter were within physiological limits. Microscopic examination revealed an extensive inflammatory infiltration of the entire CNS by angiocentric lymphohistiocytic aggregates with mild-to-moderate invasion of the perivascular neuroparenchyma in all 10 cats examined (Figure 1A and B). The distribution of the infiltrates nearly corresponded to the density of parenchymal blood vessels with the grey matter of the cerebellum, brainstem, diencephalon and basal nuclei, and the white-grey matter transition of the cerebral cortex being the most prominently affected areas in all 10 cats examined.
Figure 1.
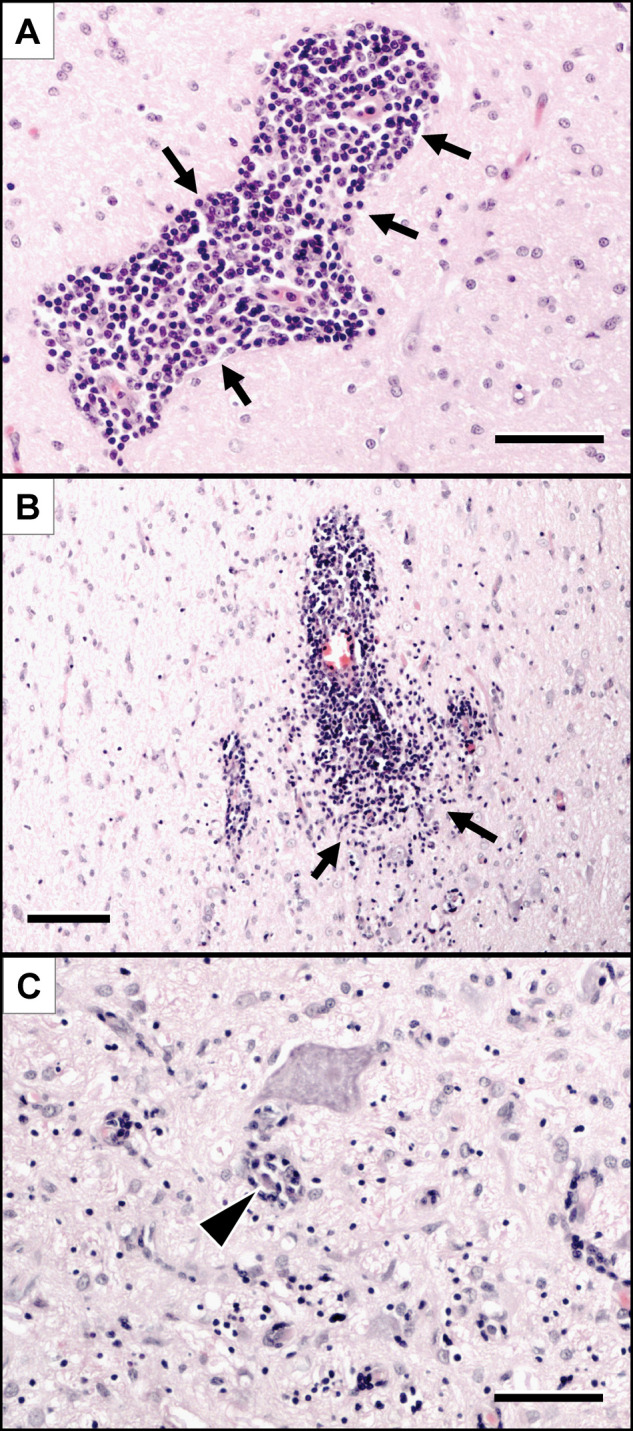
Histopathological changes observed in the brain of the cats included in this study.
Lymphohistiocytic infiltrates (arrows) are either confined to the Virchow-Robin spaces (A) or diffusely invade the perivascular neuroparenchyma (B). Further immune cells are occasionally centred on degenerating neurons (C, arrowhead). Haematoxylin-eosin stain. Scale bars: A and C: 50 µm; B: 100 µm
The leptomeninges were involved to a lesser extent than the neuroparenchyma. The inflammatory infiltrates followed the course of the blood vessels into the Virchow-Robin spaces (Figure 1A). Larger blood vessels most frequently presented with perivascular cuffing only, whereas the parenchyma was infiltrated from the small capillaries and venules (Figure 1B). There was no direct infiltration of the superficial neuroparenchyma through the pial membrane. These findings were consistent in all 10 cats examined.
The inflammatory infiltration of the neuroparenchyma was accompanied by a moderate-to-marked astrogliosis and astocytosis, microglial activation and multifocal erythrophagocytosis. Moreover, diffuse lymphohistiocytic aggregates occasionally co-localised with neuronophagic foci (Figure 1C).
Viral inclusion bodies, parasites and intralesional microorganisms were not seen. Notably, the ventricular surfaces, subependyma and choroid plexuses, as well as the cerebellar white matter, were unaffected. Immunohistochemistry and RT-PCR were negative for all infectious agents tested in all 10 investigated cats (including the cat seropositive for FIV).
Further immunohistochemical phenotyping of the inflammatory infiltrates showed a predominance of CD3-positive T cells, with CD20/CD79-positive B cells and occasional histiocytes/macrophages expressing lysozyme and MAC387 invading the Virchow Robin spaces and neuroparenchyma to a lesser extent (Figure 2). Notably, throughout the foci a significant expression of the interferon-inducible Mx protein was identified via immunohistochemistry. The marker was thus expressed by lymphocytes, as well as by vascular endothelium, intralesional astrocytes and neurons (Figure 3).
Figure 2.
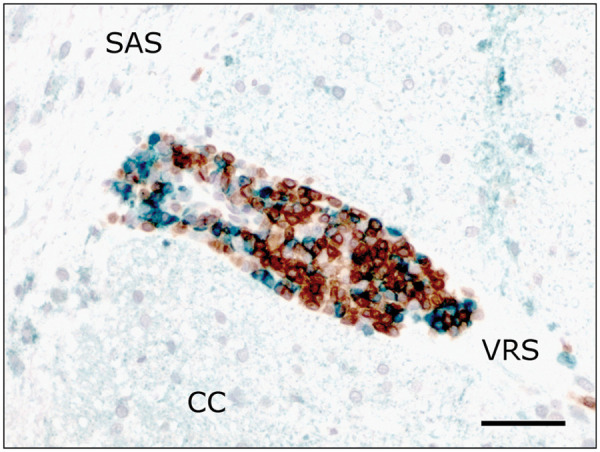
Combined immunolabelling for T-cells (CD3; brown) and B-cells (CD20; green). Perivascular lymphocytic cuffs are composed of both cell types with predominance of T-cells. CC = cerebral cortex; SAS = subarachnoid space; VRS = Virchow-Robin space. Scale bar: 50 µm
Figure 3.
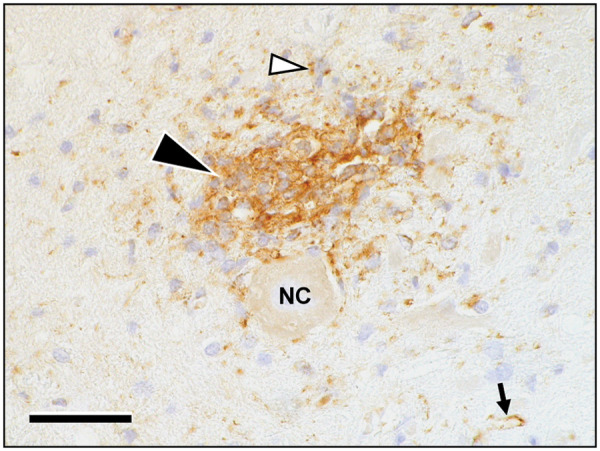
The area of infiltration intensely stains immunopositive for Mx-protein (black arrowhead). The protein is expressed mainly in immune cells and astrocytes, including their cellular processes (white arrowhead). Moreover, the antigen is present in endothelial cells of intra- and perilesional brain capillaries (small arrow). Chromagen: diaminobenzidine (brown) with haematoxylin counterstain (pale blue). NC = nerve cell. Scale bar: 50 µm
Discussion
The late age at onset (mean 9 years), the very slow progression of signs (mean 11 months), the peculiar clinical signs (particularly the posture of the tail and the gait) and, when available, the exclusion of other aetiologies and the consistent histological and immunohistochemical findings suggest that the cats included in this study were affected by the same unique, previously unreported, condition. In cats previously reported with lymphohistiocytic meningoencephalomyelitis of unknown aetiology, the onset of neurological signs is commonly at a young age (2 years or less) and progression is generally acute to subacute.2,6 In addition, the peculiar posture of the tail observed in all the cats included in this study has not been previously reported in cats with lymphohistiocytic meningoencephalomyelitis. The spastic gait is another peculiar feature of the cats described in this study. A possible explanation for this peculiar gait and tail posture is lack of extrapyramidal (especially medullary reticulospinal tract) inhibition of the spinal cord gamma and alpha neurons to the appendicular and tail muscles. 10 Of the feline lymphohistiocytic meningoencephalomyelitis reported to date, the only one that has some similarities to the disease described in this study is the so called ‘staggering disease’ reported in Sweden and Austria.11,12 Both diseases occur sporadically in cats from specific geographical regions (north-east Scotland and the areas around Lake Malaren in central Sweden, as well as a region north-east of Vienna, respectively).11,12 All the cats included in our study, and most of the cats reported with ‘staggering disease’, belong to the rural population accustomed to hunting birds and rodents.13–15 Therefore, it can be speculated that the aetiological agent may be transmitted from these animals to cats. Classical BDV is believed to be the cause of ‘staggering disease’ in the Swedish cats based on detection of BDV-specific antigens and nucleic acids in the CNS of affected cats using immunohistochemistry, in situ hybridization, RT-PCR and real-time RT-PCR, and based on isolation of a supposedly feline variant of BDV from CNS tissue. 11 In Austrian ‘staggering disease’ cases, however, demonstration of BDV in CNS samples was not successful. 12 Low level BDV-specific antigen has also been detected from the brain of a 1-year-old Belgian cat with progressive neurological signs (very similar to those of the Swedish cats with ‘staggering disease’) for 4 weeks prior to euthanasia. However, viral RNA was not detected in the CNS of this Belgian cat. 16 BDV infection has been reported in cats with and without neurological signs worldwide although, a direct aetiological role of BDV in cats with neurological signs has not been established.17,18 BDV was not detected in any of the investigated cats included in our study by means of serology (one cat), CSF PCR (one cat) and immunohistochemistry (10 cats). The antisera we used for immunodetection were directed at viral nucleoprotein p40 and phosphoprotein p24, which are common target antigens for diagnosis of BDV in horses and cats. 19 In many cats affected by ‘staggering disease’, an aetiological diagnosis has not been achieved. It has been speculated that virus persistence in cats with Borna disease-like meningoencephalitis is associated with loss of antigenicity and, therefore, negative immunodetection. 20 Hence, there is controversy on the diagnosis and relative significance of BDV infection in animals. 17 Detection in the CNS of BDV by immunohistochemistry or of BDV RNA by in situ hybridization, or both, in combination with the clinical and histological findings is considered the most reliable method of confirming BDV meningoencephalomyelitis.17,21 ‘Staggering disease’ has been reported to affect males more frequently than females,6,15 while no gender predisposition was observed in the cats included in our study. In a study on 25 cats with ‘staggering disease’, the mean age at diagnosis was 4.8 years (range 1–12 years) 14 and a recent review article on BDV in Sweden reports an age at diagnosis of 1–4 years in most cats. 11 This is quite different from the age at onset of signs of the cats in this study (mean 9 years; range 4.5–14 years). Also, progression of signs appears quite different being up to 4 weeks in the majority of the cats with ‘staggering disease’ 14 and ranging from 6 to 24 months (mean 11 months) in the cats in our study. None of the cats with ‘staggering disease’ has been reported with the peculiar tail posture observed in all the cats included in our study and it is unclear whether the so called ‘staggering’ gait is similar to the spastic gait observed in all the cats included in our study. Some of the cats with ‘staggering disease’ have been reported to develop pelvic limb paralysis, which has not been observed in any of the cats included in our study. Other clinical signs reported in cats with ‘staggering disease’ that have not been observed in any of the cats described in our study include tremors, seizures, inability to retract the claws, pruritus, fever and constipation.13,14 Behavioural changes commonly occur in cats with forebrain disease and have been reported in both diseases. The type of histological lesions is consistent with lymphohistiocytic meningoencephalomyelitis in cats with ‘staggering’ disease, as well as in the cats included in our study; however, the distribution of the infiltrates in the CNS is not entirely the same. In cats with ‘staggering disease’, the inflammatory lesions are most pronounced in the thalamus, mesencephalon, caudal colliculus, basal nuclei, hippocampus and olfactory bulb. The cerebral cortex is moderately affected and the cerebellar parenchyma is minimally affected, while cerebral and cerebellar meningitis is usually prominent.13,14 In the cats included in our study, the most prominently affected areas are the grey matter of the cerebellum, brainstem, diencephalon and basal nuclei, and the white-grey matter transition of the cerebral cortex. The leptomeninges are involved to a far lesser extent than the neuroparenchyma. The morphology and spatial pattern of the infiltrates observed in the cats included in our study resemble the chronic progressive CNS inflammation triggered by paramyxoviruses in dogs (old dog encephalitis associated with CDV), and in humans (measles virus).22–24 Chronic progressive neurological disease (seizures, myoclonus, ataxia and paresis) associated with encephalomyelitis caused by CDV has been reported in large felids. 25 Neuropathological findings included non-suppurative meningoencephalomyelitis, extensive multifocal demyelination, necrosis and inclusion bodies. 25 In the cats included in our study, demyelination, necrosis and inclusion bodies were not observed, and CDV infection was excluded by standard immunohistochemistry. In the meningoencephalomyelitis associated with paramyxoviruses, as well as with ‘staggering’ disease, the infiltrates are considered to represent a prolonged immunopathological sequela rather than a protective mechanism directed against the infection. The same pathobiology may apply to the lymphohistiocytic meningoencephalomyelitis of the cats included in our study. Hence, we tried to investigate the immunoresponse further. The immunohistochemical phenotyping of the inflammatory infiltrates in the CNS shows a predominance of T lymphocytes in the cats included in our study, as well as in cats with ‘staggering’ disease.20,26 B-cells and histiocytes are also involved, albeit to a lesser extent. Hence, the infiltration pattern is rather non-specific, indicating a T-cell driven immunoreaction only. The expression of Mx-protein enables narrowing the panel of immune responses to the action of type I interferons and interferon lambda. 9 In dogs, Mx protein has been identified immunohistochemically in neurons, astrocytes and microglial cells of brains affected by infectious and non-infectious inflammatory encephalitides. The panel of infective aetiologies in canine brains showing Mx protein expression ranged from viruses to fungi. Non-infectious encephalitides included granulomatous meningoencephalomyelitis, necrotising meningoencephalitis of Pug dogs, and necrotising encephalitis of the Yorkshire Terrier and Maltese breeds. 9 Mx-protein expression in dogs with non-infectious encephalitides was less widespread and intense than in dogs with confirmed viral infection. 9 In the cats described in our study, non-viral organisms were excluded histologically. Even though the immunohistochemical Mx-protein signal was widespread and very strong throughout the brain of the cats included in our study, therefore suggesting a viral aetiology, 9 we cannot rule out an immune-mediated disease (possibly associated with an unidentified infective or environmental immunogenic trigger) at the present stage.
Mx protein is a dynamin-like GTPase—up-regulation of which resembles an intracellular defence mechanism that interferes specifically with translocation of viruses across the cellular compartments and with post- translational modification of nucleocapsides. Evidence of the cytoplasmic Mx protein subtype, as in the present study, is predominantly seen in viruses that replicate within the cytoplasm and are dependent on sophisticated nucleocytoplasmic transfer mechanisms. 27 Hence, the slowly progressive feline lymphohistiocytic meningoencephalomyelitis described in this study may be caused by a yet unidentified RNA virus emerging from prey species. As demonstrated in many other species, Mx protein expression is not always protective which may be explained by an intrinsic viral Mx protein resistance or simply the expression lagging behind viral replication.27,28
The main limitations of this study are the lack of histological and immunohistochemical investigations in all cats and the fact that an aetiological agent could not be identified. Two cats were still alive at the time of the study and some of the owners did not give permission for post-mortem examination of the euthanased cats. Further work is in progress to attempt identification of the causative agent by means of random PCR studies and panmicrobial microarrays in order to screen for unknown infectious agents. 29
In conclusion, the late age at onset (mean 9 years), the very slow progression of signs (mean 11 months), the peculiar clinical signs (particularly the rigid extension of the tail and the spastic and ataxic gait), and, when available, the exclusion of other aetiologies and the consistent histological and immunohistochemical findings suggest that the 21 cats included in this study were affected by the same unique, previously unreported condition.
Acknowledgments
The authors would like to acknowledge Mark Leggat at Morven Veterinary practice, Alford, Scotland, for contributing cases to the study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
Supplementary Video 1
Characteristic stiff extended tail posture and spastic and ataxic gait in a 10-year-old male neutered domestic shorthair cat.
References
- 1. Bradshaw JM, Pearson GR, Gruydd-Jones TJ. A retrospective study of 238 cases of neurological disorders of the cat. J Comp Pathol 2004; 131: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rand JS, Parent J, Percy D, Jacobs R. Clinical, cerebrospinal fluid, and histological data from twenty-seven cats with primary inflammatory disease of the central nervous system. Can Vet J 1994; 35: 103–110. [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen NC. A review of feline infectious peritonitis and feline enteric coronavirus infections. Part 2. Feline Pract 1983; 13: 5–20. [Google Scholar]
- 4. De Lahunta A, Glass EN. Veterinary neuroanatomy and clinical neurology. 3rd ed St Louis: Saunders, 2009. [Google Scholar]
- 5. Summers BA, Cummings JF, De Lahunta. Veterinary Neuropathology. St Louis: Mosby, 1995. [Google Scholar]
- 6. Gunn-Moore D. Infectious diseases of the central nervous system. Vet Clin North Am Small Anim Pract 2005; 35: 103–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwab S, Herden C, Seeliger F, et al. Non-suppurative meningoencephalitis of unknown origin in cats and dogs: an immunohistochemical study. J Comp Pathol 2007; 136: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res 2002; 30: 1292–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter BF, Ambrus A, Storts RW. Immunohistochemical evaluation of mx protein expression in canine encephalitides. Vet Pathol 2006; 43: 981–987. [DOI] [PubMed] [Google Scholar]
- 10. King AS. Physiological and clinical anatomy of the domestic mammals, vol 1: central nervous system. Oxford: Blackwell Science, 2004. [Google Scholar]
- 11. Wensman JJ, Berg M, Berg AL. Experiences of Borna disease virus infection in Sweden. APMIS (Suppl) 2008; 116: 46–49. [DOI] [PubMed] [Google Scholar]
- 12. Nowotny N, Weissenbock H. Description of feline non- suppurative meningoencephalomyelitis (‘staggering disease’) and studies of its etiology. J Clin Microbiol 1995; 33: 1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berg AL. Borna disease in cats. In: Bonagura JD. (ed) Kirk’s current veterinary therapy XIII: small animal practice. Philadelphia: WB Saunders, 2000, pp 976–78. [Google Scholar]
- 14. Lundgren AL. Feline non-suppurative meningoencephalomyelitis. A clinical and pathological study. J Comp Pathol 1992; 107: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berg AL, Reid-Smith R, Larsson M, Bonnett B. Case control study of feline Borna disease in Sweden. Vet Rec 1998; 27: 715–717. [DOI] [PubMed] [Google Scholar]
- 16. De Bosschere H, Roels S, Vanopdenbosch E, et al. Staggering disease in a cat: the first case of Borna disease virus infection in a Belgian cat. Intern J Appl Res Vet Med 2004; 2: 189–194. [Google Scholar]
- 17. Greene CE, Berg AL. Miscellaneous viral infections, Borna disease meningoencephalomyelitis. In: Greene CE. (ed) Infectious diseases of the dog and cat. 3rd ed. London: Saunders-Elsevier, 2006, pp 165–167. [Google Scholar]
- 18. Njaa BL. Emerging viral encephalitides in dogs and cats. Vet Clin North Am Small Anim Pract 2008; 38: 863–878. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura Y, Watanabe M, Kamitani W, et al. High prevalence of Borna disease virus in domestic cats with neurological disorders in Japan. Vet Microbiol 1999; 70: 153–169. [DOI] [PubMed] [Google Scholar]
- 20. Lundgren AL, Lindberg R, Ludwig H, Gosztonyi G. Immunoreactivity of the central nervous system in cats with a Borna disease-like meningoencephalomyelitis (staggering disease). Acta Neuropathol 1995; 90: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wensman JJ, Thoren P, Hakhverdyan M, et al. Development of a real-time RT-PCR assay for improved detection of Borna disease virus. J Virol Methods 2007; 143: 1–10. [DOI] [PubMed] [Google Scholar]
- 22. Axthelm MK, Krakowa S. Experimental old dog encephalitis (ODE) in a gnotobiotic dog. Vet Pathol 1998; 35: 527–534. [DOI] [PubMed] [Google Scholar]
- 23. Bernard A, Fevre-Montange M, Bencsik A, et al. Brain structures selectively targeted by canine distemper virus in a mouse model infection. J Neuropathol Exp Neurol 1993; 52: 471–480. [DOI] [PubMed] [Google Scholar]
- 24. Vandevelde M, Kristensen B, Braund KG, et al. Chronic canine distemper virus encephalitis in mature dogs. Vet Path 1980; 17: 17–29. [DOI] [PubMed] [Google Scholar]
- 25. Blythe LL, Schmitz JA, Roelke M, Skinner S. Chronic encephalomyelitis caused by canine distemper virus in a Bengal tiger. J Am Vet Med Assoc 1983; 183: 1159–1162. [PubMed] [Google Scholar]
- 26. Berg AL, Johannisson A, Johansson M, et al. Peripheral and intracerebral T cell immune response in cats naturally infected with Borna disease virus. Vet Immunol Immunopathol 1999; 68: 241–253. [DOI] [PubMed] [Google Scholar]
- 27. Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae). J Biol Chem 1999; 274: 4370–4376. [DOI] [PubMed] [Google Scholar]
- 28. Benfield CT, Lyall JW, Tiley LS. The cytoplasmic location of chicken mx is not the determining factor for its lack of antiviral activity. PLoS One 2010; 5: e12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clem AL, Sims J, Telang S, et al. Virus detection and identification using random multiplex (RT)-PCR with 3’-locked random primers. Virol J 2007; 4: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]


