Abstract
Adrenal function may be altered in animals with hyperthyroidism. The aim of the study was to assess adrenal function of hyperthyroid cats (n = 17) compared to healthy cats (n = 18) and cats with chronic diseases (n = 18). Adrenal function was evaluated by adrenocorticotropic hormone (ACTH) stimulation test and the urinary cortisol to creatinine ratio (UCCR) was determined. Length and width of both adrenal glands were measured via ultrasound. Hyperthyroid cats had significantly higher cortisol levels before and after stimulation with ACTH than the other groups. However, the UCCR was not elevated in hyperthyroid cats. The size of the adrenal glands of hyperthyroid cats was not significantly different from the size of those of healthy cats. The results indicate that cats with hyperthyroidism have a higher cortisol secretory capacity in a hospital setting. The normal size of the adrenal glands suggests that cortisol levels may not be increased permanently.
Introduction
Various studies suggest that hyperthyroidism influences adrenal function.1–12 However, little is known about the interaction of thyroid and adrenal function in hyperthyroid cats. Liu et al found that the adrenal glands of 8/23 hyperthyroid cats showed nodular hyperplasia of the zona glomerulosa and fasciculata. 13 To assess the effect of non-adrenal illness on adrenal function by a combined dexamethasone suppression adrenocorticotropic hormone (ACTH) stimulation test, Zerbe et al compared groups of healthy, diabetic and sick non-diabetic cats. The group of sick non-diabetic cats included 50% hyperthyroid cats. They found a trend for higher cortisol concentrations in cats with hyperthyroidism, but the differences were not statistically significant between groups. 14 In another study, cats with hyperthyroidism were found to have a significantly elevated urinary corticoid to creatinine ratio. 15
The aims of this study were to assess cortisol levels in hyperthyroid cats compared with healthy cats and cats with chronic diseases, to measure the urinary cortisol to creatinine ratio (UCCR) and to assess adrenal gland size in the different disease groups.
Materials and methods
Animals
Fifty-three cats were included in the study. Based on a sample size determination and a clinically relevant margin of 55 nmol/l in cortisol concentration before and after the application of ACTH, 17 animals per group were calculated to achieve a power of 80% and an α of 0.05 (calculated using BiAS: http://www.bias-online.de).
The study was conducted as a prospective trial including 53 client-owned cats. It was approved by the ‘Regierung von Oberbayern’. Animals were presented to the Clinic of Small Animal Medicine of the LMU University of Munich with various signs of disease or for preventive health care. Consent forms were signed by all owners before their cats entered the study. Only patients that were 8 years or older were included. Cats that had been treated with glucocorticoids in the last 3 months were excluded from the study. Cats were assigned to three groups: healthy cats (n = 18), cats with hyperthyroidism (n = 17) and cats with other chronic diseases (n = 18). Healthy cats showed no history or clinical signs of illness and results of a total blood count and biochemistry profile were within normal limits. Thyroxine levels were within the reference interval. Hyperthyroid cats showed clinical signs of hyperthyroidism, such as weight loss, polyphagia, polydipsia, polyuria, diarrhoea and vomiting, and had a palpable thyroid gland, as well as elevated thyroxine (T4) and free thyroxine (fT4) levels. Hyperthyroid cats under treatment were only included if they were still symptomatic and had elevated T4 levels. Chronically ill cats (not hyperthyroid) had the habitus of a chronically ill patient, such as a dull haircoat or emaciation, or had shown signs of illness for more than 4 weeks. Cats in this group had chronic nephropathies (n = 6), chronic gastrointestinal disease (n = 4), neoplasia (n = 3), diabetes mellitus (n = 2), chronic respiratory disease (n = 2) and blood-loss anaemia caused by chronic flea infestation (n = 1). They had T4 and fT4 values within, or below, the normal range. T4 values of hyperthyroid cats were significantly higher than those of healthy and chronically ill cats (P <0.001). Mean values were 8.1 ± 3.0 µg/dl for hyperthyroid cats, 1.8 ± 0.7 µg/dl for healthy cats and 1.6 ± 0.6 µg/dl for cats with chronic disease. There was no significant difference between healthy cats and cats with chronic diseases. fT4 values were significantly higher in hyperthyroid cats (7.7 ± 2.3 ng/dl) than in chronically ill cats (1.7 ± 0.6 ng/dl, P <0.001).
Although the aim was to create groups with an equal age range, hyperthyroid cats were significantly older than healthy cats (P <0.001) and cats with chronic diseases (P = 0.001). Cats with chronic disease were significantly older than healthy cats (P = 0.003). The mean age of hyperthyroid cats was 15.2 ± 2.8 years, of healthy cats it was 10.2 ± 2.3 years and of cats with chronic disease it was 12.4 ± 1.3 years.
Study design
Cats were allowed to acclimatise to the clinic environment before any procedures were performed. History was taken for every patient and a physical examination was conducted. T4 and fT4 levels were measured in every cat and a complete blood count and serum biochemistry profile was performed. Complete blood counts and biochemistry profiles were performed by standard laboratory methods. T4 concentrations were determined by Diagnostic Reagents Inc (DRI) immunoassay (Modular Analytics, Hitachi/Roche, Tokio/Risch, Japan/Switzerland). fT4 was assessed by equilibrium dialysis and radioimmunoassay.
To assess adrenal function, the UCCR was determined, an ACTH stimulation test was performed and the width and length of the adrenal glands were measured. Urine samples were obtained by cystocentesis in the morning of the examination day before the ACTH stimulation test. For the UCCR, cortisol was determined by chemiluminescence immunoassay (Immulite 2000, Siemens, Berlin/Munich, Germany) and creatinine was measured by Jaffé’s method (Modular Analytics, Hitachi/Roche, Tokio/Risch, Japan/Switzerland).
After cystocenthesis, an ACTH stimulation test was performed: 0.125 mg of tetracosactide (Synacthen; Novartis Pharma GmbH, Nuremberg, Germany) was administered intravenously. Blood samples were taken at 0 and 60 min as discussed by Schoeman et al 16 and DeClue et al. 17 Blood cortisol levels were determined by electrochemiluminescence immunoassay (Elecsys 2010; Roche Diagnostics AG, Risch, Switzerland). All assays were set up according to the manufacturer’s instructions.
After the ACTH-stimulation test, all 53 cats underwent a complete abdominal ultrasound examination without sedation. Ultrasound was performed with the LOGIQ P6 (GE Healthcare, Chalfont St. Giles, UK) using an 11-mHz linear transducer. Adrenal glands were examined as previously described. 18 Maximum length and width of adrenal glands was measured by an experienced examiner (AW).
Statistical analysis
SPSS Statistics version 17.0 (IBM Deutschland GmbH, Munich, Germany) was used for statistical analysis. Distributions of values of parameters were assessed visually with box-and-whisker plots and Q–Q plots for normality and analysed using one-way ANOVA and least significant difference (LSD) post-hoc tests to test for differences between groups. Correlation between two parameters was analysed using Pearson’s correlation coefficient. Statistical significance was set at P ≤0.05.
Results
Blood cortisol levels
Baseline cortisol levels were significantly higher in cats with hyperthyroidism (251.7 ± 145.7 nmol/l) than in healthy cats (129.2 ± 70.0 nmol/l, P = 0.001) and cats with chronic diseases (121.3 ± 79.4, P <0.001). There was no statistically significant difference between healthy cats and cats with chronic diseases (P = 0.819). Cortisol levels 60 min after application of tetracosactide were significantly higher in hyperthyroid cats (369.4 ± 144.4 nmol/l) than in healthy cats (256.3 ± 81.6 nmol/l, P = 0.003) and cats with chronic diseases (220.6 ± 85.5 nmol/l, P <0.001). No significant difference was found between healthy cats and cats with chronic diseases (P = 0.321) (Figure 1).
Figure 1.
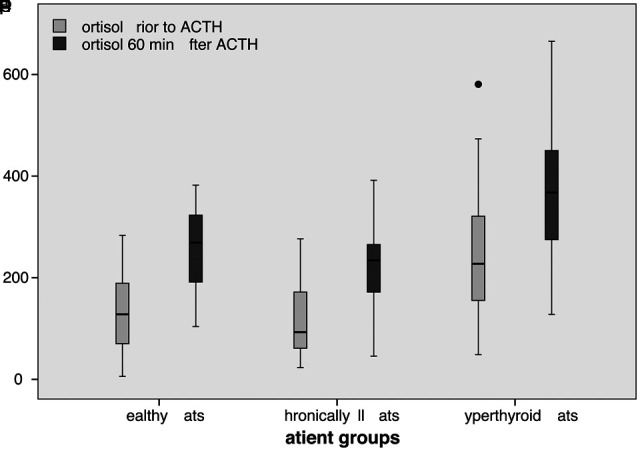
Blood cortisol levels (nmol/l) prior to and 60 min after application of tetracosactide in hyperthyroid, chronically ill and healthy cats
There was no significant difference in delta cortisol between hyperthyroid cats and healthy (P = 0.719) or chronically ill cats (P = 0.482), or between healthy cats and cats with chronic diseases (P = 0.283). Median values were 117.8 ± 81.3 nmol/l for hyperthyroid cats, 127.1 ± 75.2 nmol/l for healthy cats and 99.4 ± 73.8 nmol/l for cats with chronic diseases.
A correlation between total T4 concentration and baseline cortisol concentration (r = 0.634, P = 0.001) was seen (Figure 2). fT4 was also correlated with baseline cortisol concentration (r = 0.505, P = 0.013).
Figure 2.
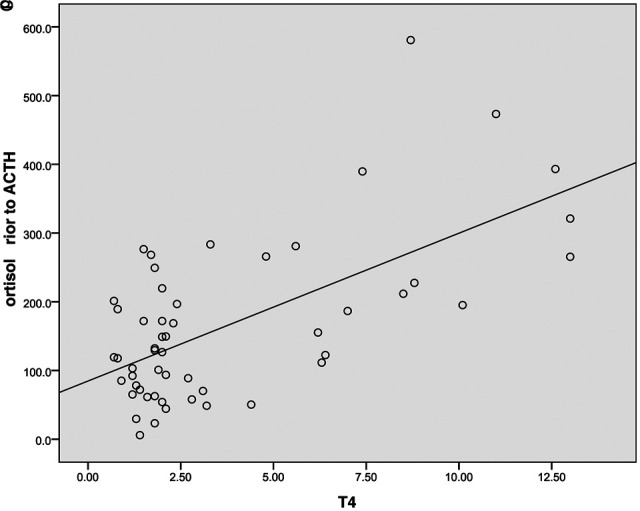
Correlation between total T4 (µg/dl) and baseline cortisol concentration (nmol/l) in hyperthyroid, chronically ill and healthy cats
Urinary cortisol to creatinine ratio
Mean values and standard deviation for the UCCR were 4.12 ± 3.95 x10-5 for hyperthyroid cats, 2.60 ± 2.98 x10-5 for healthy cats and 2.53 ± 2.14 x10-5 for cats with chronic diseases. It was neither significantly higher in hyperthyroid cats than in healthy cats (P = 0.154) or in cats with chronic diseases (P = 0.135), nor did the values of healthy and chronically ill cats differ significantly (P = 0.941).
Ultrasonographical measurement of adrenal glands
All but one left adrenal gland of one hyperthyroid cat could be visualised and measured (n = 105). There was no significant difference in the lengths of the right or left adrenal gland between groups (P >0.4). The width of the right adrenal glands in hyperthyroid cats (0.41 ± 0.11 cm) was not significantly different from healthy (0.40 ± 0.09, P = 0.807) or chronically ill cats (0.38 ± 0.08 cm, P = 0.272). There was also no significant difference between healthy cats or cats with chronic diseases (P = 0.384).
The width of the left adrenal gland of cats with hyperthyroidism (0.42 ± 0.09 cm) was not significantly different from healthy cats (0.39 ± 0.06 cm, P = 0.317). Cats with chronic diseases (0.37 ± 0.06 cm, P = 0.041) had significantly smaller adrenal glands than hyperthyroid cats. There was no difference between healthy and chronically ill cats (P = 0.317) (Figure 3). There was no correlation between the cats’ weight and the length or width of either adrenal gland (r ≤0.222, P >0.05).
Figure 3.
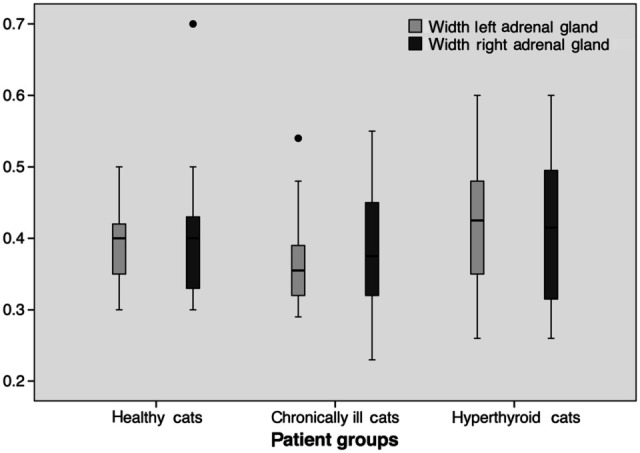
Width of both adrenal glands (cm) in hyperthyroid, chronically ill and healthy cats
Discussion
High cortisol levels have been reported in humans and rats with hyperthyroidism. Gallagher et al demonstrated that hyperthyroid human patients had more cortisol secretory episodes per day and the duration of cortisol secretion was longer than in healthy individuals. Therefore, higher amounts of cortisol were produced. 1 Boler et al showed that chronic administration of high doses of T4 resulted in high basal corticosterone levels in rats. 19 Findings in the present study indicate that cats with hyperthyroidism in a hospital environment also have higher cortisol levels than healthy cats and cats with chronic diseases.
Although most hyperthyroid individuals seem to have an elevated cortisol response, there are studies in rats9,19 and humans7,8 that indicate that an opposite scenario may also occur. It has been suggested that the contradicting results in the studies that assess adrenal function in hyperthyroidism could originate from differences in duration of the hyperthyroid state. Johnson et al treated thyroidectomised rats with T4 once a day subcutaneously for 7 and 60 days. These ‘long-term hyperthyroid rats’ (60 days) showed significantly lower cortisol levels after stimulation with ACTH than controls but did not show a difference in delta cortisol. ‘Short-term hyperthyroid rats’ (7 days), however, had a higher cortisol response to ACTH. 9 If the results of the present study were to be compared with the results of the study mentioned above, the cats would have to be classified as ‘long-term hyperthyroid’. As hyperthyroidism occurred naturally in these cats, the duration of disease most certainly exceeded 7 days. In the present study, there was no significant difference of delta cortisol in hyperthyroid cats compared to healthy cats, but in contrast to the study of Johnson et al, higher cortisol levels in hyperthyroid cats after stimulation with ACTH were found. A reason for this difference could be that in all rats hyperthyroidism had been induced experimentally and it is likely that relatively high doses of T4 were administered. Of course, there could also be differences between species.
Recent literature published on humans suggests that permanently elevated cortisol levels caused by chronic diseases are associated with osteoporosis, hypertension, diabetes mellitus, susceptibility to infections and depression. High cortisol levels may lead to increased mortality in humans with chronic diseases. 20 Elevated cortisol levels could, therefore, be regarded as a negative prognostic indicator in humans. As elevated cortisol levels were found in the hyperthyroid cats under hospital conditions in the present study, it would be interesting to explore whether hyperthyroid cats also have high cortisol levels in their home environment and whether these levels also decrease life expectancy in cats. De Lange et al 15 found high UCCRs after collecting urine of cats at home, which could be a indicator for high cortisol levels in a home environment.
Although adrenal function was increased and blood cortisol levels were elevated, the UCCR was not increased in the present study. This finding is in contrast to the findings of de Lange et al. 15 A reason for high blood cortisol level without an elevated UCCR could be either that the excreted metabolites in the urine were inappropriately measured by the assay or that the excess cortisol was not excreted in the urine. The method by which cortisol was measured does not cross react with most natural cortisol metabolites (e.g., cortison, 11-deoxycortisol, corticosterone). It has been shown that only 1.85% (median) of administered [3H] cortisol is excreted through the urine in cats and that cats mainly excrete conjugated cortisol and/or its metabolites through the urine, 21 which were not measured in the assay. Measuring UCCR with the Immulite 2000 (chemiluminescence immunoassay) in order to assess adrenal function in cats may not provide reliable results. Therefore, a limitation of this study is that the UCCR was not measured by a radioimmunoassay that would be able to also detect cortisol metabolites. It has also been shown that cats excrete administered steroid hormones almost entirely in the faeces. 22 This leads to the conclusion that the use of UCCR might not be very helpful in assessing adrenal function in cats.
There was no statistically significant difference in the width of the adrenal glands of hyperthyroid and healthy cats. Only the left adrenal gland of chronically ill cats was significantly smaller than that of hyperthyroid cats but not than that of healthy cats. This significant difference was only obtained for the left adrenal gland but not for the right one. This could be caused by the fact that the ultrasonographical access to the right adrenal gland is usually more difficult. 23 Another possibility is that the left adrenal gland reacts to changes in adrenal function more sensitively than the right adrenal gland. Wenger et al found that the size of the left and right adrenal gland in dogs with primary hypoadrenocorticism is affected differently by cortisol levels. 24
Owing to the significantly higher cortisol levels of hyperthyroid cats, an increase in the size of the adrenal glands would be expected, as the thickness of the adrenals is mainly determined by the cortex. The zona fasciculate, which is responsible for cortisol production, forms the major fraction of the cortex. However, there was no difference in the width of both adrenal glands between hyperthyroid and healthy cats. A possible explanation for the elevated cortisol levels (measured under hospital conditions) in the cats of the present study without enlarged adrenal glands could be that hyperthyroid cats do not have constantly high cortisol levels, but exhibit a higher cortisol secretory capacity to stressful situations. Results of this study imply that the width of the adrenal glands measured during ultrasound examination cannot be used as an adjunctive diagnostic tool in assessing hyperthyroidism in cats. Although a positive correlation between bodyweight and adrenal gland length was found in dogs, 24 this could not be demonstrated in cats examined in this study. The less pronounced difference of body weights in cats in general could serve as an explanation.
In conclusion, it can be said that hyperthyroid cats have high cortisol levels before and after stimulation with ACTH under hospital conditions. However, despite their elevated blood cortisol levels, they did not have thicker adrenal glands than healthy cats when assessed by ultrasonography. This could mean that hyperthyroid cats do not have permanently elevated cortisol levels, but only react with high cortisol levels to stressful situations. Future studies are needed to examine cortisol levels in hyperthyroid cats in their home environment.
Acknowledgments
We would like to thank Mrs Baerbel Garner for her help with hormone measurements.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1. Gallagher TF, Hellman L, Finkelstein J, Yoshida K, Weitzman ED, Roffwarg HD, et al. Hyperthyroidism and cortisol secretion in man. J Clin Endocrinol Metab 1972; 34: 919–927. [DOI] [PubMed] [Google Scholar]
- 2. Kamilaris TC, DeBold CR, Johnson EO, Mamalaki E, Listwak SJ, Calogero AE, et al. Effects of short and long duration hypothyroidism and hyperthyroidism on the plasma adrenocorticotropin and corticosterone responses to ovine corticotropin-releasing hormone in rats. Endocrinol 1991; 128: 2567–2576. [DOI] [PubMed] [Google Scholar]
- 3. Linquette M, Lefebvre J, Racadot A, Cappoen JP. [Proceeding: Production rate, metabolic clearance rate and mean plasma concentration of cortisol in hyperthyroidism (author’s transl.)]. Ann Endocrinol (Paris) 1975; 36: 35–36. [PubMed] [Google Scholar]
- 4. Lizcano F, Salvador J. Effects of different treatments for hyperthyroidism on the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 2008; 35: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 5. Moghetti P, Castello R, Tosi F, Zenti MG, Magnani C, Bolner A, et al. Glucose counterregulatory response to acute hypoglycemia in hyperthyroid human subjects. J Clin Endocrinol Metab 1994; 78: 169–173. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez-Franco F, Fernandez L, Fernandez G, Cacicedo L. Thyroid hormone action on ACTH secretion. Horm Metab Res 1989; 21: 550–552. [DOI] [PubMed] [Google Scholar]
- 7. Tsatsoulis A, Johnson EO, Kalogera CH, Seferiadis K, Tsolas O. The effect of thyrotoxicosis on adrenocortical reserve. Eur J Endocrinol 2000; 142: 231–235. [DOI] [PubMed] [Google Scholar]
- 8. Mishra SK, Gupta N, Goswami R. Plasma adrenocorticotropin (ACTH) values and cortisol response to 250 and 1 microg ACTH stimulation in patients with hyperthyroidism before and after carbimazole therapy: case- control comparative study. J Clin Endocrinol Metab 2007; 92: 1693–1696. [DOI] [PubMed] [Google Scholar]
- 9. Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Experimentally-induced hyperthyroidism is associated with activation of the rat hypothalamic-pituitary-adrenal axis. Eur J Endocrinol 2005; 153: 177–185. [DOI] [PubMed] [Google Scholar]
- 10. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 1993; 22: 263–277. [PubMed] [Google Scholar]
- 11. Ingbar SH. Management of emergencies. IX. Thyrotoxic storm. N Engl J Med 1966; 274: 1252–1254. [DOI] [PubMed] [Google Scholar]
- 12. Mazzaferri EL, Skillman TG. Thyroid storm. A review of 22 episodes with special emphasis on the use of guanethidine. Arch Intern Med 1969; 124: 684–690. [DOI] [PubMed] [Google Scholar]
- 13. Liu SK, Peterson ME, Fox PR. Hypertropic cardiomyopathy and hyperthyroidism in the cat. J Am Vet Med Assoc 1984; 185: 52–57. [PubMed] [Google Scholar]
- 14. Zerbe CA, Refsal KR, Peterson ME, Armstrong PJ, Nachreiner RF, Schall WD. Effect of nonadrenal illness on adrenal function in the cat. Am J Vet Res 1987; 48: 451–454. [PubMed] [Google Scholar]
- 15. de Lange MS, Galac S, Trip MR, Kooistra HS. High urinary corticoid/creatinine ratios in cats with hyperthyroidism. J Vet Intern Med 2004; 18: 152–155. [DOI] [PubMed] [Google Scholar]
- 16. Schoeman JP, Evans HJ, Childs D, Herrtage ME. Cortisol response to two different doses of intravenous synthetic ACTH (tetracosactrin) in overweight cats. J Small Anim Pract 2000; 41: 552–557. [DOI] [PubMed] [Google Scholar]
- 17. DeClue AE, Martin LG, Behrend EN, Cohn LA, Dismukes DI, Lee HP. Cortisol and aldosterone response to various doses of cosyntropin in healthy cats. J Am Vet Med Assoc 2011; 238: 176–182. [DOI] [PubMed] [Google Scholar]
- 18. Nyland TG, Mattoon JS, Herrgesell EJ, Wisner ER. Adrenal glands. In: Nyland TG, Mattoon JS. (eds). Small animal diagnostic ultrasound. St Louis, MO: Saunders, 2001, pp 196–206. [Google Scholar]
- 19. Boler RK, Moore NA. Depression of adrenocortical function by pharmacology doses of thyroxine in intact and unilaterally adrenalectomized rats. Horm Res 1982; 16: 209–218. [DOI] [PubMed] [Google Scholar]
- 20. Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf) 2009; 71: 779–786. [DOI] [PubMed] [Google Scholar]
- 21. Goossens MM, Meyer HP, Voorhout G, Sprang EP. Urinary excretion of glucocorticoids in the diagnosis of hyperadrenocorticism in cats. Domest Anim Endocrinol 1995; 12: 355–362. [DOI] [PubMed] [Google Scholar]
- 22. Taylor W. Steroid metabolism in the cat. Biliary and urinary excretion of metabolites of [4-14C]cortisone. Biochem J 1969; 113: 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmer C, Horauf A, Reusch C. Ultrasonographic examination of the adrenal gland and evaluation of the hypophyseal-adrenal axis in 20 cats. J Small Anim Pract 2000; 41: 156–160. [DOI] [PubMed] [Google Scholar]
- 24. Wenger M, Mueller C, Kook PH, Reusch CE. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet Rec 2010; 167: 207–210. [DOI] [PubMed] [Google Scholar]


