Abstract
Hypertrophic cardiomyopathy is an inherited disease in some feline breeds including the Maine Coon and Ragdoll. In these breeds, distinct causative genetic mutations have been identified. The two breeds appear to have slightly different clinical presentations, including age of diagnosis. The observation that these two breeds may have different clinical presentations, as well as different genetic mutations, suggests that hypertrophic cardiomyopathy is a diverse disease in the cat. Hypertrophic cardiomyopathy is poorly described in the Sphynx. The objective of this study was to phenotypically characterize Sphynx hypertrophic cardiomyopathy and to evaluate for a familial etiology. Records of 18 affected cats (11 female, seven male) were evaluated. Age of affected cats ranged from 0.5 to 7 years (median, 2 years). Four affected cats were from a single family and included an affected cat in each of four generations (three females, one male). Further studies are warranted to evaluate for a causative mutation and better classify the phenotypic expression.
Introduction
Hypertrophic cardiomyopathy is the most common form of heart disease in the cat.1,2 It is an adult onset disease with a reported age of diagnosis of 5–6 years (range of 3 months to 17 years).2–4 Although the disease is classically characterized by interventricular septal and/or left ventricular posterior wall hypertrophy, variations can be observed, including hypertrophy of the papillary muscles5–7 and the presence of dynamic left ventricular outflow tract obstruction associated with systolic anterior motion of the mitral valve (SAM). 8 These clinical variations could suggest the presence of multiple forms of hypertrophic cardiomyopathy in the cat.
Hypertrophic cardiomyopathy is an inherited disease in human beings and in some breeds of cats including the Maine Coon and Ragdoll.9–12 An autosomal dominant pattern of inheritance is reported most commonly in both human beings and the Maine Coon.9,13 In the Maine Coon and the Ragdoll breed, distinct genetic mutations for hypertrophic cardiomyopathy have been identified.12,14,15 In both breeds, the genetic mutation has been identified in the cardiac myosin binding protein C gene but the unique nucleotide change and the location of the mutation within the gene appears to be breed specific. Additionally, the two breeds appear to have slightly different clinical presentations, including age of diagnosis. Ragdoll cats have been reported to have a mean age of diagnosis as young as 15 months, while the Maine Coon has a mean age of diagnosis of 2.6 years, although large studies are lacking.10,11 The observation that these two breeds may have different ages of onset, as well as the fact that they have two different genetic mutations, may support the theory that hypertrophic cardiomyopathy is a diverse disease in the cat. 16
The Sphynx breed has been reported to have a predisposition for development of hypertrophic cardiomyopathy that may suggest a heritable nature. 17 The Sphynx is a fairly new breed with most of the breed expansion occurring after the 1960s. The identification of hypertrophic cardiomyopathy in this breed is fairly recent and specific aspects of the disease in this breed are not well described. 18 The objective of this study was to phenotypically characterize Sphynx hypertrophic cardiomyopathy and to evaluate for a possible familial etiology.
Materials and methods
The records of Sphynx cats that had a minimum of a screening echocardiogram (ECG) at Washington State University College of Veterinary Medicine were evaluated, as well as the reported ECG examinations of Sphynx cats performed by an American College of Veterinary Internal Medicine (ACVIM) board-certified cardiologist that were provided by Sphynx owners for study. Three generation pedigrees were obtained when available.
Phenotypic characterization
Medical records were evaluated and information was retrieved including age, gender and standard ECG measurements including interventricular (IVS) and left ventricular posterior wall (LVPW) thicknesses at systole (IVSS, LVPWS) and diastole (IVSD, LVPWD) and the left atrial to aortic ratio by M-mode. 19 When available, wall thickness was taken as the average of three measurements; however, some examiners provided only a maximum measurement for the IVS and the LVPW. Cats were classified as affected as defined by an IVSD or LVPWD measurement greater than 6.0 mm. ECG examinations were further evaluated for the presence of systolic anterior motion of the mitral valve and/or papillary muscle hypertrophy.
Familial evaluation
Pedigrees were evaluated with respect to presence of clearly defined unaffected or affected cats. Unaffected status was defined by an IVSD and/or LVWD measurement <5.5 mm at 9 years of age. Affected status was defined as stated above. Cats with a LVPWD and/or IVSD measurements between 5.51 and 5.99 mm were considered equivocal and not included in pedigree analysis. Pedigrees were analyzed with Pedigree Viewer (http://www-personal.une.edu.au/~bkinghor/pedigree.htm) to evaluate for a possible mode of inheritance.
Results
Eighteen cats met the criteria for affected. Eleven cats (seven intact, four spayed) were female and seven were male (four intact, three castrated). The age of the affected cats ranged from 0.5 to 7 years, with a median of 2 years.
ECG findings included a mean IVSD of 6.0 mm (range 3.9–8.0), a mean IVSS of 8.5 mm (6.7–10.5), a LVPWD of 6.6 mm (5.0–9.2) and a LVPWS of 9.0 mm (7.5–10.4). Four cats were considered affected based on an interventricular septum greater than 6.0 mm, eight cats based on a left ventricular posterior wall measurement greater than 6.0 mm and four cats based on hypertrophy of both walls. In two affected cats only, a value for the LVPWD (6.3 mm) or IVSD (6.0 mm) was recorded.
Eight cats had systolic anterior motion of the mitral valve and four were noted to have subjectively prominent papillary muscles. Two affected cats had notations of prominent moderator bands.
Pedigree analysis
Pedigrees were available on nine of the affected cats. Four affected cats were from the same family (Figure 1). The male proband of this family died suddenly before 8 years of age but a necropsy was not performed and cause of death is unknown. An affected cat was observed in each of four generations within this family and included three females and one male cat. Two of the affected cats had one parent that was classified as unaffected. With an affected cat in each generation, both males and female cats affected, and the presence of an unaffected parent in at least some cases, the inheritance is suggestive of an autosomal dominant mode of inheritance; however, this can not be conclusively determined at this time.
Figure 1.
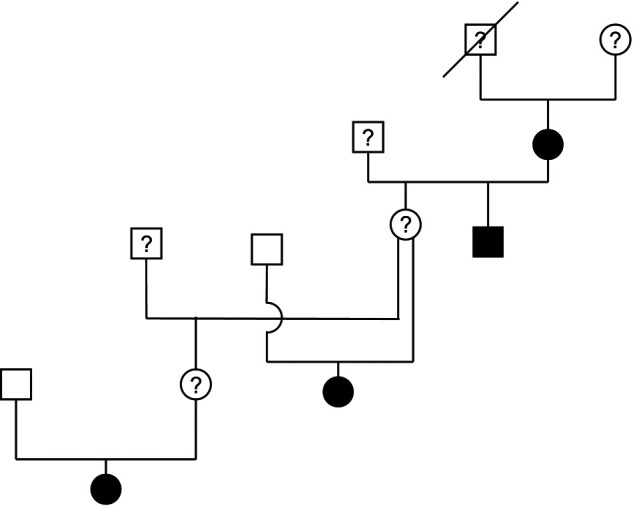
Family of Sphynx cats with hypertrophic cardiomyopathy. Four affected cats were from the same family and an affected cat was observed in each of four generations. Two of the affected cats had one parent that had been documented as unaffected. The male proband (represented by square symbol with a line across) of this family died suddenly before 8 years of age but a necropsy was not performed and cause of death is unknown. Circles represent females, square represent males. Solid black symbols represent cats with hypertrophic cardiomyopathy; solid white symbols represent unaffected cats; symbols with a question mark represent cats that were not available for evaluation
Discussion
Sphynx cats appear to have a form of hypertrophic cardiomyopathy similar to that described in other breeds with systolic anterior motion of the mitral valve and prominent papillary muscles. The disease appears to be a familial disease, at least in some cases, based on the presence of multiple affected cats within a family. Although the mode of inheritance is consistent with an autosomal dominant mode of inheritance as is observed in the Maine Coon cat and in human beings,9,13 a prospective breeding study would be required to further classify the exact mode of inheritance. Further studies are warranted to investigate a causative mutation amongst the affected population, as previous studies involving affected Sphynx cats did not identify either the Maine Coon or Ragdoll mutation. 15
Similar to the general population, the results of this study have shown that, at the time of diagnosis, the severity of hypertrophic cardiomyopathy in Sphynx cats varies from mild to severe. Sphynx cats in this study, however, were diagnosed with hypertrophic cardiomyopathy at a younger age than what has been previously reported for the general feline population with a median age at the time of diagnosis of 2.0 years, compared with 5–6 years.2,3
The retrospective nature of this study resulted in some limitations, but still provides useful information regarding hypertrophic cardiomyopathy in Sphynx cats. Despite using measurements from accepted ECG views, examinations were performed by several ACVIM board-certified cardiologists making it difficult to standardize subjective findings. Many of the included cats were found during breeder evaluations and it is possible that the age of onset may be biased by early screening. Breeders typically evaluate asymptomatic cats regularly; the general pet-owning population will not typically request an ECG for their cat unless advised by a veterinarian because clinical signs are seen or a murmur is ausculted. An additional limitation is that only initial ECG examinations were evaluated and follow-up examinations may have shown further changes in the measurements of affected cats, as well as shown any progression of equivocal or normal cats to affected which could help clarify the mode of inheritance. For that reason, any equivocal cats were excluded from the pedigree evaluation and cats were not classified as unaffected until they were at least 9 years of age — 2 years older than the oldest affected cat in our study. Finally, pedigrees were not available for every cat included and, consequently, there were sires and dams whose ECG status was not known.
Hypertrophic cardiomyopathy appears to be a familial disease in the Sphynx cat. The age of onset in this breed may be younger than that seen in the general cat population. This observation, as well as the previously noted finding that Sphynx cats with hypertrophic cardiomyopathy do not have the Maine Coon or Ragdoll mutation, 15 supports the consideration of Sphynx hypertrophic cardiomyopathy as a unique form of this disease. Further studies are warranted to evaluate for a causative mutation and better classify the phenotypic expression.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1. Riesen SC, Kovacevic A, Lombard CW, Amberger C. Prevalence of heart disease in symptomatic cats: an overview from 1998 to 2005. Schweiz Arch Tierheilkd 2007; 149: 65–71. [DOI] [PubMed] [Google Scholar]
- 2. Ferasin L, Sturgess CP, Cannon MJ, et al. Familial idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002; 220: 202–207. [DOI] [PubMed] [Google Scholar]
- 4. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc 1992; 201: 613–618. [PubMed] [Google Scholar]
- 5. Liu SK, Roberts WC, Maron BJ. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiology 1993; 72: 944–951. [DOI] [PubMed] [Google Scholar]
- 6. Van Vleet JF, Ferrans VJ, Weirich WE. Pathologic alterations in hypertrophic and congestive cardiomyopathy of cats. Am J Vet Res 1980; 41: 2037–2048. [PubMed] [Google Scholar]
- 7. Adin DB, Diley-Poston L. Papillary muscle measurements in cats with normal echocardiographs and cats with concentric left ventricular hypertrophy. J Vet Intern Med 2007; 21: 737–741. [DOI] [PubMed] [Google Scholar]
- 8. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy; an animal model of human disease. Circulation 1995; 92: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 9. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in Maine Coon cats: an animal model of human disease. Circulation 1999; 99: 3172–3180. [DOI] [PubMed] [Google Scholar]
- 10. Lefbom BK, Rosenthal S, Tyrell W, et al. Severe hypertrophic cardiomyopathy in 10 young Ragdoll cats. J Vet Intern Med 2001; 15: 308. [Google Scholar]
- 11. Sampedrano CC, Chetboul V, Mary J, et al. Prospective echocardiographic and tissue Doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin binding protein C gene: a specific analysis of the heterozygous state. J Vet Intern Med 2009; 23: 91–99. [DOI] [PubMed] [Google Scholar]
- 12. Meurs KM, Sanchez X, David RM, et al. Identification of a missense mutation in the cardiac myosin binding protein C gene in a family of Maine Coon cats with hypertrophic cardiomyopathy. Hum Mol Genet 2005; 14: 3587–93. [DOI] [PubMed] [Google Scholar]
- 13. Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol 2001; 33: 655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meurs KM, Norgard MM, Ederer MM, et al. A substitution mutation in the myosin binding protein C gene in Ragdoll cats. Genomics 2007; 90: 261–264. [DOI] [PubMed] [Google Scholar]
- 15. Mary J, Chetboul V, Sampedrano CC, et al. Prevalence of the MYBPC3-A31P mutation in a large European feline population and association with hypertrophic cardiomyopathy in the Maine Coon breed. J Vet Cardiol 2010; 12: 155–161. [DOI] [PubMed] [Google Scholar]
- 16. Meurs KM, Norgard MM, Kuan M, et al. Analysis of 8 sarcomeric candidate genes for feline hypertrophic cardiomyopathy mutations in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2009; 23: 840–843. [DOI] [PubMed] [Google Scholar]
- 17. MacDonald KA. Hypertrophic cardiomyopathy. In: Cote E, MacDonald KA, Meurs KM, Sleeper MM. (eds). Feline cardiology. Chichester: Wiley-Blackwell, 2011, pp 103–175. [Google Scholar]
- 18. Sphynx. In: Simon & Schuster’s Guide to Cats. New York: Simon & Schuster, 1983, pp 39. [Google Scholar]
- 19. Belanger MC. Echocardiography. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine, 7th ed. St Louis: Saunders Elsevier, 2010, pp 415–431. [Google Scholar]


