Abstract
This retrospective study was designed to assess the effect of pimobendan on the median survival time (MST) of cats with non-taurine responsive dilated cardiomyopathy (DCM). Thirty-two client-owned cats with a left ventricular internal dimension at end systole (LVIDs) >14 mm, a fractional shortening (FS) <28% and a lack of response to taurine therapy were included over a 9-year period (2001–2010). These cats were divided into pimobendan (n=16) and non-pimobendan (n=16) treatment groups. All cats received standard treatment with frusemide, taurine and benazepril or enalapril. Nine cats in the non-pimobendan group also received digoxin. The MST of the pimobendan group (49 days; range 1 to >502 days) was four times that of the non-pimobendan group (12 days; 1 to 244 days). The difference in survival between the two groups was statistically significant (P = 0.048). Hypothermia and FS <20% were associated with a poor prognosis. No adverse effects to pimobendan were noted.
Dilated cardiomyopathy (DCM) is an uncommon condition in cats that can result in congestive heart failure (CHF), aortic thromboembolism (ATE), arrhythmias and sudden death. In 1987 taurine deficiency was identified as a major cause of feline DCM. 1 Subsequent supplementation of commercial cat food with taurine has drastically reduced, but not eliminated, the incidence of feline DCM. 2 Other proposed aetiological factors include myocardial dysfunction secondary to toxic insult, infectious myocarditis, metabolic errors, immune-mediated disease, infiltrative disease, chronic volume overload, chronic tachyarrhythmias, end-stage hypertrophic cardiomyopathy and microvascular injury. Genetic links have also been proposed in cats and established in humans, mice and Portuguese Water dogs.3–5 Non-taurine responsive feline DCM is often referred to as idiopathic as the cause of the myocardial dysfunction is rarely apparent at the time of diagnosis. The aetiology of DCM may also be multifactorial, given that experimental induction of taurine deficiency in cats failed to result in overt DCM.1,6,7
The prognosis of feline DCM depends on the response to taurine supplementation. Predicting whether an individual is likely to respond to taurine is problematic. Taurine deficient diets may be difficult to identify as bioavailability may vary with heat processing, potassium depletion and acidification. 8 Whole blood taurine assays are thought to be more reliable than plasma taurine concentrations; however, false positive results may still be obtained with prolonged fasting or anorexia, and false negative results may be obtained with postprandial sampling, a recent dietary change or recent thromboembolism.2,9,10 Myocardial taurine concentrations also fail to correlate with the development of overt DCM. 7 Concurrent central retinal degeneration (secondary to taurine deficiency) is an inconsistent finding.2,10
Given the limitations of the tests used to identify taurine deficiency, taurine supplementation is recommended in all cats with DCM regardless of the whole blood (or plasma) taurine concentration.2,10,11 Assessment of the response to taurine supplementation appears to be the most reliable method of identifying taurine deficiency. Clinical improvement is typically observed within 2 weeks, however, significant echocardiographic improvement is not usually detected until after the first 4 weeks of treatment.10,11 Those cats which respond to taurine supplementation and survive greater than 30 days, have a favourable prognosis as a complete recovery is anticipated.1,10,11 The prognosis for non-taurine responsive cats is grave, with a median survival time (MST) of just 11 days. 12
Historically, treatment options for non-taurine responsive DCM cats have been limited. Congestive signs are controlled via diuretics and angiotensin converting enzymes (ACE) inhibitors. Thoracocentesis and oxygen supplementation may also be required. Digoxin, a positive inotrope, may have a small, but significant, positive impact on left ventricular (LV) systolic function, but it does not appear to have a beneficial effect on survival.11,13 Pimobendan, a calcium sensitiser and phos-phodiesterase inhibitor with positive inotropic, lusitropic and vasodilatory effects, significantly improves survival times in dogs with DCM.14,15 While several abstracts have reported that pimobendan therapy is well tolerated in cats, studies evaluating the effect of pimobendan on survival are lacking.16,17 The purpose of this study is to assess the effect of pimobendan on the survival of cats with non-taurine responsive DCM.
Materials and methods
The medical records of client-owned cats diagnosed with overt DCM between February 2001 and July 2010 at the Melbourne Veterinary Specialist Centre, Melbourne, Australia were retrospectively reviewed. Inclusion criteria included M-mode echocardiographic evidence of LV dilation (LV internal dimension at end systole, LVIDs >14 mm) and systolic dysfunction (fractional shortening, FS <28%),2,11,12 clinical signs attributable to DCM (including CHF and/or ATE) and an intention to treat. Exclusion criteria included a clinical or echocardiographic response to taurine therapy, prior treatment with pimobendan, evidence of congenital heart disease and a lack of follow-up.
Diagnostic evaluation
The signalment, history and clinical findings were reviewed in all cats along with haematological, radiographic and electrocardiographic (ECG) findings where available. Echocardiography was performed by one of three internal medicine specialists (Drs D Merrett BVSc, MVS, FACVSc, R Labuc BVSC, MVS, FACVSc and co-author P Bennett, BVSc, FACVSc, DACVIM) using one of two ultrasound machines (GE Healthcare LOGIQ5 Pro or Toshiba Corevision). Standard echocardiography using 2D, M-mode, colour flow and pulse wave Doppler was performed. 18 Between 2001 and 2005, left atrial (LA) and aortic outflow (AO) Dimensions were measured in the right parasternal short axis M mode view, but from 2005 onwards these parameters were measured in the right parasternal long axis 2D view with the image optimised for LA and LV outflow tract respectively.18,19 LV dimensions were measured from the right parasternal short axis view in M mode at the level of the chordae tendineae. An average of at least three leading edge measurements was recorded. If sedation was required to facilitate echocardiography, a combination of butorphanol (Dolorex; Intervet), acepromazine (ACP 2; Delvet), diazepam (Pamlin; Parnell) and/or ketamine (Ketamine; Parnell) was administered to effect.
Treatment and outcome
Treatment methods were also reviewed. All cats received standard therapy with oral frusemide (Lasix-M; Sanofi-Aventis), taurine (Taurine powder; Musashi) and either benazepril (Fortekor; Novartis) or enalapril (Enalfor; Merial). Pimobendan (Vetmedin; Boehringer Ingelheim) was administered to 80% of cats diagnosed after 2005. As pimobendan is not registered for use in cats, it was administered with the informed consent of the owners. Some cats in the non-pimobendan group also received digoxin (Lanoxin-BG; Sigma Pharmaceuticals) as a positive inotrope. Response to treatment and outcome was determined by a review of patient records and phone communication with owners. Survival times were calculated from the time of diagnosis to the time of death.
Statistical analysis
The comparisons of survival times, prognostic factors and differences between the groups at the time of diagnosis were statistically assessed using a commercially available software program (Statsdirect statistical software version 2.7.0). To assess differences between the groups prior to treatment, Mann Whitney U tests were performed on the parametric data and Fisher’s exact tests were used for dichotomous data. Survival data were collated using Kaplan Meier plots. Median survival times and Brookmeyer-Crowley 95% confidence intervals (95% CI) were determined. Given that the proportional hazards assumption was satisfied, differences in survival were assessed using the log-rank test with right censoring. Statistical significance was indicated by a P value <0.05.
Results
A search of the medical records between 2001 and 2010 revealed 35 cats that met the inclusion criteria. Three cats were subsequently excluded because of a clinical and echocardiographic response to taurine (n = 2) and a lack of follow-up (one). None of the assessed cats had any evidence of congenital heart disease. Of the 32 cats included in the study, 16 were treated with pimobendan.
Signalment
The majority of cats were mixed breeds (15 domestic shorthair, five domestic longhair, two domestic mediumhair), but Burmese (four), Siamese (two), British Shorthair (one), Somali (one), Russian Blue (one) and Ragdoll (one) were also represented. Two-thirds were male (20 neutered, one intact), the remainder were de-sexed females. Median age was 10 years (range 3–16). Weight ranged from 2.8–7.75 kg with a median of 4.7 kg.
History
All cats were being fed a wide range of commercial cat foods and home-cooked diets. Most cats had indoor and outdoor access. A range of pre-existing medical conditions were identified including; feline idiopathic cystitis (n = 4), prior hyperthyroidism treated with radioactive iodine (two), trauma (two), bronchitis (two), chronic low-grade renal disease (one) and herpes keratitis (one). None of the cats had any prior history of cardiac disease. All cats were euthyroid at the time of presentation.
Presenting signs
All cats presented with clinical signs attributable to DCM including an increase in respiratory effort (n = 28), ATE (three), ascites (two) and collapse (one). The median heart rate (HR) was 180 bpm (range 116–250). Thoracic auscultation revealed abnormalities in 31/32 cats (96.9%) including a gallop sound (n = 23), systolic murmur (11), muffled heart sounds (one) and an arrhythmia (nine). The median respiratory rate was 40 bpm (range 18–80).
Diagnostic investigation
Eighteen cats required sedation to facilitate echocardiography. Valvular insufficiency was evident in 22 cats (11 mitral and tricuspid, 10 mitral, one tricuspid). Spontaneous echogenic contrast (smoke) was visible within the left atrium of three cats. A pleural effusion was evident in 22 cats. Pericardial effusion (n = 4) and ascites (three) were also observed. Radiographic findings included cardiomegaly in 13 cats and pulmonary oedema in 11 cats. Of nine cats with audible arrhythmias, seven had ECGs performed. These revealed ventricular premature contractions (n = 3), paroxysmal ventricular tachycardia (one), atrial premature contractions (one) and supraventricular tachycardia (two).
Treatment
All cats received frusemide (5–20 mg PO q8–12h as needed, median dose 1.9 mg/kg; range 0.9–3 mg/kg q8–12h), taurine (250 mg PO q12h) and either benazepril (n = 31) or enalapril (n = 1) therapy (1.25–5 mg PO q24h, median dose 0.48 mg/kg; range 0.32–0.89 mg/kg). Two cats also received spironolactone (Aldactone; Pfizer: 12.5 mg PO q24h, range 2.6–3.5 mg/kg) and aspirin (Aspirin; Mayne Pharma International: 25 mg PO q72h, range 4.4–6.8 mg/kg). Fifteen cats required thoracocentesis and 11 required oxygen therapy. Sixteen cats received pimobendan (0.65–1.25 mg PO q12h, 1 h before food, median dose 0.26 mg/kg q12h; range 0.16–0.37 mg/kg) and nine cats received digoxin (31.25 µg PO q24h, median 7 µg/kg; range 4.85–11.1 µg/kg). Seven cats did not receive a positive inotrope. None of the cats receiving pimobendan received digoxin. Prior to presentation at the specialist centre, 17 cats (eight cats in the pimobendan group and nine cats in the non-pimobendan group) received frusemide therapy for 1–5 days (median 2 days) and four cats (one in the pimobendan group and three in the non-pimobendan group) received an ACE inhibitor for 2–4 days (median 2 days).
Differences between the non-pimobendan and pimobendan groups
Assessment of variance between the pimobendan and non-pimobendan groups revealed a significant increase in the LA and LA:AO in the pimobendan group at the time of diagnosis (median 24.3 vs 20.6, P = 0.038 and median 3.21 vs 2.76, P = 0.007, respectively). Fourteen cats in the pimobendan group had echocardiographic evidence of valvular insufficiency compared to eight in the non-pimobendan group (P = 0.03). No other variables were statistically different between the two groups (Table 1).
Table 1.
Comparison of the baseline clinical variables between the non-pimobendan and pimobendan treatment groups
| Variable | Non-pimobendan group Median (range) | Pimobendan group Median (range) | P value |
|---|---|---|---|
| LVIDs | 17.55 (14.0–22.4) | 17.6 (14.3–26) | 0.48 |
| LVIDd | 20.65 (15.6–25.7) | 22.15 (17.7–28.5) | 0.26 |
| IVSd | 3.4 (2.5–5.7) | 4.15 (2.7–5.4) | 0.19 |
| LVFWd | 4.6 (2.6–6.2) | 4.5 (1.9–5.4) | 0.33 |
| FS | 14.35 (3–26) | 16.9 (7–27) | 0.23 |
| LA | 20.4 (11–27.5) | 24.3 (15–31.4) | 0.038* |
| LA/AO | 2.77 (1.6–3.35) | 3.21 (2.24–5.24) | 0.007* |
| ATE | 2 | 1 | 0.61 |
| Heart rate | 180 (116–200) | 200 (120–250) | 0.17 |
| Temperature | 37.6 (34.1–39.8) | 37.7 (33–39.3) | 0.56 |
| Bodyweight | 4.38 (2.8–6.44) | 4.83 (3.4–7.75) | 0.36 |
| Age | 10 (4–15) | 10 (3–18) | 0.72 |
| Female | 7 | 4 | 0.30 |
| Male | 9 | 12 | 0.30 |
| Valvular insufficiency | 8 | 14 | 0.03* |
| Gallop | 14 | 9 | 0.06 |
Denotes P values <0.05
LVIDs = left ventricular internal dimension at end systole, LVIDd = left ventricular internal dimension at end diastole, IVSd = interventricular septal thickness at end diastole, LVFWd = left ventricular free wall thickness at end diastole, FS = fractional shortening, LA = left atrial dimension, AO = aortic outflow, ATE = aortic thromboembolism
Outcomes
Each of the 32 cats demonstrated persistent/recurrent clinical signs and/or echocardiographic evidence of DCM despite medical treatment. In the non-pimobendan group, all 16 of the cats died. A total of seven episodes of ATE were recorded in four cats (two at the time of diagnosis and five following the instigation of treatment). Twelve were euthanased due to refractory CHF (8/16) or ATE (4/16) and four died suddenly (presumed to be cardiac related). The survival times ranged from 1 day to 244 days with a MST of 12 days (95% CI; 4–33 days, see Figure 1).
Figure 1.
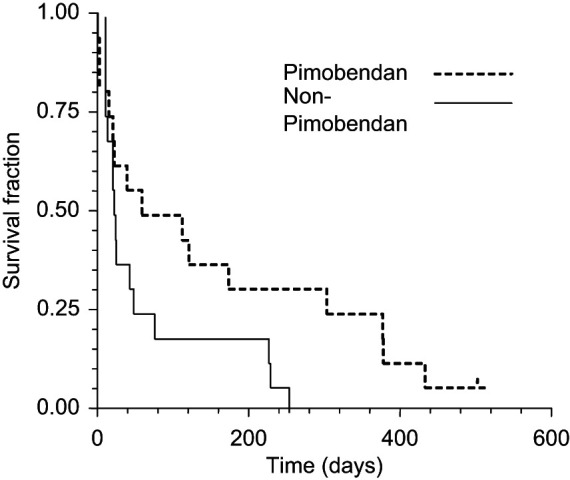
Survival times of cats treated with (n = 16) and without (n = 16) pimobendan. Cats in the pimobendan group had a significantly longer survival (P = 0.048) than those in the non-pimobendan group
Of the 16 cats in the pimobendan group 15 died. One cat remained alive at the time of writing (502 days after diagnosis) and it was censored from the outcome analysis. Two episodes of ATE were reported. One cat demonstrated clinical evidence of ATE within 24 h of diagnosis and a second cat developed an ATE 111 days into treatment. Eight cats were euthanased due to refractory CHF (n = 5), ATE (two) and seizure activity (one). Sudden death was observed in seven cats (presumed cardiac related). The survival times ranged from 1 day to >502 days. The median survival time of the pimobendan group was 49 days (95% CI; 11–164 days). The difference in survival between the pimobendan and non-pimobendan groups was statistically significant (P = 0.048). No adverse effects were reported.
Prognostic indicators
Clinical and echocardiographic variables were compared to survival in an attempt to identify prognostic indicators. Hypothermia at presentation (<37.2°C) was associated with reduced survival (P = 0.0081). A low fractional shortening (<20%) at presentation was also associated with a reduced survival (P = 0.0045). Other variables (including LVIDs, LVIDd, IVSd, LVFWd, LA, LA:AO, ATE, BW, age, sex, HR, digoxin and pimobendan dose) were also assessed, but none had a statistically significant effect on survival.
Discussion
Pimobendan appears to have a positive effect on the survival of cats with non-taurine responsive DCM. While the MST of cats in the non-pimobendan group was consistent with that of previous studies (12 days), a four-fold increase in the MST of cats in the pimobendan group (49 days) was observed. 12 Similar to previous studies, digoxin did not appear to have a significant effect on survival.11,13
As it currently stands, this is the largest study of non-taurine responsive, feline DCM. 12 Unfortunately, the rarity of DCM in cats prevents the accumulation of large sample numbers, and post hoc analysis of the power of this study was low (0.22). The retrospective nature of this study led to a lack of blinding, standardisation and randomisation of the treatment groups and, as a consequence, the results should be interpreted with caution. While 80% (16/20) of the cats diagnosed after 2005 were included in the pimobendan group, it appears unlikely that their improved survival is a function of advances in supportive care and/or monitoring over the study period because cats in the non-pimobendan group that were also diagnosed after 2008 had poor survival times (MST of 6.5 days).
Significant differences were evident between the two treatment groups at the time of diagnosis. LA and LA:AO were increased in the pimobendan group when compared to the non-pimobendan group. The significance of this difference is difficult to interpret because the method of measuring the LA and AO changed in 2005 from the right parasternal short axis M mode to the right parasternal long axis 2D view. While this change in methodology may artificially increase the LA:AO ratio in cats assessed after 2005 (80% of these cats were in the pimobendan group), LA measurements tend to be smaller when measured in the long axis 2D view. Consequently, the change in methodology should not have lead to an increase in both LA:AO and LA unless a genuine increase in LA dilation was observed. 19
As DCM progresses, dilation of the atrioventricular annulus can also lead to valvular insufficiency.12,20 Fourteen (87.5%) of the pimobendan cats had echocardiographic evidence of valvular insufficiency, compared to eight (47%) in the non-pimobendan group. While this difference may have reflected improved echocardiographic detection of valvular insufficiency via the use of a more advanced ultrasound and improved technique in the second half of this study, the concurrent increase in LA:AO suggests an increased incidence rather than enhanced detection. While none of these variables had an adverse effect on survival, the increase in LA, LA:AO and valvular insufficiency in the pimobendan group may suggest that these cats had more advanced disease (with annular dilation). None of the affected cats had a history of a pre-existing heart murmur or echocardiographic evidence of valvular pathology to suggest that the valvular insufficiency and DCM were secondary to chronic valvular disease (eg, dysplasia or endocardiosis), although this remains a differential.
While the degree of LA dilation was not a prognostic indicator in this study, a positive correlation between the incidence of ATE and the degree of LA dilation has previously been reported. 21 Despite the apparent increase in LA dimension in the pimobendan group, the incidence of ATE was reduced (n = 2) compared to the non-pimobendan group (n = 5), but this difference was not statistically significant. In vitro studies using feline platelet rich plasma, have demonstrated that pimobendan exerts an anti-platelet aggregation effect (thought to be mediated by inhibition of thromboxane A2). 22 Pimobendan may also theoretically reduce the risk of thrombus formation by improving LA functional indices (including LA appendage inflow/outflow velocities). 23 Unfortunately, the low numbers of ATE observed in this study prevented assessment of the effect of pimobendan on the incidence of ATE in cats with DCM.
Aspirin was administered to two cats in the non-pimobendan group. Both of these cats presented with ATE at the time of diagnosis and both experienced recurrent ATE despite aspirin therapy. Prophylactic aspirin therapy was not administered to any other cats because of a distinct lack of clinical data to support its routine use in feline cardiomyopathy. 24 While ATE has been associated with reduced survival in studies of cats with hypertrophic cardiomyopathy and dilated cardiomyopathy, ATE did not have an adverse effect on survival in this study.11,21,24,25
Hypothermia at the time of presentation was associated with reduced survival, which is consistent with previous studies of cats, dogs and humans with heart disease.11,15 Severe systolic dysfunction (FS <20%) was also identified as a negative prognostic indicator. While reduced FS has been associated with a poor outcome in humans with DCM, this finding has not been previously reported in cats. 26 Right ventricular internal dimension at diastole (RVIDd), hospitalisation and concurrent hyperthyroidism have been previously identified as prognostic indicators in feline DCM, but these factors were not assessed in this study. 11 In dogs with DCM, ascites, atrial fibrillation and a young age at presentation have also been identified as negative prognostic indicators.27, 28 While none of the cats assessed in this study had atrial fibrillation, the presence of a dysrhythmia was not associated with reduced survival. Age and the presence of ascites did not affect outcome. While previous studies have indicated that body weight correlates with survival in cats, dogs and humans with heart disease, this trend was not observed in this study. 29
Where possible thoracic radiographs were performed post thoracocentesis. While 22 cats had evidence of a pleural effusion on ultrasound, 14 had evidence of residual pleural effusion on thoracic radiographs. Five of the cats with low volume residual pleural effusion had radiographic evidence of concurrent cardiomegaly and pulmonary oedema. The presence of a moderate volume pleural effusion in the remaining nine cats prevented accurate radiographic assessment, and consequently the true incidence of cardiomegaly and pulmonary oedema could have been higher than previously stated.
While diets were not standardised, all cats received taurine supplements throughout their treatment. Primary taurine deficiency appears unlikely in this population of cats given that all cats continued to exhibit congestive signs, died suddenly or were euthanased due to cardiac related issues despite taurine supplementation. Cats which demonstrated a clinical and/or echocardiographic response to taurine supplementation were excluded from this study. Follow-up echocardiography was not routinely performed and consequently a partial taurine response cannot be excluded. Interpretation of follow-up echocardiography may have been difficult because improvement may be due to either pimobendan or taurine therapy. Unfortunately, the poor prognosis of these cats meant that there was not enough time to trial taurine therapy prior to the introduction of pimobendan.
Strict inclusion criteria were applied to enable comparison with previous survival studies.2,11,12 While echocardiography was performed using sedation in 19 cats, previous studies have indicated that, while sedation may decrease FS, LVIDs is unaffected. Consequently sedation should not lead to incorrect diagnosis of DCM when two strict criteria (LVIDs >14 mm and FS <28%) are applied concurrently.30–32 Echocardiographic error was also minimised by recording an average of at least three measurements.
While 22/32 cats in this study had echocardiographic evidence of valvular insufficiency, 2/32 cats had tachyarrhythmias and 1/32 cats had evidence of mild, focal hypertrophy of the left ventricular free wall (6.2 mm, IVSDd 5.6 mm), it is not known whether these changes relate to the cause or effect of DCM. 33 Given that post-mortem examinations were not performed, the inciting cause of DCM and cause of death were not confirmed.
An increased incidence of DCM has previously been reported in DSH, Siamese, Persians, Himalayans and Abyssinians, but no breed predisposition was observed in this study.2,34 The percentage of domestic mixed breed (68.75%), Burmese (12.5%) and Siamese (6.25%) cats with DCM was similar to that of the general population presenting to this referral centre (66%, 7% and 4%, respectively). More male than female cats were included in this study, but the proportion is not statistically different to the hospital population (P = 0.18).
Pimobendan has demonstrated positive inotropic and balanced vasodilatory effects in cats during experimental studies. 32 In dogs, pimobendan is thought to have a very rapid onset of action (<2 h) following oral administration and consequently, it is indicated for used for acute stabilisation of decompensated heart failure. 36 An abstract presented at the European College of Veterinary Internal Medicine (ECVIM) 2010 reported that the pharmacokinetics of pimobendan in healthy cats following a single oral dose (0.23–0.35 mg/kg) was very similar to that previously reported in dogs, although the maximum plasma concentration was substantially higher and the elimination phase was approximately three times as long. 37 Given that such a rapid onset of action is anticipated with pimobendan therapy, survival data was collected on the basis of an intention to treat.
In this study, the dose of pimobendan therapy was extrapolated from the canine dose range and limited by the available tablet/capsule size. 38 Compliance was improved via the administration of the chewable tablets rather than the capsules (which tended to adhere to the roof of the mouth).
Pimobendan use in cats with perceived systolic dysfunction has been only previously been reported in abstract format. The first abstract (ECVIM 2007), cited pimobendan use in 11 cats (1.25 mg/cat q12h) with congestive heart failure secondary to dilated (n = 7), hypertrophic (three) and restrictive (one) cardiomyopathy. 16 No adverse effects were noted and reported survival times ranged from 9–585 days. A second abstract presented at ACVIM 2010, reported mild adverse responses in 5/161 cats treated with pimobendan (0.08–0.42 mg/kg q 12 h) in combination with a range of other medications (including frusemide, ACE inhibitors and anti-thrombolytic agents). 17 These adverse effects included agitation (n = 2), anorexia (n = 1), vomiting (n = 1) and constipation (n = 1). The treated cats were reported to have congestive heart failure secondary to hypertrophic (n = 62), unclassified (55) and dilated (27) cardiomyopathy but the diagnostic criteria were not specified. The median survival time of all cats treated with pimobendan was 102 days (range 9–870 days). Survival benefit could not be assessed in these abstracts due to the inclusion of a range of cardiomyopathies and a lack of a control population, however, both reports concluded that pimobendan therapy was well tolerated in cats. No adverse effects were observed during this study.
Conclusions
Pimobendan therapy appears to improve the survival of cats with non-taurine responsive DCM. While pimobendan therapy appears to be well tolerated in cats, determination of the pharmacokinetics of pimobendan in cats is also needed to ensure that the dose and dose frequency used in this study is adequate. Further investigation of the effect of pimobendan on the incidence of ATE and the survival of cats with DCM is also warranted.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Pion PD, Kittleson MD, Rogers QR, Morris JG. Myocardial failure in cats associated with low plasma taurine: A reversible cardiomyopathy. Science 1987; 237: 764–8. [DOI] [PubMed] [Google Scholar]
- 2. Pion PD, Kittleson MD, Thomas WP, et al. Clinical findings in cats with dilated cardiomyopathy and relationship of findings to taurine deficiency. J Am Vet Med Assoc 1992; 201: 267–74. [PubMed] [Google Scholar]
- 3. Lawler DF, Templeton AJ, Monti KL. Evidence for genetic involvement in feline dilated cardiomyopathy. J Vet Intern Med 1993; 7: 383–7. [DOI] [PubMed] [Google Scholar]
- 4. Møller DV, Andersen PS, Hedley P, et al. The role of sarcomere gene mutations in patients with idiopathic dilated cardiomyopathy. European J Human Genetics 2009; 17: 1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werner P, Raducha MG, Prociuk U, et al. A novel locus for dilated cardiomyopathy maps canine chromosome 8. Genomics 2008; 91: 517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Novotny MJ, Hogan PM, Flannigan G. Echocardiographic evidence for myocardial failure induced by taurine deficiency in domestic cats. Can J Vet Res 1994; 58: 6–12. [PMC free article] [PubMed] [Google Scholar]
- 7. Fox PR, Sturman JA. Myocardial taurine concentrations in cats with cardiac disease and in healthy cats fed taurine-modified diets. Am J Vet Res 1992; 53: 237–40. [PubMed] [Google Scholar]
- 8. Dow SW, Fettman MJ, Smith KR, et al. Taurine depletion and cardiovascular disease in adult cats fed a potassium-depleted acidified diet. Am J Vet Res 1992; 52: 402–5. [PubMed] [Google Scholar]
- 9. Pion PD, Lewis J, Green K, et al. Effect of meal-feeding and food deprivation on plasma and whole blood taurine concentrations in cats. J Nutrition 1991; 121: S177–78. [DOI] [PubMed] [Google Scholar]
- 10. Sisson DD, Knight DH, Helsinki C, et al. Plasma taurine concentrations and M-mode echocardiographic measures in healthy cats and in cats with dilated cardiomyopathy. J Vet Intern Med 1991; 5: 232–8. [DOI] [PubMed] [Google Scholar]
- 11. Pion PD, Kittleson MD, Thomas WP, et al. Response of cats with dilated cardiomyopathy to taurine supplementation. J Am Vet Med Assoc 1992; 201: 275–83. [PubMed] [Google Scholar]
- 12. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atkins CE, Snyder PS, Keene BW, et al. Efficacy of digoxin for treatment of cats with dilated cardiomyopathy. J Am Vet Med Assoc 1990; 196: 1463–8. [PubMed] [Google Scholar]
- 14. Luis Fuentes V, Corcoran B, French A, et al. A double-blind, randomized, placebo-controlled study of pimobendan in dogs with dilated cardiomyopathy. J Vet Intern Med 2002; 16: 255–61. [DOI] [PubMed] [Google Scholar]
- 15. O’Grady MR, Minors SL, O’Sullivan ML, Horne R. Effect of pimobendan on case fatality rate in Doberman Pinschers with congestive heart failure caused by dilated cardiomyopathy. J Vet Intern Med 2008; 22: 897–904. [DOI] [PubMed] [Google Scholar]
- 16. Sturgess CP, Ferasin L. Clinical efficacy of pimobendan in 11 cats with systolic heart failure [ECVIM Abstract]. J Vet Intern Med 2007; 21:1443–4. [Google Scholar]
- 17. MacGregor JM, Rush JE, Laste N, et al. Use of pimobendan in 161 cats (2006–2009) [ACVIM Abstract]. J Vet Intern Med 2010; 24: 692. [Google Scholar]
- 18. Bonagura JD, Luis Fuentes V. Echocardiography. In: Ettinger SJ, Feldman EC. eds. Textbook of veterinary internal medicine. Diseases of the dog and cat, 5th edn. Philadelphia, PA: WB Saunders, 2000: 834–73. [Google Scholar]
- 19. Abbott JA, MacLean HN. Two-dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med 2006; 20: 111–9. [DOI] [PubMed] [Google Scholar]
- 20. Soderberd SF, Boon JA, Wingfield WE, Miller CW. M-Mode echocardiography as a diagnostic aid for feline cardiomyopathy. Vet Radiology 1983; 24: 66–73. [Google Scholar]
- 21. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002; 220: 202–7. [DOI] [PubMed] [Google Scholar]
- 22. Bitterman H, Smith BA, Lefer AM. Use of the novel cardiotonic and vasodilator agent pimobendan in traumatic shock. Drug Research 1988; 38: 1389–93. [PubMed] [Google Scholar]
- 23. Kent AM, Bonagura JD, Scansen BA, et al. Effects of atenolol, ivabradine and pimobendan on left atrial function: An echocardiographic study in healthy cats. [ACVIM Abstract]. J Vet Intern Med 2010; 24: 695. [Google Scholar]
- 24. Lunsford KV, Mackin AJ. Thromboembolic therapies in dogs and cats: An evidence-based approach. Vet Clin Small Anim 2007; 37: 579–609. [DOI] [PubMed] [Google Scholar]
- 25. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985-1989). J Am Vet Med Assoc 1992; 201: 613–8. [PubMed] [Google Scholar]
- 26. Nagaoka H, Isobe N, Kubota S, et al. Myocardial contractile reserve as a prognostic determinant in patients with idiopathic dilated cardiomyopathy without overt heart failure. Chest 1997; 11: 344–50. [DOI] [PubMed] [Google Scholar]
- 27. Tildholm A, Svensson H, Sylven C. Survival and prognostic factors in 189 dogs with dilated cardiomyopathy. J Am Anim Hosp Assoc 1997; 33: 364–68. [DOI] [PubMed] [Google Scholar]
- 28. Calvert CA, Pickus CW, Jacobs GJ, Brown J. Signalment, survival, and prognostic factors in Doberman pinschers with end-stage cardiomyopathy. J Vet Intern Med 1997; 11: 323–6. [DOI] [PubMed] [Google Scholar]
- 29. Finn E, Freeman LM, Rush JE, Lee Y. The relationship between body weight, body condition, and survival in cats with heart failure. J Vet Intern Med 2010; 24: 1369–74. [DOI] [PubMed] [Google Scholar]
- 30. Jacobs G, Knight DH. M-Mode echocardiographic measurements in non-anaesthestized healthy cats: Effects of body weight, heart rate, and other variables. Am J Vet Res 1985; 46: 1705–11. [PubMed] [Google Scholar]
- 31. Jacobs G, Knight DH. Change in M-mode echocardiographic values in cats given ketamine. Am J Vet Res 1985; 46: 1712–3. [PubMed] [Google Scholar]
- 32. Fox PR, Bond BR, Peterson ME. Echocardiographic reference values in healthy cats sedated with ketamine hydrochloride. Am J Vet Res 1985; 46: 1479–84. [PubMed] [Google Scholar]
- 33. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplant Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807–16. [DOI] [PubMed] [Google Scholar]
- 34. Liu SK, Tashjian RJ, Patnaik AK. Congestive heart failure in the cat. J Am Vet Med Assoc 1970; 156: 1319–30. [PubMed] [Google Scholar]
- 35. Von Meel JC. Cardiovascular effects of the positive inotropic agents pimobendan and sulmazole in vivo. Drug Research 1985; 35: 284–8. [PubMed] [Google Scholar]
- 36. Miller MW, Adams R. Digitalis, positive inotropes and vasodilators. In: Riviere J, Papich MG. eds. Veterinary pharmacology and therapeutics, 9th edn. Ames, IA: Wiley-Blackwell, 2009: 557. [Google Scholar]
- 37. Hanzlicek A, Margiocco M, Gehring R, et al. Pharmacokinetics of oral pimobendan in healthy cats: a single dose study [ECVIM Abstract]. J Vet Intern Med 2011; 24: 1562. [Google Scholar]
- 38. Boehringer Ingelheim. Vetmedin 1.25 mg chewable tablets for dogs [Drug Leaflet]. North Ryde, NSW, Australia. [Google Scholar]


