Abstract
A 13-year-old female spayed domestic shorthair cat presented for investigation of decreased appetite and increased serum liver enzyme concentrations. An abdominal ultrasound revealed multiple sessile hyperechoic structures along the luminal aspect of the gall bladder wall and a mildly enlarged liver with hyperechoic nodules. Cholecystectomy was performed and biopsies were obtained by laparotomy. Histopathologic examination with immunohistochemistry was consistent with a diagnosis of small-cell lymphoma of T cells within the gall bladder, liver and small intestine. Clonality testing confirmed the diagnosis. The cat remains clinically stable 23 months after institution of treatment with prednisolone, chlorambucil and ursodeoxycholic acid. This is the first report of small-cell lymphoma in the gall bladder of a cat.
Case Report
A 13-year-old female spayed domestic shorthair cat presented to the University of Florida Small Animal Hospital for investigation of increased concentrations of the liver enzymes, alanine transaminase (ALT) and alkaline phosphatase (ALP). The owner had noted a mild decrease in appetite 2 months before presentation. At that time, serum concentrations of ALT [452 U/l; reference interval (RI) 12–130 U/l], and ALP (174 U/l; RI 14–111 U/l) were increased. Other blood chemistries, a complete blood count (CBC) and total thyroxine concentrations proved normal; feline immunodeficiency virus antibody and feline leukemia virus antigen tests were negative. Empirical treatment with unknown doses of S-adenosylmethionine and silybin (Denamarin; Nutramax, Edgewood, MD, USA) and amoxicillin (Amoxi-Drops; Pfizer, New York, NY, USA) had resulted in no clinical improvement over the intervening 2 months. One week prior to referral, fasting bile acid concentration (125.6 µmol/l; RI <10.0 µmol/l) and feline pancreatic lipase immunoreactivity (fPLI; 18.0 µg/l; RI 0.1–3.5 µg/l) were increased. Serum concentrations of cobalamin and folate were within the RI.
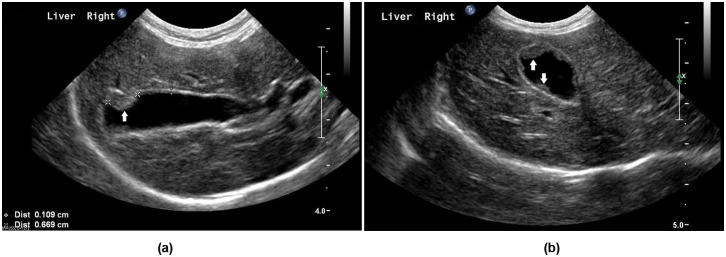
On presentation at the University of Florida, the cat’s body weight was 3.3 kg with a body condition score 1 of 4/9 and mild muscle wasting. Hepatomegaly was appreciated on palpation. Serum biochemistry showed increased liver enzymes, bilirubin and globulins (Table 1); CBC was unremarkable. An abdominal ultrasound examination identified multiple sessile hyperechoic structures up to 7 mm long, along the luminal aspect of the gall bladder wall (Figure 1). The liver had a hyperechoic echotexture and multiple, well-defined hyperechoic nodules, the largest measuring 10 mm × 8 mm × 7 mm. The spleen was mildly enlarged and heteroechoic with multiple small (<4 mm) hyperechoic nodules. The duodenal lymph nodes were considered enlarged, measuring 7 mm × 5 mm × 5 mm.
Table 1.
Pertinent laboratory results for a cat presented with a mild decrease in appetite and increased liver enzymes. ‘Week 0’ represents those results obtained on the day before surgical biopsies were collected. Chemotherapy (chlorambucil and prednisolone) was started a week later (‘week 1’), following a diagnosis of small-cell lymphoma
| Parameter (reference interval) | Week 0 | Week 1 | Week 2 | Week 3 | Month 1 | Month 3 | Month 9 | Month 18 |
|---|---|---|---|---|---|---|---|---|
| Alanine transaminase (ALT) (31–88 U/l) | 828 | 439 | 1275 | 1520 | 1680 | 748 | 482 | 360 |
| Aspartate aminotransferase (AST) (12–29 U/l) | 214 | 166 | 300 | 209 | 255 | |||
| Alkaline phosphatase (ALP) (7–42 U/l) | 130 | 135 | 133 | 129 | 167 | 241 | 75 | 137 |
| Total bilirubin (0.1–0.3 mg/dl) | 1.2 | 0.7 | 0.5 | 0.4 | 0.5 | 0.1 | ||
| Albumin (2.5–3.5 g/dl) | 3.0 | 2.4 | 3.0 | 3.0 | 3.1 | 3.0 | ||
| Globulin (2.9–5.4 g/dl) | 6.1 | 5.4 | 4.0 | |||||
| Prothrombin time (7.1–12.5 s) | 8.9 | |||||||
| Partial thromboplastin time (8.9–15.4 s) | 12.4 |
Figure 1.
Ultrasound images of the gall bladder of a cat presented with a mild decrease in appetite and increased liver enzymes. Longitudinal (A) and transverse (B) views are shown. Multiple sessile hyperechoic structures, indicated by white arrows, were noted along the luminal aspect of the gall bladder wall. The largest nodule measured 0.7 cm long (indicated by ‘x’ caliper marks on the longitudinal view). The thickness of the remainder of the gall bladder wall was unremarkable (indicated by ‘+’ caliper marks in the longitudinal view)
A laparotomy was performed under general anesthesia. Palpation of the gall bladder revealed multiple nodules within its wall and the bile duct was dilated and tortuous. A cholecystectomy was performed (Figure 2) and patency of the bile duct was confirmed. Biopsies from the liver, pancreas, stomach, duodenum, jejunum and duodenal lymph node, and aerobic and anaerobic cultures of the bile and liver were obtained. Pancreatitis was suspected based on the increased fPLI concentration, so a jejunal-through-gastrostomy feeding tube was surgically placed by passing a 5 Fr red rubber catheter (Kendall, Mansfield, MA, USA) into the jejunum through a 20 Fr pezzar catheter (Rusch, Durham, NC, USA), inserted into the stomach. The cat received cefazolin (15 mg/kg intravenously q8h; Sandoz, Princeton, NJ, USA), methadone (0.1 mg/kg intravenously q6h; Bioniche Pharma, Rosemont, IL, USA, and lactated Ringer’s solution (5 ml/kg/h intravenously; Baxter, Deerfield, IL, USA), postoperatively, and recovered uneventfully. A continuous infusion of a liquid enteral diet (Clinicare; Abbott, Abbott Park, IL, USA) was administered through the jejunal tube, starting 24 h after surgery at 1.9 ml/h and increasing to 3.8 ml/h the following day. Three days after surgery, the cat started consuming food by mouth and the jejunal tube was removed on day 4. The cat was discharged the following day with instructions to administer ursodeoxycholic acid (12.6 mg/kg by mouth q24h indefinitely: Urso; Axcan Pharma, Birmingham, AL, USA) and cephalexin (31.8 mg/kg by mouth q8h for 2 weeks; Teva Pharmaceuticals, North Wales, PA, USA). The owner was advised to supplement any food consumed by mouth by feeding through the gastrostomy tube as necessary to achieve a total caloric intake of 200 kcal/day.
Figure 2.
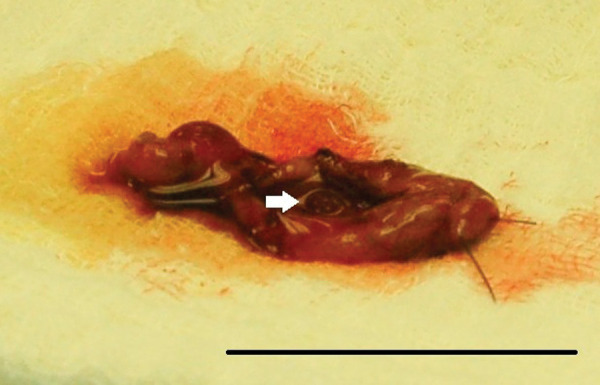
Photograph of the excised gall bladder of a cat presented with a mild decrease in appetite and increased liver enzymes. Multiple nodules within the gall bladder wall were palpated during surgery and visualized upon dissection after cholecystectomy. The white arrow indicates one of the nodules visualized on the luminal aspect of the gall bladder wall. The black reference bar measures 4 cm in length
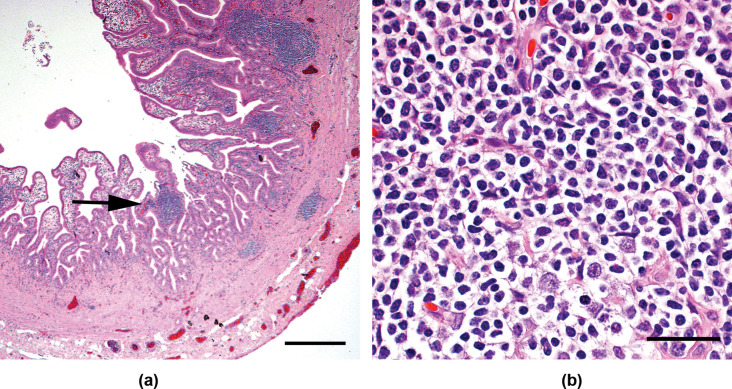
Histologic examination of the gall bladder revealed prominent villous projections extending from the mucosa into the lumen. These projections were markedly expanded by monomorphic small lymphocytes in nodular aggregates (Figure 3), with small numbers of plasma cells, macrophages, eosinophils and neutrophils. Approximately 85% of the lymphocytes were immunoreactive to CD3 antibodies, consistent with a T cell population; approximately 15% were immunoreactive to CD79a antibodies, representative of B cells. Within the liver, there was marked expansion of portal areas with small lymphocytes and rare small aggregates of lymphocytes in the sinusoids. There was extensive bile duct and arteriolar proliferation with multifocal regenerative nodules. Gastric biopsies were unremarkable, but duodenal and jejunal biopsies revealed moderate to marked expansion of the villi with small lymphocytes and a smaller population of other inflammatory cells. The liver, duodenum and jejunum had CD3 and CD79a antibody labeling patterns similar to those seen in the gall bladder. The duodenal lymph node had normal architecture with moderate expansion of the diffuse lymphatic tissue. Approximately 90% of the lymphocytes in this area were immunoreactive to CD3 antibodies, while approximately 10% were immunoreactive to CD79a antibodies. Within the pancreas, small multifocal aggregates of lymphocytes, with less organized infiltration of eosinophils and neutrophils, were identified. Polymerase chain reaction (PCR) for antigen receptor rearrangement (PARR) was performed to test for clonality 2 and a monoclonal T cell receptor (TCR) gene rearrangement was found in the cells of the gall bladder and liver, which supported a diagnosis of T cell lymphoma. An oligoclonal population of cells was identified within the jejunum, with one of the clones being identical to that seen in the gall bladder and liver. Aerobic and anaerobic cultures of the liver and bile proved negative.
Figure 3.
Photomicrographs of the gall bladder wall of a cat presented with a mild decrease in appetite and increased liver enzymes. Multifocal areas of infiltration with small, well-differentiated lymphocytes were noted. The black arrow in (A) indicates one area of lymphocytic infiltration, which is shown at greater magnification in (B). The black reference bar in (A) measures 400 µm in length; the reference bar in (B) measures 30 µm in length. The sample was stained with hematoxylin and eosin (H&E) stain
Therapy for small-cell lymphoma was initiated: chlorambucil (0.6 mg/kg by mouth q48h: Leukeran; GlaxoSmithKline, Philadelphia, PA, USA) and prednisolone (1.5 mg/kg by mouth q24h, decreased to 0.75 mg/kg by mouth q24h 4 months later: PrednisTab; Lloyd, Shenandoah, IA, USA), in addition to the previously prescribed ursodeoxycholic acid. The owner noted an improvement in appetite within the month. Liver enzyme concentrations increased 1 month after surgery but later returned to presurgery concentrations (Table 1). The gastrostomy tube was removed 8 months after surgery. Repeat abdominal ultrasound examinations performed 2 months and 18 months postoperatively revealed unchanged hepatic and splenic nodules. The patient continues to receive chlorambucil, prednisolone and ursodeoxycholic acid, and appears to be clinically stable 23 months after diagnosis.
In cats, small-cell intestinal lymphoma is a well-recognized disease and, while concurrent involvement of the liver has been reported,3–5 lymphoma of the gall bladder is extremely rare. The authors are aware of only one previous case report of feline gall bladder lymphoma in the veterinary literature, 6 and the disease in that report differs significantly from the disease described here. First, on ultrasonographic examination, multiplie sessile hyperechoic nodules protruded from the wall into the gall bladder lumen in our patient, whereas in the previous case report, the cat’s gall bladder wall was severely and diffusely thickened with abnormal hypoechoic nodules within the wall. Second, small lymphocytes predominated on histologic examination of excised tissue from our patient, whereas high-grade (lymphoblastic) gall bladder lymphoma was diagnosed in the previous case by microscopic evaluation of a fine needle aspirate of the gall bladder wall. Finally, the lymphoma in our patient was demonstrated to be of T cell origin and was associated with intestinal and hepatic lymphoma, whereas the lymphoma in the previous case report was speculated to be of B cell origin, as B cell lymphoma was concurrently identified in the urinary bladder.
In our patient, establishment of a diagnosis was dependent on a combination of histology, immunohistochemistry and clonality assays. The histologic appearance suggested a diagnosis of lymphoma, but the similarity of lymphoma to that of other lymphocytic inflammatory diseases, such as portal hepatitis or inflammatory bowel disease, and the lack of overtly neoplastic cellular morphologic characteristics, made a definitive diagnosis difficult. The increased proportion of T lymphocytes found using immunohistochemistry suggested T cell lymphoma, which is typical of feline small-cell intestinal lymphomas, 5 but this pattern has also been reported in cats with active lymphocytic cholangitis/cholangiohepatitis. 7 A diagnosis of lymphoma was only confirmed when evaluation of the gene rearrangement of the TCR showed that the cells in the gall bladder and liver originated from a single clone. A small number of clones of cells were found within the jejunum, with one identical to that in the gall bladder and liver. Oligoclonality has been reported previously in feline intestinal T cell lymphomas. 2 We suspect that the lymphocytes expanding the duodenal lymph node, and within the duodenum and pancreas, were also representative of lymphoma but clonality assays were not performed on those tissues.
The prognosis for cats with small-cell intestinal lymphoma, treated with chlorambucil and prednisolone, is considered to be excellent, with between 76% and 96% of cats achieving clinical remission and a median remission duration of 19–26 months.5,8 The cat reported here is clinically stable 23 months after diagnosis, which suggests that the presence of gall bladder involvement has not appreciably worsened the prognosis in our patient. The cause of the sustained increase in liver enzymes is unknown. Despite the current lack of clinical signs, small-cell lymphoma may still persist and remission of lymphoma cannot be confirmed without repeat hepatic and intestinal biopsies. Although considered unlikely, the persistent increase in liver enzymes may represent additional unidentified liver disease; this may also explain the hyperechogenicity of the liver on ultrasound examination, which is not typical of hepatic lymphoma. 9 The owner has declined additional biopsies at the current time because of the lack of clinical signs.
Interestingly, our patient demonstrated a hyperglobulinemia, which resolved during chemotherapy treatment. Hyperglobulinemia associated with lymphoma is typically a monoclonal gammopathy produced by a clonal proliferation of B lymphocytes which are cells actively involved in immunoglobulin production. In humans, T cell lymphoma is typically associated with a polyclonal gammopathy.10,11 The exact mechanism has not been clarified, but it is hypothesized that neoplastic T cells promote B cell differentiation and immunoglobulin production. Hyperglobulinemia can also occur secondary to inflammatory disease, typically producing a polyclonal gammopathy. Serum protein electrophoresis was not performed in this case to establish the nature of the hyperglobulinemia.
This is the first case report of small-cell lymphoma involving the gall bladder of a cat. Notable features include the unusual ultrasonographic appearance of the gall bladder and the value of clonality assays in both establishing this challenging diagnosis and confirming concurrent involvement of the liver and intestine.
Acknowledgments
The authors would like to thank Dr J Creamer, Baytree Animal Hospital, Valdosta, GA, for his contribution to the management of this case.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 2. Moore PF, Woo JC, Vernau W, Kosten S, Graham PS. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol 2005; 106: 167–78. [DOI] [PubMed] [Google Scholar]
- 3. Evans SE, Bonczynski JJ, Broussard JD, Han E, Baer KE. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447–50. [DOI] [PubMed] [Google Scholar]
- 4. Carreras JK, Goldschmidt M, Lamb M, McLear RC, Drobatz KJ, Sorenmo KU. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000). J Vet Intern Med 2003; 17: 326–31. [DOI] [PubMed] [Google Scholar]
- 5. Lingard AE, Briscoe K, Beatty JA, et al. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg 2009; 11: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geigy CA, Dandrieux J, Miclard J, Kircher P, Howard J. Extranodal B-cell lymphoma in the urinary bladder with cytological evidence of concurrent involvement of the gall bladder in a cat. J Small Anim Pract 2010; 51: 280–87. [DOI] [PubMed] [Google Scholar]
- 7. Day MJ. Immunohistochemical characterization of the lesions of feline progressive lymphocytic cholangitis/cholangiohepatitis. J Comp Pathol 1998; 119: 135–47. [DOI] [PubMed] [Google Scholar]
- 8. Stein TJ, Pellin M, Steinberg H, Chun R. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc 2010; 46: 413–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biller DS, Kantrowitz B, Miyabayashi T. Ultrasonography of diffuse liver disease. A review. J Vet Intern Med 1992; 6: 71–76 [DOI] [PubMed] [Google Scholar]
- 10. Tamaki T, Katagiri S, Kanayama Y, et al. Helper T-cell lymphoma with marked plasmacytosis and polyclonal hypergammaglobulinemia. A case report. Cancer 1984; 53: 1590–95. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe S, Shimosato Y, Shimoyama M, et al. Adult T cell lymphoma with hypergammaglobulinemia. Cancer 1980; 46: 2472–83. [DOI] [PubMed] [Google Scholar]