Abstract
Infections of the skin or subcutis of the naso-ocular region develop through two mechanisms. Cases with lesions but without concomitant signs of nasal disease probably result from cat scratch injuries. Under certain circumstances, such lacerations result in the introduction of saprophytic microorganisms in such large numbers that host defence mechanisms are overwhelmed. This results in localised, variably invasive, disease in an otherwise immunocompetent host. An unpredictable range of organisms can give rise to such infections including a variety of fungal and bacterial genera. Causal organisms will likely vary from one geography to another as a result of differences in soil type and related environmental factors. Accordingly, procurement of appropriate tissue specimens for culture and susceptibility testing is essential to guide therapy, as these cases require medical and sometimes surgical intervention in order to effect a favourable outcome. In contrast, patients with naso-ocular lesions and concurrent signs of nasal disease have a different pathogenesis. Primary infection of the sinonasal region likely results from the inhalation of infectious propagules, with the infection subsequently penetrating overlying bones to invade the subcutaneous space. These lesions are typically the result of cryptococcosis or aspergillosis and must be distinguished from invasive nasal malignancies. An approach to the investigation and treatment of these patients is presented together with photographs of representative cases.
Infections affecting the skin and subcutis of the naso-ocular region are seen from time to time in feline practice. We have investigated in excess of 20 of these cases since 1987 and the likely pathogenesis of these infections has become apparent over the years. The key finding is that infections affecting this anatomic region develop through two different mechanisms.
Cases with infection of the naso-ocular region but without concurrent nasal signs
These cases likely result from contaminated cat-scratch injuries. Presumably the claw(s) of the feline perpetrator are contaminated with viable, potentially pathogenic, saprophytic organisms. These are inoculated in such large numbers that non-specific defence mechanisms (bleeding, inflammation, neutrophilic phagocytosis, lysozyme) of the victim are overwhelmed. This results in a localised, variably invasive infection of an otherwise immunocompetent host. A wide range of microorganisms can be cultured from such cases including a variety of bacteria and fungi normally residing in soil, rotting vegetation, humus or dirt. Lesions are typically on the bridge of the nose, but they may also occur more laterally or involve the nasal planum.
In our referral centre in eastern Australia, opportunistic pathogens isolated from such cases (a total of seven cats between 1987 and 2003) have comprised the bacteria Corynebacterium pseudotuberculosis (one case; Malik et al., 1996), Mycobacterium avium (one case; Malik et al., 1998) and Nocardia nova (one case), and the fungi Cryptococcus neoformans (one case; Malik et al., 1992), Exophiala jeanselmei (two cases; including Maliket al., 1994) and Paecilomyces lilacinus (one case) (Figs. 1–7). Mycobacterium avium (Stewart et al., 1993; Fig. 8), E. jeanselmei (Bostock et al., 1982; Nuttal et al., 1990), Alternaria species (McKayet al., 2001) and Sporothrix schenkii (Kennis et al., 1994) have been reported to produce similar lesions by others. Conceptually similar mycobacterial infections (Deykin et al., 1996; Fig. 9) or mycotic lesions (Miller et al., 1983) can develop on the cornea following cat scratch abrasions. The biological behaviour of these infections depends on the virulence of the pathogen, the initial dose of organisms inoculated, the subsequent hostresponse, the effect of subsequent medical and surgical interventions and the chronicity of the lesion.
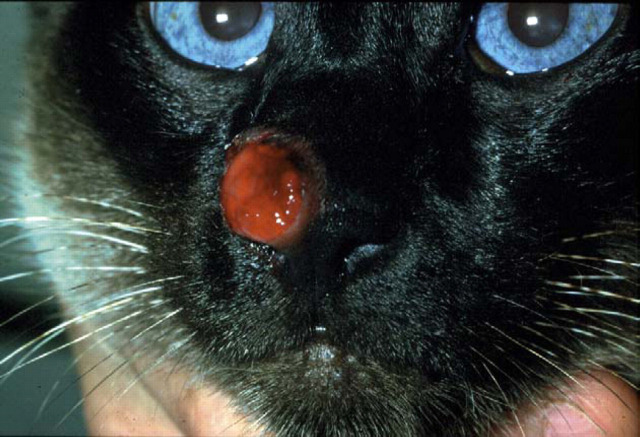
Figure 1.
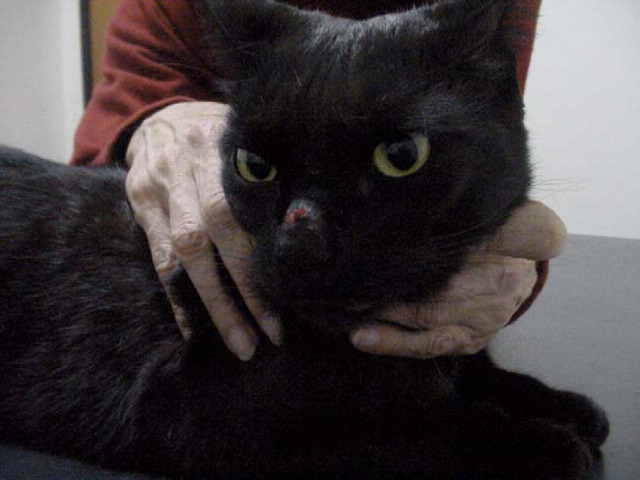
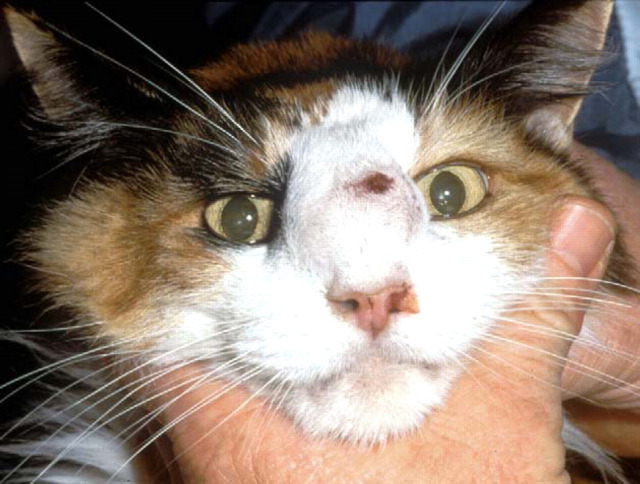
Siamese cat with localised cutaneous cryptococcosis (without nasal cavity involvement). This cat responded to fluconazole therapy (50 mg every 12 h) over several weeks, much more rapidly than is typical for cryptococcal rhinitis.
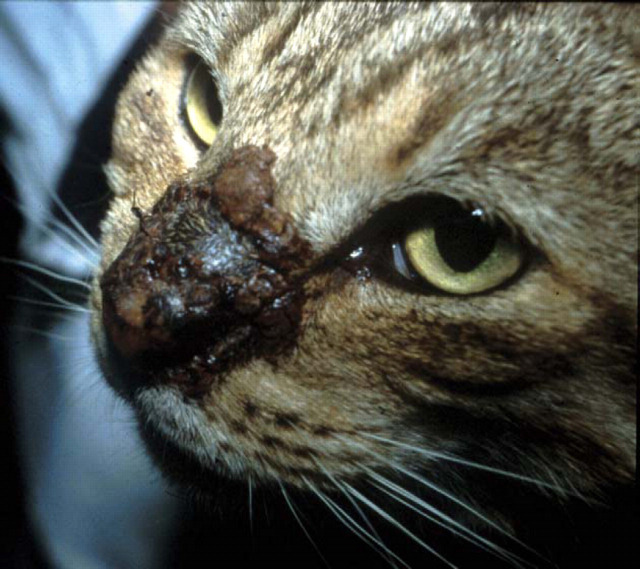
Figure 2.
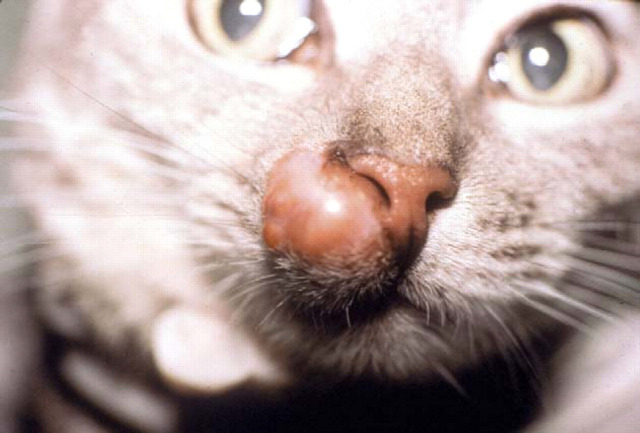
Localised phaeohyphomycosis of the nasal bridge of a domestic crossbred cat due to E. jeanselmei, a dematiaceous (pigmented) fungus. This cat failed to respond to itraconazole therapy and was euthanased because of emotional considerations. We would currently recommend cytoreductive surgery and biweekly subcutaneous amphotericin B infusions for a refractory case such as this, possibly in concert with an azole or terbinafine.
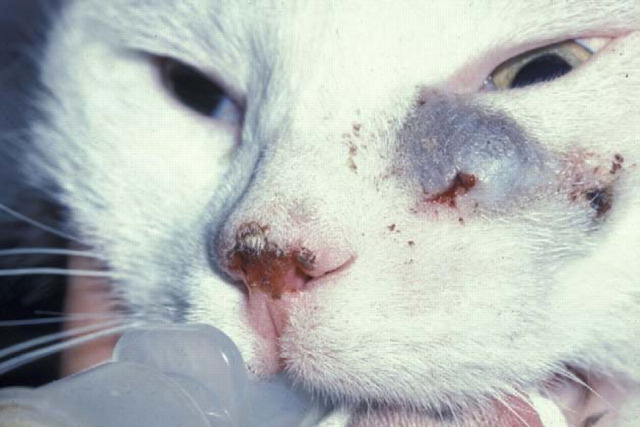
Figure 3.
Localised phaeohyphomycosis of the naso-ocular region due to E. jeanselmei. The pigment in the fungus has imparted a blue coloration to the lesion. Note also the early squamous cell carcinoma of the nasal planum. The cat was cured following surgical resection of the lesion and a long course of ketoconazole (50 mg once daily; Malik et al., 1994). We would currently recommend itraconazole, voriconazole or terbinafine rather thanketoconazole for primary therapy of an infection such as this, depending on susceptibility data and the response to therapy.
Figure 4.
Localised infection of the subcutis of thelateral nasal bridge of a domestic crossbred cat due to P. lilacinus. The patient was slowly improving on orally administered terbinafine (62.5 mg once daily) when it died as a result of vehicular trauma.
Figure 5.
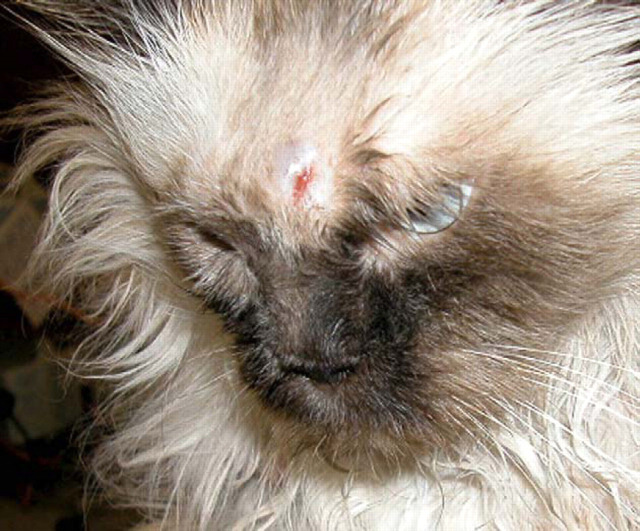
Localised pyogranulomatous infection of the skin and subcutis of the naso-ocular region due to Corynebacterium pseudotuberculosis infection (Malik et al., 1996). Two punctate draining sinus tracts, present in the centre of the lesion, are difficult to discern in this photograph. The infection resolved following cytoreductive surgery and a 3-week course of amoxycillin (100 mg every 12 h).
Figure 6.
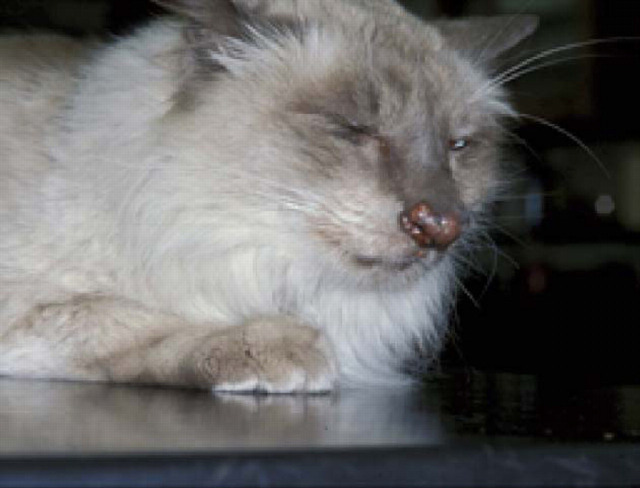
Localised Mycobacterium avium infection of the nasal bridge in a domestic crossbred cat with concurrent abdominal lymphoma. The two conditions were probably not related. The infection responded to marginal resection of the lesion and 6-weeks of oral clofazimine (25 mg once daily; Malik et al., 1998).
Figure 7.
Chronic localised N. nova infection of the nasal bridge of a FIV-positive domestic crossbred cat. This infection recurred twice following surgical resection of the lesion and appropriate long-term antimicrobial therapy (trimethoprim-sulphadiazine, doxycycline, clarithromycin) but never disseminated to other locations. The cat is currently receiving indefinite therapy with ampicillin (100 mg every 12 h).
Figure 8.
Localised nasal planum mass caused by Mycobacterium avium in a domestic crossbred cat. Thelesion was treated by surgical resection, but recurred 14 months later (Stewart et al., 1993). We would currently recommend treatment using a rifampicin (15 mg/kg daily) in concert with clarithromycin (62.5 mg twice daily) and possibly a third agent (such as clofazimine or doxycycline) for several months following cytoreductive surgery. (Photograph courtesy of Dr Stephen White.)
Figure 9.
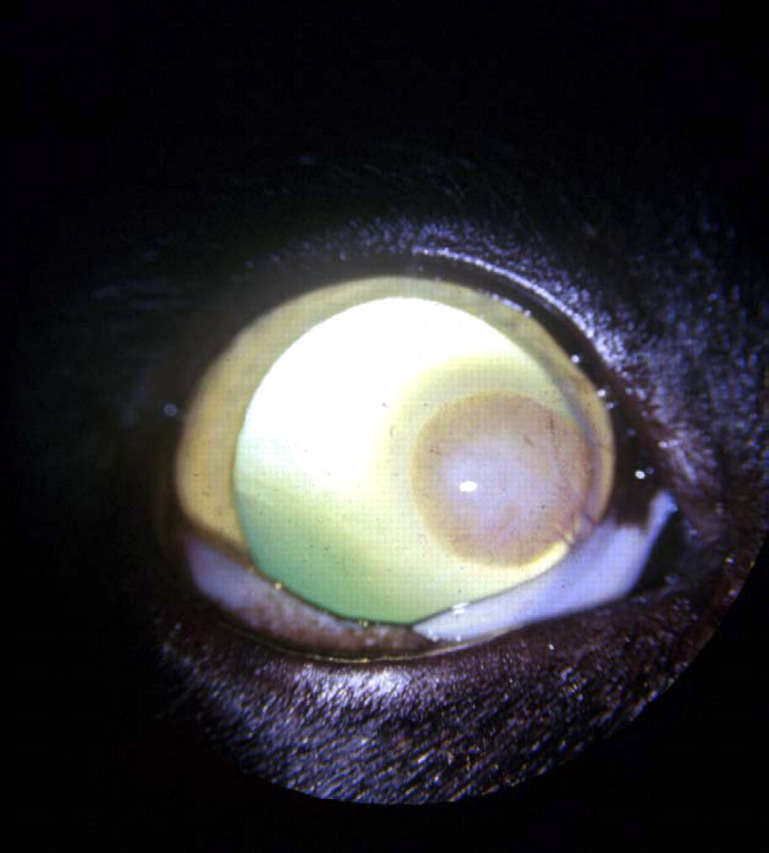
Focal keratitis due to Mycobacterium intracellulare, probably subsequent to a cat scratch injury to the cornea. The lesion resolved following keratectomy, although recurrence many months later necessitated a second surgical intervention (Deykin et al., 1996). (Photograph courtesy of Dr Anna Deykin.)
Although the precise location and appearance of lesions is quite variable from case to case, there is a relatively consistent anatomic distribution of lesions (Figs. 1–8) strongly suggestive of a cat-scratch aetiology. One differential diagnosis for florid disease at this anatomic site is insect-bite hypersensitivity (Fig. 10). However, the punctate nature of the primary lesions, frequent concurrent involvement of the ears and toes (Fig. 10inset) and characteristic eosinophilic histology distinguishes the underlying allergic basis (Mason and Evans, 1991).
Figure 10.
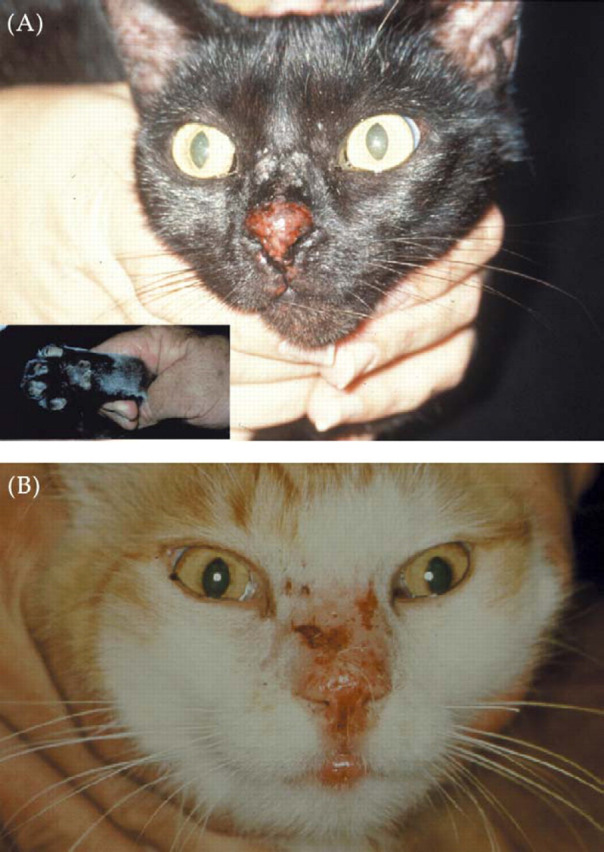
Mosquito-bite hypersensitivity of the nasal planum and nasal bridge of two different cats, (A) and (B). The primary lesion consists of multiple eosinophilic papules. Similar lesions are often present on the pinnae and/or toes (A, inset). These lesions improve rapidly without specific therapy once the inciting cause is removed, e.g., by hospitalising the cat in a mosquito-free clinic.
The main focus of this communication is to alert clinicians to the likely pathogenesis of infections in this anatomic region. Even though the saprophytic organisms isolated from these patients are generally considered to be of low virulence in most cases there is no predisposing immunodeficiency state. Thus, the infection merely reflects a breach in the integrity of normal cutaneous barriers and an especially heavy inoculum of infectious agent. Unfortunately these infections may be difficult to cure, as some causal strains are locally invasive and the region does not have an especially rich blood supply or mobile skin nearby to facilitate reconstructive surgical procedures. Furthermore, many of these saprophytic organisms demonstrate resistance to commonly used antimicrobials both in vitro and in vivo. Fungal pathogens can be especially problematic to treat.
One may speculate as to why cat scratch-related infections occur at this site rather than elsewhere. Firstly, it is a very common scratch injury site. Secondly, it is a location that cats cannot reach with their tongue. Scratch wounds elsewhere may be cleansed of potentially pathogenic microbes before an infection is established. Thirdly, the predilection area is sparsely covered by hair, so injuries from claws may penetrate more deeply into the subcutis compared to areas afforded the protection of a longer hair coat. Finally, growth of many saprophytic species may be favoured at the lower temperatures encountered at this anatomic prominence. It must be emphasised, however, that lesions attributable to a similar range of pathogenic saprophytes can develop on the body wall or distal extremities following contamination by soil or dirt from cat fight lacerations or abrasive injuries to the pads or interdigital spaces. Likewise, contaminated penetrating wounds of the caudoventral abdominal region often result in mycobacterial panniculitis of the inguinal fat pad (Malik et al., 2000).
Diagnostic work-up
Investigation of these cases typically involves obtaining representative material for cytology, histology and appropriate culture. Cytology and histology generally show pyogenic or pyogranulomatous inflammation and usually causal organisms can be visualised using special stains (DiffQuik, Gram, Ziehl-Neelsen, periodic acid Schiff, silver stains). A variety of staining techniques may be required, and in some cases an exhaustive search of smears or histological sections is needed to detect the infectious agents. Mycobacteria, fungi and Nocardia species may sometimes be detected in DiffQuik-stained smears because of a negative, rather than positive, staining reaction (Malik et al., 1998). The laboratory should be warned of the possibility of a fastidious saprophytic pathogen, as these organisms often have specific growth requirements (e.g., special culture media, reduced temperature of incubation, requirement for high carbon dioxide concentration, etc) and/or require several days or even weeks to become detectable as visible colonies in vitro. Ideally, a small portion of the biopsy specimen should also be frozen in case polymerase chain reaction techniques or additional culture studies are required at a later date (Hughes et al., 1999).
Many authorities would also recommend obtaining a minimum data base consisting of a complete blood count, serum biochemistry profile, urinalysis and possibly tests for feline immunodeficiency virus (FIV) and feline leukaemia virus before embarking on therapy. Concurrent metabolic problems such as renal insufficiency or diabetes mellitus may render the cat somewhat immunodeficient (e.g., case shown in Fig. 13), while the presence of liver or kidney dysfunction may affect the selection of the most appropriate antimicrobial agent(s) or limit doses that can be safely given (e.g., amphotericin B in cats with pre-existing renal insufficiency). A positive FIV-status does not preclude a satisfactory response to appropriate therapy, as it is generally impossible to discern the stage and impact of the FIV infection until after the cat has received appropriate therapy. Indeed, in the authors' experience, concurrent FIV infection is most often an epiphenomenon in this cohort of patients reflecting the cats' outdoor lifestyle and propensity to fight.
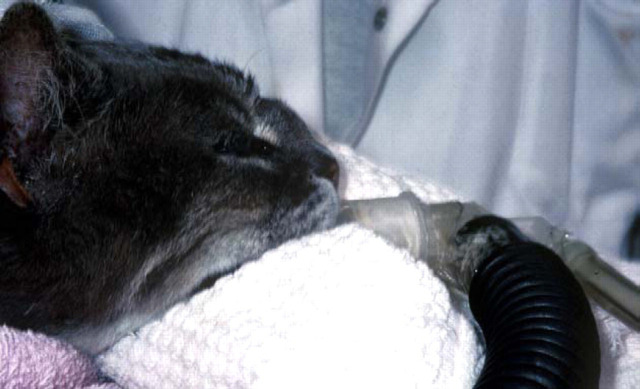
Figure 13.
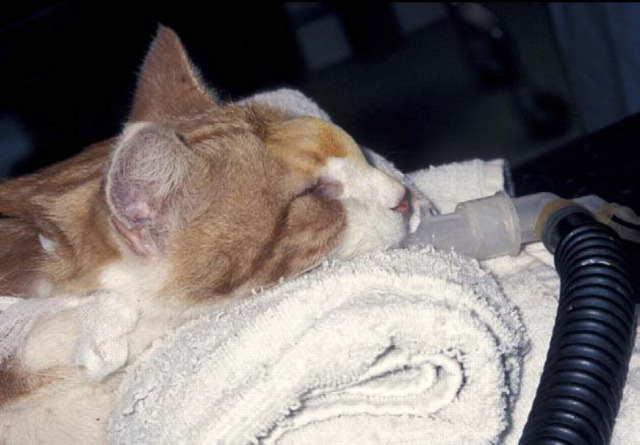
A diabetic Himalayan cat with invasive nasal aspergillosis due to Aspergillus flavus and secondary involvement of the nasal bridge/forehead. This cat developed hepatotoxicosis during itraconazole therapy (even at doses as low as 25 mg daily) but eventually responded to improved glycaemic control, twice weekly amphotericin B infusions (see Figure 11) and terbinafine (62.5 mg once daily).
Therapy
The treatment of these cases involves long courses of antimicrobials carefully selected on the basis of accurate species identification, in vitro susceptibility data (ideally from a specialist reference laboratory) and information from the human and veterinary literature available through electronic databases. Additionally, many of these patients require complete surgical excision of grossly infected tissues to assist the host's non-specific immune response.
Given the severity of the pathology in long-standing cases and the diffusion barriers resulting from tissue necrosis and fibroplasia, it is understandable that adequate levels of antimicrobials may not be achieved throughout all affected tissues. Thus, the best chance for a successful outcome for certain cats is to remove as much infected tissue as possible using en bloc resection following preliminary antimicrobial therapy which isextended into the intra-operative period and continued postoperatively. Residual microscopic foci of infection can then be targeted by the high concentrations of antibiotics achieved during and after surgery. This may be undertaken at the outset (for convenience and to minimise the number of procedures to which the patient must be subjected), or after a microbiological diagnosis has been made, e.g., by aspiration biopsy or resection of a small representative tissue specimen. In the latter scenario, it is possible to ensure that effective levels of appropriate antimicrobial agents are obtained in the peri-operative period. This may be advantageous if a major reconstructive procedure is required to remove an extensive lesion with clear margins. In some cases, surgery alone may be effective in resolving the infection (Bostock et al., 1982), although routine use of follow-up antimicrobials is strongly recommended to guard against the possibility of the surgical margin being seeded with infectious material.
Generally speaking, in the absence of complete surgical excision, these infections require treatment with long courses of antimicrobials, at least for several weeks, and typically for many months, depending on exactly which organism is involved and how much infected tissue can be safely resected at the outset. In some cases, combination therapy with two or more antimicrobial is superior to monotherapy with a single agent. Infections caused by organisms capable of intracellular survival (such as Mycobacteria species and Nocardia species) and fungi require the longest courses of therapy, and should ideally be treated not only until the lesion appears grossly normal, but for an additional period exceeding the lifespan of macrophages in the tissues, i.e., a further 2 months.
Prevention
Although the vast majority of cat scratch injuries to the face heal without any untoward sequelae, the possibility of opportunistic infections developing should be borne in mind when recommending treatment for feline patients with cat scratch injuries. Thorough cleansing of contaminated scratch wounds using saline or a dilute antiseptic (e.g., 0.05% chorhexidine) would seem prudent, followed by instillation of an ointment containing both antibacterial and antifungal agents (and without corticosteroids) and possibly a short course ofan antibiotic such as doxycycline monohydrate (5 mg/kg twice daily for 3 to 5 days). Although it is impossible to choose an agent with a spectrum sufficiently broad to cover all potentially pathological saprophytes, doxycycline has useful activity against many saprophytic mycobacteria, some Nocardia species, oropharyngeal organisms such as Pasteurella species and obligate anaerobes that may have been inoculated simultaneously via bite wounds (Love et al., 2000). Additionally, it is generally well tolerated, devoid of significant toxicity (e.g., retinotoxicity, nephrotoxicity) and available in conveniently sized tablet and paste formulations in Australia, New Zealand and South Africa (VibraVet; Pfizer Animal Health), which facilitates dosing and owner compliance. The use of a formulation containing the monohydrate salt is strongly recommended, as it is less irritant to the stomach and oesophagus than conventional human formulations using the hydrochloride salt.
Cases with naso-ocular infection and concurrent signs of nasal cavity disease
These cases are not the main focus of this communication, but are included for completeness. In these patients, the primary problem starts with infection of the nasal cavity by infectious propagules (typically spores) of saprophytic fungifiltered by the nasal passages. This may be facilitated by a pre-existing cause of nasal injury e.g. chronic post-viral rhinosinusitis. Involvement of the naso-ocular region develops subsequent to the infection spreading to the nasal planum or penetrating the overlying bones to reach the subcutis over the nasal bridge (Figs. 11–14). Most of these cases are attributable to cryptococcosis (Malik et al., 1992, 2001b; O'Brien et al., 2004) and in many of these patients the nasal planum is affected prominently (Fig. 14). We have also seen this type of disease progression with invasive aspergillosis (Fig. 13) and rhinitis caused by the termite mycoparasite Metarhizium anisopliae (Muir et al., 1998). Similar findings have also been reported in a cat with invasive bacterial rhinitis caused by an Actinomyces species (Yovich and Read, 1995).
Figure 11.

A domestic crossbred cat with invasivecryptococcal rhinitis due to Cryptococcus gattii with extensive secondary invasion of the subcutis of the nasal bridge. Most cats with this infection will respond to long courses (typically 6 to 12 months) of itraconazole (50 to 100 mg once daily) or fluconazole (33 to 50 mg twice daily). Refractory cases require twice weekly amphotericin B subcutaneous infusions (0.5 to 0.7 mg/kg in 350 ml 0.45% NaCl and 2.5% dextrose), ideally in concert with orally administered flucytosine (250 mg every 8 h), to effect a cure.
Figure 12.
A Burmese cat with invasive cryptococcal rhinitis due to Cryptococcus neoformans with early, focal invasion of the subcutis of the nasal bridge. This cat responded favourably to fluconazole (33 mg every 12 h) for several months.
Figure 14.
A domestic crossbred cat with long-standing cryptococcal rhinitis and prominent secondary involvement of the nasal planum. This cat improved markedly during preliminary itraconazole therapy but was subsequently lost to follow-up.
These cases can be investigated either by directing attention to the primary site of infection, i.e., the nasal cavity, by cytological examination of nasal swabs or washings (e.g., for budding, capsulate yeasts), serum cryptococcal antigen titre determinations, anterior/posterior rhinoscopy, cross-sectional imaging and biopsy of affected turbinates. Alternatively, needle aspirates or incisional biopsies can be obtained from the subcutaneous lesions and submitted for cytological and histological investigations and culture. Invasive mycotic rhinosinusitis is generally treated with one or a combination of antifungal agents administered systemically. Although monotherapy with azoles such as itraconazole or fluconazole is convenient for owners and effective in many patients, some cases do not respond and require amphotericin B as twice weekly subcutaneous infusions to effect a cure (Malik et al., 2001b). Unusual fungal infections may sometimes be more susceptible to other classes of antifungal agent such as terbinafine, or newer azoles such as voriconazole. Although topical therapy using clotrimazole ‘soaks’ has been used by others to treat cases such as this, the authors believe systemic therapy is preferable due to the invasive, granulomatous nature of the infection and the propensity in cats for bony erosion (including the cribriform plate) to occur in association with these infections.
The major differential diagnosis in these cases is invasive nasal neoplasia which can also breach the integrity of overlying nasal bones to invade the subcutaneous tissues of the nasal bridge and/or forehead. In our practice, lymphoma is the commonest sinonasal malignancy in the cat (Fig. 15), followed by adenocarcinoma (Fig. 16) and osteosarcoma, whereas solar-induced squamous cell carcinoma is the most common cancer of the nasal planum.
Figure 15.
A domestic crossbred cat with invasive nasal lymphoma and local invasion of the forehead. Although this particular cat did not enjoy a robust remission, many cats with nasal lymphoma can live for several years or are permanently cured by sequential multi-agent chemotherapy (Malik et al., 2001a).
Figure 16.
A ginger domestic crossbred cat with invasive nasal adenocarcinoma with local spread to the forehead/bridge of the nose.
Acknowledgments
Conversations with Dr Sue Shaw have helped to clarify our understanding of all the conditions described in this communication, while Dr George Wilkinson provided the first descriptions of many of the infections mentioned here.
References
- Bostock D.E., Cohoe P.J., Castellani P.J. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat, Journal of Comparative Pathology 92, 1982, 479–482. [DOI] [PubMed] [Google Scholar]
- Deykin A.R., Wigney D.I., Smith J.S., Young B.D. Corneal granuloma caused by Mycobacterium intracellulare in a cat, Australian Veterinary Practitioner 26, 1996, 23–26. [Google Scholar]
- Hughes M.S., Ball N.W., Love D.N., Canfield P.J., Wigney D.I., Dawson D., Davis P.E., Malik R. Disseminated Mycobacterium genavense infection in a FIV-positive cat, Journal of Feline Medicine & Surgery 1, 1999, 23–29. [DOI] [PubMed] [Google Scholar]
- Kennis R.A., Rosser E.J., Dunstan R.W. Difficult dermatologic diagnosis. [Sporotrichosis in a cat], Journal of the American Veterinary Medical Association 204, 1994, 51–52. [PubMed] [Google Scholar]
- Love D.N., Malik R., Norris J.M. Bacteriological warfare amongst cats: what have we learned about cat bite infections?, Veterinary Microbiology 74, 2000, 179–193. [DOI] [PubMed] [Google Scholar]
- McKay J.S., Cox C.L., Foster A.P. Cutaneous alternariosis in a cat, Journal of Small Animal Practice 42, 2001, 75–78. [DOI] [PubMed] [Google Scholar]
- Malik R., Gabor L., Martin P., Mitchell D.H., Dawson D.J. Subcutaneous granuloma caused by Mycobacterium avium complex infection in a cat, Australian Veterinary Journal 76, 1998, 604–607. [DOI] [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Dawson D., Martin P., Hunt G.B., Love D.N. Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings, Journal of Feline Medicine and Surgery 2, 2000, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Gabor L.J., Foster S.F., McCorkell B.E., Canfield P.J. Therapy for Australian cats with lymphosarcoma, Australian Veterinary Journal 79, 2001a, 808–817. [DOI] [PubMed] [Google Scholar]
- Malik R., Jacobs G.J., Love D.N. Cryptococcosis: new perspectives on etiology, pathogenesis, diagnosis, andclinical management. August J.R. Consultationsin Feline Internal Medicine 4, 2001b, Saunders: Philadelphia, 39–50. [Google Scholar]
- Malik R., Martin P., Davis P.E., Love D.N. Localised Corynebacterium pseudotuberculosis infection in a cat, Australian Veterinary Practitioner 26, 1996, 27–30. [Google Scholar]
- Malik R., Wigney D.I., Muir D.B. Phaeohyphomycosis due to Exophiala jeanselmei in a cat, Australian Veterinary Practitioner 24, 1994, 27–31. [Google Scholar]
- Malik R., Wigney D.I., Muir D.B., Gregory D.J., Love D.N. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole, Journal of Medical and Veterinary Mycology 30, 1992, 133–144. [DOI] [PubMed] [Google Scholar]
- Mason K.V., Evans A.G. Mosquito bite-caused eosinophilic dermatitis in cats, Journal of the American Veterinary Medical Association 198, 1991, 2086–2088. [PubMed] [Google Scholar]
- Miller D.M., Blue J.L., Winston S.M. Keratomycosis caused by Cladosporium species in a cat, Journal of the American Veterinary Medical Association 182, 1983, 1121–1122. [PubMed] [Google Scholar]
- Muir D., Martin P., Kendall K., Malik R. Invasive hyphomycotic rhinitis in a cat due to Metarhizium anisopliae , Journal of Medical and Veterinary Mycology 36, 1998, 51–54. [PubMed] [Google Scholar]
- Nuttal W., Woodgyer A., Butler S. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat, New Zealand Veterinary Journal 38, 1990, 123. [DOI] [PubMed] [Google Scholar]
- O'Brien C.R., Krockenberger M.B., Wigney D.I., Martin P., Malik R. A retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases, Medical Mycology 44, 2004, in press [DOI] [PubMed] [Google Scholar]
- Stewart L.J., White S.D., Kennedy F.A., Pavletic M.M. Cutaneous Mycobacterium avium infection in a cat, Veterinary Dermatology 4, 1993, 87–90. [Google Scholar]
- Yovich J.C., Read R.A. Nasal Actinomyces infection in a cat, Australian Veterinary Practitioner 25, 1995, 114–117. [Google Scholar]