Abstract
The present paper reports the clinical and neuropathological findings in two cats with a neuropathologically confirmed diagnosis of necrosis of the hippocampus and piriform lobe. The cats were presented because of acute onset of behavioural changes and complex partial seizures. The neurological examination suggested a forebrain lesion. The results of blood examination were within the normal range, and the cerebrospinal fluid (CSF) analysis and computed tomography (CT) scan in one cat did not show any abnormality. Despite therapy with diazepam (Valium®; Roche) there was deterioration of the clinical signs and the cats were euthanased. The neuropathological examination revealed hippocampal necrosis that included the piriform lobe.
Case report
Two domestic shorthair cats, which had never had the opportunity to meet stray cats outdoors, were referred because of the acute onset of behavioural changes and seizures. One cat (1) was a 7-year-old male, while the other (2) an 8-year-old neutered female.
In cat 1, the clinical signs started 3 days before examination and consisted of behavioural changes and seizures. The normally quiet and calm cat became hyperanxious and restless; nocturnal activity, ‘nightmares’ and continuous mewing were also noticed. The cat had clusters of complex partial seizures which lasted a few seconds and were invariably characterized by the following sequence: sitting down with extended neck, twitching of lips and eyelids, ear movements and profuse salivation. These signs were followed by masticatory movements and retching. Consciousness was slightly impaired, and return to normal condition occurred with no post-ictal phase. The frequency of the seizures progressively increased.
Cat 2 was presented because of behavioural changes. In the previous days her normal good character had suddenly turned into extremely aggressive behaviour. In response to any stimulus, the cat showed a defensive posture, piloerection, hissing and scratching paw movements at the owners. Twitching of the head and body as well as hypersalivation were considered atypical seizure activities. Clinical signs progressed to disorientation, bumping into objects, abnormal elimination, increased seizure frequency and enhanced aggressiveness.
On physical examination, the two cats were in good general condition. In cat 1, neurological examination showed behavioural changes consisting of mild disorientation and constant mewing. The cat had a normal gait, and postural and proprioceptive reactions were normal. The spinal reflexes were normal. Cranial nerve tests revealed a bilaterally decreased menace response. During the examination the cat had three short complex partial seizures identical to those previouslydescribed. Because of aggressiveness, cat 2 was sedated with medetomidine (Domitor®; Pfizer) and therefore a thorough neurological examination could not be performed. Neurological observation before sedation showed altered behaviour consisting in hyperaggressiveness, fear, piloerection and hissing. Posture was normal, while gait could not be appreciated. Menace response was bilaterally absent and pupils were midriatic and slowlyresponsive to light. General physical examination after medetomidine injection did not reveal any abnormality.
In both cases clinical signs localized a forebrain lesion.
Cell blood count and biochemical panel including electrolyte profile (AST, ALT, CK, AP, creatinine, urea, glucose, total bilirubin, fasting serum bile acids, triglycerides, total cholesterol, GGT, total proteins, albumin, albumin/globulins, calcium, phosphorus, sodium, potassium, chloride) did not show significant abnormalities in both cases. Blood ammonia was checked on cat 1 and was within the normal range. Serology for toxoplasmosis (Feline IgG antibody kit®; Medical Service) was done in cat 2 and was negative. FeLV/FIV tests (Snap Combo Plus®; Idexx) were negative for both cats. Cerebrospinal fluid (CSF) analysis including Pandy reaction, total cell count and cytomorphology as well as computed tomography (CT) of the skull, done before and after injection of contrast medium (Iodixanole-Visipaque®320, 2 ml/kg IV) were performed on cat 1. Results were normal.
Symptomatic therapy consisted of diazepam (Valium®; Roche: 1 mg/kg per os BID), which reduced seizures in both cases, but no improvement was observed in the cats' mental status. Cat 1 was euthanased 2 weeks after physical examination because of continuous mewing and restlessness. Cat 2 survived 4 months: seizure frequency was reduced, but she did not show any improvement of behavioural changes and other neurological deficits. Unfortunately, the owner very soon declined further investigations and therapeutic attempts. Euthanasia was finally elected because of the severe difficulty to manage the cat.
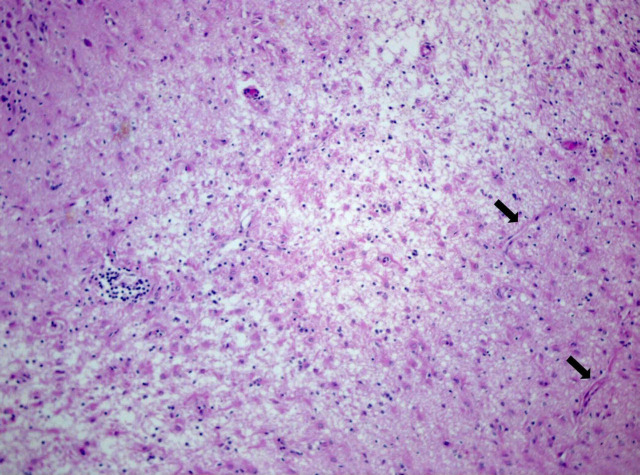
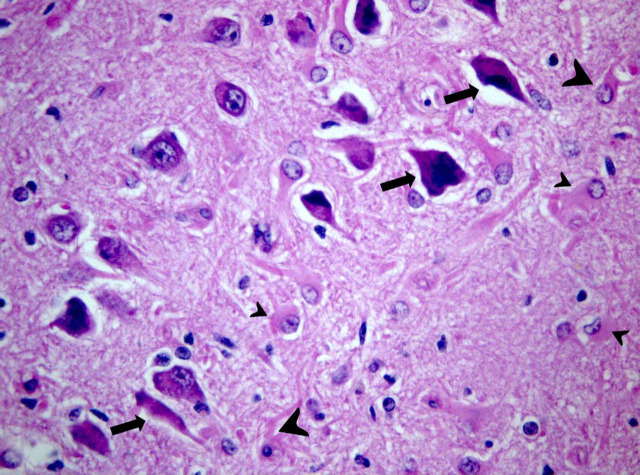
General pathology was not performed. Neuropathology of the brain showed the same lesions in the two cats. Macroscopically, no brain abnormalities were observed. Histopathological examination revealed bilateral pathologic changes selectively involving the hippocampus and piriform lobe. There was neuronal loss and necrosis in the pyramidal cell layer (Figs. 1 and 2) and at the level of the small neurons of the piriform lobe. Lesions were characterized also by the presence of hypertrophic astrocytes (gemistocytes) and fibrillary astrocytes (sclerosis) (Fig. 2). Most neurons were shrunken and had pyknotic or lytic nuclei surrounded by brightly eosinophilic cytoplasm (Fig. 2). In addition, in both cases capillary proliferation and swelling of the endothelial nuclei were prominent. In cat 2, scattered mononuclear perivascular infiltration wasnoticed at the level of hippocampus. Neuronal loss and presence of fibrillary astrocytes were slightly more represented on cat 2, probably reflecting the more chronic course of the disease.
Figure 1.
Cat brain, hippocampus, severe necrosis: loose texture, loss of neurons, capillary proliferation (arrows), mononuclear perivascular infiltration. Hematoxylin and Eosin, 4×.
Figure 2.
Cat brain, hippocampus: degeneration and loss of pyramidal cells; remaining neurons shrunken with pycnotic nuclei (arrows); swollen (small arrow heads) and fibrillary (large arrow heads) astrocytes. Hematoxylin and Eosin, 40×.
So far, to the authors' knowledge, this disease has been observed only in Switzerland. In our cats, clinical signs and neuropathological findings arealmost identical with those described in this report in 38 Swiss cats (Fatzer et al., 2000). The pathophysiology of seizures in cats is hardly understood and the presence of idiopathic epilepsy in this species is still controversial. At present, some authors believe that seizures in cats usually are due to structural brain diseases (Quesnel and Parent, 1997; Russo, 1991). In a recent study involving a large cat population, beside idiopathic epilepsy (21%) the most common causes of seizures were necrosis of hippocampus and piriform lobe (25%), metabolic/toxic encephalopathies (17%), inflammation/infection of the central nervous system (14%), and neoplastic disorders (8%)(Cizinauskas et al., 2002). Quesnel and Parent (1997)found non-suppurative meningoencephalitis and feline ischemic encephalopathy (FIE) to be the most frequent causes of seizures in their cats population. Our results give evidence that feline hippocampal necrosis, though rarely reported so far, has to be included among the possible causes of seizures in cats.
In our cats, metabolic, toxic and inflammatory aetiologies were considered among the possible differential diagnoses. In our experience, it was possible to exclude a toxic cause since the cats had no possibility to have contacts with toxic substances in their apartments. Lead poisoning was excluded mainly on the basis of environmentalfactors, absence of gastrointestinal signs and a normal CBC, and was confirmed by the lack of diffuse histopathological brain changes such as spongiosis. Hepatic encephalopathy was ruled out not only by clinicopathological data but also by the lack of Alzheimer type II astrocytes and diffuse polymicrocavitation on histopathological specimens. The CSF examination ruled out inflammatory aetiologies on cat no. 1. In feline polioencephalomyelitis, some clinical signs may fit with the signs reported in our cats. Nevertheless, ataxia and other gait abnormalities are more commonly reported for this latter disease and neuropathology shows a more disseminated polioencephalomyelitis (Vandevelde and Braund, 1979). In both cats neuropathology definitively excluded an inflammatory aetiology.
Similarly to what has been reported by Fatzeret al. (2000), neuropathological findings in our cats showed bilateral degenerative lesions confined to the hippocampus and the piriform lobe, classified as necrosis. There are some similarities between these findings and FIE. Nevertheless, FIE is characterized by a peracute onset of non-progressive and asymmetrical cerebral signs. In both diseases the pathogenesis of the lesions is not known. FIE appears to be a vascular disorder, and current pathogenetic theories include ischemia due to vasospasm from haemorrhage, e.g. caused by parasitic migration (larvae of Cuterebra sp.; Summerset al., 1995; Williams et al., 1998). Examination of the heart for cardiomyopathy has not confirmed a causal relationship (Summers et al., 1995).
In domestic animals, the relationship between seizures and brain injury has not been clarified yet; in human medicine it is accepted that severe seizures may cause ischemic neuronal necrosis in certain areas, foremost in hippocampus, amygdala, and neocortex (Summers et al., 1995). In a colony of beagles involved in a life-time study and not receiving any therapy against seizures, neuropathologic ischemic changes in the brain were found in 48.5% out of 68 dogs (Montgomery and Lee, 1983). In an old report, bilateral hippocampal necrosis has been described in a poodle that had had seizures for several weeks (Andersson and Olsson, 1959). In our cats, assuming that the lesions found in the hippocampus and piriform lobe were the cause rather than the result of seizure activity, the aetiology of the disorder remains unknown. In the Swiss study, metabolic or toxic disorders were included among the aetiological suspicions because the cats came from a restricted area and small clusters of cases were recorded in two villages (Fatzer et al., 2000). However, the authors stress that an endogenous excitotoxin is more likely to be involved than any exogenous toxin. Our report demonstrates that necrosis of the hippocampus is not affecting only cats from a definite geographic region. Further studies are required to clarify the aetiology of the disease. Nevertheless, authors emphasize that feline hippocampal necrosis has to be included among the possible aetiologies in the differential diagnosis of seizures in cats.
References
- Andersson B., Olsson S.-E. Epilepsy in a dog with extensive bilateral damage to the hippocampus, Acta Veterinaria Scandinavia 1, 1959, 98–104. [Google Scholar]
- Cizinauskas S., Fatzer R., Schenkel M., Gandini G., Jaggy A. Can idiopathic epilepsy be confirmed in cats?, Proc.15th ESVN-ECVN Annual Symposium—26–29th September 2002—Philadelphia (USA), 2002.
- Fatzer R., Gandini G., Jaggy A., Doherr M., Vandevelde M. Necrosis of hippocampus and piriform lobe in 38 domestic cats with seizures: a retrospective study on clinical and pathologic findings, Journal of Veterinary Internal Medicine 14, 2000, 100–104. [DOI] [PubMed] [Google Scholar]
- Montgomery D.L., Lee A.C. Brain damage in the epileptic beagle dog, Veterinary Pathology 20, 1983, 160–169. [DOI] [PubMed] [Google Scholar]
- Quesnel A.D., Parent J.M. Diagnostic approach and medical treatment of seizures disorders. August J.R. Consultation in Feline Internal Medicine 3, 1997, W.B. Saunders Company: Philadelphia, USA, 389–405. [Google Scholar]
- Russo M.E. Seizures. August J.R. Consultation in Feline Internal Medicine 1, 1991, W.B. Saunders Company: Philadelphia, USA, 523–526. [Google Scholar]
- Summers B.A., Cummings J.F., de Lahunta A. Veterinary Neuropathology, first ed, 1995, Mosby–Year Book: St Louis, MO, 242–246. [Google Scholar]
- Vandevelde M., Braund K.G. Polioencephalomyelitis in cats, Veterinary Pathology 16, 1979, 420–427. [DOI] [PubMed] [Google Scholar]
- Williams K.J., Summers B.A., de Lahunta A. Cerebrospinal cuterebriasis in cats and its association with feline ischemic encephalopathy, Veterinary Pathology 35, 1998, 330–343. [DOI] [PubMed] [Google Scholar]