Abstract
Proteinuria was assessed in 100 randomly selected sick cats and 22 healthy cats by means of the urine protein:creatinine ratio, a traditional urine ‘dipstick’ and a commercial ELISA-based dipstick designed to detect microalbuminuria (MA) semi-quantitatively. In addition the repeatability and reproducibility of the MA test was assessed by comparing results of five replicate tests of 26 urine samples, interpreted by two different readers. Discrepancies existed in the replicate test result in 23 and 27% of the samples examined by reader 1 and 2, respectively, and on several occasions this discrepancy was between whether the sample was ‘positive’ or ‘negative’ for MA. The inter-reader agreement was good (κ=0.75), but again discrepancies were noted and part of the reason for these problems appeared to be the necessary subjectivity in the interpretation of colour changes when reading test results. Proteinuria was significantly (P≤0.014) more prevalent in the sick than the healthy cats with 36 and 9%, respectively, having detectable MA, 34 and 5%, respectively, having a urine protein to creatinine (UPC) ratio >0.5, and 84 and 9%, respectively, having positive urine protein dipstick analysis. There was a moderate significant correlation between UPC ratio and MA concentrations (r s=0.68, P<0.0001). While 13/87 cats with a UPC ratio ≤0.5 had positive MA results, 10/84 cats with negative MA results had a UPC ratio >0.5, and none of these had evidence of lower urinary tract disease. This study confirmed that MA and proteinuria are commonly seen in cats with a variety of diseases, but they are not necessarily both elevated, and the UPC ratio can be elevated without an increase in MA results. Furthermore, some repeatability problems were demonstrated with the semi-quantitative MA test. These findings demonstrate that the semi-quantitative MA test should not be relied on as the sole determinant of proteinuria.
Urine produced by a healthy kidney contains little protein, as these molecules are largely retained at the level of the glomerulus because of their size and/or charge. Small proteins or amino acids that do pass through the normal glomerulus are mostly reabsorbed by renal tubules, or degraded by tubular epithelial cells. Persistently increased proteinuria is an abnormal finding, and in the absence of lower urinary tract disease may reflect either altered glomerular permeability, reduced tubular re-absorption; increased secretion of proteins from tubular epithelial cells or, less commonly, overflow from the circulation if there is raised serum levels of low-molecular weight proteins (Kashif et al 2003). Alterations in glomerular permeability may occur in primary glomerular disease (amyloidosis, immune-mediated glomerulonephritis, hereditary glomerulonephropathies) or as a result of glomerular capillary hypertension, or endothelial cell dysfunction (Syme and Elliot 2006).
Proteinuria (in the absence of lower urinary tract disease) is, therefore, usually a marker of renal damage or dysfunction (glomerular or tubular), although this may result from either primary or secondary renal disease. In humans, most cases of renal failure occur secondary to diabetes mellitus or essential hypertension, and there is considerable interest in the potential role of proteinuria as a cause, as well as an indicator, of progressive renal damage (Venkat 2004). Proteinuria may be measured quantitatively over 24 h, or more simply by a random urine protein to creatinine (UPC) ratio (the two showing a close correlation in both humans and cats – Monroe et al 1989; Adams et al 1992). However, evidence in humans suggests that early increases in albuminuria (microalbuminuria, MA) reflect glomerular damage undetectable by the traditional UPC ratio, and may serve as both a negative prognostic marker and also potentially contribute to renal damage in cases of renal failure (Stankeviciute et al 1998, Kashif et al 2003, Venkat 2004), although MA may also be found in conditions affecting glomerular function other than renal failure. In man, MA is defined as an albumin to creatinine ratio (expressed as mg albumin/g creatinine) in the range 30–300 (Kashif et al 2003), which equates to urinary albumin loss of 20–200 μg/min, or 20–200 μg/ml, assuming a standard urine production rate of 1 ml/min. Smaller losses are within normal ranges and greater losses are detectable by the UPC ratio (overt proteinuria). Monitoring for MA is encouraged in patients at risk of renal disease in order to allow early therapeutic intervention.
In the veterinary field, significant renal-origin proteinuria has been demonstrated in association with a variety of underlying conditions in dogs (Nichols 1994, Cook and Cowgill 1996), and there is evidence to suggest that in cases of canine chronic renal failure, proteinuria is a negative prognostic indicator (Jacob et al 2005), with a number of recent studies also evaluating MA in dogs (Jensen et al 2001, Pressler et al 2001, Vaden et al 2001, Lees et al 2002, Whittemore et al 2003).
In the cat, a UPC ratio >0.4–0.5 is accepted as abnormal (Syme and Elliott 2003b, Lees et al 2005). Proteinuria has been shown to occur in cats with immune-mediated glomerulonephritis (Nash et al 1979, Bishop et al 1991), multiple myeloma (Patel et al 2005), acute renal failure (Thrall et al 1984, Rimbeiha et al 2004), chronic renal failure (Adams et al 1992, Finco et al 1998, Syme and Elliott 2003a, Mather et al 2004), hyperthyroidism (Syme and Elliott 2001), acute pancreatitis (Hines et al 1996), drug reactions (Rottman et al 1991) and hypertension (Syme and Elliott 2003a). There is also preliminary evidence that higher levels of proteinuria correlate with reduced survival times in cats with or without renal failure (Gunn-Moore 2003, Syme and Elliot, 2003b, Walker et al 2004). Much less is known about the occurrence and significance of MA in the cat though. A commercial semi-quantitative ELISA-based test for the measurement of feline MA is available, but normal urine albumin concentrations have not been well established for feline patients. One brief report suggests that healthy cats may have an age-related increase in urinary albumin concentrations, and that cats with a wide range of medical conditions may also have elevated MA measurements (Langston 2004). However, the fact that tubulointerstitial disease (rather than glomerular disease) tends to dominate in feline renal failure (Minkus et al 1994) raises important doubts over any assumptions that the interpretation of MA in cats will necessarily be the same as in humans.
The aim of this study was three-fold. Firstly to evaluate the reliability of a commercial feline-specific urine MA dipstick (Feline E.R.D.-HealthScreen Urine Test, Heska) in a clinical setting, by assessing intra- and inter-reader variation and by evaluating changes in detected MA over time with urine sample storage; secondly, to provide information on the prevalence and degree of MA in both healthy and diseased cats from the UK; and thirdly, to assess the association between MA concentrations and the UPC ratio in healthy and sick cats.
Materials and Methods
Urine samples were collected from 100 randomly selected sick cats presented for investigations at the Small Animal Clinic at the Animal Health Trust – cats were selected on the basis that urinalysis was being undertaken as part of their clinical investigations and that sufficient urine was available for the additional investigations needed for this study. Urine was collected either by cystocentesis (most samples), from voided samples in a clean litter tray containing non-absorbent litter, or by urethral catheterisation, where this procedure was appropriate during a patient's investigations. Urine samples were also collected from 22 cats belonging to staff from the AHT or the University of Bristol, with no clinical signs of disease, which were undergoing routine health checks. Each of the 122 cats underwent a full clinical examination and where possible (58 cats) a measurement of systolic blood pressure, by Doppler ultrasonic sphygmomanometry.
Urine samples from all cats were assessed for specific gravity using a refractometer, and for routine chemistry using a commercial ‘dipstick’ (Combur9 Test; Roche). The protein pad of the dipstick contains buffered tetrabromophenol blue which acts as an indicator dye in the presence of albumin, but is much less sensitive to the presence of other proteins. Urine sediment cytological analysis was performed using standard laboratory techniques. The UPC ratio was calculated from measurements on a commercial laboratory analyser, the Konelab 20i chemistry analyser, utilising the Konelab CSF/U total protein reagent (pyrogallol red dye-binding measurement). Pyrogallol red binds to amino acid groups of proteins within urine, causing a colour change which is then detected and compared to standard (control) protein concentrations by the analyser. This assay measures total protein and is not specific for albumin. Semi-quantitative assessment of MA was made using the Feline E.R.D.-HealthScreen Urine Test (Heska), following the manufacturer's instructions. This test is designed to detect low albumin concentrations using a rapid immunoassay method. A minimum volume of 2 ml of urine is collected and the specific gravity (SG) assessed. The sample is then diluted to a standard SG of between 1.010 and 1.020 (or tested undiluted if the SG is <1.020), and the test device is placed in the sample. Interpretation of the results requires comparison of the intensity of two coloured bands that develop in the window of the test device, and readings correspond to a qualitative measurement of the degree of MA (see Fig 1). The instructions state that the result may be negative (lower coloured band appears darker than the top band), low positive (bands are of equal intensity), medium positive (top band is slightly darker than the lower band), high positive (top band much darker) or very high positive (lower band absent or very faint). Negative results indicate a lack of detectable MA (the lower limit of detection claimed to be approximately 1 mg/dl or 10 μg/ml), positive results indicate detectable MA, although no figures are given for the level of MA corresponding to ‘low’, ‘medium’, ‘high’ and ‘very high’ positive readings. To the authors' knowledge, the accuracy and sensitivity of this test has not been independently reported, but the manufacturer claims >99% specificity and a sensitivity of >95% once urine albumin concentration reaches >2 mg/dl (20 μg/ml) (source: www.Heska.com).
Fig 1.
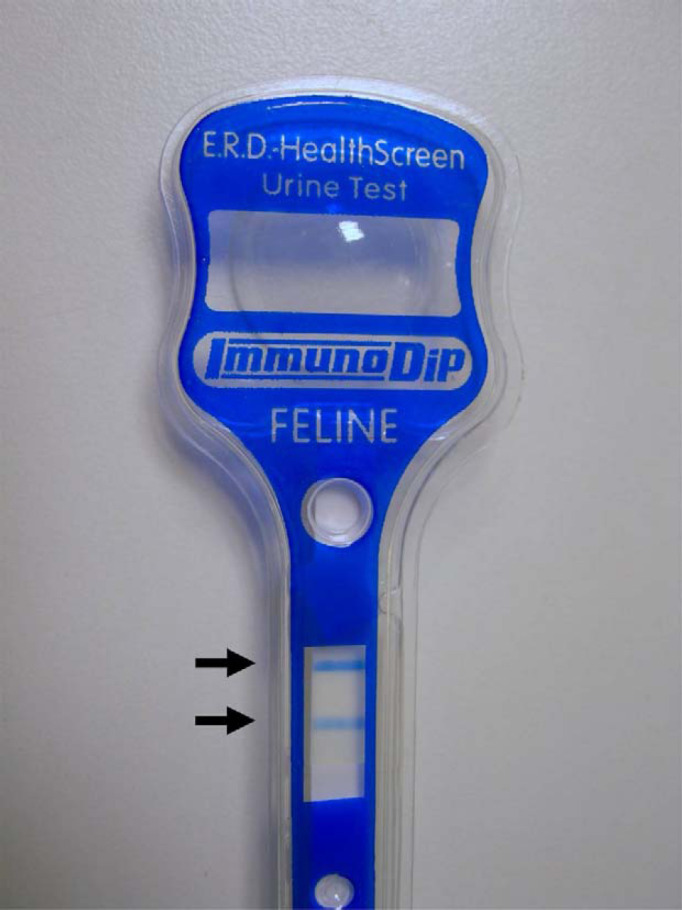
Appearance of the commercial dipstick for semi-quantitative microalbuminuria determination. The relative colour change of the two bands (dark arrows) is used to determine the quantity of albumin present.
Further clinical investigations were completed as necessary on an individual basis in the 100 sick cats. These cats were then assigned to one of eight categories of disease based on their major clinical presentation and diagnosis as follows: endocrine, gastrointestinal, neoplastic, lower urinary tract disease (LUTD), haematological, renal insufficiency, glomerulonephritis, and miscellaneous disease. All cats with cytological evidence of lower urinary tract inflammation were placed into the LUTD group.
To evaluate the reliability of the MA test kits, where sufficient urine was available from diseased and control cats (n=26) additional assays were performed. In 10 of these samples small amounts of pooled feline serum were added to ensure proteinuria spanning a wide range of values was present. Each of the 26 urine samples was split into six aliquots, one was used for measurement of the UPC ratio and five were tested for MA concentration. The results were read by two independent people who were blinded to each other's results and to the protein concentrations in the samples.
Finally, to evaluate the effect of storage on the stability of the test samples, aliquots of the 26 plain and serum-supplemented samples were stored at fridge (4°C) and room temperature, and re-tested at 6, 24 and 48 h after collection.
Statistical Analysis
For the purposes of statistical analysis, the MA results obtained in this study were converted to numerical data with a value of 0 being assigned to a negative result, 1 to a ‘low positive’, 2 to a ‘medium positive’, 3 to a ‘high positive’ and 4 to a ‘very high positive’.
The correlation between variables was assessed using the Spearman Rank correlation coefficient or simple linear regression (Pearson's correlation coefficient) as appropriate. Results of data sets were compared using the Mann–Whitney U test or the Kruskall–Wallis (with post-test comparisons between individual data sets where overall significance was achieved) as appropriate. Inter-reader agreement for the MA test was assessed using the Kappa statistic. Proportions (2× κ contingency tables) were compared using the χ2 or Fisher's exact test as appropriate. A P value of <0.05 was considered significant.
Results
Intra- and Inter-reader Variability
The results of the intra-reader variability using 26 urine samples are shown in Table 1. From these data it can be seen that the 26 samples yielded the same result for every repeat analysis on 19 (73%) occasions for reader 1, and on 20 (77%) occasions for reader 2. Five of the 26 samples yielded inconsistent results from both readers (samples 6, 7, 12, 16 and 22). On every occasion when there was a discrepancy within a set of five repeat samples, the variation observed was of one concentration gap.
Table 1.
Data from intra-reader repeatability study with two different readers
| Sample | UPC ratio | Microalbuminuria result, operator 1 | Agreement | Microalbuminuria result, operator 2 | Agreement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||||
| 1 | 0.09 | 0 | 0 | 0 | 0 | 0 | Yes | 0 | 0 | 0 | 0 | 0 | Yes |
| 2 | 0.13 | 0 | 1 | 1 | 0 | 0 | No | 0 | 0 | 0 | 0 | 0 | Yes |
| 3 | 0.16 | 0 | 0 | 0 | 0 | 0 | Yes | 0 | 0 | 0 | 0 | 0 | Yes |
| 4 | 0.19 | 0 | 0 | 0 | 0 | 0 | Yes | 0 | 0 | 0 | 0 | 0 | Yes |
| 5 | 0.21 | 0 | 0 | 0 | 0 | 0 | Yes | 0 | 0 | 0 | 0 | 0 | Yes |
| 6 | 0.21 | 1 | 1 | 1 | 2 | 2 | No | 1 | 1 | 1 | 2 | 1 | No |
| 7 | 0.25 | 1 | 1 | 0 | 0 | 1 | No | 1 | 1 | 0 | 0 | 0 | No |
| 8 | 0.27 | 0 | 0 | 0 | 0 | 0 | Yes | 0 | 0 | 0 | 0 | 0 | Yes |
| 9 | 0.29 | 2 | 2 | 2 | 2 | 2 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 10 | 0.3 | 2 | 2 | 2 | 2 | 2 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 11 | 0.3 | 2 | 2 | 2 | 2 | 2 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 12 | 0.44 | 1 | 1 | 1 | 0 | 1 | No | 1 | 1 | 0 | 0 | 0 | No |
| 13 | 0.47 | 3 | 3 | 3 | 3 | 3 | Yes | 3 | 3 | 3 | 3 | 3 | Yes |
| 14 | 0.53 | 3 | 3 | 3 | 3 | 3 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 15 | 0.57 | 1 | 1 | 1 | 1 | 1 | Yes | 1 | 1 | 1 | 1 | 1 | Yes |
| 16 | 0.73 | 1 | 1 | 2 | 1 | 1 | No | 1 | 2 | 2 | 2 | 2 | No |
| 17 | 0.79 | 3 | 3 | 3 | 3 | 3 | Yes | 3 | 3 | 3 | 3 | 3 | Yes |
| 18 | 0.99 | 2 | 2 | 2 | 2 | 2 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 19 | 1.25 | 2 | 2 | 2 | 2 | 2 | Yes | 2 | 2 | 2 | 2 | 2 | Yes |
| 20 | 1.32 | 3 | 3 | 3 | 3 | 3 | Yes | 3 | 3 | 3 | 3 | 3 | Yes |
| 21 | 1.5 | 3 | 3 | 3 | 3 | 3 | Yes | 3 | 3 | 3 | 3 | 3 | Yes |
| 22 | 1.56 | 2 | 3 | 2 | 2 | 2 | No | 1 | 2 | 2 | 2 | 2 | No |
| 23 | 1.94 | 3 | 3 | 3 | 3 | 3 | Yes | 3 | 3 | 3 | 3 | 3 | Yes |
| 24 | 3.41 | 4 | 3 | 3 | 3 | 3 | No | 3 | 3 | 3 | 3 | 3 | Yes |
| 25 | 4.93 | 4 | 4 | 4 | 4 | 4 | Yes | 4 | 4 | 4 | 4 | 4 | Yes |
| 26 | 84.51 | 4 | 4 | 4 | 4 | 4 | Yes | 3 | 4 | 3 | 3 | 4 | No |
| Non-agreement | 7 (26.9%) | 6 (23.1%) | |||||||||||
UPC=urine protein to creatinine.
To evaluate inter-reader variability, the median of the five repeat readings for each of the two readers was used. These data demonstrated significant (P<0.0001) and substantial (κ=0.748) agreement between the two readers with 21 of the 26 (81%) samples evaluated having identical readings. Where discrepancies were observed between readers, the difference was of one concentration gap. On two occasions readings differed between ‘negative’ and ‘low positive’ MA values; on one occasion between ‘low’ and ‘medium’ positives; on one occasion between ‘medium’ and ‘high’ positives, and on one occasion between ‘high’ and ‘very high’ positives.
Stability of MA Readings Over Time
Overall, there was no significant difference in the results of the 26 urine samples obtained from the analysis after storage for different lengths of time. Of those stored at room temperature, six samples (21%) had inconsistent results at different time points; on each occasion the discrepancy was one concentration gap different from the initial sample. Similarly of those stored at 4°C, there were seven (24%) that had inconsistent results, again each time the discrepancy was one concentration gap different from the initial sample. The proportion of samples that yielded discrepancies after storage at both room temperature and 4°C was not significantly different from the proportions that yielded discrepancies during intra-reader variability assessment.
Clinical Investigations
Of the 122 cats included in this study, 53 were females (one entire) and 69 were males (one entire). There was no significant difference in UPC ratios or MA concentrations between male and female cats. The ages of the cats varied between one and 17 years for the diseased cats (mean 8.4, median 9) and between one and 13 years for the healthy cats (mean 6.0, median 5). There was no significant correlation between the age and UPC ratio (r2=0.002), however, there was a poor but significant (P=0.002) correlation between age and MA concentration (rs=0.283).
Blood pressure data were available for 58 of the cats and in these the systolic pressure (measured by Doppler ultrasonic sphygmomanometry) varied between 85 and 245 mmHg (mean 144, median 140) with 11 cats having pressures in excess of 160 mmHg. There was no significant correlation between systolic blood pressure and either UPC ratio or MA concentration.
UPC Ratio and MA Concentration
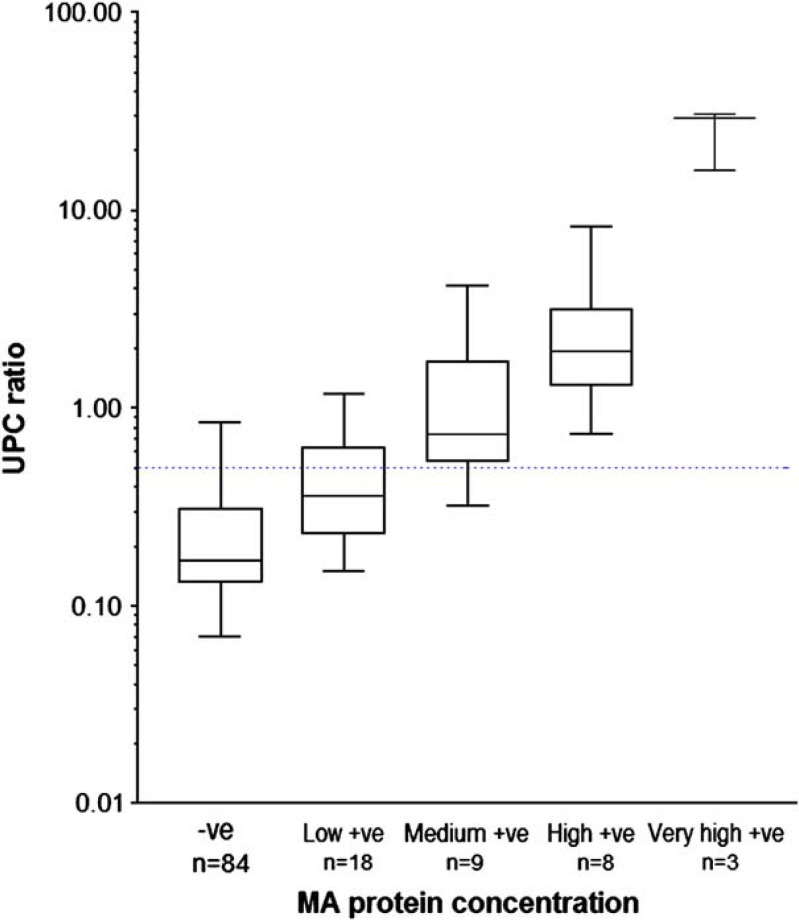
There was a moderate and significant correlation between UPC and the MA concentrations (rs=0.679, P<0.0001). Overall 84 cats had a negative MA result, 18 had a low positive result, nine a medium positive, eight a high positive and three a very high positive result. Figure 2 shows classical box and whisker plots of the UPC ratios in the different categories of MA. Further analysis of these data showed that the median UPC values in these five groups were significantly different from each other (P<0.0001), with post-test analysis demonstrating significant differences between all pairs of categories with the exception of the three highest MA categories (‘medium’, ‘high’ and ‘very high’ MA values).
Fig 2.
Box and whisker plots of urine protein to creatinine ratios found in the urine of cats with different quantities of microalbuminuria. Blue dotted line=UPC of 0.5.
UPC Ratio, MA and Proteinuria Assessed by Urine Dipstick
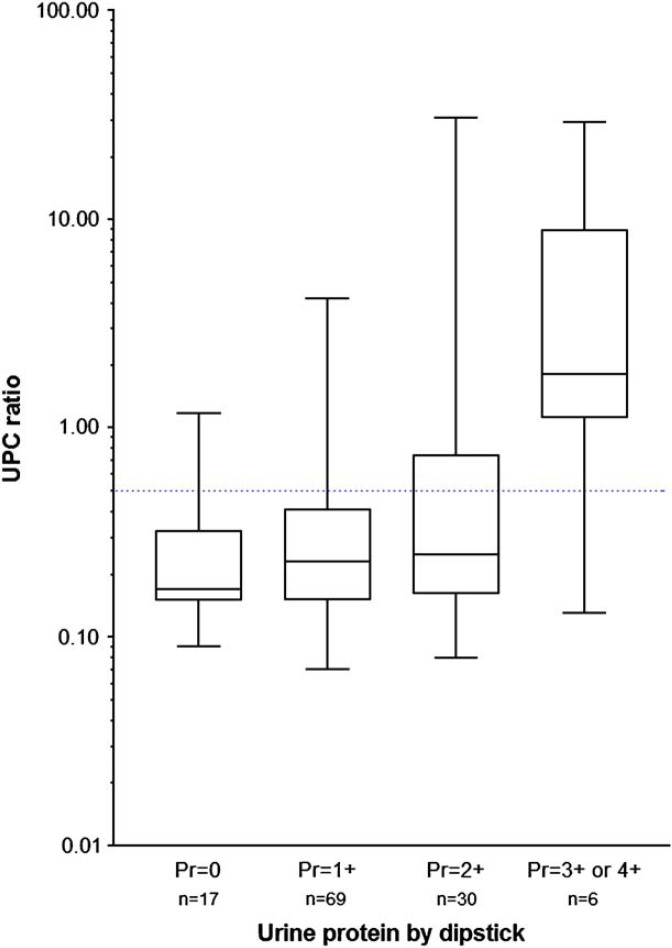
Urine protein dipstick test results correlated poorly with the UPC ratio (rs=0.281, P=0.002, Fig 3). A significantly higher (P<0.0001) proportion of diseased cats (84/100) had positive urine dipstick protein results compared with healthy cats (2/22). In total, 54 of 87 (62%) cats with a UPC ratio ≤0.5 had positive urine dipstick results and 3/35 (8%) cats with a UPC >0.5 had negative protein dipstick results (the range of UPC ratios in these three was 0.53–1.17). Protein concentration as measured by urine dipstick had a better, although still only moderate, correlation with MA results (r s =0.518, P<0.0001).
Fig 3.
Box and whisker plots of urine protein to creatinine ratios found in the urine of cats with different amounts of proteinuria measured by urine dipstick. Blue dotted line=UPC of 0.5.
Proteinuria in Healthy and Diseased Cats
Overall, 36/100 diseased cats (36%) and 2/22 healthy cats (9%) had positive MA results, whereas 34 of the diseased cats (34%) and one of the healthy cats (5%) had a UPC ratio >0.5.
Of the diseased cats, 27 were classified as having endocrine disease (22 with hyperthyroidism, two with primary hyperaldosteronism, two with diabetes mellitus and one with hyperadrenocorticism), 11 had gastrointestinal disease, nine had neoplasia, eight had LUTD, seven had haematological disorders, seven had renal failure and four had glomerulonephritis. There were 27 with miscellaneous disorders. Figure 4 shows standard box and whisker plots of the UPC ratios found in these different disease categories and in the healthy cats. Compared with healthy cats, diseased cats had significantly higher MA concentrations overall (P=0.014, although median value for both groups was 0), and significantly higher UPC ratios (P<0.0001) with median values of 0.32 (diseased cats) and 0.16 (healthy cats). Both these results remained significant when the four cats with glomerulonephritis (the four with highest levels of proteinuria) were excluded from the analysis.
Fig 4.
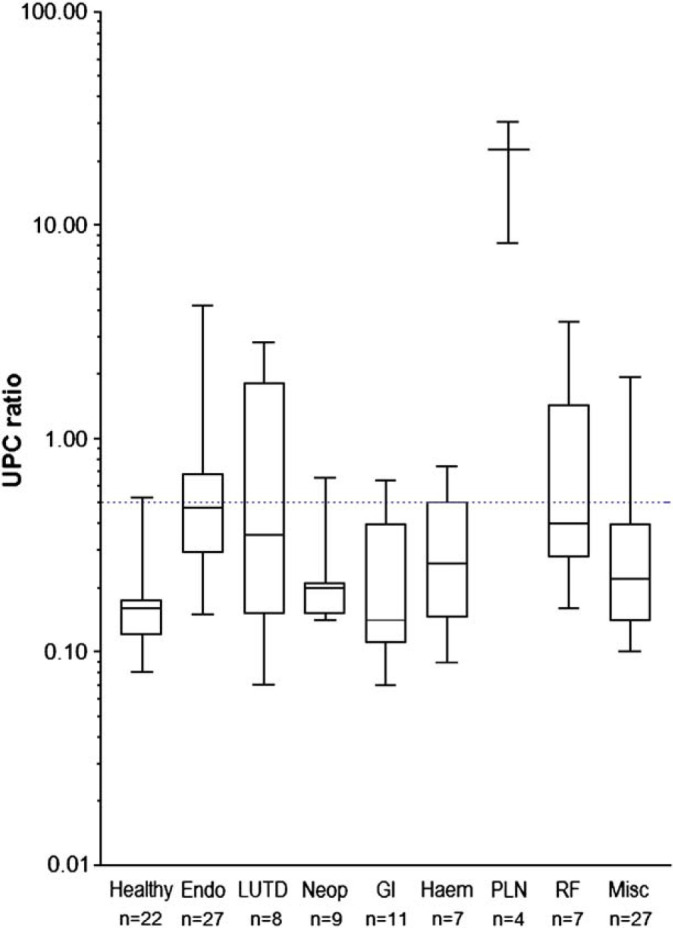
Box and whisker plots of urine protein to creatinine ratios found in the urine of healthy cats, and cats with different diseases. Blue dotted line=UPC of 0.5.
Distinguishing Normal from Abnormal Proteinuria
Using a UPC ratio of >0.5 as abnormal, overall there were 87 cats with normal UPC ratios, and 35 with elevated ratios (34 diseased cats and one healthy cat, the latter with a UPC ratio of 0.53). There were, however, discrepancies between the UPC ratios and the MA results of the cats, and these are illustrated in Table 2. Cats with high or very high MA results had UPC ratios above the normal range, as did all but one cat with a medium positive MA result (this cat had a UPC ratio of 0.32). Thus of 20 cats with a MA result of medium positive or higher, 19 (95%) had an elevated UPC ratio. However, 13/87 (15%) cats with a UPC <0.5 had positive (low or medium) MA results, while 10/84 (12%) cats with a negative MA result had a UPC >0.5, and none of the latter had evidence of any lower urinary tract disease.
Table 2.
Comparison between ‘cut-off’ values for urine protein to creatinine ratio (UPC) and microalbuminuria (MA) results
| UPC≤0.5 | 0.5<UPC≤1.0 | 1.0<UPC≤3.00 | UPC>3.0 | Total | |
|---|---|---|---|---|---|
| MA negative | 74 | 10 | 0 | 0 | 84 |
| MA low positive | 12 | 3 | 3 | 0 | 18 |
| MA medium positive | 1 | 4 | 3 | 1 | 9 |
| MA high positive | 0 | 2 | 4 | 2 | 8 |
| MA very high positive | 0 | 0 | 0 | 3 | 3 |
| Total | 87 | 19 | 10 | 6 | 122 |
Discussion
The recent availability of an easy bed-side test for the evaluation of MA in canine and feline patients has created considerable interest in this test and in the significance of a positive finding. Several papers and abstracts have reported investigations into MA in dogs (Jensen et al 2001, Pressler et al 2001, Vaden et al 2001, Grauer et al 2002, Lees et al 2002, Vaden et al 2002, Donnelly et al 2003, Gary et al 2003, Pressler et al 2003), but to the authors' knowledge there has been only one published report in cats (Langston 2004), which contains some data from the test kit manufacturer (Heska). To the authors' knowledge therefore, the present study is the first independent publication to critically assess the commercial feline MA test in a clinical setting.
The E.R.D.-HealthScreen test is quick and simple to run, but interpretation of results requires a subjective assessment of the relative intensity of two blue coloured bands (Fig 1). During the study, it became evident that it was not always easy to categorise results – variations may occur in bandwidth and/or colour intensity along its length (as was found in a number of samples), unavoidably contributing to a degree of ambiguity when selecting a result category. Such variations in colour intensity or bandwidth occurred without ‘blotched’ or broken lines that would, in accordance with the test's instructions, render the test invalid.
Intra-reader variations occurred frequently in this study, and although we found good agreement between the two readers, there were still a number of inter-reader discrepancies. This could be explained by a number of means – the test itself may have poor repeatability, the samples being evaluated could have been ‘borderline’ between two MA categories, or the subjectivity involved in interpreting the test results noted above could be a factor. While the latter appears likely to be important, additional work would be necessary to determine the influence of other factors, but this study has highlighted some concerns about the repeatability of the test results, especially as some discrepancies occurred between ‘negative’ and ‘low positive’ readings. Regrettably, one serious limitation of the current study was that an accurate quantitative measurement of urine albumin was not possible – had this been available the accuracy and precision of the semi-quantitative MA test could have been assessed. There is a clear need for this in future studies.
An additional concern in interpretation of test results in this study was that in the test kits used, there was a subtle difference between the instructions given in the package insert and the quick-reference card supplied in the kits. While the insert stated that a result is ‘very high positive’ if the lower band is either ‘very faint or absent’, the card stated that a ‘very high positive’ result equates to ‘top band very dark, bottom band absent’. This inconsistency may have contributed to discrepancies between the two readers and may explain why sample 26 (which was spiked with serum to create a very high level of proteinuria) was reported by one reader as only ‘high positive’ on 3/5 occasions.
There was very poor correlation between urine dipstick protein readings and UPC ratio, as might be expected given that urine dipsticks are semi-quantitative and are not corrected for either specific gravity or creatinine concentration. Although it has previously been reported that protein levels assessed by urine dipstick measurement do not give false negative results (Moore et al 1991), we found four cats with negative dipstick results that had elevated UPC ratios. Interestingly, there was a better and moderate association between dipstick protein measurements and MA results – this may be explained partly as the reagent within the dipstick ‘protein’ pad reacts mainly with albumin (Kashif et al 2003, Syme and Elliot 2006), and perhaps also because both tests generated discrete categorical results rather than continuous numerical data.
The 36% of sick cats with positive MA results that we found is slightly lower than the 43% reported in a previous study (Langston 2004), but still reflects a substantial proportion of clinical cases presented for investigation. Similarly the 9% of healthy cats testing positive was slightly lower than 14% in the previous study, although the number of healthy cats in our study was relatively low, and the age distribution somewhat limited. An association between MA and age has been previously reported for both dogs and cats (Donnelly et al 2003, Langston 2004), and although we found a weak association with age, there were too few healthy cats (and too few with positive MA results) to be able to assess this independently within the healthy group. Interestingly though, we found UPC ratios did not show an increase with age.
MA, UPC ratio and dipstick protein results were significantly higher in the diseased cats than the healthy cats. This has been suggested as justification for assessing proteinuria and/or albuminuria in as part of a routine health screen to detect potential underlying disease, be it renal or extra-renal in origin. However, the clinical relevance of a positive MA or elevated UPC ratio result may be difficult to interpret, due to the wide range of diseases that can result in positive test results (in addition to potential inaccuracies with the MA test). We also found a moderately good association between MA concentrations and UPC ratios in this study, which was anticipated as the smaller size of albumin (compared with other proteins) means that it is likely to be an early and major constituent of proteinuria associated with altered glomerular permeability. Certainly in man, trace proteinuria has been shown to correlate well with albuminuria (Sam et al 2003, Davidson and Smiley 1999).
However, as MA measurement is a more sensitive method of detecting urinary albumin than UPC, it would be expected that a proportion of cases would show MA in the absence of a raised UPC ratio (patients with early or mild glomerular disease). Such a situation is seen in man and has also been demonstrated in a sub-set of dogs with hereditary renal disease (Lees et al 2002) or other conditions affecting glomerular integrity (Pressler et al 2003, Whittemore et al 2003). In keeping with these observations, we found 13 cats with positive MA results that had normal UPC ratios (Table 2). However, the reliability issues with the MA test highlighted in this study potentially argue the need for further (quantitative) testing of albuminuria in those cases where a positive MA result is obtained. Additionally, although cystocentesis-induced blood contamination (the main collection method used in this study) may not significantly affect the UPC ratio (Vaden et al 2004), it is not yet known if the same is true for the MA test and this is an area that should also be addressed in future studies.
Although we expected to find some cats with positive MA results and normal UPC ratios, we also found 10 cats that had elevated UPC ratios but negative MA results. This was unexpected, and contrasts with a previous study of canine patients where negative MA results consistently predicted a normal UPC ratio (Whittemore et al 2003). As none of these cats had evidence of LUTD, the cause of this finding remains unknown. It is possible that the MA test yielded false negative results, but this assumption cannot be made without comparison with an accurate urine albumin measurement, given that the UPC ratio is not selective for albumin. Additionally the parameters that constitute true MA in cats have not yet been defined, and it is not known if the sensitivity of the test is necessarily appropriate for cats. Quantitative evaluation of albumin (and other urinary proteins) would have been very helpful to explore these discrepancies, but was beyond the scope of this study.
There were too few cases of primary renal disease in this group of cats to draw any meaningful conclusions about the association between MA, proteinuria and renal failure. However, it is of note that we found no association between systemic systolic blood pressure and either MA measurement or UPC ratio, given that in humans one of the two major recognised conditions underlying positive MA readings is essential hypertension. However, other studies have shown a correlation between renally induced systemic hypertension and MA in cats (Mather et al 2002). As there were relatively few hypertensive cats (n=11) included in the current study, this is an area that will also require further investigation.
In summary, we found that the MA test kit results correlate quite well with UPC ratios, but discrepancies between the tests exist and the reason(s) for these could not be determined in this study. Although the MA kit provides a convenient method for rapid semi-quantitative assessment of albuminuria, there were some problems with repeatability of the test, and further work is needed to verify the sensitivity of the test and whether the cut-off values employed are appropriate in cats. However, until more is known, it is difficult to draw firm conclusions about the significance of MA in feline urine, and especially its relationship to primary renal disease. In particular, the fact that MA does seem to occur in a wide variety of disease conditions raises concerns that it is not appropriate to view it as a sensitive early-stage marker of chronic renal failure. Furthermore, as tubulointerstitial disease (or a combination of this with glomerular disease) appears to be the major underlying pathophysiological mechanism in feline renal failure (Minkus et al 1994, Polzin et al 2000), it is not yet clear whether measurements of MA will offer significant advantages over the UPC ratio in the detection and monitoring of cats with renal disease.
Acknowledgements
This work was kindly supported by a grant from VetXX Ltd. The authors would also like to thank Sue Cade, Steve Dodkin, Dr Kostas Papasouliotis, Professor Tim Gruffydd-Jones, Suzanne Rudd, Anita Shwartz, and Reb Giles, for their invaluable assistance.
References
- Adams L.G., Polzin D.J., Osborne C.A., O'Brien T.D. Correlation of urine protein/creatinine ratio and 24-hour urinary protein excretion in normal cats and cats with surgically induced chronic renal failure, Journal of Veterinary Internal Medicine 6, 1992, 36–40. [DOI] [PubMed] [Google Scholar]
- Bishop S.A., Lucke V.M., Stokes C.R., Gruffydd-Jones T.J. Plasma and urine biochemical changes in cats with experimental immune complex glomerulonephritis, Journal of Comparative Pathology 104, 1991, 65–76. [DOI] [PubMed] [Google Scholar]
- Cook A.K., Cowgill L.D. Clinical and pathological features of protein-losing glomerular disease in the dog: a review of 137 cases (1985–1992), Journal of the American Animal Hospital Association 32, 1996, 313–322. [DOI] [PubMed] [Google Scholar]
- Davidson M.B., Smiley J.F. Relationship between dipstick positive proteinuria and albumin:creatinine ratios, Journal of Diabetes Complications 13, 1999, 52–55. [DOI] [PubMed] [Google Scholar]
- Donnelly R., Jensen W.A., Stinchcomb D.T. Effect of age and breed on the prevalence of microalbuminuria in dogs, Journal of Veterinary Internal Medicine 17, 2003, 406, (abstract) [Google Scholar]
- Finco D.R., Brown S.A., Brown C.A., Crowell W.A., Sunvold G., Cooper T.L. Protein and calorie effects on progression of induced chronic renal failure in cats, American Journal of Veterinary Research 59, 1998, 575–582. [PubMed] [Google Scholar]
- Gary A.T., Cohn L.A., Kerl M.E., Jenson W.A. The effects of exercise on microalbuminuria in dogs, Journal of Veterinary Internal Medicine 17, 2003, 435–436, (abstract) [DOI] [PubMed] [Google Scholar]
- Grauer G.F., Oberhauser E.B., Basaraba R.J., Lappin M.R., Simpson D.F., Jensen W.A. Development of microalbuminuria in dogs with heartworm disease, Journal of Veterinary Internal Medicine 16, 2002, 352, (abstract) [Google Scholar]
- Gunn-Moore D. Influence of proteinuria on survival time in cats with chronic renal insufficiency, Journal of Veterinary Internal Medicine 17, 2003, 405. [Google Scholar]
- Hines B.L., Salisbury S.K., Jakovlievic S., DeNicola D.B. Pancreatic pseudocyst associated with chronic-active necrotizing pancreatitis in a cat, Journal of the American Animal Hospital Association 32, 1996, 147–152. [DOI] [PubMed] [Google Scholar]
- Jacob F., Polzin D.J., Osborne C.A., Neaton J.D., Kirk C.A., Allen T.A., et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure, Journal of the American Veterinary Medical Association 226, 2005, 393–400. [DOI] [PubMed] [Google Scholar]
- Jensen W.A., Grauer G.F., Andrews J., Simpson D.F. Prevalence of microalbuminurea in dogs, Journal of Veterinary Internal Medicine 15, 2001, 300, (abstract) [Google Scholar]
- Kashif W., Siddiqi N., Dincer A.P., Dincer H.E., Hirsch S. Proteinuria: how to evaluate an important finding, Cleveland Clinic Journal of Medicine 70, 2003, 535–547. [DOI] [PubMed] [Google Scholar]
- Langston C.L. Microalbuminuria in cats, Journal of the American Animal Hospital Association 40, 2004, 251–254. [DOI] [PubMed] [Google Scholar]
- Lees G.E., Jensen W.A., Simpson D.F., Kashtan Persistent albuminuria precedes onset of overt proteinuria in male dogs with x-linked hereditary nephropathy, Journal of Veterinary Internal Medicine 16, 2002, 353, (abstract) [Google Scholar]
- Lees G.E., Brown S.A., Elliot J., Grauer G.E., Vaden S.L. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal), Journal of Veterinary Internal Medicine 19, 2005, 377–385. [DOI] [PubMed] [Google Scholar]
- Mather S., Syme H., Brown C.A., Elliot J., Moore P.A., Newell M.A., et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency, American Journal of Veterinary Research 63, 2002, 833–839. [DOI] [PubMed] [Google Scholar]
- Mather S., Brown C.A., Dietrich U.M., Munday J.S., Newell M.A., Sheldon S.E., et al. Evaluation of a technique of inducing hypertensive renal insufficiency in cats, American Journal of Veterinary Research 65, 2004, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Minkus G., Reusch C., Horauf A., Breuer W., Darbes J., Kraft W., et al. Evaluation of renal biopsies in cats and dogs – histopathology in comparison with clinical data, Journal of Small Animal Practice 35, 1994, 465–472. [Google Scholar]
- Monroe W.E., Davenport D.J., Saunders G.K. Twenty-four hour urinary protein loss in healthy cats and the urine protein–creatinine ratio as an estimate, American Journal of Veterinary Research 50, 1989, 1906–1909. [PubMed] [Google Scholar]
- Moore F.M., Brum S.L., Brown L. Urine protein determination in dogs and cats: comparison of dipstick and sulfasalycylic acid procedures, Veterinary Clinical Pathology 20, 1991, 95–97. [DOI] [PubMed] [Google Scholar]
- Nash A.S., Wright N.G., Spencer A.J., Thompson H., Fisher E.W. Membranous nephropathy in the cat: a clinical and pathological study, Veterinary Record 105, 1979, 71–77. [DOI] [PubMed] [Google Scholar]
- Nichols R. Allergic- and immune-associated diseases of the urinary tract, Veterinary Clinics of North American Small Animal Practice 24, 1994, 749–763. [DOI] [PubMed] [Google Scholar]
- Patel R.T., Caceres A., French A.F., McManus P.M. Multiple myeloma in 16 cats: a retrospective study, Veterinary Clinical Pathology 34, 2005, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler B.M., Vaden S.L., Jensen W.A., Simpson D.F. Prevalence of microalbuminuria in dogs evaluated at a referral veterinary hospital, Journal of Veterinary Internal Medicine 15, 2001, 300, (abstract) [Google Scholar]
- Pressler B.M., Proulx D.A., Williams L.E., Jensen W.A., Vaden S.L. Urine albumin concentration is increased in dogs with lymphoma or osteosarcoma, Journal of Veterinary Internal Medicine 17, 2003, 404, (abstract) [Google Scholar]
- Polzin D.J., Osborne C.A., Jacob F., Ross S. Chronic renal failure. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 5th edn, 2000, WB Saunders: London, 1634–1662. [Google Scholar]
- Rimbeiha W.K., Francis J.A., Fitzgerald S.D., Nair M.G., Holan K., Bugyei K.A., et al. A comprehensive study of Easter lily poisoning in cats, Journal of Veterinary Diagnostic Investigations 16, 2004, 527–541. [DOI] [PubMed] [Google Scholar]
- Rottman J.B., English R.V., Breitschwerdt E.B., Duncan D.E. Bone marrow hypoplasia in a cat treated with griseofulvin, Journal of the American Veterinary Medical Association 198, 1991, 429–431. [PubMed] [Google Scholar]
- Sam R., Shykh M.S., Pegorara A.A., Khalili V., Hristea I., Singh A.K., et al. The significance of trace proteinuria, American Journal of Nephrology 23, 2003, 438–441. [DOI] [PubMed] [Google Scholar]
- Stankeviciute N., Sabah S., Sing A., Shaykh M., Bakir A.A., Arruda J.A., et al. Can total urinary protein measurements predict microalbuminuria?, American Journal of Nephrology 18, 1998, 285–290. [DOI] [PubMed] [Google Scholar]
- Syme H.M., Elliott J. Evaluation of proteinuria in hyperthyroid cats, Journal of Veterinary Internal Medicine 15, 2001, 299, (abstract) [Google Scholar]
- Syme H.M., Elliott J. Urinary protein excretion in cats with renal failure and/or hypertension, Journal of Veterinary Internal Medicine 17, 2003a, 405, (abstract) [Google Scholar]
- Syme H.M., Elliott J. Relation of survival time and urinary protein excretion in cats with renal failure and/or hypertension, Journal of Veterinary Internal Medicine 17, 2003b, 405, (abstract) [Google Scholar]
- Syme H.M., Elliott J. Proteinuria. August John R. Consultations in Feline Internal Medicine vol. 5, 2006, Elsevier 2006, 415–422. [Google Scholar]
- Thrall M.A., Grauer G.F., Mero K.N. Clinicopathologic findings in dogs and cats with ethylene glycol intoxication, Journal of the American Veterinary Medical Association 184, 1984, 37–41. [PubMed] [Google Scholar]
- Vaden S.L., Jensen W.A., Longhofer S., Simpson D.F. Longitudinal study of microalbuminuria in soft-coated wheaten terriers, Journal of Veterinary Internal Medicine 15, 2001, 300, (abstract) [Google Scholar]
- Vaden S.L., Pressler B.M., Lappin M.R., Simpson D.F., Jensen W.A. Urinary tract inflammation has a variable effect on urine albumin concentrations, Journal of Veterinary Internal Medicine 16, 2002, 378, (abstract) [Google Scholar]
- Vaden S.L., Pressler B.M., Lappin M.R., Jensen W.A. Effects of urinary tract inflammation and sample contamination on urine albumin and total protein concentrations in canine urine samples, Veterinary Clinical Pathology 33, 2004, 14–19. [DOI] [PubMed] [Google Scholar]
- Venkat K.K. Proteinuria and microalbuminuria in adults: significance, evaluation, and treatment, Southern Medical Journal 97, 2004, 969–979. [DOI] [PubMed] [Google Scholar]
- Walker D., Syme H.M., Markwell P., Elliott J. Predictors of survival in healthy, non-azotaemic cats, Journal of Veterinary Internal Medicine 18, 2004, 417, (abstract) [Google Scholar]
- Whittemore J.C., Jensen W.A., Prause L., Radecki S., Gill V., Lappin M.R. Comparison of microalbuminuria, urine protein dipstick, and urine protein creatinine ratio results in clinically ill dogs, Journal of Veterinary Internal Medicine 17, 2003, 437. [Google Scholar]