Abstract
The surgical technique for removal of tentorial meningiomas is described on six cats using a unilateral temporal supracerebellar transtentorial approach. Complete gross tumour resection was achieved in four of six cats. In one cat, only subtotal resection was achieved. One cat died shortly after surgery because of extensive cerebral haemorrhage. The surgical approach, combined with cisternal or ventricular cerebrospinal fluid puncture and an open-window technique (tumour fenestration and enucleation) provided sufficient visibility and tumour accessibility without excessive manipulation of the brain parenchyma. In all patients, a postoperative transient worsening of the clinical signs was observed. The neurological signs resolved with time with the exception of blindness in two cats. All five surviving cats were monitored for a mean follow-up time of 19 months (median 20 months; range 6–30 months). All patients died or were euthanased because of tumour regrowth within the follow-up period. Although challenging, surgical treatment is a useful therapeutic measure in the treatment of cats presenting with tentorial meningiomas.
Meningiomas are thought to arise from neoplastic arachnoid cap cells, which are themselves a unique population of mesothelial-like cells. These display morphology similar to the embryological cells found at the interface of the developing arachnoid and dura within the cranial and spinal cavities. The primitive meningeal cells originate from cells of the neural crest and from mesodermal cells that migrate into the area of the developing neural tube. In adults, the arachnoid cap cells are found at the apices of arachnoid granulations and are morphologically similar, in many respects, to the cells of the arachnoid barrier (Nafe 1979, Yasargil 1994). The majority of meningiomas develop from these cells and grow as unilateral hemispherical lesions with a flat dural base. They initially grow outward into the arachnoidal spaces and onto the brain.
In humans, tumours arising from the tentorium are frequently dumbbell shaped, with both supratentorial and infratentorial components. These tumours may arise in a median, paramedian or lateral position on the tentorium (Harrison and al-Mefty 1997, Bret et al 2000). Tentorial meningiomas that are attached to the tentorial free edge have been called ‘medial tentorial meningiomas’, ‘inner ring meningiomas’ or ‘tentorial notch meningiomas’ in human medicine (Yasargil 1994, Samii et al 1996). Tentorial meningiomas are not uncommon in cats (Jaggy 2004) and arise in the transverse fissure from the inner ring of the tentorium, and less frequently, from the outer ring along the transverse sinus. Surgical treatment of medial tentorial meningiomas remains a challenge because of the close relationship of the tentorial edge to the surrounding important neurovascular structures (Fukushima et al 1991, Firsching et al 2003). Several approaches to medial tentorial meningiomas with supratentorial extension have been attempted in humans, including frontotemporal, subtemporal suboccipital and occipital interhemispheric routes. Each approach has its own inherent risk of damage to the temporal or the occipital lobe because of the need for brain retraction or cortical incision (Samii et al 1996, Kakou and Jan 1999, Firsching et al 2003).
The aim of this report, is to present a unilateral temporal supracerebellar transtentorial approach as a surgical therapeutic option in feline medial tentorial meningiomas.
Materials and Methods
Six feline tentorial meningiomas were treated surgically at the Small Animal Clinic of the Free University of Berlin between 1999 and 2005. Only meningiomas with a neuroradiologically and surgically documented implantation at the tentorium were included in this report. The records of all six cats were retrospectively reviewed for clinical presentation, neuroradiological evaluation, surgical management and outcome.
Computed Tomography
All cats were anaesthetised using induction with a combination of midazolam (Dormicum; Roche AG, Mannheim, 0.2 mg/kg IV) and propofol (Diprivan; Astra Zeneca, 4 mg/kg IV), followed by isoflurane (Isoflurane; Abbott, Germany) and oxygen inhalation. Computed tomography (CT) examination was performed with a third generation multislice scanner (General Electric Healthcare Qxi, Haan, Germany) with animals positioned in dorsal recumbency. Helical scanning at a collimation of 1.25 mm with a pitch of 3.5 was carried out and 1.25 mm slices were calculated out of the data set. The slices were transferred onto the advantage workstation 4.0 ultra (AWD) for the post-processing operations. Multiplanary reformations and maximum intensity projections were used to visualise the locations of the meningiomas within the brain. Following the first native scan, a second scan was performed following a single intravenous bolus of non-ionic iodine contrast medium (Accupaque 300, Nycomed, Ireland, 600 mg/kg).
Surgical Treatment
All cats were intravenously premedicated with methylprednisolone sodium succinate (Medrate solubile; Pfizer, 30 mg/kg) and mannitol (Braun-melsungen; Melsungen, Germany, 0.5 g/kg over 20 min) to minimise brain oedema and inflammation during surgery. Induction was achieved with midazolam (Dormicum; Roche AG, Mannheim, 0.2 mg/kg IV), fentanyl (Janssen; Neuss, Germany, 0.02 mg/kg IV) and propofol (Diprivan; Astra Zeneca, 4 mg/kg IV). Mild mechanical hyperventilation (end-tidal CO2 3–3.5%) was applied and anaesthesia was maintained with 1.5–2% isoflurane (Abbott; Wiesbaden, Germany) in oxygen. Lactated Ringer's solution (3 ml/kg/h IV) was administered throughout surgery. Prophylactic antimicrobial therapy with cefazolin (Kefzol; Medica, Aesch, Switzerland, 25 mg/kg IV) was administered immediately before induction and again at the end of surgery. Monitoring included electrocardiography and end-tidal CO2 concentrations. Postoperative analgesia was provided with buprenorphine (Temgesic; Essex, München, Germany, 0.01 mg/kg SC) and metamizol (Novalgin; Aventis Pharma, Germany, 20 mg/kg IV).
Cats were positioned in sternal recumbency with the head flexed at 60°, slightly rotated and elevated, taking care to maintain airway patency. The head was shaved from the eyebrows to the level of C2, scrubbed with chlorhexidine and disinfected with alcohol and povidone-iodine.
A horseshoe shaped incision and a unilateral temporal craniectomy, extending over the tentorium, were performed (Fig 1). After release of 2–3 ml cerebrospinal fluid (CSF) from the cisterna magna, the cerebellum became slack and shifted downward due to gravity. Minimal retraction of the cerebellum permitted sufficient visualisation of the tentorium. Moreover, a small part of the meningioma was found protruding medially from the tentorial edge in most cases. In cases with ventricular enlargement, a puncture of the intraventricular CSF (3–5 ml) was also performed. The CSF pressure was not monitored during this procedure. The osseous part of the tentorium was carefully removed under magnification using a fine bone rongeur. Small bleeding vessels were cauterised with bipolar microforceps. Larger venous structures were obliterated with methylcellulose. After removal of the tentorium, the tumour was internally decompressed through a single-window technique: the visible surface of the tumour was fenestrated and the centre of the tumour enucleated using tumour forceps through this window. The tumour margins could then be carefully dissected away from the midbrain favoured by a good cleavage plane. After removal of all blood clots and copious lavage of the craniotomy site with saline solution, the operation site was closed by suture of the temporal muscle to its attachment, and closure of subcutaneous tissue (Vicryl 3-0, Ethicon; Norderstedt, Germany) and the skin (Prolene 4-0, Ethicon; Norderstedt, Germany).
Fig 1.

Extent of the craniotomy site overlying the cerebellar tentorium depicted on a hemisection of a feline calvarium (medial view). The fenestration of the tentorium allows removal of the tumour without causing excessive pressure on surrounding tissues.
Pathological Examination
Tumours were immersed in 10% neutral buffered formalin immediately following excision. After proper fixation, samples were processed routinely in an automatic tissue processor, embedded in paraffin, sectioned at 5 μm and stained with hemalaun–eosin and Masson trichrome. Further slices underwent immunohistochemical staining for S-100, vimentin, cytokeratin, and MIB-1 (Dakocytomation, Glostrup, Denmark). Histological typing was based on the WHO classification of tumours of the nervous system of domestic animals (Koestner et al 1999). Moreover, histological growth pattern, mitotic index, MIB-labelling index and presence or absence of anaplastic features were considered indicative for tumour biology and gave rise to further classification into WHO grades I–IV as has been proposed for human tumours (Kleinhues and Cavenee 2000).
Results
Patient Population
There were four castrated male and two spayed female cats, ranging in age from 10 to 14 years in this study. Breeds affected included five domestic shorthair cats and one Persian. Only one cat had multiple meningiomas (median tentorial and left temporal).
Clincal Signs
The duration of presenting signs ranged from 3 weeks to 3 months prior to surgery. Behavioural disturbances (four cats), pacing to one side (three cats), gait ataxia (four cats), and blindness (one cat) were the most common presenting signs. Signs were more pronounced on one side in four cats. One cat was presented with anorexia and lethargy but without specific neurological signs. All cats were FeLV negative. A complete blood count, serum biochemistry and thoracic radiographs were performed in all cats. Mild anaemia (haematocrit 30%) and a mildly elevated serum creatinine (2.0 μg/dl) were each detected in one cat. The remaining results of laboratory analyses and radiographs were unremarkable.
Imaging Studies
In five cats, several nidi of calcifications were seen within the tumour. All tumours displayed homogenous contrast-medium enhancement. Meningioma size ranged from 1.3 to 2.1 cm3 (length×width×depth). In most cases, the location of the tumour extended from the centre of the brain between the two hemispheres over the corpus callosum to the caudal edge of the bony tentorium. In one cat, the meningioma displayed a rostro- and caudotentorial growth, causing a dumbbell shape of the tumour. Only one cat had multiple meningiomas (median tentorial and right temporal, Fig 2). In two cats, obstructive hydrocephalus with enlargement of the lateral ventricles was observed. Deviation of the falx cerebri was not observed.
Fig 2.
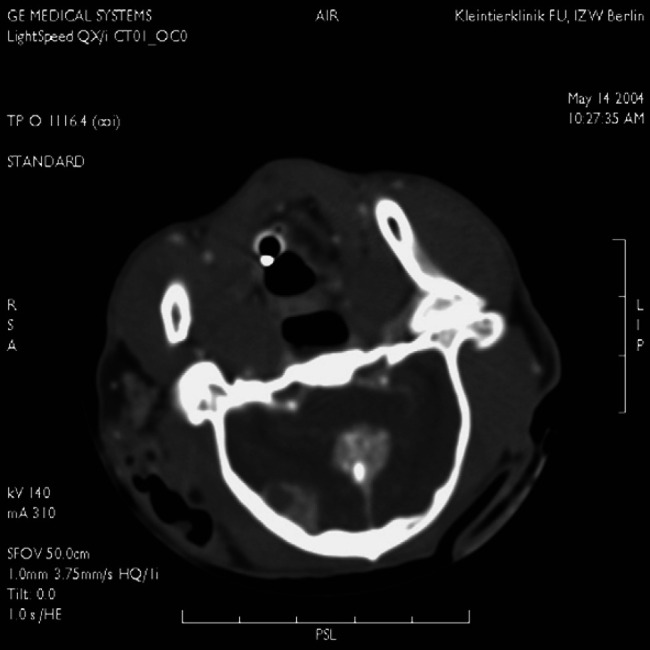
Multiple meningioma in one cat: right temporal and median tentorial localisation. The central mass arises from the meningeal layer overlying the bony end of the tentorium.
Postoperative CT revealed complete gross tumour removal in four cases (Fig 3). In one case, subtotal excision with a small interhemispheric remnant (2×2 mm) of the cranial part of the meningioma, was observed. In the last case, extensive haemorrhage illustrated by a large contrast-medium effusion within the operation site and around the brainstem, was observed. In all cases, moderate brain oedema and pneumocephalus as a consequence of iatrogenic parenchyma manipulation and removal of the space-occupying lesion, was observed. The air within the brain was resorbed within hours as the decompressed brain began to re-expand.
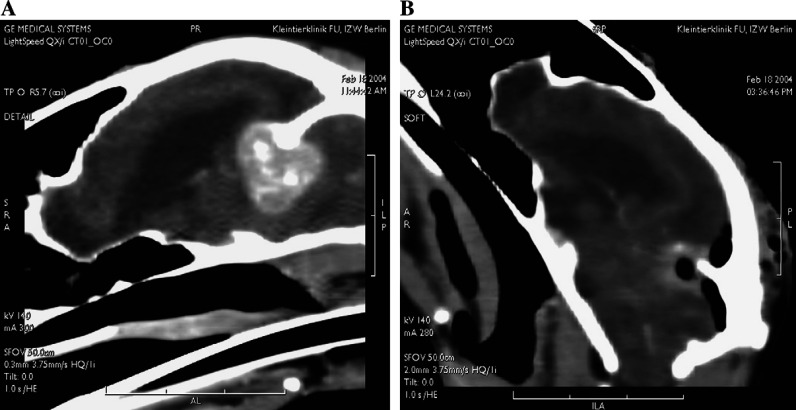
Fig 3.
Tentorial meningioma. (A) The meningioma appears as a homogenous contrast-enhancing mass attached to the tentorium. Some calcifications within the tumour and enlargement of the ventricles are evident. (B) After resection and removal of the right side of the tentorium, oedema (diffuse contrast-medium enhancement) is evident around the operation site. The resection appears to be complete, and clinical signs did not recur for 20 months after surgery.
Postoperative Management
A complete physical and neurological examination was performed on the first few days, as well as 6 weeks and 3 months after surgery. One cat died in the immediate postoperative period as a consequence of extensive intracranial bleeding due to iatrogenic laceration of a critical vascular structure. This finding was suspected on postoperative CT-scan and confirmed by necropsy. The remaining five patients experienced new or exacerbated neurological deficits in the immediate postoperative period. All cats were treated with antibiotics (cefalexin, Cefaseptin mite; Chassot, Ravensburg, Germany, 25 mg/kgPOq12h), analgesics (buprenorphine, Temgesic: München, Germany, 0.01 mg/kgSCq8h) and crystalloid infusions. Patients were discharged from the clinic 3 to 5 days after surgery. All patients received hydroxyurea (Litalir; Bristol-Myers Squibb, Germany, 20 mg/kgPO daily).
Pathology
In all cases, histomorphology and immunohistochemistry confirmed the diagnosis of a meningioma. Based on histopathological architecture, three tumours revealed features of a fibrous meningioma, two of a psammomatous meningioma and one of a transitional subtype. None of the tumours exhibited histological signs of anaplasia. The mitotic index was 0.1±0.08, and a mean of 3.1% of tumour cells stained MIB-1 positive. If analysed in accordance to human neuro-oncology, all of these features are compatible with benign WHO grade-I tumours (Kleinhues and Cavenee 2000).
Follow-Up Results
All five surviving cats were monitored during a mean follow-up period of 19 months (median 20 months, range 6–30 months). The patient with incomplete tumour resection survived 17 months with a slight persistent gait ataxia and proprioceptive deficits on the right side before being euthanased because of sudden deterioration of neurological signs. The owners declined any postmortem examination. Follow-up evaluation of the other patients revealed that most postoperative new neurological deficits had completely or almost completely resolved within 3 months of surgery. Only two cats were blind in the postoperative period, and one of these was considered blind before surgery. The vast majority of preoperative presenting signs had resolved by the long-term follow-up examinations. All patients died or were euthanased because of tumour regrowth within the follow-up period. No complications associated with the administration of hydroxyurea were observed.
Discussion
Several characteristics, including a mean age of 12.1 years and a male to female ratio of 1.5:1, are similar to previous reports of feline tentorial meningiomas. The domestic shorthair was the most common breed, most likely because of the relative frequency of this breed in the general cat population (Nafe 1979, Gordon et al 1994, Troxel et al 2003).
The surgical prognosis of intracranial meningiomas in cats is good and survival time is significantly prolonged with surgical intervention. Median postoperative survival time is 685 days (23 months) compared to 18 days for cats that are treated medically (Gordon et al 1994). Similar survival would be obtained with radiotherapy treatment. Median survival times for dogs after irradiation for meningiomas ranged from 4.9 to 16 months. Axlund et al (2002) compared the outcome in canine patients with intracranial meningiomas treated with surgical resection alone or followed by radiation therapy and found median survival times of 7 and 16 months, respectively. The same results could be expected in cats but to our knowledge no concluding comparative studies concerning cats are available at this time (Rohrer Bley et al 2005). All clients of patients in the current study declined radiation treatment because of the large distance to the nearest veterinary radiotherapy unit.
Hydroxyurea was administered to cats in this study in an attempt to diminish tumour regrowth. The rationale for this was based on in vitro studies that showed that multiplication of feline meningioma cells was slowed or completely arrested by the application of hydroxyurea, and subsequent clinical studies, corroborating this in vitro effect (Forterre et al 2000).
Several subgroups of meningiomas remain a surgical challenge because of their vicinity to critical neural and vascular structures. Tentorial meningiomas still represent complex surgical entities associated with significant mortality and morbidity (Ono et al 1984, Samii et al 1996). In the current study, one cat died because of cerebral haemorrhage. The development or aggravation of neurological deficits in the remaining five cats is likely a consequence of the manipulation necessary to remove the tumour. One cat developed blindness during surgery, which may have been due to excessive pressure on the occipital cortex during cavitation of the tumour. Even if most of the symptoms were reversible, vascular disturbances cannot be ruled out as a cause of them. The plasticity of brain tissue could also explain an almost complete recovery through compensatory adaptation after more severe lesions (Yasargil 1994).
The goal of our study was to present a surgical treatment option for medial tentorial meingiomas. The surgical approach described here allows sufficient visualisation of the tumour without excessive bone removal or traumatic manipulation of the brain tissue. In contrast, a combined rostro- and caudotentorial approach would require two craniotomy sites, as well as retraction of the occipital lobe in order to adequately visualise the craniodorsal edge of the tentorium, which was the main anchor point of the tumour in our patients. Such cranial retraction can lead to cortical hypoxia and blindness when sustained for sufficient periods of time (Yasargil 1994, Samii et al 1996, Firsching et al 2003). An isolated rostro- or caudotentorial approach would not allow sufficient exposition of the tumour and would automatically lead to excessive manipulation of brain parenchyma. The unilateral temporal supracerebellar transtentorial approach allowed removal of the tumour and its arachnoidal attachment overlying the bony tentorium through a single craniotomy site. With this technique, resection completeness may be improved by removal of the tentorium and its overlying meningeal layers, but the major advantage remains an increased accessibility to the tumour with preservation of the main vascular structures and without excessive manipulation of the brain parenchyma. Puncture of CSF increased tumour visualisation and the range of atraumatic motion within the calvarium. Puncture of CSF (ventricular, cisternal or combined) allows the brain tissue to relax, increasing atraumatic surgical manipulation possibly through reduction of the brain volume. With correct positioning of the patient (head slightly elevated and flexed at 60°), the cerebellum shifts downward following cisternal CSF puncture. No complication following cerebral collapse was detected. Haemorrhage, meningeal tearing and cerebellar herniation have been described as dramatic consequences of CSF puncture in presence of brain tumours, but not in cases of surgical puncture associated with craniotomy. The single-window technique was used to prevent iatrogenic damage to the surrounding brain tissue during cleavage and excision of the meningioma. In cats, these tumours are relatively firm and an en bloc resection of these large masses (1.3–2.1 cm3) would automatically lead to excessive compression of the surrounding parenchyma (Yasargil 1994). Instead, the most accessible area of the tumour surface is opened and the centre of the tumour is enucleated using tumour forceps through this window. The first phase of the single-window technique permits reduction of the tumour mass and requires very little space. In fact, it creates the space necessary to dissect and free the capsule or pseudocapsule from the surrounding tissue and to remove the remainder of the tumour piece by piece from the wound. Finally, copious lavage of the surgical site stops bleeding from small vessels and helps to remove floating tumour cells.
Contrast-enhanced multislice spiral CT has proved to be an excellent diagnostic tool in meningiomas. The location of the tumour could be appraised in detail using the native axial and the multiplanary reformations. This is a valuable tool for planning the operative procedure, which may possibly be enhanced by three-dimensional reconstructions (Turrel et al 1986).
In combination with the application of intravenous iodine contrast medium, CT also offers a tool with which to accurately assess the extent of the resection postoperatively. Of the six cats in this series, postoperative imagery revealed subtotal resection in only one animal. In this case, re-operation was not considered a viable option because of the very small size and deep localisation of the remnant tissue. In one case, very extensive haemorrhage was observed in the postoperative CT. This cat died shortly after surgery. No complications related to the presence of postoperative pneumocephalus were observed in our craniotomy patient population.
The theoretical goal of meningioma surgery is complete resection in order to prevent tumour recurrence. However, this goal should not be pursued at any cost and the decision to leave a small tumour fragment in cases of tumour adhesions to critical structures can be associated with a long recurrence-free survival, challenging the assertion that subtotal removal regularly leads to early tumour recurrence (Yasargil 1994). The technique described is an alternative approach allowing removal of challenging tentorial meningiomas without excessive parenchyma manipulation. Radiation treatment, in combination with this technique, should be considered to provide the longest tumour-free period.
References
- Axlund T.W., Mc Glasson M.L., Smith A.N. Surgery alone or in combination with radiotherapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002), Journal of American Veterinary Association 221, 2002, 1597–1600. [DOI] [PubMed] [Google Scholar]
- Bret P., Guyotat J., Madarassy G., Ricci A.C., Signorelli F. Tentorial meningiomas. Report on twenty-seven cases, Acta Neurochirurgica 142, 2000, 513–526. [DOI] [PubMed] [Google Scholar]
- Firsching R., Synowitz H.J., Grimm C. Pitfalls in surgery of the free tentorial edge, Zentralblatt Neurochirurgie 64, 2003, 151–158. [DOI] [PubMed] [Google Scholar]
- Forterre F., Matis U., Schrell U., Geier M., Gutmannsbauer B., Schmahl W. Intracranial meningiomas – findings, therapy and results in nine cats and one dog, Tierärztliche Praxis 28, 2000, 170–177. [Google Scholar]
- Fukushima T., Mizoguchi T., Tsuchimochi H., Matsuda T., Tsugu H., Sakamoto S., Tomonaga M., Goto K., Maehara F. Clinicopathological study of meningiomas of the tentorium and its surrounding structures, No Shinkei Geka 19, 1991, 517–524, (abstract) [PubMed] [Google Scholar]
- Gordon L.E., Thacher C., Matthiesen D.T., Joseph R.J. Results of craniotomy for the treatment of cerebral meningioma in 42 cats, Veterinary Surgery 23, 1994, 94–100. [DOI] [PubMed] [Google Scholar]
- Harrison M.J., al-Mefty O. Tentorial meningiomas, Clinical Neurosurgery 44, 1997, 451–466. [PubMed] [Google Scholar]
- Jaggy A. Grosshirn, Atlas und Lehrbuch der Kleintierneurologie, 1st edn, 2004, Schlütersche Verlag: Hannover, pp. 391–430 [Google Scholar]
- Kakou M., Jan M. Tentorial meningioma. Surgical experience with 20 cases, Neurochirurgie 45, 1999, 15–23. [PubMed] [Google Scholar]
- Kleinhues P., Cavenee W.K. WHO Classification of Tumors. Pathology and Genetics – Tumours of the Nervous System, 2000, IARC Press: Lyon, pp. 176–184 [Google Scholar]
- Koestner A., Bilzer T., Fatzer R., Schulman F.Y., Summers B.A., van Winkle T.J. Histological Classification of the Tumors of the Nervous System of Domestic Animals 2nd Series vol. V, 1999, AFIP: Washington, p. 23. [Google Scholar]
- Nafe L.A. Meningiomas in cats: a retrospective clinical study of 36 cases, Journal of American Veterinary Medical Association 174, 1979, 1224–1227. [PubMed] [Google Scholar]
- Ono M., Rhoton A.L., Barry M. Microsurgical anatomy of the region of the tentorial incisura, Journal of Neurosurgery 60, 1984, 365–369. [DOI] [PubMed] [Google Scholar]
- Bley C. Rohrer, Surnova A., Roos M., Kaser-Hotz B. Irradiation of brain tumors in dogs with neurologic disease, Journal of Veterinary Internal Medicine 19, 2005, 849–854. [DOI] [PubMed] [Google Scholar]
- Samii M., Carvalho G.A., Tatagiba M., Matthies C., Vorkapic P. Meningiomas of the tentorial notch: surgical anatomy and management, Journal of Neurosurgery 84, 1996, 375–381. [DOI] [PubMed] [Google Scholar]
- Troxel M.T., Vite C.H., Van Winkle T.J., Newton A.L., Tiches D., Dayrell-Hart B., Kapatkin A.S., Shofer F.S., Steinberg S.A. Feline intracranial neoplasia: retrospective review of 160 cases (1985–2001), Journal of Veterinary Internal Medicine 17, 2003, 850–859. [DOI] [PubMed] [Google Scholar]
- Turrel J.M., Fike J.R., LeCouteur R.A. Computerized tomographic characteristics of primary brain tumors in 50 dogs, Journal of American Veterinary Medical Association 42, 1986, 275–281. [PubMed] [Google Scholar]
- Yasargil M.G. Microneurosurgery, 1st edn, 1994, Georg Thieme Verlag: Stuttgart, New York, Vols IVa and IVb. [Google Scholar]