Abstract
The purpose of this study was to analyse retrospectively a feline population with intracranial neoplastic diseases, to document seizure patterns in these animals and to determine whether partial seizures were more frequently associated with structural brain lesions then generalised seizures. In addition, a comparison was made within the population with intracranial neoplasia between two groups of cats: one with and one without seizures. Special emphasis was given to the evaluation of tumour type, localisation and size of the lesion and its correlation with seizure prevalence. Sixty-one cats with histopathological diagnosis of intracranial tumour were identified. Fourteen cats (23%; group A) had a history of seizure(s). Forty-seven cats (77%; group B) had no history of seizure(s). Generalised tonic-clonic seizures were seen in eight cats (57%) and were the most common seizure pattern in our cats with intracranial neoplasia. Clusters of seizures were observed in six cats. Status epilepticus was observed in one patient. The mean age of the cats was 7.9 years within group A (median 8.5) and 9.3 years (median 10) within group B. The cats with lymphoma within both groups were significantly younger than cats with meningioma. In both groups meningioma and lymphoma were confirmed to be the most frequent tumour type, followed by glial cell tumours. The prevalence of the seizures in patients with glial cell tumours was 26.7%, 26.3% in patients with lymphomas and 15% in cases with meningiomas. In 33 cases (54.1%) the tumours were localised in the forebrain, 15 tumours (24.6%) were in the brainstem, four (6.6%) in the cerebellum and nine tumours (14.7%) had multifocal localisation. Parietal lobe and basal ganglia mostly affected group A. In group B tumours were most frequently located in the parietal and frontal lobes as well as in the diencephalon. A positive association was documented between the localisation of a tumour in the forebrain and seizure occurrence.
Different causes of seizures are recognized in dogs and cats. Intracranial structural diseases or metabolic causes are believed to be more frequent causes of seizures in cats while idiopathic epilepsy is considered to be rare in this species. Infectious, vascular or neoplastic conditions are the most frequent structural brain diseases to cause seizures in cats (Parent and Quesnel 1996; Quesnel et al 1997; Platt 2001).
A different pattern of seizures is reported in cats in comparison with dogs. Primary generalised seizures are uncommonly observed in feline patients while partial and complex partial seizures prevail (Parent and Quesnel 1996). It has been noted that partial seizures are more frequently associated with structural brain lesion while generalised seizures are usually registered with metabolic or toxic diseases in cats (Parent and Quesnel 1996).
Intracranial tumours in cats are less common than in dogs (Zaki and Hurvitz 1976; Vandevelde 1984). Primary nervous system tumours in cats are mostly meningiomas. Lymphomas are the most frequent secondary central nervous system (CNS) tumours in this animal species (Summers et al 1995; Vite 2005).
Clinical manifestation of tumours in CNS depends on anatomical localisation (Bagley et al 1999; Beaumont and Whittle 2000). Seizures are a very common clinical sign of brain tumours in dogs (46–51%) (McGrath 1960; Foster et al 1988; Bagley et al 1999) and humans (20–45%) (Recht and Glantz 1997; Schaller and Rüegg 2003). The presence of seizures also depends on the type of tumour: low-grade, well-differentiated gliomas have the highest incidence of seizures in humans (Beaumont and Whittle 2000). However, the pathophysiology of tumour-related seizures has been only partly understood (Schaller and Rüegg 2003; Schaller 2005). Similar data are not available in veterinary epileptology.
There are no studies describing seizure pattern in cats with intracranial neoplasia. Several case reports and one study mentioned seizure type in cats with intracranial tumours (Sarfaty et al 1987; Fondevila et al 1998; Demierre et al 2002; Barnes et al 2004). The other references did not attempt to classify the seizures (Lawson et al 1984; Gallagher et al 1993; Noonan et al 1997; Quesnel et al 1997; Darbès et al 1998; Lu et al 2003; Troxel et al 2003).
The purpose of this study was to analyse retrospectively a feline population with intracranial neoplastic diseases, to document seizure pattern in these animals and to determine whether partial seizures were more frequently associated with structural brain lesions then generalised seizures. A comparison was made between two groups of cats in a population with intracranial neoplasia: one with seizure(s) and one without. Special emphasis was placed on the evaluation of tumour type, the localisation and size of the lesion and any correlation with seizure prevalence.
Materials and Methods
The clinical records of cats with the histopathological diagnosis of intracranial neoplasia were retrospectively evaluated. All cats were examined at the Institute of Animal Neurology, University of Bern, Switzerland, during the years 1985–2003. Only records with information about history, clinical and neurological examination and histopathological diagnosis were included. The cats were divided into two groups. Group A was composed of cats with a history of seizure(s) and group B comprised cats without reported seizures.
Data about breed, gender, age and history including the cat's environment; its age when the first seizure was observed; seizure pattern including frequency, duration and type of seizures; and clinical findings were all retrospectively evaluated.
Seizures were classified as partial, complex partial or generalised, based on clinical manifestation. Generalised seizures were characterised by bilateral and symmetrical motor activity, with or without accompanying autonomous signs such as salivation, urination and defecation and with or without loss of consciousness. Unilateral or asymmetrical motor signs without impairment of consciousness characterised simple partial seizures. Complex partial seizures were diagnosed when unilateral or asymmetrical motor signs occurred together with impaired consciousness, or when a purely transient and involuntary change in behaviour was observed, characterised by jumping, unprovoked biting and attacking of real or imaginary objects, and/or blindly running into objects. Secondary generalisation of simple or complex partial seizures was noted. Status epilepticus was defined as continuous epileptic activity or the presence of two or more separate seizure episodes of more than 30 min duration with no return to consciousness between them. Seizure clusters were diagnosed when two or more isolated seizures were observed during a 24-h period with the patient regaining consciousness between them. In order to define seizure frequency, the individual number of seizures per patient was counted. Seizures in clusters were counted as separate seizure episodes.
Seizures were primarily observed and described by owners. A detailed questionnaire was used during the first visit of the owner to our hospital in an attempt to record the animal's seizure history. In the seizuring cats, the owner was to describe only the individual seizure episodes, including the duration of the ictal and post-ictal phase and the frequency of the seizures. If the information given by the owner was incomplete, specific questions were asked (ie, convulsions on one or more limb, on the facial musculature, behaviour, consciousness, vegetative components, etc) to gain a complete picture of the type of the seizure. In some cats seizures were observed by the first opinion vet before the patient was referred to our hospital, and in three animals the seizure was documented during hospitalisation. In three cats the seizures were also filmed by the owner and then analysed by the neurologist in our hospital.
Diagnostic work comprised a clinical and neurological examination, a biochemistry profile, haematology, CSF examination and diagnostic imagines (X-ray, CT or MRI). Full diagnostics were not completed in every case. In all but two tumours a definitive histopathological diagnosis was performed at postmortem examination. In two animals from group B the definitive diagnosis was confirmed postoperatively after a craniotomy had been completed on the removed mass.
General pathology and neuropathology results were noted. The type of intracranial neoplasia and the localisation and size of the lesion were compared between the two groups of cats.
Tumours diagnosed as lesions occupying a single space were localised to one of the following brain sections: parietal, frontal, temporal, occipital or pyriform lobes, basal nuclei, diencephalon, mesencephalon, medulla oblongata and cerebellum. Any multifocal localisation was also registered, if three or more of the above-mentioned brain areas were affected by separate focal tumours (for example, lymphoma infiltrating frontal, parietal and temporal lobes and basal ganglia). An anatomical description of brain areas included the forebrain (cerebral cortex and basal ganglia), the brainstem (di-, mes- and myelencephalon) and the cerebellum. Tumour size was defined as the largest diameter of the solid mass. For statistical comparison astrocytoma, oligodendroglioma, gliomatosis cerebri, glioblastoma and ependymoma were put together in the group of glial cell tumours.
Frequencies and descriptive statistics were derived for age, breed, gender, environment, type and frequency of the seizure, the time from the first seizures or other first signs to presentation, the main complaint of the owner, the type of tumour, and the localisation and size of the lesion. The Kruskal–Wallis One-way ANOVA on Ranks was used to correlate the average age of the cats in groups A and B with the tumours and also to compare the study groups (A and B) with respect to the average interval from the appearance of the first sign to presentation and the incidence of the types of intracranial tumours. This test was also used to compare tumour size between both groups. The association between seizure occurrence (x/n) and the localisation of the lesion (x/y) was calculated using Fisher's exact test. Multifocal lesions were not included in these calculations. Confidence intervals (CI) were made as percentages owing to the small population of cats with intracranial tumours examined. Statistical analyses were performed with NCSS computer software (www.ncss.com). For all statistical analyses, P-values<0.05 were considered significant.
Results
Sixty-one cats with histopathological diagnosis of intracranial tumour were identified. Fourteen cats (23%) had a history of seizure(s) and comprised group A. Forty-seven cats (77%) with neoplastic brain disease had no history of seizure(s) and were included in group B. The distribution of types of tumours in both groups is presented in Table 1.
Table 1.
Numbers of cats with intracranial neoplasia within group A (cats with seizures) and group B (cats without seizures)
| Type of neoplasia | Group A | Group B | Groups A & B |
|---|---|---|---|
| Meningioma | 3 (4.9%) | 17 (27.9%) | 20 (32.8%) |
| Lymphoma | 5 (8.2%) | 14 (23%) | 19 (31.1%) |
| Astrocytoma | 3 (4.9%) | 6 (9.8%) | 9 (14.8%) |
| Metastatic tumours | 1 (1.6%) | 2 (3.3%) | 3 (4.9%) |
| Gliomatosis cerebri | 0 | 2 (3.3%) | 2 (3.3%) |
| Ependymoma | 0 | 2 (3.3%) | 2 (3.3%) |
| Osteosarcoma | 0 | 2 (3.3%) | 2 (3.3%) |
| Glioblastoma | 0 | 1 (1.6%) | 1 (1.6%) |
| Oligodendroglioma | 1 (1.6%) | 0 | 1 (1.6%) |
| Olfactory neuroblastoma | 1 (1.6%) | 0 | 1 (1.6%) |
| Hypophyseal adenocarcinoma | 0 | 1 (1.6%) | 1 (1.6%) |
| Total | 14 (23%) | 47 (77%) | 61 (100%) |
Group A
Data on the history and seizure pattern of cats from group A are summarised in Table 2. The mean age of the cats was 7.9 years (median 8.5 years, range 2–13 years). The mean age of the cats with lymphoma was 5.6 years (median 5 years), with meningioma 10.3 years (median 11 years) and with glial cell tumours 8.5 years (median 8 years). Domestic shorthair cats were the most frequently encountered (11/14; 78.6%; CI 57–100%). Seizure was the first sign observed by owners in nine cases (64.3%) and in eight cases (57.1%) the seizures were the main reason for visiting a veterinarian. The mean time between the first seizure and presentation was 66.4 days (median 14 days; range 1–365 days). In three cats other neurological deficits were noted 1 week (cats 1 and 3) and 3 weeks (cat 14) before the occurrence of the first seizure. The mean time between the first sign and presentation was 68.9 days (median 17.5 days; range 1–365 days). The two groups were compared with one another to establish any variations in the mean time between the first sign to presentation and the type of tumour. The mean time between the first sign to presentation in meningioma patients from both groups (A+B) was significantly longer (89.2 days) than in lymphoma (16.6 days) (P=0.0002) (Fig 1).
Table 2.
Summary of sex, age, breed, history and seizure characteristics of 14 cats from group A (cats with intracranial neoplasia and seizures)
| Cat 1–14 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Age (years) | 2 | 2 | 5 | 8 | 11 | 9 | 11 | 11 | 5 | 9 | 13 | 7 | 8 | 10 |
| Sex | m | f | m | mc | mc | m | fn | fn | f | mc | mc | fn | f | f |
| Breed | DSH | DSH | Persian | Birman | DSH | DSH | Angora | DSH | DSH | DSH | DSH | DSH | DSH | DSH |
| Environment | out | out | out | in | out | out | in | out | out | out | in | out | out | out |
| Seizure was first historical sign | no | yes | no | yes | yes | yes | yes | yes | yes | no | yes | yes | no | no |
| Seizure was primary presenting complaint | no | yes | no | no | no | no | yes | yes | yes | yes | yes | yes | no | yes |
| Interval between first seizure and presentation | 7D* | 1D | 1D* | 14D | 1D | 60D | 14D | 365D | 21D | 30D | 14D | 365D | 30D | 1D* |
| Type of seizure | CP | G | G | G+P | G | CP | CP | G | G | P+G | CP→G | G | G | G |
| Number of seizures | 4 | 3 | 5 | 1G+xP | 1 | 10 | 8 | 4 | 5 | 6 | 2 | 12 | 4 | 1 |
| Frequency of seizures | >3/W | – | – | 1/2W | – | 1/W | >3/W | 2/Y | 1–2/W | 1–2/W | 1/W | 1/4W | 1/W | – |
| Cluster/status epilepticus | – | C (3) | C (5) | C (xP) | – | – | – | C (3) | – | C (5) | – | SE | – | C |
| Seizure duration (min) | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 4 | 2 | 1 | 5 | 1 | – | 1 |
| Histopathological localisation | basal nc. le | parietal le | multifocal | diencephalon | frontal ri | parietal ri | parietal ri | temporal le | parietal le | basal nc. le | basal nc. le | pyriform l. ri | frontal bs | multifocal |
| Tumour type | L | L | L | L | L | M | M | M | A | A | A | ODG | ONB | BC |
m=male, mc=male castrated, f=female, fn=female neutered, DSH=domestic shorthair, out=outdoor cat, in=indoor cat, D=day, W=week, Y=year, P=simple partial seizure, CP=complex partial seizure, G=generalised seizure, 1G+xP=1 generalised and many (x) partial seizures, CP→G=complex partial seizure with secondary generalisation, C=cluster, SE=status epilepticus, >3/W=more than three seizures in 1 week, 1/2W=one seizure per 2 weeks, le=left, ri=right, nc.=nuclei, l.=lobe, L=lymphoma, M=meningioma, A=astrocytoma, ODG=oligodendroglioma, ONB=olfactory neuroblastoma, BC=bronchogenic carcinoma (brain metastases).
The other neurological deficits was already seen 1–3 weeks before the first seizure was present.
Fig 1.
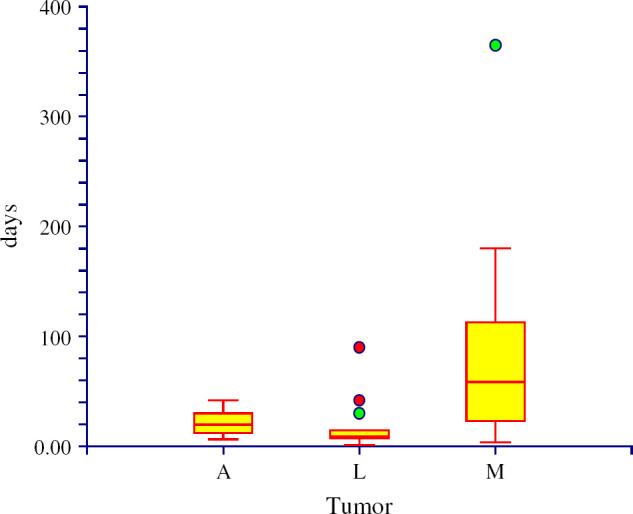
The time between the first sign and the presentation within groups A+B (A=cats with seizure, B=cats without seizure). Comparison between the three most presented tumours: A=astrocytoma, L=lymphoma, M=meningioma. The mean time from the first sign to the presentation between the meningioma (89.2 days) and lymphoma (16.6 days) was significantly different (P=0.0002).
The incidence of seizure in all cats with intracranial tumours was 23% (14/61; CI 12.4–33.5%). Generalised tonic-clonic seizures were seen in eight cats (57.1%; CI 31.2–83%). Both generalised and partial seizures were observed in two cats (14.3%; CI 0–32.6%). Three cats suffered exclusively complex partial seizures (21.4%; CI 0–42.9%). One cat had partial complex seizures with secondary generalisation (7.1%; CI 0–20.6%). Clusters of seizure were observed in six cats (43%; CI 16.9–68.8%). One cat (case 4) had clusters of partial seizures characterised by motor activity of the masticatory and facial muscles. In four cats (cases 2, 3, 8 and 14) clusters of generalised seizures and, in cat 10, clusters of both simple partial and generalised seizures were noted. Single separate seizures were observed prior to the cluster in four cats (cases 4, 8, 10 and 14). In two cats (cases 2 and 3) only seizure clusters were observed. Cat 2 died at home after the third seizure. No prior abnormalities were noted in this cat. Cat 3 was euthanased at the request of the owner because of seizure clusters. Status epilepticus was observed in one patient (cat 12). This cat had 12 generalised seizures during the year before presentation in our clinic and was under therapy with phenobarbital (3 mg/kg, bid).
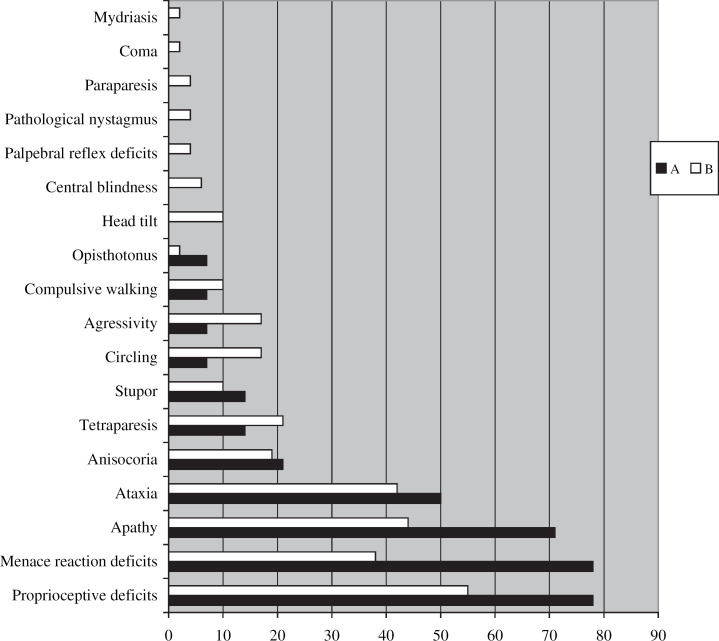
All living cats examined at our clinic (13/14) showed neurological deficits, which allowed accurate neuro-anatomical localisation. Clinical signs relating to neoplastic conditions were noted, including nasal discharge in a cat with olfactory neuroblastoma and abnormal lung sounds in a cat with bronchogenic carcinoma. Objective information about cat 2 is missing as animal died at home after a cluster of seizures. Neurological abnormalities are compared with group B in Fig 2. No cat from group A was diagnosed ante-mortem. Ten cats were euthanased after clinical assessment at the owners' request.
Fig 2.
Prevalence (x%) of the neurological deficits (y) in cats from group A (cats with seizures) and group B (cats without seizures).
Group B
The mean age of cats from group B was 9.3 years (median 10 years, range 1–16 years). The mean age of cats with lymphoma was 7.7 years (median 7 years), with meningioma 10.6 years (median 11 years) and with glial cell tumours 7.3 years (median 8 years). The cats with lymphoma were significantly younger than the cats with meningioma (P=0.04). Domestic shorthair cats also dominated in group B (42/47; 89.4%; CI 80.5–98.2%). There were also three Siamese, one Persian and one Abyssinian cat. Nine cats were intact males (19.1%), 16 were castrated males (34.1%), 12 were intact females (25.5%), and 10 were spayed females (21.3%). The mean time from the first signs to presentation in cats from group B was 42.1 days (median 21 days; range 2–365 days) and comparison with group A did not highlight any statistical differences.
An ante-mortem diagnosis was performed on four cats with meningioma (using MRI and/or CT examination). A pituitary tumour and brain metastasis were strongly suspected in another cat after a CT scan. Craniotomy was successfully performed in two meningioma cases and both cats were alive at the time of writing this manuscript. The postoperative observation periods were 2 and 3.5 years in these animals.
Pathological Examination, Groups A and B
The mean age of all cats (groups A and B) was 8.9 years (median 9 years). A general pathological examination was performed in 11 cases from group A (cats 1–4, 6, 7, 10–14). Significant changes revealed under general pathological examination included a mass in the ethmoidal and frontal sinuses, which was later identified as an olfactory neuroblastoma (cat 13), as well as nodules of bronchoepithelial carcinoma in lungs, kidneys and in the fifth right intercostal muscles in cat 14.
General pathology within group B was performed on all but four cats (two meningioma cases are still alive after craniotomies and, in two meningioma patients, only brain neuropathology was performed). Generalised lymphoma was diagnosed in 10 cats involving abdominal as well as retroperitoneal lymph nodes, kidneys and liver. Mammary gland carcinoma was diagnosed in a cat with metastasis of this tumour in the cerebellum. Results from general pathology carried out on the remaining cats from group B were normal.
Meningioma and lymphoma were the most frequently confirmed tumours. Primary intracranial lymphoma was diagnosed in seven cases (3=group A; 4=group B). The third most common intracranial tumour in our study was astrocytoma. All glial cell tumours were represented in 24.6% (15/61; CI 13.8–35.4%) cases. The prevalence of seizures in glial cell tumours was 26.7% (4/15; CI 4–49%), 26.3% (5/19; CI 6.5–46.1%) in lymphomas, and 15% (3/20; CI 0–30.6%) in meningiomas.
The neuro-anatomical localisation of the tumours is summarised in Table 3. In 33 cases (54.1%; CI 41.6–66.6%) the tumours were localised in the forebrain. Brainstem localisation was noted in 15 tumours (24.6%; CI 13.8–35.4%) and, in four animals (6.6%; CI 0.3–12.8%), the cerebellum was affected. In nine cases (14.7%; CI 5.9–23.6%) multifocal localisation was recorded. Eleven cats (11/14; 78.6%; CI 57–100%) from group A and 22 animals (22/47; 46.8%; CI 32.5–61.1%) from group B had a single lesion localised only in the forebrain. The forebrain was affected in all cats with multifocal tumour localisation. The parietal lobe (4/14; 28.6%; CI 4.9–52.2%) and basal nuclei (3/14; 21.4%; CI 0–42, 9%) were most frequently affected within group A. The parietal and frontal lobes as well as the diencephalon were the most frequent tumour localisations within group B (8/47; 17%; CI 6.3–27.8%).
Table 3.
Histopathological localisation of the tumours diagnosed in cats from group A (cats with seizures) and from group B (cats without seizures)
| Localisation | All tumours | M | L | G | ONB | PA | MC | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A+B | A | B | A | B | A | B | A | B | A | B | B | |
| Forebrain | 11 | 22 | 33 | 3 | 12 | 3 | 2 | 4 | 7 | 1 | 0 | 0 | 0 | 1 |
| Parietal lobe | 4 | 8 | 12 | 2 | 5 | 1 | 1 | 2 | 1 | |||||
| Frontal lobe | 2 | 8 | 10 | 3 | 1 | 2 | 3 | 1 | ||||||
| Basal nuclei | 3 | 2 | 5 | 1 | 2 | 2 | ||||||||
| Temporal lobe | 1 | 2 | 3 | 1 | 2 | |||||||||
| Occipital lobe | 0 | 2 | 2 | 2 | ||||||||||
| Pyriform lobe | 1 | 0 | 1 | 1 | ||||||||||
| Diencephalon | 1 | 8 | 9 | 3 | 1 | 2 | 2 | 1 | ||||||
| Cerebellum | 0 | 4 | 4 | 2 | 1 | 1 | ||||||||
| Mesencephalon | 0 | 3 | 3 | 1 | 1 | 1 | ||||||||
| Medulla oblongata | 0 | 3 | 3 | 2 | 1 | |||||||||
| Multifocal | 2 | 7 | 9 | 1 | 6 | 1 | 1 | |||||||
| Total | 14 | 47 | 61 | 3 | 17 | 5 | 14 | 4 | 11 | 1 | 1 | 1 | 2 | 2 |
M=meningioma, L=lymphoma, G=glial cell tumour, ONB=olfactory neuroblastoma, PA=pituitary adenocarcinoma, MC=metastatic carcinoma, OS=osteosarcoma.
The prevalence of seizuring animals with neoplasia in the forebrain was 33.3% (11/33; CI 17.2–49.4%). A positive association between localisation of a tumour in the forebrain and seizure occurrence was found (P<0.05). The animals with multifocal lesions were not included in these calculations.
The mean size of the focal lesions in 11 cats from group A was 1.09 cm (median 1.25 cm; range 0.5–2 cm). When compared with the mean size of the tumours in group B, no statistical difference was found (mean 1.35 cm; median 1.6 cm; range 0.2–3 cm).
Discussion
The pathophysiology of tumour-related seizures has been only partly understood. The pathophysiological basis of seizures associated with brain tumours is related to different biochemical, anatomical and physiological changes that shift the balance between intracortical inhibitory and excitatory mechanisms toward excitation (Beaumont and Whittle 2000; Schaller and Rüegg 2003; Schaller 2005). The peritumoral tissue is of particular interest, as it seems to have a greater relationship to the generation, maintenance and propagation of seizures. A number of different mechanisms have been proposed that may influence tumour-induced epileptogenesis, including disturbances in peritumoral amino acids levels, metabolic imbalance, oedema, pH alterations, and morphological changes in the neuropil (Schaller and Rüegg 2003; Nakase and Naus 2004; Schaller 2005).
The average age of our cats with intracranial neoplasia was similar to that in some of the previous reports (Lawson et al 1984; Sarfaty et al 1987; Noonan et al 1997; Quesnel et al 1997; Fondevila et al 1998; Demierre et al 2002; Lu et al 2003). Other authors have documented the median age of cats with brain tumours as 10 or 11.3 years (Dewey et al 2000; Troxel et al 2003). Our cats were younger (median 9 years), most probably because of a relatively higher number of lymphomas. The median age of dogs with intracranial tumours was 9 years (Bagley et al 1999; Dewey et al 2000). Bagley et al (1999) reported that seizures with intracranial neoplasia were seen in 32% of dogs 8 years of age or younger and in 65% of dogs older than 8 years. In our study 50% cats with seizures were 8 years old or younger. In humans, there is bimodal age distribution of brain tumours, with the first peak incidence in children (meduloblastoma, pilocytic astrocytoma), and a second, larger peak in adults aged 45–70 years (glioma, glioblastoma and meningioma) (Lantos et al 2002).
Domestic shorthair cats are generally predominant in cat populations and no breed predisposition for particular types of tumour has been reported till now. Troxel et al (2003) reported a male:female ratio of 1, 5:1 for the occurrence of the intracranial neoplasia in cats. In humans, gliomas and embryonal tumours occur more frequently in the male population, whereas meningiomas preferentially affect women (Lantos et al 2002).
Seizures are often the first sign of intracranial tumours; they frequently predate other symptoms or diagnosis by many years in humans (Beaumont and Whittle 2000). Sixteen months was the mean time from the first seizure to diagnosis of a tumour in one human study (Liigant et al 2001). The situation in animals is often considered to be different: animals with brain tumours usually exhibit neurological abnormalities at the time of presentation (Bagley and Gavin 1998; Bagley et al 1999; Dewey et al 2000). Exceptions might be tumours of the olfactory or rostral frontal lobe (Foster et al 1988; Smith et al 1989). Neurological abnormalities observed between our two groups of cats, with and without seizures, were similar to and comparable with the previous reports (Dewey et al 2000; Troxel et al 2003). All but one cat with seizures (group A) were abnormal on neurological examination. The mean interval between the first sign and presentation was significantly longer in meningioma (89.2 days) than in lymphoma (16.6 days) patients. Our result and the results from the previous study (Troxel et al 2003) most probably reflect the quicker growth of lymphoma as compared with meningioma. Meningiomas are slow-growing tumours, and about 50% of meningiomas do not manifest any clinical signs in cats. The diagnosis is therefore confirmed incidentally during a pathological examination (Summers et al 1995; Lu et al 2003).
The incidence of seizures in our population (23%) was similar to that found in the other retrospective study (22.5%) (Troxel et al 2003). The incidence of seizures in dogs with intracranial neoplasia was reported in 46–51% of affected animals (McGrath 1960; Foster et al 1988; Bagley and Gavin 1998; Bagley et al 1999). Seizures occur in approximately 20–45% of adult humans (Recht and Glantz 1997; Liigant et al 2001; Schaller and Rüegg 2003) and in 1–10% of children with brain tumours (Wojcik-Draczkowska et al 2003).
The type of intracranial tumour is an important factor in seizure genesis. Humans with low-grade, well-differentiated gliomas experience seizures most frequently (89–90% in oligodendrogliomas and 60–66% in astrocytomas) (Beaumont and Whittle 2000). Liigant et al (2001) and Oberndorfer et al (2002) also reported seizures in astrocytomas (42%, 69%), oligodendrogliomas (53%, 50%) and mixed gliomas (62%, 65%). Glial cell tumours are not generally very common in cats but a similar tendency towards seizure prevalence in these tumours has been observed. The highest incidence of seizures was in astrocytoma patients (3/9; 33, 3%) amongst our cats. The incidence of seizures in all glial cell tumours was 26.7% (4/15). A Troxel study (2003) also recorded high incidences of seizures in cats with astrocytoma (4/6), oligodendroglioma (3/6) and ependymoma (3/7). These findings have to be interpreted cautiously as only a small number of animals were available for evaluation in both reports. On the other hand, seizures were a very common sign in cats with lymphoma (10/14; 71, 4%) in the other study (Noonan et al 1997). Seizure prevalence in our lymphoma patients was the second highest (5/19; 26%) after the glial cell tumours. Three of five cats with intracranial lymphoma from group A suffered from primary lymphoma. Primary lymphoma was less frequent amongst non-seizuring cats (4/14). Similar observations have been made in humans. The number of seizures in metastatic tumours in brain parenchyma was lower than in patients with a primary brain tumour (Recht and Glantz 1997; Oberndorfer et al 2002; Wojcik-Draczkowska et al 2003). It has been reported that seizures occur in approximately 26–41% of humans with intracranial meningioma (Beaumont and Whittle 2000; Lieu and Howng 2000). Unfortunately, to the knowledge of the present authors, no information is available about seizure prevalence in different tumour types amongst the canine population.
Intracranial tumours are frequently localised in the forebrain of dogs, cats and humans (Zaki and Hurvitz 1976; Vandevelde 1984; Recht and Glantz 1997; Bagley et al 1999; Troxel et al 2003). Our results support this notion. The cerebral cortex is the primary element in the generation of epileptic seizure, but seizure can also originate in the thalamo-cortical interactive system or in the brainstem (Fisher et al 2005). Not every cortical area has the same predisposition towards the development of epileptic activity. The temporal lobe, primary motor and primary sensory cortical area, the frontal parasagittal region of the supplementary motor area and the secondary somatic sensory areas have the lowest thresholds for producing seizures, whilst the occipital region has a much higher threshold (Beaumont and Whittle 2000). The frontal lobes (44%), olfactory region (20%) and parietal lobes (20%) were more often associated with seizures in dogs (Bagley et al 1999). The parietal and frontal cortex was also the most common localisation of tumours in our cats. None of the tumours within group A were localised in the occipital lobe. The incidence of tumours in the basal ganglia was higher in our seizuring group.
Little is known about the relationship between the seizure type and the intracranial lesion localisation in companion animals. Most available knowledge is taken from human epileptology or experimental studies and applied to cats and dogs in veterinary medicine. In humans, temporal lobe lesions frequently cause complex partial seizures, and partial motor and/or sensory seizures are common with lesions affecting the primary motor or sensory fronto-parietal cortex (Beaumont and Whittle 2000). The present authors have reviewed literature describing seizure types in cats with intracranial neoplasia. Seizure type was mentioned only sporadically in some reports (Sarfaty et al 1987; Fondevila et al 1998; Demierre et al 2002; Barnes et al 2004). Partial and complex partial seizures are usually reported as the most common type of seizures in cats and are usually associated with structural brain lesion (Parent and Quesnel 1996; Kline 1998). In some studies of feline epilepsy, generalised seizures dominated (Quesnel et al 1997; Barnes et al 2004). Generalised seizures were the most common seizure pattern in our cats with intracranial neoplasia. Both complex partial (2/4) and generalised (2/4) seizures have been observed in cats with parietal lobe tumours; predominantly complex partial seizures have been observed in all three cats with a tumour in the basal nuclei; and exclusively generalised seizures were seen in both of our cats with frontal lobe tumours. In the absence of video recordings, slow-motion analyses of convulsions, or ictal EEG recordings, it is difficult to interpret the seizure type accurately. Some of the seizures from the present study were observed in the hospital by the authors directly or filmed by the owner and then analysed in the hospital by a neurologist. Nevertheless, the short and/or subtle initial focal manifestation of the primary epileptic focus might have gone unnoticed, and the total number of the primary generalised seizures in our population might have been over diagnosed.
Interestingly, in humans the age of the patient is important in the type of the seizure the patient is suffering, as secondary generalised seizures were mainly observed in children, while simple and complex partial seizures were more frequently noted in adults (Wojcik-Draczkowska et al 2003). We compared the type of seizure in two age groups: cats of 8 years and younger, and those older than 8 years. In both groups partial and generalised seizures were diagnosed. Younger group included more individuals with exclusively generalised seizures (5/7) while partial seizures prevailed in the older group (4/7). Nevertheless, no statistical significance was observed.
Seizure clusters or status epilepticus usually document progression of the clinical signs in patients with intracranial neoplasia. Status epilepticus due to intracranial neoplasia in dogs and humans ranges between 3.6% and 15% (Towne et al 1994; DeLorenzo et al 1995; Bateman and Parent 1999; Knake et al 2001; Platt and Haag 2002; Gandini et al 2003). Status epilepticus was observed in just one cat in our study but clusters were very common amongst our cats (42.9%). Occurrence of status epilepticus and seizure clusters is most probably multifactorial and depends on the tumour type and localisation, the exhaustion of brain compensatory mechanisms due to increasing tumour volume, the development of vasogenic oedema, the induction of obstructive hydrocephalus and eventual tumour-associated acute haemorrhage (Bagley 1996; Moore et al 1996; Dewey et al 2000). Secondary hydrocephalus and haemorrhage were not observed in any of our cats, whether they suffered from seizures or not. This finding indicates that other factors are playing a more important role in seizure genesis than these two possible secondary complications.
The present authors believe that a documented case history and clinical findings together with a histopathological diagnosis of intracranial tumours can help to advance our understanding of the pathogenesis of tumour-associated epilepsy. The study suggests that generalised seizures are the most common seizure pattern in cats with intracranial neoplasia. Our results also clearly indicate that anatomical tumour location in the cerebral cortex and basal nuclei, regardless of the size or biological nature of the tumour, has a marked correlation with the generation of epileptic activity. Similar findings in the case histories, interictal neurological deficits, tumour size and type in both our groups of cats indicate that the answer to the question as to why some of the cats suffer seizures and some not may well be hidden in the cellular interaction between the tumour and the surrounding nerve cells. Individual predisposition to seizures should be not underestimated. To solve the problem, we shall have to await the development and classification of suitable animal models able to demonstrate the correlation between clinical manifestation and physiological changes (Beaumont and Whittle 2000).
References
- Bagley R.S. Pathophysiologic sequelae of intracranial diseases, Veterinary Clinics of North America Small Animal Practice 26, 1996, 711–733. [PubMed] [Google Scholar]
- Bagley R.S., Gavin P.R. Seizures as a complication of brain tumours in dogs, Clinical Techniques in Small Animal Practice 13 (3), 1998, 179–184. [DOI] [PubMed] [Google Scholar]
- Bagley R.S., Gavin P.R., Moore M.P., Silver G.M., Harrington M.L., Conors R.L. Clinical signs associated with brain tumours in dogs: 97 cases (1992–1997), Journal of American Veterinary Medical Association 215 (6), 1999, 818–819. [PubMed] [Google Scholar]
- Barnes H.L., Chrisman C.L., Mariani C.L., Sims M., Alleman A.R. Clinical signs, underlying cause, and outcome in cats with seizures: 17 cases (1997–2002), Journal of American Veterinary Medical Association 225 (11), 2004, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Bateman S.W., Parent J.M. Clinical findings, treatment, and outcome of dogs with status epilepticus or cluster seizures: 156 cases (1990–1995), Journal of American Veterinary Medical Association 215 (10), 1999, 1463–1468. [PubMed] [Google Scholar]
- Beaumont A., Whittle I.R. The pathogenesis of tumor associated epilepsy, Acta Neurochir 142, 2000, 1–15. [DOI] [PubMed] [Google Scholar]
- Darbès J., Majzoub M., Breuer W., Hermanns W. Large granular lymphocyte leukemia/lymphoma in six cats, Veterinary Pathology 35, 1998, 370–379. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R.J., Pellock J.M., Towne A.R., Boggs J.G. Epidemiology of status epilepticus, Journal of Clinical Neurophysiology 12 (4), 1995, 316–325. [PubMed] [Google Scholar]
- Demierre S., Bley T., Botteron C., Fatzer R., Jaggy A. Intrakranielle astrozytome bei acht katzen: Klinische und pathologische befunde, Schweizerischer Archiv Tierheilkunde 144, 2002, 66–73. [DOI] [PubMed] [Google Scholar]
- Dewey C.W., Bahr A., Ducoté J.M., Coates J.R., Walker M.A. Primary brain tumours in dogs and cats, Compendium of Continuing Education for the Practicing Veterinarian 22, 2000, 756–762. [Google Scholar]
- Fisher R.S., van Boas W. Emde, Blume W., Elger C., Genton P., Lee P., Engel J., Jr. Epileptic seizure and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE), Epilepsia 46 (4), 2005, 470–472. [DOI] [PubMed] [Google Scholar]
- Fondevila D., Vilafranca M., Pumarola M. Primary central nervous system T-cell lymphoma in a cat, Veterinary Pathology 35, 1998, 550–553. [DOI] [PubMed] [Google Scholar]
- Foster E.S., Carrillo J.M., Patnaik A. Clinical signs of tumours affecting the rostral cerebrum in 43 dogs, Journal of Veterinary Internal Medicine 2, 1988, 71–74. [DOI] [PubMed] [Google Scholar]
- Gallagher J.G., Berg J., Knowles K.E., Williams L.L., Bronson R.T. Prognosis after surgical excision of cerebral meningiomas in cats: 17 cases (1986–1992), Journal of American Veterinary Medical Association 203 (10), 1993, 1437–1440. [PubMed] [Google Scholar]
- Gandini G., Fluehmann G., Brini E., Cizinauskas S., Jaggy A. (2003) Status epilepticus in the dog: retropective evaluation of 41 cases. Proceedings to 16th Annual Symposium of European Society of Veterinary Neurology.
- Kline K.L. Feline epilepsy, Clinical Techniques in Small Animal Practice 13 (3), 1998, 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knake S., Rosenow F., Vescovi M., Oertel W.H., Mueller H.H., Wirbatz A., Katsarou N., Hamer H.M. Incidence of status epilepticus in adults in Germany: a prospective, population-based study, Epilepsia 42 (6), 2001, 714–718. [DOI] [PubMed] [Google Scholar]
- Lantos P.L., Louis D.N., Rosenblum M.K., Kleihues P. Tumours of the nervous system, 7th edn, Greennfield's Neuropathology Vol. II, 2002, Arnold: London, pp. 767–1052 [Google Scholar]
- Lawson D.C., Burk R.L., Prata R.G. Cerebral meningioma in the cat: diagnosis and surgical treatment of 10 cases, Journal of American Animal Hospital Association 20, 1984, 333–342. [Google Scholar]
- Lieu A.S., Howng S.L. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors, Epilepsy Research 38 (1), 2000, 45–52. [DOI] [PubMed] [Google Scholar]
- Liigant A., Haldre S., Oun A., Linnamagi U., Saar A., Asser T., Kaasik A.E. Seizure disorders in patients with brain tumours, European Neurology 45 (1), 2001, 46–51. [DOI] [PubMed] [Google Scholar]
- Lu D., Lamb R.L., Targett M.P. Concurrent benign and malignant multiple meningiomas in a cat: clinical, MRI and pathological findings, Veterinary Record 152, 2003, 780–782. [DOI] [PubMed] [Google Scholar]
- McGrath J.T. Intracranial neoplasms, Neurologic Examination of the Dog, 2nd edn, 1960, Lea & Febiger: Philadelphia, pp. 148–195 [Google Scholar]
- Moore M.P., Rodney S., Bagley R.S., Harrington M.L., Gavin P.R. Intracranial tumours, Veterinary Clinics of North America Small Animal Practice 26, 1996, 759–777. [DOI] [PubMed] [Google Scholar]
- Nakase T., Naus C.C. Gap junctions and neurological disorders of the central nervous system, Biochimica Biophysica Acta 1662 (1–2), 2004, 149–158. [DOI] [PubMed] [Google Scholar]
- Noonan M., Kline K.L., Meleo K. Lymphoma of the central nervous system: a retrospective study of 18 cats, Compendium of Continuing Education for the Practicing Veterinarian 19 (4), 1997, 497–504. [Google Scholar]
- Oberndorfer S., Schmal T., Lahrmann H., Urbanis S., Lindner K., Grisold W. The frequency of seizures in patients with primary brain tumours or cerebral metastasis. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Keiser Franz Josef Hospital, Vienna, Wien Klin Wochenschr 114, 2002, 911–916. [PubMed] [Google Scholar]
- Parent J.M., Quesnel A.D. Seizures in cats, Veterinary Clinics of North America: Small Animal Practice 26 (4), 1996, 811–825. [PubMed] [Google Scholar]
- Platt S.R. Feline seizure control, Journal of American Animal Hospital Association 37, 2001, 515–517. [DOI] [PubMed] [Google Scholar]
- Platt S.R., Haag M. Canine status epilepticus: a retrospective study of 50 cases, Journal of Small Animal Practice 43, 2002, 151–153. [DOI] [PubMed] [Google Scholar]
- Quesnel A.D., Parent J.M., McDonell W., Percy D., Lumsden J.H. Diagnostic evaluation of cats with seizure disorders: 30 cases (1991–1993), Journal of American Veterinary Medical Association 210 (1), 1997, 65–71. [PubMed] [Google Scholar]
- Recht L.D., Glantz M. Neoplastic diseases. Engel J., Pedley T.A. Epilepsy: A Comprehensive Textbook, 1997, JB Lippincott Co.: Philadelphia, 2579–2585. [Google Scholar]
- Sarfaty D., Carrillo J.M., Patnaik A.K. Cerebral astrocytoma in four cats: clinical and pathological findings, Journal of American Veterinary Medical Association 191, 1987, 976–978. [PubMed] [Google Scholar]
- Schaller B. Influence of brain tumor-associated pH changes and hypoxia on epileptogenesis, Acta Neurologica Scandinavia 111, 2005, 75–83. [DOI] [PubMed] [Google Scholar]
- Schaller B., Rüegg S.J. Brain tumor and seizures: pathophysiology and its implications for treatment revisited, Epilepsia 44 (9), 2003, 1223–1232. [DOI] [PubMed] [Google Scholar]
- Smith M.O., Turrel J.M., Bailey C.S., Gain G.R. Neurologic abnormalities as the predominant signs of neoplasia of the nasal cavity in dogs and cats: seven cases (1973–1986), Journal of American Veterinary Medical Association 195, 1989, 242–245. [PubMed] [Google Scholar]
- Summers B.A., Cummings J.F., de Lahunta A. Tumours of the Central Nervous System, Veterinary Neuropathology, 1995, Mosby: St. Louis, pp. 351–401 [Google Scholar]
- Towne A.R., Pellock J.M., DeLorenzo R.J. Determinant of mortality in status epilepticus, Epilepsia 35 (1), 1994, 27–34. [DOI] [PubMed] [Google Scholar]
- Troxel M.T., Vite C.H., Van Winkle J., Newton A.L., Tiches D., Dayrell-Hart B., Kapatkin A.S., Shofer F.S., Steinberg S.A. Feline intracranial neoplasia: retrospective rewiew of 160 cases (1985–2001), Journal of Veterinary Internal Medicine 17, 2003, 850–859. [DOI] [PubMed] [Google Scholar]
- Vandevelde M. (1984) Brain tumors in domestic animals: an overview. Abstract for Conference on Brain Tumours in Man and Animals. National Institute of Environmental Health Sciences, Research Triangle Park, NC. [Google Scholar]
- Vite C.H. Neoplasia of the nervous system. Vite C.H. Braund's Clinical Neurology in Small Animals – Localization, Diagnosis and Treatment, 2005, IVIS: Ithaca, New York. [Google Scholar]
- Wojcik-Draczkowska H., Mazurkiewitcz-Beldzinska M., Mankowska B., Dilling-Ostrowska E. Epileptic seizures as a manifestation of brain tumours: clinical and electroencephalographic correlations, Przegl Lek 60 (1), 2003, 42–44. [PubMed] [Google Scholar]
- Zaki F.A., Hurvitz A.I. Spontaneous neoplasms of the central nervous system of the cat, Journal of Small Animal Practice 17, 1976, 773–782. [DOI] [PubMed] [Google Scholar]