Abstract
In 155 cats, both with and without clinical signs of hyperthyroidism, total thyroxine (TT4) concentrations were compared to a sensitive, semi-quantitative thyroid palpation technique. On the basis of TT4 concentrations, 23 of the 155 cats were classified as hyperthyroid. The size of individual thyroid glands was scored between ‘0’ (non-palpable) and a maximum of ‘6’. One or more enlarged thyroid glands (score >0) were palpated in 22 of the 23 hyperthyroid cats and in 78 of the 132 euthyroid cats. However, none of the 132 euthyroid cats had a thyroid lobe score of greater than ‘3’ whereas 18 of the 23 hyperthyroid cats had a thyroid lobe score of ‘4’ or greater, and in two of the five that had scores below ‘4’ there was evidence of intrathoracic functional thyroid tissue on scintigraphy.
Hyperthyroidism is considered the most common endocrine disorder of cats, with histopathological examination of affected tissues indicating that the vast majority of cases are caused by functional thyroid adenomas, and a small proportion (1–2%) being caused by thyroid carcinoma (Turrel 1988, Feldman 1996). Despite its prevalence, the underlying cause of hyperthyroidism in the cat remains unknown (Peterson 1999), although current theories include immunological, nutritional, and environmental factors that either alone, or in combination, lead to molecular aberrations that contribute to the disease (Kass et al 1999, Hammer et al 2000).
A diagnosis of hyperthyroidism is usually made using a combination of clinical signs, thyroid palpation, appropriate laboratory tests and/or imaging findings. Characteristic clinical signs include weight loss, polyphagia, vomiting, diarrhoea, polyuria, polydipsia, hyperactivity and an unkempt hair coat (Peterson 1999). The laboratory test most commonly employed to confirm a diagnosis is the determination of serum total thyroxine concentration (TT4). Free T4 (fT4) testing has also been employed, but is less suitable as a screening test due to elevated levels in some euthyroid cats (Mooney et al 1996, Peterson et al 2001). Nevertheless, fT4 concentrations may help in the diagnosis of occult disease, as may thyroid function tests such as the triiodothyronine suppression test (Peterson et al 1990). 99mTc scanning is recommended when the results of blood tests are equivocal (Peterson 1999).
Thyroid palpation is a standard part of a physical examination and can be an important aid in the diagnosis of hyperthyroidism. Although its accuracy will inevitably vary from one clinician to another, based on natural ability, technique, and experience, it has been reported that more than 90% of hyperthyroid cats have palpably enlarged thyroid glands (Peterson et al 1983, Thoday & Mooney 1992). Recently, however, a study has reported on the presence of non-functional thyroid adenomatous hyperplasia in cats (Chaitman et al 1999), and, to the authors' knowledge, there are currently no published reports on the prevalence of palpable goitre in cats of different ages and its relationship to TT4 values.
The purpose of this study was, therefore, to undertake systematic thyroid palpation in a group of cats both with and without clinical signs of hyperthyroidism seen at a first opinion hospital and to relate these findings to serum assay of TT4.
Materials and methods
Data collection
Patient records of cats presented for examination to Acres North Animal Hospital, San Antonio, TX from July to November 1999 were reviewed. Data were obtained from 155 cats in which thyroid palpation results (using a standard sized technique) were recorded and correlated with TT4 values. Clinical signs that prompted the examination were also recorded. In many cases, no clinical signs were present (ie, wellness examinations), or the presenting clinical signs were not indicative of hyperthyroidism, or the diagnosis was of non-thyroidal disease. The earliest data were collected on cats whose examination dated back to 1994. However, the vast majority of the data was derived from examinations during the 12 months prior to the close of the study period. The data also include cats whose initial examination occurred during the course of the study. Therefore, some data are retrospective, and others are prospective.
Thyroid palpation technique
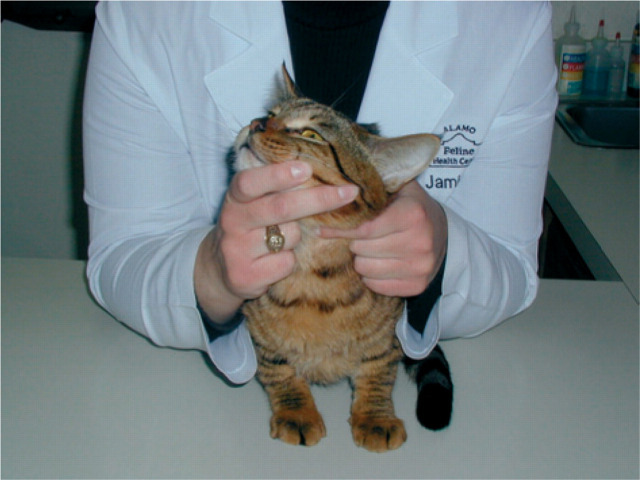
The authors employed a sensitive, semiquantitative thyroid palpation technique to detect thyroid enlargement. Each cat was placed in a standing position, with the clinician directly behind it. To palpate the right thyroid lobe, the cat's head was held with the clinician's left hand, the chin was elevated to 45° from the horizontal and the head was turned 45° to the left from the vertical. The tip of the clinician's right index finger was placed in the groove formed by the trachea and the right sternothyroideus muscle just below the larynx, and then moved ventrally down the groove to the thoracic inlet (see Fig 1). If the thyroid lobe was enlarged, a characteristic ‘pop’ was felt as the thyroid lobe briskly moved cranially after the clinician's finger passed its caudal extent. If the thyroid lobe was not felt on the first occasion, a second pass was made down the groove, and if still not palpated, a third pass was made after releasing and repositioning the head (which invariably resulted in a slightly different position, sometimes resulting in a positive palpation). If no lobe was palpable after the three procedures, a final palpation attempt was made using more finger contact (ie, the flat part of the third phalanx) with the cat's neck which permitted palpation of a few enlarged lobes that had previously been undetected (often apparently medial to the sternothyroideus muscles in cats with marked weight loss and advanced thyroid disease). The process was repeated in reverse for the cat's left side. All thyroid palpations were undertaken by one clinician (GDN), and results were only recorded when cats were co-operative and allowed the systematic examination to be performed.
Fig. 1.
Positioning for palpation. Patient positioning was the basis for the palpation technique used in this study. To palpate the left thyroid lobe, the cat's chin was raised at a 45° angle and turned 45° to its right. The clinician's left index finger was placed in the groove between the trachea and the muscles to its left at the level of the larynx. The finger was moved downward to the cat's thoracic inlet.
Each thyroid lobe size was scored semiquantitatively on an arbitrary scale from 0 to 6. Cats with non-palpable lobes were scored as ‘0’. A ‘1’ was assigned to a lobe that was just barely palpable, and a ‘6’ was assigned to a lobe approximately 2.5 cm or greater in length. The other values were assigned proportionally to measurements between ‘1’ and ‘6.’
Statistical analysis
Data were entered into a computer database (Microsoft Works for Windows V 4.0, Microsoft Corporation, Bellevue, WA, USA) and subsequently transferred to a statistical software package (SPSS Version 9.0, SPSS Inc., Chicago, IL, USA) for analysis by one of the authors (VJA). The results are reported as median, minimum, and maximum as appropriate for data that are not normally distributed and include ordinal level data for thyroid size scores. Median was used as a measure of central tendency to describe the distribution of a set of values around a value at or near the middle of the data set. Pairwise Mann-Whitney U tests for multiple comparisons were then performed to assess differences in lobe palpation scores. All statistical tests were two-tailed and a P value of <0.05 was considered to indicate statistical significance.
Results
Based on the results of the TT4 assays, 132 (85%) of the cats were classified as euthyroid, and 23 (15%) as hyperthyroid. However, 100 (65%) of the 155 cats had palpable thyroid nodules (49% left only, 23% right only, and 28% bilateral). Thus, thyroid enlargement was detected in 22 (96%) of the 23 hyperthyroid cats and 78 (59%) of the euthyroid cats. The cats with palpable nodules and TT4 values in the normal range had an age range of 5 to 21 years with a median age of 12.3 years. The cats with palpable nodules and TT4 values above the normal range had an age range of 9 to 18 years with a median age of 13.6 years. Summary results of the hyperthyroid and euthyroid groups are presented in Table 1. A Mann-Whitney U test shows that these two groups (palpation scores of ‘0’ to ‘3’ vs ‘4’ to ‘6’) have significantly different TT4 values (P<0.0001). None of the cats classified as euthyroid had clinical signs strongly suggestive of hyperthyroidism. Of the 76 with palpable thyroid lobes, sizes ranged from ‘1’ to ‘3’. Twenty of them had bilateral involvement; and the frequency of bilateral enlargement increased, as the lobes got larger (see Table 2).
Table 1.
Comparison of TT4 values and palpation scores of 155 cats
| No. of Cats | TT4∗ | TT4 range (nmol/l) | TT4 median (nmol/l) | Palpation score of largest lobe 0–3 | Palpation score of largest lobe 4–6 |
|---|---|---|---|---|---|
| 132 | Normal | 10.3–47.6 | 26.5 | 132 | 0 |
| 23 | >Normal | 60.5–306.3 | 147.8 | 5 | 18 |
TT4 reference range = 10.2–51.5 nmol/l.
Table 2.
Comparison of lobe sum and TT4 medians of 100 cats with thyroid lobe enlargement
| Lobe sum | Largest lobe | Number of cats | TT4 range (nmol/l) | Median TT4 (nmol/l) |
| 1 | 1 | 34 | 14.2–37.3 | 25.7 |
| 2 | 1 | 6 | 15.4–30.9 | 24.5 |
| 2 | 2 | 23 | 15.4–42.5 | 25.7 |
| 3 | 2 | 8 | 20.6–46.3 | 28.3 |
| 3 | 3 | 5∗ | 16.7–128.7∗ | 47.6∗ |
| 4 | 2 | 3 | 33.5–61.8 | 33.5 |
| 4 | 3 | 2 | 29.6–46.3 | 38.6 |
| 4 | 4 | 5 | 68.2–100.4 | 74.6 |
| 5 | 3 | 1 | 60.5∗∗ | 60.5∗∗ |
| 5 | 4 | 1 | 99.1∗∗ | 99.1∗∗ |
| 5 | 5 | 3 | 136.4–213.6 | 139.0 |
| 6 | 4 | 2 | 82.4–203.3 | 142.9 |
| 6 | 6 | 3 | 150.6–306.3 | 208.5 |
| 7 | 4 | 1 | 236.8∗∗ | 236.8∗∗ |
| 7 | 5 | 1 | 149.3∗∗ | 149.3∗∗ |
| 8 | 4 | 1 | 158.3∗∗ | 158.3∗∗ |
| 12 | 6 | 1 | 193.0∗∗ | 193.0∗∗ |
Note: TT4 reference range = 10.2–51.5 nmol/l.
Includes a cat with an enlarged intrathoracic thyroid lobe and a TT4 of 128.7 nmol/l.
TT4 constant (n=1 in each case).
Of the 23 cats classified as hyperthyroid, five had a score of ‘3’ or less for their largest thyroid lobe. Details of these five cats are provided in Table 3; all had some signs consistent with hyperthyroidism. One of these cats was lost to follow-up, and in another (presented with nasal discharge) a diagnosis of nasal adenocarcinoma was made, and thyroid histology was not undertaken. In one cat, thyroidectomy was performed and histology revealed a thyroid adenoma, and in the remaining two cats (both of which had TT4 values greater than 120 nmol/l and typical signs of hyperthyroidism) technetium 99M scanning demonstrated a large intrathoracic mass in both cases. The scan on the cat with a cervical mass scored as a ‘3’ did not show an increase in function of the cervical mass, as determined by the degree of isotope uptake.
Table 3.
Cats with elevated thyroid values and palpation scores less than ‘4’
| Left lobe score | Right lobe score | Largest lobe score | TT4 value (nmol/l) | Notes |
| 0 | 3 | 3 | 52.8 | Weight loss; lost to follow up |
| 3 | 2 | 3 | 60.5 | WL/PU/PD/murmur HP: thyroid adenoma |
| 2 | 2 | 2 | 61.8 | Weight loss HP: nasal adenocarcinoma |
| 3 | 0 | 3 | 128.7 | Tech scan: intrathoracic thyroid adenoma |
| 0 | 0 | 0 | 139.0 | Tech scan: intrathoracic thyroid adenoma |
Note: TT4 reference range=10.2–51.5 nmol/l.
WL=weight loss; PU=polyuria; PD=polydipsia; HP=histopathology; Tech=technetium 99M.
Table 2 summarises the findings by the sum of thyroid lobe sizes and the maximum lobe size in 98 cats. It can be seen that there is a trend for higher TT4 values both as the maximum lobe size increases and as the lobe sum score increases, although the correlation appears to be better for the former. For example, the median TT4 values when the lobe sum score is ‘4’ and the largest lobe size is ‘4’ (74.6 nmol/l, n=5) compared to when the lobe sum is ‘4’ but composed of individual lobes smaller than ‘4’ (33.5 nmol/l, n=5) are significantly different (P=0.008).
Discussion
There were two difficulties anticipated in the development of this study. Firstly, TT4 values are known to fluctuate in normal and hyperthyroid cats (Peterson et al 1987). Since only one TT4 determination was made for each cat, some error is inevitable. However, the inclusion of a substantial number of cats in this study largely circumvents this limitation. Secondly, the authors are not aware of a practical way to objectively measure thyroid lobe size in the typical private practice setting. Palpation was therefore employed, as this can be used routinely in a clinical setting. However, as palpation is inherently subjective, a careful, reproducible protocol was developed in this study for semiquantitative thyroid palpation. This protocol was performed by a single clinician (GDN) in all cats to maximise consistency of the results.
Total T4 determination offers the best combination of accuracy and practicality for routine screening for hyperthyroidism in cats and was employed in this study. It is recognised that a small proportion of hyperthyroid cats may have TT4 levels within the reference range and require further techniques to define their thyroid status (Peterson et al 2001). While additional testing would have been ideal as a routine in the cats reported here, particularly in cats with ‘high-normal’ TT4 values or physical findings consistent with hyperthyroidism, that was outside the scope of this study in all but two cases. In those 99mTc scanning was justified on the basis of disparity between palpation and TT4 determinations. Nevertheless, the results of this study confirm that thyroid palpation can be an important adjuvant modality in the diagnosis of hyperthyroidism, although clearly considerable practice is needed in perfecting the described technique. Furthermore, considerable care is needed in its interpretation.
Employing the sensitive, semi-quantitative technique for palpation, it was demonstrated that all cats with palpation scores of ‘4’, ‘5,’ and ‘6’ had elevated TT4 values (and clinical signs) consistent with a diagnosis of hyperthyroidism. However, palpation also permitted detection of thyroid enlargement in cats without signs of hyperthyroidism or elevated TT4 values, suggesting that euthyroid cats may have palpable goitre and that the presence of a goitre alone is a poor indicator of the presence of symptomatic hyperthyroidism. The significance of these findings will be the subject of a subsequent paper.
The effects of thyroid lobe enlargement on TT4 values were shown to be more dependent on the largest lobe size than on the sum of the two lobe sizes. Thus, when the largest lobe was scored as ‘4’ or higher, the TT4 was consistently elevated. However, addition of the sizes of two lobes of ‘3’ or less to make a sum of ‘4’ or more did not produce the same effect on the TT4.
Three cats (Table 3) had thyroid enlargement scored at less than ‘4’ with marginally elevated TT4 values (52.8–61.8 nmol/l). Additional functional tests could not be performed on these cats although the histopathology undertaken in one of them confirmed the presence of a thyroid adenoma. These cats were considered to be hyperthyroid (based on the TT4 values) and illustrate that even with semi-quantitative thyroid palpation, complete distinction between ‘normal’ and ‘hyperthyroid’ associated tissue is not possible. Two other cats had thyroid enlargements scored at less than ‘4’ but had substantially elevated TT4 levels (>120 mmol/l, Table 2). In both these cases scintigraphy revealed large intrathoracic uptake of technetium. Thus, combined sensitive thyroid palpation and TT4 estimation can be extremely valuable in increasing the index of suspicion for intrathoracic thyroid disease. This has important therapeutic implications, as an intrathoracic thyroid adenoma is best treated by radioiodine therapy rather than routine thyroidectomy.
In conclusion, this study demonstrated that a significant number of euthyroid cats have a palpable goitre. It is tantalising to speculate that these cases, in time, will develop increasingly functional thyroid adenomas and subsequently develop symptomatic hyperthyroidism; this theory is yet to be proved. However, sensitive, semi-quantitative thyroid palpation was valuable in distinguishing hyperthyroid from euthyroid cats (based on TT4 determination) and thus can be useful in assessing the significance of the presence of a goitre.
References
- Chaitman J, Hess R, Senz R, van Winkle T, War C. (1999) Thyroid adenomatous hyperplasia in euthyroid cats. Journal of Veterinary Internal Medicine 13, 242 (Abstract). [Google Scholar]
- Feldman EC, Nelson RW. (1996) Feline hyperthyroidism (thyrotoxicosis). In: Canine and Feline Endocrinology and Reproduction (2nd edn) Feldman EC, Nelson RW. (eds). Philadelphia: WB Saunders, pp. 118–166. [Google Scholar]
- Hammer KB, Holt DE, Ward CR. (2000) Altered expression of G proteins in thyroid gland adenomas obtained from hyperthyroid cats. American Journal of Veterinary Research 61, 874–879. [DOI] [PubMed] [Google Scholar]
- Kass PH, Peterson ME, James LJ. (1999) Evaluation of environmental, nutritional, and host factors in cats with hyperthyroidism. Journal of Veterinary Internal Medicine 13(4), 323–329. [DOI] [PubMed] [Google Scholar]
- Mooney CT, Little CJ, Macrae AW. (1996) Effect of illness not associated with the thyroid gland on serum total and free thyroxine concentrations in cats. Journal of the American Veterinary Medicine Association 15, 2004–2008. [PubMed] [Google Scholar]
- Peterson ME. (1999) Hyperthyroidism. In: Textbook of Veterinary Internal Medicine (5th edn) Ettinger SJ, Feldman EC. (eds). Philadelphia: WB Saunders, pp. 1400–1419. [Google Scholar]
- Peterson ME, Graves TK, Gamble DA. (1990) Triiodothyronine (T3) suppression test — an aid to the diagnosis of mild hyperthyroidism in cats. Journal of Veterinary Internal Medicine 4, 233–238. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Kintzer PP, Cavanagh PG, Fox PR, Ferguson DC, Johnson GF, Becker DV. (1983) Feline hyperthyroidism: pre-treatment clinical and laboratory evaluation of 131 cases. Journal of the American Veterinary Medicine Association 183, 103–110. [PubMed] [Google Scholar]
- Peterson ME, Melian C, Nichols R. (2001) Measurement of serum concentrations of free thyroxine, total thyroxine and total triiodothyronine in cats with hyperthyroidism and with nonthyroidal disease. Journal of the American Veterinary Medicine Association 218, 529–536. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Graves TK, Cavanagh I, Turrel JM, Feldman EC, Nelson RW. (1987) Serum thyroid hormone concentrations fluctuate in cats with hyperthyroidism. Journal of Veterinary Internal Medicine 1(2), 142–148. [DOI] [PubMed] [Google Scholar]
- Thoday KL, Mooney CT. (1992) Historical, clinical and laboratory features of 126 hyperthyroid cats. Veterinary Record 131, 257–264. [DOI] [PubMed] [Google Scholar]
- Turrel JM, Feldman EC, Nelson RW. (1988) Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986). Journal of the American Veterinary Medicine Association 193, 359–364. [PubMed] [Google Scholar]