Abstract
Staphylococcal enterotoxin B (SEB) is a superantigen that causes mass proliferation of murine Vβ8+ T cells via major histocompatibility complex (MHC) class II molecules and leads to their apoptosis or anergy. SEB also stimulates other MHC class II-bearing cells to proliferate and secrete cytokines, some of which might enhance early host defenses against urinary tract infections (UTIs). We investigated the effect of SEB administration on the course of an induced Escherichia coli UTI in mice. Treatment with SEB 3 or 7 days before the infection had no effect on UTI resolution. However, when SEB was administered at the time of infection, bacterial colonization in the bladders was reduced at time points between 6 h and 3 days. This reduction was not due to a physiological effect, such as increased urinary glycosaminoglycans, or altered pH, nor was SEB bactericidal for the inoculum. Cytokine production in the spleens and bladders of SEB-treated and/or infected mice was evaluated by reverse transcription-PCR. SEB treatment resulted in increased levels of interleukin-2 (IL-2), IL-4, IL-6, and IL-10 mRNAs in the spleen and IL-1α, IL-6, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha transcripts in the bladder. Also, liver cells from SEB-treated mice expressed IL-6 mRNA, which induces the production of acute-phase proteins. These data indicate that SEB treatment in vivo leads to enhanced UTI resolution through a mechanism that may include direct stimulation of effector cells in the bladder, the action of cytokines induced in the spleen, or cytokine-mediated induction of acute-phase proteins.
Six to ten percent of women suffer from recurrent, uncomplicated urinary tract infections (UTIs) caused predominantly by Escherichia coli (15, 29). Host characteristics of this unusually susceptible population are not completely defined, but it has been noted that women with recurrent UTIs have diminished antibody responses to some E. coli antigens (13). Whereas the immunological basis of this observation is not completely understood, hyporesponsiveness may be due to the absence of T helper subsets required for induction of anti-E. coli B-cell responses.
Bacterial superantigens are known to stimulate T-cell populations through a series of events that begins with their binding to major histocompatibility complex (MHC) class II molecules outside the conventional antigen binding site. Superantigens are then presented by class II molecules to a specific subset of T cells, causing mass proliferation followed by apoptosis or anergy of that T-cell subset. Staphylococcal enterotoxin B (SEB) specifically stimulates the Vβ3+, Vβ12+, Vβ14+, Vβ15+, Vβ17+, and Vβ20+ T-cell subsets in humans (17) and the Vβ8+ T-cell subset in mice (22). The effects of SEB on the immune system begin with the secretion of cytokines at 0 to 24 h, leading to the clonal expansion of Vβ8+ T cells (in mice) from 1 to 3 days followed by deletion and anergy within 3 days (3, 4, 9, 16, 24, 35). We investigated the effect of SEB treatment in vivo on the resolution of an induced E. coli UTI and observed that SEB given prior to inoculation with E. coli did not affect subsequent UTI resolution, whereas SEB administered concurrently with the inoculum enhanced UTI resolution. Further studies on SEB-treated mice revealed increased production of cytokines in the spleen and bladder and of interleukin-6 (IL-6) in the liver.
MATERIALS AND METHODS
Mice.
Female, 8- to 10-week-old BALB/c mice were purchased from Harlan Sprague-Dawley, Indianapolis, Ind.
UTI induction.
Mice were infected, as described previously (12), with the clinically isolated uropathogenic E. coli strain 1677 (34). Briefly, a catheter was inserted into the bladder of an anesthetized mouse, and a dose of 108 1677 CFU in phosphate-buffered saline was delivered in a 20-μl volume. The intensity of infection was determined by dilution plating bladder homogenates on Levine’s EMB agar (Difco) and calculating the log10 (CFU/milligram of bladder).
SEB administration.
Fifty micrograms of SEB (Sigma) was administered intraperitoneally (i.p.) in 100 μl of RPMI 1640 medium (Mediatech, Fisher Scientific) (36) at either 3 or 7 days prior to UTI induction or concurrently with the bladder inoculation. The purity of the SEB was verified by the manufacturer by sodium dodecyl sulfate-gel electrophoresis and was reported to have 31% protein and less than 0.2% SEA in a certificate of analysis.
Toxicity testing.
Strain 1677 was prepared the same as for UTI induction, and 108 cells were cultured with various amounts of SEB (0 to 50 μg) in a total volume of 120 μl at 37°C for 30 or 60 min. The cell suspension was dilution plated on EMB agar to calculate the log10 (CFU/milliliter).
Measurement of urinary GAG and pH.
Urine was collected from SEB-treated and untreated mice (10 mice per group) at 2, 4, and 6 h after i.p. injection by gently pressing on the lower abdomen and was pooled for each group. Glycosaminoglycan (GAG) levels were assayed as described previously (33) and were normalized to creatinine levels. pH levels were obtained by pipetting urine onto pH indicator paper (Whatman).
Detection of cytokine mRNA expression by reverse transcription-PCR (RT-PCR).
Spleens, bladders, and livers were removed at 6, 12, and 24 h from animals in different treatment groups, immediately snap-frozen in liquid nitrogen, and stored at −70°C. Total RNA was prepared by using the Ultraspec RNA isolation system (Biotecx, Houston, Tex.) according to the manufacturer’s instructions, with the addition of QIAshredders (Qiagen) after cell lysis to remove cellular debris. The quality of RNA was confirmed by spectrophotometry (A260/A280) and agarose gel electrophoresis. In a total volume of 30 μl, 1.5 μg of RNA was reverse transcribed with 2.5 μM oligo(dT) primer (Research Genetics, Huntsville, Ala.)–1 mM deoxynucleoside triphosphate (Promega)–450 U of Moloney murine leukemia virus reverse transcriptase (Promega) at 37°C for 60 min followed by 95°C for 5 min. Equal amounts of cDNA from identical treatment groups were pooled. Cytokine detection PCR primer sequences for IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p40, granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), transforming growth factor β (TGF-β), tumor necrosis factor alpha (TNF-α), and β-actin were published previously (8) and synthesized by Research Genetics. With the β-actin primers as an internal control, each reaction mixture contained 0.25 μM each β-actin and cytokine primers, 1.5 μl of cDNA, 200 μM deoxynucleoside triphosphates, 2 mM MgCl2, and 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus) and was amplified for 24 to 30 cycles of 45 s at 95°C, 1.5 min at 60°C, and 1.5 min at 72°C, followed by a final extension for 7 min at 72°C. Negative controls with omission of cDNA were used. PCR products were separated on a 2% agarose gel and visualized by staining with SYBR Green I (FMC, Rockland, Maine). Photographs were taken with a DC40 Kodak digital science camera and analyzed with Electrophoresis Documentation Analysis software (Kodak, Rochester, N.Y.).
Statistical analysis.
Infection resolution data were analyzed by analysis of covariance on rank transformed data (30).
RESULTS
Effects of SEB treatment on resolution of induced UTIs.
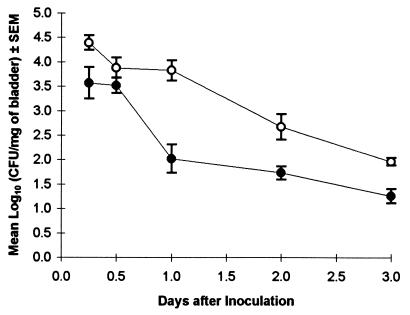
When animals were pretreated with SEB 3 or 7 days before infection induction, the expanded population of Vβ8+ T cells presumably had undergone apoptosis or become unresponsive (3, 22, 35). Hypothetically, with an incomplete T-cell repertoire, E. coli may be able to establish a more intense bladder infection. SEB pretreatment at 3 or 7 days before inoculation had no effect on bladder colonization compared to control mice (Table 1). However, when SEB was given at the time of inoculation, the infection progressed in a host with proliferating T cells and abnormally high cytokine levels. In this environment, the UTI resolved more rapidly than in non-SEB-treated mice (Fig. 1). Mice given SEB had significantly lower numbers of E. coli in the bladder at 6 h, 1 day, 2 days, and 3 days (P = 0.01, 0.0001, 0.001, and 0.0001, respectively).
TABLE 1.
SEB pretreatment does not increase susceptibility to an induced UTI
| Day of SEB (50 μg i.p.) pretreatment | Mean log10 (CFU/mg of bladder) ± SEMa
|
|
|---|---|---|
| Assay day 1b | Assay day 3c | |
| −7 | 3.96 ± 0.22 | 1.95 ± 0.08 |
| −3 | 3.56 ± 0.22 | 2.29 ± 0.26 |
| None | 3.82 ± 0.09 | 2.15 ± 0.16 |
Infection induced on day 0 with 108 strain 1677 cells.
P > 0.7, SEB-treated animals compared to controls; six mice per treatment group.
P > 0.2, SEB-treated animals compared to controls; 13 to 20 mice per treatment group with repeat experiments pooled.
FIG. 1.
SEB injection given concurrently with UTI induction accelerates infection resolution. Mice were given 50 μg of SEB i.p. (•) at the time of intravesical inoculation with 108 CFU of strain 1677. Control animals (○) received the bacterial inoculum only. A total of 8 to 24 mice were assayed per time point when repeat experiments were pooled.
Physiological effects of SEB.
While it is most likely that the observed effect of SEB on UTI resolution was mediated by stimulated T cells or their products, or other MHC class II+ cells, it is possible that SEB is bactericidal or causes other physiological effects that could lead to decreased E. coli colonization of the bladder. To rule out direct toxicity of SEB for E. coli, we cultured strain 1677 with fivefold dilutions of SEB from 50 to 0 μg and measured bacterial viability after 30- and 60-min incubations. For all dilutions of SEB tested, the mean log10 (CFU/ml) ± standard error of the mean (SEM) were 8.86 ± 0.04 and 7.65 ± 0.10, compared to 8.91 and 7.76 in the control cultures lacking SEB, after the 30- and 60-min incubations, respectively. Therefore, we concluded that SEB had no significant bactericidal effect on strain 1677.
To assess possible effects of SEB on bladder physiology that might decrease bacterial colonization, we measured urine pH and bladder GAG, which are thought to play a role in UTI resistance (19, 26). Urine was collected from mice injected with SEB and untreated controls at 2, 4, and 6 h postinjection and assayed for GAG and pH levels. Mean GAG levels were 13.3 ± 3.0 and 15.7 ± 1.6 μg of GAG/mg of creatinine ± SEM for all three time points for SEB-treated mice and untreated controls, respectively, indicating no significant rise in urinary GAG levels following exposure to SEB. Similarly, there was no change in the urinary pH levels. The mean pH values (± SEM) for SEB-treated and untreated mice were 6.5 ± 0.3 and 6.2 ± 0.2, respectively.
Cytokine mRNA expression following SEB administration.
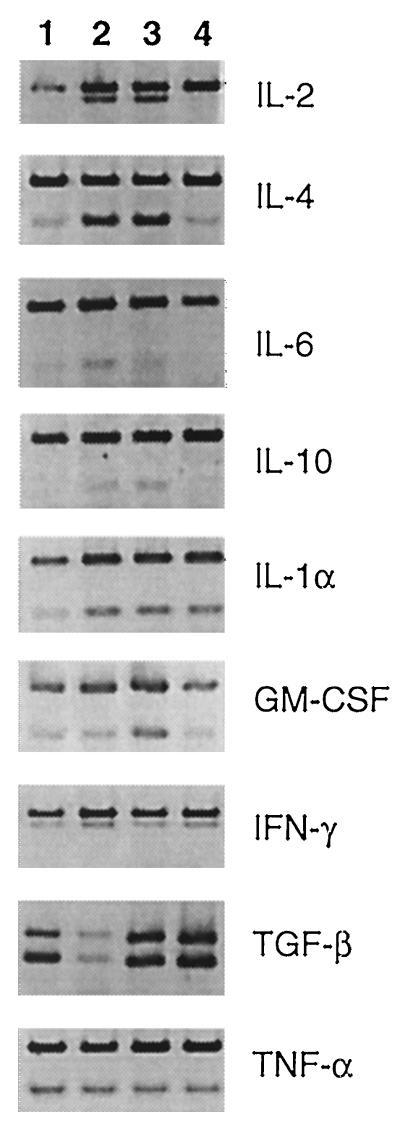
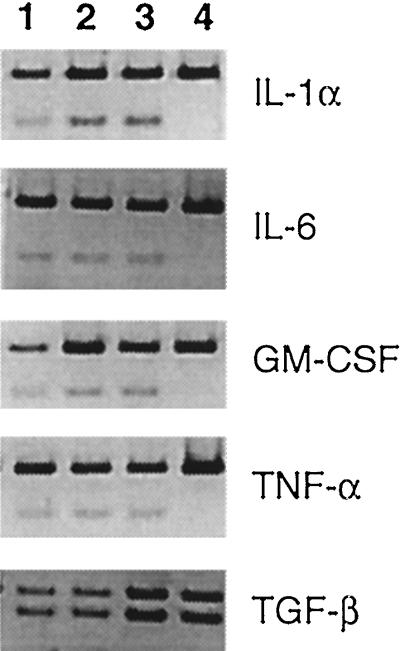
In the absence of physiological evidence for SEB-enhanced UTI resolution, we investigated possible immunological effects. When SEB and E. coli inoculum were given at the same time, an initial decrease in the level of bladder colonization occurred within 6 h, and the greatest reduction in bacterial numbers was seen in the first 24 h (Fig. 1). Because cytokines induced by SEB could be responsible for this early effect, cytokine profiles were obtained from spleen cells to examine cytokine regulation of trafficking immune cells, and cells in the bladder, to determine local cytokine production at the site of infection. All cytokine mRNA levels were considered relative to the β-actin transcript (Fig. 2 and 3, upper band in each lane). The results for the 6-, 12-, and 24-h time points did not differ in either the spleen or bladder (data not shown). Spleen cells of SEB-treated mice displayed an mRNA cytokine profile typical of cells stimulated by SEB (Fig. 2) (9, 32). IL-2 and IL-4 expression was upregulated significantly, and IL-6 and IL-10 levels were slightly increased. IL-1α, GM-CSF, IFN-γ, TGF-β, and TNF-α were produced constitutively. IL-4 was the only cytokine detected above control levels in animals receiving an infection only. IL-5 and IL-12 were not induced in any treatment group (data not shown).
FIG. 2.
Cytokine mRNA expression levels in spleen cells following SEB treatment in vivo and/or induced UTI. Lane 1, infection only; lane 2, SEB plus infection; lane 3, SEB only; lane 4, untreated control. The upper band in each lane is the β-actin transcript. Results from the 6-, 12-, and 24-h time points did not significantly differ; therefore, a representative gel from each group is shown.
FIG. 3.
Cytokine mRNA expression levels in bladder cells following SEB treatment in vivo and/or induced UTI. Lane 1, infection only; lane 2, SEB plus infection; lane 3, SEB only; lane 4, untreated control. The upper band in each lane is the β-actin transcript. Results from the 6-, 12-, and 24-h time points did not significantly differ; therefore, a representative gel from each group is shown.
The presence of cytokines in the spleen have an undetermined effect on UTI resolution, and so additionally, we examined cytokine mRNA expression in the bladder (Fig. 3). IL-1α, IL-6, GM-CSF, and TNF-α were expressed by cells in the bladder of an SEB-treated mouse. No other cytokines were detected (data not shown), with the exception of TGF-β, which was expressed constitutively in all experimental groups. The cytokine mRNA expression pattern was the same in all three experimental groups, indicating that the administration of SEB has mimicked the bladder’s natural response to UTI.
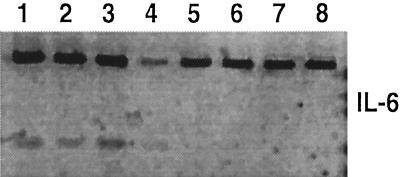
Another mechanism of early host defense is the IL-6-stimulated secretion of acute-phase proteins in the liver (14). We compared the livers of SEB-treated mice and untreated controls for IL-6 mRNA expression 12 h postinjection. IL-6 transcription in the liver was upregulated 12 h after SEB administration (Fig. 4).
FIG. 4.
IL-6 mRNA expression levels in liver cells 12 h after SEB treatment in vivo. Lanes 1 to 4, RT-PCR products from four individual mice receiving 50 μg of SEB i.p.; lanes 5 to 8, RT-PCR products from four untreated control mice. The upper band is the β-actin transcript.
DISCUSSION
The administration of SEB in vivo has allowed us to examine several aspects of host response to UTIs. SEB belongs to a family of superantigens, secreted by certain bacteria such as staphylococci, that are among the body’s natural flora. We originally postulated that a woman with recurrent UTIs may have had asymptomatic staphylococcal infections during which SEB was secreted, and exposure to the superantigen created an unresponsive population of T cells purportedly necessary for the resolution of UTIs. Our experiments examining this hypothesis demonstrated that pretreatment with SEB did not affect the course of UTI resolution as postulated, indicating that the Vβ8+ T-cell subset may not play a major role in host defense against UTIs. To be more conclusive, we would need to confirm that the SEB treatment conditions used here and by others (3, 22, 35) led to depletion of Vβ8+ T cells. In contrast to pretreatment, when SEB and E. coli infection were given concurrently, there was an approximate 90% decrease in the level of bladder colonization at 6 h and 1 to 3 days after infection in SEB-treated mice compared to untreated controls. This finding would be consistent with an SEB-induced release of stored cytokines within 6 h, followed by de novo cytokine synthesis. It is worth mentioning that the animals injected with SEB may also be receiving an amount of SEA less than 0.1 μg. Future experiments will involve testing low doses of purified SEA in our system to reveal any role it may have in UTI resolution.
After possible physiological effects of SEB (bactericidal effects, lowered urinary pH, or GAG increase) were discounted, we examined components of early host defense that could be stimulated by SEB treatment to accelerate resolution of UTIs. In this regard, it is conceivable that enhanced clearance of bacteria in the bladder could be initiated by cytokines produced either by spleen cells or locally by cells in bladder tissue. We first determined the types of cytokines produced in the spleen that could be transported to the bladder. Spleen cells from SEB-treated mice produced primarily IL-2 and IL-4 and to a lesser extent IL-6 and IL-10. Potential effects of IL-2 and IL-4 include stimulation of T and B cells and differentiation and growth of many cell types in the spleen (14). IL-6 and IL-10 expression may lead to proliferation of numerous cell types and enhance B-cell proliferation, respectively (14). All four of these cytokines can be made by splenic T and B cells, in addition to many other cell types (14, 25). Our results are in agreement with a previous report on cytokines stimulated by SEB treatment in vivo in which IL-2, IL-4, IL-10, and IFN-γ were detected in splenic CD4+ T cells within 2 to 48 h by RT-PCR (9, 32). Similar results were seen by others in lymph node cells and serum (11, 20). Because IFN-γ message was also present our untreated controls, we were unable to determine if IFN-γ transcription was upregulated by SEB.
SEB can also produce local effects in the bladder, since it can migrate through all organs in a few hours (24). To evaluate this, we looked for increased cytokine mRNA expression in bladder cells following i.p. injection of SEB. IL-1α, IL-6, GM-CSF, and TNF-α mRNA were expressed above control levels in the bladder. The bladder has numerous cell types that are able to secrete these cytokines upon stimulation. These include epithelial cells (14), macrophages (14, 25), mast cells (25), dendritic cells (14), neutrophils (5), natural killer (NK) cells (25), and γδ T cells (21, 25). Furthermore, several studies have shown that SEB stimulates not only Vβ8+ T cells but also many other effector cells bearing MHC II receptors belonging to the nonadaptive immune response repertoire, including γδ T cells (27) and NK cells. In vitro experiments revealed that after 24 h, SEB directly stimulated cytokine secretion (6) and killer activity of NK cells, which had enhanced antibacterial activity against E. coli strains including a pyelonephrogenic strain (10). The cytokines that we detected belong to a family that act collectively to enhance the inflammatory response, recruit effector cells, and resist infection (18). For example, IL-1β and TNF-α were shown to have nonspecific anti-Pseudomonas activity in a granulocytopenic mouse model (1), and IFN-γ, TNF-α, and IL-6 were protective in mycobacterial infections (2). Mast cells were shown to release stored TNF to help confine bacterial infections (7), modulate neutrophil influx, and enhance bacterial clearance in murine acute septic peritonitis (23). Other researchers have shown that urinary IL-6 levels increased within 2 h after exposure to E. coli and that E. coli can stimulate epithelial cells to initiate release of neutrophil chemoattractants (31).
We also found induction of IL-6 message in liver cells from mice treated with SEB, suggesting that production of acute-phase proteins may have been stimulated and thus could have played a role in accelerating infection resolution (14).
Our data of cytokine mRNA profiles suggest a cascade of SEB-stimulated immunological events leading to enhanced bacterial clearance from the bladder. First, we detected mRNA for a group of inflammation-enhancing cytokines in the bladder (IL-1α, IL-6, GM-CSF, and TNF-α), and this family of cytokines, along with SEB-stimulated effector cells (possibly NK or γδ T cells) could be responsible for the early (6 h) reduction of E. coli in the bladder. Second, the production of IL-2, IL-4, IL-6, and IL-10, inferred from transcripts in the spleen, may contribute to infection resolution at later time points (1 to 3 days). Conceivably, these cytokines would activate T and B cells in the spleen, which could then enter the circulation, travel to the bladder, and enhance bacterial clearance. Third, IL-6 mRNA detected in liver cells of SEB-treated mice may stimulate secretion of acute-phase proteins, which could travel in the serum to the bladder, bind bacteria, and promote bacterial phagocytosis through opsonization (28). Whereas this proposed model needs to be verified, the current studies on SEB administration in vivo have allowed us to begin identifying some early host defense mechanisms necessary to resolve UTIs.
ACKNOWLEDGMENTS
We thank Dennis Heisey for performing the statistical analyses and Steve Klodd for technical assistance.
This work was supported by NIH grant DK 44378.
REFERENCES
- 1.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520–525. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- 3.Baschieri S, Lees R K, Lussow A R, MacDonald H R. Clonal anergy to staphylococcal enterotoxin B in vivo: selective effects on T cell subsets and lymphokines. Eur J Immunol. 1993;23:2661–2666. doi: 10.1002/eji.1830231041. [DOI] [PubMed] [Google Scholar]
- 4.Bell S J D, Buxser S E. Staphylococcal enterotoxin B modulates Vβ8+ TcR-associated T-cell memory against conventional antigen. Cell Immunol. 1995;160:58–64. doi: 10.1016/0008-8749(95)80009-8. [DOI] [PubMed] [Google Scholar]
- 5.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 6.D’Orazio J A, Burke G W, Stein-Streilein J. Staphylococcal enterotoxin B activates purified NK cells to secrete IFN-γ but requires T lymphocytes to augment NK cytotoxicity. J Immunol. 1995;154:1014–1023. [PubMed] [Google Scholar]
- 7.Echtenacher B, Männel D N, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 8.Eckmann L, Fierer J, Kagnoff M F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- 9.Florquin S, Amraoui Z, Abramowicz D, Goldman M. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol. 1994;153:2618–2623. [PubMed] [Google Scholar]
- 10.Garcia-Peñarrubia P, Koster F T, Kelley R O, McDowell T D, Bankhurst A D. Antibacterial activity of human natural killer cells. J Exp Med. 1989;169:99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimeno R, Codony-Servat J, Plana M, Rodriguez-Sanchez J L, Juarez C. Stat1 implication in the immune response to superantigens in vivo. J Immunol. 1996;156:1378–1386. [PubMed] [Google Scholar]
- 12.Hopkins W J, Hall J A, Conway B P, Uehling D T. Induction of UTI by intraurethral inoculation with Escherichia coli: refining the murine model. J Infect Dis. 1995;171:462–465. doi: 10.1093/infdis/171.2.462. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins W J, Xing Y, Dahmer L A, Balish E, Uehling D T. Western blot analysis of anti-Escherichia coli serum immunoglobulins in women susceptible to recurrent urinary tract infections. J Infect Dis. 1995;172:1612–1616. doi: 10.1093/infdis/172.6.1612. [DOI] [PubMed] [Google Scholar]
- 14.Janeway C A, Jr, Travers P. Immunobiology: the immune system in health and disease. 2nd ed. New York, N.Y: Current Biology Ltd./Garland Publishing Inc.; 1996. p. 7:26. [Google Scholar]
- 15.Johnson J R, Stamm W E. Urinary tract infections in women: diagnosis and treatment. Ann Intern Med. 1989;111:906–917. doi: 10.7326/0003-4819-111-11-906. [DOI] [PubMed] [Google Scholar]
- 16.Koide Y, Uchijima M, Yoshida A, Yoshida T. Effect of staphylococcal enterotoxin B-induced anergy on cytokine gene expression: anergy-sensitive and resistant mRNA expression. J Interferon Cytokine Res. 1996;16:225–236. doi: 10.1089/jir.1996.16.225. [DOI] [PubMed] [Google Scholar]
- 17.Kotzin B L, Leung D Y M, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, Moreno de Alborán I, Gonzalo J A, Martínez-A C. Immunoregulation by cytokines. Crit Rev Immunol. 1993;13:163–191. [PubMed] [Google Scholar]
- 19.Kunin C M. Urinary tract infections: detection, prevention, and management. 5th ed. Baltimore, Md: The Williams & Wilkins Co.; 1997. pp. 338–340. [Google Scholar]
- 20.Litton M J, Sander B, Murphy E, O’Garra A, Abrams J S. Early expression of cytokines in lymph nodes after treatment in vivo with Staphylococcus enterotoxin B. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 21.Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström M. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–2312. [PubMed] [Google Scholar]
- 22.MacDonald H R, Baschieri S, Lees R K. Clonal expansion precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 23.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–79. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 24.Miethke T, Wahl C, Heeg K, Wagner H. The pathophysiology of bacterial superantigens in vivo. In: Thibodeau J, Sékaly R, editors. Bacterial superantigens: structure, function and therapeutic potential. R. G. Austin, Tex: Landes Co.; 1995. pp. 160–179. [Google Scholar]
- 25.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 26.Parsons C L, Greenspan C, Mulholland S G. The primary antibacterial defense mechanisms of the bladder. Investig Urol. 1975;13:72–76. [PubMed] [Google Scholar]
- 27.Ramesh N, Horner A, Ahern D, Geha R S. Bacterial superantigens induce the proliferation of resting γ/δ receptor bearing T cells. Immunol Investig. 1995;24:713–724. doi: 10.3109/08820139509060700. [DOI] [PubMed] [Google Scholar]
- 28.Roitt W, Brostoff J, Male D. Immunology. St. Louis, Mo: The C. V. Mosby Co.; 1985. pp. 1.2–1.3. [Google Scholar]
- 29.Sanford J P. Urinary tract symptoms and infection. Annu Rev Med. 1975;26:485–498. doi: 10.1146/annurev.me.26.020175.002413. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute Inc. SAS/STAT user’s guide, version 6. 4th ed. Vol. 1. Cary, N.C: SAS Institute; 1989. p. 943. [Google Scholar]
- 31.Svanborg C, Agace W, Hedges S, Lindstedt R, Svensson M L. Bacterial adherence and mucosal cytokine production. Ann N Y Acad Sci. 1994;730:162–181. doi: 10.1111/j.1749-6632.1994.tb44247.x. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi I, Nakagawa I, Xu L, Hamada S. IL-12 rescues galactosamine-loaded mice from lethal shock triggered by staphylococcal enterotoxin. Biochem Biophys Res Commun. 1995;217:74–80. doi: 10.1006/bbrc.1995.2747. [DOI] [PubMed] [Google Scholar]
- 33.Uehling D T, Kelley E, Hopkins W J, Balish E. Urinary glycosaminoglycan levels following induced cystitis in monkeys. J Urol. 1988;139:1103–1105. doi: 10.1016/s0022-5347(17)42796-0. [DOI] [PubMed] [Google Scholar]
- 34.Uehling D T, Hopkins W J, Jensen J, Balish E. Vaginal immunization against induced cystitis in monkeys. J Urol. 1987;137:327–329. doi: 10.1016/s0022-5347(17)44015-8. [DOI] [PubMed] [Google Scholar]
- 35.Williams O, Aroeira L S, Martínez-A C. Absence of peripheral clonal deletion and anergy in immune responses of T cell-reconstituted athymic mice. Eur J Immunol. 1994;24:579–584. doi: 10.1002/eji.1830240313. [DOI] [PubMed] [Google Scholar]
- 36.Wood A C, Todd I. Staphylococcus enterotoxin B toxicity in BALB/c mice: effect of T cells, plasma cytokine levels and biochemical markers. FEMS Immunol Med Microbiol. 1995;11:91–98. doi: 10.1111/j.1574-695X.1995.tb00094.x. [DOI] [PubMed] [Google Scholar]