Abstract
Oral glucosamine was compared to a placebo for the management of cats with feline idiopathic cystitis (FIC) in a randomised, double-blinded, placebo-controlled, study. Forty cats with a history of recurrent cystitis due to FIC were divided into two groups and treated daily per os with either 125 mg N-acetyl glucosamine or a placebo for six months. Owners graded their cats' clinical signs at the beginning and end of the study, and kept daily diaries documenting signs of cystitis using visual analogue scales. Further episodes of cystitis were seen in 26 (65%) of the cats during the study. Affected cats experienced a mean of five recurrences (range 1–19) with each recurrence lasting a mean of four days (range 1–64 days).
There were no significant differences between the two groups when considering the owners assessments of the mean health score (P > 0.5), the average monthly clinical score (P = 0.22) or the average number of days with clinical signs (P = 0.28). Two cats suffered from such severe recurrent urethral obstruction that they were euthanased; they were both in the placebo group. Compared to the start of the study the majority of cats in both groups improved significantly (P < 0.001) (mean health score of each group at the start was 0.5 ± SD 0.5, compared to glucosamine 4.4 ± 0.7 and placebo 3.9 ± 1.6 at the end). This is believed to have occurred because the owners of 36 of the 40 cats (90%) started feeding more canned cat food. The urine specific gravity at the start of the trial was significantly higher (mean 1.050 ± SD 1.007) than when reassessed one month later (1.036 ± 1.010, P < 0.01).
Introduction
The term feline lower urinary tract disease (FLUTD) describes a collection of conditions that can affect the bladder and/or urethra of cats. However, the majority (55–69%) of cases are idiopathic (Barsanti et al., 1996; Buffington et al., 1997; Kruger et al., 1991; Lekcharoensuk et al., 2001) and research over the last 30 years has failed to find a consistent cause for the inflammation. A recent hypothesis suggests that feline idiopathic cystitis (FIC) may result, in part, from alterations in the protective glycosaminoglycan (GAG) layer that lines the bladder. In this, and other respects, FIC has similarities to interstitial cystitis (IC) in humans (Buffington et al., 1996b, 1999).
A thin layer of GAG covers the bladder urothelium. This layer helps to prevent microbes and crystals from sticking to the bladder lining and limits the transepithelial movement of urine proteins and other solutes (Lilly and Parsons, 1990; Parsons, 1994; Parsons and Mulholland, 1978;Parsons et al., 1979 Parsons et al., 1990; Pantazopoulos et al., 1990). Qualitative and quantitative changes in the GAG layer and an associated increase in urothelial permeability have been found in humans with IC (Akcay and Konukoglu, 1999; Parsons, 1994; Wei et al., 2000) and cats with FIC (Buffington et al., 1996a; Gao et al., 1994). The increased permeability allows noxious substances within the urine to pass through the urothelium where they may cause inflammation (Gao et al., 1994). It is not currently known what causes the alteration in the GAG layer.
A number of experimental studies have shown that it is possible to reduce transitional cell injury using exogenous forms of GAG (including heparin, hyaluronic acid, and pentosan polysulphate sodium [PPS; a semi-synthetic low molecular weight heparin GAG analogue]) (Lilly and Parsons, 1990; Nickel et al., 1998; Pantazopoulos et al., 1990; Parsons et al., 1990; Parsons, 1994).
Clinical studies have shown that oral or intravesicular administration of PPS (Elmiron™) may be beneficial in the management of human IC. Given orally it has resulted in subjective and objective improvements, with remission of clinical signs in 28 to 63% of humans with IC (Bade et al., 1997; Fritjofsson et al., 1987; Holm-Bentzen et al., 1987; Hwang et al., 1997; Mulholland et al., 1990; Parsons et al., 1983, 1993; Parsons and Mulholland, 1987). Intravesicular hyaluronic acid and oral d-glucosamine (which is a precursor for GAG) have also resulted in moderate to significant beneficial responses (Morales et al., 1997; Strohmaier et al., 1989). Anecdotally, oral glucosamine, oral PPS, and parenteral PPS (Cartrophen™) have been used with apparent success in some cases of FIC. Adverse effects are uncommon, however, they may consist of prolonged prothrombin time, epistaxis, gingival bleeding, alopecia, abdominal pain, diarrhoea, nausea and, possibly, in the case of glucosamine, insulin resistance (Monauni et al., 2000; Mulholland et al., 1990).
The aim of this study was to determine whether the addition of a food supplement containing the GAG precursor N-acetyl glucosamine could reduce the severity, or recurrence rate, of clinical signs in cats with recurrent FIC.
Material and methods
Cats with a history of recurrent dysuria, pollakiuria, and haematuria were recruited from the referral cases of the Feline Clinic of the University of Edinburgh Small Animal Hospital, between February 2001 and May 2002. For inclusion, each cat had to have experienced a minimum of two episodes of FLUTD within the preceding six months. All cases were subject to a minimum assessment of history, physical examination, evaluation of serum urea and creatinine concentrations, routine urinalysis (performed within 30 min of collection), urine culture, survey abdominal radiography, double contrast cystography and retrograde urethrography. Cases were considered to have idiopathic cystitis when there was no evidence of urolithiasis, bacterial infection, bladder neoplasia or congenital deformity. Cats with occasional crystals within their urine were included but those with aggregates of crystals were excluded.
Forty cats with FIC were recruited and randomly assigned to the blinded treatment groups. Each group contained 20 cats. Each cat received either a placebo (cellulose) or 125 mg of N-acetyl glucosamine (Cystease™, Ceva Animal Health) by mouth each day. The owners had to commit to giving the medication daily for six months, and to keeping a treatment diary so that this could be confirmed. They were also given detailed notes on FLUTD that discussed the current understanding of the pathogenesis of FIC. The cats were not to be given any other sources of glucosamine or GAG during the study. Other medical treatments could be given, according to medical indications, and noted on the diaries.
The owners were asked to grade the severity of their cat's signs at both the start and the end of the study using a health score scale of 0 (very severe cystitis) to 5 (normal cat). The grades were compared using the Mann–Whitney rank-sum test with continuity correction, with significance determined at P ≤ 0.05 (Glantz, 1992).
During the study the owner's were asked to keep a record of ‘cystitis events’, recording every day that their cat showed signs of cystitis and defining the severity of each of the signs on standard 10 cm long visual analogue scales (VAS). The signs that they were asked to record were (i) increased frequency of urination, (ii) straining while urinating, (iii) crying out while urinating, (iv) the presence of blood in the urine (macroscopic haematuria), (v) urination outside the litter box, (vi) increased grooming around the perineum, and (vii) altered behaviour (defined by the owner as increased aggression/fear/hiding, etc.). The average monthly clinical score was determined for each group by calculating the total ‘cystitis events’ score from the VAS for each cat for each month of the trial (i.e. first month, second month, etc.), then averaging the scores for all 20 cats. The results were compared using the Mann–Whitney rank-sum test with continuity correction, with significance determined at P ≤ 0.05. The average number of days with clinical signs was determined by calculating the total number of days with clinical signs for all of the cats within one group, then dividing by 20. Results were compared using unpaired 2-tailed Student's t test, with significance determined at P ≤ 0.05.
Each owner was asked to collect a urine sample from their cat (using a litter box filled with pebbles) after one month of the trial. The urine samples were frozen immediately by the owners (at −20 °C), and then shipped on ice to the University of Edinburgh. The mean urine specific gravity for the cats at the start of the study was compared to the mean urine specific gravity after one month of the study using paired 2-tailed Student's t test, with significance determined at P≤0.05.
Results
Twenty-nine of the 40 cats (72.5%) were domestic shorthaired cats, four (10%) were domestic longhaired cats, six (15%) were Persians, and one (2.5%) was a Burmese. Thirty-one (77.5%) were neutered males, nine (22.5%) were neutered females. Six (19%) of the cats were males referred with apparent urethral obstruction. The mean age of the cats was six years (range 1–14 years). Cats were similarly distributed in both treatment groups.
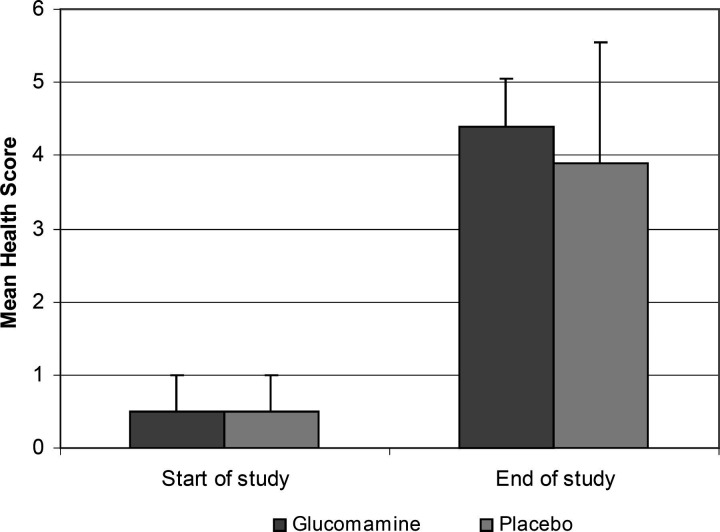
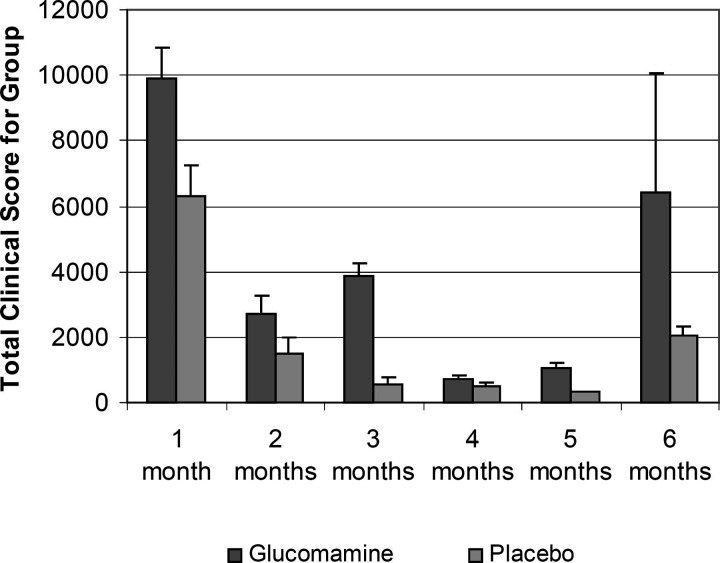
There were no significant differences between the two groups. Although the owners assessments suggested that the glucosamine-treated cats achieved a slightly greater improvement by the end of the study (mean health score 4.4 ± SD 0.7) compared to the placebo group (3.9 ± 1.6), there was no significant difference when compared using the Mann–Whitney rank-sum test with continuity correction (P > 0.5) (Fig. 1). There were no significant differences between the two groups when assessed by the average monthly clinical scores (glucosamine 4113 ± 3509, placebo 1872 ± 2273, P = 0.22) (Fig. 2) or the average number of days with clinical signs (glucosamine 15 ± 29, placebo 7.2 ± 11, P = 0.28). The chance of Type II statistical error (β), giving false negative results, was calculated to be <10%, that is when α = 0.05 (i.e. significance was determined at P≤0.05), n = 20 in each group, and looking for a change in the mean test population of approximately one times the standard deviation. Two cats in the placebo group suffered from such severe recurrent urethral obstruction that they were euthanased. No adverse effects to treatment were observed in either group. None of the cats were given any medications that could have affected their urine specific gravity.
Figure 1.
The figure shows a graph of the mean health scores at the start and the end of the study. The owner's were asked grade the severity of their cat's signs using the scale from 0 (very severe signs of cystitis) to 5 (normal cat). The error bars show the standard deviation. There is no statistical difference between the groups.
Figure 2.
The figure shows a graph of the mean monthly clinical scores against time for the six months study. The error bars show the standard deviation. There is no statistical difference between the groups.
Further episodes of cystitis were seen in 26 of the 40 cats (65%) during the study. Affected cats experienced a mean of five recurrences (median 2.5, range 1–19) with each recurrence lasting a mean of four days (median 3, range 1–64 days).
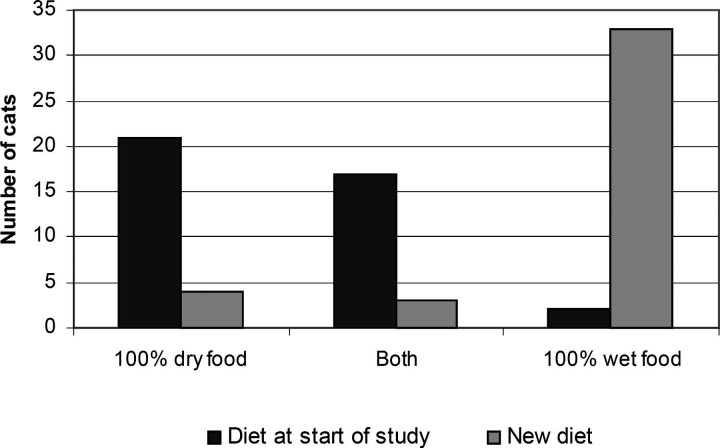
Compared to the start of the study the majority of cats in both groups improved significantly (P < 0.001) (mean health score of each group at the start was 0.5 ± SD 0.5, compared to glucosamine 4.4 ± 0.7 and placebo 3.9 ± 1.6 at the end). At the start of the study, 21 of the 40 cats (52.5%) were fed solely on dry cat food. A further 17 cats (42.5%) were fed at least half of their daily food intake as dry cat food. Only two of the cats (5%) were fed solely on canned cat food (Fig. 3). On starting the trial, 36 (90%) of the owners increased the amount of canned cat food in their cat's diet, such that the cats were eating at least 50% of their food intake as canned food. In 33 of the cats (82.5%) canned cat food became the only food fed. There was no difference in the initial or the altered diet between the two groups. The urine specific gravity at the start of the trial was significant higher (mean 1.050 ± SD 1.007) than when reassessed one month later (1.036 ± 1.010, P < 0.01).
Figure 3.
The figure shows a graph of the amounts of dry and wet food fed at the start of the study and after the owners' made non-authorised changes to their cat's diets. All 40 cats have been grouped together as there was no difference between the groups.
Discussion
Male cats are significantly over-represented in this study. This may be because male cats with recurrent FIC have an increased risk of urinary tract obstruction, and this may prompt referral to a university hospital. A male predisposition has previously been observed in cats with FIC (Krugeret al., 1991; Lekcharoensuk et al., 2001), however, these studies were also performed at university hospitals. In contrast, other studies have found no sexual predisposition in cats with FLUTD (Buffington et al., 1997; Jones et al., 1997; Willeberg, 1984).
During the six month trial period recurrent cystitis was seen in 65% of the cats in this study. In contrast, cats with FIC in a previous one-year study experienced a recurrence rate of only 11 to 39% (depending on whether they were fed a canned or dry diet) (Markwell et al., 1999). A 39% recurrence rate was also seen in separate one-year study (Barsanti et al., 1982). It is unclear why the cats in the current study suffered more recurrence of disease. It may reflect the severity of the cases. In addition, it may also have resulted from close observation by the owners that lead to recognition of mild episodes of disease. This study counted mild clinical signs such as increases in the frequency of urination, urinating outside the litter box, grooming of the perineum, and/or altered behaviour as an episode of recurrence. These findings suggest that cats with recurrent FIC may be suffering from many more episodes of bladder discomfort than was previously appreciated. It is interesting to note the variation of recurrence rate (from 0–19 recurrences), and the variation of the duration of these recurrences (mean four days; range 1–64 days). The latter finding is in agreement with other studies that have found that recurrence of clinical signs is usually self-limiting, typically lasting for 5–7 days (Barsanti et al., 1982).
Given the apparent usefulness of oral GAG supplementation in some humans with IC, the poor response of cats with FIC was disappointing. There are a number of possible reasons for this. There is some debate as to the overall significance of changes in the GAG layer in the pathogenesis of IC (Elbadawi, 1997; Erickson et al., 1997) and it may be that its role in FIC has been over-emphasised. Also, even in humans with IC the response to treatment has been very variable (Bade et al., 1997; Fritjofsson et al., 1987; Holm-Bentzen et al., 1987; Hwang et al., 1997; Morales et al., 1997; Mulholland et al., 1990; Parsons et al., 1983, 1993; Parsons and Mulholland, 1987; Strohmaier et al., 1989), and different forms of GAG have been shown to have differing urothelial protective properties (Gill et al., 1982). For example, in rabbits, intravesicular PPS and heparin have been shown to be more effective than hyaluronic acid (Nickel et al., 1998). It is not known which forms of GAG may be most effective in cats, so it is possible that different forms may have given different results. In addition, optimum dosages have not been determined so extrapolation from human studies may have resulted in inadequate dosage. It is also possible that the wrong variables were measured. In humans with IC, it is pain that resolves first. It is only after six to eight months of treatment that the frequency of urination reduces and the volume of each urination increases (Parsons, 1994). It is therefore possible that our six-month study may have been insufficiently long to see a significant difference between the groups.
Interestingly, four of the cats in the treatment group have been unable to stop medication. Every attempt to cease giving the glucosamine has resulted in recurrence of clinical signs, which have resolved on reintroduction of the medication. While this does not prove the efficacy of the product it does suggest that it may be more beneficial in some cats than others.
In retrospect, giving the owners notes about FLUTD and the possible pathogenesis of FIC was a complicating factor. The notes stated that increasing water turnover may be of considerable benefit in the management of cats with FIC. These were very committed owners, so they all tried to increase their cat's daily water consumption by feeding a higher percentage of canned cat food. Prior to starting the study 95% of the cats were being fed at least 50% of their diet as dry cat food, however, within a month over 80% were being fed solely on canned cat food. As a similar percentage of each group changed their diet it did not affect the comparison of the glycosamine with the placebo.
Increasing water turnover can significantly reduce the proportion of cats with FIC that experience recurrence of clinical signs (Buffington and Chew, 1999). In one study, only 11% of cats fed on canned cat food had recurrent signs, compared to 39% of cats fed dry cat food (Markwell et al., 1999). In that study, the urine specific gravity of the cats fed on a dry diet decreased from an average of 1.050 to an average of 1.030 when fed on canned food (Markwell et al., 1999). A similar decrease was noted in the current study, where urine specific gravity decreased from 1.050 to 1.036 within the first month of the study. This coincided with the change in the diet, and with the initial fall in the mean monthly clinical scores. The decrease in the specific gravity may be of benefit because it is associated with reduced concentrations of urinary solutes, reduced urine osmolality, and increased urine volume.
It has previously been noted that cats with FIC tend to be fed significantly more dry food than cats without FIC. In one study nearly 60% of cats with FIC ate solely dry food, and an additional 17% ate 75% or more dry food (Buffington et al., 1997). The current study showed similar results at presentation, with 52.5% of the cats eating solely dry food, and a further 42.5% eating at least 50% dry food. While this does not show that dry food causes FIC, it implies that it may unmask or aggravate it. Given that so many cats with FIC do eat large amounts of dry food the most simple treatment option (and perhaps the most effective) is to change their diet from a dry to a canned food.
The only other explanation for the overall improvement in both groups would be a profound ‘placebo effect’. Giving a capsule each day and paying attention to the cat by looking for signs of cystitis may have resulted in the cats' receiving more attention from their owners' than prior to the study. If increased owner interaction was associated with increased stroking and petting it could have acted to calm the cats. Stress has been suggested as a possible trigger for both IC in humans and FIC in cats (Elbadawi, 1997; Jones et al., 1997; Kalkstein et al., 1999). However, while it is possible that the cats in the study were less stressed than they had been prior to the study the owners of the cats all denied spending significantly more time with them.
In addition to the N-acetyl glucosamine the capsules (Cystease™) also contained small amounts of alfalfa, pepsin, bromelain and Lactobacillus acidophilus. Their inclusion in the capsules is theoretically aimed at supporting gastrointestinal function and promoting the absorption of the N-acetyl glucosamine. They were not believed to have any effect on bladder function.
In conclusion, while this study was not able to show that oral GAG supplementation gave significant clinical benefit to cats with FIC; it did underline the importance of feeding these cats on canned diets. Further studies could involve analysing the GAG content of urine samples to see if cats that appear to gain most benefit from glucosamine have the most severe alterations in their urine GAG content. If this is found to be the case, assessing cats for their urine GAG content could help in tailoring treatment to particular cats. It will also be important to determine which types of canned diet have the most beneficial effects.
Acknowledgement
D. Gunn-Moore's lectureship is funded by Nestlé Purina. This study was supported by Ceva Animal Health and Vetri-Science Laboratories. The authors would like to thank all members of the University of Edinburgh Hospital for Small Animals for their assistance with the cases, the referring veterinary surgeons for referring the cases and the owners for taking part in the study.
References
- Akcay T., Konukoglu D. Glycosaminoglycans excretion in interstitial cystitis, International Urology and Nephrology, 31 (4, 1999, 431–435. [DOI] [PubMed] [Google Scholar]
- Bade J.J., Laseur M., Nieuwenburg A., Van der Weele L.T., Mensink H.J. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis, British Journal of Urology, 79, 1997, 168–171. [DOI] [PubMed] [Google Scholar]
- Barsanti J.A., Finco D.R., Shotts E.B., Ross L. Feline urological syndrome: further investigation into therapy, Journal of the American Animal Hospital Association, 18, 1982, 387–390. [Google Scholar]
- Barsanti J.A., Brown J., Marks A., Reece L., Greene C.E., Finco D.R. Relationship of lower urinary tract signs to seropositivity for feline immunodeficiency virus in cats, Journal of Veterinary Internal Medicine, 10 (1, 1996, 34–38. [DOI] [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J. Diet therapy in cats with lower urinary tract disorders, Veterinary Medicine, 94 (7, 1999, 626–630. [Google Scholar]
- Buffington C.A.T., Blaisdell J.L., Binns S.P., Woodworth B.E. Decreased urine glycosaminoglycan excretion in cats with interstitial cystitis, Journal of Urology, 155 (5, 1996a, 1801–1804. [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J., DiBartola S.P. Interstitial cystitis in cats, Veterinary Clinics of North America: Small Animal Practice, 26 (2, 1996b, 317–326. [DOI] [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J., Kendall M.S., Scrivani P.V., Thompson S.B., Blaisdell J.L., Woodworth B.E. Clinical evaluation of cats with nonobstructive urinary tract diseases, Journal of the American Veterinary Medicine Association, 210 (1, 1997, 46–50. [PubMed] [Google Scholar]
- Buffington C.A.T., Chew D.J., Woodworth B.E. Feline interstitial cystitis, Journal of the American Veterinary Medicine Association, 215 (5, 1999, 682–687. [PubMed] [Google Scholar]
- Elbadawi A. Interstitial cystitis: A critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis, Urology, 49 (Supplement 5A, 1997, 14–40. [DOI] [PubMed] [Google Scholar]
- Erickson D.R., Ordille S., Martin A., Bhavanandan V.P. Urinary chondroitin sulfates, heparan sulfate and total sulfated glycosaminoglycans in interstitial cystitis, Journal of Urology, 157 (1, 1997, 61–64. [PubMed] [Google Scholar]
- Fritjofsson A., Fall M., Juhlin R., Persson B.E., Ruutu M. Treatment of ulcer and non-ulcer interstitial cystitis with sodium pentosanpolysulfate; a multicenter trial, Journal of Urology, 138 (3, 1987, 508–512. [DOI] [PubMed] [Google Scholar]
- Gao X., Buffington C.A.T., Au J.L.S. Effect of interstitial cystitis on drug absorption from urinary bladder, Journal of Pharmacology and Experimental Therapeutics, 271 (2, 1994, 818–823. [PubMed] [Google Scholar]
- Gill W.B., Jones K.W., Ruggiero K.J. Protective effects of heparin and other sulfated glycosaminoglycans on crystal adhesion to injured urothelium, Journal of Urology, 127 (1, 1982, 152–154. [DOI] [PubMed] [Google Scholar]
- Glantz S.A. Primer of Bio-statistics, third ed., 1992, McGraw-Hill, Inc: New York. [Google Scholar]
- Holm-Bentzen M., Jacobsen F., Nerstrom B., Lose G., Kristensen J.K., Pedersen R.H., Krarup T., Feggetter J., Bates P., Barnard R., Larsen S., Hald T. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease, Journal of Urology, 138 (3, 1987, 503–507. [DOI] [PubMed] [Google Scholar]
- Hwang P., Auclair B., Beechinor D., Diment M., Einarson T.R. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis, Urology, 50 (1, 1997, 39–43. [DOI] [PubMed] [Google Scholar]
- Jones B.R., Sanson R.L., Morris R.S. Elucidating the risk factors of feline lower urinary tract disease, New Zealand Veterinary Journal, 45, 1997, 100–108. [DOI] [PubMed] [Google Scholar]
- Kalkstein T.S., Kruger J.M., Osborne C.A. Feline idiopathic lower urinary tract disease. Part II. Potential causes, Compendium of Continuing Education for the Practising Veterinarian, 21 (2, 1999, 148–154. [Google Scholar]
- Kruger J.M., Osborne C.A., Goyal S.M., Wickstrom S.L., Johnston G.R., Fletcher T.F., Brown P.A. Clinical evaluation of cats with lower urinary tract disease, Journal of the American Veterinary Medicine Association, 199, 1991, 211–216. [PubMed] [Google Scholar]
- Lekcharoensuk C., Osborne C.A., Lulich J.P. Epidemiologic study of risk factors for lower urinary tract diseases in cats, Journal of the American Veterinary Medicine Association, 218 (9, 2001, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Lilly J.D., Parsons C.L. Bladder surface glycosaminoglycans is a human epithelial permeability barrier, Surgical Gynecology and Obstetrics, 171 (6, 1990, 493–496. [PubMed] [Google Scholar]
- Markwell P.J., Buffington C.A.T., Chew D.J., Kendell M.S., Harte J.G., DiBartola S.P. Clinical evaluation of commercially available urinary acidification diets in the management of idiopathic cystitis in cats, Journal of the American Veterinary Medicine Association, 214 (3, 1999, 361–365. [PubMed] [Google Scholar]
- Monauni T., Zenti M.G., Cretti A., Daniels M.C., Targher G., Caruso B., Caputo M., McClain D., Del Prato S., Giaccari A., Muggeo M., Bonora E., Bonadonna R.C. Effects of glucosamine infusion on insulin secretion and insulin action in humans, Diabetes, 49, 2000, 926–935. [DOI] [PubMed] [Google Scholar]
- Morales A., Emerson L., Nickel J.C. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis, Urology, 49 (supplement 5A, 1997, 111–113. [DOI] [PubMed] [Google Scholar]
- Mulholland S.G., Hanno P., Parsons C.L., Sant G.R., Staskin D.R. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study, Urology, 35 (6, 1990, 552–558. [DOI] [PubMed] [Google Scholar]
- Nickel J.C., Downey J., Morales A., Emerson L., Clark J. Relative efficiency of various exogenous glycosaminoglycans in providing a bladder surface permeability barrier, Journal of Urology, 160, 1998, 612–614. [PubMed] [Google Scholar]
- Pantazopoulos D., Karagiannakos P., Sofras F., Kostakopoulos A., Deliveliotis C., Dimopoulos C. Effect of drugs on crystal adhesion to injured urothelium, Urology, 36 (3, 1990, 255–259. [DOI] [PubMed] [Google Scholar]
- Parsons C.L. The therapeutic role of sulphated polysaccharides in the urinary bladder, Urology Clinics of North America, 21 (1, 1994, 93–100. [PubMed] [Google Scholar]
- Parsons C.L., Mulholland S.G. Bladder surface mucin. Its antibacterial effect against various bacterial species, American Journal of Pathology, 93 (2, 1978, 423–432. [PMC free article] [PubMed] [Google Scholar]
- Parsons C.L., Mulholland S.G. Successful therapy of interstitial cystitis with pentosanpolysulfate, Journal of Urology, 138, 1987, 513–516. [DOI] [PubMed] [Google Scholar]
- Parsons C.L., Mulholland S.G., Anwar H. Antibacterial activity of bladder surface mucin duplicated by exogenous glycosaminoglycan (heparin), Infection and Immunity, 24 (2, 1979, 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C.L., Schmidt J.D., Pollen J.J. Successful treatment of interstitial cystitis with sodium pentosanpolysulfate, Journal of Urology, 130, 1983, 51–53. [DOI] [PubMed] [Google Scholar]
- Parsons C.L., Boychuk D., Jones S., Hurst R., Callahan H. Bladder surface glycosaminoglycans: An epithelial permeability barrier, Journal of Urology, 143, 1990, 139–142. [DOI] [PubMed] [Google Scholar]
- Parsons C.L., Benson G., Childs S.J., Hanno P., Sant G.R., Webster G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate, Journal of Urology, 150 (3, 1993, 845–848. [DOI] [PubMed] [Google Scholar]
- Strohmaier W.L., Quack M., Wilbert D.M., Bichler K.H. [Therapy of interstitial and radiogenic cystitis with d-glucosamine], Helvetica Chimica Acta, 56 (3, 1989, 323–325. [PubMed] [Google Scholar]
- Wei D.C., Politano V.A., Selzer M.G., Lokeshwar V.B. The association of elevated urinary total to sulphated glycosaminoglycan ratio and high molecular mass hyaluronic acid with interstitial cystitis, Journal of Urology, 163, 2000, 1577–1588. [PubMed] [Google Scholar]
- Willeberg P. Epidemiology of naturally occurring feline urological syndrome, Veterinary Clinics of North America: Small Animal Practice, 14 (3, 1984, 455–469. [DOI] [PubMed] [Google Scholar]