Abstract
This case report documents clinical and molecular findings in two littermate kittens of the Japanese domestic cat with GM2 gangliosidosis variant 0. Analysis included detailed physical, magnetic resonance imaging, biochemical, pathological and genetic examinations. At first, these littermate kittens showed typical cerebellar signs at approximately 2 months of age. About 2 months later, they progressively showed other neurological signs and subsequently died at about 7 months of age. Magnetic resonance imaging just before the death showed an enlarged ventricular system, T1 hyperintensity in the internal capsule, and T2 hyperintensity in the white matter of the whole brain. Histological findings suggested a type of lysosomal storage disease. Biochemical studies demonstrated that the kittens were affected with GM2 gangliosidosis variant 0, and a DNA assay finally demonstrated that these animals were homozygous for the mutation, which the authors had identified in a different family of the Japanese domestic cat. The findings in the present cases provide useful information about GM2 gangliosidosis variant 0 in Japanese domestic cats.
Lysosomal storage diseases are a group of genetic diseases of cellular metabolism caused by a deficiency of certain catabolic reaction(s) within the lysosomal catabolic pathway (March 1996). GM2 gangliosidosis is a lysosomal disease caused by the excessive accumulation of GM2 ganglioside and related glycolipids in lysosomes, especially in the lysosomes of neurons; they are inherited as autosomal recessive traits (Gravel et al 2001). Sandhoff disease is a variant (variant 0) of GM2 gangliosidosis and is caused by deficiencies of β-hexosaminidase A and B. A common component of these two enzymes, β-subunit, is coded by the HEXB gene, and mutations of this gene can cause Sandhoff disease. Tay–Sachs disease (B variant) and GM2 activator protein deficiency (AB variant) are other main variants of GM2 gangliosidosis.
In domestic animals, GM2 gangliosidosis has been reported in dogs (Bernheimer and Karbe 1970, Gambetti et al 1970, Cummings et al 1985, Yamato et al 2002), cats (Cork et al 1977, Neuwelt et al 1985, Yamato et al 2004a, Martin et al 2005), pigs (Read and Bridges 1968, Pierce et al 1976) and deer (Fox et al 1999). The diseases in a golden retriever (Yamato et al 2002) and in three families of cat, shorthaired domestic cats (Cork et al 1977), Korat cats (Neuwelt et al 1985) and Japanese domestic cats (Yamato et al 2004a), are animal models of human Sandhoff disease. Recently, a novel causative mutation in a family of Japanese domestic cats has been identified as a single nucleotide substitution from C to T at nucleotide position 667 (667C>T) of the open reading frame of the feline HEXB gene (Kanae et al 2007), which was preceded by the identification of two different mutations in Korat cats (Muldoon et al 1994) and domestic shorthaired cats (Martin et al 2004). The molecular basis of canine disease has not been demonstrated.
This case report documents the clinical, magnetic resonance (MR) imaging, biochemical, pathological and genetic findings of GM2 gangliosidosis in two littermate kittens of Japanese domestic cat with no family history of the disease. This report provides useful information for veterinary clinicians.
In 2002, two stray apparent littermate kittens of the Japanese domestic cat with ataxia (one male and another female), which appeared to be about 2 months old, were brought to the department of Veterinary Radiology, Nippon Veterinary and Life Science University, Musashino city, Tokyo. The blood cell counts and serum chemistry appeared to be within normal ranges. Neurological examinations demonstrated cerebellar ataxia, hypermetria, pendular nystagmus, intension tremor and loss of menace response without any other abnormal observable signs such as postural reactions and spinal reflexes. As these neurological signs hardly changed over 2 months after the initial examination, we first suspected that the kittens might have the well-known cerebellar hypoplasia caused by intrauterine infection of feline parvovirus (FPV) (Lorenz and Kornegay 2004).
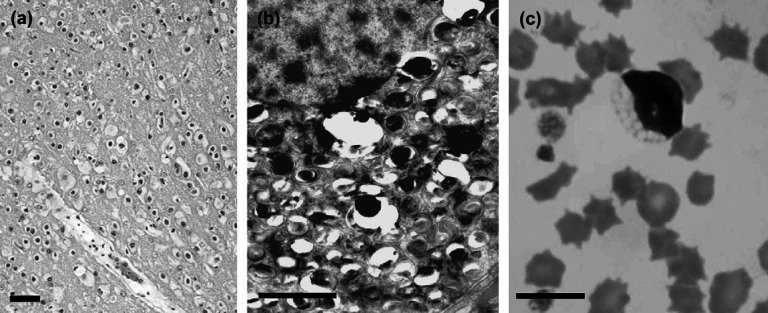
However, the female kitten (case 1) began to show other neurological signs such as loss of proprioception and postural reactions, and hyper spinal reflexes at about 4 months of age. Case 1 also showed signs of dehydration caused by difficulty in drinking and eating, as well as anaemia with an indeterminate cause (haematocrit 25 to 17%), respiratory acidosis (blood pH 7.26, PvCO2: 63.8 mmHg), and hypothermia (34.8°C) at about 6 months of age. Despite symptomatic therapy, none of the signs showed any improvement over the course of treatment. Case 1 was humanely euthanased and subsequently submitted for necropsy. Although there were no abnormal gross lesions visually present, further histological examination demonstrated swollen neurons with eosinophilic inclusion throughout the central nervous system (Fig 1a). Ultrastructurally, membranous cytoplasmic bodies were seen in the neurons (Fig 1b), suggesting a type of lysosomal disease, for example, GM1 or GM2 gangliosidosis or sphingomyelinosis (Niemann–Pick disease).
Fig 1.
(a) Histopathology of a kitten (case 1) with GM2 gangliosidosis. Light micrograph of the cerebral cortex (H&E stain). All neuronal cells have swollen cell bodies with eosinophilic inclusions. Bar=100 μm. (b) Electron micrograph of a neuron from case 1. A number of membranous cytoplasmic bodies (MCBs) were found. Bar=2 μm. The unclear appearance of MCBs is due to improper fixation. (c) Lymphocyte with abnormal vacuoles acquired from a blood smear from case 2 with GM2 gangliosidosis. Bar=10 μm.
The male kitten (case 2) had also begun to show progressive neurological signs including hypothermia and dysuria about 1 week after the death of case 1. When case 2 reached about 7 months of age, intracranial MR images were obtained and microscopic analysis of cell morphology in blood and bone marrow smears was performed. Concentrations of GM1 and GM2 gangliosides in cerebrospinal fluid (CSF) (Satoh et al 2004, Yamato et al 2004b) and activities of β-hexosaminidase (isozymes A and B) (Suzuki 1978, Yamato et al 2004b) and β-galactosidase (Suzuki 1978, Yamato et al 2000) were measured. Using formalin-fixed brain samples of case 1, ganglioside composition was determined using high performance thin-layer chromatography (HPTLC) (Yamato et al 2000, 2004a).
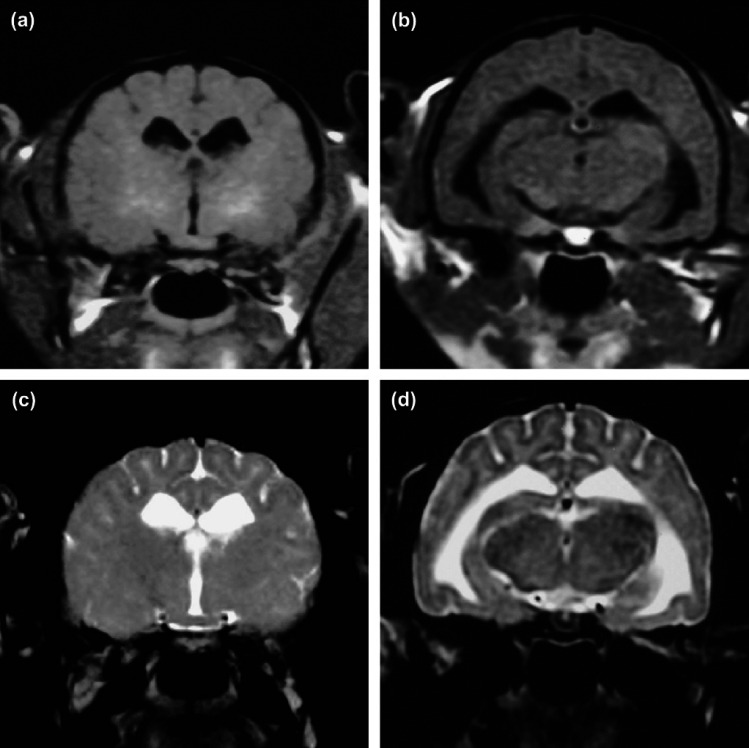
MR images from case 2 demonstrated mild hyperintensity on T1-weighted (T1W) image in the internal capsule (Fig 2a) and ventricular enlargement (Fig 2b), and hyperintensity on T2-weighted (T2W) image in the white matter of the whole forebrain (Fig 2c and d). Gadodiamide (Omniscan, Daiichi) did not enhance the lesions on T1W image (data not shown). CSF analysis showed marked increase of GM2 ganglioside concentration (97.9 nmol/l; reference 0.2–0.5 nmol/l) and mild elevation of GM1 ganglioside concentration (36.6 nmol/l; reference 2.1–21.6 nmol/l). Microscopic analysis of blood and marrow smears demonstrated cytoplasmic vacuolation of lymphocytes in blood (Fig 1c), and macrophages and myelocytes in bone marrow (data not shown). Activities of β-hexosaminidase A and B in leukocytes and plasma were deficient while β-galactosidase activity was normal (Table 1). HPTLC analysis of formalin-fixed brain samples from case 1 showed a marked increase of GM2 ganglioside but not GM1 ganglioside (Table 2).
Fig 2.
Magnetic resonance imaging of a kitten (case 2) with GM2 gangliosidosis. (a, b) Transverse T1-weighted imaging at the level of the thalamus and hippocampus. (c, d) Transverse T2-weighted imaging at the same level as (a) and (b). Mild hyperintensity on T1-weighted image in the internal capsule (a) and ventricular enlargement (b), and hyperintensity on T2-weighted image in the white matter of whole forebrain (c and d).
Table 1.
Leukocytes and plasma lysosomal enzyme activity
| Lysosomal enzymes* | Case 2 | Control † | ||
|---|---|---|---|---|
| Leukocytes | Plasma | Leukocytes | Plasma | |
| β-Hexosaminidase A | 3.1 | 0.4 | 2269±592 | 107±77 |
| β-Hexosaminidase B | 0.0 | 0.0 | 582±288 | 5.5±4.0 |
| β-Galactosidase | 45.7 | 10.6 | 51.0±15.3 | 6.5±3.2 |
Activities of β-hexosaminidase and β-galactosidase were measured using 4-methylumbelliferyl N-acetyl-β-d-glucosaminide and 4-methylumbelliferyl β-d-galactoside, respectively. The activities are expressed as nmol/h/mg protein for leukocytes and nmol/h/ml in the case of plasma.
Control data were obtained from 13 to 19 clinically normal cats. The values are shown as the mean±standard deviation.
Table 2.
Brain tissue ganglioside composition from a control cat and the kittens with GM2 gangliosidosis
| Gangliosides* | Case 1 (formalin-fixed) | Case 2 (fresh) | Control† (formalin-fixed) |
|---|---|---|---|
| Cerebrum | |||
| GM2 | 1188 | 2505 | 0.5 |
| GM1 | 145 | 280 | 132 |
| GD1a | 350 | 209 | 301 |
| GD1b | 172 | 119 | 129 |
| GT1b | 158 | 95 | 187 |
| Cerebellum | |||
| GM2 | 521 | 288 | 0.2 |
| GM1 | 57 | 10 | 33 |
| GD1a | 55 | 15 | 42 |
| GD1b | 69 | 11 | 172 |
| GT1b | 91 | 5 | 205 |
Values are expressed as nmol of N-acetylneuraminic acid per gram wet weight of tissue.
Control cat died of idiopathic cardiomyopathy.
Case 2 survived until about 7 months of age while receiving symptomatic treatment including heat-insulation, compulsory feeding, and compressive urination and defecation. Immediately after death, necropsy and HPTLC analysis of fresh brain samples were performed (Table 2). Findings confirmed that case 2 had the same disease as its littermate, case 1.
Three years after the death of cases 1 and 2, a DNA assay recently developed to detect the 667C>T mutation (Kanae et al 2007) was performed using preserved liver specimens. Consequently both animals were homozygous for the mutation (data not shown).
In many lysosomal diseases, the main clinical signs are progressive and lethal neurological signs. At early stage of these diseases, affected animals usually show typical cerebellar signs including ataxia, hypermetria, intension tremor and so on. These early manifestations might be attributable to the fact that those specific signs are easier to notice or because cerebellar neurons are more susceptible to the damage caused by lysosomal diseases. In this report, the two affected kittens showed progressive neurological dysfunctions, but first manifested only the typical cerebellar signs, which promoted a misdiagnosis of the kittens as FPV-induced cerebellar hypoplasia.
In this report, the affected kittens were Japanese domestic cats, and their clinical features including clinical course and lymphocyte vacuolation were similar to those in the first case of Japanese domestic cat reported previously (Yamato et al 2004a) although their survival periods (6–7 months) were relatively short compared with that of the first case (10 months). In addition, the pathological and biochemical features in the present cases were also similar to those in the previous case of GM2 gangliosidosis variant 0. Swollen neurons with membranous cytoplasmic bodies, GM2 ganglioside accumulation in the brain and CSF, and deficient β-hexosaminidase activities in leukocytes and plasma were common features between the present and previous cases. Furthermore, the molecular basis in the present cases was also the same as that in the previous case. Clinical, pathological and biochemical findings in the present cases provide useful information about GM2 gangliosidosis variant 0 in Japanese domestic cats.
There was no familial information in the present cases, but the animals might be related to the previously reported cat family with GM2 gangliosidosis (Yamato et al 2004a) because both the present and previous cases were homozygous for the same mutation. This suggests that these cats may have a common ancestor. All the affected cats were found in Tokyo, but it seemed to be impossible for cats from the two areas to have come into contact with each other, which suggests that the mutant allele (667C>T) is distributed widely in the population of Japanese domestic cats at least in the area around Tokyo. In addition, a DNA test detecting this mutation may be useful for all randomly mixed-breed cats in Japan because Japanese domestic cats are non-purebred cats found anywhere in Japan.
In general, MR imaging can be a non-invasive and useful method for evaluating the intracranial lesions even in neurodegenerative diseases. Several reports regarding MR imaging in GM2 gangliosidosis have been published in humans (Koelfen et al 1994, Mugikura et al 1996, Grosso et al 2003) and animals (Kroll et al 1995, Matsuki et al 2005). In human cases of GM2 gangliosidosis, abnormal signal intensities from the thalamus and basal ganglion, and cerebral atrophy seem to be the most common findings. Abnormalities on MR images correlate with the clinical form or stage of the disease rather than with the biochemical variant of the enzymatic defect. Therefore, the characteristics of MR findings are helpful for a diagnosis of GM2 gangliosidosis, but are not used for determining the variant form of the disease, ie, a definitive diagnosis.
Matsuki et al (2005) reported MR findings of a dog with GM2 gangliosidosis variant 0. The MR imaging of the dog brain displayed bilateral T2 hyperintensity and T1 hypointensity in the nucleus caudatus, and mild atrophy of the cerebral cortex. However, Kroll et al (1995) reported MR findings of Korat cats with GM2 gangliosidosis and described that the typical MR finding, hyperintensity of white matter on T2W image, became most apparent in 7.5 to 8 weeks (about 2 months) of age. The MR findings of the present cases were consistent with those of the Korat cats. In addition, hyperintensity of white matter on T2W image was also observed in the first case of Japanese domestic cat with GM2 gangliosidosis when it was 6 months of age (personal communication, Satoru Matsunaga, the University of Tokyo). Therefore, T2 hyperintensity in the white matter of the whole forebrain may be specific to feline GM2 gangliosidosis. However, the enlarged ventricular system observed in a 7-month-old kitten does not seem typical in feline GM2 gangliosidosis because this has not been observed in Korat cats (Kroll et al 1995). Although data from a larger number of affected cats is needed to specify the characteristics of feline GM2 gangliosidosis, MR image can be considered a useful tool for diagnosing this disease.
Acknowledgements
This paper was supported in part by Grants-in-Aid for Scientific Research (No. 16380210, O.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and by the project ‘Development of Preventive Veterinary Medicine using Genome and Proteome Analyses’ of Nippon Veterinary and Life Science University (T.A.), which was also funded by MEXT.
References
- Bernheimer H., Karbe E. Morphologische und neurochemische Untersuchungen von 2 Formen der amaurotischen Idiotie des Hundes: Nachweis einer G-Gangliosidose, Acta Neuropathologica 16, 1970, 243–261. [DOI] [PubMed] [Google Scholar]
- Cork L.C., Munnell J.F., Lorenz M.D., Murphy J.V., Baker H.J., Rattazzi M.C. GM2 ganglioside lysosomal storage disease in cats with β-hexosaminidase deficiency, Science 196, 1977, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Cummings J.F., Wood P.A., Walkley S.U., De Lahunta A., Deforest M.E. GM2 gangliosidosis in a Japanese spaniel, Acta Neuropathologica 67, 1985, 247–253. [DOI] [PubMed] [Google Scholar]
- Fox J., Li Y.T., Dawson G., Alleman A., Johnstrude J., Schumacher J., Homer B. Naturally occurring GM2 gangliosidosis in two Muntjak deer with pathological and biochemical features of human classical Tay–Sachs disease (type B GM2 gangliosidosis), Acta Neuropathologica 97, 1999, 57–62. [DOI] [PubMed] [Google Scholar]
- Gambetti L.A., Kelly A.M., Steinberg S.A. Biochemical studies in a canine gangliosidosis, Journal of Neuropathology and Experimental Neurology 29, 1970, 137–138. [Google Scholar]
- Gravel R.A., Kaback M.M., Proia R.L., Sandhoff K., Suzuki K. GM2 gangliosidosis. Scriver C.R., Beaudet A.L., Sly W.S., Valle D. The Metabolic and Molecular Bases of Inherited Disease, 8th edn, 2001, McGraw-Hill: New York, 3827–3876. [Google Scholar]
- Grosso S., Farnetani M.A., Berardi R., Margollicci M., Galluzzi P., Vivaewlli R., Morgese G., Ballestri P. GM2 gangliosidosis variant B1 neuroradiological findings, Journal of Neurology 250, 2003, 17–21. [DOI] [PubMed] [Google Scholar]
- Kanae Y., Endoh D., Yamato O., Hayashi D., Matsunaga S., Ogawa O., Maede Y., Hayashi M. Nonsense mutation of feline β-hexosaminidase β-subunit (HEXB) gene causing Sandhoff disease in a family of Japanese domestic cats, Research in Veterinary Science 82 (1), 2007, 54–60. [DOI] [PubMed] [Google Scholar]
- Koelfen W., Freund M., Jaschke W., Koenig S., Schultze C. GM-2 gangliosidosis (Sandhoff's disease): two year follow-up by MRI, Neuroradiology 36, 1994, 152–154. [DOI] [PubMed] [Google Scholar]
- Kroll R.A., Pagel M.A., Roman-Goldstein S., Barkovich A.J., D'Agostino A.N., Neuwelt E.A. White matter changes associated with feline GM2 gangliosidosis (Sandhoff disease): correlation of MR findings with pathologic and ultrastructural abnormalities, American Journal of Neuroradiology 16, 1995, 1219–1226. [PMC free article] [PubMed] [Google Scholar]
- Lorenz M.D., Kornegay J.N. Ataxia of the head and the limbs. Lorenz M.D., Kornegay J.N. Handbook of Veterinary Neurology, 4th edn, 2004, Saunders: Philadelphia, 219–244. [Google Scholar]
- March P.A. Degenerative brain disease, Veterinary Clinics of North America: Small Animal Practice 26, 1996, 945–971. [PubMed] [Google Scholar]
- Martin D.R., Krum B.K., Varadarajan G.S., Hathcock T.L., Smith B.F., Baker H.J. An inversion of 25 base pairs causes feline GM2 gangliosidosis variant, Experimental Neurology 187, 2004, 30–37. [DOI] [PubMed] [Google Scholar]
- Martin D.R., Cox N.R., Morrison N.E., Kennamer D.M., Peck S.L., Dodson A.N., Gentry A.A., Griffin B., Rolsma M.D., Baker H.J. Mutation of the GM2 activator protein in a feline model of GM2 gangliosidosis, Acta Neuropathologica 110, 2005, 443–450. [DOI] [PubMed] [Google Scholar]
- Matsuki N., Yamato O., Kusuda M., Maede Y., Tsujimoto H., Ono K. Magnetic resonance imaging of GM2-gangliosidosis in a golden retriever, Canadian Veterinary Journal 46, 2005, 275–278. [PMC free article] [PubMed] [Google Scholar]
- Mugikura S., Takahashi S., Higano S., Kurihara N., Kon K., Sakamoto K. MR findings in Tay–Sachs disease, Journal of Computer Assisted Tomography 20, 1996, 551–555. [DOI] [PubMed] [Google Scholar]
- Muldoon L.L., Neuwelt E.A., Pagel M.A., Weiss D.L. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease), American Journal of Pathology 144, 1994, 1109–1118. [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E.A., Johnson W.G., Blank N.K., Pagel M.A., Maslen-McClure C., McClure M.J., Wu P.M. Characterization of a new model of GM2-gangliosidosis (Sandhoff's disease) in Korat cats, Journal of Clinical Investigation 76, 1985, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K.R., Kosanke S.D., Bay W.W., Bridges C.H. Animal model of human disease: GM2 gangliosidosis, American Journal of Pathology 83, 1976, 419–422. [PMC free article] [PubMed] [Google Scholar]
- Read W.K., Bridges C.H. Cerebrospinal lipodystrophy in swine. A new disease model in comparative pathology, Pathologia Veterinaria 5, 1968, 67–74. [DOI] [PubMed] [Google Scholar]
- Satoh H., Yamato O., Asano T., Yamasaki M., Maede Y. Increased concentration of GM1-ganglioside in cerebrospinal fluid in dogs with GM1- and GM2-gangliosidoses and its clinical application for diagnosis, Journal of Veterinary Diagnostic Investigation 16, 2004, 223–226. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Enzymic diagnosis of sphingolipidoses, Methods in Enzymology 50, 1978, 456–488. [DOI] [PubMed] [Google Scholar]
- Yamato O., Matsuki N., Satoh H., Inaba M., Ono K., Yamasaki M., Maede Y. Sandhoff disease in a golden retriever dog, Journal of Inherited Metabolic Disease 25, 2002, 319–320. [DOI] [PubMed] [Google Scholar]
- Yamato O., Matsunaga S., Takata K., Uetsuka K., Satoh H., Shoda T., Baba Y., Yasoshima A., Kato K., Takahashi K., Yamasaki M., Nakayama H., Doi K., Maede Y., Ogawa H. GM2-gangliosidosis variant 0 (Sandhoff-like disease) in a family of Japanese domestic cats, Veterinary Record 155, 2004a, 739–744. [PubMed] [Google Scholar]
- Yamato O., Ochiai K., Masuoka Y., Hayashida E., Tajima M., Omae S., Iijima M., Umemura T., Maede Y. GM1 gangliosidosis in shiba dogs, Veterinary Record 146, 2000, 493–496. [DOI] [PubMed] [Google Scholar]
- Yamato O., Satoh H., Matsuki N., Ono K., Yamasaki M., Maede Y. Laboratory diagnosis of canine GM2-gangliosidosis using blood and cerebrospinal fluid, Journal of Veterinary Diagnostic Investigation 16, 2004b, 39–44. [DOI] [PubMed] [Google Scholar]