Abstract
Inflammatory polyps of the feline middle ear and nasopharynx are non-neoplastic masses that are presumed to originate from the epithelial lining of the tympanic bulla or Eustachian tube. The exact origin and cause are unknown, however, it is thought that inflammatory polyps arise as a result of a prolonged inflammatory process. It is unclear whether this inflammation initiates or potentiates the development and growth of inflammatory polyps. Cats with inflammatory polyps typically present with either signs of otitis externa and otitis media or with signs consistent with upper airway obstruction. Traditional diagnostics involve imaging of the tympanic bulla either with skull radiographs or computed topography (CT). Treatment consists of traction and avulsion of the polyp with or without ventral bulla osteotomy (VBO) to remove the epithelial lining of the tympanic bulla. The three cases described here are unusual manifestations or presentations of feline inflammatory polyps that address the following issues: (1) concurrent otic and nasopharyngeal polyps, (2) potential association with chronic viral infection, (3) polyp development in the contralateral middle ear, (4) CT appearance of the skull following VBO, and (5) development of secondary pulmonary hypertension.
Case 1
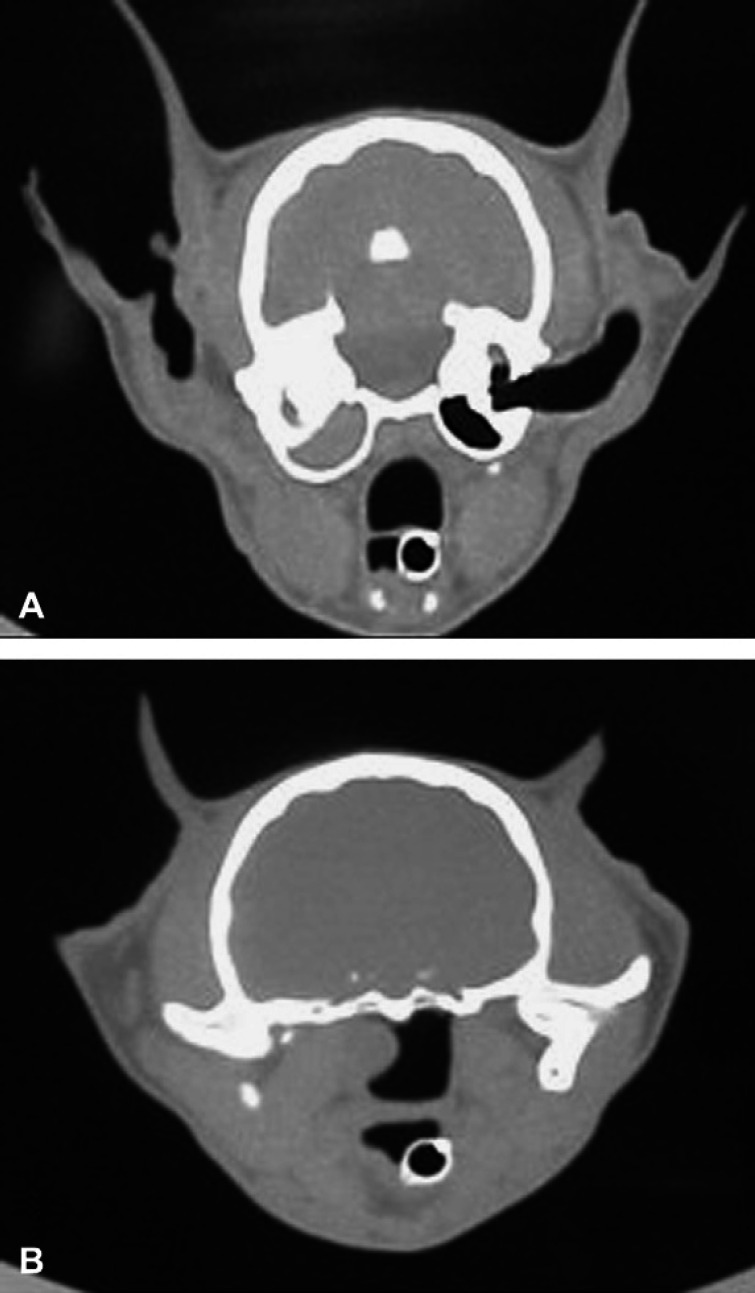
A 12-week-old, female cat was purchased from Laboratory Animal Resources at Colorado State University and shown to be negative for feline herpesvirus 1 (FHV-1) and calicivirus by throat culture, negative for feline leukemia virus antigen in serum, and negative for antibodies against FHV-1, calicivirus, panleukopenia, and feline immunodeficiency virus. This cat had been vaccinated three times with a modified live FVRCP vaccine and housed in isolation until sequentially challenged with the USDA challenge strains of panleukopenia virus, calicivirus, and FHV-1 at approximately 3 years of age (Lappin et al 2002). Sterilizing immunity against panleukopenia virus was present, but mild clinical signs of acute calicivirus and acute FHV-1 infections consisting of rhinitis and conjunctivitis occurred post-challenge. The signs of acute rhinitis waned over a 3–4 week period, but mild chronic rhinitis persisted and was presumed to relate to calicivirus or FHV-1 infection. The cat was ovariohysterectomized and adopted to a private home where she lived exclusively indoors as the only cat in the family. The new owner reported that chronic upper respiratory congestion and intermittent sneezing persisted in the 6 months after adoption. At that time an otic discharge was noted and the cat was presented to Colorado State University Veterinary Medical Center (CSU-VMC) for evaluation at approximately 3.5 years of age. The only significant physical examination abnormality was the presence of a mass in left horizontal ear canal. A computed topography (CT) scan of the skull was performed and on transverse images through the tympanic bullae, the left tympanic bulla and horizontal external ear canal were opacified with fluid-dense material (Fig 1A). Caudal to the left tympanic bulla, a soft tissue mass was noted extending into the nasopharynx (Fig 1B). The cat went to surgery for a left ventral bulla osteotomy (VBO). Both compartments of the bulla were entered and a large amount of purulent material was identified. Debridement of the dorsal compartment resulted in removal of a 1 cm polyp-like mass from the external ear canal. Subsequent debridement resulted in retrieval of a 2 cm polyp-like mass. Histopathology confirmed both masses to be inflammatory polyps. Following surgery, the nasopharynx was evaluated with a flexible endoscope and no polyp or tissue remnants were found. The cat recovered well from anesthesia without any neurological deficits.
Fig 1.
(A) Transverse CT image at the level of the tympanic bulla demonstrating soft tissue density within the left bulla and extending into the horizontal ear canal. (B) Transverse CT image at the level of the nasopharynx demonstrating a soft tissue intensity mass protruding into the left nasopharyngeal area.
This case describes a bilobed polyp that extended through the tympanic membrane into the horizontal external ear canal and through the Eustachian tube into the nasopharyngeal area. Previous retrospective studies have shown this to be an uncommon occurrence (Faulkner and Budsberg 1990, Trevor and Martin 1993, Anderson et al 2000). Therefore, in cats that present with signs of otitis externa or otitis media, further evaluation of the nasopharyngeal area is also warranted. The nasopharyngeal mass in this cat was not observed on routine entubation for general anesthesia. Thorough evaluation of the nasopharynx can be evaluated by digital palpation of the soft palate, retraction of the soft palate with a spay hook or stay sutures, visual inspection of the dorsal nasopharynx using a dental mirror, or use of a flexible fiberoptic endoscope.
It has been proposed that inflammatory polyps arise secondary to chronic inflammation (Rogers 1988, Veir et al 2002). As cultures for aerobic bacteria are generally negative and organisms are not usually seen on histopathology, a viral cause has been proposed. The inflammatory polyps described in case 1 developed after chronic FHV-1 and calicivirus infections, which may support the hypothesis that these masses occur secondary to chronic viral infection in some cats. However, to date neither FHV-1 nor calicivirus have been amplified from feline inflammatory polyp tissue (Veir et al 2002). The failure to amplify FHV-1 DNA or calicivirus RNA from the polyps may have related to the formalin fixation, which decreases sensitivity of the assays or the timing of the assays. For example, a herpesvirus is thought to be responsible for green sea turtle fibropapillomatosis, a syndrome that histologically resembles feline inflammatory polyps (Herbst et al 1998). In sea turtles, herpesvirus antigen is detected by immunohistochemistry more commonly in the most immature, experimentally induced tumors (47%) than older, spontaneously occurring tumors (7.5%) (Herbst et al 1998). Thus, it is possible that FHV-1 or calicivirus infection is responsible for initiation of feline inflammatory polyps, but by the time clinical signs are noted, the organisms have been cleared. It is also possible that FHV-1 or feline calicivirus were present in the tissues, but the assays utilized were not sensitive enough. Evaluating other FHV-1 and calicivirus gene targets may demonstrate a viral association with feline inflammatory polyps.
Case 2
A 1.5-year-old male-castrated, domestic shorthair was presented to a local veterinarian for evaluation of fever (41.1°C; 106°F) and intermittent lethargy of 3 weeks' duration. The owner had also noticed that the cat was unable to blink the right eye. The cat was prescribed clindamycin (10 mg/kg, PO, q12h) (Antirobe; Pfizer Animal Health) and was hospitalized for 4 days with minimal signs of improvement. The cat was referred to CSU-VMC. Abnormalities noted on physical examination included elevated rectal body temperature (39.4°C; 103.0°F), right-sided lagophthalmos, mild right-sided head tilt, and lethargy. The cat had been vaccinated within the last 6 months for panleukopenia, calicivirus, FHV-1, and rabies virus. At 12 weeks of age, the cat had a 1-month history of clinical signs consistent with upper respiratory tract infection.
Serologic tests for Toxoplasma gondii IgM, T gondii IgG, feline leukemia virus antigen, and feline immunodeficiency virus antibody were negative. A complete blood count and serum biochemical panel were within normal limits. While awaiting test results, enrofloxacin (5 mg/kg PO q24h) (Baytril; Bayer Corporation) was administered to improve the antibiotic spectrum against Gram-negative organisms. The owner was instructed to apply sterile ophthalmic ointment to the right eye every 4–6 h to improve corneal hydration. One week later, the cat was readmitted as horizontal nystagmus had developed 3 days earlier. The right-sided head tilt and lagophthalmos persisted and mild ataxia had developed.
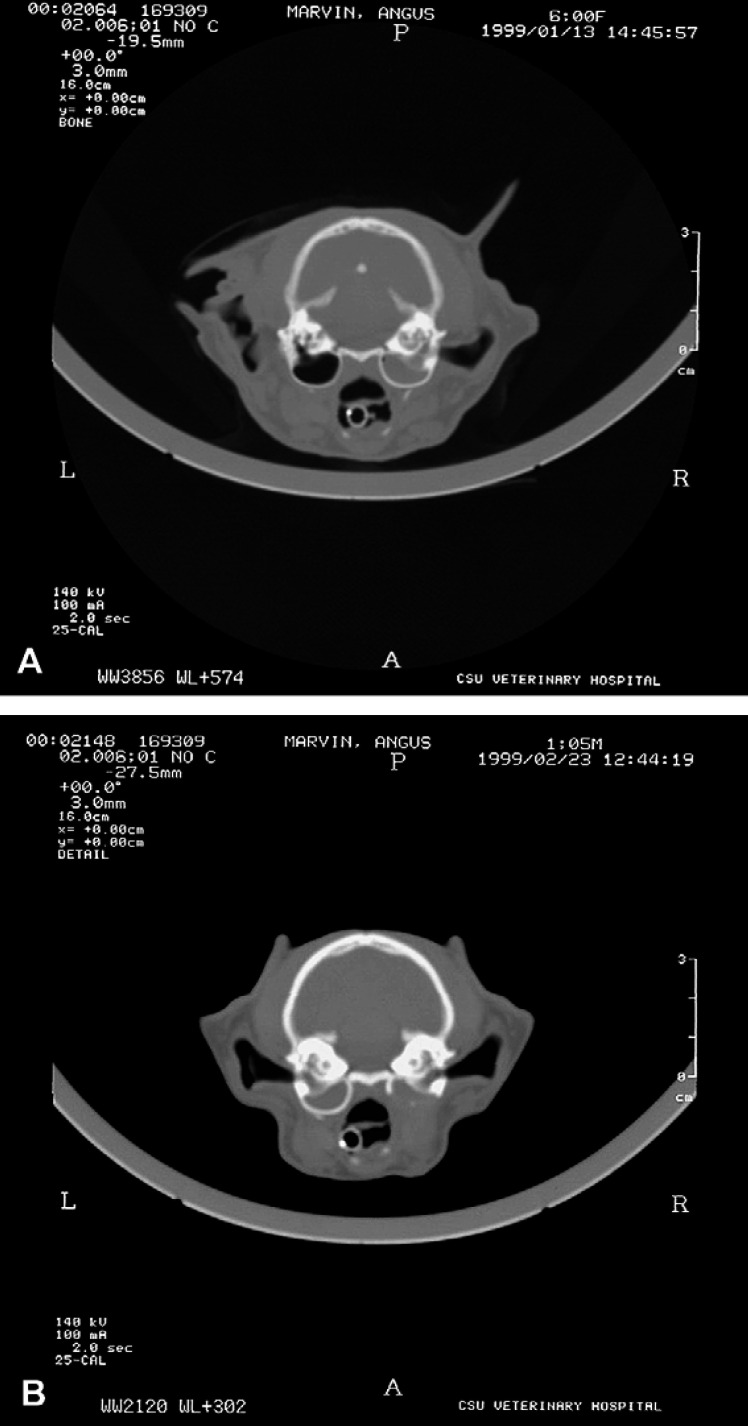
A CT scan was performed and transverse images of the skull showed a soft tissue opacity completely filling the right tympanic bulla (Fig 2A). There was also thickening of the right tympanic bulla wall and sclerosis of the bones in the right petrous temporal area. On initial review, no other abnormalities were identified. These results were consistent with chronic otitis media and possibly otitis interna of the right ear.
Fig 2.
(A) Transverse CT image at the level of the tympanic bulla. Soft tissue opacity completely fills the right tympanic bulla and the wall is thickened. The left tympanic bulla is normal. (B) Transverse CT image at the level of the tympanic bulla. There is an increased soft tissue opacity within the left tympanic bulla and a soft tissue opacity within the ostectomized right tympanic bulla.
A right-sided VBO was performed. Aerobic and anaerobic cultures, antimicrobial susceptibility testing, histopathology, FHV-1 polymerase chain reaction (PCR) assay and calicivirus RT-PCR assay were performed on the purulent and polyp-like material removed from within the tympanic bulla. The cat had an uneventful recovery postoperatively. Cultures were negative and viral DNA or RNA were not amplified. Histopathological examination of tissue removed from the bulla confirmed an inflammatory polyp with severe suppurative inflammation. As the fever had resolved, organisms were not cultured, and organisms were not seen histologically, the cat was sent home without antibiotics. The head tilt and ataxia slowly resolved, the cat regained the ability to blink the right eye, and the fever did not recur.
Eighteen days after surgery, the owner presented the cat for evaluation of a 2-day duration of acute lethargy, decreased appetite, mild ataxia, left-sided head tilt, and inability to close the left eye. An abbreviated biochemical panel was within normal limits. A CT scan was repeated and it revealed a soft tissue opacity mass within the left tympanic cavity and thickening of the wall of the tympanic bulla (Fig 2B). When compared to the CT examination performed 3 weeks prior, a board-certified radiologist confirmed that the left side was normal at that time. The osteotomy of the right tympanic bulla was confirmed and a soft tissue density was identified within the residual bulla. The cat was taken to surgery and a left-sided VBO was performed. A large mass was removed and submitted for histopathology, as well as aerobic and anaerobic culture and antimicrobial susceptibility testing. The mass was identified histologically as an inflammatory polyp. Cultures were negative. Postoperative recovery was uneventful and the cat was discharged. Clinical signs slowly improved and at 2 months postoperatively the only clinical sign was a mild head tilt to the left.
Approximately 3 months after the second surgery, the cat was presented for evaluation of anorexia, depression, and failure to close the right eye. CT scan revealed increased soft tissue density in the area that remained of both the right and left tympanic bulla. A tentative diagnosis of bacterial otitis media was made and the cat was discharged on cefadroxil (50 mg PO q12h) (Cefa-Drops; Fort Dodge) to be given for 1 month. Clinical signs of disease resolved and the cat has been clinically normal for 19 months.
This cat initially had a unilateral inflammatory polyp that induced facial nerve palsy and signs of vestibular disease. Disease recurred in the opposite ear within 1 month of successful treatment of the first polyp. The first CT scan showed no overt evidence of disease on the contralateral side, therefore this case demonstrates that a polyp may develop in a very short period of time. The actual rate of polyp development is still unknown. To our knowledge, this is the first case of confirmed contralateral recurrence. Previous studies report the occasional occurrence of bilateral polyps, but for the most part feline inflammatory polyps tend to be unilateral (Faulkner and Budsberg 1990, Kapatkin et al 1990, Trevor and Martin 1993, Anderson et al 2000). Kapatkin et al (1990) describe one cat with a nasopharyngeal polyp that had an aural polyp removed 18 months previously, however, it is unclear whether this occurred on the same or opposite side (Kapatkin et al 1990). Veir et al (2002) describe two cats with contralateral recurrence. However, one of those cases is the cat described in this report. The other cat was evaluated with only skull radiographs at the original presentation. Skull radiographs have been shown to have a 25% false negative rate for diagnosis of otitis media, therefore a reasonable assumption could be made that there was possible bilateral disease present at that time (Remedios et al 1991).
This report also documents the CT appearance of the feline tympanic bulla following VBO. It was originally thought that VBO would promote extensive regrowth of soft tissue into the tympanic cavity (McAnulty et al 1995). However, this was found not to be the case as experimental studies in normal dogs with VBO found that 75% would completely reform the tympanic bulla within 6 weeks of surgery by formation of fibrous connective tissue lined with cuboidal epithelium (McAnulty et al 1995). Reduced tympanic bulla volume by soft tissue was seen in only 33% of dogs and ranged from 20 to 40% impingement of soft tissue through the osteotomy site. Radiographic evaluation of five dogs after VBO performed to treat otitis media found partial to complete reformation of the bulla in all dogs (Molt and Walker 1997). No difference was seen between the dogs that had resolution of clinical signs and the one dog that had unsatisfactory results. Therefore, the value of radiographs to assess the recurrence of otitis media in dogs following VBO is still unknown; and the use of CT for diagnosis of recurrent otitis media has not been evaluated. For the cat in this report, follow-up CT scans found the tympanic bulla to be completely filled with a soft tissue or fluid density. This is consistent with changes seen in another cat at our hospital that underwent bilateral VBO for otitis media; bilateral tympanic cavity fluid or soft tissue proliferation was documented on CT scan 2 months postoperatively, but the cat had no clinical signs. Based on information from these two cases, it is impossible to distinguish polyp recurrence, otitis media, or fibrous tissue ingrowth on CT scan following VBO.
Case 3
A 1-year-old male Siamese cat was presented to a local veterinarian with the complaint of respiratory difficulty. The cat had a 6-month history of chronic upper respiratory infection, as well as recent episodes of acute collapse. On physical examination the cat was in severe respiratory distress manifested by inspiratory and expiratory stertor, open-mouth breathing, and cyanosis. Thoracic auscultation detected a third heart sound, but no murmur was heard. Jugular pulses were present. A complete blood count, biochemical profile, and urinalysis were performed and the only significant abnormality was a moderate leukocytosis (27.5×103/μl; normal=6.5–17.5×103/μl). Thoracic radiographs showed right-sided cardiomegaly. A cursory oral examination revealed a large membranous mass in the nasopharynx. The cat was referred to CSU-VMC for further evaluation.
Physical examination revealed a small, thin cat with a poor hair coat and body condition, with severe respiratory stridor. A grade III/VI systolic right sternal heart murmur was ausculted at that time. Further handling or examination resulted in exacerbation of respiratory difficulties, therefore the cat was placed in a cage with 40% oxygen supplementation. The cat was anesthetized and an oral endotracheal tube was placed with moderate difficulty. A CT scan found fluid in both tympanic bulla and a large mass that occupied the entire nasopharynx rostral to the tympanic bulla. The external ear canals were within normal limits. With the cat in dorsal recumbency, two monofilament stay sutures were placed in the soft palate to facilitate visualization of the nasopharyngeal mass. The mass was grabbed with Allis tissue forceps and removed with traction. Mild hemorrhage in the pharynx was noted. A flexible endoscope was used to further evaluate the nasopharynx and above the soft palate, which found no residual tissue. Histopathology of the mass confirmed an inflammatory polyp. VBO was not performed at this time and the cat recovered from anesthesia.
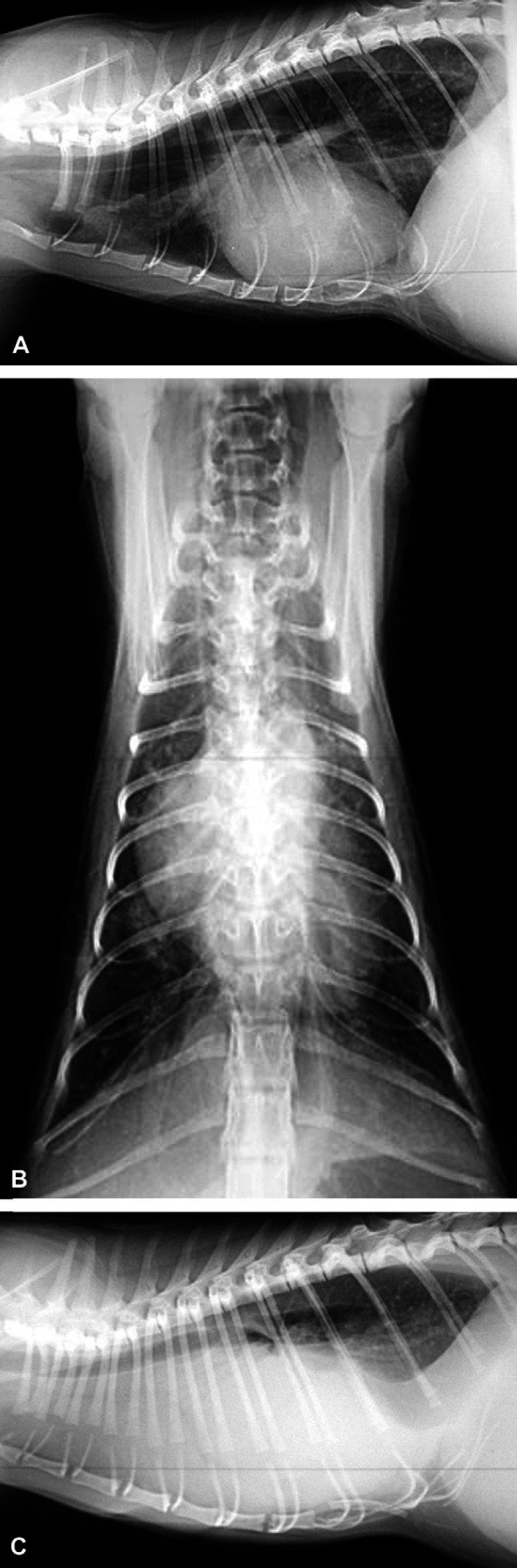
The following day, the cat was somewhat improved with only a mild inspiratory stridor that increased when stressed. Thoracic radiographs and cardiology work-up were performed. Thoracic radiographs showed cardiomegaly with a vertebral heart sum of 10 (normal=7.5±0.3) (Litster and Buchanan 2000) (Fig 3A and B). The thoracic esophagus was dilated and there was an interstitial and alveolar pattern involving the right cranial lung lobes consistent with aspiration pneumonia. There was also a peribronchial and interstitial pattern involving the caudodorsal lung fields. The pulmonary arteries to the caudal lung lobes were enlarged and tortuous. Serological tests for Diroflilaris immitus antibodies and antigen were negative. Electrocardiography showed a right-axis shift. Echocardiography revealed moderate right ventricular hypertrophy and dilation, severe right atrial dilation and moderate tricuspid valve insufficiency. No septal defects or other congenital abnormalities were noted and the tricuspid valve leaflets appeared to be normal. The velocity of the regurgitant jet across the tricuspid wave was 4.5 m/s, which is equivalent to a pressure gradient of 81 mmHg consistent with moderate to severe pulmonary hypertension. A bubble study was performed to rule out a reverse patent ductus arteriosus; the study was negative.
Fig 3.
(A, B) Lateral and ventrodorsal thoracic radiographs demonstrating cardiomegaly, megaesophagus, peribronchial and interstitial lung lobe pattern, and enlarged and tortuous pulmonary arteries in the caudal lung lobes. (C) Lateral thoracic radiograph showing findings consistent with moderate to severe pleural effusion.
The cat was sent home on prednisolone and antibiotics were continued as described. The plan was to reassess the cat in 2–3 months and consider VBO surgery at that time. However, the cat returned to CSU-VMC 2 months later in severe respiratory distress. An abbreviated physical examination noted a unilateral purulent nasal discharge. Thoracic auscultation revealed muffled heart sounds and harsh lung sounds bilaterally. A single lateral thoracic radiograph was performed that found moderate to severe pleural effusion (Fig 3C). Approximately 200 ml of straw colored fluid was removed from the right side of the thoracic cavity by thoracocentesis. Repeat echocardiography demonstrated no change from the previous examination. Complete blood count documented a slightly elevated packed cell volume (3%; normal=33–50%). Biochemical profile showed a severely elevated bicarbonate (44 mEq/l; normal=14–21 mEq/l). A room air venous blood gas found a severely elevated PCO2 (85.9 mmHg; normal=35–45 mmHg) with a normal pH (7.34; normal=7.3–7.4). With no other explanation for the elevated bicarbonate, a diagnosis was made of severe respiratory acidosis with compensatory metabolic alkalosis (Wingfield et al 1994). The cat was started on enalapril (1.25 mg PO q24h) (Enacard; Merial), spironolactone (6.25 mg PO q24h) and furosemide (6 mg IV q12h) (Lasix; Intervet).
Over the next 3 days, there was only mild improvement in the cat's condition. The cat was anesthetized and oropharyngeal examination found recurrence of a large nasopharyngeal polyp. The polyp was again removed and bilateral ventral bulla osteotomies were performed. Samples of the bulla contents were taken for histopathology and aerobic and anaerobic bacterial culture. The cat had a very poor recovery from surgery and suffered respiratory arrest 5 h postoperatively. Per the owner, resuscitation efforts were not performed and the cat died. Necropsy findings included bilateral ventricular hypertrophy, right side worse than left, and severe right atrial dilation. The lungs had lesions of patchy edema, congestion, and prominent activated alveolar macrophage accumulation consistent with long-standing impaired pulmonary circulation.
Although primary pulmonary hypertension has not been identified in veterinary medicine, secondary pulmonary hypertension occurs as a result of increased pulmonary blood flow, chronic elevations of left atrial pressure, or increased pulmonary vascular resistance (Tancreadi 1992). Aside from obstructive or obliterative diseases of the pulmonary vasculature, increased pulmonary vascular resistance can also result from hypoxic vasoconstriction. Hypoxemina and hypercarbia that develop from upper airway obstruction are known to be potent mediators of pulmonary vasoconstriction (Meyrick and Perkett 1989). Development of pulmonary hypertension and cor pulmonale secondary to chronic upper airway obstruction is a well-documented phenomenon in human medicine, particularly in pediatric patients (Blum and McGowan 2004). Although upper airway obstruction occurs commonly in small animals, the prevalence of secondary pulmonary hypertension in these animals is unknown.
Definitive diagnosis of pulmonary hypertension requires cardiac catheterization, however, it is most commonly diagnosed through an accumulation of less invasive clinical information. In the case described here, characteristic signs of pulmonary hypertension were obvious on thoracic radiographs and echocardiography. Doppler echocardiography is used for estimation of pulmonary artery pressure. A high-velocity regurgitant jet across the tricuspid valve in the absence of right ventricular outlet obstruction generally indicates pulmonary hypertension. The modified Bernoulli equation is used to estimate the pulmonary artery pressure. A peak tricuspid regurgitant velocity ≥2.8 m/s estimates a systolic pulmonary artery pressure ≥32 mmHg (Johnson et al 1999). The cat described in this report had a regurgitant flow across the tricuspid valve of 4.5 m/s. Diagnosis of pulmonary hypertension in cats is rare, and has only been previously reported in cats with either right-to-left shunting patent ductus arteriosus or pulmonary thromboembolism (Jeraj et al 1978, Sottiaux and Franck 1999, Connolly et al 2003). To our knowledge, this is the first report of a cat with pulmonary hypertension associated with chronic upper airway obstruction. Although this cat is unusual, the case demonstrates that survey thoracic radiographs of cats with inflammatory polyps should be considered to rule out concurrent lower airway disease.
References
- Anderson D.M., Robinson R.K., White R.A. Management of inflammatory polyps in 37 cats, The Veterinary Record 147, 2000, 684–687. [PubMed] [Google Scholar]
- Blum R.H., McGowan F.X., Jr. Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implication, Pediatric Anesthesia 14, 2004, 75–83. [DOI] [PubMed] [Google Scholar]
- Connolly D.J., Lamb C.R., Boswood A. Right-to-left shunting patent ductus arteriosus with pulmonary hypertension in a cat, The Journal of Small Animal Practice 44, 2003, 184–188. [DOI] [PubMed] [Google Scholar]
- Faulkner J.E., Budsberg S.C. Results of ventral bulla osteotomy for the treatment of middle ear polyps in cats, Journal of the American Animal Hospital Association 26, 1990, 496–499. [Google Scholar]
- Herbst L.H., Greiner E.C., Erhart L.M., Bagley D.A., Klein P.A. Serological association between spirorchiadiasis, herpesvirus infection, and fibropapillomatosis in green turtles from Florida, Journal of Wildlife Diseases 34, 1998, 496–507. [DOI] [PubMed] [Google Scholar]
- Jeraj K., Ogburn P., Lord P.F., Wilson J.W. Patent ductus arteriosis with pulmonary hypertension in a cat, Journal of the American Veterinary Medical Association 172, 1978, 1432–1436. [PubMed] [Google Scholar]
- Johnson L., Boon J., Orton E.C. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992–1996, Journal of Veterinary Internal Medicine 13, 1999, 440–447. [DOI] [PubMed] [Google Scholar]
- Kapatkin A.S., Matthiesen D.T., Noone K.E., Church E.M., Scavelli T.E., Parnaik A.K. Results of surgery and long-term follow-up in 31 cats with nasopharyngeal polyps, Journal of the American Animal Hospital Association 26, 1990, 387–392. [Google Scholar]
- Lappin M.R., Andrews J., Simpson D., Jensen W.A. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cat, Journal of the American Veterinary Medical Association 31, 2002, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Litster A.L., Buchanan J.W. Vertebral scale system to measure heart size in radiographs of cats, Journal of the American Veterinary Medical Association 216, 2000, 210–214. [DOI] [PubMed] [Google Scholar]
- McAnulty J.F., Hattel A., Harvey C.E. Wound healing and brain stem auditory evoked potentials after experimental ventral bulla osteotomy in dogs, Veterinary Surgery 24, 1995, 9–14. [DOI] [PubMed] [Google Scholar]
- Meyrick B.O., Perkett E.A. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli, American Review of Respiratory Disease 140, 1989, 186–189. [DOI] [PubMed] [Google Scholar]
- Molt D.E., Walker L. Radiographic appearance of the middle ear after ventral bulla osteotomy in five dogs with otitis media, Veterinary Radiology 28, 1997, 182–184. [DOI] [PubMed] [Google Scholar]
- Remedios A.M., Fowler J.D., Pharr J.W. A comparison of radiographic versus surgical diagnosis of otitis media, Journal of the American Animal Hospital Association 27, 1991, 183–188. [Google Scholar]
- Rogers K.S. Tumors of the ear canal, Veterinary Clinics of North America: Small Animal Practice 18, 1988, 859–868. [DOI] [PubMed] [Google Scholar]
- Sottiaux J., Franck M. Pulmonary embolism and cor pulmonale in a cat, The Journal of Small Animal Practice 40, 1999, 88–91. [DOI] [PubMed] [Google Scholar]
- Tancreadi R.G. Pulmonary vascular disease: primary pulmonary hypertension, Cardiovascular Clinics 22, 1992, 1113–1124. [PubMed] [Google Scholar]
- Trevor P.B., Martin R.A. Tympanic bulla osteotomy for treatment of middle-ear disease in cats: 19 cases (1984–1991), Journal of the American Veterinary Medical Association 202, 1993, 123–128. [PubMed] [Google Scholar]
- Veir J.K., Lappin M.R., Foley J.E., Getzy D.M. Feline inflammatory polyps: historical, clinical, and PCR findings for feline calici virus and feline herpesvirus-1 in 28 cases, Journal of Feline Medicine and Surgery 4, 2002, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield W.E., Van Pelt D.R., Hackett T.B., Martin L., Salman M.D. Usefulness of venous blood in estimating acid–base status of the seriously ill dog, Journal of Veterinary Emergency and Critical Care 4, 1994, 23–27. [Google Scholar]