Abstract
A 2-year-old, 4 kg, healthy, domestic shorthair female cat presented with ulcerated subcutaneous nodules on the commissures of its mouth. The cat was negative for feline leukaemia virus and feline immunodeficiency virus. Skin mycobacteriosis was diagnosed after detection of numerous acid-fast bacilli in Ziehl Neelsen-stained smears from the ulcers. Feline leprosy was suspected following preliminary polymerase chain reaction results: positive for Mycobacterium genus but negative for Mycobacterium tuberculosis and Mycobacterium avium complexes. Mycobacterium lepraemurium was later identified following DNA sequence analysis of the 5′ end of the 16S rRNA gene and the 16S–23S internal transcribed spacer region. Microscopic lesions consisted of pyogranulomas containing mainly large foamy macrophages with 10–100 intra-cellular acid-fast bacilli per field. The cat was cured after surgery and a 14-week course of clofazimine (30 mg daily) and clarithromycin (50 mg twice daily).
A 2-year-old female domestic shorthair cat originating from the island of Kythira, Greece, was referred with skin nodules on each commissure of its mouth. Although located south of Athens, Kythira has a humid and cool climate during winter: between November and April the average temperature is 13°C and the average monthly rainfall is 72 l/m2 (Datclim 2006). On this rural island rodents are numerous and the owners remembered seeing the cat hunting and eating rats. The location of the lesions was compatible with rodent bites.
One large (10 by 7 mm) wet and red ulcerated nodule was present on the left side of the mouth. Two smaller ulcerated nodules of 5 mm length were on the right side (Fig 1). The lesions developed over a 10-day period and did not respond to an injection of dexamethasone dimethylbutyrate (0.5 mg) given by a colleague who suspected them to be an eosinophilic granuloma. Clinical examination was otherwise normal, with a body weight of 4 kg.
Fig 1.
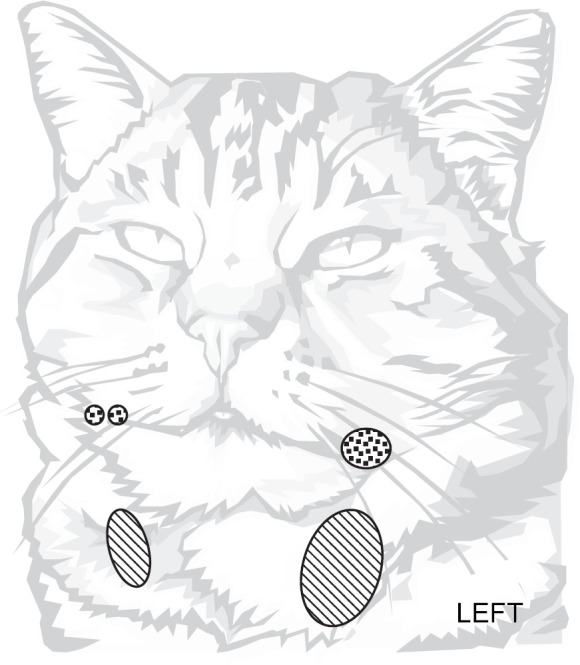
Dots: primary nodules, hash: secondary nodules.
Numerous acid-fast bacilli were clearly observed in smears of the ulcer stained with a Kinyoun-modified Ziehl Neelsen method (Kent and Kubica 1985). The nodules were completely and widely excised: half of each nodule was fixed in 10% formalin for histology, and the other half refrigerated prior to mycobacterial polymerase chain reaction studies and culture. Post-surgery the cat was prescribed 2 months of marbofloxacin (5 mg daily).
After a month of medication, the owners stopped the treatment for 3 days. This led to a rapid and severe recurrence, with numerous aggregated subcutaneous nodules appearing in both pharyngeal chains, on the left side it measured 1.5 cm long. Nodules did not ulcerate. Thoracic radiographs were unremarkable and tests for feline leukaemia virus and feline immunodeficiency virus were negative. A second surgery was performed and a different antimicrobial regimen was selected: clofazimine (30 mg daily) and clarithromycin (50 mg twice daily). The follow-up protocol included monitoring for potential hepatic side effects of clofazimine, with assessment being made every 2 weeks.
The cat tolerated the drugs well: it vomited just once on the second day, and its appetite and body weight remained stable throughout the 14 weeks of treatment. After 2 weeks the surgical incisions had healed, and only a small swelling was palpable in the left retro-pharyngeal area. Alkaline phosphatase (ALKP reference 13–116 IU/l) and alanine transaminase (ALT reference 20–85 IU/l) values were 40 and 27 IU/l, respectively. Healing was complete 4 weeks after surgery, with only a small solitary nodule of about 2 mm remaining. ALT and ALKP values were still unremarkable. Seven weeks after surgery palpation was normal. No recurrence has been observed since then (over 1.5 years).
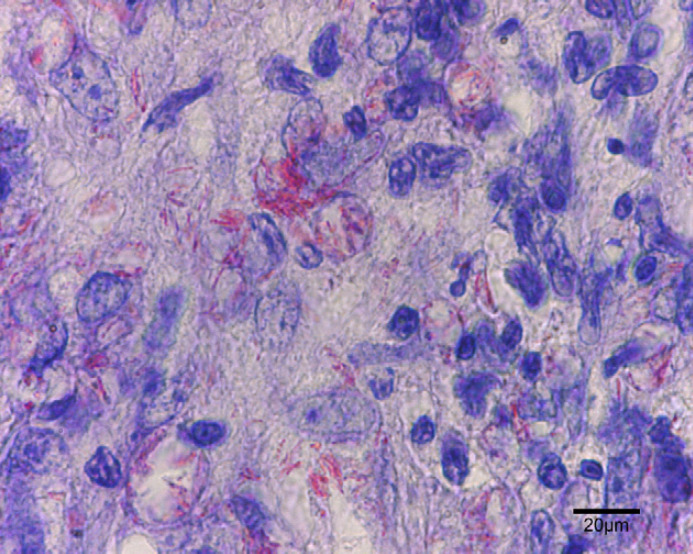
At the surface of the ulcer (Fig 2), 1–10 acid-fast bacilli were seen in every field. Most were within the cytoplasm of large macrophages and few were extracellular. Bacilli measured 4–9 μm in length, had for some of them a beaded morphology and were sometimes aggregated. In tissue sections, nodules (Fig 3) contained numerous pyogranulomas of several hundred micrometres that infiltrated the upper dermis, hypodermis and subcutis. In the area of the ulcer, erosion of the epidermis was observed with exfoliating granuloma. Granulomas (Fig 4) were composed mainly of aggregated macrophages with large microvacuolated (foamy) cytoplasm; some areas (Fig 5) were infiltrated with polynuclear neutrophil cells but no caseous necrosis was observed. Some lymphocytes and plasma cells were observed at the periphery of the granulomas. Ziehl Neelsen staining (Fig 6) revealed 10–100 acid-fast bacilli per field. Bacilli were mostly aggregated within the cytoplasm of macrophages. Similar lesions have been described in the ‘feline multi-bacillary lepromatous leprosy syndrome’ that can be caused by M lepraemurium, M avium or M AJ294746 (Davies et al 2006).
Fig 2.
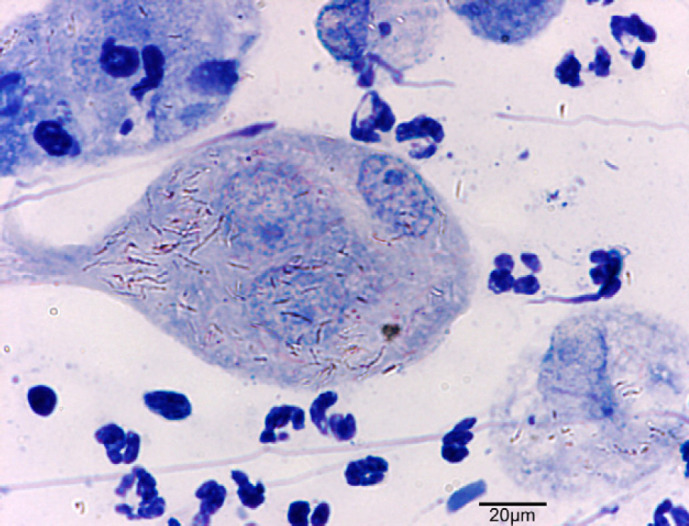
Fig 3.
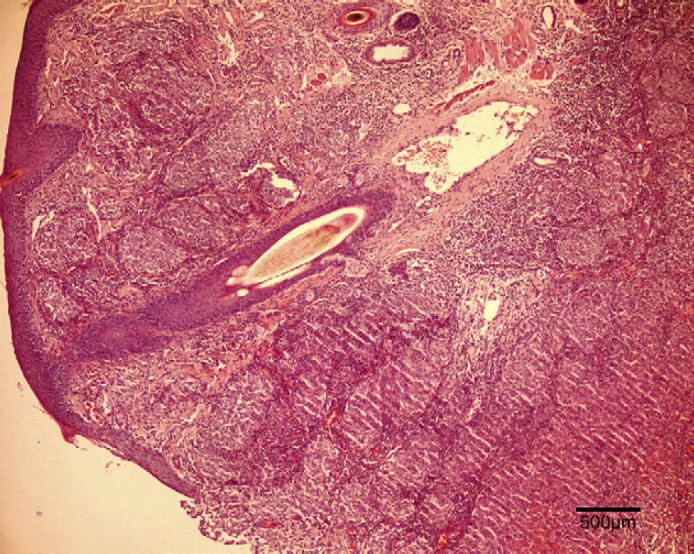
Fig 4.
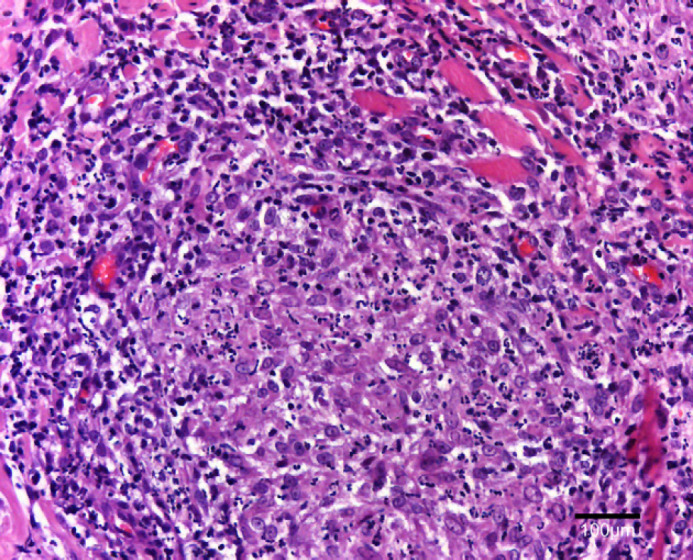
Fig 5.
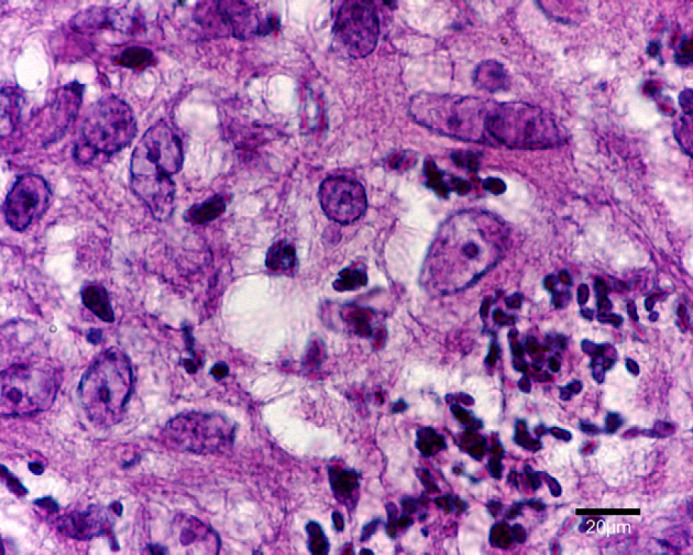
Fig 6.
For mycobacterial culture, fresh refrigerated tissue was sliced with a scalpel blade and homogenized by ‘mechanical squashing’ in the presence of 4% sulphuric acid as decontaminating solution (Thorel 1976). After neutralisation with 6% sodium hydroxide, samples were inoculated with Lowenstein-Jensen and Coletsos egg based solid media as well as Bactec MGIT 960 liquid medium. Cultures were negative at 3 months.
For DNA analysis 2 ml of tissue homogenate as prepared for bacteriology, were centrifuged for 5 min at 8000 rpm, washed several times in TE buffer, resuspended in 200 μl TE, shaken at 6 m/s for 45 s and finally processed with the High Pure PCR Template (Roche) for DNA extraction. Five microlitres of extract were used for polymerase chain reaction (PCR). Amplification was performed based on sequences specific for the M tuberculosis complex (Hermans et al 1990), M avium complex (van Soolingen et al 1998) and Mycobacterium species 16S rRNA (Huard et al 2003) sequences. No products were obtained for the PCR assays specific for the M tuberculosis and M avium complexes, however, a product (strong positive) was obtained for Mycobacterium species generic PCR.
16 s RNA sequencing was performed on the 5′ end region of the 16S rRNA gene which contains the hypervariable regions A and B (Tortoli 2003). A search of the RIDOM database (Harmsen et al 2002) was performed using the nucleotide sequence obtained, and a 100% match was obtained with the sequence of M lepraemurium. As the search also gave 99.55% similarity with the sequence from M avium, the ITS region was amplified and sequenced using the primers SP1 and SP2 (Roth et al 2000). A RIDOM search performed using the 219 bp amplicon again gave 100% similarity with the entry for M lepraemurium, thus confirming the identity of the infecting organism.
Published sensitivity data for M lepraemurium is scarce because the bacteria are difficult to grow (Malik et al 2002). Therefore, to choose from the published empirical protocols is difficult. We chose clofazimine/clarithromycin combination therapy. Clofazimine has a good reputation and few side effects in practice (Malik et al 2002), and has good intra-cellular distribution, especially within macrophages (Holdiness 1989). The difficulty in its administration relates to the need to reformulate this lipid soluble molecule which is presented in soft spherical (50 mg) or oval (100 mg) capsules (Lamprene; Novartis). We manufactured 30 mg size 4 capsules, easily swallowed by cats. The original capsules were cut with a scalpel blade on one side and the gel content recovered by applying pressure. The operator opened enough capsules to obtain 3 g of clofazimine. The gel was ‘liquefied’ in a ceramic bowl with few drops of ethyl ether. Silica powder (20 ml) was incorporated gradually while stirring, in order to make a 0.15 g/ml clofazimine mix. Mixing was continued until the ethyl ether had evaporated and the operator obtained a homogeneous colour that indicated proper mixing. The powder mix was then placed into the capsule filler with size 4 capsules.
Clarithromycin has been used to successfully treat some cats with leprosy and a dose-dependent efficacy relationship has been observed in one case (Malik et al 2002). Like clofazimine, this drug has intra-cellular distribution (Rodvold 1999). In addition, it is the first line antibiotic with a clear dose-dependent efficacy relationship for the treatment of M avium in humans (Nuermberger and Grosset 2004), and has been used successfully for treatment of disseminated M avium infection in young cats (Baral et al 2006).
Rifampicin was not chosen as it has a disappointing in vitro activity against M lepraemurium (Portaels et al 1982) especially when it is compared to the resistance standard for tuberculosis of 1 μg/ml (Hugo et al 1989). In addition rifampicin is inactive against M avium in the mouse model of infection (Nuermberger and Grosset 2004), and is not wise to combine with clarithromycin (Rodvold 1999).
Fluoroquinolones had failed when used as a first choice antibiotic earlier in this case.
Prior to our first consultation, the ulcer had been open for 2 weeks. The family members had taken no precaution towards this unsuspected source of infection and kept close contact with the cat. The two family cats slept in the same basket, frequently groomed each other and had play-fights. To date, no infection has been observed in the family members or pets, reinforcing the epidemiological observation that this disease is not naturally transmissible between cats, or from cats to other species (Rojas-Espinosa and Lovik 2001).
Acknowledgements
We thank Dr Rachel Dean at Bristol Veterinary School for early discussions, at AFSSA-LERPAZ, Maisons-Alfort, France: Sylvie Hénault, for DNA extraction and PCR, and Claudine Karoui for culture. Mr Colleta Rouen, France for the ‘ancient’ chemistry recipe for reformulation of clofazimine capsules. Ditri Papalexis Paris, France for the weather data.
References
- Baral R.M., Metcalfe S.S., Krockenberger M.B., Catt M.J., Barrs V.R., McWhirter C., Hutson C.A., Wigney D.I., Martin P., Chen S.C., Mitchell D.H., Malik R. Disseminated Mycobacterium avium infection in young cats: overrepresentation of Abyssinian cats, Journal of Feline Medicine and Surgery 8, 2006, 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datclim 2006, Hellenic National Meteorological Service: Greece, Athens.
- Davies J.L., Sibley J.A., Myers S., Clark E.G., Appleyard G.D. Histological and genotypical characterization of feline cutaneous mycobacteriosis: a retrospective study of paraffin embedded tissues, Veterinary Dermatology 17, 2006, 155–162. [DOI] [PubMed] [Google Scholar]
- Harmsen D., Rothganger J., Frosch M., Albert J. RIDOM: ribosomal differentiation of medical microorganisms database, Nucleic Acids Research 30, 2002, 416–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P.W., van Soolingen D., Dale J.W., Schuitema A.R., McAdam R.A., Catty D., van Embden J.D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis, Journal of Clinical Microbiology 28, 1990, 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdiness M.R. Clinical pharmacokinetics of clofazimine. A review, Clinical Pharmacokinetics 16, 1989, 74–85. [DOI] [PubMed] [Google Scholar]
- Huard R.C., de Oliveira Lazzarini L.C., Butler W.R., van Soolingen D., Ho J.L. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions, Journal of Clinical Microbiology 41, 2003, 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo D., Levy-Frebault V., Thorel M.F. Titrages de resistance aux antibiotiques. Pasteur Institut. Methodes de laboratoire pour mycobacteriologie clinique, 1989, Institut Pasteur: Paris, 71. [Google Scholar]
- Kent P.T., Kubica G.P. Public health mycobacteriology. US Department of Health and Human Services. A Guide for the Level III Laboratory, 1985, Public Health Service, CDC: Atlanta, USA. [Google Scholar]
- Malik R., Hughes M.S., James G., Martin P., Wigney D.I., Canfield P.J., Chen S., Mitchell D.H., Love D.N. Feline leprosy: two different clinical syndromes, Journal of Feline Medicine and Surgery 4, 2002, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuermberger E., Grosset J. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections, European Journal of Clinical Microbiology and Infectious Diseases 23, 2004, 243–255. [DOI] [PubMed] [Google Scholar]
- Portaels F., Pattyn S.R., Francken A. In vitro sensitivity of Mycobacterium lepraemurium for antimycobacterial drugs, Arzneitmittel-Forschlung 32, 1982, 1123–1124. [PubMed] [Google Scholar]
- Rodvold K.A. Clinical pharmacokinetics of clarythromycin, Clinical Pharmacokinetics 37, 1999, 385–398. [DOI] [PubMed] [Google Scholar]
- Rojas-Espinosa O., Lovik M. Mycobacterium leprae and Mycobacterium lepraemurium infections in domestic and wild animals, Revue Scientifique et Technique OIE 20, 2001, 219–251. [DOI] [PubMed] [Google Scholar]
- Roth A., Reischl U., Streubel A., Naumann L., Kroppenstedt R.M., Habicht M., Fischer M., Mauch H. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S–23S rRNA gene spacer and restriction endonucleases, Journal of Clinical Microbiology 38, 2000, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soolingen D., Bauer J., Ritacco V., Leão S. Cardoso, Pavlik I., Vincent V., Rastogi N., Gori A., Bodmer T., Garzelli C., Garcia M.J. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization, Journal of Clinical Microbiology 36, 1998, 3051–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel M.F. Action de l'acide sulfurique à 4% sur les diverses espèces de Mycobactéries, Annales de Biologie Clinique 34, 1976, 431–435. [PubMed] [Google Scholar]
- Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s, Clinical Microbiology Reviews 16, 2003, 319–354. [DOI] [PMC free article] [PubMed] [Google Scholar]