Abstract
Q fever is a worldwide zoonotic disease caused by Coxiella burnetii. Although traditionally associated with livestock exposure, human infection has also been documented from contact with parturient cats. The goal of this study was to determine the prevalence of C burnetii DNA in uterine and vaginal tissues from healthy client-owned and shelter cats of north-central Colorado using polymerase chain reaction assay. Coxiella burnetii was not amplified from vaginal samples of any cat or uterine biopsies of shelter cats. However, a nucleotide sequence with 99% homology to C burnetii DNA was amplified from four of 47 (8.5%) uterine biopsies of client-owned cats. This study demonstrates that clinically normal cats in north-central Colorado can harbor C burnetii. Care should be taken when attending to parturient cats and contact with parturient secretions should be avoided. Additional studies are indicated to further characterize the role of cats in zoonotic Q fever.
Qfever is a worldwide disease of domestic animals and humans which is caused by the rickettsial organism Coxiella burnetii. C burnetii is a Gram-negative, obligate intracellular, small, pleomorphic bacteria with a complicated life cycle (Maurin and Raoult 1999, Kazar 2005). This organism infects a wide variety of animal species and endemic infections exist worldwide. Ticks are involved in the natural cycle of C burnetii but are not thought to transmit the organism to people (Kazar 2005). Traditionally, Q fever infection has been associated with livestock species, with organisms found in very high concentrations in the placenta, reproductive tract and parturient secretions of cattle, sheep and goats (Maurin and Raoult 1999, Berri et al 2001). Although animal infections are often subclinical, abortion and stillbirths are fairly common, and pneumonia, conjunctivitis and hepatitis have been rarely reported in ruminants (Berri et al 2001). Subclinical animal infections are often chronic, with organism shedding in feces, urine and parturient secretions (Babudieri 1959, Harris et al 2000, Berri et al 2001).
Humans most commonly acquire C burnetii infection via inhalation of parturient secretions from subclinically infected host animals, although cases have been reported from ingestion of contaminated dairy products (Maurin and Raoult 1999). Human to human transmission has also been reported (Milazzo et al 2001). The majority of infected humans are asymptomatic or present with non-specific, flu-like signs which may never be diagnosed. Human infection most commonly manifests as pneumonia or hepatitis, although endocarditis, meningoencephalitis and other less common presentations have been reported (Marrie 1995, Maurin and Raoult 1999). Chronic human infection has been linked to a post-Q fever fatigue syndrome (post-QFS) including symptoms such as fatigue, myalgia, arthralgia, sleep disturbance, fever and headache (Marmion et al 1990, 1996, Ayres et al 1998, Wildman et al 2002).
Although traditionally associated with livestock contact, zoonotic transmission of Q fever to humans has been reported from other domestic animals as well (Komiya et al 2003a). Cats have been recognized as a potentially important domestic reservoir of C burnetii, and several reports have documented cases of human Q fever resulting from exposure to parturient cats (Kosatsky 1984, Langley et al 1988, Marrie et al 1985, 1988a, 1988b, 1989, Pinsky et al 1991). Antibodies against C burnetii have been detected in the serum of cats in North America (Randhawa et al 1974, Willeberg et al 1980, Higgins and Marrie 1990, Case et al 2006). Antibodies against C burnetii have also been detected among cats in Japan and Korea (Komiya et al 2003b), as well as in Africa (Matthewman et al 1997), suggesting that cats may be an important worldwide source of potential human exposure. The presence of C burnetii antibodies in cat serum indicates past exposure but does not necessarily prove current infection. In 1998, C burnetii was cultured from vaginal samples of some pet cats in Japan (Nagaoka et al 1998), providing the first evidence of active feline infection to our knowledge.
Because the organism is difficult to culture, amplification of C burnetii DNA by polymerase chain reaction (PCR) has also been used to document infection in both domestic animals (Lorenz et al 1998, Berri et al 2000, Brennan and Samuel 2003) and humans (Stein and Raoult 1992, Kato et al 1998, Hakamata et al 2002, Fournier and Raoult 2003, Fenollar et al 2004). To our knowledge, C burnetii infection of domestic cats in North America has not been documented using a PCR assay. The purpose of the present study was to assess the prevalence of C burnetii DNA in vaginal and uterine samples of clinically normal cats in north-central Colorado using a PCR assay.
Materials and methods
Animals
The study group was comprised of 50 shelter and 47 client-owned, intact, female cats presented to the Veterinary Teaching Hospital at Colorado State University between June 2002 and October 2003 for elective ovariohysterectomy. Most cats were estimated to be less than 3 years old, however, exact mean age could not be determined because many in the shelter group were stray cats. Domestic shorthair and longhair breeds were represented. All cats were clinically normal on presentation, and only one cat (a healthy pregnant female in the shelter group) had evidence of vaginal discharge. The protocol for all procedures in the study was approved by the Animal Care and Use Committee of Colorado State University.
Sample collection and DNA isolation
Feline patients were anesthetized for elective ovariohysterectomy according to standard procedure by the anesthesia section of the Veterinary Teaching Hospital at Colorado State University. While under general anesthesia and prior to ovariohysterectomy, vaginal samples were obtained with a calcium alginate fiber tipped ultra-fine aluminum applicator swab (Fisher, Hampton, NH, USA). The vaginal sample was eluted from the swab by pulse vortexing with 200 μl sterile phosphate buffered saline (PBS) for 10 s, followed by 30 min room temperature incubation and an additional 10 s pulse vortex. Swabs were then disposed of in an approved infectious waste container and were stored at −70°C until DNA isolation. After ovariohysterectomy, the uterus was collected and stored at −70°C. Prior to DNA isolation, the uterus was thawed and a 3 mm by 3 mm piece was cut from the tissue. Vaginal sample DNA was isolated by use of the QIAamp DNA blood kit (QIAGEN, Valencia, CA, USA), while uterine sample DNA was isolated using the DNeasy tissue kit (QIAGEN). Vaginal sample DNA was quantitated using spectrophotometric analysis and DNA was shown to be present in every sample. Uterine sample DNA was not quantitated due to the large amount of tissue used in the isolation process. Vaginal DNA and uterine DNA were stored at −20°C until use. Coxiella burnetii positive control DNA was obtained from commercially available immunofluorescence assay slides containing both phase 1 and 2 antigens (Focus Technologies, Cypress, CA, USA). DNA from the slides was eluted with sterile PBS, purified with the QIAamp DNA blood kit (QIAGEN) and stored at 4°C until use.
PCR assay
Samples were amplified in an adaptation of a previously reported PCR assay using the following primers targeting the repetitive transposon-like region: Trans1 (5′-TAT GTA TCC ACC GTA GCC AGT C-3′) and Trans2 (5′-CCC AAC AAC ACC TCC TTA TTC-3′) (Berri et al 2000). The expected product of amplification with these primers was 687 bp in length. The PCR reaction was performed on 2.5 μl of each prepared sample in a total volume of 25 μl. The final reaction mixture consisted of 2 μmol/l each primer, 0.2 mmol/l each deoxynucleoside triphosphate, 3 mmol/l MgCl2, 1× Taq polymerase buffer (10 mmol/l Tris–HCl (pH 8.3), 50 mmol/l KCl) and 2.5 U Amplitaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA).
The PCR reactions were amplified using a Perkin Elmer 480 thermocycler (Applied Biosystems). Samples were subjected to touchdown PCR assay consisting of five cycles of denaturation at 94°C for 30 s, annealing at 66–61°C (decreasing 1° with every cycle) for 1 min and an extension at 72°C for 1 min. This was followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 61°C for 30 s and extension at 72°C for 1 min. Negative (sterile water) and positive (C burnetii DNA) control templates were run with each PCR assay to serve as internal controls. A 10 μl sample of each PCR product was analyzed by gel electrophoresis on a 1.5% Tris Borate EDTA agarose gel run at 120 V for 1 h, stained with ethidium bromide, visualized with an ultraviolet transilluminator and photographed.
Sequencing of PCR products and sequence analysis
The positive PCR products were excised from the gel and purified using the QIAquick gel extraction kit (QIAGEN). PCR products were sequenced in both directions on an ABI Prism 3100 genetic analyzer with the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) by Macromolecular Resources of Colorado State University in Ft. Collins, Colorado. The sequencing results were then blasted through the NBCI online program (http://www.ncbi.nlm.nih.gov/BLAST).
Results
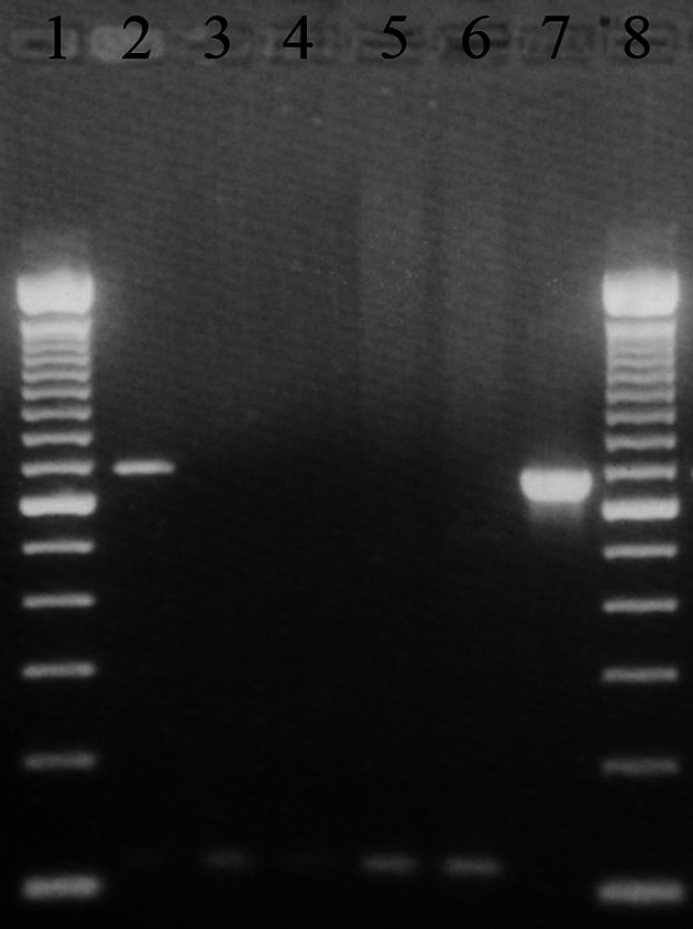
Amplification of Coxiella burnetii positive control DNA resulted in the expected 687 bp product (Fig. 1). No product was generated by using sterile water instead of DNA as a template. When estimating the sensitivity threshold of the PCR assay, positive PCR products were obtained from the 10−4 dilution of C burnetii positive control DNA (equivalent to 3 pg of DNA). In order to identify the amount of C burnetii DNA required in a tissue sample before DNA isolation for a PCR amplicon to be generated, known negative tissue samples were spiked with C burnetii DNA before DNA isolation. In this manner, PCR amplicons were obtained with an average of 1.2 ng DNA (range between 0.12 ng and 12 ng).
Fig 1.
Gel electrophoresis demonstrating Coxiella burnetii PCR. Lane 1: 100 bp DNA ladder. Lane 2: positive feline uterine sample with 687 bp amplicon. Lanes 3–5: negative feline uterine samples. Lane 6: negative control (sterile water template). Lane 7: positive control (C burnetii DNA). Lane 8: 100 bp DNA ladder.
Coxiella burnetii DNA was not amplified from the vaginal samples of any cat or the uterine biopsies of shelter cats. However, C burnetii DNA was amplified from four of 47 (8.5%) uterine biopsies of client-owned cats. Sequencing results obtained from all PCR products had 99% homology to NBCI C burnetii accessions AE016966.1, AEO16965.1 and AE16960.1.
Discussion
Coxiella burnetii is acknowledged as one of the world's most important zoonotic agents because of the wide geographic distribution, large host range and high infectious potential of the organism. In addition to many acute manifestations of illness, human C burnetii infection has been linked to long lasting symptoms similar to those seen in chronic fatigue syndrome (post-QFS). Although most human post-QFS cases have been associated with livestock exposure (Marmion et al 1990, 1996, Ayres et al 1998, Wildman et al 2002), in one study, approximately one-third of Japanese patients reported their main animal contact was with cats (Arashima et al 2004). In addition, C burnetii DNA was detected in two dogs and a cat kept by these patients, suggesting the pets could have been the source of the human infections. While C burnetii antibodies have been detected in serum of a small number of cats in North America (Randhawa et al 1974, Willeberg et al 1980, Higgins and Marrie 1990, Case et al 2006), to our knowledge, this is the first study to document the presence of C burnetii DNA in samples from cats in this region.
All uterine samples positive for C burnetii DNA in this study were from client-owned cats. This is in contrast to a serologic study in Asia which demonstrated that the antibody positive rate in stray cats was significantly higher than that in pet cats (Komiya et al 2003b). As two different testing methodologies and two different regions of the world were assessed in the studies, the results cannot be directly compared. In future prospective studies, concurrent assessment of C burnetii serum antibody titers and PCR assay detection of C burnetii DNA would aid in the determination of prevalence rates. The sample size in the current study was small, precluding statistical comparison between groups, and so failure to detect C burnetii DNA from shelter cats may only have been due to chance. It is unknown where the C burnetii DNA positive cats in this study acquired the infection as the housing history and source of the cats were unknown. It is possible that the cats were infected with C burnetii from contact with their queens or other cats in crowded environments like pet stores or shelters before being adopted into private homes. If the cats were allowed outdoors, C burnetii infection could have been acquired as adults from contact with infected vectors or transport hosts.
Based on the size of the uterine tissues digested and spectrophotometric assessment of the vaginal samples, we believe all samples assessed in this study contained DNA. Based on titration studies, the C burnetii PCR assay utilized here was sensitive, with a detection limit of 3 pg C burnetii DNA. However, when applied to vaginal samples and uterine tissue, it is possible that the presence of PCR inhibitors or other factors lessened the sensitivity of the assay, potentially leading to false negative results in some samples. In future prospective studies, C burnetii culture and PCR assays should be performed concurrently in an attempt to further determine the sensitivity of the assay. The failure to amplify C burnetii DNA from vaginal swabs from uterine sample positive cats may only reflect different sensitivities; a greater amount of total DNA was available from the uterine samples. However, the discordant results may also indicate site-specific preference for organism residence and replication because of differences between vaginal and uterine epithelia. It is also possible that organism numbers vary in the different genital tract tissues dependent on the state of the estrous cycle. For example, vaginal swabs in sheep were positive for C burnetii DNA in 44% and 6% of the animals tested at parturition and 5 weeks after parturition, respectively (Berri et al 2001). While C burnetii has been cultured from vaginal swabs from cats in Japan, the stage of the estrous cycle of the positive cats was unknown (Nagaoka et al 1998).
During pregnancy, C burnetii is known to colonize the placenta in large numbers (Baca and Paretsky 1983). Coxiella burnetii can be cultured from the uterus of cats for 10 weeks after parturition (Higgins and Marrie 1990) and the urine of cats for 2 months after experimental infection (Babudieri 1959). The organism is very resistant to desiccation and a single inhaled organism can cause infection (Babudieri 1959). In the study described here, urogenital abnormalities were not detected in any of the cats with C burnetii DNA in uterine tissues. However, the stage of the estrous cycle for these cats is unknown, and future prospective studies should correlate relationship of the stage in the estrous cycle with susceptibility to infection with C burnetii. Thus, our results indicate that apparently healthy cats could serve as a source of human C burnetii infection. However, as we did not amplify C burnetii DNA from the vaginal swabs of any subjects, we believe these cats are an unlikely source of human infection. This hypothesis is supported by the fact that most cases of human Q fever associated with cat contact have been those associated with parturient cats.
In conclusion, we believe that the findings of this study support the recommendations that care should be taken when attending to parturient cats and direct contact with parturient secretions and uterine tissues of cats should be avoided (Brown et al 2005). The results of this and previous studies also suggest that early ovariohysterectomy of cats may be indicated, particularly in cats to be adopted into homes with immune suppressed individuals.
References
- Arashima Y., Kato K., Komiya T., Kumasaka K., Matsukawa Y., Murakami M., Takahashi K., Ikeda T., Arakawa Y. Improvement of chronic nonspecific symptoms by long-term minocycline treatment in Japanese patients with Coxiella burnetii infection considered to have post-Q fever fatigue syndrome, Internal Medicine 43, 2004, 49–54. [DOI] [PubMed] [Google Scholar]
- Ayres J.G., Flint N., Smith E.G., Tunnicliffe W.S., Fletcher T.J., Hammond K., Ward D., Marmion B.P. Post-infection fatigue syndrome following Q fever, Quarterly Journal of Medicine 91, 1998, 105–123. [DOI] [PubMed] [Google Scholar]
- Babudieri B. Q fever: a zoonosis, Advances in Veterinary Science 5, 1959, 81. [Google Scholar]
- Baca O., Paretsky D. Q fever and Coxiella burnetii: a model for host–parasite interactions, Microbiological Reviews 47, 1983, 127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berri M., Laroucau K., Rodolakis A. The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction, Veterinary Microbiology 72, 2000, 285–293. [DOI] [PubMed] [Google Scholar]
- Berri M., Souriau A., Crosby M., Crochet D., Lechopier P., Rodolakis A. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep, Veterinary Record 148, 2001, 502–505. [DOI] [PubMed] [Google Scholar]
- Brennan R.E., Samuel J.E. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay, Journal of Clinical Microbiology 41, 2003, 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.R., Elston T.H., Evans L., Glaser C., Gulledge M.L., Jarboe L., Lappin M.R., Marcus L.C. Feline zoonoses guidelines from the American Association of Feline Practitioners, Journal of Feline Medicine and Surgery 7, 2005, 243–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J.B., Chomel B., Nicholson W., Foley J.E. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies, Journal of Feline Medicine and Surgery 8, 2006, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenollar F., Fournier P.E., Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection, Journal of Clinical Microbiology 42, 2004, 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P.E., Raoult D. Comparison of PCR and serology assays for early diagnosis of acute Q fever, Journal of Clinical Microbiology 41, 2003, 5094–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y., Ishikawa Y., Nagaoka H., Akiyama M. Prevalence and clinical characterization of Coxiella burnetii infection in patients with protracted low-grade fever, Journal of the Japanese Association for Infectious Diseases 76, 2002, 901–910. [DOI] [PubMed] [Google Scholar]
- Harris R.J., Storm P.A., Lloyd A., Arens M., Marmion B.P. Long-term persistence of Coxiella burnetii in the host after primary Q fever, Epidemiology and Infection 124, 2000, 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D., Marrie T.J. Seroepidemiology of Q fever among cats in New Brunswick and Prince Edward Island, Annals of the New York Academy of Sciences 590, 1990, 271–274. [DOI] [PubMed] [Google Scholar]
- Kato K., Arashima Y., Asai S. Detection of Coxiella burnetii specific DNA in blood samples from Japanese patients with chronic non-specific symptoms by nested polymerase chain reaction, FEMS Immunology and Medical Microbiology 21, 1998, 139–144. [DOI] [PubMed] [Google Scholar]
- Kazar J. Coxiella burnetii infection, Annals of the New York Academy of Sciences 1063, 2005, 105–114. [DOI] [PubMed] [Google Scholar]
- Komiya T., Sadamasu K., Toriniwa H., Kato K., Arashima Y., Fukushi H., Hirai K., Arakawa Y. Epidemiological survey on the route of Coxiella burnetii infection in an animal hospital, Journal of Infection and Chemotherapy 9, 2003a, 151–155. [DOI] [PubMed] [Google Scholar]
- Komiya T., Sadamasu K., Kang M., Tsuboshima S., Fukushi H., Hirai K. Seroprevalence of Coxiella burnetii infections among cats in different living environments, Journal of Veterinary Medical Science 65, 2003b, 1047–1048. [DOI] [PubMed] [Google Scholar]
- Kosatsky T. Household outbreak of Q fever pneumonia related to a parturient cat, Lancet 324, 1984, 1447–1449. [DOI] [PubMed] [Google Scholar]
- Langley J.M., Marrie T.G., Covert A., Waag D.M., Williams J.C. Poker players' pneumonia: an urban outbreak of Q fever following exposure to a parturient cat, New England Journal of Medicine 319, 1988, 354–356. [DOI] [PubMed] [Google Scholar]
- Lorenz H., Jager C., Willems H., Baljer G. PCR detection of Coxiella burnetii from different clinical specimens, especially bovine milk, on the basis or DNA preparation with a silica matrix, Applied and Environmental Microbiology 64, 1998, 4234–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmion B.P., Ormsbee R.A., Kyrkou M., Wright J., Worswick D.A., Izzo A.A., Esterman A., Feery B., Shapiro R.A. Vaccine prophylaxis of abattoir-associated Q fever: eight years experience in Australian abattoirs, Epidemiology and Infection 104, 1990, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmion B.P., Shannon M., Maddocks I., Storm P., Penttila I. Protracted debility and fatigue after acute Q fever, Lancet 347, 1996, 977–978. [DOI] [PubMed] [Google Scholar]
- Marrie T.J., Haldane E.V., Faulkner R.S., Kwan C., Grant B., Cook F. The importance of Coxiella burnetii as a cause of pneumonia in Nova Scotia, Canadian Journal of Public Health 76, 1985, 233–236. [PubMed] [Google Scholar]
- Marrie T.J., MacDonald A., Durant H., Yates L., McCormick L. An outbreak of Q fever probably due to contact with a parturient cat, Chest 93, 1988a, 98–103. [DOI] [PubMed] [Google Scholar]
- Marrie T.J., Durant H., Williams J.C., Mintz E., Waag D.M. Exposure to parturient cats: a risk factor for acquisition of Q fever in maritime, Canadian Journal of Infectious Disease 158, 1988b, 101–108. [DOI] [PubMed] [Google Scholar]
- Marrie T.J., Langille D., Papukna V., Yates L. Truckin' pneumonia – an outbreak of Q fever in a truck repair plant probably due to aerosols from clothing contaminated by contact with newborn kittens, Epidemiology and Infection 102, 1989, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T.J. Coxiella burnetii (Q fever) pneumonia, Clinical Infectious Diseases 21, 1995, S253–S264. [DOI] [PubMed] [Google Scholar]
- Maurin M., Raoult D. Q fever, Clinical Microbiology Reviews 12, 1999, 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthewman K., Kelly P., Hayter D., Downie S., Wray K., Bryson N., Rycroft A., Raoult D. Exposure of cats in southern Africa to Coxiella burnetii, the agent of Q fever, European Journal of Epidemiology 13, 1997, 477–479. [DOI] [PubMed] [Google Scholar]
- Milazzo A., Hall R., Storm P.A., Harris R.J., Winslow W., Marmion B.P. Sexually transmitted Q fever, Clinical Infectious Diseases 33, 2001, 399–402. [DOI] [PubMed] [Google Scholar]
- Nagaoka H., Sugieda M., Akiyama M., Nishina T., Akahane S., Fujiwara K. Isolation of Coxiella burnetii from the vagina of feline clients at veterinary clinics, Journal of Veterinary Medical Science 60, 1998, 251–252. [DOI] [PubMed] [Google Scholar]
- Pinsky R.L., Fishbein D.B., Greene C.R., Gensheimer K.F. An outbreak of cat-associated Q fever in the United States, Journal of Infectious Diseases 164, 1991, 202–204. [DOI] [PubMed] [Google Scholar]
- Randhawa A.S., Dieterich W.H., Jolley W.B., Hunter C. Coxiellosis in pound cats, Feline Practice 6, 1974, 37–38. [Google Scholar]
- Stein A., Raoult D. Detection of Coxiella burnetii by DNA amplification using polymerase chain reaction, Journal of Clinical Microbiology 30, 1992, 2462–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman M.J., Smith E.G., Groves J., Beattie J.M., Caul E.O., Ayres J.G. Chronic fatigue following infection by Coxiella burnetii (Q fever): 10-year follow-up of the 1989 UK outbreak cohort, Quarterly Journal of Medicine 95, 2002, 527–538. [DOI] [PubMed] [Google Scholar]
- Willeberg P., Ruppanner R., Behymer D.E., Haghighi S., Kaneko J.J., Franti C.E. Environmental exposure to Coxiella burnetii: a sero-epidemiologic survey among domestic animals, American Journal of Epidemiology 111, 1980, 437–443. [DOI] [PubMed] [Google Scholar]