Abstract
An 18-week-old male domestic long-hair kitten was presented with a history of polyuria and polydipsia for several weeks. The general condition was unremarkable, but the kitten was considerably smaller than expected for the age and showed cataracts in both eyes. Serum glucose concentrations were persistently elevated and based on clinical findings and an elevated serum fructosamine concentration, a diagnosis of diabetes mellitus was established.
Diabetes mellitus is not commonly diagnosed in young kittens, nor are cataracts recognised as a frequent feature of this disease in cats. The cataracts progressed in spite of the insulin therapy and the kitten was euthanised 10 weeks after referral. Histopathological examination of the pancreas revealed few and small islets of Langerhans compared to the examination of pancreas from a healthy kitten of the same age. Histopathological changes in the eyes included cataracts affecting both cortex and nucleus.
Diabetes mellitus is a common endocrine disease in adult, middle-aged to old cats (Panciera et al 1990, Thoresen & Grøndalen 1995). Cases of diabetes mellitus in young cats are rarely described (Woods et al 1994, Root et al 1995, Barnett & Crispin 1998). In dogs, diabetes mellitus is associated with a high incidence of bilateral cataracts that occur secondary to aberrations in carbohydrate metabolism, but this phenomenon is not a frequent feature of diabetes mellitus in cats. Feline cataract, when occurring, is almost always secondary to other eye diseases (Barnett & Crispin 1998).
In this report, a case of diabetes mellitus with concomitant bilateral cataracts in a kitten is presented and the quite uncommon features of the disease in this case are discussed.
Case history
An 18-week-old male domestic long-hair kitten, weighing 1.8 kg, was referred to The Norwegian School of Veterinary Science with a history of polyuria and polydipsia of 3 to 4 weeks duration. The kitten was small compared to the littermates, although with normal body proportions (Fig 1). Its appetite had all along been normal. Vaccination status and worming protocol were fulfilled satisfactorily.
Fig 1.
Domestic long-hair kitten, 18-week-old. The kitten is smaller than expected but shows normal body proportions.
On clinical examination the kitten was alert and active. Body temperature was normal and pulse and respiration frequencies were within normal limits. The peripheral lymph nodes were palpably normal. Mucous membrane colour and capillary refill times were normal. No other abnormal clinical findings were found apart from the ocular changes.
Eye examination
The eyes were of normal size with no signs of intraocular inflammation. Direct and indirect pupillary light reflexes were normal in both eyes. Menace responses were inconsistent and difficult to evaluate. Intraocular pressures were 12 and 16 mmHg in the left and right eye, respectively. The cataracts in both eyes were immature and affected both nucleus and cortex (Fig 2a Fig 2b), but permitted examination of a normal fundus. During the following 2 months the cataracts progressed further, with the right eye most severely affected.
Fig 2a, b.
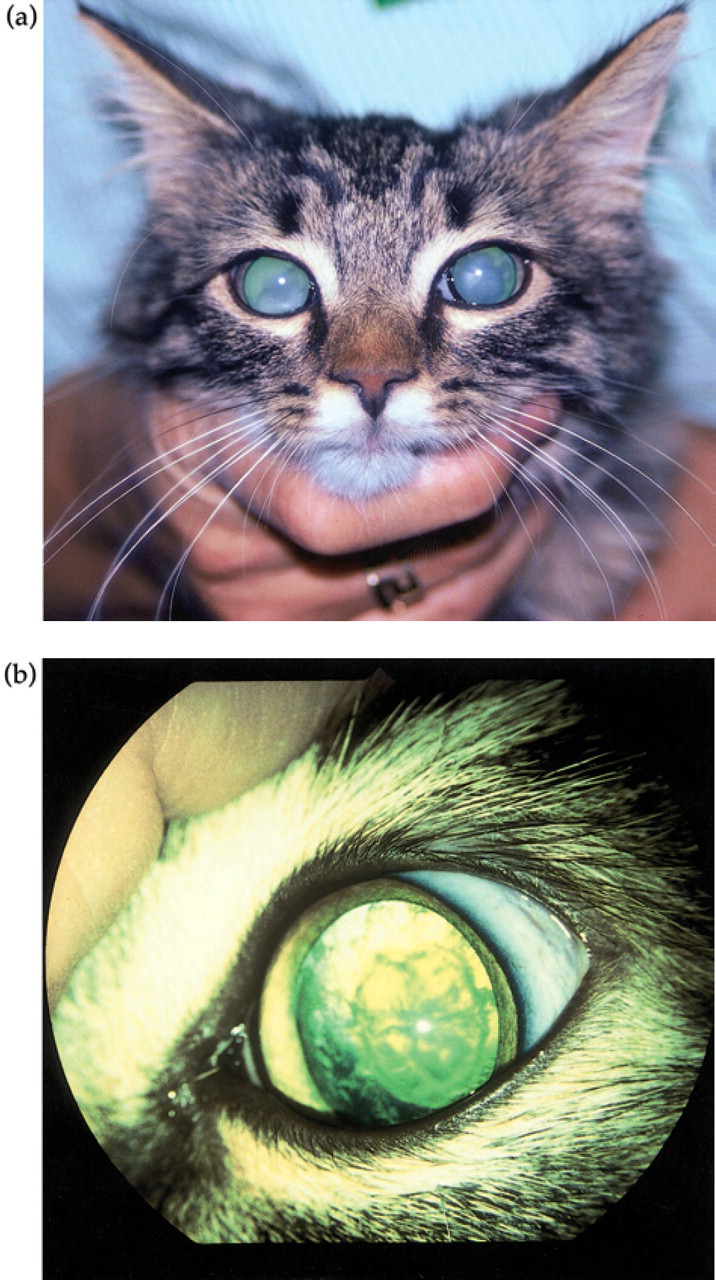
Bilateral immature cataract in the 18-week-old kitten.
Clinical pathology
A blood sample was drawn from the jugular vein and a urine sample was collected by cystocentesis. Abnormal findings of clinical significance included glucosuria, hypocalaemia and increased serum concentrations of alkaline phosphate, inorganic phosphate, glucose, and fructosamine.
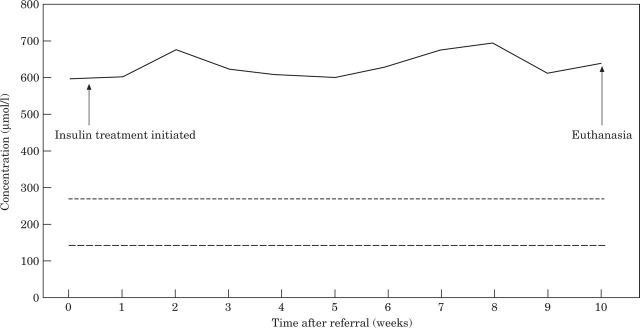
The results of the haemogram, biochemical profile and urinalysis at the time of referral are listed in Tables 1, 2, and 3. The results of weekly serum fructosamine measurements during the 10 weeks treatment period are given in Fig 3.
Table 1.
Haemogram
| Parameter | Result | Reference range | Unit |
|---|---|---|---|
| RBC | 6.6 | (5.0–10.0) | T/L |
| HGB | 99 | (80–150) | g/L |
| HCT | 0.32 | (0.24–0.45) | L/L |
| MCV | 48.1 | (39–50) | fL |
| MCHC | 311 | (320–360) | g/L |
| RDW | 13.9 | (13.0–17.0) | % |
| HDW | 16.8 | (13.0–28.0) | g/L |
| PLT | Adequate | (190–400) | G/L |
| MPV | 6.8 | (5.5–8.5) | fL |
| WBC | 7.3 | (5.5–15.4) | G/L |
| NEUT | 3.8 | (2.5–12.5) | G/L |
| LYMP | 3.1 | (1.5–7.0) | G/L |
| MONO | 0.1 | (0–0.9) | G/L |
| EOS | 0.3 | (0–0.5) | G/L |
| BAS | 0.0 | (0–0.1) | G/L |
| LUC | 0.0 | na § | G/L |
Not applicable.
Table 3.
Urinalysis (sample collected by cystocentesis)
| Parameter | Result | Reference range |
|---|---|---|
| Specific gravity | 1.028 | (1.035–1.060) |
| Color | Pale | na § |
| Turbidity | Clear | na § |
| pH | 6.5 | (5.5–7.0) |
| Glucose | Positive | (Negative) |
| Ketones | Negative | (Negative) |
| Bilirubin | Negative | (Negative) |
| Protein | Negative | (Negative) |
| Occult blood | Negative | (Negative) |
| WBC/hpf * | 2 | (0–3) |
| RBC/hpf * | 3 | (0–3) |
| Casts | Negative | (Negative) |
| Crystals | Negative | (Negative) |
| Bacteria | Negative | (Negative) |
| Culture | Negative | (Negative) |
High power field.
Not applicable.
Fig 3.
Serum fructosamine concentrations (—–) measured weekly during the 10-week treatment period. Upper reference limit,—-; lower reference limit,—.
Radiology
Left and right lateral and dorsoventral abdominal radiographs demonstrated no radiological abnormal findings.
Post-mortem examination
The kitten was euthanised 10 weeks after referral due to failure to respond to insulin treatment (Insulatard® NovoLet®, 0.8 IU/kg bw twice daily) and the progression of the bilateral cataracts. An autopsy was performed and the eyes were immediately removed and placed in 10% unbuffered formalin. The liver was enlarged and had a light brown colour with multiple, small red areas. Pancreas was of normal size and the middle portion had focal white spots with diameter up to 1 mm.
Tissue specimens were collected from various organs, including liver, kidneys and several sections of pancreas. The excised samples were fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin. Sections werecut 3 to 5 μm thick and stained with haematoxylin and eosin (HE). Sections of pancreas were also stained with Congo red. Immunohistochemistry for insulin was performed on sections of pancreatic tissue. A guinea-pig polyclonal antibody against porcine insulin (DAKO A/S, Glostrup, Denmark) was used in an avidin-biotin complex (ABC) staining technique (Hsu et al 1981). Pancreas from a healthy 6-month-old cat was used as a positive control.
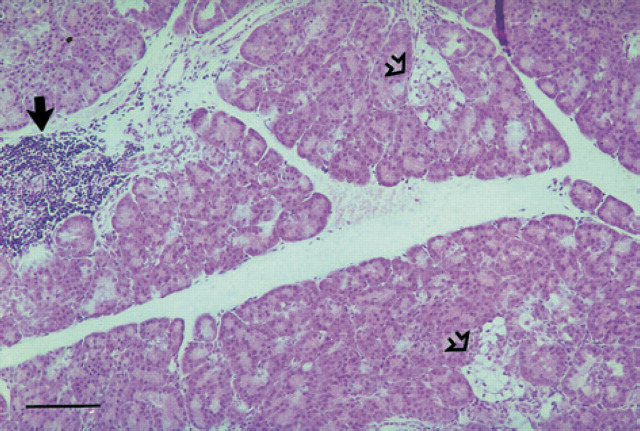
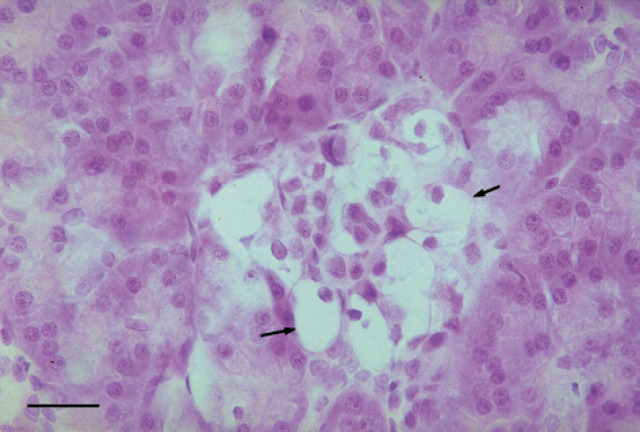
Few pancreatic islets were recognised in HE stained sections of the diabetic kitten (Fig 4) when compared with the control cat. The islet cells in the diabetic kitten showed degeneration with swollen, pale and vacuolated cytoplasm and peripherally located small nuclei (Fig 5).
Fig 4.
Pancreas, 18-week-old kitten with diabetes mellitus. Pancreatic islets contain several vacuolated cells (open arrows). Focal interstitial aggregates of leucocytes (black arrow) are present. Haematoxylin and eosin. Bar=100 μm.
Fig 5.
Pancreas, 18-week-old kitten with diabetes mellitus. Higher magnification of a pancreatic islet with degeneration. Some islet cells have pale, vacuolated cytoplasm and peripherally located, small nuclei (arrows). Haematoxylin and eosin. Bar=25 μm.
Table 2.
Biochemistry profile
| Parameter | Result | Reference range | Unit |
|---|---|---|---|
| AST | 38 | (0–60) | U/L |
| ALT | 87 | (0–75) | U/L |
| ALP | 302 | (0–90) | U/L |
| CK | 505 | (0–580) | U/L |
| Amylase | 263 | (0–800) | U/L |
| Protein (total) | 71 | (60–82) | g/L |
| Albumin | 31 | (25–39) | g/L |
| Globulins | 40 | (26–50) | g/L |
| Urea | 8.3 | (5.0–10.0) | mmol/L |
| Creatinine | 75 | (75–180) | μmol/L |
| Bile acids | 10 | (0–5) | μmol/L |
| Bilirubin (total) | 1 | (0–4) | μmol/L |
| Cholesterol | 8.4 | (1.5–6.0) | mmol/L |
| Glucose | 23.5 | (3.5–9.0) | mmol/L |
| Fructosamine | 600 | (146–271) | μmol/L |
| Inorganic phosphate | 3.6 | (1.0–2.8) | mmol/L |
| Calcium | 2.9 | (2.2–2.9) | mmol/L |
| Sodium | 153 | (150–165) | mmol/L |
| Potassium | 3.4 | (3.7–5.8) | mmol/L |
| Chloride | 113 | (112–129) | mmol/L |
Amyloid was not present in the islets. The interstitial tissue of pancreas had multifocal infiltration with lymphocytes, neutrophils and histiocytes and moderate proliferation of fibrous tissue (Fig 4). The changes were most prominent close to the pancreatic ducts. Hyperplasia of duct epithelium was present in some areas.
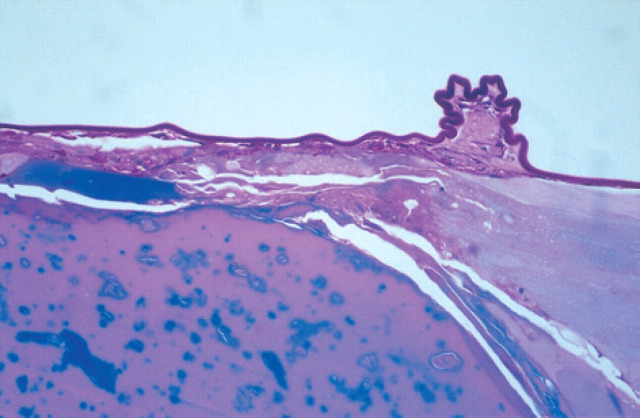
Sections stained for insulin with the ABC procedure in the diabetic kitten (Fig 6a) showed positive reactivity in a few islet cells, compared with the control cat where more cells stained positive for insulin (Fig 6b). Microscopic examination of the liver and kidneys showed fatty degeneration of centrolobular hepatocytes and renal epithelial cells of the Henle's loop. Histopathological changes in the eyes were limited to the lens. There was evidence of migration of lens epithelial cells beneath the surface of the posterior capsule, and in an area of capsular wrinkling fibrous metaplasia was present (Fig 7). The cortical changes were dramatic and include membranolysis of lens fibre cells and liquefaction of lens proteins. The anterior capsule and subcapsular cortex showed equatorial vacuolisation and degeneration of the lens epithelium, with associated subcapsular cortical membranolysis and liquefaction (Fig 8).
Fig 6.
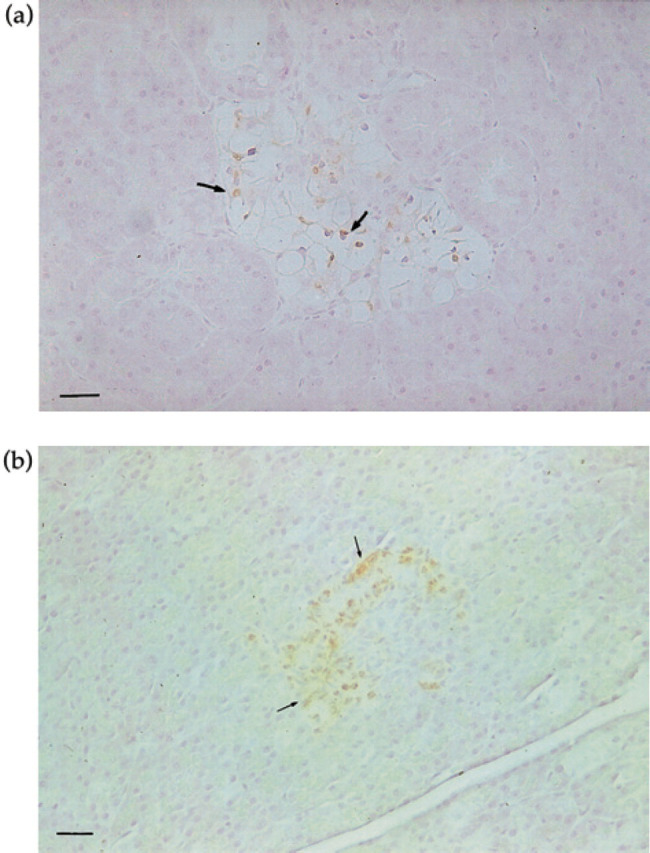
(a). Pancreas, of 18-week-old kitten with diabetes mellitus, stained for insulin. A few islet cells are positive for insulin (arrows). ABC staining technique, haematoxylin counterstain. Bar=25 uμm. (b) Pancreas of a 6-month-old control kitten, stained for insulin. Several islet cells show reactivity for insulin (arrows). ABC staining technique, haematoxylin counterstain. Bar= 25 μm.
Fig 7.
Posterior equatorial lens capsule showing capsular wrinkling, indicative of liquefaction and leakage of cataract proteins. Periodic acid-Schiff stain x200.
Fig 8.
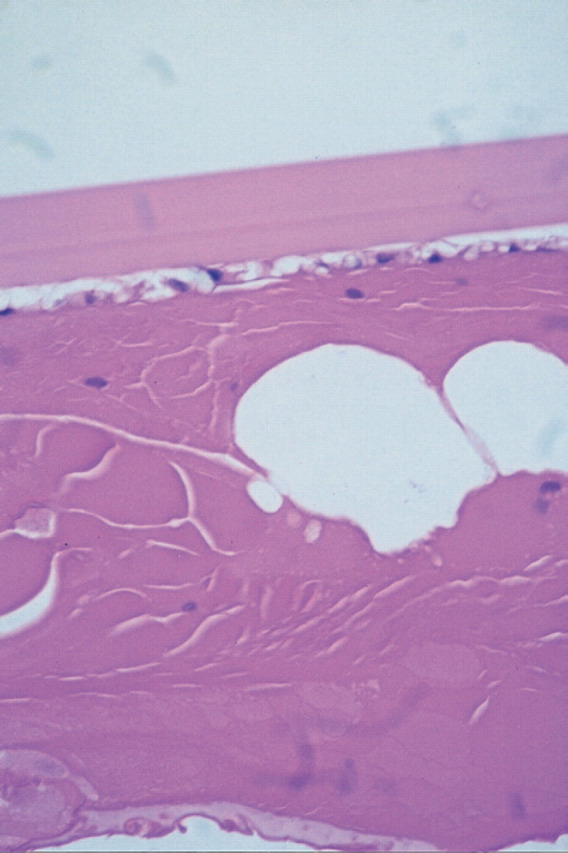
The anterior lens capsule and subcapsular cortex. Haematoxylin and eosin x400.
Discussion
The problems identified during the initial database collection in this case includes polyuria and polydipsia, glucosuria, small but proportional body size, bilateral cataracts, hypocalaemia and significantly increased levels of serum alkaline phosphate, inorganic phosphate, glucose, and fructosamine.
In this case, the presented problem list justifies the suspicion of diabetes mellitus. The single diagnostic criterion for diabetes mellitus is persistent hyperglycaemia. However, one single demonstration of hyperglycaemia might be due to several other causes than diabetes mellitus. Especially in cats, stress and drug induced short-term hyperglycaemia is a well-known phenomenon (Feldberg & Symonds 1980, Hsu & Hemborough 1982, Weiland et al 1984, Opitz 1990, Crenshaw et al 1996).
Measurements of serum fructosamine (glycated serum proteins) and glycated haemoglobin are increasingly used to replace plasma glucose concentration to diagnose diabetes mellitus and to monitor the response to insulin treatment in several species (Baker et al 1983, Salemans et al 1987, Akol et al 1992, Reusch et al 1993, Thoresen & Bredal 1996). These measurements are not affected by transient changes in glucose concentration. An initially, pre-treatment elevated serum fructosamine concentration usually verifies a persistent hyperglycaemia. Another major factor that may affect the serum fructosamine concentration, besides the average serum glucose concentration is the serum albumin concentration (Dolhofer & Wieland 1980, Iberg & Flückiger 1986). In this kitten protein status was normal, implying that the elevated serum fructosamine concentration was caused by a persistent alteration in plasma glucose concentration only.
Chronically elevated serum fructosamine concentrations in this kitten for the 10 weeks treatment period reflect a continuing persistent hyperglycaemia in spite of the insulin administration. Verifying that the kitten received the insulin in a proper manner, and that the insulin was stored as recommended, the results indicate insulin resistance. This may result from problems occurring before the interaction of insulin with its receptor, at the receptor, or at steps distal to the interaction of insulin and its receptor (Ihle & Nelson 1991). Pre-receptor problems reduce free metabolically active insulin concentration and include increased insulin degradation and insulin-binding antibodies. Circulating insulin-binding antibodies were not measured in the present case. In dogs and cats, receptor and post-receptor abnormalities are usually caused by obesity or a disorder causing excessive secretion of a diabetogenic hormone, ie, acromegaly and hyperadrenocorticism. Obesity was certainly not the case, and no other disease involving diabetogenic hormones was tested for. In the cat, acromegaly is caused by a pituitary tumour that secretes excess growth hormone. In the present case, no clinical signs indicative of acromegaly were present and acromegaly is less likely to be suspected in a cat that lacks typical conformational alterations. In cases suspected to suffer from acromegaly, a definite diagnosis requires documentation of increased baseline serum growth hormone or IGF-1 concentrations. However, due to lack of consistent clinical signs and that such measurements are not readily available, they were not performed.
Hyperadrenocorticism is rare in the cat (Watson & Herrtage 1998). Without any clinical signs suggestive of the disease, further testing was not done.
Cataracts were present in both eyes. Signs of other eye diseases were not observed. Primary cataract is an infrequent entity in the cat. Cataract associated with multiple ocular defects, including microphthalmia, occasionally occurs in cats (Peiffer & Gelatt 1974), but in the present case the eyes were considered to be of normal size with no concomitant ocular defects. Primary congenital cataracts and suspected hereditary cataracts have been reported (Peiffer & Gelatt 1975, Schwink 1986, Rubin 1986), but are considered uncommon in domestic cats. Diabetic cataract has been described as rare in the cat and is certainly much less common than in the dog, and of slower onset. Reports on diabetic cataract in very young cats are few. Barnett & Crispin (1998) reported diabetic cataract in a 22-week-old Chinchilla cross Persian male kitten, and James (1960) reported cataract as one of the findings in a 7-year-old domestic short-hair cat. Initial lens changes in diabetic cataract in cats, when occurring, are described as equatorial lens vacuoles (Glaze & Gelatt 1999). This is in accordance with the findings in the present case report. In a study by Taylor et al (1997) diabetic canine cataractous lenses were completely opaque in both the cortex and nucleus. It has been reported that formation of diabetic cataract is mainly due to osmotic swelling and the rupture of lens fibre cells caused by reduction of the excess glucose via the hexose monophosphate shunt resulting in intracellular accumulation of sorbitol (Kinoshita 1965, Peiffer et al 1977). The question why diabetic cataracts in cats are so infrequent has not yet been answered. A possible explanation for the species difference may be related to differences in aldose reductase activity. Aldose reductase catalyses the reduction of glucose to sorbitol. Thus, diabetic cataractogenesis may be dependent not only on the amount of glucose available but the level of lens aldose reductase activity as well. Studies have shown that there is a species difference in lens aldose reductase levels corresponding with the susceptibility to form cataracts (Datiles & Kinoshita 2000). In addition to the osmotic effect of sorbitol, there is evidence that sorbitol also directly interacts with lens proteins (Basher & Roberts 1995), causing amino acid modification and lens protein insolubility with ensuing cataract formation. Glycation may bring about a conformational change in the proteins such that the buried sulphydryl groups become exposed (Whikehart 1994). One of the susceptible amino acids is lysine that can react with a carbonyl group to form what is known as a Schiff base. The most important sources of free carbonyl groups in cells are sugars; hence, the process is referred to as glycation. Should the Schiff base progress to form a more stable Amadori product, the end result will also be unfolding of proteins and cross-linking into aggregates. Aggregates arising from sugar-protein adduct formation have been called advanced glycation end products (AGEs) (Brownlee et al 1988). It has also been reported that the glycation makes the proteins more sensitive to oxidative stress caused by lowered pH and visible light (Kamei 1991). The fact that the insolubility of the lens proteins is accelerated by glucose in the presence of visible light suggests that light may enhance the effects of glucose on the lens protein aggregation and insolublisation.
Insolublisation of the lens proteins by glucose is also increased under acidic conditions. The pH level in blood is decreased in diabetic acidosis and glucose may therefore exhibit a more effective action to insolublise the lens proteins in diabetic patients. Based on the case history and the clinical signs the cataracts were considered most likely to be secondary to diabetes mellitus.
Microscopic examination of pancreas showed few and atrophic islets, indicating that a hypoplasia may be present in addition to degeneration. Normally, the distribution and number of islets vary in different parts of pancreas and also with age (Bencosme & Liepa 1955, Jubb 1993, Xu et al 1999). However, sections from 10 different areas of pancreas were examined and only a few islets were identified, supporting the diagnosis of hypoplasia of pancreatic islets. In contrast, islets were more numerous in various parts of pancreas of the age-matched control.
The vacuolar degeneration, which was present in islet cells of this kitten, is in accordance with findings reported in diabetes in dogs and cats (Atkins et al 1988, Jubb 1993). Amyloid deposits in the islets were not observed in the present case, although amyloidosis of the islets is reported as a common feature of feline diabetes (O'Brien et al 1986). Findings consistent with a moderate interstitial pancreatitis were observed in the diabetic kitten, but the acinar, exocrine part of pancreas was apparently normal. Therefore, it seems unlikely that an interstitial inflammatory reaction has contributed to the lesions of the islets. Diabetes mellitus in a kitten showing bilateral cataracts is uncommon. The pathological findings showed that this case of diabetes mellitus was apparently due to pancreatic islet hypoplasia. The development of bilateral cataracts is considered most likely secondary to diabetes mellitus.
References
- Akol KG, Waddle JR, Wilding P. (1992) Glycated hemoglobin and fructosamine in diabetic and nondiabetic cats. Journal of the American Animal Hospital Association 28, 227–231. [Google Scholar]
- Atkins CE, LeCompte PM, Chin HP, Hill JR, Ownby CL, Brownfield MS. (1988) Morphologic and immunocytochemical study of young dogs with diabetes mellitus associated with pancreatic islet hypoplasia. American Journal of Veterinary Research 49, 1577–1581. [PubMed] [Google Scholar]
- Baker J, O'Connor JP, Metcalf P, Lawson MR, Johnson R. (1983) Clinical usefulness of estimation of serum fructosamine concentration as a screening test for diabetes mellitus. British Medical Journal 287, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett KC, Crispin SM. (1998) Feline Ophthalmology. An Atlas Text. Philadelphia: WB Saunders, pp 114–116. [Google Scholar]
- Basher AW, Roberts SM. (1995) Ocular manifestations of diabetes mellitus: Diabetic cataracts in dogs. Veterinary Clinics of North America: Small Animal Practice 25, 661–676. [DOI] [PubMed] [Google Scholar]
- Bencosme SA, Liepa E. (1955) Regional differences of the pancreatic islet. Endocrinology 57, 588–593. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Cerami A, Vlassara H. (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. New England Journal of Medicine 318, 1315–1321. [DOI] [PubMed] [Google Scholar]
- Crenshaw KL, Peterson ME, Heeb LA, Moroff SD, Nichols R. (1996) Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. Journal of Veterinary Internal Medicine 10, 360–364. [DOI] [PubMed] [Google Scholar]
- Datiles MB, Kinoshita JH. (2000) Pathogenesis of Cataracts. In: Duane's Clinical Ophthalmology on CD-ROM, Vol 1, Chapter 72B. Tasman W, Jaeger EA. (eds) Hagerstown: Lippincott, Williams & Wilkins. [Google Scholar]
- Dolhofer R, Wieland OH. (1980) Increased glycosylation of serum albumin in diabetes mellitus. Diabetes 29, 417–422. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Symonds HW. (1980) Hyperglycaemic effect of xylazine. Journal of Veterinary Pharmacology and Therapeutics 3, 197–202. [Google Scholar]
- Glaze MB, Gelatt KN. (1999) Feline ophthalmology. In: Veterinary Ophthalmology. Gelatt KN. (ed.) Philadelphia: Lippincott, Williams & Wilkins, pp. 997–1052. [Google Scholar]
- Hsu SM, Raine L, Fanger H. (1981) Use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry 29, 577–580. [DOI] [PubMed] [Google Scholar]
- Hsu WH, Hemborough FB. (1982) Intravenous glucose tolerance test in cats: Influenced by acetylpromazine ketamine morphine thiopental and xylazine. American Journal of Veterinary Research 43, 2060–2061. [PubMed] [Google Scholar]
- Iberg N, Flückiger R. (1986) Nonenzymatic glycosylation of albumin in vivo. The Journal of Biological Chemistry 261, 13542–13545. [PubMed] [Google Scholar]
- Ihle SL, Nelson RW. (1991) Insulin resistance and diabetes mellitus. Compendium on Continuing Education for the Practising Veterinarian 13, 197–205. [Google Scholar]
- James OD. (1960) Spontaneous diabetes mellitus in the cat (Letter). Veterinary Record 72, 630. [Google Scholar]
- Jubb KVF. (1993) The pancreas. In: Pathology of Domestic Animals. Volume 2. Jubb KVF, Kennedy PC, Palmer N. (eds) San Diego: Academic Press Inc, pp. 407–424. [Google Scholar]
- Kamei A. (1991) Possible process of insolubilization of lens proteins—direct effect of glucose. Experimental Eye Research 52, 369–374. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH. (1965) Cataracts in galactosemia. Investigative Ophthalmology 4, 768–99. [PubMed] [Google Scholar]
- O'Brien TD, Hayden DW, Johnson KH, Fletcher TF. (1986) Immunohistochemical morphometry of pancreatic endocrine cells in diabetic normoglycaemic glucose-intolerant and normal cats. Journal of Comparative Pathology 96, 357–369. [DOI] [PubMed] [Google Scholar]
- Opitz M. (1990) Stress hyperglycaemia in cats. Berliner und Munchener Tierarztliche Wochenschrift 103, 151–158. [PubMed] [Google Scholar]
- Panciera DL, Thomas CB, Eicker SW, Atkins CE. (1990) Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). Journal of American Veterinary Medical Association 197, 1504–1508. [PubMed] [Google Scholar]
- Peiffer RL, Gelatt KN. (1974) Cataracts in the cat. Feline Practice 4, 34–38. [Google Scholar]
- Peiffer RL, Gelatt KN. (1975) Congenital cataracts in a Persian kitten. Veterinary Medicine Small Animal Clinician 70, 1334–1335. [PubMed] [Google Scholar]
- Peiffer RL, Gelatt KN, Gwin RM. (1977) Diabetic cataracts in the dog. Canine Practice 2, 18–22. [Google Scholar]
- Reusch CE, Liehs MR, Hoyer M, Vochezer R. (1993) Fructosamine. A new parameter for diagnosis and metabolic control in diabetic dogs and cats. Journal of Veterinary Internal Medicine 7, 177–182. [DOI] [PubMed] [Google Scholar]
- Root MV, Johnson KH, Allen TA, Johnston SD. (1995) Diabetes mellitus associated with pancreatic endocrine insufficiency in a kitten. Journal of Small Animal Practice 36, 416–420. [DOI] [PubMed] [Google Scholar]
- Rubin LF. (1986) Hereditary cataracts in Himalayan cats. Feline Practice 16, 14–15. [Google Scholar]
- Salemans THB, Van Dieijen-Visser MP, Brombacher PJ. (1987) The value of HbA1 and fructosamine in predicting impaired glucose tolerance—an alternative to OGTT to detect diabetes mellitus or gestational diabetes. Annals of Clinical Biochemistry Liverpool 24, 447–452. [DOI] [PubMed] [Google Scholar]
- Schwink K. (1986) Posterior nuclear cataracts in two Birman kittens. Feline Practice 16, 31–34. [Google Scholar]
- Taylor VL, Peiffer RL, Costello MJ. (1997) Ultrastructural analysis of normal and diabetic cataractous canine lenses. Veterinary & Comparative Ophthalmology 7, 117–125. [Google Scholar]
- Thoresen SI, Bredal WP. (1996) Serum fructosamine: Clinical usefulness in feline diabetes mellitus. Journal of Small Animal Practice 37, 64–68. [DOI] [PubMed] [Google Scholar]
- Thoresen SI, Grøndalen J. (1995) Diabetes mellitus in dogs and cats—a review (Diabetes mellitus hos hund og katt—en oversikt med vekt på diagnostikk og behandling). Norsk Veterinaertidsskrift 2, 101–111. [Google Scholar]
- Watson PJ, Herrtage ME. (1998) Hyperadrenocorticism in six cats. Journal of Small Animal Practice 39, 175–184. [DOI] [PubMed] [Google Scholar]
- Weiland A, Molbak I, Madsen J. (1984) Xylazine (Rompun). A sedative with a hyperglycaemic effect in the dog and cat. Dansk Veterinaertidsskrift 67, 221–223. [Google Scholar]
- Whikehart DR. (1994) Carbohydrates. In: Biochemistry of the eye. Whikehart DR. (ed.) Boston: Butterworth-Heinemann, pp 53–86. [Google Scholar]
- Woods JP, Panciera DL, Snyder PS, Jackson MW, Smedes SL. (1994) Diabetes mellitus in a kitten. Journal of the American Animal Hospital Association 30, 177–180. [Google Scholar]
- Xu RJ, Wang T, Zhang SH. (1999) Functional structure and growth of the pancreas in postnatal growing animals. In: Biology of the Pancreas in Growing Animals. Developments in Animal and Veterinary Sciences 28. Pierzynowski SG, Zabielski R. (eds). Amsterdam: Elsevier, pp 15–26. [Google Scholar]