Abstract
Prenatal feline fetal growth and utero-placental development were ultrasonographically evaluated using an ultrasound scanner with a 10 MHz sector probe. Uterus, placenta, embryo, fetus and fetal membranes in 16 pregnant cats were monitored during the course of pregnancy; 13 subjects underwent an ovariectomy on specific days while three subjects went to term. Various anatomic structures, fixed in Carson-buffered formalin, were sectioned and then compared to ultrasound images.
By ultrasound examination it is possible to evaluate every stage of the fetal development; the gestational chamber can be seen on the 10th and the embryo inside the chamber on the 14th day. By the 20th day it is possible to evaluate all the fetal membranes, and later it is possible to appreciate organs and structures such as the stomach, intestine, eyes (crystalline lens), kidneys and the cerebral choroid plexi, on the 30th, 40th, 50th, 39th and 40th day respectively.
Based on our observations, it will be simpler to locate anomalies of development or pathologies during ultrasound examination of pregnant queens.
Cat owners are becoming increasingly demanding whenever veterinary care is necessary for their pets. The excellent quality of modern ultrasound equipment permits sophisticated diagnostic examinations to be performed on pet animals. However, interpretation of ultrasound images may sometimes be hampered by a lack of knowledge of normal ultrasonographic anatomy. While the different stages of embryonal and fetal growth have been widely observed in different species of domestic and laboratory animals, the literature on the cat is scarce and incomplete. The studies of Davidson et al (1986) and Grof et al (1993) do not provide sufficient data in order to correctly follow the entire pregnancy from the ultrasonographic point of view.
Knowledge of ultrasonographic anatomy of feline fetal growth would allow identification of developmental defects as well as a more accurate clinical monitoring of the normal course of pregnancy. The objective of this study was to perform sequential ultrasound examinations in queens at different stages of pregnancy in order to collect anatomic data on prenatal development in cats.
Materials and methods
Sixteen privately owned, 2–5–year-old, pregnant crossbred cats weighing between 3.0–3.5 kg were used for this study. The date of mating was noted for each queen. Three of the 16 queens were hospitalised for the entire pregnancy and fed a commercial diet. In these three animals, daily abdominal ultrasound examinations were performed from day 4 until parturition using an ESAOTE BIOMEDICA AU530 unit equipped with a 10 MHz sector probe.
The remaining 13 cats were presented for elective ovariohysterectomy at various stages of pregnancy to the Small Animal Hospital of the Veterinary School of the University of Bologna (Italy). These subjects underwent ultrasound examination and were then ovariohysterectomised on the 10th, 14th, 16th, 17th, 18th, 20th, 22nd, 25th, 30th, 35th, 45th, 50th and 60th day of pregnancy, respectively.
Queens were anesthetised with 20 mg/kg ketamine HCl (Ketavet 50, Farmaceutici Gellini) and 0.5 mg acetylpromazine (Prequillan, Fatro Spa) and ovariohysterectomy was performed routinely. Once the pregnant uterus was removed from the abdominal cavity, it was fixed in formalin buffered Carson, sectioned and then used for an anatomic study.
The ultrasound evaluation was carried out on the following structures: uterus, placenta, embryo, fetus and fetal membranes. For each one of these structures, the following ultrasonographic data were recorded: echographic appearance, time of the ultrasound appearance (expressed with the modal) and development during the course of the entire pregnancy (considering the day of mating as day zero) for the 13 ovariohysterectomised queens.
Results
In Table 1, results are reported for the number of pregnant queens undergoing ultrasonographic examination on each day of pregnancy and the number of gestational chambers, embryos and fetuses studied.
Table 1.
The number of pregnant queens undergoing ultrasonographic examination on each day of pregnancy, and the number of gestational chambers, embryos and fetuses studied
| Days of pregnancy | Number of pregnant queens undergoing ultrasonographic exam on each day of pregnancy* | Number of uteri collected on elective ovariohysterectomy | Number of gestational chambers/embryos/foetuses studied * |
|---|---|---|---|
| 4th to 9th | 3 | 12 | |
| 10th | 3 + 1 | 1 | 12+3 |
| 11th to 13th | 3 | 12 | |
| 14th | 3+1 | 1 | 12+2 |
| 15th | 3 | 12 | |
| 16th | 3+1 | 1 | 12+5 |
| 17th | 3+1 | 1 | 12+2 |
| 18th | 3+1 | 1 | 12+4 |
| 19th | 3 | 12 | |
| 20th | 3+1 | 1 | 12+3 |
| 21th | 3 | 12 | |
| 22th | 3+1 | 1 | 12+3 |
| 23th to 24th | 3 | 12 | |
| 25th | 3+1 | 1 | 12+4 |
| 26th to 29th | 3 | 12 | |
| 30th | 3+1 | 1 | 12+3 |
| 31th to 34th | 3 | 12 | |
| 35th | 3+1 | 1 | 12+4 |
| 36th to 44th | 3 | 12 | |
| 45th | 3+1 | 1 | 12+3 |
| 46th to 49th | 3 | 12 | |
| 50th | 3+1 | 1 | 12+3 |
| 51th to 59th | 3 | 12 | |
| 60th | 3+1 | 1 | 12+4 |
Where two figures are given, the first refers to the three queens monitored through to parturition and the second to queens that underwent elective ovariohysterectomy.
Utero-gestational chamber-placenta
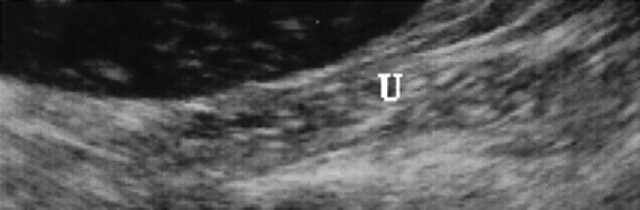
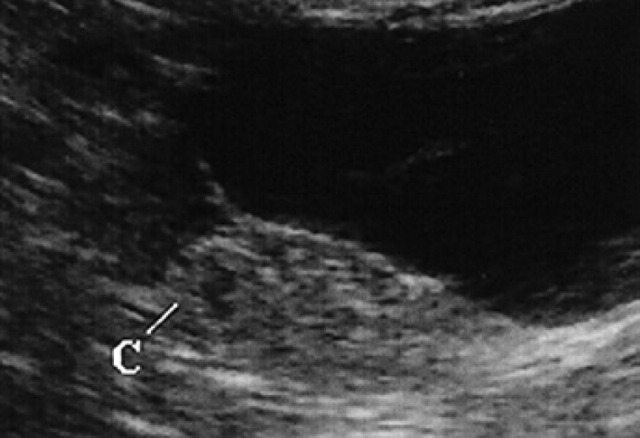
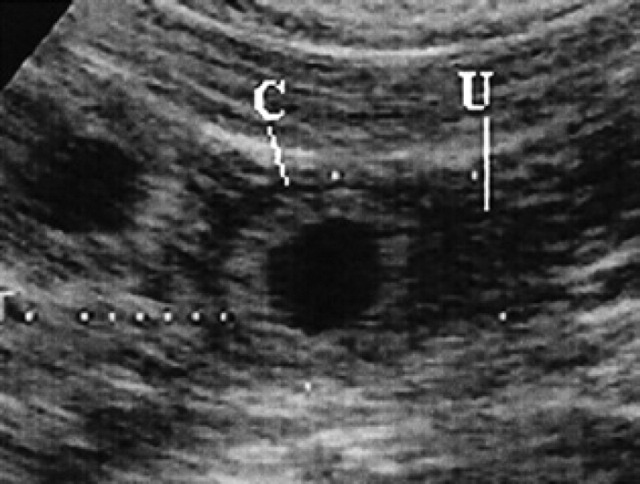
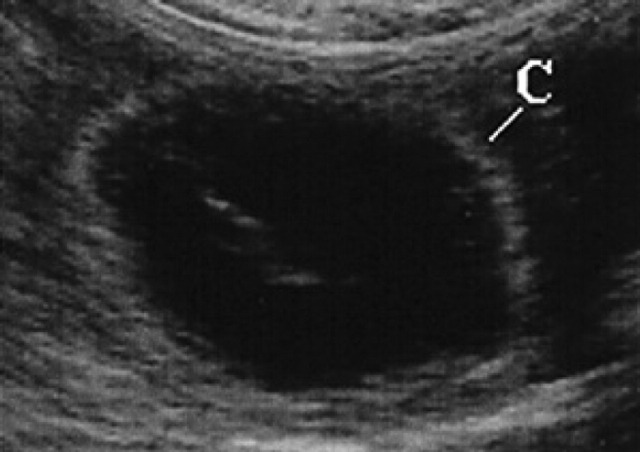
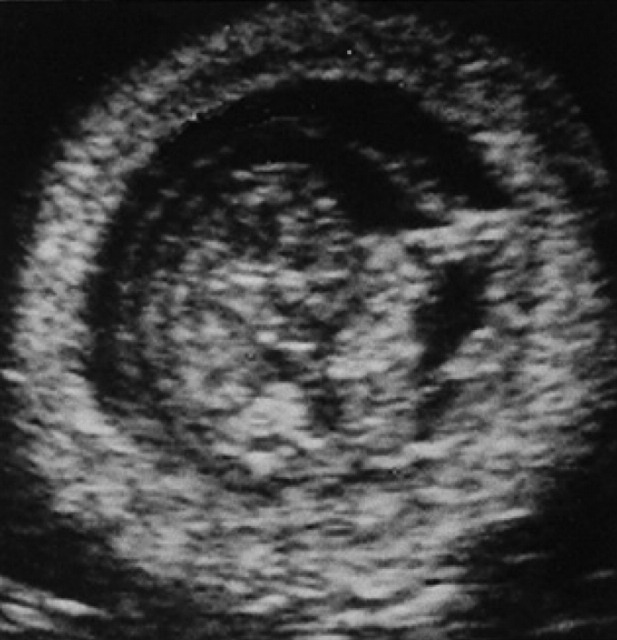
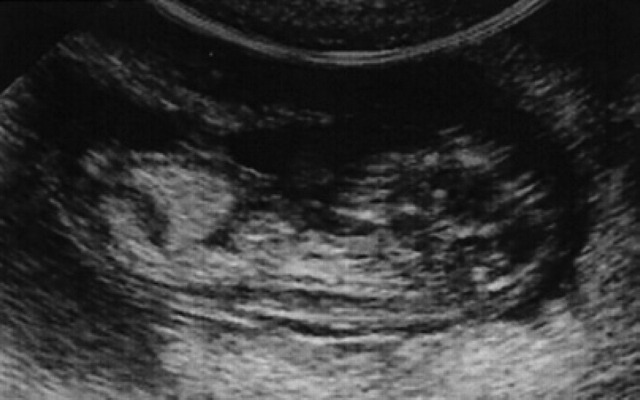
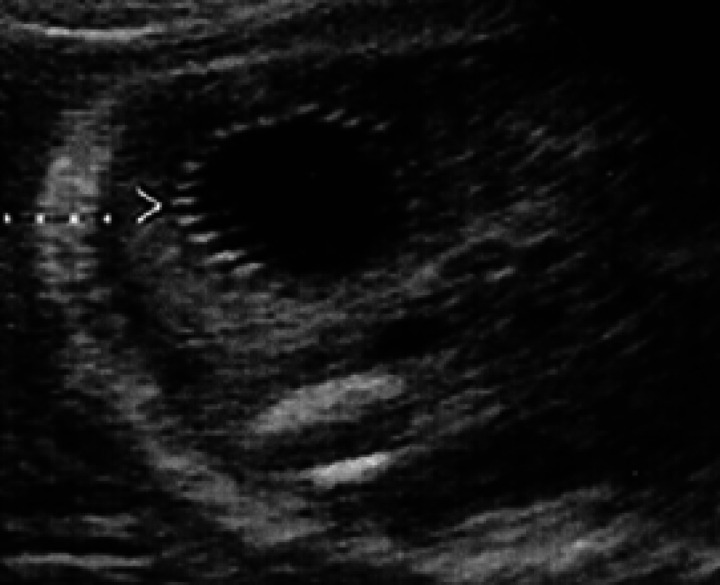
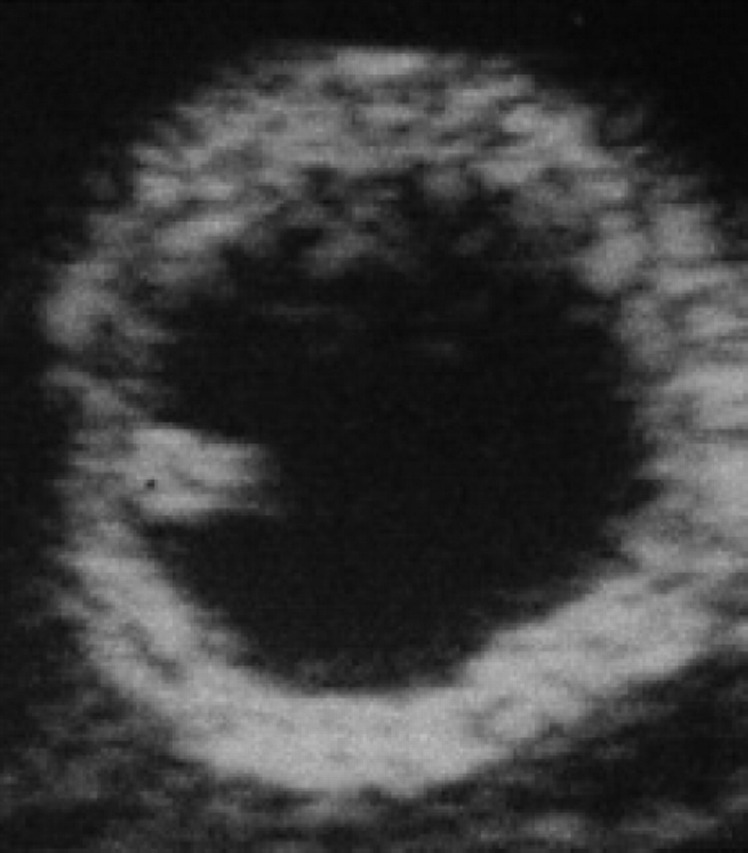
During diestrus and early pregnancy (day 4) (Fig 1), uterine horns show a hypoechoic endometrium with an increase in volume and edema, presumably indicating a physiological hyperplasia (Fig 2). On the 10th day of pregnancy it was possible to identify the gestational chambers, which appear as circular anechoic structures. At this stage, placentas and embryos are still not visible and the gestational chamber is occupied exclusively by the yolk sac (Fig 3). On the 14th day of pregnancy, the gestational chamber was still circular with a hyperechoic wall with respect to the uterine wall (Fig 4). On the 16th day of pregnancy, the developing placenta shows, at the level of the gestational chamber, a layered structure which is represented by two hyperechoic lines separated by one hypoechoic line (Fig 5).
Fig 1.
7th day of pregnancy: ultrasound longitudinal section of the uterus. The uterine horn (U), dorsal with respect to the bladder, shows a hypoechoic endometrium with increased volume.
Fig 2.
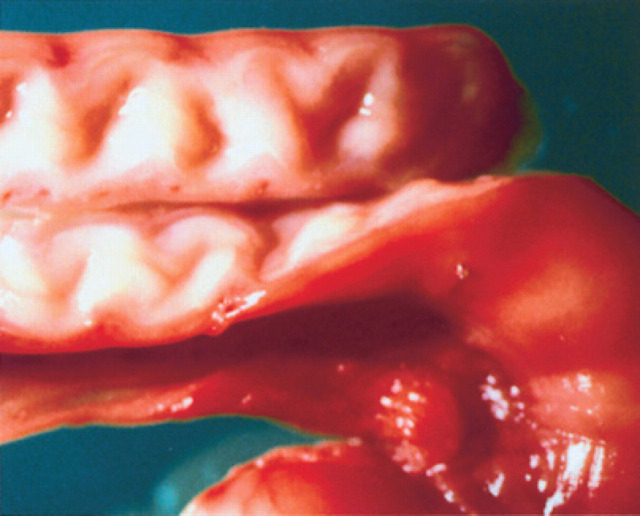
Early diestrus/pregnancy: note the characteristic spiraled aspect of the endometrium.
Fig 3.
10th day of pregnancy: ultrasound transverse section of the uterus. In the left horn, a gestational chamber (C) can be seen.
Fig 4.
14th day of pregnancy: ultrasound longitudinal section of the uterus. The circular gestational chamber (C) shows a wall, which is hyperechoic with respect to that of the uterus (U).
Fig 5.
16th day of pregnancy: ultrasound section of the uterus. The embryo appears as a hyperechoic spot, protruding into the cavity of the gestational chamber itself.
On the 18th day of pregnancy, the gestational chamber is still circular, while on the 20th day, it has the classical ‘lemon’ form (Fig 6). On the 30th-35th day, the stratified aspect of the uterus is still easily visible; this appearance is maintained until the end of pregnancy, although from day 40–45 on it was more difficult to appreciate.
Fig 6.
20th day of pregnancy: ultrasound longitudinal section of the uterus. The gestational chamber (C) presents the classic lemon shape; inside, some parts of the fetal teguments are barely visible.
Gestational chambers appear spherical, with a progressively increasing diameter between days 10 and 30 of pregnancy, after which they tend to become more elongated, progressively losing their spherical shape.
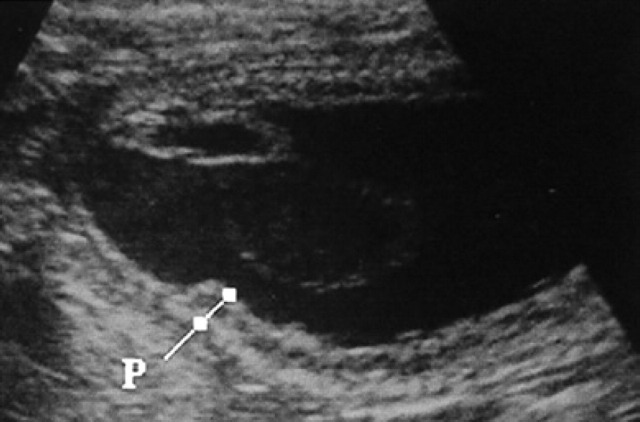
On day 45, the uterus shows a uniform cylindrical shape and, from now on, its external shape remains unchanged. The placenta zonata can only be clearly distinguished from the 25th day of pregnancy as a structure (Fig 7) occupying the entire surface of the gestational chamber with the exception of the two poles; it appears hyper-echoic at the level of its internal and external surfaces while it is hypoechoic in the centre (Fig 8).
Fig 7.
25th day of pregnancy: ultrasound longitudinal section of the uterus. The placenta zonata (P) is hyperechoic at the level of its internal and external surfaces, while it is hypoechoic in the centre.
Fig 8.
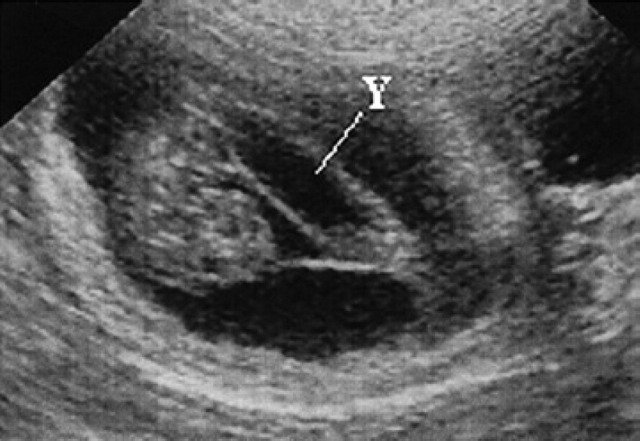
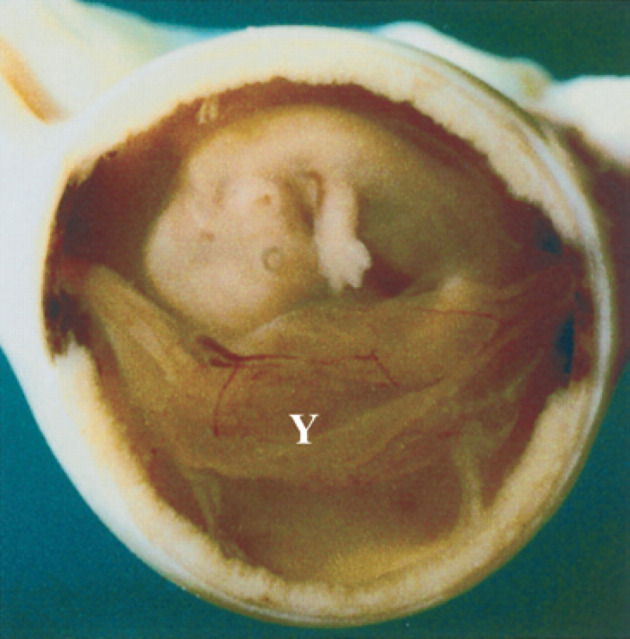
25th day of pregnancy: ultrasound longitudinal section of the uterus. The yolk sac (Y), ventral with respect to the fetus, has assumed a tubular form and is fixed to the poles of the gestational chamber.
Like the uterus, the placenta is easily visualised until about day 35, after which, even if well developed, it is difficult to appreciate.
Ultrasonographic differences were not observed in the structure of the placenta from its initial development until the end of pregnancy.
Embryo and fetus
On the 14th day of pregnancy, the embryo appears as a thickening of the uterine wall on the internal aspect of the gestational chamber. On the 16th day, it appears as a protruding hyperechoic spot in the cavity of the gestational chamber (Figs 5, 9).
Fig 9.
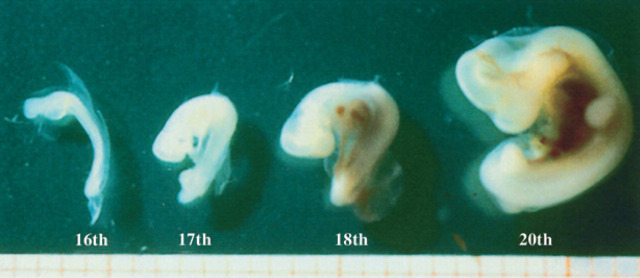
Embryos on the 16th-17th, 18th-20th day of pregnancy.
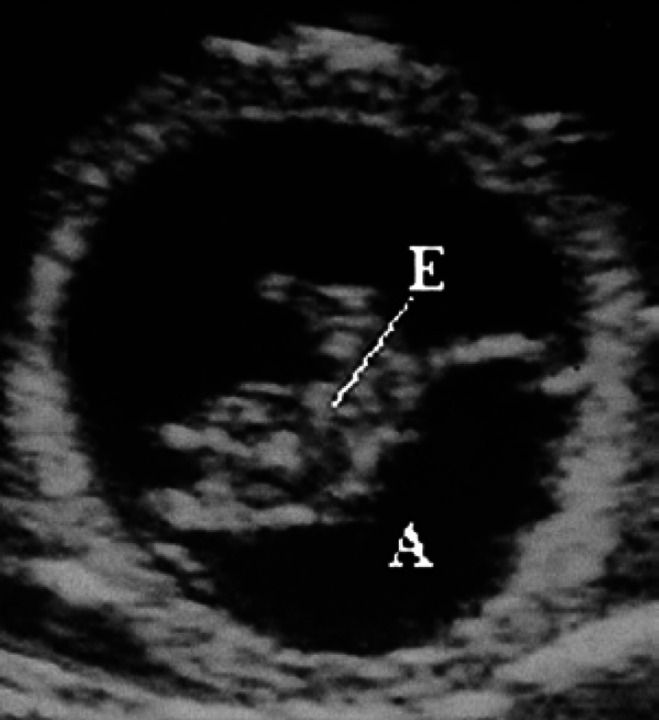
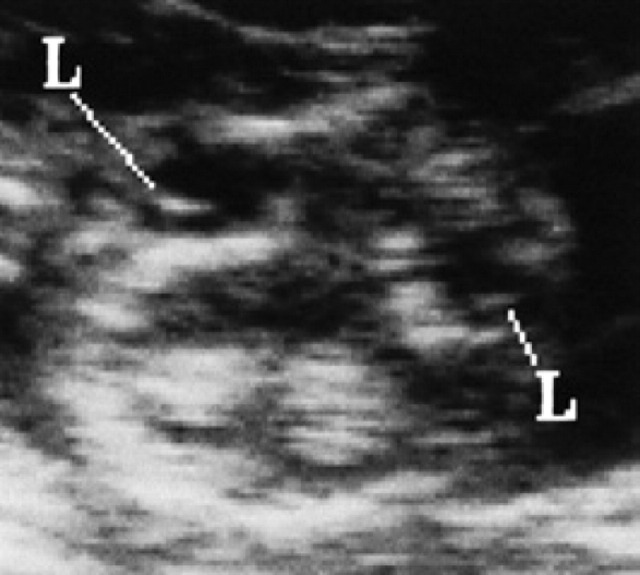
On the 17th day of pregnancy, the embryo appears clearly separated from the wall of the gestational chamber, but it is still in its proximity. Its ultrasonographic appearance is still not well defined, even though it is clearly beginning to become curvilinear in shape (Figs 10, 9). On the 18th day of pregnancy, the embryo is now C-shaped with the buds of the head and the trunk and the buds of the hyperechoic thoracic limbs (Figs 11, 12, 9).
Fig 10.
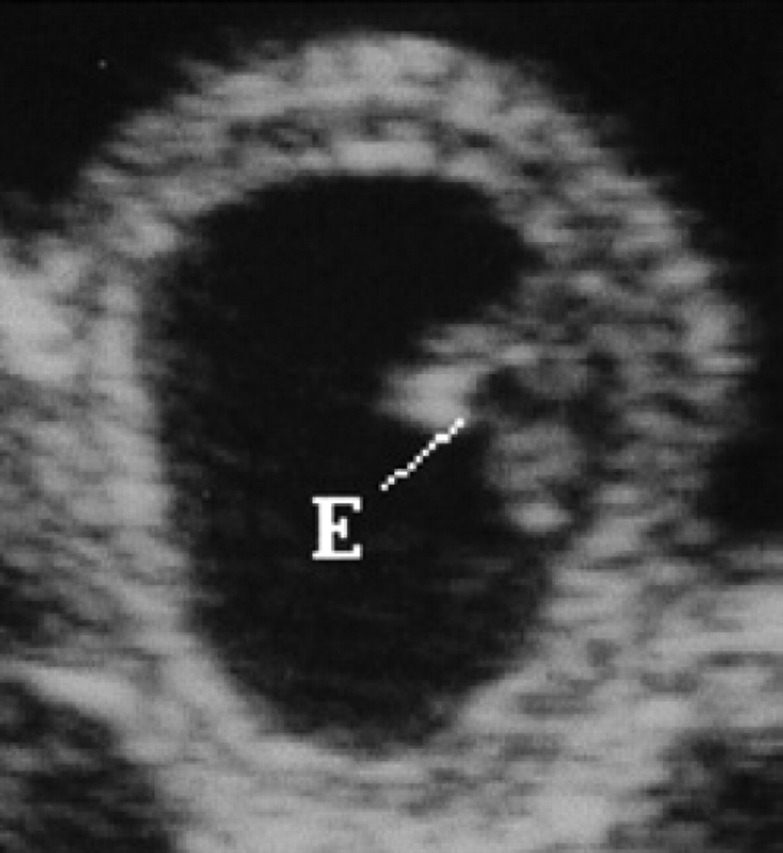
17th day of pregnancy: ultrasound section of the gestational chamber. The embryo (E) appears separated from the wall of the gestational chamber, but still near it.
Fig 11.
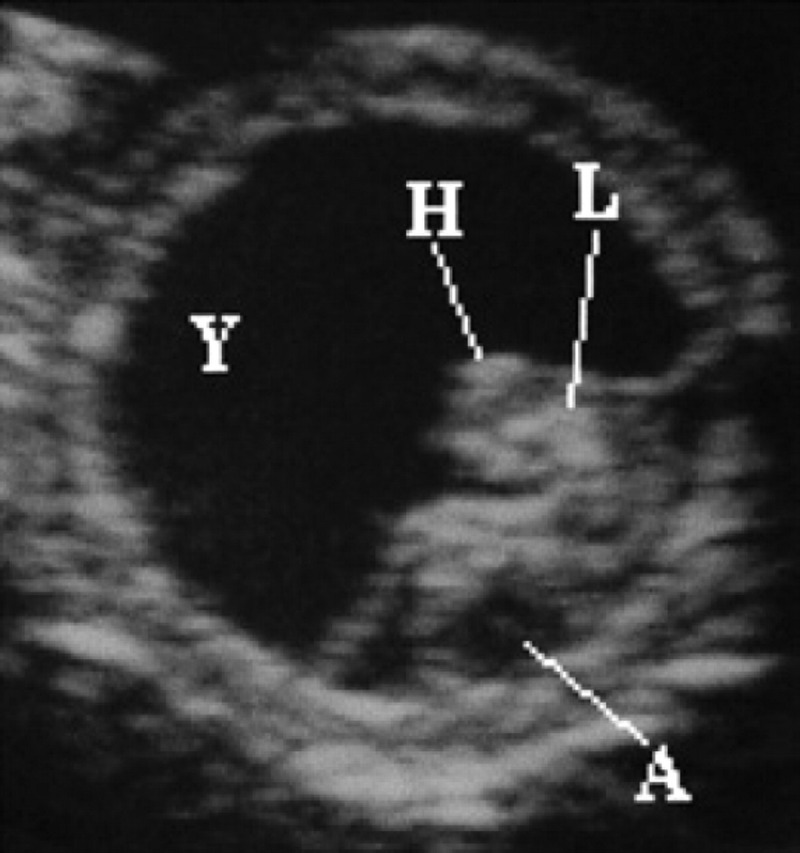
18th day of pregnancy: ultrasound section of the gestational chamber. The embryo has assumed the shape of a C; buds of the head (H) and trunk and the buds of the thoracic limbs (L) are recognisable and hyperechoic. The yolk sac (Y), ventral, almost completely fills the gestational chamber while the allantoid (A), caudally, appears as a small circle-shaped anechoic area.
Fig 12.
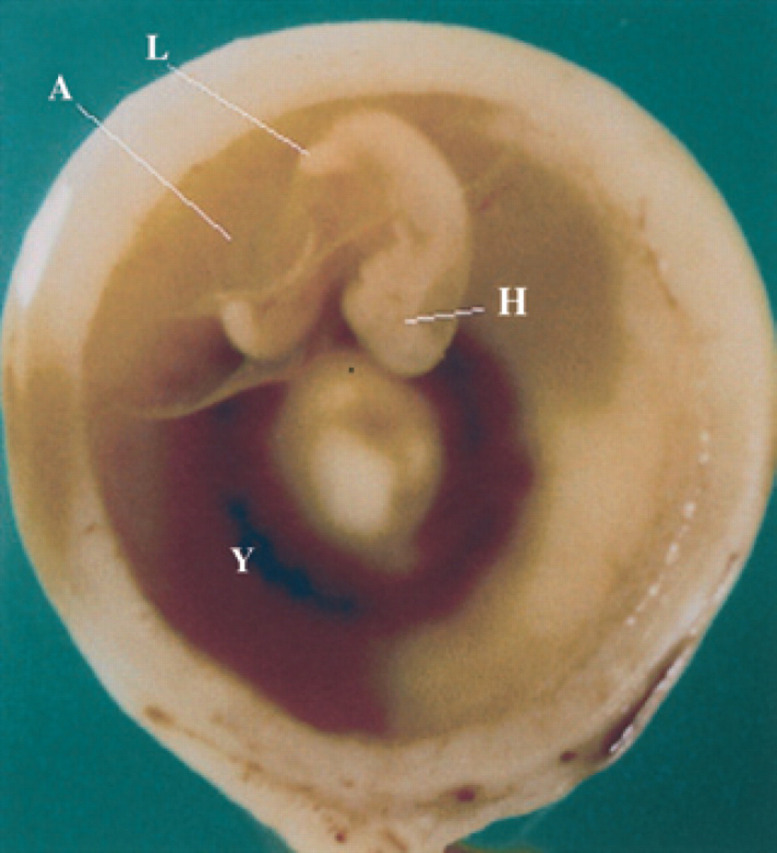
18th day of pregnancy: gestational chamber fixed and seen in cross-section. Anatomic aspect of Fig 18.
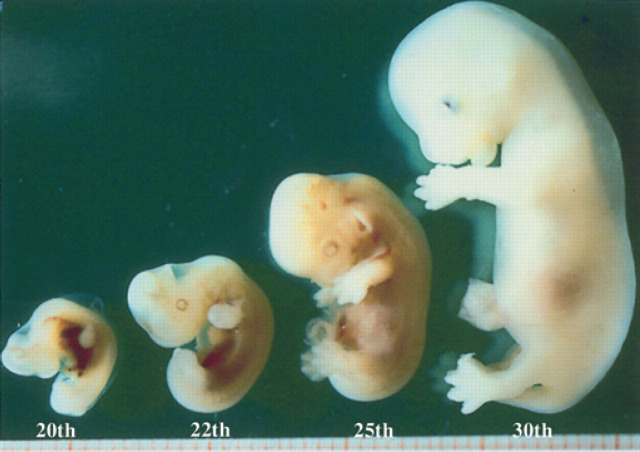
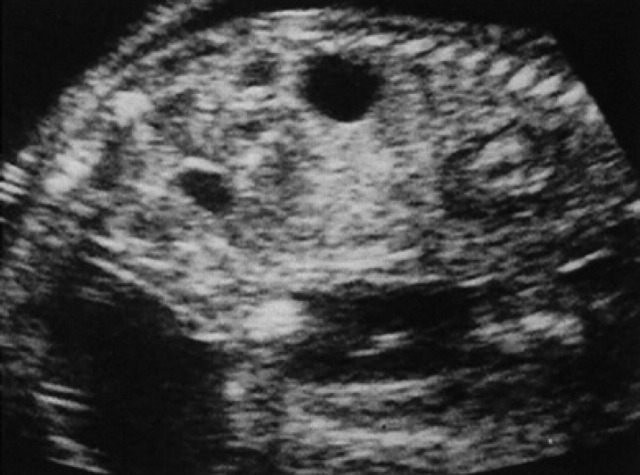
On the 20th day of pregnancy, following the development of the allantoids, the embryo, still folded onto itself, is found in the centre of the gestational chamber (Figs 13, 9). On the 22nd day of pregnancy, the embryo is located near the wall opposite the gestational chamber. On the 30th day of pregnancy, within the limits of the fetal body, a dorsal, sagittal, tubular structure with a hyperechoic wall and an anechoic content is observed, corresponding to the primitive neural tube (future spinal cord), and an anechoic area at the level of the head which represents the primitive encephalic vesicle/primary brain vesicle (future cerebral ventricles) (Veggetti, 1987)(Fig 14). Macroscopically, the fetus begins to assume its definitive form at about the 26th day; the thoracic limbs, the buds of the posterior ones and the bud of the auricle are visible (Fig 15). In the formalin fixed fetus, which has been sectioned longitudinally, the heart, lungs and liver can be observed (Fig 16). Ultrasono-graphically these organs are identifiable as well-defined structures the fetal body after the 30th day of pregnancy.
Fig 13.
20th day of pregnancy: ultrasound section of the gestational chamber. Following the development of the allantoid (A), the embryo (E) is found in the centre of the gestational chamber.
Fig 14.
25th day of pregnancy: ultrasound longitudinal section of the fetus. The fetus presents an anechoic area at the level of the head and a dorsal sagittal tubular structure having hyperechoic walls and anechoic content.
Fig 15.
Embryos on the 20th and 22nd day of pregnancy and fetuses on the 25th and 30th day of pregnancy.
Fig 16.
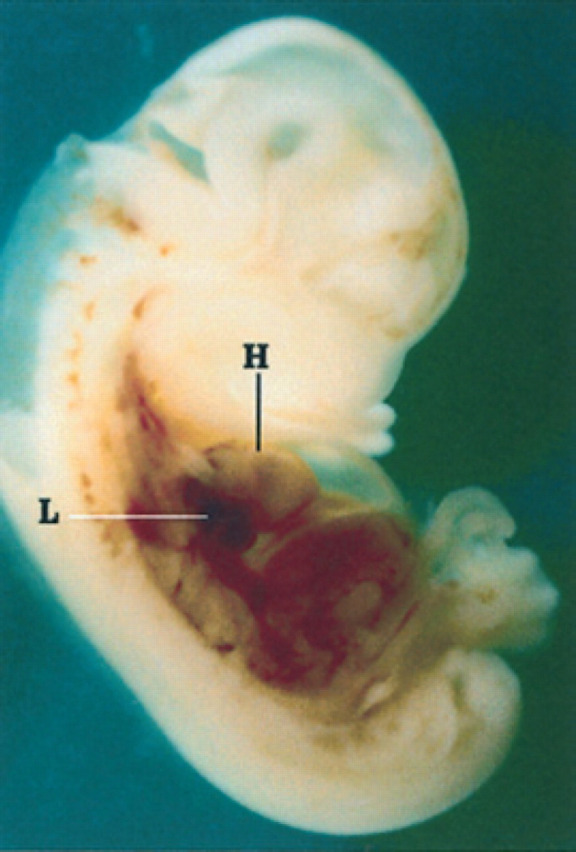
25th day of pregnancy: fetus fixed and sectioned longitudinally. The heart (H) and lungs (L) in the chest and the liver in the abdomen can be distinguished.
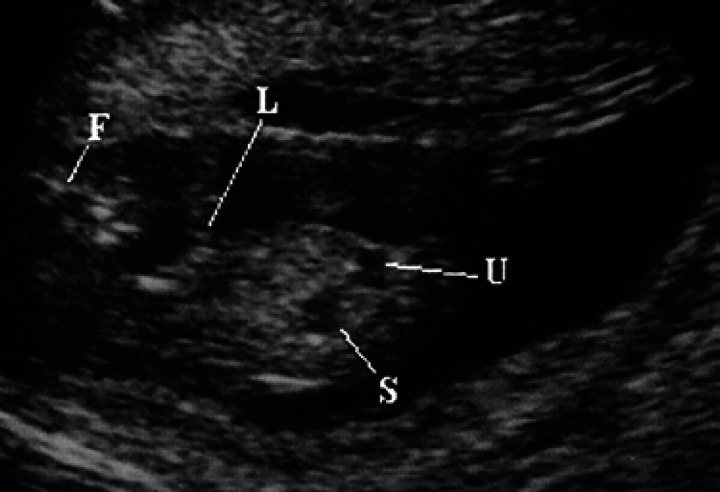
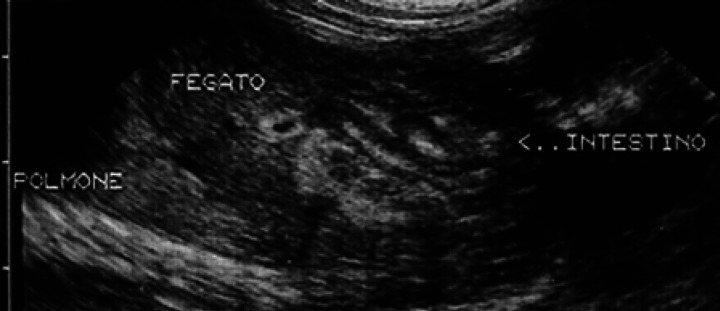
On the 30th day of pregnancy, in the area of the thoracic limbs and the fetal head, small hyper-echoic areas without an acoustic shadow can be seen which correspond to the buds of some facial bones and some diaphyses of the long bones of the thoracic limbs (Fig 17). Moreover, on the 29th-30th day, it is possible to identify the urinary bladder, which appears as a punctiform anechoic area situated cranially to the pelvic limbs and near the umbilical cord (Fig 17). Its dimensions vary according to its state of repletion. The stomach is presented as a small anechoic area situated dorsally with respect to the bladder and the liver (Fig 17); the latter occupies almost the entire abdominal cavity (Fig 18).
Fig 17.
30th day of pregnancy: ultrasound longitudinal section of the fetus. In the limits of the thoracic limbs (L) and the face (F) of the fetus, small hyperechoic areas, without shadow cone, which correspond to the buds of some facial bones and the diaphysis of the long bones of the thoracic limbs. The urinary bladder (U) and the stomach (S) appear as anechoic areas.
Fig 18.
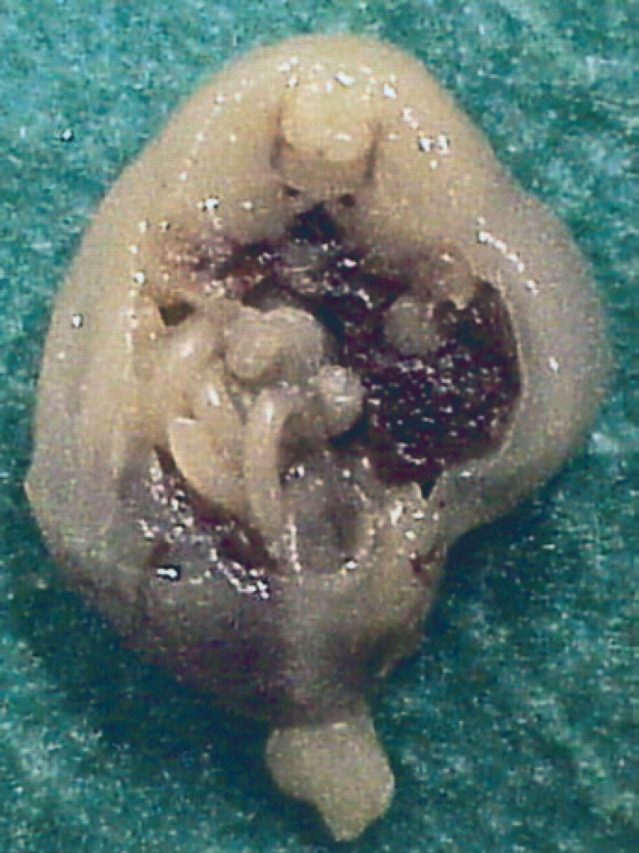
30th day of pregnancy: fetus seen in a cross section which passes cranially to the pelvic cavity. The intestine is limited to a small area situated on the left with respect to the liver.
The fetal heart is visible on the 16th-17th day, and on its dorsal aspect, the pulmonary lobes which appear hyperechoic with respect to the liver parenchyma (Fig 19). In this period, weak lateral movements of the body are often observed.
Fig 19.
30th day of pregnancy: ultrasound longitudinal section of the fetus. In the abdomen, the liver is visible. In the chest, the heart can be seen and, dorsally to this, the pulmonary lobes which appear hyperechoic with respect to the hepatic parenchyma.
Macroscopically, the shape of the fetal body is becoming similar to the definitive one (Fig 15). On the 35th day, the pads of the feet are visible.
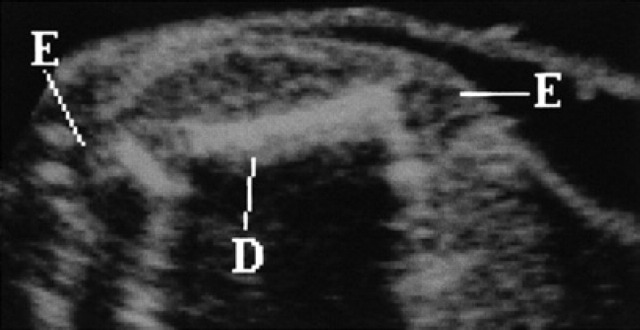
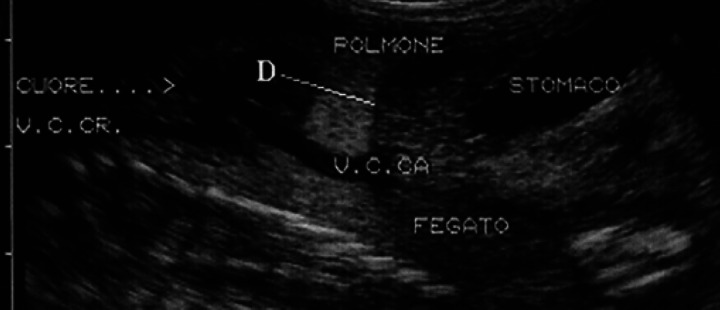
Starting from the 40th day, the spinal column appears sub-divided into vertebrae (Fig 20). On the 42nd day, all the bones are hyperechoic and, on the 50th day, they show a posterior shadow cone; the diaphysis of the long bones is hyper-echoic, while the epiphysis is seen as hypoechoic area until the end of the pregnancy (Fig 21).
Fig 20.
45th day of pregnancy: ultrasound longitudinal section of the fetus. The metameric structure of the spinal column is evident.
Fig 21.
50th day of pregnancy: ultrasound section of the fetus. The femoral diaphysis (D) is hyperechoic with shadow cone. The epiphysis (E) appears hypoechoic until the end of the pregnancy.
The first fetal movements (back-flexion) can be seen on the 33rd day; from the 37th day, movements of flexion and extension of the limbs, hands, head, neck as well as acts of swallowing can be seen. On the 50th day, frequent movements of the tongue and opening of the mouth as well as rhythmic movements of the diaphragm of a hiccuping type are also visible.
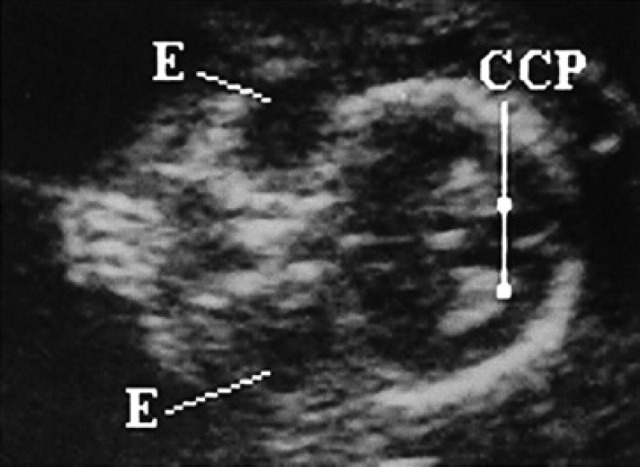
On the 35th day, at the level of the head, two circular anechoic areas corresponding to the eyes can be seen. Within these, and in a rostral position, the crystalline lens is seen as a hyper-echoic structure on the 50th day (Fig 23). On the 40th day, the cerebral choroid plexi become visible as two hyperechoic symmetrical structures with respect to the surrounding brain (Fig 22).
Fig 23.
50th day of pregnancy: ultrasound frontal section of the head of the fetus. Inside the eye, the hyperechoic crystalline lens (L) can be seen.
Fig 22.
45th day of pregnancy: ultrasound frontal section of the head of the fetus. The eyes (E) are presented as anechoic areas; on the right, the cerebral choroid plexi (CCP) are evident, and hyperechoic.
Differentiation between the cardiac chambers is only possible after the 50th day. The large cardiac vessels first appear on the 42nd day as tubular anechoic structures, located ventrally to the spinal column and in contact with the heart and liver. In the same period, the umbilical vessels can be seen as they pass from the umbilical wall towards the liver (Fig 24). The kidneys are seen for the first time on the 39th day as two elliptical areas situated ventro-laterally to the spinal column and isoechoic with the liver. Until the 50th day, it is not possible to distinguish the renal cortex from the medulla on ultrasound. The kidneys assume their definitive structure only towards the 60th day (Fig 26).
Fig 24.
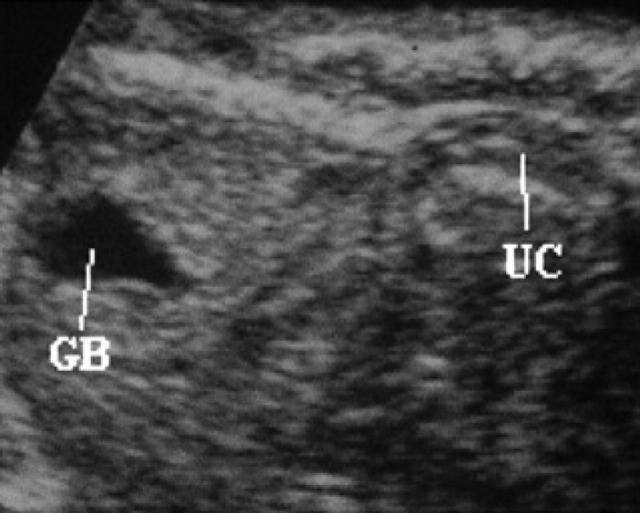
60th day of pregnancy: ultrasound longitudinal section of the fetus. The anechoic area on the left corresponds to the gall-bladder (GB); inside the umbilical cord (UC), the vessels that branch off to the liver can be seen.
Fig 26.
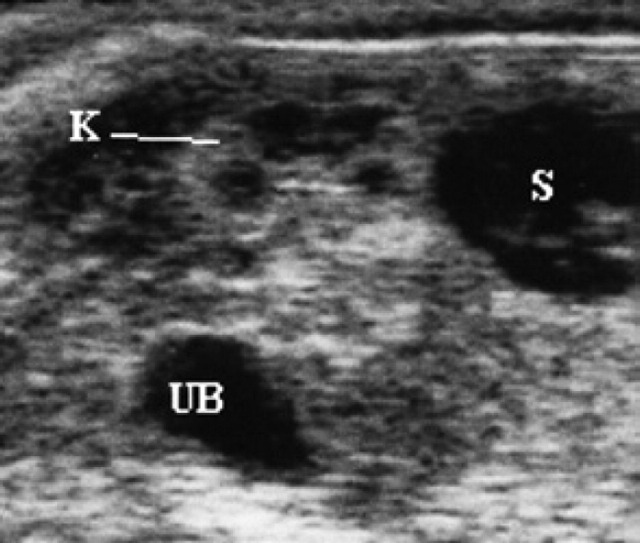
60th day of pregnancy: ultrasound longitudinal section of the fetus. The kidney (K) has already acquired the characteristic ultrasound sub-division into cortex and medulla. To its right, the stomach (S) is present and ventrally, the urinary bladder (UB).
On the 49th day, the progressively developing stomach, passes from a dorsal position to a caudal position with respect to the liver (Figs 27, 28). The stomach can be observed as an anechoic area of increasing dimensions. On the 54th day, hyperechoic speckling, probably referable to the rugal folds, can be seen on its internal wall (Fig 29). The gall-bladder is visible as an anechoic area (Fig 24).
Fig 27.
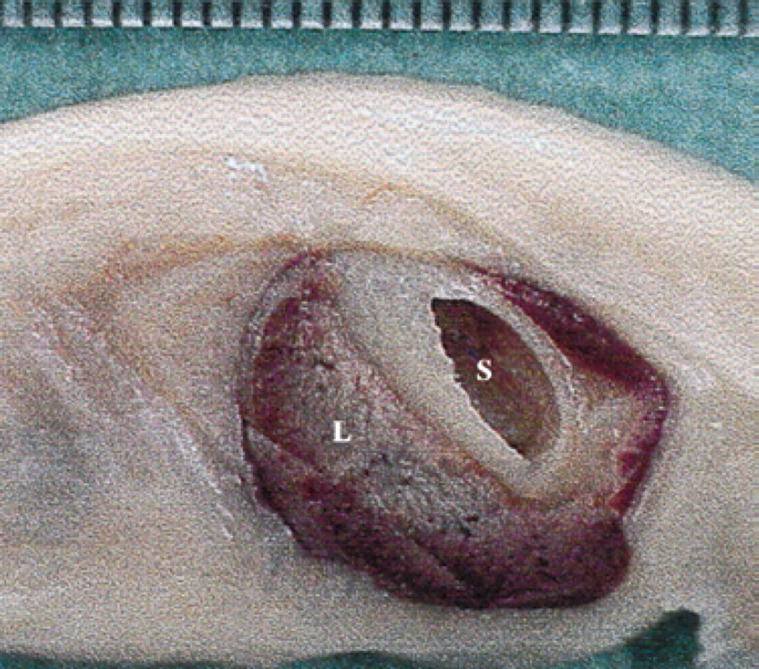
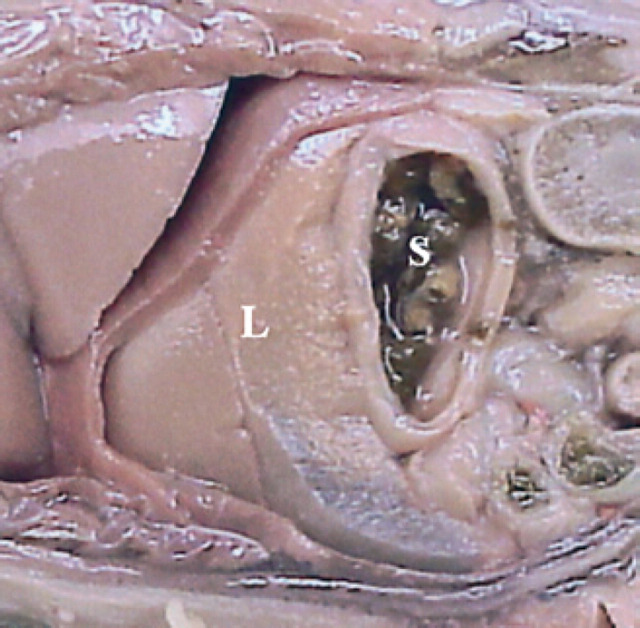
45th day of pregnancy: fetus in longitudinal section, parallel to the median plane. The stomach (S) is situated dorsally to the liver (L).
Fig 28.
50th day of pregnancy: fetus in longitudinal section, parallel to the median plane. The stomach (S) is situated caudally to the liver (L).
Fig 29.
55th day of pregnancy: ultrasound cross-section of the fetus. The internal wall of the stomach shows a hyper-echoic speckling corresponding to the gastric rugal folds.
The small and large intestine is hardly visible until the 40th day when an area hypoechoic to the liver, and of about equal dimensions is visualised. In 45-day fixed fetuses, the liver is not observed in the more caudal sections of the abdomen, but remains confined to the section made cranially to the umbilical cord. The intestine presents an echostructure similar to the adult only after day 54, when it is possible to see stratification of the intestinal wall (Fig 30).
Fig 30.
55th day of pregnancy: ultrasound longitudinal section of the fetus. The intestine presents an echostructure similar to the adult one.
The liver always appears hypoechoic compared to the lungs. On the 50th day, a hypoechoic line cranial to the liver can be seen, and this separates the latter from the lungs and can be considered to be the diaphragm (Fig 31).
Fig 31.
55th day of pregnancy: ultrasound longitudinal section of the fetus. The liver always shows an ecogenicity inferior to the lungs. A hypoechoic line is visible with respect to the liver, referable to the diaphragm (D).
Fetal membranes
On the 10th day of pregnancy, the gestational chamber is occupied exclusively by the yolk sac (Fig 3). On the 17th day of pregnancy, it is possible to distinguish the bud of the allantoid in the fixed embryo (Fig 9). On the 18th day of pregnancy, ventrally to the embryo, the yolk sac is present which almost completely fills the gestational chamber; the allantois is visible caudally to the embryo and appears as a small anechoic area having a circular form (Fig 11). In the gestational chamber, fixed and sectioned transversally, the embryo, the ventral yolk sac and the allantois can be seen in Figs 12, 9.
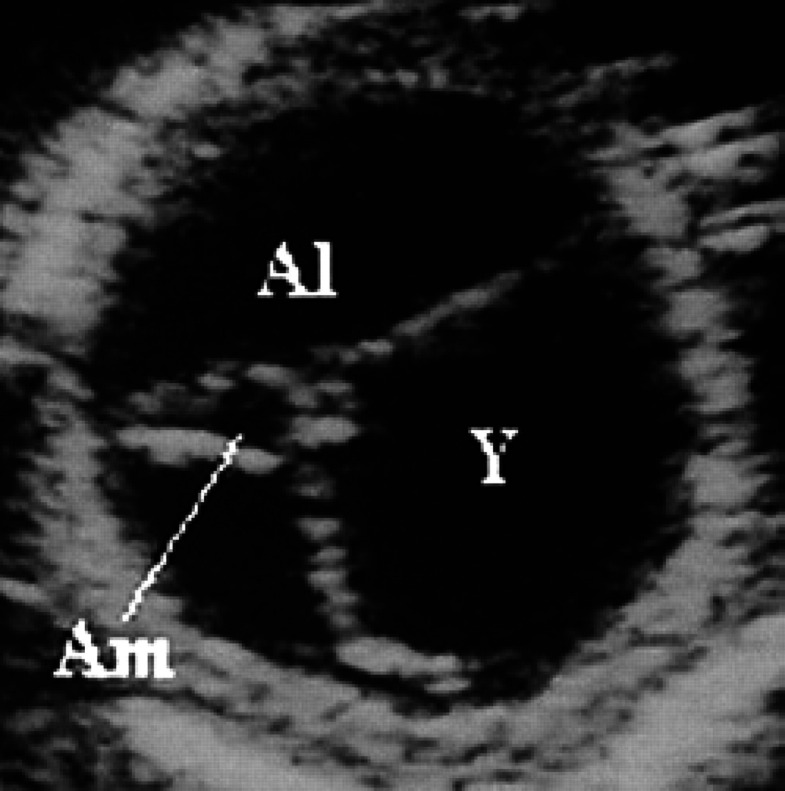
On the 20th day of pregnancy, the allantois and the yolk sac have about the same volume while the amnion appears as a small anechoic area (Fig 32). On the 22nd day of pregnancy a yolk sac smaller than the amnion can be observed ventrally to the amnion itself. The rest of the gestational chamber is occupied by the allantois. All the fetal membranes can be distinguished macroscopically. On the 25th day of pregnancy, the amnion and the yolk sac have approximately the same volume; the latter has, by now, assumed a tubular form and is fixed to the poles of the gestational chamber (Fig 33, 8).
Fig 32.
20th day of pregnancy: ultrasound section of the gestational chamber. The allantoid (Al) and the yolk sac (Y) have about the same volume; the amnion (Am) appears as a small anechoic area.
Fig 33.
25th day of pregnancy: gestational chamber fixed and sectioned longitudinally. The fetus, wrapped in the amnion, ventrally presents the yolk sac (Y) which is tubular and fixed to the poles of the gestational chamber.
All the fetal membranes can be easily seen on ultrasound until the 30th day, and, with some difficulty, until the 45th day of pregnancy. However, at the 35th day, they appear to be billowing and not very taut. Therefore, their shape is different to the earlier stages of pregnancy.
The yolk sac, which already appears to have a reduced volume after the 30th day, becomes barely visible and is always found in a ventral position with respect to the fetus. From about the 40th day it cannot be seen at all.
From the 45th day, the amnion can be seen only when scanning between the two limbs, between the tail and the limbs or between the head and the limbs. After the 50th day, the allantoic fluid is visible only in small areas around the fetus (Fig 25).
Fig 25.
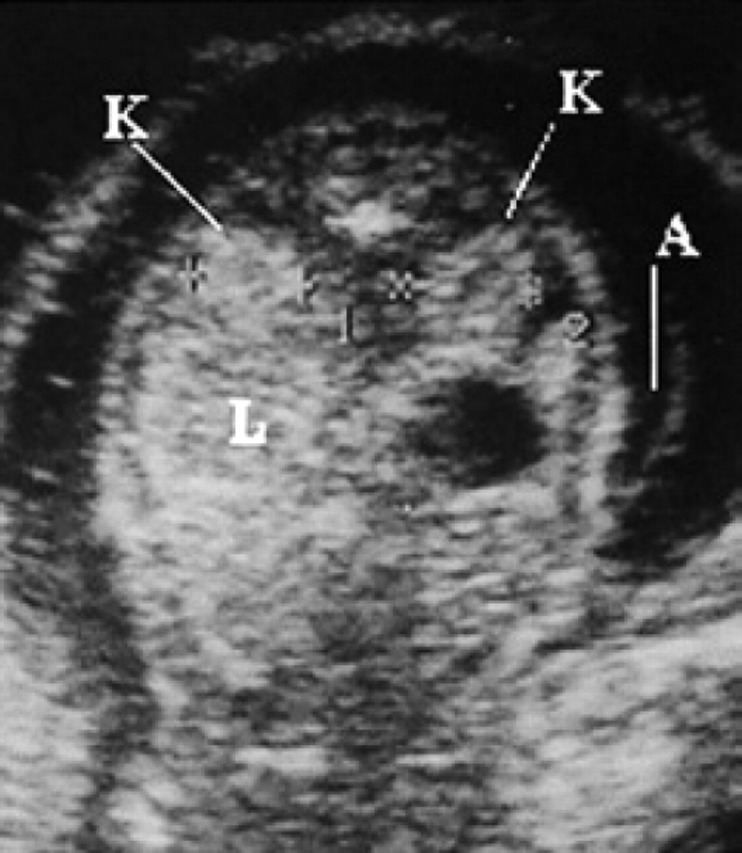
45th day of pregnancy: ultrasound transversal section of the fetus. The kidneys (K), situated laterally to the vertebrae, are isoechoic to the liver (L). The hypoechoic circular area corresponds to the stomach. The amnion (A), containing little fluid, is visible behind the fetus.
From about the 55th day, small dimension mobile echoic structures, probably meconium, can be seen inside the amnion. Ultrasonographic aspects of the development of some fetal and extra-fetal structures are reported in Tables 2 and 3.
Table 2.
Time of ultrasound appearance and subsequent development of some fetal and extra-fetal structures during the first 30 days of pregnancy in cats
| Days after mating | |||
|---|---|---|---|
| Mode | Range | ||
| Gestational chamber | Spherical shape | 10 | 10–11 |
| Lemon shape | 20 | 19–21 | |
| Uterine wall | Hypoechic endometrium | 4 | 3–5 |
| Layers of the placenta | 16 | 15–17 | |
| Placenta zonata | 25 | 24–26 | |
| Position of the embryo | On the wall of the gestational chamber | 14 | 13–16 |
| Protruding into the gestational chamber | 16 | 15–17 | |
| Separated from the wall of the chamber | 17 | 16–18 | |
| In the centre of the gestational chamber | 20 | 19–21 | |
| Embryo axis parallel to the horn axis | 26 | 25–28 | |
| Fetal teguments | Yolk sac | 10 | 10–11 |
| Allantoid | 18 | 17–19 | |
| Volume yolk sac=volume allantoid | 20 | 19–21 | |
| Amnion | 20 | 20–21 | |
| Volume amnion=volume yolk sac | 25 | 24–25 | |
| Tubular yolk sac (longitudinal section) | 25 | 25–27 | |
| Embryo and fetus | Heartbeat | 16–17 | 16–18 |
| C-form (head and trunk) | 18 | 17–19 | |
| Buds of the thoracic limbs | 18 | 17–19 | |
| Definitive form | 26 | 24–27 | |
| Sagittal dorsal tube | 30 | 26–30 | |
| Anechoic area at the level of the head | 30 | 26–30 | |
| Stomach (punctiform) | 30 | 29–30 | |
| Bladder (punctiform) | 29–30 | 29–32 | |
| Hyperechoic lung vs liver | 30 | 29–32 | |
| Umbilical cord | 30 | 30–32 | |
| Hyperechoic skeleton (long bones, thoracic limbs and head) | 30 | 30–33 | |
| Fetal movements (laterally) | 30 | 30–34 | |
Table 3.
Time of ultrasound appearance and subsequent development of some fetal and extra-fetal structures after the 30th day of pregnancy in cats
| Days after mating | |||
|---|---|---|---|
| Mode | Range | ||
| Stomach | Dorsal to the liver | 30 | 30–32 |
| Caudal to the liver | 49 | 48–50 | |
| Rugal folds | 54 | 54–57 | |
| Intestine | Appearance (identifiable) | 40 | 38–42 |
| Layered structure | 54 | 52–56 | |
| Eyes | Appearance (identifiable) | 35 | 35–39 |
| Crystalline lens | 50 | 47–50 | |
| Skeleton | Vertebrae | 40 | 35–40 |
| Ossification: long bone diaphysis | 50 | 48–50 | |
| Movements | Dorso-ventral flexion | 33 | 32–35 |
| Limbs, neck and head | 37 | 37–40 | |
| Hiccups, mouth and tongue movements | 50 | 48–52 | |
| Kidneys | Isoechoic with the liver | 39 | 38–41 |
| Cortex and medulla | 50 | 48–50 | |
| Cardio-vascular apparatus | Cardiac chambers | 50 | 48–50 |
| Major vessels | 42 | 40–44 | |
| Diaphragm | 50 | 50–56 | |
| Footpads | 35 | 33–35 | |
| Choroid plexi | 40 | 38–42 | |
Discussion
Ultrasonographic imaging of the uterus, placenta and fetal membranes provides useful information on the developmental state of the embryo and the fetus, especially in the first half of pregnancy. From day 30 of pregnancy on, the buds of the organs assume their definitive structure and the fetus grows progressively, becoming more easily identified on ultrasound investigations.
According to Davidson et al (1986), the presence of a hypoechoic endometrium can be observed both in pregnant and pseudo-pregnant cats, due to physiological endometrial hyperplasia associated with high plasma concentrations of progesterone. Our findings support this observation.
The use of a 10 MHz sector probe allowed an earlier confirmation of pregnancy (10 days after mating) than previously reported in the literature (Davidson et al, 1986; Grof et al, 1993). Comparison of the gestational chambers permitted a positive diagnosis of pregnancy, but as spontaneous embryonic resorption is frequent in the early stages of gestation, pregnancy must be subsequently confirmed on day 20 to 25. With regard to the stratified ultrasound appearance of the placenta, the intermediate hypoechoic line is probably caused at least initially, by the presence of an edematous and hyperplastic endometrium (due to the high concentrations of plasma progesterone). Later, with the development of the placenta itself, it is probably due to increased local vascularisation.
We did not observe any apparent variation in the echostructure of the placenta throughout the entire duration of pregnancy. In contrast, in humans, the placenta is staged from 1 to 3 according to the degree of maturity; and through this staging and other parameters, it is possible to evaluate fetal well being (Ziviello & Bazzocchi 1994).
Until the 26th day, the conceptus does not always present its major axis parallel to the major axis of the gestational chamber and, therefore, of the uterine horn; only when it has reached a certain length and the umbilical cord is well-formed, do the two axes become parallel and remain that way until the end of the pregnancy.
Before the 30th day, the different organs are still poorly delimited from an ultrasound perspective in as much as they have a very similar echogenicity. In order to distinguish between them, it is necessary to carry out longitudinal scans of the fetus where they can be seen simultaneously, making it easier to distinguish one from the other.
The homogeneity of the ultrasonographic appearance of the various tissues is probably attributable to various factors, including the large quantity of water contained in all the tissues (many of which are still not completely differentiated), that they are still developing, and the presence of fat and large vessels, which cannot be visualised, using ultrasound, in the first 30 days of pregnancy.
Observation of cerebral choroid plexi the 40th day of pregnancy in this study occurred later in comparison to observations made in dogs (Valocky et al, 1997). The eye was first observed on the 35th day, which was later than previously reported by Grof et al (1993). In the literature, there are no data on the ultrasonographic appearance of the fetal crystalline lens, which we observed on the 50th day.
The movements of the body, limbs and head were observed with increasing frequency towards the end of pregnancy. In all cases, this could be readily stimulated applying delicate pressure with the ultrasound probe on the abdomen of the queen.
According to our findings, ultrasound detection of skeletal calcification is complete by the 50th day, later than that reported by Grof et al (1993). This difference could be due to the fact that feline bones produce weak acoustic shadows and are therefore more difficult to identify during the initial phases of development (Grof et al 1993).
The urinary bladder was observed on the 29th-30th day, which is earlier than reported by Yeager et al (1992) and Nyland & Mattoon (1995). Its dimensions varied according to the state of repletion, as has been observed in dogs (England et al 1990). For this reason, urinary bladder size cannot be used to evaluate gestational age.
In accordance with bibliographical data, from the 50th day, beginning with the differentiation of the cortex and the medulla, kidneys can be observed more easily and their dimensions recorded.
The allantois and the amnion first appear on ultrasound scans on the 18th and the 20th days, respectively, and increase in volume as the pregnancy progresses while the yolk sac, already visible on the 10th day, progressively decreases until it reaches extremely small dimensions which present its observation on ultrasound.
On the 30th day, the fetal membranes become billowing assuming different shapes, this is due to a loss of internal tension caused by the thinning of the uterine wall.
With slight pressure using a probe, a deformation of the gestational chamber is produced which can cause the billowing of the membranes and/or the removal of liquid, which is displaced at a distance from the probe itself.
Acknowledgements
The help of Prof. Stefano Romagnoli, Department of Veterinary Clinical Sciences, University of Pisa, Italy in reviewing the manuscript is gratefully acknowledged.
References
- Davidson AP, Nyland TG, Tsutsui T. (1986) Pregnancy diagnosis with ultrasound in the domestic cat. Veterinary Radiology 27:4, 109–114. [Google Scholar]
- England GCW, Allen WE, Porter DJ. (1990) Studies on canine pregnancy using B-mode ultrasound: Development of the conceptus and determination of gestational age. Journal of Small Animal Practice 31, 324–329. [Google Scholar]
- Grof D, Poulsen Nautrup C, Wissdorf FH. (1993) Sonographisch-anatomische Trach-tigkeitsdiagnostik bei del Katze. Kleintierpraxis 38: 11, 733–744. [Google Scholar]
- Nyland TG, Mattoon JS. (1995) Ultrasonography of the Genital System. In: Veterinary Diagnostic Ultrasound, 1st edn. Philadelphia: WB Saunders Co., 146–151. [Google Scholar]
- Valocky I, Iuchtman A, Lazar G, Kacmarik J, Sevcik A, Jr, Lukan M, Csicsai G. (1997) Applications of ultrasonographic biometry in the pregnant bitch: Estimation of foetal development. Folia Veterinaria 41:3–4, 117–122. [Google Scholar]
- Veggetti A. (1987) Corso di Embriologia per gli Studenti di Medicina Veterinaria. 2a ed. Bologna: Societa Editrice Esculapio, 47–49. [Google Scholar]
- Yager AE, Mohammed HO, Mayers Wallen V, Vannerson L, Concannon PW. (1992) Ultrasonographic appearance of the uterus, placenta, fetus and fetal membranes throughout accurately timed pregnancy in Beagles. American Journal of Veterinary Research 53: 3, 342–351. [PubMed] [Google Scholar]
- Ziviello M, Bazzocchi M. (1994) Ecografia, Volume 2, Casa Editrice Gnocchi, 759–761. [Google Scholar]