Abstract
This study investigated relationships between plasma leptin, insulin concentrations, insulin sensitivity and glucose tolerance in lean and overweight cats. Leptin concentrations were measured in 16 cats during glucose tolerance tests before and after gaining weight, and after feeding a test meal in overweight cats.
An important finding of this study is that in both lean (r=-0.79) and overweight (r=-0.89) cats, the higher the leptin concentrations, the more insulin resistant the cat, independent of the degree of adiposity. Leptin concentrations at baseline and after consuming a meal tended to be higher in overweight cats with glucose intolerance, compared to overweight cats with normal glucose tolerance, although the difference was not significant. After feeding the test meal to overweight cats in the early morning, plasma leptin concentrations initially decreased before subsequently rising to peak 15 h later, which coincided with late evening. The leptin peak occurred 9 h after the insulin peak following ingestion of the test meal.
Importantly, this study suggests that increased leptin concentrations may contribute to the diminished insulin sensitivity seen in overweight cats. Alternatively, the compensatory hyperinsulinaemia found with insulin resistance in overweight cats could stimulate leptin production.
Introduction
Obesity in cats and humans is associated with multiple abnormalities of glucose metabolism including insulin resistance, hyperinsulinaemia, impaired glucose tolerance and diabetes (Kolterman et al 1980, Nelson et al 1990, Panciera et al 1990, Biourge et al 1997, Appleton et al 2001a). The mechanism by which obesity leads to insulin resistance and increases the risk of diabetes is unclear, but may involve molecules secreted from fat such as the hormone leptin (Cohen et al 1996, Müller et al 1997). Secretion of leptin from adipose tissue is increased in proportion to body fat mass in cats, humans and rodents (Maffei et al 1995, Considine et al 1996, Appleton et al 2000, Backus et al 2000). Fasting plasma leptin concentrations increased more than three times (7.88±4.02 ng/ml HE vs 24.5±12.1 ng/ml HE) in cats which gained an average of 1.9 kg or 44% body weight (Appleton et al 2000). We found that plasma leptin was most strongly correlated with body weight in lean cats and with body fat percent or total fat mass in overweight cats (Appleton et al 2000).
It is postulated that leptin is a mediator of some of the metabolic consequences of obesity. Insulin resistance is associated with increased leptin concentrations in lean and obese humans (Larsson et al 1996, Segal et al 1996, Haffner et al 1997, Nyholm et al 1997, Zimmet et al 1998). Logically, the relationship between leptin and insulin sensitivity may not be causal, but be related to fat mass, as leptin concentrations are proportional to fat mass, and obesity causes insulin resistance. However, these studies showed that this relationship occurs independent of fat mass.
There is other evidence indicating that insulin resistance and leptin may be causally related. In vitro, leptin has been shown to desensitise liver and fat cells to insulin (Cohen et al 1996, Walder et al 1997, Mueller et al 1998). If the same is true in vivo, elevated leptin levels could contribute to the pathophysiology of insulin resistance in obesity. Alternatively, the relationship between insulin resistance and leptin might be secondary to a stimulatory influence of insulin on leptin synthesis and secretion. This hypothesis is supported by several reports. Firstly, insulin has been shown to stimulate leptin synthesis in human and rodent adipocytes in vitro (Saladin et al 1995, Kolaczynski et al 1996, Wabitsch et al 1996). Secondly, plasma leptin concentrations in humans increase when plasma insulin is increased, for example, within 4–6 h of consuming high carbohydrate meals (Mohamed-Ali et al 1996, Coppack et al 1997, Dallongeville et al 1998, Havel et al 1999) or in response to glucose or insulin infusions (Sonnenberg et al 1996, Utriainen et al 1996, Saad et al 1998a). Thus, the hyperinsulinaemia that accompanies insulin resistance in obesity may stimulate leptin production.
Little is known about the relationship between leptin, obesity, and the metabolism of glucose and insulin in cats. Studies in other species suggest that there is an inter-relationship between leptin, insulin resistance and also with diabetes. This study was undertaken to determine if plasma leptin concentrations are associated with insulin concentrations, insulin sensitivity or glucose tolerance in cats before and after weight gain, and also, to study the effects of consumption of a meal on plasma leptin in overweight cats.
Materials and methods
Plasma glucose, insulin and leptin concentrations were measured during glucose tolerance and insulin sensitivity tests in 16 cats before and after 44% weight gain. These hormones were also measured after weight gain during a meal response test. Before and after weight gain, relationships between plasma leptin, glucose and insulin concentrations, and indices of glucose tolerance and insulin sensitivity were evaluated after controlling for differing levels of adiposity.
The protocol for this study and the care and handling of these animals were approved by the Animal Experimentation Ethics Committee of the University of Queensland.
Animals
Before and after weight gain, cats (six castrated males and 10 spayed females) were assessed as healthy by clinical examination and routine haematological and serum biochemical analyses. Accurate ages were unknown, however all were estimated by visual assessment and examination of dentition to be young adults between 1 and 5 years of age.
After 10 months of ad lib feeding energy dense diets, mean weight gain was 1.9 kg per cat (44.2%) (Appleton et al 2001a). Mean initial body weight of the cats was 4.37±0.76 which increased significantly to 6.28±1.26 kg after weight gain (Appleton et al 2001a). Body condition score and body mass index were also significantly higher after weight gain (Appleton et al 2001a). Six cats were classified as overweight and 10 classified as obese based on having a body condition score of 4 or 5 respectively (Sunvold & Bouchard 1998). Body composition was estimated by dual energy X-ray absorptiometry (DEXA) scans after weight gain, and percentage body fat content was above 30% in all cats (41.3%±3.96, range 34.2–48.7), classing them as overweight or obese (Butterwick 2000).
Metabolic characteristics determined from the glucose tolerance and insulin sensitivity tests in these cats before and after weight gain have been previously reported and include significantly lower insulin sensitivity and glucose effectiveness, and significantly higher insulin concentrations and indices (Appleton et al 2001a). In addition, seven of the 16 cats became glucose intolerant with weight gain (Appleton et al 2001a).
Experimental protocol
Intravenous glucose tolerance and insulin sensitivity tests were performed in the mornings on alternate days in each cat, at least 24 h after jugular catheter placement under general anaesthesia (Appleton et al 2001b). Food was removed 12 h prior to commencement of each metabolic test. No cat underwent more than one test per 24-h period. During testing, cats were housed individually. After completion of initial testing, cats were transferred to group housing and offered a combination of two extruded foods with high energy density (448 and 480 kilocalories of metabolisable energy per 100 g). The composition of the diets were 33% protein, 22.3% fat, 30.2% carbohydrate; and 40% protein, 26.6% fat, 17.2% carbohydrate as fed, respectively. These diets were fed ad libitum for an average of 10.5±1.1 months (range, 9–12 months) to promote weight gain. After weight gain cats were again individually housed, insulin sensitivity and glucose tolerance tests were repeated, and meal response tests were performed over 18 h on a separate day.
Glucose tolerance tests
During the glucose tolerance test, plasma glucose, insulin and leptin concentrations were measured at baseline and at 2, 5, 10, 15, 30, 45, 60, 90 and 120 min after glucose infusion (Appleton et al 2001b). Glucose half-life (T1/2) was calculated by linear regression analysis of the semilogarithmic plot of glucose concentration vs time between 15 and 90 min after glucose administration (Link & Rand 1998). Areas under the glucose and insulin curves (AUC) were calculated by the trapezoidal method (Rowland & Tozer 1989). The curves were calculated from above the lower limit of sensitivity for the individual assays which was 3 mg/dl for glucose, and 3 μU/ml for insulin. Insulin secretion in response to the glucose infusion was estimated as the incremental area above baseline for each cat during the first 10 min, representing first phase insulin response, the first 60 min and for the second 60 min of the test, which has been used as a measure of second phase insulin response (Nelson et al 1990, Biourge et al 1997).
After weight gain, cats were divided into two groups based on their glucose tolerance status. Impaired glucose tolerance (n=7) was defined as being present when one or more of the values for T1/2 or plasma glucose concentrations at 0, 60, 90 or 120 min during a glucose tolerance test, exceeded the upper limit of the population 95% tolerance intervals (Appleton et al 2001a). The tolerance intervals for these values were determined previously using the same protocol in 32 clinically healthy, normal weight cats, 16 of which were used in the current study (Appleton et al 2001b). The upper limit of the normal reference range for T1/2 was 74 min, and for glucose concentrations at 0, 60, 90 and 120 min were 104, 223, 163, and 108 mg/dl, respectively (Appleton et al 2001b). Body fat percent and total fat mass were not significantly different between cats that maintained normal glucose tolerance after weight gain (n=9), and cats that developed glucose intolerance (n=7).
Insulin sensitivity tests with minimal model analysis
The insulin-modified, frequently sampled intravenous glucose tolerance test with minimal modelling analysis (Bergman et al 1979, Finegood et al 1990), was used to determine the insulin sensitivity index (SI) (Appleton et al 2001a). This index is a measure of how a given change in plasma insulin can increase the clearance of plasma glucose (Beard et al 1986). An increasing index value indicates that the subject is more insulin sensitive. Other parameters determined from the test included the acute insulin response to glucose (AIRg) and the glucose-mediated glucose disposal or glucose effectiveness (SG), defined as the increase in fractional disappearance of glucose per unit increase in glucose at basal insulin (Finegood & Tzur 1996).
Meal response tests
Meal response tests (Appleton et al 2001a), were performed only after weight gain. Plasma glucose, insulin and leptin concentrations were determined at baseline and at 1, 2, 4, 6, 8, 10, 12, 15 and 18 h after cats were fed a test meal containing 50% of their calculated normal daily food intake. After the meal, cats remained fasted for the 18-h test duration. Test meals were fed between 06.15 and 06.30 in the morning. Post-prandial sampling began at 07.30 h, which was 1 h after cats finished the test meal, and the last blood sample was obtained at 00.30 h the following morning. The test meal contained 448 Kcal per 100 g metabolisable energy and consisted of 30.2% carbohydrate, 33% protein, 22.3% fat and 1.6% crude fibre. Areas under the glucose, insulin and leptin curves (AUC) were calculated by the trapezoidal method (Rowland & Tozer 1989). The curves were calculated from above the lower limit of sensitivity for the individual assays which was 0.3 mg/dl for glucose, 3 μU/ml for insulin and 1 ng/ml HE for leptin.
Sample handling and analysis
All samples were handled similarly. After collection, blood samples were placed into chilled, sterile EDTA vacuettes containing the proteinase inhibitor, aprotinin (Trasylol; Bayer Australia Ltd, Pymble, NSW) added to the vacuettes at 0.05 ml per ml of blood. After centrifugation for 8 min at 1500 g, each plasma sample was split, placed into a 500 μl vials, and stored at −70°C until assayed.
Glucose concentrations were measured in plasma using an automated glucose analyser (YSI 2300 Stat Plus; Yellow Springs Instrument Company, Yellow Springs, OH). Insulin was measured using a commercially available kit, validated for use in cats (Phadeseph Insulin RIA; Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden), (Lutz & Rand 1993). Plasma leptin concentrations were determined with a radioimmunoassay (Multi-species Leptin Radioimmunoassay Kit; Linco Research Inc, St Charles, MO). This kit was developed to quantitate leptin in plasma or serum from several species and has been validated for use in cats (Backus et al 2000). The multi-species leptin antibody in the kit was raised against human leptin and human leptin standards are used in the assay. The manufacturers therefore recommend using ng/ml Human Equivalent or ng/ml HE as the unit of measure.
Statistical analysis
Unless otherwise stated, statistical analyses were performed using a statistical software package (Sigmastat, Version 2.0 for windows; SSPS Software, Chicago, IL). Pearson product moment correlation tests were used to measure the strength of association between plasma leptin, glucose, insulin concentrations and glucose tolerance and insulin sensitivity indices. When comparing leptin concentrations between cats with impaired or normal glucose tolerance, Students t-tests were performed.
Partial regression analysis was performed with SAS (Proc REG, Version 8.0 for windows, SAS Institute Inc, Cary, NC), to examine relationships between plasma leptin concentrations, glucose, insulin and indices of glucose tolerance and insulin sensitivity, independent of adiposity. Before weight gain, body weight and body mass indexes were used as measures of adiposity. After weight gain, measures of adiposity used in statistical analysis included body weight, body mass indexes, and DEXA-derived body fat percent and total body fat mass.
Analysis of covariance (ANCOVA) was performed using SAS (Proc GLM, Version 8.0 for windows, SAS Institute Inc) for the dependent variable leptin. The independent variables used in the model were the presence or absence of glucose intolerance. To adjust for the influence of obesity, each measure of adiposity was included as an independent covariate in the regression analysis.
To investigate whether there were significant changes from baseline in the serial measurements of glucose, insulin or leptin concentrations during the glucose tolerance and meal response tests, paired t-tests were performed. All data are reported as mean±1 standard deviation (SD), followed by range in parentheses. A P value of <0.05 was considered significant.
Results
Glucose tolerance test
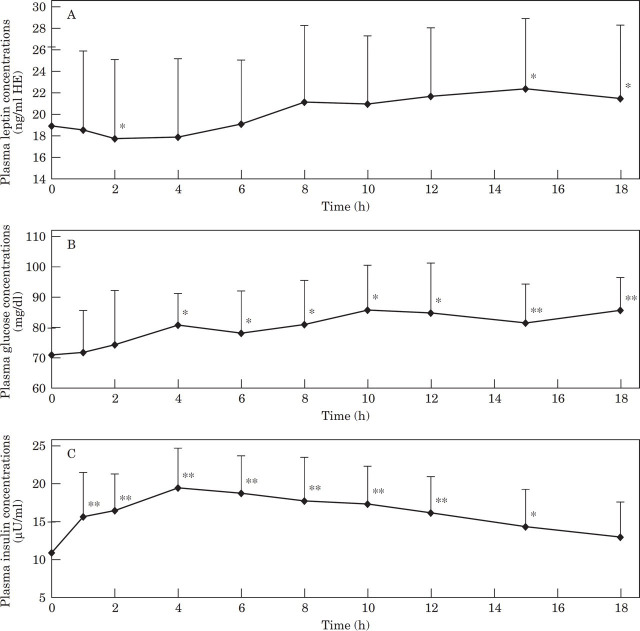
Plasma leptin concentrations were significantly lower than baseline at most time points during the glucose tolerance test both before, and after weight gain, in contrast to the increased glucose and insulin concentrations throughout the test (Fig 1).
Fig 1.
Mean and standard deviation of plasma leptin (A), glucose (B) and insulin (C) concentrations during a glucose tolerance test in 16 cats before and after gaining an average of 44% body weight. Significantly (*P<0.05. **P<0.001) different from baseline concentration. Lean cats, —♦—; obese cats —□—.
In lean cats, fasting plasma leptin concentrations were significantly related to glucose tolerance status as measured by fasting glucose concentrations (r=0.50), and area under the glucose curve after glucose infusion (r=0.69). After controlling for the influence of body weight, these relationships strengthened (r=0.80 and r=0.81, respectively). There was no relationship between fasting leptin and insulin concentrations or derived insulin parameters in lean cats.
In contrast, in overweight cats there was no relationship detected between fasting plasma leptin and parameters of glucose tolerance including fasting plasma glucose, glucose half-life (T1/2) or area under the glucose curve. Also in contrast to lean cats, fasting leptin and insulin parameters were related in overweight cats. After gaining weight, cats with the highest fasting plasma leptin concentrations, had the highest fasting insulin concentrations (r=0.63), area under the insulin curve (r=0.74), and insulin response during the second hour after glucose infusion (r=0.54). After controlling for the effect that body fat percent has on increasing both insulin secretion and leptin concentrations, the relationship with area under the insulin curve and second phase insulin secretion remained significant and were strengthened (increased from r=0.74 to r=0.86 and from r=0.54 to r=84, respectively).
When lean, there was a non-significant trend toward higher baseline leptin concentrations in cats that developed glucose intolerance after gaining weight, compared with those that maintained normal glucose tolerance (8.96±5.34 ng/ml HE and 7.03±2.64 ng/ml HE, respectively). Baseline leptin concentrations in overweight cats also tended to be higher in those with glucose intolerance compared to those with normal glucose tolerance (27.7±13.4 ng/ml HE and 22.1 ±11.1 ng/ml HE, respectively) however, the difference was not significant. After adjusting for body fat percent or total fat mass, this trend was no longer evident.
Insulin sensitivity test
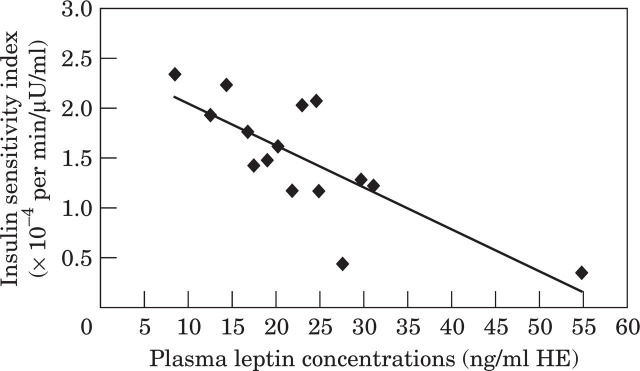
In lean cats, the lower a cat's insulin sensitivity, the higher the fasting leptin concentrations (r= −0.50), a relationship which strengthened after controlling for the effect of body weight (r= −0.79). After cats gained weight, decreased insulin sensitivity (insulin resistance) was also strongly related to elevated leptin concentrations, even after controlling for the influence of body fat percent (r= −0.89) (Fig. 2). However, this relationship was not evident in all cats. Initially, there appeared to be no association between fasting plasma leptin and the insulin sensitivity index in cats after weight gain (r= −0.42, P=0.10). However, the results from one cat appeared to greatly distort the relationship. This cat was atypical in that despite gaining a relatively large amount of weight (1.9 kg or 37% of its bodyweight), its insulin sensitivity did not diminish, its fasting plasma insulin concentration decreased, and it did not develop glucose intolerance. In fact this cat's insulin sensitivity actually improved by 30% (1.92×10−4 min−1/μU/ml to 2.49×10−4 min−1/μU/ml) and its fasting insulin diminished by 12% (11.4 μU/ml to 10.2 μU/ml) with weight gain. If the data from this cat were treated as an anomaly and removed, a strong inverse relationship was evident between leptin and insulin sensitivity in all other cats, which was independent of adiposity (Fig 2).
Fig 2.
Linear regression between baseline plasma leptin concentrations and the insulin sensitivity index (SI) in 15 cats after the removal of one inconsistant data point. The correlation coefficient was −0.77 (P<0.001) prior to controlling for the effect of body fat percent.
After controlling for body fat percent in overweight cats, the higher the leptin concentrations, the larger the acute insulin response to glucose (r=0.92). Leptin concentrations were not associated with glucose effectiveness (SG) either before or after weight gain.
Meal response test
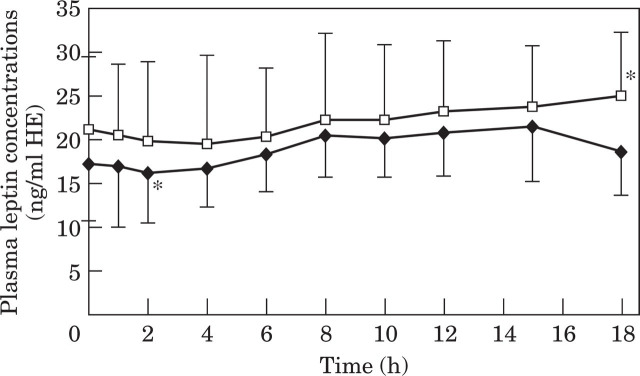
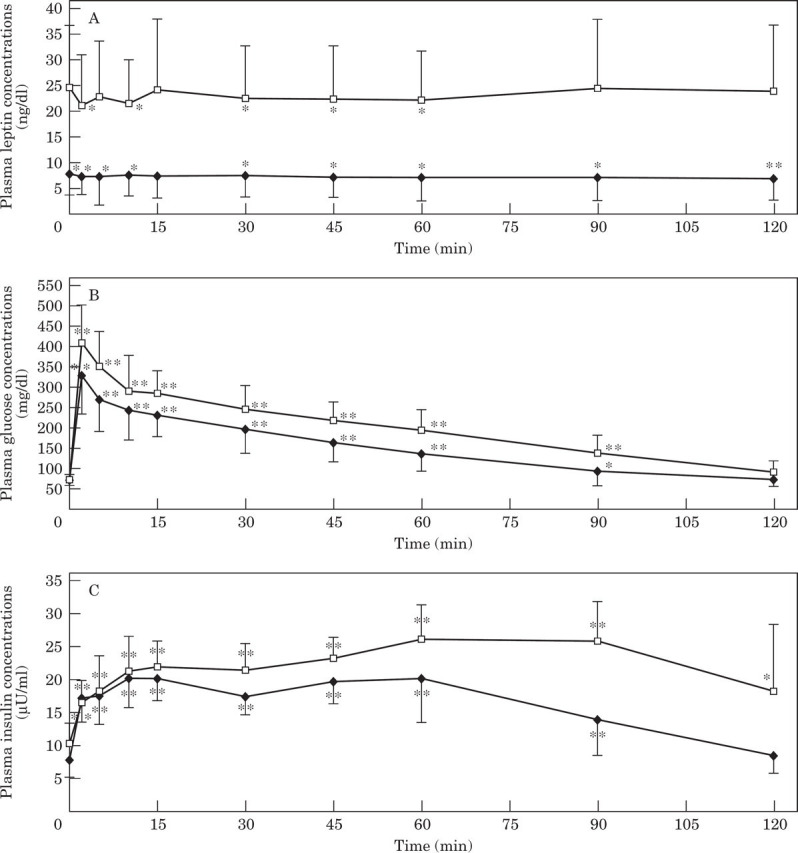
As expected, both glucose and insulin concentrations increased in response to consuming a test meal (average 154 kcal consumed per cat over 15 min), (Fig. 3). In contrast, plasma leptin concentrations initially decreased between baseline and 2 h after the meal, before subsequently increasing until they were significantly higher than baseline 15 and 18 h after the test-meal (Fig. 3). The modal times for peak absolute leptin, glucose and insulin concentrations were 15 h, 18 h and 6 h, respectively. There was a non-significant trend for plasma leptin concentrations to be higher at all sampling times before and after consuming a meal in glucose intolerant cats compared with overweight cats with normal glucose tolerance (Fig. 4). This resulted in overweight glucose intolerant cats having a larger (14%) area under the leptin curve than glucose tolerant cats, however the difference was not significant (22,611 ng/ml. min HE and 19,817 ng/ml. min HE, respectively).
Fig 3.
Mean and standard deviation of plasma leptin (A), glucose (B) and insulin (C) concentrations during a meal response test in 16 obese cats. Significantly (*P<0.05, **P<0.001) different from baseline concentration.
Fig 4.
Mean and standard deviation of plasma leptin concentrations over 18 h during a meal response test in nine obese cats with normal glucose intolerance (NGT, —♦—) and in seven obese cats with impaired glucose tolerance (IGT, —□—). *Significantly different from baseline concentrations.
Discussion
The most important new finding in this study is that in both lean and overweight cats, the higher the leptin concentrations, the more insulin resistant the cat. In both cats and humans, increasing body fat is accompanied by insulin resistance and a compensatory increase in insulin secretion (Kolterman et al 1980, Nelson et al 1990, Appleton et al 2001a), as well as elevated leptin concentrations (Maffei et al 1995, Appleton et al 2000, Backus et al 2000). It could therefore be argued that the relationship between increased leptin and reduced insulin sensitivity is confounded by their shared relationship with body fat. However, partial regression analysis adjusting for body weight in lean cats and body fat percent in overweight cats, revealed that the association between these factors not only remained, but was strengthened. Similarly in humans, high plasma leptin levels are associated with insulin resistance, regardless of the level of adiposity (Segal et al 1996, Nyholm et al 1997, Zimmet et al 1998). These data suggest that leptin overproduction may contribute to the diminished insulin sensitivity seen in overweight cats and humans or alternatively, the compensatory hyperinsulinaemia found with insulin resistance could stimulate leptin production.
Interestingly, the relationship between elevated plasma leptin levels and reduced insulin sensitivity, after adjusting for body fat percent, did not initially appear to be significant in overweight cats. On further examination of the data, it was evident that one cat's basal insulin concentration did not increase with weight gain, its insulin sensitivity did not diminish, and it maintained normal glucose tolerance. In contrast, the elevated fasting leptin concentration in this cat (47.04 ng/ml HE), was entirely expected considering it gained 1.9 kg, resulting in an obese body weight of 7.1 kg, 56% of which was adipose tissue. Leptin levels have consistently been shown to correlate with body fat in humans, rodents and recently, also in cats (Lönnqvist et al 1995, Maffei et al 1995, Considine et al 1996, Appleton et al 2000, Backus et al 2000). Re-analysing the data without this cat, demonstrated a strong relationship (r= −0.89), between diminished insulin sensitivity and elevated leptin concentrations independent of the percentage body fat percent.
The inconsistent data from this one cat is similar to what is reported in humans. Although most humans develop insulin resistance with obesity, obesity does not always result in the development of insulin resistance, and in fact, some obese humans are quite insulin sensitive (Taniguchi et al 2000). This cat's phenotype may be better suited to the modern lifestyle of excessive food intake and physical inactivity than the majority of cats, which develop insulin resistance and hyperinsulinaemia with obesity.
Although we have demonstrated a strong relationship between leptin and insulin resistance in cats, it is still unclear whether leptin is causally involved in this relationship. In obese humans, increased secretion of leptin from adipose tissue has been postulated to be a mechanism by which weight gain causes insulin resistance (Taylor et al 1996). In vitro, leptin has been shown to impair important metabolic actions of insulin in isolated rat fat cells including stimulation of glucose transport, glycogen synthase, lipogenesis and protein synthesis (Müller et al 1997). Leptin also inhibits insulin binding to insulin receptors in isolated rat fat cells (Walder et al 1997). Although the exact mechanism of leptin-induced insulin resistance is unknown, it is postulated to occur early in the insulinsignalling cascade, close to the insulin receptor, and may involve signalling factors (Müller et al 1997). In hepatocytes, leptin was found to attenuate some of insulin's signals and reduce suppression of gluconeogenesis (Cohen et al 1996). If these effects are also present in vivo, they represent mechanisms by which increased leptin levels could contribute to the pathophysiology of insulin resistance in obesity and thus possibly to type 2 diabetes mellitus.
A recent study suggests that glucose transport into fat cells may be an important determinant of insulin sensitivity in muscle and liver cells (Able et al 2001). Mice that were genetically modified to have selective depletion of the glucose transporter GLUT 4 in fat cells, had markedly reduced insulin-stimulated glucose uptake, not only in fat cells, but also in muscle and liver (Able et al 2001). Importantly, humans with obesity or type 2 diabetes have also been found to have a selective decrease in the levels of GLUT 4 in fat cells (DeFronzo 1997, Shepherd & Kahn 1999). Based on the study in mice, it was postulated that when insulin-stimulated glucose uptake into fat cells is impaired, fat cells release a molecule that secondarily induces insulin resistance in muscle and liver. Shortly after this report, a new protein called resistin was identified, which is secreted by fat cells and causes insulin resistance in muscle and liver (Steppan et al 2001). Resistin is postulated to be one of the key factors mediating the insulin resistance of obesity. It is interesting to speculate whether the reported in vitro effect of leptin to impair glucose transport into adipocytes is the trigger for the release of resistin, and whether this occurs as a result of leptininduced depletion of GLUT 4 in fat cells. If this mechanism occurs, it would link leptin, resistin and obesity-induced insulin resistance. While the exact connection between obesity and insulin resistance is still to be elucidated, these studies indicate that adipocyte-secreted proteins such as leptin, resistin, and/or other fat-factors secreted from GLUT 4-depleted adipocyte cells are involved.
Studies in humans indicate that insulin has a role in chronic, but not acute regulation of leptin concentrations. There appears to be a lag time of between 4 and 6 h before the impact of consuming a high carbohydrate meal (Mohamed-Ali et al 1996, Coppack et al 1997, Dallongeville et al 1998, Havel et al 1999), or infusions of glucose or insulin (Sonnenberg et al 1996, Utriainen et al 1996, Saad et al 1998a), can be seen on leptin secretion in humans. This delay can extend out to 12–16 h if a lower glucose infusion rate is used (Sonnenberg et al 1996). Thus, the magnitude and the promptness of the response of plasma leptin to feeding and subsequent insulin release in humans, appears to be dependent on the dose and duration of exposure to glucose and insulin (Sonnenberg et al 1996). In our study, insulin secretion induced by feeding did not appear to affect leptin secretion in overweight cats until 15–18 h after consumption of the meal. The test meal in our study contained 30% carbohydrate (as fed basis), and cats consumed only 50% of their daily energy requirements. This relatively small meal stimulus may explain the longer post-prandial delay in leptin increase in our study, compared with studies in humans fed high carbohydrate meals. Also, cats have been shown to have a smaller and more prolonged increase in post-prandial glucose, compared to humans and dogs (Bouchard & Sunvold 2000), which may delay leptin secretion. In normal cats, glucose and insulin concentrations did not return to baseline until 18 h after feeding, compared to between 4 and 6 h in humans and dogs (Bouchard & Sunvold 2000). Based on human data, leptin concentrations in the cats would have been expected to fall at 15–18 h rather than increase, as the cats were fasted for 18 h after they consumed the test meal. In humans extended fasting is associated with a significant decline in plasma leptin concentrations (Sonnenberg et al 1996, Pratley et al 1997). Therefore, the significant post-prandial increase in leptin concentrations seen in our overweight cats at 15–18 h is likely due to the delayed postprandial increase in insulin concentrations. Our results suggest that insulin is involved in increasing leptin secretion in cats, as it is in other species. Thus, conditions characterised by insulin resistance and hyperinsulinaemia such as obesity, would be expected to have concurrent hyperleptinaeima.
Based on studies in other species, it would be unlikely to observe any direct effects of acute elevations of glucose or insulin on plasma leptin during the relatively short, 2-h duration of the glucose tolerance test. A physiological explanation for the acute decline (commencing at 2 min after glucose infusion) in plasma leptin concentrations during the glucose tolerance test is that the osmotically active glucose dose may have resulted in haemodilution, thus causing a relative lowering of plasma leptin concentrations throughout the test.
Studies in humans and rats have demonstrated that leptin secretion follows a diurnal rhythm, rising nocturnally to peak around midnight and the early morning, before declining gradually to reach a late morning or early afternoon nadir (Saladin et al 1995, Sinha et al 1996, Saad et al 1998b). This nocturnal rise of leptin appears to be related to meal-induced insulin excursions in humans (Laughlin & Yen 1997, Saad et al 1998b), and does not occur if the subjects are fasted (Boden et al 1996). In our study, cats consumed their test meal between 06.15 to 06.30 h. Subsequently, leptin concentrations decreased during the morning, before rising back to baseline and eventually peaking 15–18 h after the meal was consumed. This variation coincides with a daytime nadir and a night time peak similar to that seen in humans. The initial fall in leptin levels after eating may simply be reflective of a normal diurnal rhythm, with the levels continuing to decline during the morning from a nocturnal peak. Thus when cats are meal-fed, plasma leptin secretion may also undergo diurnal variations related to food intake. However, additional studies in cats are required to investigate this theory further.
Although this study has shown a relationship between leptin and insulin resistance independent of adiposity, it is not known whether leptin is causally involved. If future studies prove leptin to contribute to insulin resistance, research efforts may be directed at decreasing excessive leptin secretion in an attempt to minimise insulin resistance. For instance, diets that result in the lowest 24-h post-prandial insulin and glucose excursions would be expected to reduce daily leptin secretion. Studies have shown that humans consuming a high carbohydrate, low fat diet (60% and 20% respectively) have higher overall insulin and glycaemic responses, and higher 24 h leptin secretion compared with subjects consuming low carbohydrate, high fat diets (20% and 60% respectively), (Havel et al 1999).
Some brands of dry cat food regularly incorporate carbohydrate at this level in their formulations. Studies in cats have also shown that feeding diets with starch sourced from sorghum, corn or barley result in lower insulin responses than those based on rice (Bouchard & Sunvold 2000), and may therefore result in lower leptin concentrations. Furthermore, vitamin A supplementation at levels well below the AAFCO recommended maximum, has been shown to minimise weight gain and to reduce plasma leptin levels by 41% in cats fed a high-fat diet (Scarpace et al 2000, American Feed Control Officials Inc., 2000 No. 915). It is therefore possible that formulating a diet with lower overall carbohydrate content and/or changing to a starch source such as sorghum or corn, in combination with vitamin A supplementation, may result in lower overall leptin secretion, thereby improving insulin sensitivity. Further studies in cats are required to explore this theory.
Conclusion
This is the first study to demonstrate that increased plasma leptin concentrations are associated with decreased insulin sensitivity in cats, independent of adiposity. Whether this relationship involves elevated leptin concentrations contributing to the insulin resistance in obesity, or whether insulin resistance and its associated hyperinsulinaemia stimulate leptin secretion is unknown.
The change in plasma leptin concentrations in response to feeding overweight cats may reflect a delayed influence of meal-stimulated insulin secretion, possibly with a superimposed diurnal rhythm. However, species differences exist for leptin regulation, and mechanisms of control in cats are currently unknown.
In summary, these results suggest that there may be a possible physiological role for leptin in the link between obesity and insulin resistance. Further studies are needed to determine whether the hyperleptinaemia/insulin resistance relationship has a role in the natural history of obesity and type 2 diabetes.
Acknowledgements
Appreciation is expressed to Rachael Price, Emma Bennett and Rebekah Wilson for their assistance with animal care and data collection, to Lyn Knott for her help with sample analysis, to Jan Priest for her statistical advice and to The Iams Company for funding this project.
References
- Able DE, Peroni O, Kim JK, Kim Y, Boss O, Hadro E, Minnemann T, Shulman S, Kahn BB. (2001) Adipose-selective targeting of the GLUT 4 gene impairs insulin action in muscle and liver. Nature 409, 729–733. [DOI] [PubMed] [Google Scholar]
- Appleton DJ, Rand JS, Sunvold GD. (2000) Plasma leptin concentrations in cats: Reference range, effect of weight gain, and relationship with adiposity as measured by dual energy X-ray absorptiometry. Journal of Feline Medicine and Surgery 2, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton DJ, Rand JS, Sunvold GD. (2001a) Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain. Journal of Feline Medicine and Surgery 3, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton DJ, Rand JS, Sunvold GD, Priest J. (2001b) Determination of reference values for glucose tolerance, insulin tolerance and insulin sensitivity tests in clinically normal cats. American Journal of Veterinary Research 62, 630–636. [DOI] [PubMed] [Google Scholar]
- Backus RC, Havel PJ, Gingerich RL, Rogers QR. (2000) Relationship between serum leptin immunoreactivity and body fat mass as estimated by use of a novel gas-phase fourier transform infrared spectroscopy deuterium dilution method in cats. American Journal of Veterinary Research 61, 796–801. [DOI] [PubMed] [Google Scholar]
- Beard JC, Bergman RN, Ward WK, Porte DJ. (1986) The insulin sensitivity index in nondiabetic man: Correlation between clamp-derived and IVGTT-derived values. Diabetes 35, 362–369. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C. (1979) Quantitative estimation of insulin sensitivity. American Journal of Physiology 236, E667–E677. [DOI] [PubMed] [Google Scholar]
- Biourge V, Nelson RW, Feldman EC, Willits NH, Morris JG, Rogers QR. (1997) Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. Journal of Veterinary Internal Medicine 11, 86–91. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Mozzoli M, Ryan I. (1996) Effect of fasting on serum leptin in normal human subjects. Journal of Clinical Endocrinology and Metabolism 81, 3419–3423. [DOI] [PubMed] [Google Scholar]
- Bouchard GF, Sunvold GD. (2000) Effect of dietary carbohydrate source on postprandial plasma glucose and insulin concentration in cats. In: Recent Advances in Canine and Feline Nutrition. Reinhart GA, Carey DP. (eds). Wilmington, Ohio; Orange Frazer Press, pp. 91–101. [Google Scholar]
- Butterwick R. (2000) Considerations for weight reduction programs. The 2000 Purina Nutrition Forum, pp. 78. [Google Scholar]
- Cohen B, Novick D, Rubenstein M. (1996) Modulation of insulin activities by leptin. Science 275, 1185–1188. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MP, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. (1996) Serum immunoreactive leptin concentrations in normal-weight and obese humans. New England Journal of Medicine 334, 292–295. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Stanner S, Rawesh A, Goodrick S, Mohamed Ali V. (1997) In vivo adipose tissue leptin production before and after a high carbohydrate meal (Abstr.). International Journal of Obesity 21(Suppl 2), S33. [Google Scholar]
- Dallongeville J, Hecquet B, Lebel P, Edme J-L, Le Fur C, Fruchart J-C, Auwerx J, Romon M. (1998) Short term response of circulating leptin to feeding and fasting in man: Influence of circadian cycle. International Journal of Obesity 22, 728–733. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. (1997) Pathogenesis of type 2 diabetes: Metabolic and molecular implications for identifying diabetes genes. Diabetes Reviews 5, 177–269. [Google Scholar]
- Finegood DT, Hramiak IM, Dupre J. (1990) A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. Journal of Clinical Endocrinology and Metabolism 70, 1538–1549. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Tzur D. (1996) Reduced glucose effectiveness associated with reduced insulin release: An artifact of the minimal-model method. American Journal of Physiology 271, E485–E495. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Mykkäänën L, Karhapää P, Rainwater DL, Laakso M. (1997) Leptin concentrations and insulin sensitivity in normoglycaemic men. International Journal of Obesity 21, 393–399. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Townsend R, Chaump L, Teff K. (1999) High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 48, 334–341. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Nyce NR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. (1996) Acute and chronic effect of insulin on leptin production in humans. Diabetes 45, 699–701. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Insel J, Saekow M, Olefsky JM. (1980) Mechanisms of insulin resistance in human obesity. Journal of Clinical Investigation 65, 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Elmståhl S, Ahrén B. (1996) Plasma leptin levels correlate to islet cell function independently of body fat in postmenopausal women. Diabetes 45, 1580–1584. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Yen SSC. (1997) Hypoleptinemia in women athletes: Absence of diurnal rhythm with amenorrhea. Journal of Clinical Endocrinology and Metabolism 82, 318–321. [DOI] [PubMed] [Google Scholar]
- Link KRJ, Rand JS. (1998) Reference values for glucose tolerance and glucose tolerance status in cats. Journal of the American Veterinary Medical Association 213, 492–496. [PubMed] [Google Scholar]
- Lönnqvist F, Arner P, Nordfors L, Schalling M. (1995) Overexpression of the obese (ob) gene in adipose tissue of obese human subjects. Nature Medicine 1, 950–953. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1993) Comparison of five radioimmunoassay kits for the measurement of feline insulin. Research in Veterinary Science 55, 64–69. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. (1995) Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine 1, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick SJ, Stanner SA, Rawesh A, Yudkin JS, Coppack SW. (1996) Adipose tissue leptin production at fasting and after a high carbohydrate meal (Abstr.). Diabetic Medicine 13, S19.8894476 [Google Scholar]
- Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ. (1998) Evidence that glucose metabolism regulates plasma leptin secretion from cultured rat adipocytes. Endocrinology 139, 551–558. [DOI] [PubMed] [Google Scholar]
- Müller G, Ertl J, Gerl M, Preibisch G. (1997) Leptin impairs metabolic actions of insulin in rat adiposites. Journal of Biological Chemistry 272, 10585–10593. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Himsel CA, Feldman EC, Bottoms GD. (1990) Glucose tolerance and insulin response in normal-weight and obese cats. American Journal of Veterinary Research 51, 1357–1362. [PubMed] [Google Scholar]
- Nyholm B, Fisker S, Lund S, Møller N, Schmitz O. (1997) Increased circulating leptin concentrations in insulin-resistant first-degree relatives of patients with non-insulin-dependent diabetes mellitus: Relationship to body composition and insulin sensitivity but not to family history of non-insulin-dependent diabetes mellitus. European Journal of Endocrinology 136, 173–179. [DOI] [PubMed] [Google Scholar]
- Panciera DL, Thomas CB, Eiker SW, Atkins CE. (1990) Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). Journal of the American Veterinary Medical Association 197, 1504–1508. [PubMed] [Google Scholar]
- Pratley RE, Nicolson M, Bogardus C, Ravussin E. (1997) Plasma leptin responses to fasting in Pima Indians. American Journal of Physiology 273, E644–E649. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. (1989) Assessment of Area. In: Clinical Pharmacokinetics: concepts and applications. Philadelphia, Lea and Febiger, pp. 459–471. [Google Scholar]
- Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V. (1998a) Physiological insulinemia acutely modulates plasma leptin. Diabetes 47, 544–549. [DOI] [PubMed] [Google Scholar]
- Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, Boyadjian R, Steil GM. (1998b) Diurnal and ultradian rhythmicity of plasma leptin: Effects of gender and adiposity. Journal of Clinical Endocrinology and Metabolism 83, 453–459. [DOI] [PubMed] [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. (1995) Transient increase in obese gene expression after food intake or insulin administration. Nature 377, 527–529. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Kumar MV, Bouchard GF, Sunvold GD. (2000) Dietary vitamin A supplementation: Role in obesity and leptin regulation in the dog and cat. In: Recent Advances in Canine and Feline Nutrition. Reinhart GA, Carey DP. (eds). Wilmington, Ohio, Orange Frazer Press, pp. 103–111. [Google Scholar]
- Segal KR, Landt M, Klein S. (1996) Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 45, 988–991. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB. (1999) Glucose transporters and insulin action-implications for insulin resistance and diabetes mellitus. New England Journal of Medicine 341, 248–257. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Herman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JC. (1996) Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. Journal of Clinical Investigation 97, 1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GE, Krakower RG, Hoffman RG, Maas DL, Hennes MMI, Kissebah AH. (1996) Plasma leptin concentrations: Effects of extended fasting and stepwise increases in glucose infusions (Abstr.). Obesity Research 4, 13S. [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. (2001) The hormone resistin links obesity to diabetes. Nature 409, 307–312. [DOI] [PubMed] [Google Scholar]
- Sunvold GD, Bouchard GF. (1998) Assessment of obesity and associated metabolic disorders. In: Recent Advances in Canine and Feline Nutrition Volume 2. Reinhart GA, Carey DP. (eds). Wilmington, Ohio, Orange Frazer Press, pp. 135–148. [Google Scholar]
- Taniguchi A, Fukushima M, Sakai M, Doi K, Arakawa H, Nagasaka S, Tokuyama K, Nakai Y. (2000) The role of body mass index and triglyceride levels in identifying insulinsensitive and insulin-resistant variants in Japanese non-insulin-dependent diabetic patients. Metabolism: Clinical and Experimental 49, 1001–1005. [DOI] [PubMed] [Google Scholar]
- Taylor SI, Barr V, Reitman M. (1996) Does leptin contribute to diabetes caused by obesity? Science 274, 1151–1152. [DOI] [PubMed] [Google Scholar]
- Utriainen T, Malmström R, Mäkimattila S, Yki-Järvinen H. (1996) Supraphysiological hyperinsulinaemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes 45, 1364–1366. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H. (1996) Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes 45, 1435–1438. [DOI] [PubMed] [Google Scholar]
- Walder K, Filippis A, Clarke S, Zimmet P, Collier G. (1997) Leptin inhibits insulin binding in isolated rat adipocytes (Abstr.). Diabetologia 40, A176. [DOI] [PubMed] [Google Scholar]
- Zimmet PZ, Collins VR, de Courten MP, Hodge AM, Collier GR, Dowse GK, Alberti KGMM, Tuomilehto J, Hemraj F, Gareeboo H, Chitson P, Fareed D. (1998) Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. International Journal of Obesity 22, 171–177. [DOI] [PubMed] [Google Scholar]