Abstract
This retrospective study describes the clinicopathological findings in five cats with soft tissue mineralisation of interdigital spaces and footpads. Paw disease was the reason for veterinary consultation in three out of five cats. All cats had laboratory findings suggestive of renal failure and high solubility product [calcium x phosphorus]. In all cases, cytological examination of paw lesions was suggestive of calcinosis. The results of our study agree with two previous case reports of paw calcification in the cat, suggesting a metastatic pathogenesis and a correlation between paw mineralisation and renal failure.
This retrospective study includes five client-owned cats. Age, sex, breed, history, principal presenting signs, abnormal clinical findings and follow-up were recorded and are summarised in Table 1.
Table 1.
Signalment, history, principal clinical findings, follow-up and significant laboratory results in five cats with cutaneous calcinosis of paw
| Signalment | Cat 1 | Cat 2 | Cat 3 | Cat 4 | Cat 5 |
|---|---|---|---|---|---|
| DLH, FS, 13y | DSH, NM, 18m | DSH, NM, 7y | DSH, FS, 5y | DSH, FS, 3y | |
| History and reasons for veterinary consultation | Chronic renal failure diagnosed 6 months before. Generalised paw disease and lameness | Lameness, reluctant to move, progressive enlargement of all footpads, ulcerated interdigital nodules on all paws, anorexia and polyuria/polydipsia | Ulcerated nodules on paws noted two months before, anorexia and depression, polyuria/polydipsia | Progressive weight loss, anorexia and finally vomiting | Two months history of severe protein-losing nephropathy. Progressive weight loss, anorexia and finally vomiting |
| Principal clinical findings | Poor body condition, 5–7% dehydrated, pale mucous membranes, enlargement of all paws and footpads, firm and painful ulcerated nodules on the ventral aspect of paws | Poor body condition, unable to maintain a standing position, pale mucous membranes, painful footpads, firm ulcerated interdigital and footpads nodules | Poor body condition, hypothermic, severely dehydrated (10–12% estimated), pale mucous membrane, several hard nodules in interdigital spaces of all paws | Poor body condition, 5–7% dehydrated, pale mucous membranes, firm ulcerated nodules in interdigital spaces and footpads of all paws | Poor body condition, bilateral symmetrical nephromegaly, small (up to 5 mm) white nodules in multiple footpads |
| Follow-up | Euthanased after 4 months | Slight improvement of paw disease after 4 weeks. Euthanized after 4 months | Euthanased after 2 weeks | Unchanged after 3 months | Euthanased after 2 months |
| Significant laboratory results | |||||
| PCV (RR 30–45%) | 27.3 | 18.3 | 18.0 | 15.1 | 27.9 |
| RBC × 1012/1 (RR 5.0–10.0) | 5.98 | 3.4 | 3.8 | 2.6 | 5.0 |
| Urea (RR 5–11 mmol/l) | 29.9 | 31.3 | 83.7 | 32.3 | 55.8 |
| Creatinine (RR 30–130 μmol/l) | 194.5 | 221 | 459.7 | 283 | 654.2 |
| Phosphorus (RR 0.5–1.6 mmol/l) | 2.6 | 2.3 | 3.6 | 4.1 | 9.4 |
| Calcium (RR 2.0–2.75 mmol/l) | 3.4 | 2.6 | 2.7 | 2.1 | 1.5 |
| Solubility product * | 111.5 | 72.4 | 119.8 | 104 | 178.7 |
| [calcium × phosphorus] | |||||
| (RR<70) | |||||
| PTH (pmol/l) | 19.6 (RR 0.1–5.0) | NP | NP | 7.1 (RR 0.1–1.5) | 1.1 (RR 0.1–1.5) |
| Urinalysis (relevant findings) | USG 1.026 (RR 1.035–1.060) | USG 1.025 (RR 1.035–1.060) | NP | USG 1.015 (RR 1.035–1.060) | USG 1.019 (RR 1.035–1.060) Persistent proteinuria on urine dipstick (Combur Test, Roche) † |
DSH, domestic shorthaired cat; DLH, domestic longhaired cat; SF, spayed female; NM, neutered male; y, years; m, months; RR, reference range; USG, urine specific gravity; NP, not performed.
Product is calculated with calcium and phosphorus concentration expressed in mg/dl.
protein >5 g/1.
Paw disease was the primary complaint in cats 1, 2 and 3. Cat 3 was evaluated because of anorexia, severe weight loss and polyuria/polydipsia. However, the referring veterinarian had evaluated this cat two months earlier for the presence of several ulcerated interdigital nodules. These lesions did not improve after three different courses of oral antibiotic treatments with amoxicillin/clavulonate, enrofloxacin and cefalexin respectively. Cats 4 and 5 were presented because of progressive weight loss, anorexia and finally vomiting and paw lesions were observed only during physical examination. In cases 1 through 4, owners were unable to ascertain the onset of paw disease, whereas in cat 5 paw mineralisation occurred in the end-stage of a protein losing nephropathy (PLN).
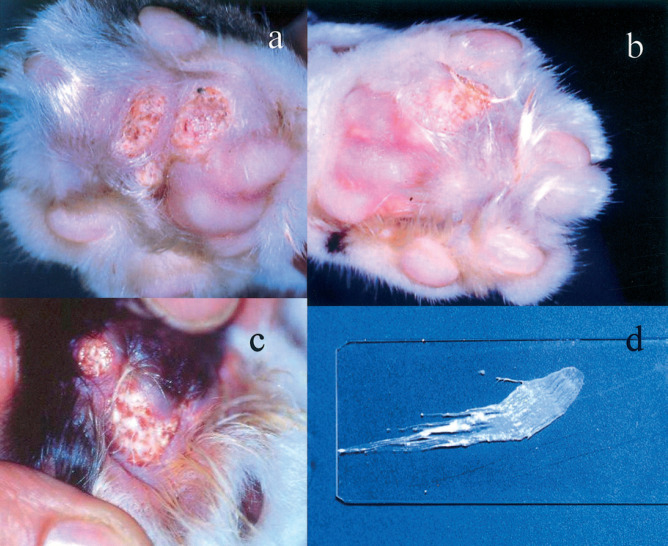
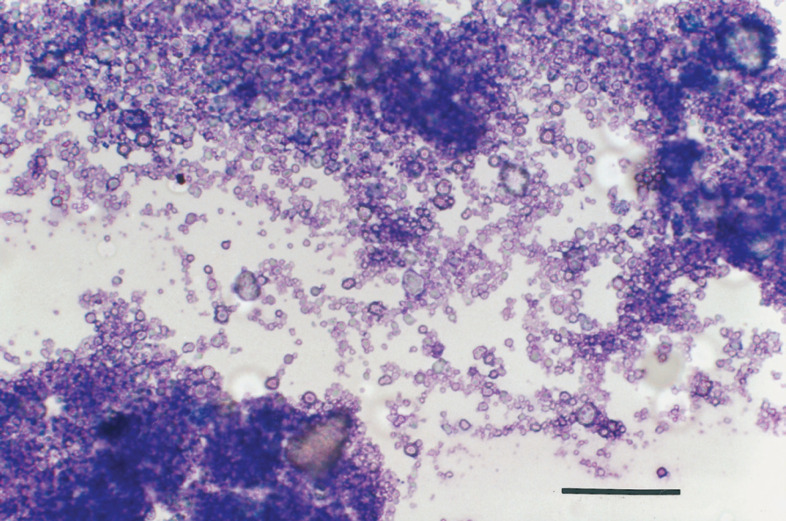
Paw calcifications appeared as intact or ulcerated nodules involving multiple interdigital spaces and footpads (Fig 1a-c). These nodules ranged from a few millimetres to 2 cm in size and were firm on palpation. A white to grey, purulent-like, pasty material drained from the palmar or plantar side in several lesions, except for cat 5, the nodules of which were small (less than 5 mm) and intact. In all cases multiple samples were taken from several paws to obtain smears for cytology. Material from these lesions was obtained by fine needle aspiration biopsy, scraping and imprint. All smears were stained with a Romanowsky-type stain (May-Grünwald-Giemsa or Hemacolor®; Merck). A diagnosis of cutaneous calcinosis was based on microscopic appearance of cytological smears. During preparation, smears gave a gritty sensation. Once dried on slides, smears had a chalky macroscopic appearance (Fig 1d). Microscopically, they showed a large amount of amorphous granular, dark-grey to bluish particulate material, along with larger fragments of glassy refractile material that failed to take up any stain (Fig 2). This was consistent with calcium salts deposits. Rare inflammatory cells (neutrophils, macrophages and spindle cells) were found in some smears. Extracellular bacteria (cocci and rods) were observed in smears obtained by imprint. Occasional giant multinucleated cells were found in samples from cat 4. Cytological findings were suggestive of cutaneous calcinosis in all cases. Cats 3, 4 and 5 also had cutaneous histopathology of paws performed on biopsy material or after necropsy. On histology, multiple spherical loculi demarcated by a fibrous stroma were seen. These loculi contained an amorphous, granular basophilic, von Kossa-positive material. Fibrous stroma sometimes contained lymphoid, epithelioid and occasional multi-nucleated cells. These findings were consistent with a histological diagnosis of calcinosis circumscripta.
Fig 1.
Paw calcifications in the cat. (a) Through (c) multiple interdigital, ulcerated nodules are evident. Sometimes they discharged a white, pasty material (c). Once dried on a slide, the smear had a chalky-like appearance (d).
Fig 2.
Photomicrograph of a smear obtained by fine needle aspiration biopsy from an interdigital nodule in a cat with paw calcifications. A large amount of amorphous granular, dark-grey to bluish particulate material is evident, along with larger fragments of glassy refractile material that failed to take up any stain. This is suggestive of calcium salts deposits (400 x. Hemacolor® stain, Merck; bar equal to 50 μm).
Complete blood count and serum biochemistry were performed on all cats, while urinalysis was performed on cats 1, 2, 4 and 5. Significant laboratory findings are reported in Table 1. All cats had laboratory results suggestive of chronic renal failure (CRF). In cat 5 renal failure was associated with a PLN. All cats had anaemia, azotaemia and hyperphosphataemia. In all cases the solubility product [calcium x phosphorus] [SP(CaxP)] was above 70. Cat 5 had also persistent mild hypoalbuminaemia, ranging from 19 g/l to 23 g/l on several evaluations (normal range 25–40 g/l). Urinalysis (evaluated by commercial dipstick, see Table 1) showed evidence of significant proteinuria in only one cat (cat 5), and urine sediment was unremarkable in all four cases examined. Serum parathyroid hormone (PTH) level was determined in cats 1, 4 and 5: it was above the reference range in cats 1 and 4, and in the normal range in cat 5. Results of testing for feline immunodeficiency virus and feline leukaemia virus were available for cats 4 and 5 and they were negative.
Lateral and dorsopalmar radiographs of front feet were performed on cat 4: diffused soft tissue mineralization of footpads and interdigital space was evident. Thoraco-abdominal radiographs were performed on cats 4 and 5 and they showed soft tissue mineralization in thoracic and abdominal aorta and in the gastric wall (only cat 4). An irregular, 1.5 to 3 cm subcutaneous interscapular area of calcification was palpable and radiographically evident in both cats 4 and 5. In cat 4, a fine needle aspiration of this area revealed the same acellular material observed in cytological smears from paws. In cat 5, this lesion was examined only by postmortem histopathology and consisted of multifocal to coalescing areas of calcification with necrosis of subcutis and muscular layers.
Cats were treated with a combination of intravenous (IV) and subcutaneous (SQ) cristalloids fluids such as 0.9% saline solution or lactated Ringer's solution (cats 1, 3, 4 and 5), ranitidine (Ranidil; Menarini) 2 mg/kg/12 h IV, SQ or orally (cats 3, 4 and 5), aluminium hydroxide (Diplogel; Formenti) 10 mg/kg/8 h orally (cat 3), prednisone (Predsolan; Schering-Plough) 2 mg/kg/24 h SQ (cat 5), and benazepril (Fortekor; Ciba-Geigy) 0.5 mg/kg/24 h orally (cat 5). In all cases, a low phosphorus/low protein diet was prescribed. However, food intake was considered unsatisfactory in all cats. A moderate improvement in paw disease was noted only in cat 2 four weeks after presentation: nodules were reduced in size and lameness was less evident. In the other cats, the lesions remain unchanged. Owners requested euthanasia in all cases but four, from two weeks to four months after initial presentation. At the time of writing (three months after diagnosis) cat 4 is still alive. Necropsy could be performed only in case 5. Confluent plaques of calcification of the tunica media of the aorta and other main arteries, end-stage kidney with multifocal tubular calcification, alveolar emphysema with diffuse calcification of arteries and capillaries and mild multifocal hepatic necrosis with subacute to chronic inflammatory infiltration were the relevant findings.
In the cat, paws and footpads diseases can be caused by localised or systemic disorders and local or metastatic neoplasm. Reported causes in literature are: inflammatory or immune-mediated diseases (microbial, fungal or immune-mediated pododermatitis; plasmacellular pododermatitis; allergic pododermatoses; eosinophilic granuloma complex; uremic vasculitis; ‘drug eruption’; parasitic pododermatoses), irritant contact pododermatitis, endocrinopathies (hypothyroidism, Cushing's syndrome, diabetes mellitus) and neoplasm: primary (papillomas, squamous cell carcinoma, tricoepithelioma, fibrosarcoma, fibrous malignant histiocytoma) or metastatic (e.g. from lung carcinoma) (Gauguère et al 1992). This paper shows that cutaneous calcinosis of paws should also be considered as an additional differential diagnosis.
Cutaneous calcifications can be classified as dystrophic, metastatic, iatrogenic or idiopathic (Walsh & Fairley 1995, Jackson & Barber 1998, Schaer et al 2001). Dystrophic calcification occurs as a result of local tissue damage (Walsh & Fairley 1995). In dogs and cats, this can occur in foreign body granulomas, interdigital pyoderma, demodicosis, follicular cysts, pilomatrixomas, hyperadrenocorticism and diabetes mellitus (Scott et al 1995, Schaer et al 2001). Metastatic calcifications are associated with disorders of calcium and phosphorus homeostasis leading to a SP(CaxP) greater than 70 (Walsh & Fairley 1995, Jackson & Barber 1998, Schaer et al 2001). Blood vessels, lung, gastric mucosa, kidneys, cutaneous and subcutaneous tissues may be involved (Schaer et al 2001). Any disorders that cause hypercalcaemia and/or hyperphosphataemia (hypervitaminosis D, lymphomas, multiple myeloma, carcinomas, renal diseases, systemic blastomycosis) can be responsible for metastatic calcification (Schaer et al 2001). Iatrogenic calcinosis cutis has been described as a result of percutaneous injection of calcium containing drug (calcium gluconate) in one dog (Schaer et al 2001) and one cat (Ruopp 2001). If no cause can be determined, cutaneous calcification is considered idiopathic (Walsh & Fairley 1995, Jackson & Barber 1998).
Complex derangement in calcium, phosphorus and Vitamin D3 metabolism frequently occurs in feline chronic renal diseases, resulting in hyperphosphataemia and high PTH serum levels. (Barber & Elliott 1998, Barber et al 1999; Polzin et al 2000). In a recent study, 84% of 80 cats affected by CRF had high PTH levels (Barber & Elliott 1998). In cats 1 and 4, renal secondary hyperparathyroidism (RHPTH) was confirmed by high PTH level, whereas in cat 5 PTH was in the normal range.
In our series, all cats had moderate to severe renal failure, high SP(CaxP) and cutaneous calcification involving only the paws. The association between paw mineralization and renal failure, found in our study, agree with previous reported cases of footpads calcification in seven dogs (Cordy 1967, Legendre & Dade 1974, Kowaliewich & Hawkins 1992, Gross 1997) and in two cats (Bohmer et al 1991, Jackson & Barber 1998). In fact, calcinosis of footpads has been reported only once in a dog not affected by CRF (Stampley & Bellah 1990). Our findings suggest a metastatic pathogenesis for paw mineralisation, possibly supported by a traumatic dystrophic effect on the feet that could occur during stance and gait, as suggested by Jackson & Barber (1998).
Serum calcium was normal in cat 2, 3 and 4, and low in cat 5. Soft tissue calcifications can also occur with low-normal serum calcium, if phosphataemia is high enough to lead to an elevated SP(CaxP), as in the cases reported by Legendre & Dade (1974) and by Bohmer et al (1991).
In the case reported by Jackson & Barber (1998), paw calcifications regressed three months after the cat had been put on a low phosphorus/low protein diet (Feline Low Protein Diet; Waltham), and SP(CaxP), phosphataemia and PTH level decreased significantly. Four out of the five cats described in our report had, however, a poor prognosis. This could be explained by insufficient intake of specific diets (all cats) and by an advanced stage of the renal disease (cats 3 and 5).
Subcutaneous calcification in the interscapular space observed in cats 4 and 5, could reflect a phenomenon termed calciphylaxis in humans (Walsh & Fairley 1995, Essary & Wick 2000). This condition is a kind of metastatic calcification that can be found also with a SP(CaxP) less than 70 (Walsh & Fairley 1995). Calciphylaxis can occur after exposing a patient to a sensitising agent (e.g., PTH, vitamin D, high calcium-phosphate product) and then administering topical or systemic products (e.g., metallic salts, egg albumin, corticosteroids)(Walsh & Fairley 1995). The subcutaneous interscapular area was used for parenteral drug administration in both cats 4 and 5.
Calcinosis was diagnosed by cytology alone in cats 1 and 2 and by both cytology and histopathology in cats 3, 4 and 5. The presence of refractile, granular, basophilic or colourless, heterogeneous material as depicted in Fig 2, is considered cytologically diagnostic for calcium deposits in human medicine. (Solans et al 1997, Gupta et al 1998, Deshpande & Munshi 1999). Differential diagnosis of abundant calcium mineral in fine needle aspiration biopsy samples includes calcifications associated with neoplasm, granulomatous infection and calcinosis circumscripta. The absence of suspected neoplastic and chronic inflammatory cells on cytopathology, along with appropriate clinical setting, support the diagnosis of calcinosis secondary to CRF and RHPTH in humans (Solans et al 1997, Gupta et al 1998, Deshpande & Munshi 1999). In some smears (in particular from cat 4), mixed inflammatory cells were present (neutrophils, macrophages, spindle cells and multinucleated giant cells). Histopathology of cats 3, 4 and 5 showed that calcium deposits were surrounded by a foreign body reaction. Therefore, mixed inflammatory cells were not unexpected in cytological samples.
Aknowledgements
The authors thank Dr Luca Magnoni and Dr Mariella Ferla for referring case 4 and for their assistance.
References
- Barber PJ, Elliott J. (1998) Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. Journal of Small Animal Practice 39, 108–116. [DOI] [PubMed] [Google Scholar]
- Barber PJ, Rawlings JM, Markwell PJ, Elliott J. (1999) Effect of phosphate restriction on renal secondary hyperpara-thyroidism in the cat. Journal of Small Animal Practice 40, 62–70. [DOI] [PubMed] [Google Scholar]
- Bohmer E, Hanichen T, Lohss E. (1991) Cutaneous calcinosis of the foodpads in a cat. Tierarztliche Praxis 19, 88–95. [PubMed] [Google Scholar]
- Cordy DR. (1967) Apocrine cystic calcinosis in dogs and its relationship to chronic renal disease. The Cornell Veterinarian 42, 107–118. [PubMed] [Google Scholar]
- Deshpande A, Munshi M. (1999) Calcinosis cutis: diagnosis by aspiration cytology—A case report. Diagnostic Cytopathology 21, 200–202. [DOI] [PubMed] [Google Scholar]
- Essary LR, Wick MR. (2000) Cutaneous calciphylaxis. An underrecognized clinicopathologic entity. American Journal of Clinical Pathology 113, 280–287. [DOI] [PubMed] [Google Scholar]
- Gauguere E, Hubert B, Delabre C. (1992) Feline pododermatosis. Veterinary Dermatology 3, 1–12. [DOI] [PubMed] [Google Scholar]
- Gross TL. (1997) Calcinosis circumscripta and renal dysplasia in a dog. Veterinary Dermatology 8, 27–32. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Naran S, Cheung YK. (1998) Fine—needle aspiration cytology of soft—tissue calcinosis presenting as an enlarging mass in the chest wall. Diagnostic Cytopathology 19, 465–467. [DOI] [PubMed] [Google Scholar]
- Jackson HA, Barber PJ. (1998) Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. Journal of Small Animal Practice 39, 495–497. [DOI] [PubMed] [Google Scholar]
- Kowaliewich NJ, Hawkins EC. (1992) Calcinosis circumscripta involving the metatarsal region in a dog with chronic renal failure. Canadian Veterinary Journal 33, 465–466. [PMC free article] [PubMed] [Google Scholar]
- Legendre AM, Dade AW. (1974) Calcinosis circumscripta in a dog. Journal of the American Veterinary Medical Association 164, 1192–1194. [PubMed] [Google Scholar]
- Polzin DJ, Osborne CA, Jacob F, Ross S. (2000) Chronic renal failure. In: Textbook of veterinary internal medicine (5th edn) Ettinger SJ, Feldman EC. (eds). Philadelphia: W. B. Saunders Company, pp. 1634–1662. [Google Scholar]
- Ruopp JL. (2001) Primary hypoparathyroidism in a cat complicated by suspected iatrogenic calcinosis cutis. Journal of the American Animal Hospital Association 37, 370–373. [DOI] [PubMed] [Google Scholar]
- Schaer M, Ginn PE, Fox LE, Leon J, Ramirez FM. (2001) Severe calcinosis cutis associated with treatment of hypoparathyroidism in a dog. Journal of the American Animal Hospital Association 37, 364–369. [DOI] [PubMed] [Google Scholar]
- Scott DW, Miller WH, Griffin GE. (1995) Neoplastic and non-neoplastic tumors. In: Muller & Kirk's small animal dermatology (5th edn). Philadelphia: W. B. Saunders Company, pp. 1115–1117. [Google Scholar]
- Solans EP, Bakhos R, Castelli MJ, Gattuso P. (1997) Fine needle aspiration biopsy of calcinosis cutis. Acta Cytologica 41, 590–592. [DOI] [PubMed] [Google Scholar]
- Stampley A, Bellah JR. (1990) Calcinosis circumscripta of the metacarpal pad in a dog. Journal of the American Veterinary Medical Association 196, 113–114. [PubMed] [Google Scholar]
- Walsh JS, Fairley JA. (1995) Calcifying disorders of the skin. Journal of the American Academy of Dermatology 33, 693–706. [DOI] [PubMed] [Google Scholar]