Abstract
An 18-month-old domestic short-haired neutered male cat presented with a nodular dermal thickening on a digit. Biopsy demonstrated pyogranulomatous inflammation with moderately frequent acid-fast bacilli. A member of the Mycobacterium terrae complex was isolated. There was no evidence of systemic involvement. Treatment was initiated with enrofloxacin, rifampicin and clarithromycin. After 2 months there was no longer any clinically apparent dermal thickening. Treatment was continued for a further 3 months using enrofloxacin and rifampicin.
Case history
The patient was an 18-month-old neutered male domestic short-haired indoor/outdoor cat which the owners had purchased as a kitten. He was regularly dewormed and vaccinated against feline leukaemia virus, feline herpesvirus, feline calicivirus and feline parvovirus.
The cat originally presented with a swollen left hind paw which was assumed to be the result of an infected cat bite. There was no response to empirical treatment with clindamycin (Antirobe; Upjohn) at 5.5 mg/kg orally q12h for 7 days, and only a very slight improvement in response to marbofloxacin (Marbocyl; Vetoquinol) at 2 mg/kg orally q24h for 10 days.
The cat was re-examined 19 days later. He was in good body condition. There was a nodular dermal thickening dorsal to the 5th digit of the left hind limb (approximately 2 cm by 1 cm), with marked ulceration and a draining sinus. Mild discomfort was evident on firm palpation. No other abnormalities were detected.
The differential diagnoses for an ulcerated dermal nodule with a draining sinus include foreign body granuloma, mycobacterial granuloma, eosinophilic granuloma, chronic bacterial infection, mycotic infection or neoplasia. Actinomycosis, nocardiosis and actinobacillosis are rare in the UK.
Radiography of the foot revealed soft tissue swelling but no other abnormalities. Under general anaesthesia the lesion was explored surgically but no foreign body was found. The draining tract was flushed with saline and a sample of the nodule was fixed in formalin and prepared for histology (Fig 1a and Fig 1b). Histology demonstrated pyogranulomatous inflammation with moderately frequent acid-fast bacilli (AFB) present within foci of necrosis. The bacteria measured 4–12 μm, were slender and appeared beaded at about 2 μm intervals. Some appeared curved or serpentine in profile (Fig 1b). Mycobacterial granuloma was diagnosed.
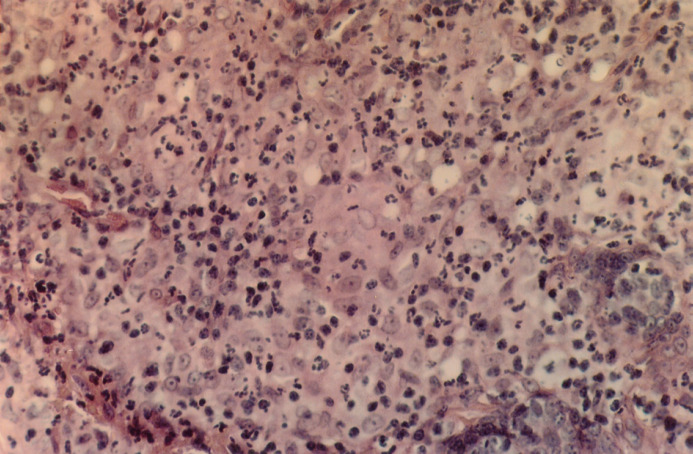
Fig 1a.
Haemotoxylin and Eosin (x100). Histological examination of 5 μm sections stained with haematoxylin and eosin (H&E) showed expansion of the dermis and subcutis by a mass of pyogranulomatous inflammation. The histiocytes have a moderate amount of pale staining to eosinophilic cytoplasm and have hypochromic nuclei, often with a single nucleolus.
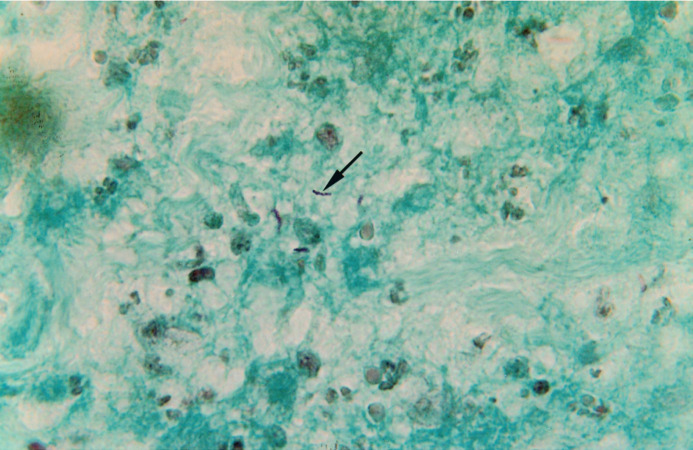
Fig 1b.
Ziehl-Neelsen (x250). Further sections were stained by the Ziehl Neelsen (ZN) technique and these demonstrated the presence of moderately abundant acid-fast bacilli within the foci of necrosis (arrow). These bacilli appear to form short chains.
Subsequent to the histology results, other investigations were performed to determine the presence or absence of any systemic involvement. Haematology demonstrated mild haemoconcentration and biochemistry revealed a mild increase in urea concentration (12.9 mmol/l; reference range 3.5–8.0). These were attributed to mild dehydration following an overnight fast. There was also a mildly elevated alkaline phosphatase activity (114 iu/l; reference range 0–40). ELISA testing was negative for FeLV antigen and FIV antibodies (SpeedCat FeLV/FIV; Vetlab), and the feline coronavirus antibody titre was <10. Thoracic radiographs, performed under general anaesthetic, were within normal limits. Further tissue samples were taken at this stage for mycobacterial culture.
Following homogenisation, the tissue sample was treated with 4% sodium hydroxide to remove contaminants, neutralised in phosphate buffer (pH 6.8) and centrifuged. One millilitre of deposit was then inoculated into the appropriate Bactec bottle and cultured at 37°C in the Bactec 9000 automated liquid culture system (Becton Dickinson). Cultures flagged positive for AFB after 17 days and tested negative for the M. avium—M. intracellulare (MAI) and M. tuberculosis (TB) complexes using DNA specific probes (Accuprobe Genprobe).
Growth was visible on solid Lowenstein-Jensen slopes after 58 days. The isolate grew at 25°C and 37°C, thus ruling out tuberculosis complex. It was non-pigmented, resistant to p-nitrobenzoic acid, thiacetazone and thiophene-2-carboxylic acid hydrazide. Growth was microaerophilic. The isolate again tested negative for MAI complex. Since a small number of MAI strains will give negative probe results, thin layer chromatography of the mycobacterial lipids was performed excluding MAI complex (Marks, Jenkins and Schaeffer 1971). These characteristics placed the organism in the Mycobacterium terrae complex (Marks 1976). Final identification took 5 months. Antibacterial sensitivity testing was not performed.
Final diagnosis was a mycobacterial granuloma in a presumed immune-competent host, with no apparent systemic involvement.
Treatment was initiated subsequent to the second biopsy procedure with enrofloxacin (Baytril; Bayer) at 5 mg/kg orally q24h, rifampicin (Rifadin Syrup; Hoechst Marion Roussel Ltd) at 10 mg/kg orally q24h and clarithromycin (Klaricid; Abbotts) at 5 mg/kg orally q12h. After 2 months the clarithromycin was stopped and the other two drugs continued for a further 3 months.
There was a gradual reduction in the size of the nodule over the first two months of treatment until no dermal thickening could be detected. There was no further recurrence and the cat appears healthy at the time of writing (30 months after the completion of treatment).
Discussion
Skin lesions on the distal limbs of cats are a very common presentation in practice, frequently caused by bacterial infection following a bite or other traumatic wound. The cat in this case was initially assumed to have such an infection and it was only after it failed to respond to antibiotic treatment that further possibilities were investigated. Histology provided a diagnosis of mycobacterial granuloma. It would have been prudent to have taken samples for bacterial and mycotic culture and sensitivity testing at the time of initial biopsy, as well as retaining some frozen tissue in case molecular techniques such as polymerase chain reaction (PCR) became necessary.
Mycobacterium is a genus of morphologically similar, aerobic, environmentally resistant, non spore forming, acid-fast bacteria which have a wide variation in host affinity and potential for pathogenicity (Greene 1995). The ability to survive intracellularly and the presence of lipids and glycolipids within the cell wall result in granulomatous to pyogranulomatous inflammation in the host tissues.
Three groups of mycobacterial skin disease are recognised in cats (Gunn-Moore et al 1996). Classical tuberculosis is caused by M bovis and M tuberculosis. Diseases caused by M bovis and M tuberculosis pose a significant zoonotic risk. Disease is usually localised to the respiratory and gastrointestinal tracts and associated lymph nodes, although nodular skin lesions are not uncommon. However, currently in the UK most cases of cutaneous mycobacterial disease are caused by M microti or ‘microti-like’ organisms (previously called tuberculous syndrome (Gunn-Moore and Shaw 1997)). Lesions are usually cutaneous nodules that may have draining sinuses or ulcerate, sometimes with local lymphadenopathy. There may be occasional systemic involvement. Feline leprosy is generally thought to be caused by M. lepraemurium, the causative agent of rat leprosy (Lemarie 1999), and is characterised by single or multiple cutaneous nodules which sometimes ulcerate. M. lepraemurium is difficult to culture using standard techniques, and use of molecular techniques such as PCR have suggested that other mycobacterial organisms may be involved in some cases of presumptive feline leprosy (Hughes et al 1997, Malik et al 2002). The third group are the opportunistic mycobacteria which are saprophytic organisms found in soil, water and decaying vegetation, and are usually non pathogenic. Most cases of feline disease in this category are caused by ‘rapidly-growing’ organisms such as M chelonae, M fortuitum, M smegmatis and M phlei, which usually cause a panniculitis in the inguinal region (Green et al 1998, Malik et al 2000). As access to appropriate culture techniques improves, it is likely that more of the slow-growing organisms such as the M terrae complex will be implicated in feline disease.
The age of this cat and gross appearance of the lesion would have been consistent with M lepraemurium infection, and the possibility of culture of M terrae as a contaminant could not be ruled out. Although organism length is not a recognised method for differentiating AFB, descriptions of M lepraemurium indicate it usually measures 2–6 μm (Wilkinson 1977, Malik et al 2002) whereas the bacillus in this case measured 4–12 μm. In addition, M terrae is rarely cultured in the reference laboratory suggesting that it is not a common contaminant of clinical samples. Unfortunately there was no tissue left for retrospective PCR analysis, which may have confirmed the diagnosis.
The cutaneous forms of mycobacterial disease often arise at the common ‘fight and bite’ sites. As in this case, it is probable that the opportunistic organisms gain entry through such wounds. There is considerable overlap between the appearance of lesions making culture essential for definitive diagnosis in order to assess zoonotic potential and to aid in therapeutic decisions. Nontuberculous mycobacteria are generally considered to pose little in the way of zoonotic risk (Malik et al 2001). Although tuberculosis complex was considered unlikely given the negative results with the DNA probe on day 17, it could not be definitively ruled out for several weeks due to the slow growth of the organism in this case. Potential zoonotic risks, particularly to immunosuppressed individuals, were therefore discussed with the cat's owner prior to commencing treatment.
The M terrae complex includes M terrae, M nonchromogenicum, and M triviale (Preheim 1999). To the authors' knowledge, there are no previously reported cases of such infection in cats. In man, members of the complex have been associated with pulmonary infection (Tonner and Hammond 1989, Peters and Morice 1991, Spence and Ferris 1996) and tenosynovitis (Ridderhof et al 1991, Smith et al 2000). Disseminated infection has been reported in a patient with advanced human immunodeficiency virus disease (Carbonara et al 2000), which may have relevance for cats that are suffering from feline immunodeficiency virus (FIV), feline leukaemia virus (FeLV), or are immunosuppressed for other reasons. The cat in this case was negative for FIV and FeLV, and there was no apparent systemic spread.
Blood was obtained prior to treatment to assess the general health of the cat. Granulomatous disease has been associated with hypercalcaemia in cats, which may warrant specific treatment (Mealey et al 1999). The serum calcium concentration was normal in this case. The reason for the elevated alkaline phosphatase is unclear. It may reflect a degree of cholestasis. Barbiturates and other drugs can cause induction of alkaline phosphatase although the enzyme has a very short half life in cats (Duncan 1998), so it is unlikely that the thiopentone administered at the first anaesthetic 2 weeks previously would have been responsible. Further investigations could have included ongoing monitoring of liver enzymes, measurement of bile acids, ultrasonography and possibly liver biopsy but these were not carried out due to financial constraints.
Since culture and final identification of the mycobacterial species could take up to several months, empirical treatment with a combination of antituberculous drugs was initiated following the second biopsy procedure (Gunn-Moore and Shaw 1997). There was no clinical evidence of side effects from the treatment. Enrofloxacin has been recently associated with retinal degeneration in cats (Gelatt et al 2001). Both rifampicin and clarithromycin can cause erythema particularly of the pinnae, and rifampicin is also known to be potentially hepatotoxic in humans (Gunn-Moore et al 1996), as well as causing induction of liver enzymes. Financial constraints precluded the monitoring of liver enzymes in this case.
Current recommendations for specifically treating M terrae infections in people include a macrolide antibiotic such as clarithromycin, ethambutol and one other antituberculous drug. Aminoglycoside therapy and surgery should also be considered (Smith et al 2000). En-bloc surgical resection and reconstruction is often recommended in conjunction with antibacterial therapy for treatment of nontuberculous mycobacterial granulomas in cats (Malik et al 2001), and would have been considered in this case if the lesion had not resolved.
The prognosis for cats with opportunistic mycobacterial skin infections is generally good if appropriate treatment is given (Malik et al 2000, Malik et al 2001). Some infections appear to respond to enrofloxacin or doxycycline alone (Studdert and Hughes 1992, Malik et al 2000). However, single agent treatment may encourage development of resistance, which could potentially endanger human patients. Some lesions have been reported to resolve even after treatment with drugs associated with in vitro resistance (Peters and Morice 1991), and spontaneous remission of mycobacterial lesions is also known to occur (Roccabianca et al 1996, Spence and Ferris 1996). Hence it is possible that in some cases the apparent response to antibiotics is coincidental.
References
- Carbonara S, Tortoli E, Costa D, Monno L, Fiorentino G, Grimaldi A, Boscia D, Rollo MA, Pastore G, Angaranao G. (2000) Disseminated Mycobacterium terrae infection in a patient with advanced human immunodeficiency virus disease. Clinical Infectious Diseases 30, 831–835. [DOI] [PubMed] [Google Scholar]
- Duncan J. (1998) Clinical biochemistry. In: Manual of Small Animal Clinical Pathology. Davidson M. (ed). Cheltenham: BSAVA, pp 61–85. [Google Scholar]
- Gelatt KN, van der Woerdt A, Ketring KL, Andrew SE, Brooks DE, Biros DJ, Denis HMM, Cutler TJ. (2001) Enrofloxacin-associated retinal degeneration in cats. Veterinary Ophthalmology 4, 99–106. [DOI] [PubMed] [Google Scholar]
- Greene CE. (1995) Bacterial Diseases. In: Textbook of Veterinary Internal Medicine, (4th edn) Ettinger SJ, Feldman EC. (eds). Philadelphia: WB Saunders, pp 367–376. [Google Scholar]
- Greene CE, Gunn-Moore DA, Lewis DY, Kunkle GA. (1998) Mycobacterial infections. In: Infectious Diseases of the Dog and Cat (2nd edn). Greene CE. (ed). Philadelphia: WB Saunders. pp 313–325. [Google Scholar]
- Gunn-Moore DA, Jenkins PA, Lucke VM. (1996) Feline tuberculosis: a literature review and discussion of 10 cases caused by an unusual mycobacterial variant. Veterinary Record 138, 53–58. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D, Shaw S. (1997) Mycobacterial disease in the cat. In Practice 19, 493–501. [Google Scholar]
- Hughes MS, Ball NW, Beck L-A, de Lisle GW, Skuce RA, Neill SD. (1997) Determination of the etiology of presumptive feline leprosy by 16S rRNA gene analysis. Journal of Clinical Microbiology 35, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarie SL. (1999) Mycobacterial dermatitis. Veterinary Clinics of North America: Small Animal Practice 29, 1291–1301. [DOI] [PubMed] [Google Scholar]
- Malik R, Hughes MS, James G, Martin P, Wignye DI, Canfield PJ, Chen SCA, Mitchell DH, Love DN. (2002) Feline leprosy: two different clinical syndromes. Journal of Feline Medicine and Surgery 4, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Hughes MS, Love DN. (2001) Nontuberculous mycobacterial diseases. In: Consultations in Feline Internal Medicine 4. August JR. (ed). Philadelphia: WB Saunders, pp 221–232. [Google Scholar]
- Malik R, Wigney DI, Dawson D, Martin P, Hunt GB, Love DN. (2000) Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings. Journal of Feline Medicine and Surgery 2, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J, Jenkins PA, Shaeffer WB. (1971) Thin-layer chromatography of mycobacterial lipids as an aid to classification: technical improvements. Tubercle 52, 219. [DOI] [PubMed] [Google Scholar]
- Marks J. (1976) A system for the examination of tubercle bacilli and the Mycobacteria. Tubercle 57, 207. [DOI] [PubMed] [Google Scholar]
- Mealey KL, Willard MD, Nagode LA, Helman RG. (1999) Hypercalcemia associated with granulomatous disease in a cat. Journal of the American Veterinary Medical Association 215, 959–962. [PubMed] [Google Scholar]
- Peters E, Morice R. (1991) Miliary pulmonary infection caused by Mycobacterium terrae in an autologous bone marrow transplant patient. Chest 100, 1449–1450. [DOI] [PubMed] [Google Scholar]
- Preheim LC. (1999) Other non-tuberculous mycobacteria and M. bovis. In: Tuberculosis and Nontuberculous Mycobacterial Infections (4th edn). Schlossberg D. (ed). Philadelphia: WB Saunders, pp 398–405. [Google Scholar]
- Ridderhof JC, Wallace RJ, Kilburn JO, Butler WR, Warren NG, Tsukamura M, Steele LC, Wong ED, et al. (1991) Chronic tenosynovitis of the hand due to Mycobacterium nonchromogenicum: Use of high performance liquid chromatography for identification of isolates. Reviews of Infectious Diseases 13, 857–864. [DOI] [PubMed] [Google Scholar]
- Roccabianca P, Caniatti M, Scanziani E, Penati V. (1996) Feline Leprosy: Spontaneous Remission in a Cat. Journal of the American Animal Hospital Association 32, 189–193. [DOI] [PubMed] [Google Scholar]
- Smith DS, Lindholm-Levy P, Huitt GA, Heifets LB, Cook JL. (2000) Mycobacterium terrae: Case reports, literature review, and in vitro susceptibility testing. Clinical Infectious Diseases 30, 444–453. [DOI] [PubMed] [Google Scholar]
- Spence TH, Ferris VM. (1996) Spontaneous resolution of a lung mass due to infection with Mycobacterium terrae. Southern Medical Journal 89, 414–416. [DOI] [PubMed] [Google Scholar]
- Studdert VP, Hughes KL. (1992) Treatment of opportunistic mycobacterial infections with enrofloxacin in cats. Journal of the American Veterinary Medical Association 201, 1388–1390. [PubMed] [Google Scholar]
- Tonner JA, Hammond MD. (1989) Pulmonary disease caused by Mycobacterium terrae complex. Southern Medical Journal 82, 1279–1282. [DOI] [PubMed] [Google Scholar]
- Wilkinson GT. (1977) Feline Leprosy. In: Current Veterinary Therapy IV, Kirk RW. (ed). Philadelphia; WB Saunders, pp 569–571. [Google Scholar]