Diastolic function has traditionally taken a back seat to systolic function, partly because systolic failure is easier to recognise as a cause of cardiac dysfunction, but possibly also because diastolic dysfunction is much harder to define. Systolic function was once considered complex, though with hindsight these complexities were more related to the problems of measuring true contractility rather than the mechanisms of systolic function itself. In truth, systolic function might be thought simple in comparison with diastolic function.
Diastole: definition
Diastole is usually defined as the period from aortic valve closure to mitral valve closure (Opie 1998). It may be simplified into two main components: relaxation, and compliance (or distensibility). There are many individual forces that will influence diastolic properties, including systolic function, heart rate, preload, and afterload. Left atrial (LA) function, right ventricular (RA) function, and pericardial restraint can also influence left ventricular (LV) diastolic function, and both rhythm and PR interval can affect the pattern of filling. The dominant forces influencing early diastolic filling may be very different from those influencing late diastole. Early diastolic filling usually dominates in the normal individual, with an additional filling increment associated with atrial contraction. Relaxation and compliance will be considered separately in turn.
LV relaxation
The molecular basis for LV relaxation is a decrease in cross-bridge formation between actin and myosin, and re-uptake of calcium ions from the cytosol into the sarcoplasmic reticulum. Relaxation is an energy-consuming, ATP-requiring process, and is impeded in energy-deficient states such as ischaemia. An increase in cAMP and protein kinase A activity is involved in both mechanisms, via troponin-I in the case of decreased cross-bridge formation, and phosphorylation of phospholamban in the re-uptake of calcium (Apstein & Morgan 1994). These two processes actually start as soon as LV pressure starts to decline, after peak systole. Once LV pressure falls below aortic diastolic pressure, a period of isovolumic pressure decline follows, where both atrioventricular and semilunar valves are closed. As LV pressure declines to levels below left atrial (LA) pressures, the mitral valve opens, and early rapid (passive) filling begins. Changes in relaxation are the dominant influence in diastolic function in normal individuals, as relaxation may be improved with increased sympathetic tone (increased cAMP), resulting in a more rapid fall in LV pressure and a shorter isovolumic relaxation period. Relaxation may be impaired with ischaemia, LV hypertrophy, and many other cardiac diseases, so that relaxation may not be completed during early diastole, and may still be incomplete at the end of diastole when severely impaired. Delayed relaxation usually results in a shift in LV filling towards the end of diastole, instead of the more usual situation where most filling occurs during early rapid filling.
Compliance
Compliance refers to the passive properties of the LV, and is the reciprocal of stiffness. It can be defined as the rate of increase in LV volume for a given increase in pressure, with more compliant ventricles distending more readily without an increase in pressure (Little & Downes 1990).
The rate of change in LV volume with pressure varies with LV volume, so that already-full ventricles are less compliant (and thus distend less easily) than partially filled ones. This means that compliance rather than relaxation is normally more important in late diastole. Compliance may be affected by myocardial tissue characteristics, with interstitial fibrosis having an adverse effect on distensibility. Chamber geometry may also influence compliance, as thicker LV walls are harder to distend than normal walls. The end-diastolic volume will influence LV stiffness, as a ventricle becomes harder to fill the more it becomes distended, even without a change in hypertrophy or myocardial tissue characteristics. Incomplete relaxation may intrude into late diastole, where the effect will also be to produce a stiffer LV.
Other factors affecting diastolic filling
Heart rate has a significant influence, as short diastolic periods may result in one wave of LV filling, rather than an early and atrial component. Rhythm may also have a profound influence, such as the loss of atrial contraction associated with atrial fibrillation. LA factors are also important. Increased left atrial pressures will tend to increase early diastolic filling, which can mask delayed relaxation. Reduced LA systolic function can reduce the contribution of atrial filling.
Assessment of diastolic function
The traditional technique for assessing diastolic function is measurement of intracardiac pressures with cardiac catheterisation. A high fidelity micromanometer catheter is necessary for measurement of relaxation. The isovolumic time constant of relaxation (tau) is obtained by fitting the isovolumic portion of LV pressure decline to a monoexponential equation and calculating the natural log of the slope, and is generally considered to be the best index of relaxation (Weiss et al 1976, Constable et al 1999). Assessment of compliance requires simultaneous measurement of LV dimensions and pressure, which can be achieved using conductance catheters. A pressure-volume curve can be constructed, to allow measurement of compliance. Both techniques are invasive, and would require anaesthesia in small animals.
In human clinical medicine, non-invasive assessment of diastolic function is now generally made using echocardiography (Nishimura & Tajik 1997, Appleton et al 2000). Although it is not usually possible to make the load-independent assessments provided by cardiac catheterisation, clinically useful information may still be obtained. Over the past 10–15 years, increasingly sophisticated echocardiographic methods have been developed to allow the clinician to infer information about relaxation, compliance, and filling pressures (Garcia et al 1998, Ommen et al 2000, Nagueh et al 1997). Trans-mitral flow velocities measured by pulsed wave Doppler echocardiography offer insights into LA-LV pressure gradients. Though influenced by LA pressure, the isovolumic relaxation time (IVRT) is a measure of relaxation. Pulmonary venous flow patterns are influenced by mean LA pressure and LV compliance. Pulsed wave Doppler tissue imaging allows measurement of mitral annulus velocities, which are less influenced by load than blood flow velocities, and the flow propagation velocity of LV filling (measured by colour M-mode Doppler echocardiography) can yield information about LV relaxation with relatively little influence from load (Garcia et al 2000).
Diastolic filling patterns
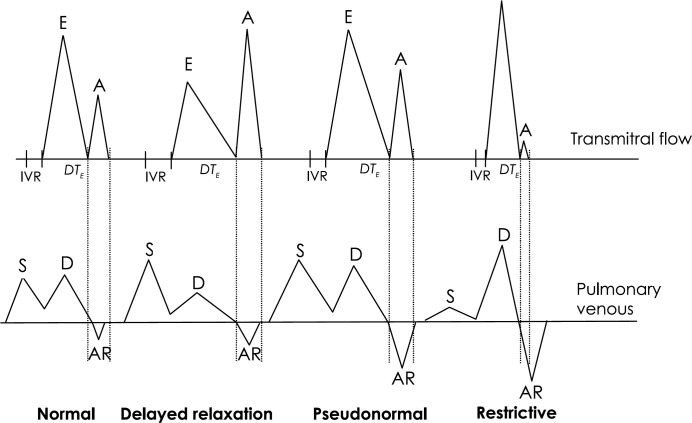
A progression in the pattern of LV filling has been documented in a number of human cardiac conditions and animal models of heart disease (Appleton & Hatle 1992, Klein et al 1990). In normal young adults, transmitral flow occurs mainly in early diastole, with a fairly short isovolumic relaxation time (IVRT). With the onset of early heart disease (or increasing age), relaxation becomes impaired, so that early diastolic filling is reduced. With a delayed relaxation pattern of filling, IVRT is increased, and the ratio of transmitral early filling velocity (E) to atrial filling velocity (A) is reduced. With more advanced heart disease, the effect of increased LA pressure overcomes the resistance to early filling from delayed relaxation, and the proportion of early to late diastolic filling resembles a more normal pattern. This pseudonormal phase may be associated with increased reversal of flow in the pulmonary veins during atrial contraction. As LA pressures increase, and LV compliance decreases, there is a shift towards a restrictive pattern of filling. High LA pressures result in rapid early filling that abruptly decelerates, as the low LV compliance opposes further filling. This is characterised by a high E:A ratio, short mitral E wave deceleration time (DTE) and short IVRT. The duration of the transmitral A wave may be shorter than the duration of the pulmonary venous atrial reversal wave, as it is easier for blood to exit the LA via the pulmonary veins than the stiff LV.
Doppler tissue imaging measurements of mitral annulus motion also show a progression, without any pseudonormalisation with increasing LA pressures. Early annulus velocities (Ea) are reduced with delayed relaxation, and remain reduced with increasing severity of heart disease. The slope of LV flow propagation (Vp) is reduced with delayed relaxation, and appears to be relatively unaffected by increased preload. The ratio of early mitral filling to flow propagation velocity (E/Vp) may relate to LV filling pressures (Garcia et al 1997).
Diastolic function in myocardial disease
Unfortunately, diastolic dysfunction in feline cardiomyopathies has been studied by only a few groups (Bright et al 1999, Gavaghan et al 1999), although much work has been done in human cardiomyopathies (Rihal et al 1994, St Goar et al 1991, Oki et al 1995, Fujimoto et al 1995, David et al 1989, Maron et al 1987).
Hypertrophic cardiomyopathy (HCM)
HCM usually results in abnormal relaxation, which may be a result of a fundamental abnormality of calcium-handling, as delayed calcium transients can be documented even in single cell preparations. The LV hypertrophy and small artery changes may impair LV perfusion, so that ischaemia contributes to the abnormal relaxation. Chamber compliance may also be abnormal in the presence of severe hypertrophy, myofibre disarray, and interstitial fibrosis. The superimposition of arrhythmias may further worsen the situation, so that cats with HCM may have multiple factors for their diastolic dysfunction. Delayed relaxation forces tend to dominate in most human patients with HCM, with a delayed relaxation pattern the most common finding. Even high left atrial pressures may fail to overcome the high early LV pressures associated with incomplete relaxation.
Restrictive cardiomyopathy (RCM)
Although relaxation may also be impaired in RCM, the severe LV myocardial changes result in an extremely stiff ventricle, and the loss of compliance precludes much filling in late diastole. Concurrent LA pressures are generally high, so that early rapid filling predominates, with abrupt deceleration into an uncompliant LV chamber. As expected, a restrictive pattern of filling is usually seen.
Dilated cardiomyopathy (DCM)
Relaxation abnormalities generally accompany (and may often precede) systolic dysfunction. A characteristic progression in diastolic filling patterns has been documented in human patients with myocardial failure, beginning with delayed relaxation as the dominant problem, and progressing to a more complex picture with increased LA pressures and decreased LV compliance.
Prognosis
Prognosis has been shown to be worse in patients with DCM exhibiting abnormal compliance patterns, although if diastolic filling is favourably affected by therapy then an improved outcome can be expected (Rihal et al 1994, Shen et al 1992). Prognosis appears to be more difficult to assess in human HCM patients, as so many have a delayed relaxation pattern of filling, even with high filling pressures. Alteration of filling patterns may be seen with successful therapy, and this may be useful in assessing the response to treatment (Temporelli et al 1998).
Treatment for feline HCM
Therapy for cats with HCM is most often based on diltiazem or atenolol, with congestive signs often controlled with frusemide, with or without enalapril. There have been few published studies of therapy in feline HCM, and treatment is usually empirical (Bright et al 1991).
Diltiazem
As a calcium channel antagonist with cardiac specificity, diltiazem has negative chronotropic effects with some negative inotropic effects, but minimal vascular effects. It has been quoted as having a positive lusitropic effect (improving relaxation), although this may not be a direct effect. The effect of slowing heart rate may be to improve the time for ventricular filling, and the modest negative inotropic effects may reduce myocardial oxygen consumption. It does not appear to be very effective at reducing dynamic left ventricular outflow tract gradients.
Atenolol
The β1 blocking effects result in negative chronotropic and inotropic effects, and there may be direct adverse effects on relaxation. However, the direct effects on relaxation may be offset by the same beneficial effects seen with diltiazem (increase in time for ventricular filling and reduction in myocardial oxygen consumption). The negative inotropic effects may be more pronounced than with diltiazem, and may reduce dynamic outflow tract obstruction more effectively than diltiazem (Wey & Kittleson 2000). Atenolol might also be better at reducing ischaemic effects. An increase in dosage, however, may lead to subnormal systolic function and LV dilation.
Monitoring effects of therapy with echocardiography
Therapy should be geared to the underlying functional disturbance, and this information is best obtained with echocardiography (Modersohn et al 1993, Brutsaert et al 1993). In simple terms, effective therapy should reduce signs of pulmonary oedema on radiographs, and reduce LA size when measured by echocardiography. This is not always achievable, and only subtle changes may be evident. A reversal of the normal progression in diastolic filling pattern may indicate a reduction in filling pressures, which is one of the primary aims of therapy (whatever the type of myocardial disease). Pseudonormal patterns can pose problems when serial exams are not available (as they may be difficult to distinguish from normal transmitral filling patterns), although normal patterns are highly unlikely in the presence of clear structural changes.
Until the results of large scale controlled studies are available, it is difficult for the clinician to choose between the two standard therapies. It may be that therapy should be tailored to the individual, and echocardiographic assessment of diastolic filling patterns can help us choose the ideal therapy for each patient.
References
- Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD. (2000) The echo-Doppler evaluation of left ventricular diastolic function. A current perspective. Cardiology Clinics 18, 513–546. [DOI] [PubMed] [Google Scholar]
- Appleton CP, Hatle LK. (1992) The natural history of left ventricular filling abnormalities: assessment by two-dimensional and Doppler echocardiography. Echocardiography 9, 437–457. [Google Scholar]
- Apstein CS, Morgan JP. (1994) Cellular mechanisms underlying left ventricular diastolic dysfunction. In Left Ventricular Diastolic Dysfunction and Heart Failure, Gaasch WH, Lewinter MM. (eds) Lea & Febiger, Philadelphia, pp. 3–24. [Google Scholar]
- Bright JM, Golden AL, Gompf RE, Walker MA, Toal RL. (1991) Evaluation of the calcium channel-blocking agents diltiazem and verapamil for treatment of feline hypertrophic cardiomyopathy. J Vet Intern Med 5, 272–282. [DOI] [PubMed] [Google Scholar]
- Bright JM, Herrtage ME, Schneider JF. (1999) Pulsed Doppler assessment of left ventricular diastolic function in normal and cardiomyopathic cats. J Am Anim Hosp Assoc 35, 285–291. [DOI] [PubMed] [Google Scholar]
- Brutsaert DL, Sys SU, Gillebert TC. (1993) Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol 22, 318–325. [DOI] [PubMed] [Google Scholar]
- Constable P, Muir W, III, Sisson D. (1999) Clinical assessment of left ventricular relaxation. J Vet Intern Med 13, 5–13. [PubMed] [Google Scholar]
- David D, Lang RM, Neumann A, Sareli P, Marcus R, Spencer KT, Borow KM. (1989) Comparison of Doppler indexes of left ventricular diastolic function with simultaneous high fidelity left atrial and ventricular pressures in idiopathic dilated cardiomyopathy Am J Cardiol 64, 1173–1179. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Parker KH, Xiao HB, Inge KSK, Gibson DG. (1995) Early diastolic left ventricular inflow pressures in normal subjects and patients with dilated cardiomyopathy. Reconstruction from pulsed Doppler echocardiography Br Heart J 74, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD. (1997) An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 29, 448–454. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Smedira NG, Greenberg NL, Main M, Firstenberg MS, Odabashian J, Thomas JD. (2000) Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: Animal and human validation. J Am Coll Cardiol 35, 201–208. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Thomas JD, Klein AL. (1998) New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 32, 865–875. [DOI] [PubMed] [Google Scholar]
- Gavaghan BJ, Kittleson MD, Fisher KJ, Kass PH, Gavaghan MA. (1999) Quantification of left ventricular diastolic wall motion by Doppler tissue imaging in healthy cats and cats with cardiomyopathy Am J Vet Res 60, 1478–1486. [PubMed] [Google Scholar]
- Klein AL, Hatle LK, Taliercio CP, Taylor CL, Kyle RA, Bailey KR, Seward JB, Tajik AJ. (1990) Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 16, 1135–1141. [DOI] [PubMed] [Google Scholar]
- Little WC, Downes TR. (1990) Clinical evaluation of left ventricular diastolic performance. Progress in Cardiovascular Diseases 32, 273–290. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. (1987) Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 10, 733–742. [DOI] [PubMed] [Google Scholar]
- Modersohn D, Walde T, Bruch L. (1993) Diastolic heart function—pathophysiology, characterization, and therapeutic approaches. Clin Cardiol 16, 850–858. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, Tajik AJ. (1997) Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol 30, 8–18. [DOI] [PubMed] [Google Scholar]
- Oki T, Fukuda N, Iuchi A, Tabata T, Kiyoshige K, Manabe K, Kageji Y, Sasaki M, Hama M, Yamada H. (1995) Evaluation of left ventricular diastolic hemodynamics from the left ventricular inflow and pulmonary venous flow velocities in hypertrophic cardiomyopathy. Jpn Heart J 36, 617–627. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A Comparative Simultaneous Doppler-Catheterization Study. Circulation 102, 1788–1794. [DOI] [PubMed] [Google Scholar]
- Opie LH. (1998) Ventricular function. In The Heart, Physiology from Cell to Circulation, Opie LH. (ed.) Lippincott-Raven, Philadelphia, pp. 343–389. [Google Scholar]
- Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. (1994) Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation 90, 2772–2779. [DOI] [PubMed] [Google Scholar]
- Shen WF, Tribouilloy C, Rey J-L, Baudhuin J-J, Boey S, Dufosse H, Lesbre J-P. (1992) Prognostic significance of Doppler-derived left ventricular diastolic filling variables in dilated cardiomyopathy. Am Heart J 124, 1524–1533. [DOI] [PubMed] [Google Scholar]
- St Goar FG, Masuyama T, Alderman EL, Popp RL. (1991) Left ventricular diastolic dysfunction in end-stage dilated cardiomyopathy: simultaneous Doppler echocardiography and hemodynamic evaluation. J Am Soc Echocardiogr 4, 349–360. [DOI] [PubMed] [Google Scholar]
- Temporelli PL, Corra U, Imparato A, Bosimini E, Scapellato F, Giannuzzi (1998) Reversible restrictive left ventricular diastolic filling with optimized oral therapy predicts a more favorable prognosis in patients with chronic heart failure. J Am Coll Cardiol 31, 1591–1597. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Frederiksen JW, Weisfeldt ML. (1976) Hemodynamic determinants of the time course of fall in canine left ventricular pressure. Journal of Clinical Investigation 58, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey AC, Kittleson MD. (2000) Comparison of the efficacy of intravenous diltiazem and esmolol to reduce left ventricular outflow tract velocity and heart rate in cats with hypertrophic obstructive cardiomyopathy. J Vet Intern Med 14, 335. [Google Scholar]