Abstract
Objective:
To determine the impact of various aerosol mitigation interventions and to establish duration of aerosol persistence in a variety of dental clinic configurations.
Methods:
We performed aerosol measurement studies in endodontic, orthodontic, periodontic, pediatric, and general dentistry clinics. We used an optical aerosol spectrometer and wearable particulate matter sensors to measure real-time aerosol concentration from the vantage point of the dentist during routine care in a variety of clinic configurations (eg, open bay, single room, partitioned operatories). We compared the impact of aerosol mitigation strategies (eg, ventilation and high-volume evacuation (HVE), and prevalence of particulate matter) in the dental clinic environment before, during, and after high-speed drilling, slow–speed drilling, and ultrasonic scaling procedures.
Results:
Conical and ISOVAC HVE were superior to standard-tip evacuation for aerosol-generating procedures. When aerosols were detected in the environment, they were rapidly dispersed within minutes of completing the aerosol-generating procedure. Few aerosols were detected in dental clinics, regardless of configuration, when conical and ISOVAC HVE were used.
Conclusions:
Dentists should consider using conical or ISOVAC HVE rather than standard-tip evacuators to reduce aerosols generated during routine clinical practice. Furthermore, when such effective aerosol mitigation strategies are employed, dentists need not leave dental chairs fallow between patients because aerosols are rapidly dispersed.
Certain dental procedures generate high concentrations of aerosols in a patient’s mouth, which may increase the risk for transmission of respiratory pathogens to dental healthcare professionals (DHP) and other patients. Most aerosols are generated and emitted from 2 sources: (1) high-speed, high-frequency dental instruments that use water for cooling, and (2) the patient’s bodily fluids, including blood, saliva, dental plaque, and food debris.1
Previous studies show that dentists and patients are exposed to >10,000 bacteria/m3, and the risk of inhaling infectious aerosols during dental procedures may be high.2,3 Dentists and other DHPs may inhale up to 0.014 μL of saliva over a 15-minute peak exposure period, and up to as much as 0.12 μL of saliva in extreme instances.4 Several studies have examined emissions during dental procedures. Ultrasonic scaling has been associated with an increased burden of bacterial contamination.5,6 In dental clinics, aerosols can remain prevalent in the surrounding environment for as long as 30 minutes after a procedure has been performed.7–9 However, these studies only provide daily average values; these investigators did not consider the impact of aerosol mitigation interventions, and they did not measure exposure from the vantage point of the dentist or other DHPs.10,11
Preliminary data derived from mannequin experiments suggest that the risk posed by aerosols can be reduced with high-volume evacuation (HVE), rubber dam isolation, flushing water lines at the beginning of the workday and between patients, preprocedural patient mouth rinses, and appropriate use of personal protective equipment by DHP.1,12–15 However, these studies were not performed during actual patient care and lack realtime particle monitoring necessary to determine the effectiveness of these interventions.
In this study, we used real-time optical aerosol instruments (1) to characterize aerosol emissions from the vantage point of dentists and other DHP during common dental procedures, (2) to compare the effectiveness of commonly used aerosol mitigation interventions including HVE, and (3) to establish the duration over which aerosols persist in the dental clinic environment following an aerosol-generating procedure.
Materials and methods
We collected data on aerosols generated during procedures in pediatric dentistry, general dentistry, orthodontic, periodontic, and endodontic clinics in St. Louis, Missouri, from July to October 2020. Aerosol sensors were used to collect data. Wi-fi hotspots were deployed to transmit data from dental clinics to the data “cloud,” allowing the research team to monitor environmental aerosol concentrations in real-time.
Experimental system
Dental clinics
Each dental clinic had a different configuration. Pediatric and general dental operatories had a single-room layout. Endodontic and periodontic clinics had semiprivate operatories with partial wall barriers between dental chairs. The orthodontic clinic included a large multioperator clinic space (~35 m × 20 m × 20 m). In each dental clinic setting, the patient was accompanied by a dentist and up to 2 additional DHP depending on the complexity of the procedure to be performed. Supplementary Figure 1 shows a typical dental clinic setting with the location of the aerosol sensors used for the study.
We evaluated the following dental procedures: high-speed drilling during debonding of orthodontic brackets; enamel and dentin cutting during cavity and crown preparation; slow speed drilling for finishing cavity preparation, polishing, and trimming during crown preparation; removal of dentin and soft tissues during endodontics; and ultrasonic scaling during teeth cleaning. Dental suction was used in all configurations with 8.2 mm tip with flow rate 74 standard cubic feet per minute at 7.0 Hg (2095.44 LPM; Henry Schein 1400 RAMVAC standard model).
Instrumentation
A MINIMA wearable particulate matter sensor (Applied Particle Technology, St. Louis, MO) worn on the chest pocket of the dentist’s coat was used to measure the particulate matter concentration exposure of dentists.16,17 Start and end times for the dental procedures were recorded by dental clinic staff. The particulate matter sensors recorded every 15 seconds; data were transmitted wirelessly in real-time to a cloud-based dashboard for visualization and temporal analysis. The number concentration of aerosols generated during each dental procedure was also measured using an optical aerosol spectrometer (Model 11C, GRIMM Aerosol Technik, Ainring, Germany) that allows particle detection from 220 nm to 32 μm.18 The inlet of the optical aerosol spectrometer was positioned 20 cm in front of and 10 cm above the patient’s mouth without interruption of the procedures performed by the dentist.
Test plan
The test plan (Table 1) comprised 4 objectives for measuring the aerosol emission during the following dental procedures: highspeed drilling; slow-speed drilling; prophylaxis; and ultrasonic scaling in different dental clinic settings.
Table 1.
Aerosol Measurement Experiments
| Test No. | Objective | Description | Dentistry Type | Result |
|---|---|---|---|---|
| I | The effect of type of dental procedure on aerosol emission using tip HVE mitigation | 1. High-speed drill used for 10 minutes. 2. Slow-speed drill used for 2 minutes. 3. Prophy used for 5 minutes. 4. Ultrasonic scaler used for 15 minutes |
(A) Orthodontic (B) Pediatric (C) Endodontic (D) Periodontic |
Figure 1 |
| II | Effect of aerosol mitigation strategy on aerosol emission during operation on patient | 1.Use of high-speed drill with minimal ventilation in dental space 2. Use of high-speed drill with Conical HVE 3. Use of high-speed drill with tip HVE 4. Use of ultrasonic scaler with tip HVE 5. Intermittent use of Ultrasonic scaler with isovac 6. Continuous use of Ultrasonic scaler with isovac |
(A) Orthodontic (D) Periodontic |
Figure 2 |
| III | Temporal variation of aerosol emission during operation on patient | 1. High-speed drill | (A) Orthodontic (E) General |
Figure 3 |
| IV | Effect of operation location in oral cavity on aerosol emission | 1. High-speed drill with tip HVE on anterior teeth 2. High-speed drill with tip HVE on posterior teeth 3. Ultrasonic scaler with tip HVE on anterior teeth 4. Ultrasonic scaler with tip HVE on posterior teeth |
(A) Orthodontic (B) Pediatric (D) Periodontic |
Supplementary Figure 3 |
Data analysis
The number concentration obtained from the optical aerosol spectrometer was used to generate the size number distribution of aerosols emitted during each dental procedure.19 The number concentration used for the size distribution function calculation was the difference between the number concentration measured during the procedure and the number concentration measured before the start of procedure (background data).
The size distribution function of the aerosols (ndp was calculated using the following equation:
| (1) |
where is the number concentration of the particles measured by optical aerosol spectrometer for a particular size range in the range dp1 to dp2. The size distribution of aerosols is important because it estimates the number of particles in a desired size range. The total number concentration of particles in a specific size range is obtained by integrating the size distribution function over infinitesimally small particle size with the upper and lower limits as maximum and minimum particle size.
Results
The effect of type of dental procedure on aerosol emission using tip HVE mitigation
Figure 1 represents the size distribution of the aerosols generated during the use of high-speed drilling in orthodontics, pediatric dentistry, and endodontics, as well as the use of an ultrasonic scaler in periodontics during routine patient care. All procedures utilized standard-tip HVE (Supplementary Fig. 2a) to mitigate aerosol spread in the environment. The data represent the average number concentration of aerosols emitted during the entire duration of each procedure using the dental handpiece on a patient as a function of log of the size of the aerosols. Data from use of slow-speed drill and prophylaxis are not presented because no aerosols were observed. The use of a high-speed drill in orthodontics demonstrated emission of the highest concentration of particles at 2 modes (0.28 μm and 0.35 μm), whereas the remaining dental procedures only showed a single mode at 0.28 μm. Many aerosols were generated in orthodontics for the particle size.
Fig. 1.
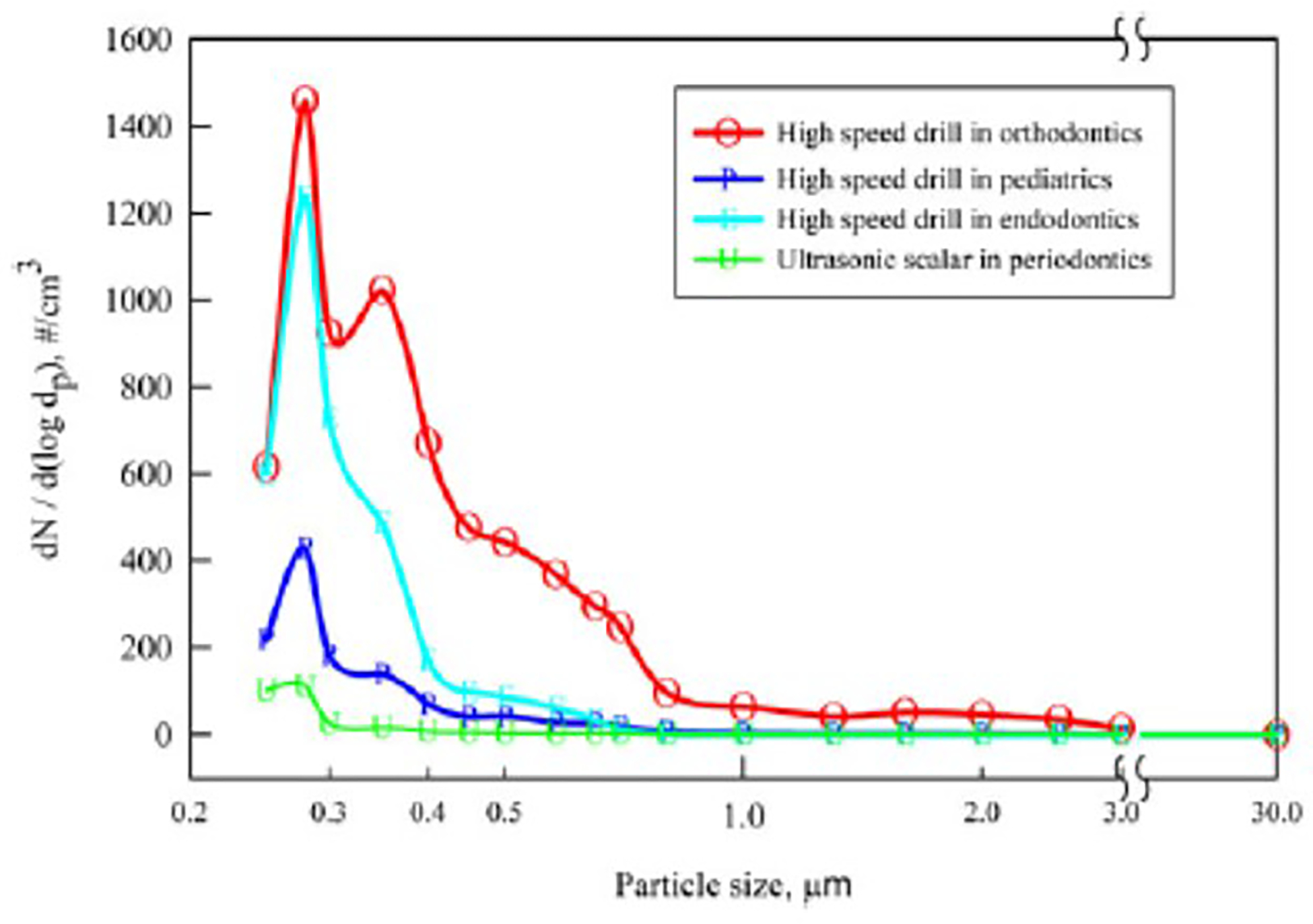
Size distribution of aerosols generated during different dental procedures usingtip HVE mitigation:The concentration and size distribution of aerosols generated by slow speed drilling in pediatrics were also measured but were close to background levels and are therefore not shown (for clarity).
Aerosol emissions from high-speed drilling in orthodontics were higher than from high-speed drilling in endodontics and pediatrics. The average concentration of particles in the range of 250 nm to 1 μm that were emitted was as high as 340 particles per cm3 of air during 10 minutes of high-speed drilling in orthodontics. The number concentrations of the particle were obtained by multiplying the size distribution function with the desired size range as per the equation 1: 170 particles per cm3 of air during 10 minutes of high-speed drilling in endodontics, 60 particles per cm3 of air during 10 minutes of high-speed drilling for in pediatrics, and 15 particles per cm3 of air ultrasonic scaling for 15 minutes in periodontics.
Effect of aerosol mitigation strategy on aerosol emission during operation on patient
The aerosol emissions among different mitigation strategies employed by dentists are presented in Figure 2. The concentration of aerosol was highest when using a high-speed drill in a dental operatory space with little ventilation. Conical HVE had higher efficiency for removing aerosol plumes than the standard-tip HVE (Supplementary Fig. 2b).
Fig. 2.
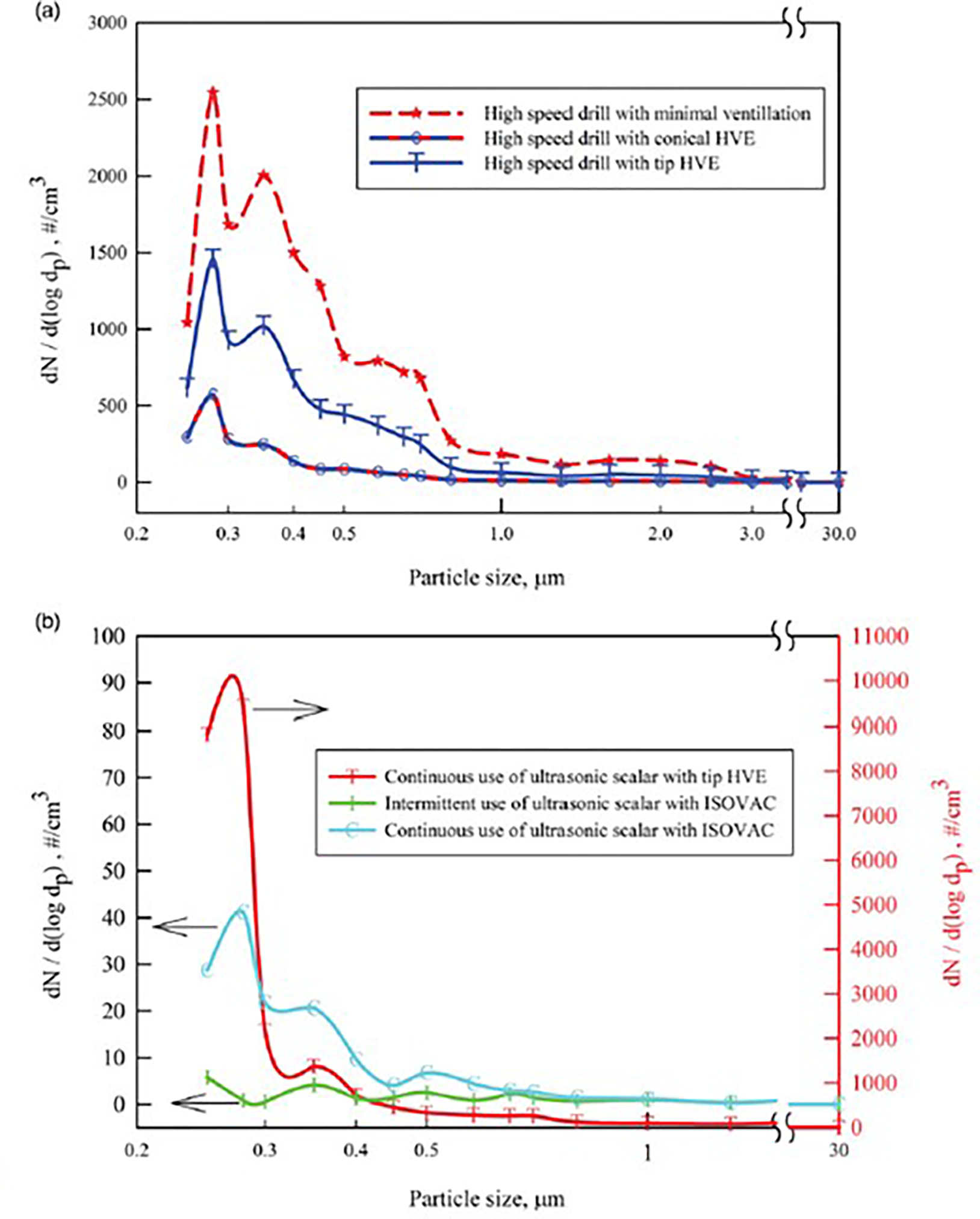
Size distribution of the aerosol emissions measured using different aerosol mitigation strategies.
The ISOVAC dental isolation adapter used with HVE was also evaluated during ultrasonic scaler use (Supplementary Fig. 2c). Compared to standard-tip HVE, ISOVAC reduced aerosol emissions by a factor >1,000 during intermittent ultrasonic scaler use, and when continuous scaling was performed with ISOVAC HVE, aerosol emissions were very low but were higher than intermittent ultrasonic use during the procedure.
Temporal variation of aerosol emission during operation on patient
The real-time variation in PM2.5 concentration was measured using portable low-cost particulate matter sensors. PM2.5 refers to the concentration of particles with a diameter <2.5 micrometers, considered to be of the greatest health concern and most likely to be inhaled into the lower respiratory tract 19. Figure 3b depicts PM 2.5 concentration during a procedure performed in a closed single patient room. During this observation, the dentist was exposed to PM2.5 concentrations as high as 350 μg/m3 when HVE was partially used during the procedure. In contrast, Figure 3a demonstrates lower PM2.5 concentrations (peak ~50 μg/m3 ) during high-speed drilling in a large open multioperator space.
Fig. 3.
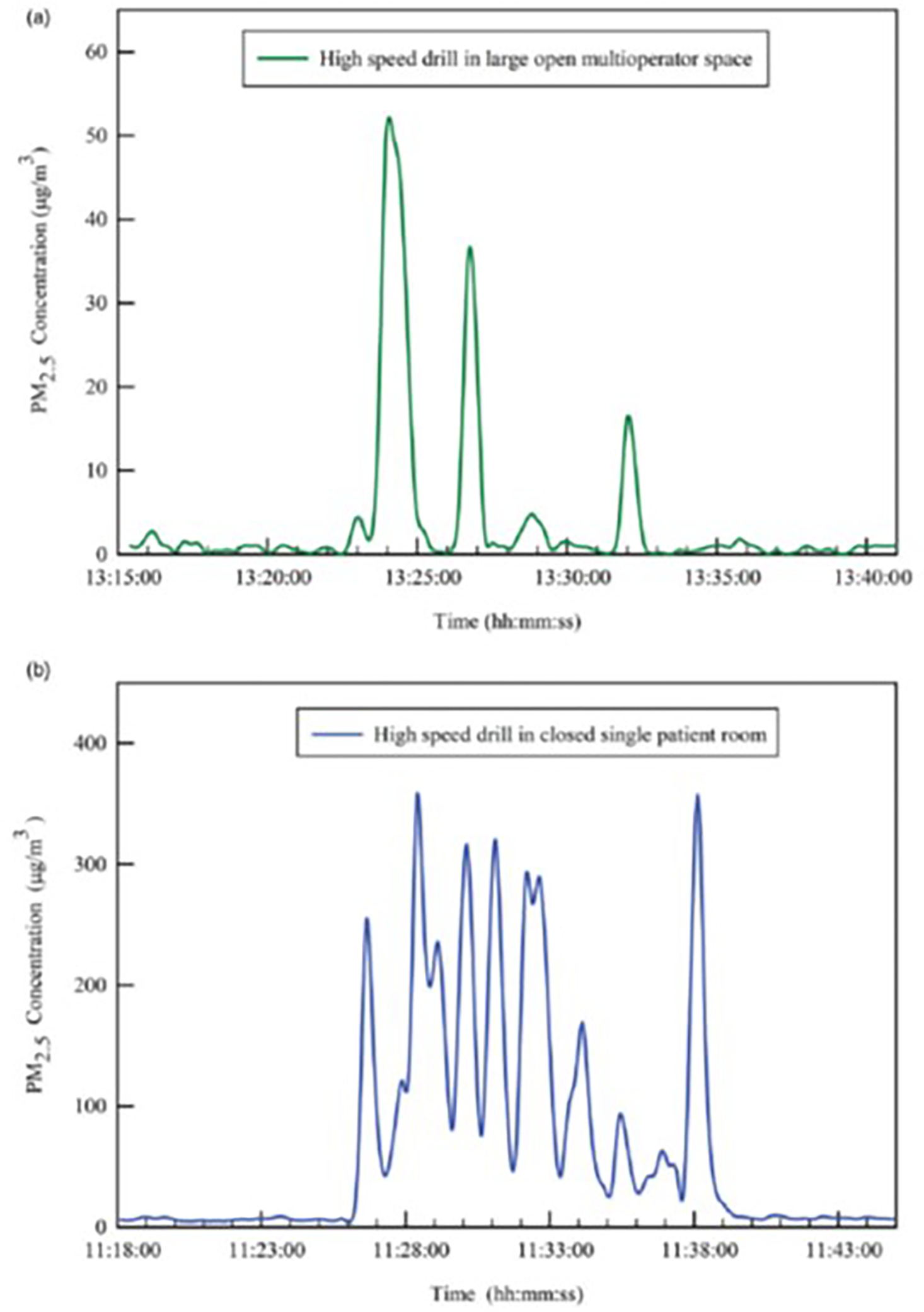
Temporal variation of PM2.5 concentration aerosols before,during and after dental procedures PM2 5 refers to the concentration of particles with a diameter <2.5μm.Note the different y-axis in 2 images.
Discussion
To our knowledge, this is the first study to evaluate continuous, real-time monitoring of particulate matter concentrations during real-world dental procedures. By using portable, wearable, aerosol sensors on DHPs and an optical aerosol spectrometer placed in close proximity to the patient, this study provides the most accurate representation of DHP aerosol exposure reported to date.
The largest aerosol concentrations were generated by ultrasonic scaling and high-speed drilling of anterior teeth (Supplementary Fig. 3). This was evident during dental procedure on upper front teeth because a huge amount of water is splashed back into the atmosphere surrounding patient and DHP with large space available for HVE to reduce the aerosols resulting in reduced efficiency of aerosol capture. In comparison, the other locations in the mouth more likely limit the spread of aerosols with HVE. HVE significantly reduced the aerosol concentration during all types of dental procedures sampled. Conical HVE was more effective in reducing aerosol concentrations than standard-tip HVE, but ISOVAC HVE was the most effective form of aerosol mitigation. Procedures performed in a closed, single-patient room demonstrated slightly higher concentrations of aerosols than those performed in larger, open, and ventilated clinic spaces. However, in general, we also observed that dental aerosols, when present, appeared transient, regardless of dental clinic configuration. Thus, fallow time can be reduced to 5 minutes, which likely occurs during routine patient care.
Further investigation of dental aerosols remains necessary. During the time of measurements, the humidity outdoors was 60%, and the humidity indoors at the measurement site ranged between 40% and 55%. The focus of this study was on indoor environments, where the environment is climate controlled. If the environments were not climate controlled, the findings would indeed be seasonal. Recent articles show that humidity <30% results in evaporation of water in less than a few seconds. In such situations, the aerosols after water evaporation can linger around in the environment for longer intervals. At high humidity levels (>90%), hygroscopic growth of the particle occurs, resulting in large particle sizes that settle in less time.20,21 Even with aggressive aerosol mitigation interventions, dentists and other DHPs remain exposed to a small concentration of aerosols. Additionally, the emitted aerosols were < 1 μm in size, well within the region where lower respiratory tract deposition could occur. Such aerosols could pose an infectious disease threat to DHPs should they harbor respiratory pathogens.
Preliminary data on swab samples of splatter from the environment in dental settings identified severe acute respiratory coronavirus virus 2 (SARS-CoV-2).20 However, these investigators made only limited measurements of dental aerosols. Aerosols < 10 μm can remain suspended in air for several hours before depositing on surfaces22; thus, pathogens may still be present in the air.23 These investigators did not use microbiologic culture to determine viability of pathogens detected. Concurrent measurement of pathogen concentrations in aerosol emissions needs to be conducted to provide a more accurate evaluation of infection risk during dental procedures.
A bimodal distribution of particle size was seen in some situations (eg, orthodontic high-speed drilling), suggesting the possibility of 2 different sources of particles. The larger particle sizes measured during procedures primarily consisted of water droplets, and these were also visually observed. Further chemical characterization is necessary to differentiate between aerosols emanating from the patient and those from dental equipment. A recently published study also measured the size distribution of aerosols during different dental procedures, and most particles were in the 52 nm– 72 nm range at a sampling height of 60 cm.24 However, larger droplets and particles were not found. Emissions varied from patient to patient. This finding highlights the importance of real-time monitoring of aerosol emissions. Slow-speed drilling and prophylaxis did not emit significant aerosols, and thus HVE may not be required during these procedures. However, this observation was made under conditions in which the patient was not coughing, which is known to generate aerosols.25
Conical HVE is likely more efficient in reducing emissions from high-speed drilling than standard-tip HVE because of the relatively large surface area available for conical HVE to evacuate aerosols from the dental environment. Our data demonstrated that the addition of ventilation further prevented accumulation of aerosols in the dental environment. This was largely due to the air dilution effect and transportation of aerosols away from the immediate dental setting. Use of ISOVAC HVE during ultrasonic scaling provided better aerosol mitigation than standard-tip HVE. Aerosol mitigation of ultrasonic scaling procedures was best when ISOVAC HVE and an intermittent scaling approach were used together. This combination helped by providing enough time for aerosols generated to be evacuated. DHPs who anticipate performing ultrasonic scaling for a prolonged period of time may wish to turn off the scaler intermittently during routine care to allow aerosols to dissipate.
Supplementary Material
Acknowledgments.
For their assistance with wearing low-cost particulate matter sensors and scheduling the aerosol measurements during the dental operations, the authors acknowledge Dr. Andrew Kim and Dr. Emily Hahn from St. Louis Children’s Hospital; Dr. Elio Reyes Rosales, Dr. Craig Rhodes, Dr. Ki Beom Kim, Dr. John Hatton from Center for Advanced Dental Education, St. Louis University; Shequita Hargrove from Deer Creek Dental, O’Fallon, Missouri. The authors also acknowledge Candace Miller for help transporting the aerosol instruments to different dental clinics. In addition, the authors thank Drs. Dena Fisher, Gregg Gilbert, Mary Ann McBurnie, the National Dental PBRN Practitioner Executive Committee for their assistance reviewing and providing feedback on the preliminary drafts of our research report.
Financial support.
The study was supported by the National Institute for Dental and Craniofacial Research of the National Institutes of Health (grant no. U19DE028717, subawards X01DE030403 and K23DE029514). In addition, this study was also supported in part by the Foundation for Barnes-Jewish Hospital and their generous donors, and the Washington University Institute of Clinical and Translational Sciences which is, in part, supported by the National Institutes of Health/National Center for Advancing Translational Sciences (CTSA grant no. UL1TR002345). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest. All authors report no conflicts of interest relevant to this article.
Supplementary material. To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.26
References
- 1.Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc 2004;135: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutil S, Mériaux A, de Latrémoille M-C, et al. Measurement of airborne bacteria and endotoxin generated during dental cleaning. J Occup Environ Hyg 2008;6:121–130. [DOI] [PubMed] [Google Scholar]
- 3.Kumar PS, Subramanian K. Demystifying the mist: sources of microbial bioload in dental aerosols. J Periodontol 2020; 91:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett AM, Fulford MR, Walker JT, et al. Microbial aerosols in general dental practice. Br Dental J 2020;189:664–667. [DOI] [PubMed] [Google Scholar]
- 5.Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc 1998; 129:1241–1249. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman MF, Menso L, Steinfort J, et al. Atmospheric contamination during ultrasonic scaling. J Clin Periodont 2004;31:458–462. [DOI] [PubMed] [Google Scholar]
- 7.Checchi V, Bellini P, Bencivenni D, Consolo U. COVID-19 dentistryrelated aspects: a literature overview. International Dental Journal COVID-19 interactive map. Johns Hopkins University website. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed January 15, 2020. [Google Scholar]
- 8.Epstein JB, Chow K, Mathias R. Dental procedure aerosols and COVID-19. Lancet Infect Dis 2020;21:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res 2020;99: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gioda A, Hanke G, Elias-Boneta A, Jiménez-Velez B. A pilot study to determine mercury exposure through vapor and bound to PM10 in a dental school environment. Toxicol Indust Health 2007;23:103–113. [DOI] [PubMed] [Google Scholar]
- 11.Helmis CG, Tzoutzas J, Flocas HA, et al. Indoor air quality in a dentistry clinic. Sci Total Environ 2007;377:349–365. [DOI] [PubMed] [Google Scholar]
- 12.Balanta-Melo J, Gutiérrez A, Sinisterra G, et al. Rubber dam isolation and high-volume suction reduce ultrafine dental aerosol particles: an experiment in a simulated patient. Appl Sci 2020;10:6345. [Google Scholar]
- 13.Eliades T, Koletsi D. Minimizing the aerosol-generating procedures in orthodontics in the era of a pandemic: current evidence on the reduction of hazardous effects for the treatment team and patients. Am J Orthod Dentofacial Orthop 2020;158:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallier C, Williams DW, Potts AJC, Lewis MAO. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br Dental J 2010;209:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holliday R, Allison JR, Currie CC, et al. Evaluating contaminated dental aerosol and splatter in an open plan clinic environment: implications for the COVID-19 pandemic. J Dentist 2021;105:103565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Mattewal SK, Patel S, Biswas P. Evaluation of nine low-cost-sensor– based particulate matter monitors. Aerosol Air Qual Res 2020;20:254270. [Google Scholar]
- 17.Applied particle technology website. https://appliedparticletechnology.com/. Accessed September 10, 2020. [Google Scholar]
- 18.Sousan S, Koehler K, Hallett L, Peters TM. Evaluation of the alphasense optical particle counter (OPC-N2) and the grimm portable aerosol spectrometer (PAS-1.108). Aerosol Sci Tech 2016;50:1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Hoboken, NJ: John Wiley & Sons; 1999. [Google Scholar]
- 20.Ahlawat A, Wiedensohler A, Mishra SK. An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol Air Qual Res 2020;20:1856–1861. [Google Scholar]
- 21.Yang W, Marr LC. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol 2012;78:6781–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meethil AP, Saraswat S, Chaudhary PP, Dabdoub SM, Kumar PS. Sources of SARS-CoV-2 and other microorganisms in dental aerosols. J Dent Res 2021;100:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhawan S and Biswas P. Aerosol Dynamics model for estimating the risk from short-range airborne transmission and inhalation of expiratory droplets of SARS-CoV-2. Environ Sci Tech 2021;55:8987–8999. [DOI] [PubMed] [Google Scholar]
- 24.Polednik B Exposure of staff to aerosols and bioaerosols in a dental office. Building and Environment 2021;187:107388. https://www.sciencedirect.com/science/article/pii/S0360132320307575. [Google Scholar]
- 25.Klompas M, Baker M, Rhee C. What is an aerosol-generating procedure? JAMA Surgery 2021;156:113–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


