Abstract
Cytotoxic necrotizing factor type 1 (CNF1), a 110-kDa toxin-like protein from pathogenic Escherichia coli strains, induces an actin cytoskeleton reorganization consisting of the formation of prominent stress fibers by permanent activation of the small GTP-binding protein Rho. Since p21Rho regulates tight-junction permeability and perijunctional actin reorganization in epithelial intestinal cells (A. Nusrat, M. Giry, J. R. Turner, S. P. Colgan, C. A. Parkos, E. Lemichez, P. Boquet, and J. L. Madara, Proc. Natl. Acad. Sci. USA 92:10629–10633, 1995), we used polarized T84 epithelial intestinal cell monolayers to examine whether CNF1 could affect microvillus structure, transepithelial resistance, and polymorphonuclear leukocyte (PMN) transmigration. Incubation of T84 cells with CNF1 did not influence transepithelial resistance, suggesting that barrier function and surface polarity were not affected by the toxin. However, CNF1 effaced intestinal cell microvilli and induced a strong decrease of PMN transepithelial migration in either the luminal-to-basolateral or the basolateral-to-luminal direction. CNF1 could thus be a virulence factor exhibiting a new type of combined activity consisting of effacing of microvilli and occlusion of the epithelial barrier to PMNs. Attenuated transepithelial migration of PMNs could result in the enhanced growth and protection of luminal bacteria.
Certain pathogenic Escherichia coli strains, associated with gastroenteritis or urinary tract infections, elaborate a toxin-like protein named cytotoxic necrotizing factor 1 (CNF1) (3). CNF1 induces a strong dermonecrotic action in the rabbit after intradermal injection, a high lethality when injected into mice, and the formation of large multinucleated cells in tissue culture (3). Activity of CNF1 on cultivated cells is due to a remarkable reorganization of the actin cytoskeleton consisting mainly of the accumulation stress fibers (10). CNF1 stimulates formation of actin stress fibers by permanently activating the GTP-binding protein Rho (11). The Rho GTP-binding protein belongs to a family of small GTPases involved in actin cytoskeleton reorganization in response to growth factors (17). This family includes Rho, which, in Swiss 3T3 cells, controls actin stress fiber formation and the assembly of focal adhesion points (32); Rac, which regulates actin membrane ruffling (33); and Cdc42, which determines formation of filopodia (27).
After cell membrane translocation, CNF1 deamidates Rho glutamine 63 into glutamic acid (12, 35). Glutamine 63 is a critical residue involved in the GTP hydrolysis activity of most of the p21 small GTPases (4). Modification of Rho glutamine 63 into glutamic acid by CNF1 leads to a dominant active form of Rho, inducing prominent actin stress fiber formation (10, 12, 35). Rho exerts its activity on the actin cytoskeleton by inducing bundling and contractibility of actin microfilaments as follows. Calcium/calmodulin-dependent activation of the myosin 2 light-chain kinase (MLCK), leading to smooth muscle contraction (by association of phosphorylated myosin with actin filaments), is normally rapidly counteracted by the myosin light-chain dephosphorylation mediated by the myosin light-chain phosphatase (MLCP). This mechanism allows smooth muscle relaxation. Rho-GTP activates the Ser/Thr kinase Rho kinase (Rock) (16), which phosphorylates MLCP, inhibiting the phosphatase activity of this enzyme. Rho activity thus reinforces the calcium-dependent phosphorylation of the myosin light chain, inducing a prolonged smooth muscular contraction (14).
Nonkeratinizing epithelia occlude paracellular spaces from the epithelial surface by means of tight junctions (or occluding junctions) located at the apical portion of the lateral membrane (21). The existence of tight junctions in the intestinal epithelium impedes the movement of molecules between the lumen and the basolateral space (19, 21). The dynamic linkage of occluding junctions to the actin cytoskeleton organization was demonstrated by the fact that treatment of polarized T84 cells with cytochalasin D influenced the paracellular-pathway permeability (18, 22). In polarized epithelia, the Rho GTP-binding protein regulates filamentous actin organization preferentially at the apical pole of polarized intestinal epithelial cells and thus influences the permeability of tight junctions (28). Recently, Rho has been shown to control both tight and adherens junctions in MDCK cells (37) and cadherin interactions in keratinocytes (2). It thus appears that Rho is a pivotal regulatory protein in epithelial cell interactions.
In the alimentary tract, migration of polymorphonuclear leukocytes (PMNs) across epithelial linings is a hallmark of disorders ranging from idiopathic inflammatory bowel disease to bacterial enterocolitis (39). PMNs stimulated by bacterial chemoattractants transmigrate by the paracellular space through epithelia and enter the luminal space, where they can exert their scavenging activities toward virulent microorganisms. PMNs must force the occluding junction to reach the luminal space (26). Pathogenic bacteria may have exploited the PMN barrier effect of tight junctions to increase their virulence. Indeed, inhibition of the Rho GTPase by bacterial toxins such as Clostridium difficile toxins A and B is associated with inflammatory bowel diseases such as pseudomembranous colitis (7).
In this work we present evidence that CNF1 is able to efface intestinal microvilli and to decrease the transepithelial migration of PMNs. This could ultimately lead to proliferation of CNF1-producing E. coli in the luminal space by providing a better nutrient supply to bacteria and by decreasing the number of phagocytic cells that microbial pathogens would encounter.
MATERIALS AND METHODS
Source of CNF1.
Highly purified CNF1, used throughout this work, was prepared as described previously (8).
Tissue culture and electrophysiology.
T84 cells (American Type Culture Collection) (passages 65 to 90), a human colonic carcinoma cell line (5), were grown and maintained as confluent monolayers on collagen-coated permeable supports as described previously with detailed modifications (23). T84 cells were grown as monolayers in a 1:1 mixture of Dulbecco-Vogt modified Eagle’s medium and Ham’s F-12 medium supplemented with 15 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (pH 7.5), 14 mM NaHCO3, 40 mg of penicillin per ml, 90 mg of streptomycin per ml, 8 mg of ampicillin per ml, and 5% newborn calf serum. Monolayers were grown on 0.33-cm2 ring-supported polycarbonate filters (Costar, Cambridge, Mass.) and utilized 6 to 14 days after plating. Steady-state resistance was reached in 4 to 6 days, with variability largely related to cell passage number. Monolayers received one weekly feeding following the initial plating. Confluent monolayers on permeable supports were constructed to permit either apical-to-basolateral migration of PMNs (inserts) or basolateral-to-apical migration of PMNs (inverted inserts) as previously described (15, 23).
To assess currents, transepithelial potentials, and resistances, a commercial voltage clamp (Bioengineering Department, University of Iowa) was employed and interfaced with an equilibrated pair of calomel electrodes submerged in saturated KCl along with a pair of Ag-AgCl electrodes submerged in Hanks’ balanced salt solution (HBSS). Agar bridges were used to interface the electrode with the solutions on either side of the monolayers (one calomel and one Ag-AgCl electrode in each well), and measurements of short-circuit current (Isc) and resistance were made as detailed elsewhere (15, 23). Isc and transepithelial resistance were monitored during the period of PMN transmigration.
Preparation of human PMNs.
PMNs were isolated from whole blood by using a gelatin-sedimentation technique (23). Briefly, whole blood, anticoagulated with citrate-dextrose, was centrifuged at 300 × g for 20 min at 20°C. The plasma and buffy coat were removed, and the gelatin-cell mixture was incubated at 37°C for 30 min to eliminate contaminating erythrocytes. Residual erythrocytes were then lysed with isotonic ammonium chloride. After being washed in HBSS without Ca2+ or Mg2+, the cells were counted and resuspended at 5 × 107 PMNs/ml. PMNs (95% pure) with 98% viability (estimated by dye exclusion) were used for experiments within 1 h after isolation.
PMN transmigration assay.
PMN transmigration experiments were performed at 37°C on 0.33-cm2 inserts or inverts with human PMNs isolated as described above. For transmigration experiments PMNs were added at final concentrations of 2 × 106 and 1 × 106 for the apical-to-basolateral direction (inserts) and the basolateral-to-apical direction (inverts), respectively. Transmigration of PMNs was initiated by applying either 1.0 μM (inserts) or 0.1 μM (inverts) N-formyl-Met-Leu-Phe to the lower reservoir for 15 min to allow a transepithelial chemotactic gradient prior to the addition of PMNs. These gradients ensured that roughly equivalent numbers of PMNs migrated across inserts and inverts (23). Transepithelial resistances and Iscs were then monitored over the 120-min course of transmigration.
Transmigration of PMNs was assayed by quantification of the azurophil granule marker myeloperoxidase as previously described (23). Briefly, after transmigration, T84 monolayers were rapidly cooled to 4°C, washed with HBSS and solubilized in 0.5% Triton X-100-containing HBSS. The pH was adjusted to 4.2 with 0.1 M Na citrate (pH 4.2), and myeloperoxidase activity was assayed by the addition of an equal volume of 1 mM 2.2′-azino-di-(3-ethyl)dithiazoline sulfonic acid and 10 mM H2O2 in 100 mM citrate buffer (pH 4.2). To quantitate PMNs which transmigrated through the monolayer into the lower reservoir, 10% Triton X-100 was added directly to the reservoir and assayed as described above. Color development was quantitated on a microtiter plate reader at 405 nm.
Incubation of T84 monolayers or PMNs with CNF1 and agonist stimulation.
T84 monolayers were incubated at 37°C with 10−10 M CNF1 in culture medium for 16 h under sterile conditions. CNF1-incubated monolayers were kept at 37°C and washed three times with warm HBSS before PMN transmigration experiments were performed. Experiments involving the response of CNF1-treated monolayers were conducted with 10 μM forskolin in HBSS or 100 μM carbachol in warm HBSS. Peak Isc responses were measured. PMNs were incubated in HBSS for 4 h at 37°C, and subsequently, transmigration of PMNs was initiated. Viability was assessed by trypan blue exclusion.
Immunofluorescence.
Fluorescence staining of control and CNF1-treated (10−10 M CNF1 for 16 h) T84 monolayers was performed before PMN transmigration. T84 monolayers grown on plastic or permeable filters were rinsed extensively in HBSS and processed for F-actin staining as follows. Cells were fixed with 3.7% paraformaldehyde (in phosphate-buffered saline [PBS], pH 7.4) for 20 min at room temperature and rinsed three times for 5 min each in buffer containing 0.2% gelatin and 0.25% Triton X-100. Cells were then incubated for 45 min in the dark with 500 nM rhodamine-phalloidin diluted in PBS (Molecular Probes, Junction City, Oreg.). Fluorescence staining of ZO-1 was performed for control and CNF1-treated T84 monolayers. Polyclonal antibodies to ZO-1 were purchased from Zymed (San Francisco, Calif.). T84 monolayers were washed in HBSS, fixed in methanol at 0°C for 20 min, and incubated with the primary antibody diluted at 2 μg/ml in HBSS containing 0.2% gelatin and 0.075% saponin in a humidified chamber. Monolayers were washed extensively in HBSS and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (Sigma), diluted at 0.5 μg/ml, for 60 min. All monolayers were washed five times in HBSS, mounted on glass slides in a phenylenediamine-glycerol-PBS medium, and observed and photographed with an epifluorescence-equipped photomicroscope.
Electron microscopy studies.
After removal from the inserts, the T84 monolayers were fixed with 2% freshly prepared formaldehyde in 0.1 M Na cacodylate (pH 7.4) for 1 h at 4°C. Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated through graded alcohol solutions, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas were thin sectioned, mounted on copper mesh grids, and stained with uranyl acetate and lead citrate. Ultrathin sections were examined on a Jeol 1200 XII electron microscope. The numbers of microvilli seen per epithelial cell were counted in a random section on 20 10−10 M CNF1-treated and untreated T84 cells.
Data presentation.
Resistance time courses were compared by two-factor analysis of variance. Myeloperoxidase assay data and numbers of microvilli per epithelial cell were compared by Student’s t test. Values are expressed as the means and standard errors of the means (SEM) for n experiments.
RESULTS
CNF1 induces reorganization of the T84 cell actin cytoskeleton.
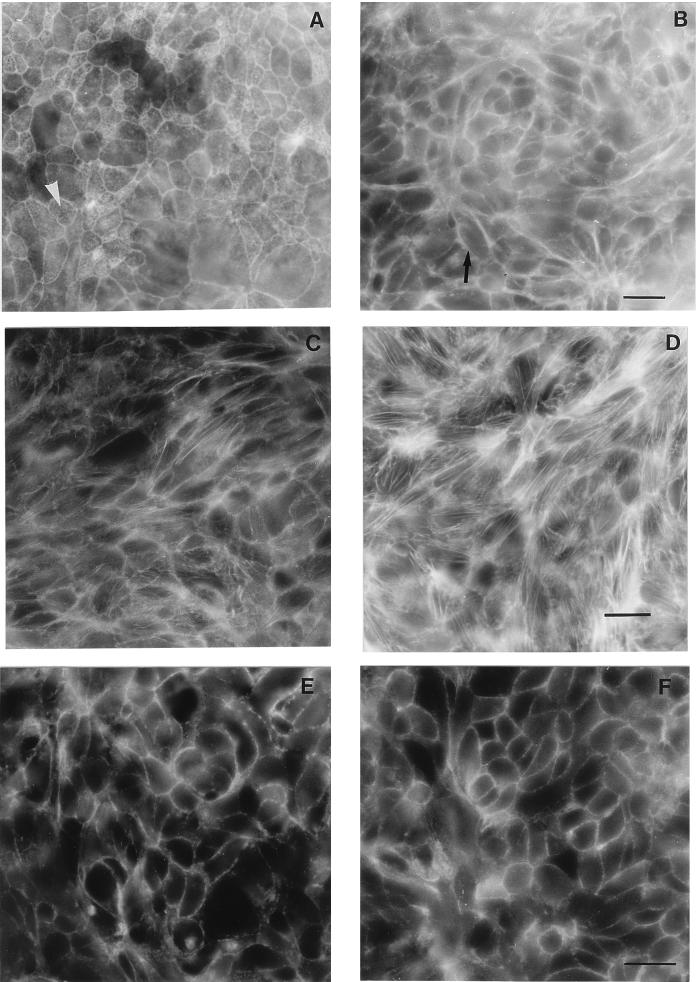
Since it has been shown that CNF1 induces a prominent F-actin reorganization in most cultured cells (10), we tested the effect of this toxin (10−10 M for 16 h) on the T84 cell actin cytoskeleton. Formation of actin stress fibers was observed on the basolateral face of CNF1-treated T84 cells compared with control preparations (Fig. 1C and D). In addition, a disappearance of the punctuate fluorescence corresponding to microvilli, in the control preparation, was observed on the apical surface of CNF1-treated cells (Fig. 1A and B). Inhibition of Rho activity by the Clostridium botulinum toxin C3 transferase in T84 epithelial cells leads to dissociation of the tight-junction-associated protein ZO-1 (28). Treatment of T84 cells with CNF1 did not modify the immunofluorescent pattern of ZO-1 (Fig. 1E and F). Together these observations are indicative of a major remodelling of F-actin structures, including disorganization of microvilli at the apical surface and formation of stress fibers at the basolateral face in CNF1-treated cells without tight-junction disruption. Thus, as previously reported (2, 28, 37), Rho plays a major role in the regulation of F-actin organization in polarized epithelial cells.
FIG. 1.
F-actin and ZO-1 fluorescent staining of T84 cell monolayers incubated with or without CNF1. (A and C) F-actin contents of apical and basolateral faces, respectively, of control cells. (B and D) F-actin contents of apical and basolateral faces, respectively, of cells treated with CNF1 (10−10 M) for 16 h. The arrow in panel B shows the absence of F-actin microvilli, whereas the arrowhead in panel A shows F-actin present in microvilli. (E and F) Distribution of the junctional protein ZO-1 in T84 control cells and in T84 cells exposed to CNF1 (10−10 M) for 16 h, respectively. Bars, 10 μm.
CNF1 induces effacing of the microvillus structure.
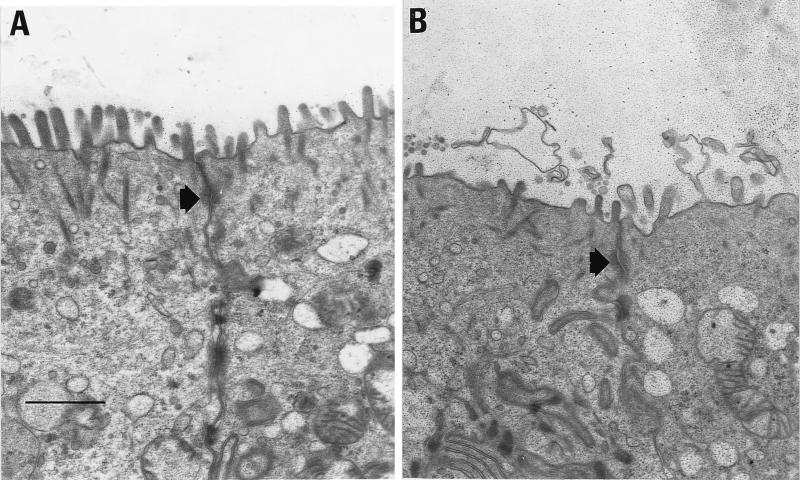
Using transmission electron microscopy, we next examined T84 cells after incubation with CNF1 (10−10 M for 16 h). CNF1-treated T84 cells exhibited effacing of the brush border compared to untreated preparations (Fig. 2A and B). The number of microvilli seen per cell was considerably reduced in CNF1-treated cells in comparison with untreated T84 epithelial cells (10 ± 5 versus 28 ± 9, for treated and untreated cells, respectively [P < 0.01]). The few microvilli still present on CNF1-treated cells were generally short. Tight junctions appeared to be unaffected in CNF1-treated cells compared with control preparations (Fig. 2A and B, arrows). We can conclude from these observations that actin reorganization, induced by CNF1 activation of Rho, affects organization of microfilaments in microvilli.
FIG. 2.
Electron microscopic study of T84 cell monolayers incubated in the presence or absence of CNF1. (A) Section of a T84 monolayer not treated with CNF1; (B) section of a T84 monolayer incubated with CNF1 (10−10 M) for 16 h. The arrows indicate positions of tight junctions. Bar, 5 μm.
Incubation of T84 cells with CNF1 does not modify epithelial permeability.
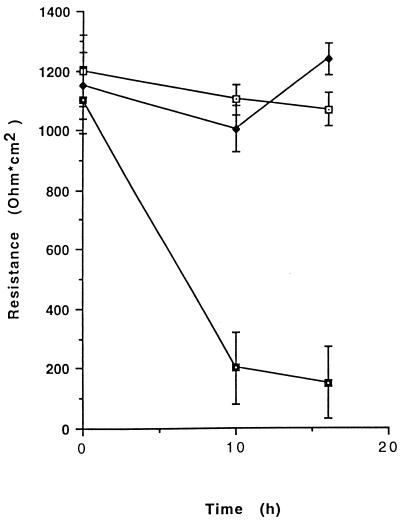
T84 cell monolayers were incubated with 10−10 M purified CNF1, and transepithelial resistance was measured at the onset of CNF1 addition and after 10 and 16 h. As shown in Fig. 3, CNF1 did not significantly alter the barrier function of epithelial monolayers, since a transepithelial resistance of 1,070 ± 123 Ω/cm2 was found for CNF1-treated T84 cells, versus 1,250 ± 115 Ω/cm2 for control untreated monolayers, after 16 h.
FIG. 3.
Measurements of transepithelial resistances in T84 cell monolayers after 10 and 16 h of incubation with either 1 × 10−10 M CNF1 or 2 × 10−3 mM EDTA. Incubation of confluent monolayers with CNF1 (open squares) showed no effect on transepithelial resistance during the course of incubation compared to results for controls (solid diamonds). Monolayers responded normally to EDTA (solid squares) by decreasing junctional resistances. Results are presented as means ± SEM for five experiments.
CNF1-treated monolayers showed a normal Isc response to cyclic AMP agonist stimulation by forskolin (10 μM) and to the Ca2+ agonist carbachol (100 μM). Indeed, control values were 45 ± 2.9 and 65.8 ± 3.4 μA/cm2 for forskolin and carbachol, respectively, whereas after incubation of cells with 10−10 M CNF1 for 16 h, values of 43.9 ± 2.9 and 67.8 ± 2.7 μA/cm2 were obtained (the differences are not significant) (data not shown).
Incubation of T84 monolayers with CNF1 alters the N-formyl-Met-Leu-Phe-induced PMN transmigration.
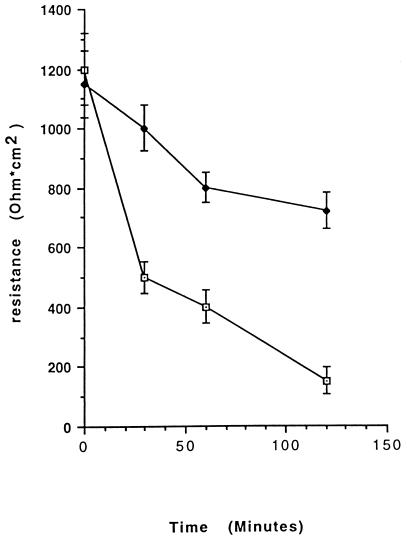
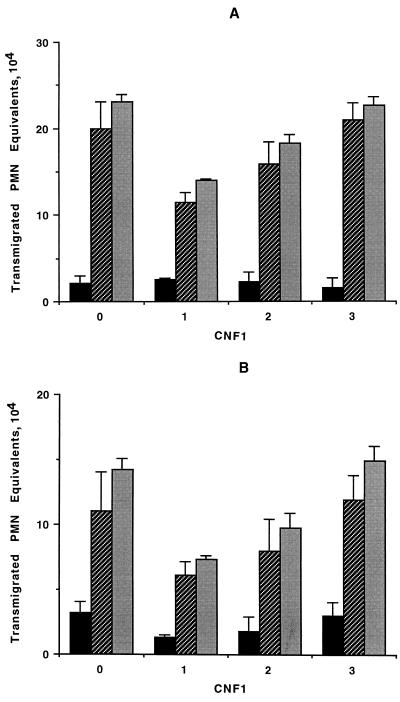
Incubation of T84 monolayers with 10−10 M CNF1 for 16 h significantly reduced the decrease in transepithelial resistance observed with a luminal-to-basolateral-directed PMN transmigration (Fig. 4). As evident in Fig. 5, incubation of monolayers with CNF1 (10−10 M for 16 h) decreased the efficiency of PMN transmigration as assessed by myeloperoxidase measurement, both in the basolateral-to-luminal (Fig. 5A) and luminal-to-basolateral (Fig. 5B) directions. Incubation of monolayers with CNF1 decreased the total number of PMNs transmigrating the monolayers into the reservoirs, since 23.4 × 104 ± 2.7 × 104 PMN cell equivalents were found in control untreated preparations whereas 11.7 × 104 ± 1.9 × 104 PMN cell equivalents were observed with 10−10 M CNF1-treated monolayers after 120 min of PMN migration in the basolateral-to-luminal direction (P < 0.01) (Fig. 5A). Parallel experiments with the luminal-to-basolateral direction yielded similar results (14.2 × 104 ± 3.1 × 104 versus 7.1 × 104 ± 2.3 × 104 PMN cell equivalents for control and 10−10 M CNF1-treated monolayers, respectively [P < 0.01]) (Fig. 5B). In both cases the blocking effect of CNF1 was dose dependent, with more than 50% inhibition at the maximum concentration used (10−10 M).
FIG. 4.
Apical-to-basolateral transmigration of 2 × 106 PMNs across confluent T84 monolayers in the presence (solid diamonds) or absence (open squares) of 10−10 M CNF1. Monolayers incubated with CNF1 demonstrated less of a decrease in the transepithelial resistance during the course of transmigration than did controls. Results are presented as means ± SEM for 6 to 12 monolayers.
FIG. 5.
Myeloperoxidase assay of PMNs in the monolayers (black bars) and reservoirs (hatched bars) after 2 h of transmigration. Grey bars, total. T84 monolayers were incubated with no CNF1 (bars 0) or with 10−10 M (bars 1), 10−11 M (bars 2), or 10−12 M (bars 3) CNF1. (A) Transmigration of PMNs in the physiological (basolateral-to-apical) direction was diminished in a concentration-dependent manner in the presence of CNF1. (B) Transmigration of PMNs in the nonphysiological (apical-to-basolateral) direction was also attenuated in a concentration-dependent manner in monolayers incubated with CNF1. Data are pooled from 6 to 12 individual monolayers for each condition, and results are expressed as means ± SEM.
Incubation of PMNs with CNF1 does not block PMN migration across epithelial cell monolayers.
Some CNF1 molecules, still associated with CNF1-treated T84 cell monolayers after the last washing step terminating toxin incubation, could act directly on PMNs and might modify their intrinsic mobility. To rule out this possibility, PMNs were incubated for 4 h with various concentrations of CNF1 and tested for epithelial transmigration. CNF1-treated PMNs migrated across epithelial monolayers (apically-to-basolaterally directed) similarly to untreated control PMNs (14.7 × 104 ± 2.1 × 104 versus 12.6 × 104 ± 0.6 × 104 PMN cell equivalents for control and 10−10 M CNF1-treated PMNs, respectively [the differences are not significant]) (Fig. 6). CNF1 thus acts on epithelial cells and not directly on PMNs to decrease their transmigration.
FIG. 6.
Myeloperoxidase assay of PMNs in the monolayers (black bars) and reservoirs (hatched bars) after 2 h of transmigration (apical-to-basolateral direction). Grey bars, total. PMNs were incubated for 4 h prior to transmigration either without CNF1 (bars 0) or with 10−10 M (bars 1), 10−11 M (bars 2), or 10−12 M (bars 3) CNF1. Results are presented as means ± SEM for 6 to 12 monolayers.
DISCUSSION
Transmigration of PMNs across intestinal epithelia involves a series of dynamic interactions between epithelial cells and PMNs. Although emphasis on such interactions has largely focused on PMNs (29, 30), it is now becoming increasingly clear that epithelial cells are far from passive participants in this process (15). In this study we showed that the toxin CNF1, a virulence factor produced by some pathogenic E. coli strains, might decrease the transepithelial migration of PMNs through remodelling of the cell actin cytoskeleton without affecting tight-junction permeability.
As previously described, the basolateral cytoskeletal cortex of intestinal epithelial cells can influence the migration of neutrophils (15). CNF1 could, by reorganization of the F-actin basolateral cytoskeleton cortex, decrease the number of transmigrated PMNs. However, we have shown that the role of the cytoskeletal cortex in the rate of PMN transmigration is strictly directional and that it did not influence the basolateral-to-luminal-directed migration of PMNs (15). Conversely, CNF1 acts on PMN transmigration in both the nonphysiological and physiological directions.
The Rho GTP-binding protein in T84 intestinal epithelial cells is efficiently ADP-ribosylated by the C. botulinum C3 transferase exoenzyme (28). ADP-ribosylation of Rho has been shown to result in marked selective effects on apical cytoskeletal F-actin, enhanced tight-junction permeability, perijunctional ring loss, and ZO-1 displacement from tight junctions to cytosol (28, 37) The regulatory influences of Rho thus might have functional consequences on epithelial barrier function.
C. difficile toxins A and B monoglycosylate a threonine residue present in the effector domain of the Rho GTP-binding protein (7). Glycosylation of Rho inactivates the protein by inhibiting the active Rho-GTP protein from binding its effector(s). Toxin A- or B-induced Rho inactivation provokes the reorganization of microfilaments in epithelial cells, which is followed by a drop in epithelium resistance (25) and later by massive accumulation of PMNs in the intestinal lumen (7, 31). Conversely, CNF1 induces a covalent modification of Rho, which maintains the small GTP-binding protein in an activated state promoting formation of actin stress fibers in the cell. Rho bound to GTP induces activation of Rock, which induces phosphorylation of MLCP, resulting in inhibition of this enzyme from dephosphorylating myosin light chains. This leads to the prolonged activity of the calcium/calmodulin-dependent MLCK, inducing smooth muscle contraction (16). By this mechanism Rho potentiates the Ca2+-induced phosphorylation of myosin light chains, resulting in the prolonged contraction of smooth muscles (14, 36). This mechanism might account for the regulatory influence exerted by Rho, activated by CNF1 through deamidation of glutamine 63, on the paracellular-pathway permeability to PMNs.
CNF1-treated T84 monolayers demonstrated a marked loss of apical microvilli. Effacement of the intestinal brush border has also been observed when enteropathogenic strains of E. coli colonize intestinal epithelia, providing an important mechanism of bacterial pathogenesis (6, 13).
By activating the Rho GTP-binding protein, CNF1 might provide several advantages to the colonizing bacteria. For instance, it could reduce, by reducing the ability of PMNs to cross the paracellular pathway, the number of neutrophils entering the lumen, where CNF1-producing bacteria are attached and growing. Also, by effacing microvilli CNF1 would make a flat apical surface of intestinal cells, allowing (i) better adherence and (ii) a better supply of nutrients to bacteria by having reduced the activity of microvillar absorption. By triggering membrane folding, as already reported for the invasive bacterium Shigella flexneri (1, 38), CNF1 could promote bacterial cell invasion, allowing microbes to replicate intracellularly and/or to transcytose to the blood (8). However, CNF1 has been found only associated with the bacterial cytosol and is not secreted by E. coli in culture (3, 8). The CNF1 gene neither shows an amino-terminal signal sequence for its release in the external medium by the Sec-dependent general pathway of secretion (secretion type II) (34) nor is organized in an ABC transporter operon like that involved in the secretion of the α hemolysin by E. coli (secretion type I) (34). How can a cytosolic bacterial protein such as CNF1 affect eukaryotic cells? Certain virulence factors from gram-negative bacteria, such as the invasion proteins from Shigella (Ipa proteins), Yersinia (Yop proteins), or Salmonella (Inv proteins), are secreted by a mechanism triggered by cell contact named the type III secretion system (9, 24). We have shown that CNF1 could, in certain conditions, be massively released from the uropathogenic strain E. coli J96 by cell contact (1a), indicating that CNF1 is probably secreted by E. coli via a type III mechanism.
In conclusion, CNF1, through permanent activation of the Rho GTP-binding protein, may be a virulence factor exerting new mechanisms of pathogenicity.
ACKNOWLEDGMENTS
We extend special thanks to Mireille Mari for excellent electron microscopic technical assistance.
This work was supported in part by the Fondation Pour La Recherche Médicale, Paris, France (to P.H.).
Footnotes
Corresponding author. Mailing address: INSERM Unité 364, Faculté de Médecine de Nice, Avenue de Valombrose, 06107 Nice, Cedex 02, France. Phone: (33) 4 92 03 77 07. Fax: (33) 4 93 81 94 56. E-mail: hofman@unice.fr.
REFERENCES
- 1.Adam T, Giry M, Boquet P, Sansonetti P. Rho-dependent membrane folding causes Shigella entry into cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 1a.Boquet, P., et al. Unpublished data.
- 2.Braga V M M, Machesky L M, Hall A, Hotchin N A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caprioli A, Falbo V, Roda L G, Ruggeri F M, Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological alterations. Infect Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chardin P. Structural conservation of Ras-related proteins and its functional implications. In: Dickey B F, Birnbaumer L, editors. Handbook of experimental pharmacology, GTPases in biology. Heidelberg, Germany: Springer Verlag; 1993. pp. 159–176. [Google Scholar]
- 5.Dharmasathaphorn K, McRobert J A, Mandel K G, Tisdale L D, Masui H. A human colonic tumor cell that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:6204–6208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichel-Streiber C, Boquet P, Sauerborn M, Thelestam M. Large clostridial toxins, a family of glycosyl transferases modifying small GTP-binding proteins. Trends Microbiol. 1996;4:375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]
- 8.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanié L, Oswald E, Boquet P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 9.Finlay B, Cossart P. Exploitation of mammalian cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentini C, Arancia G, Caprioli A, Falbo V, Ruggeri F M, Donelli G. Cytoskeletal changes induced in Hep-2 cells by the cytotoxic necrotizing factor of Escherichia coli. Toxicon. 1988;26:1047–1056. doi: 10.1016/0041-0101(88)90203-6. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of p21 Rho by glutamine deamidation. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 13.Galan J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Gong M C, Iisuka K, Nixon G, Browne J P, Eccleston A, J F, Sugai M, Kobayashi S, Somlyo A V, Somlyo A P. Role of guanine nucleotide-binding protein Ras family or trimeric proteins or both in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofman P, D’Andrea L, Carnes D, Colgan S P, Madara J L. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am J Physiol. 1996;271:312–320. doi: 10.1152/ajpcell.1996.271.1.C312. [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–247. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 17.Machesky L M, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- 18.Madara J L, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absortive cells: further evidence that the cytoskeleton may influence paracellular permeability. J Cell Biol. 1986;102:2125–2135. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madara J L. Intestinal absortive cell tight junctions are linked to cytoskeleton. Am J Physiol. 1987;253:171–175. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- 20.Madara J L, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 1987;253:854–861. doi: 10.1152/ajpcell.1987.253.6.C854. [DOI] [PubMed] [Google Scholar]
- 21.Madara J L, Stafford J, Barenberg D, Carlson S. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol. 1988;254:416–423. doi: 10.1152/ajpgi.1988.254.3.G416. [DOI] [PubMed] [Google Scholar]
- 22.Madara J L. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madara J L, Colgan S, Nusrat A, Delp C, Parkos C. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial monolayers transmigration. J Tissue Culture Methods. 1992;14:209–216. [Google Scholar]
- 24.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence-type III secretion and pathogenicity islands. Emerging Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore R, Pothoulakis C, Lamont T, Carlson S, Madara J L. C. difficile toxin A increases intestinal permeability and induces Cl− secretion. Am J Physiol. 1990;258:165–172. doi: 10.1152/ajpgi.1990.259.2.G165. [DOI] [PubMed] [Google Scholar]
- 26.Nash S, Stafford J, Madara J L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobes C D, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 28.Nusrat A, Giry M, Turner J R, Colgan S P, Parkos C A, Lemichez E, Boquet P, Madara J L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkos C P, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkos C A, Colgan S P, Liang T W, Nusrat A, Bacarra A E, Carnes D K, Madara J L. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price A B, Davies D R. Pseudomembranous colitis. J Clin Pathol. 1977;30:1–12. doi: 10.1136/jcp.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridley A J, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–400. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 33.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 34.Salmond G P C. Secretion of extracellular virulence factors by plant pathogenic bacteria. Annu Rev Physiopathol. 1994;32:181–200. [Google Scholar]
- 35.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–728. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 36.Tai Y H, Flicks S A, Levine, Madara J L, Sharp G W G, Donowitz M. Regulation of tight junction resistance in T84 monolayers by elevation in intracellular Ca++: a protein kinase C effect. J Membr Biol. 1996;149:71–79. doi: 10.1007/s002329900008. [DOI] [PubMed] [Google Scholar]
- 37.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watarai M, Kamata Y, Kosaki S, Sasakawa C. Rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J Exp Med. 1997;185:281–292. doi: 10.1084/jem.185.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yardley J H. Recent developments in the therapy of inflammatory bowel disease. Proceedings of a symposium. Baltimore, Md: Johns Hopkins University; 1986. Pathology of idiopathic inflammatory bowel disease and relevance of specific cell findings: an overview; pp. 3–9. [Google Scholar]