Abstract
The neurotoxicity of epsilon-toxin, one of the major lethal toxins produced by Clostridium perfringens type B, was studied by histological examination of the rat brain. When the toxin was injected intravenously at a lethal dose (100 ng/kg), neuronal damage was observed in many areas of the brain. Injection of the toxin at a sublethal dose (50 ng/kg) caused neuronal damage predominantly in the hippocampus: pyramidal cells in the hippocampus showed marked shrinkage and karyopyknosis, or so-called dark cells. The dark cells lost the immunoreactivity to microtubule-associated protein-2, a postsynaptic somal and dendric marker, while acetylcholinesterase-positive fibers were not affected. Timm’s zinc staining revealed that zinc ions were depleted in the mossy layers of the CA3 subfield containing glutamate as a synaptic transmitter. The cerebral blood flow in the hippocampus was not altered significantly before or after administration of the toxin, as measured by laser-Doppler flowmetry, excluding the possibility that the observed histological change was due to a secondary effect of ischemia in the hippocampus. Prior injection of either a glutamate release inhibitor or a glutamate receptor antagonist protected the hippocampus from the neuronal damage caused by epsilon-toxin. These results suggest that epsilon-toxin acts on the glutamatergic system and evokes excessive release of glutamate, leading to neuronal damage.
Epsilon-toxin, produced by Clostridium perfringens type B and D strains, is the most potent clostridial toxin after botulinum and tetanus neurotoxins (34). It is secreted as an inactive prototoxin of 311 amino acids with a molecular weight of 32,700 (19), and the prototoxin is converted to the active form through cleavage in both the N- and C-terminal regions after treatment with proteases such as trypsin, chymotrypsin, and a zinc metalloprotease produced by the type B and D strains (28). The two strain types producing epsilon-toxin are etiologic agents of severe and rapidly fatal enterotoxemia in domestic animals, although they differ in the host range and also in that hemorrhagic colitis is accompanied by lamb dysentery caused by beta-toxin-producing type B. The mortality rates with both infections can be as high as 100%, and their outbreak is of great economic importance (5, 34).
Clinical signs, such as retraction of the head, opisthotonus, convulsions, agonal struggling, hazard roaming, and head pressing, are often observed during the chronically progressive course of the enterotoxemia (40). Characteristic neurologic features have also been reported for an experimental animal model: muscular incoordination, tremor, and pleurothotonos developed after the toxin was injected intravenously (i.v.) into a mouse (15). Pathological changes caused by the toxin were observed mainly in the brain (10, 14). Liquefactive necrotic foci are formed in the brains of affected animals (6), and epsilon-toxin intoxication is characterized by the occurrence of focal-to-diffuse necrotic brain lesions (7). Thus, a primary target of the toxin is considered to be the central nervous system.
Very few data are available on the mode of action of epsilon-toxin. Although epsilon-toxin has recently been demonstrated to exhibit cytotoxicity to the Madin-Darby canine kidney (MDCK) cell line through formation of a large membrane complex (36), the mechanism underlying enterotoxemia-associated brain lesions remains unknown (34). Based on the observation that perivascular edema occurred in the brains, hearts, and lungs of mice administered the toxin, damage to the vascular endothelium and impairment of the cardiorespiratory function have been implicated in the brain damage caused by epsilon-toxin intoxication (7, 10, 11). However, the fact that i.v. injected epsilon-toxin accumulates preferentially in the brain (29) cannot be explained simply by such toxicity toward the vascular endothelium. A high-affinity binding site for epsilon-toxin, which has been suggested to be on a sialoglycoprotein, exists in the synaptosomal membranes in the brain (30), and some drugs acting on the central nervous system reduce the lethality of the toxin in mice (31).
These histological and biochemical results may imply that epsilon-toxin exhibits neurotoxicity through a direct effect on a certain region with toxin-binding sites, although it is also possible that the toxin impairs the vascular endothelium and thereby causes brain edema depending on the dose of the toxin. Taking into account all of these possibilities, we have histologically examined the damage to the rat brain after i.v. administration of the toxin at various doses. With a low dose, neuronal damage occurred exclusively in the hippocampus, while with a high dose, it occurred extensively. Examination of this preferential neurotoxicity of epsilon-toxin toward the hippocampus forms the basis of this report. We characterized the epsilon-toxin-induced hippocampal lesions by means of histochemical and immunochemical methods. We also examined the effects of a glutamate release inhibitor and a glutamate receptor antagonist on the hippocampal damage caused by the toxin. Our results indicated that epsilon-toxin exhibits preferential neurotoxicity toward the hippocampus by increasing glutamatergic transmission in the area. The significance of these results with respect to the potent neurotoxicity of epsilon-toxin is discussed.
MATERIALS AND METHODS
Preparation of epsilon-toxin.
Epsilon-prototoxin was prepared from cultures of C. perfringens type B NCIB 10691 and purified by ammonium sulfate precipitation, gel filtration, and anion- and cation-exchange chromatographies, as described previously (28). Activation of epsilon-prototoxin by crude trypsin, which contained residual activity of chymotrypsin, was performed essentially in the same manner as described previously (28). Briefly, 3 μg of the purified prototoxin was incubated with 75 μg of trypsin (1:250) (Difco Laboratories, Detroit, Mich.) in 30 μl of 0.1 M phosphate buffer (pH 8.0) at 37°C for 30 min and then diluted with a Bacto Peptone-saline solution (1% Bacto Peptone [Difco Laboratories] in 0.25% NaCl).
Animals and toxin administration.
Female Sprague-Dawley rats weighing 250 to 350 g were housed under a 12-h day-night cycle with free access to food and water. The rats were injected i.v. through a tail vein with the activated epsilon-toxin or the vehicle (1% Bacto Peptone-saline solution containing trypsin). The rats were deeply anesthetized with barbiturate (65 mg/kg) at 4 or 24 h postinjection (p.i.). They were killed by transcardial perfusion with 0.1 M phosphate-buffered saline (PBS) for 3 min and then with ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 15 min. The perfusion and fixation were performed through a 21-gauge needle, which was connected to a reservoir held at a height of 120 cm (25, 42), to avoid artificial neuronal alterations (3). Glutaraldehyde (4%) in 0.12 M phosphate buffer (pH 7.4) containing 0.1% sodium sulfide was used as a fixative for Timm’s sulfide-silver reaction. The brains were removed and postfixed at 4°C in the same fixative for at least a further 24 h and then immersed in 20% sucrose at 4°C until they sank. The brains were serially sectioned in the coronal plane at a thickness of 20 μm with a vibratome (Dosaka EM Co., Kyoto, Japan) and then processed for histology. The lethality of epsilon-toxin to rats was determined by injecting 25, 50, 100, and 200 ng of the activated epsilon-toxin per kg of body weight i.v. to a group of four rats and then recording death occurring by 24 h. For the histological examination by means of hematoxylin-eosin (HE) staining, acetylcholinesterase (AChE) staining, and immunohistochemical staining and also for the observation of neurologic features and behavioral abnormalities, the rats were injected with epsilon-toxin (25, 50, or 100 ng/kg; n = 6, 14, and 14, respectively) or the vehicle (n = 10) and then sacrificed at the indicated times. Subsets of these groups were used in each experiment as described below. For the other examinations, rat groups different from those described above were used separately in each experiment (see below).
Histology of HE-stained sections.
Histological examination with HE staining was done for rats which were injected with epsilon-toxin (25, 50, or 100 ng/kg; n = 6, 12, and 8, respectively) and then sacrificed at the indicated times. The severity of brain lesions was graded by the histological assessments described by Finnie (10) and Auer et al. (1) with the following modifications: + indicates weak damage (presence of <10% shrinking and karyopyknotic neurons [so-called dark cells] and/or mild vacuolation of the neuropil), ++ indicates moderate damage (presence of 11 to 50% dark cells and/or moderate vacuolation of the neurophil), and +++ indicates marked damage (presence of >50% dark cells and severe vacuolation of the neuropil or massive necrosis). Lesions in each brain region were judged at the following levels: the hippocampus and thalamus at 3.3 mm posterior to the bregma, the striatum at the widest point of septal nuclei (approximately 0.26 mm posterior to the bregma); the cerebral cortex, paraventricular area of lateral ventricles, and corpus callosum at the level of the subfornical organ (approximately 1.4 mm posterior to the bregma); and the substantia nigra and cerebellum at 5.8 and 10.3 mm posterior to the bregma, respectively. The distance to the bregma was according to the rat brain atlas of Paxinos and Watson (33).
Histological examination by other staining.
The sections from the above-described group (given epsilon-toxin at 50 ng/kg) were subjected to further detailed histological examination by means of AChE staining (n = 6 for toxin group; n = 4 for control group) and microtubule-associated protein-2 (MAP-2) immunohistochemical staining (n = 5 for toxin group; n = 4 for control group). AChE staining based on the thiocholine method was performed to investigate the damage to cholinergic afferent fibers (13, 16, 24). Briefly, sections were incubated overnight at 37°C in Koelle medium (6 mM acetylthiocholine, 9 mM cupric ion, and 16 mM glycine in 50 mM acetate buffer at pH 5), followed by development in sulfide solution for 1 min. Ethopropazine and silver nitrate were used as the inhibitor of nonspecific esterase and the enhancer of the sulfide reaction product, respectively, for the thiocholine method. To investigate the damage to the neuronal soma and dendrites, MAP-2 immunostaining was performed (20, 23). Briefly, free-floating sections were rinsed in 10 mM PBS (pH 7.4) three times for 5 min each and then incubated in PBS containing 0.3% Triton X-100 for 60 min. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 30 min. The sections were incubated in PBS containing 1% bovine serum albumin for 10 min and then with a mouse monoclonal anti-MAP-2 antibody (1:100) (Chemicon International, Temecula, Calif.) overnight at 4°C. After five rinses with PBS, the sections were incubated with a biotinylated second antibody for 30 min at room temperature. The sections were then incubated with an avidin-biotin-peroxidase complex for 1 h at room temperature according to the supplier’s recommendations (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, Calif.). The sections were finally reacted with 3,3′-diaminobezidine (Sigma Chemical Co., St. Louis, Mo.) and H2O2. All sections were dehydrated and mounted on gelatin-coated slides. The investigation was focused mainly on the hippocampus, but other regions of the brain were also examined.
Zinc (Zn2+) staining based on the Neo-Timm method (9, 22) was performed to investigate the damage to the mossy fibers in the hippocampus. Rats (n = 7 for the group given toxin at 50 ng/kg; n = 4 for the control group) different from those described above were used because of the difference in fixation procedures between this and other staining. Briefly, the sections were incubated with the physical developer (13.2% gum arabic, 1.7% citric acid, 0.57% hydroquine, and 0.073% silver lactate in distilled water) for 2 h at 26°C, followed by incubation with 5% sodium thiosulfate for 30 min. After AChE and Neo-Timm staining, the sections were lightly counterstained with cresyl violet.
Inhibition of the glutamatergic system.
Three chemical agents (riluzole, MK-801, and CNQX) and saline only, as a control, were injected 30 min before 100 ng of epsilon-toxin per kg was injected i.v. (n = 8 per group). Riluzole (2-amino-6-trifluoromethoxybenzothiazole) (Research Biochemicals Inc., Natick, Mass.), an inhibitor of presynaptic glutamate release (1, 16), was first dissolved in 0.1 N HCl at a concentration of 20 mg/ml and then diluted five times in saline, followed by injection intraperitoneally (i.p.) at a dose of 8 mg/kg. MK-801 (hydrogen maleate) (Research Biochemicals), a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist (43), was diluted in saline to 2 mg/ml and then injected i.p. at a dose of 3 mg/kg. CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) (Research Biochemicals), a competitive non-NMDA receptor antagonist (17), was diluted in dimethyl sulfoxide to 100 μmol/ml and then injected intraventricularly (right lateral ventricle) (100 nmol). Four hours after toxin injection, the animals were perfused and fixed. Their brains were serially sectioned in the coronal plane at a thickness of 20 μm with a vibratome, and then the sections were stained with HE. The damage to the hippocampus was quantitated by an observer who was blind as to the experimental protocol by counting dark cells in the hippocampal pyramidal layer in one section, in both hemispheres, corresponding roughly to 3.3 mm posterior to the bregma. To determine the influence of the hypothermia induced by the glutamate antagonist on the experimental results, the brain temperatures in the groups given epsilon-toxin only (100 ng/kg, i.v.), MK-801 (3 mg/kg) with epsilon-toxin, and MK-801 only were monitored (n = 4 per group). The rats were anesthetized with barbiturate (30 mg/kg), and then a small hole was drilled in the cranium (0.5 mm anterior to the bregma and 3.0 mm to the right of the midline) after placement of the rats in a stereotaxic apparatus. A 5-mm cannula (18-gauge needle) was lowered onto the dural surface and secured with methacrylate glue and dental cement. One or two days following the implant surgery, the rats were briefly anesthetized with halothane while wireless brain probes (model XM-FH; Mini-Mitter Co., Inc., Sunriver, Oreg.) were inserted and secured in the cannula. MK-801 or saline was injected 30 min before toxin injection. The brain temperature was monitored before and after the toxin administration.
Measurement of cerebral blood flow.
To determine whether epsilon-toxin causes brain ischemia, which contributes to brain damage, the blood flow in the dorsal hippocampus was measured by laser-Doppler flowmetry (LDF) (BRL-100; Bio Research Inc., Nagoya, Japan) with a needle-type probe as previously reported (26). Briefly, the rats were anesthetized with barbiturate (30 mg/kg) and then placed in a stereotaxic apparatus. A median sagittal skin incision was made to expose the skull, and then a drill was used to bore a burr hole 3.3 mm posterior to the bregma and 2.0 mm to the right of the midline, according to Paxinos and Watson’s atlas (33), leaving the dura intact. An 18-gauge guide cannula attached to a stereotaxic holder (3.0 mm in length) was placed in the small hole in the skull over the hippocampus without penetrating the dura. The cannula was secured to the skull with methacrylate glue and dental cement. On the following day, the rats were anesthetized with 1.0% halothane in a 2:1 mixture of nitrous oxide and oxygen via a face mask and then placed in the stereotaxic apparatus. The rectal temperature was monitored continuously and kept close to 37°C with a thermistor-regulated servo-controlled heating blanket (CMA 150; Carnegie Medicine, Stockholm, Sweden). An LDF probe of 0.5 mm in diameter was directed through the guide cannula to the dorsal hippocampus, 2.5 mm deep into the skull. Steady-state baseline values were recorded before epsilon-toxin injection, and hippocampal blood flow was expressed as a percentage of the average of six baseline measurements taken at 5-min intervals prior to toxin injection. The rats were injected with epsilon-toxin (50 and 100 ng/kg; n = 5 for both groups) or the vehicle (n = 4), and then the blood flow in the dorsal hippocampus was monitored with the probe for 4 h after toxin administration. Evidence of bleeding following probe placement was uncommon, and when it occurred, the rat was excluded from the study.
Statistics.
All data were expressed as means ± standard errors of the means (SEM). Hippocampal blood flow data were analyzed by the Kruskal-Wallis test followed by the Mann-Whitney U test for individual group comparisons. The number of damaged neurons and the brain temperature were analyzed by one-way analysis of variance followed by Scheffé’s test. A probability level of <0.05 was considered to be statistically significant.
RESULTS
For the purpose of this study, i.e., characterization of the neurotoxicity of epsilon-toxin at a sublethal or minimal lethal dose, an attempt was made to determine the lethal dose of the toxin in rats by injecting twofold serially diluted epsilon-toxin i.v. into four rats per group. All four rats died after the administration of the toxin at a dose of 100 or 200 ng/kg, while two of the four rats injected with the toxin at a dose of 50 ng/kg died and all four rats injected with a dose of 25 ng/kg remained alive. Thus, the minimal lethal dose of epsilon-toxin in rats was roughly estimated to be in the range of 50 to 100 ng/kg. The lethality of epsilon-toxin toward rats seems to be similar to that toward mice, since the 50% mouse lethal dose of epsilon-toxin activated by crude trypsin was previously determined to be approximately 70 ng/kg (28).
Rats injected with 50 or 100 ng of the toxin per kg developed the following neurologic features. All 14 rats, when injected with the dose of 100 ng/kg, manifested upper body tremor, rigidity of the limbs, hypersensitivity, and muscular incoordination as early symptoms and then hypotonus and paralysis, which started in the lower extremities and then proceeded to the upper part of the body. Of the 14 rats injected with 50 ng of epsilon-toxin per kg, 10 showed the same symptoms. In contrast, none of the six rats injected with 25 ng of the toxin per kg showed any significant behavioral abnormality.
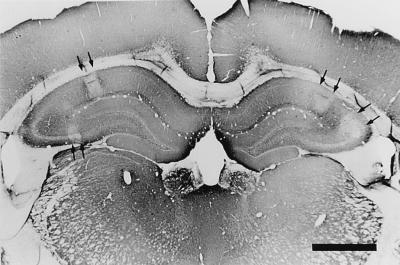
Histological examination of the brain was performed at 4 h p.i. with epsilon-toxin (Table 1 and Fig. 1). When epsilon-toxin was injected i.v. into six rats at the dose of 25 ng/kg, no pathological change was observed, except for mild hippocampal damage in one rat (data not shown). When the dose of the toxin was increased to 50 ng/kg, neuronal damage was observed frequently in the hippocampus and cortex but rarely in other regions. Epsilon-toxin intoxication at the dose of 100 ng/kg caused more severe neuronal damage in the hippocampus and cortex and mild but significant neuronal damage in other areas, such as the thalamus, cerebellum, striatum, and corpus callosum. The histology at 24 p.i. with 50 ng/kg revealed that all regions other than the substantia nigra were more or less affected. Serious damage (scored as +++) was observed in the hippocampus in one of five rats and three of eight rats with the doses of 50 and 100 ng/kg, respectively. However, none of the rats showed serious (+++) damage in the cortex. Furthermore, loss of MAP-2 immunoreactivity, which can serve as a marker for cells dying after neuronal insult (2), was clearly observed in the hippocampus but not in the other regions at 4 h p.i. with 50 ng of toxin per kg (Fig. 1). These results indicate that the rat hippocampus and cortex are highly sensitive to epsilon-toxin, with the former being more sensitive than the latter. Therefore, the subsequent study was focused on characterization of the neurotoxicity of epsilon-toxin toward the hippocampus.
TABLE 1.
Differences in damage caused by epsilon-toxin intoxication in different regions of the rat brain
| Brain region | No. of rats with the indicated lesion severitya after injection of epsilon-toxin atb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 ng/kg, 4 h (n = 7)
|
100 ng/kg, 4 h (n = 8)
|
50 ng/kg, 24 h (n = 5)
|
||||||||||
| + | ++ | +++ | Total positive | + | ++ | +++ | Total positive | + | ++ | +++ | Total positive | |
| Cerebral cortex | 4 | 1 | 0 | 5 | 5 | 2 | 0 | 7 | 2 | 1 | 1 | 4 |
| Corpus callosum | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 1 | 2 | 2 | 5 |
| Striatum | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 0 | 3 |
| Hippocampus | 3 | 1 | 1 | 5 | 3 | 2 | 3 | 8 | 1 | 1 | 2 | 4 |
| Thalamus | 1 | 0 | 0 | 1 | 6 | 0 | 0 | 6 | 1 | 2 | 0 | 3 |
| Substantia nigra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paraventricular area of lateral ventricles | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 3 | 1 | 0 | 4 |
| Cerebellum | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 1 | 0 | 3 |
The severity of lesions is expressed as +, ++, or +++ as described in Materials and Methods.
Rats were injected with the indicated dose of epsilon-toxin and sacrificed at the indicated times p.i.
FIG. 1.
Section of a rat brain at 4 h p.i. with 50 ng of epsilon-toxin per kg stained by means of the MAP-2 immunohistochemical reaction. The regions indicated by arrows are areas of the hippocampus which lost MAP-2 immunoreactivity due to the neuronal damage caused by epsilon-toxin intoxication. Bar, 1 mm.
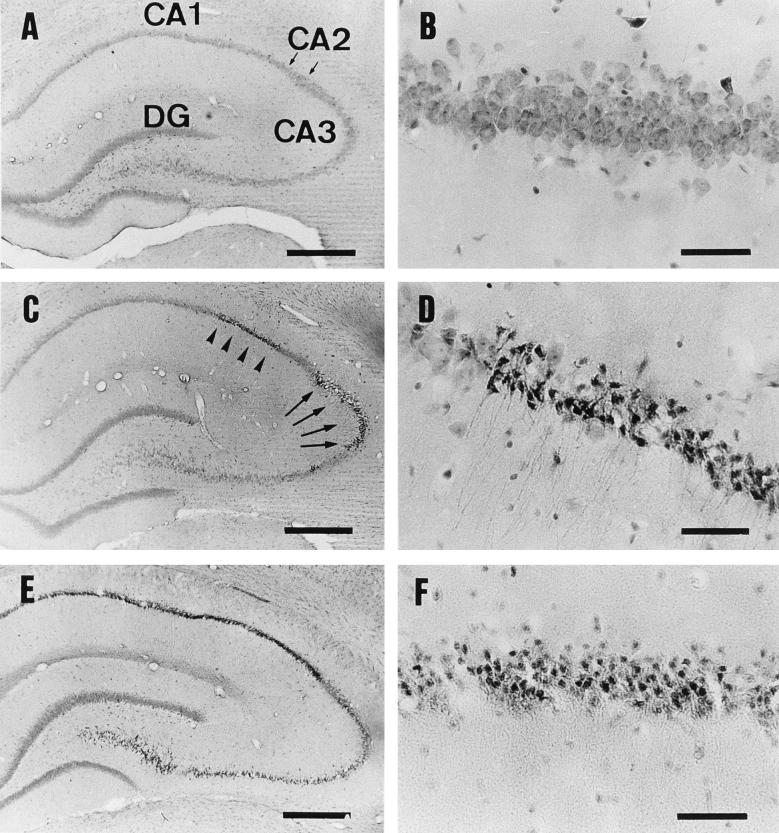
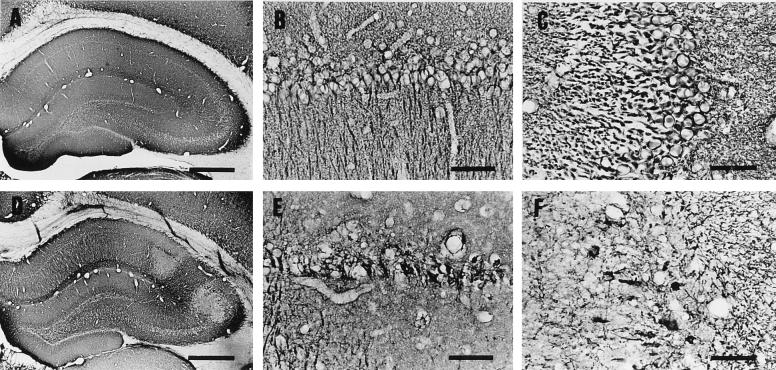
Pyramidal cells in the hippocampus shrank and exhibited karyopyknosis (so-called dark cells) at 4 h p.i. with 50 ng of epsilon-toxin per kg (Fig. 2C and D). With this dose, dark cells appeared most frequently in the CA1 and CA3 subfields but rarely in the CA2 subfield or dentate gyrus. Almost all the pyramidal cells in the hippocampus were damaged with the dose of 100 ng/kg (Fig. 2E). At 24 h p.i., they had changed to eosinophilic cells (Fig. 2F). MAP-2 immunoreactivity was evident in the dendrites and neuronal perikarya of the hippocampal pyramidal cells in a control rat, as described previously (20, 23) (Fig. 3A, B, and C). In contrast, this MAP-2 immunoreactivity was lost in a rat injected with 50 ng of epsilon-toxin per kg (Fig. 3D, E, and F).
FIG. 2.
HE-stained sections of the hippocampus. Rats were injected with the vehicle alone (A and B), 50 ng of epsilon-toxin per kg (C, D, and F), or 100 ng of epsilon-toxin per kg (E) and then sacrificed at 4 h (A through E) or 24 h (F) p.i. (A) The areas denoted CA1, CA2 (an area between two small arrows), CA3, and DG are the CA1, CA2, and CA3 subfields and the dentate gyrus in the control injected with vehicle alone, respectively. (B) Higher magnification of panel A, showing the appearance of intact pyramidal cells in the CA1 subfield. (C) Appearance of the hippocampus at 4 h p.i. with 50 ng of epsilon-toxin per kg, showing the damage to the CA1 (arrowheads) and CA3 (arrows) subfields. (D) Higher magnification of panel C, showing so-called dark cells in the CA1 subfield. (E) Appearance of the hippocampus at 4 h p.i. with 100 ng of epsilon-toxin per kg. Note that almost all of the pyramidal cells, but not the dentate gyrus, are damaged. (F) Appearance of pyramidal cells in the CA1 subfield at 24 h p.i. with 50 ng of epsilon-toxin per kg. Note that the dark cells have transformed into eosinophilic cells. Bars, 500 μm (A, C, and E) and 50 μm (B, D, and F).
FIG. 3.
MAP-2 immunostaining of the hippocampus in a rat injected with the vehicle alone (A through C) or 50 ng of epsilon-toxin per kg (D through F). The rats were sacrificed at 4 h p.i. Panels B and C are higher magnifications of the CA1 and CA3 subfields, respectively, in panel A. Panels E and F are higher magnifications of the CA1 and CA3 subfields, respectively, in panel D. Note that MAP-2 immunoreactivity was lost in the dendrite and perikarya of dark cells (D, E, and F). Bars, 500 μm (A and D) and 50 μm (B, C, E, and F).
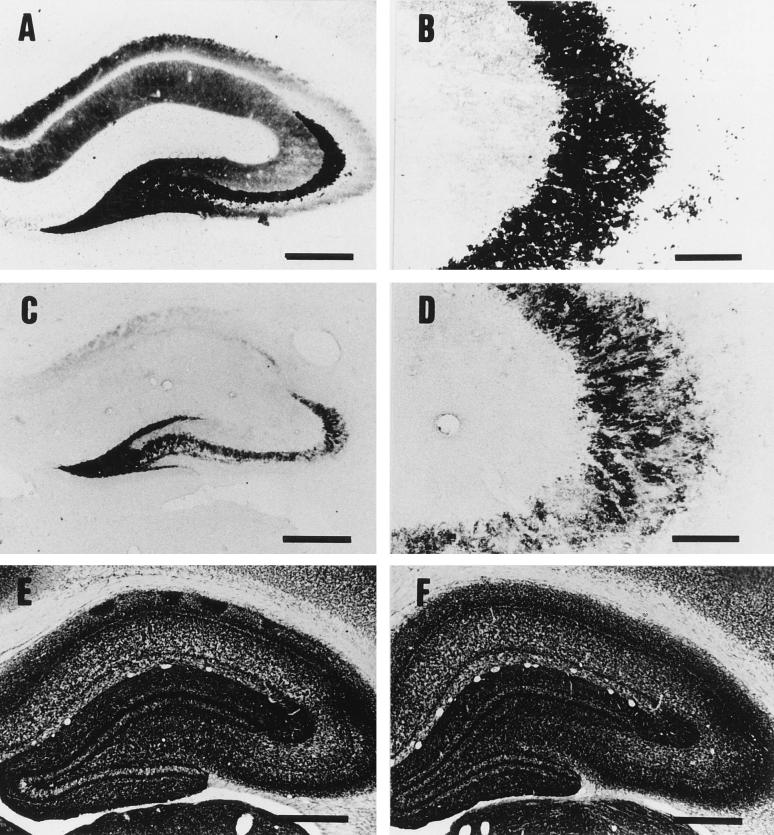
In order to determine whether epsilon-toxin exhibits differential neurotoxicity toward the glutamatergic and cholinergic systems, a sample at 4 h p.i. with 50 ng of epsilon-toxin per kg was examined by means of Timm’s zinc and AChE staining. The intensity of zinc staining decreased in many regions of the hippocampus but most prominently in the mossy fiber layers of the CA3 subfield (Fig. 4C and D). On the other hand, the density and distribution of AChE-positive fibers were unaffected by the epsilon-toxin intoxication, as shown in Fig. 4F.
FIG. 4.
Timm’s zinc staining (A through D) and AChE staining (E and F) of the hippocampus. Rats were injected with the vehicle alone (A, B, and E) or 50 ng of epsilon-toxin per kg (C, D, and F) and then sacrificed at 4 h p.i. Panels B and D are higher magnifications of the CA3 subfields in panels A and C, respectively. Note that epsilon-toxin intoxication decreased the intensity of zinc staining in the hippocampus (C), especially in the mossy fiber layers of the CA3 subfield (D), while it did not change the density or distribution of AChE-positive fibers (E and F). Bars, 500 μm (A, C, E, and F) and 100 μm (B and D).
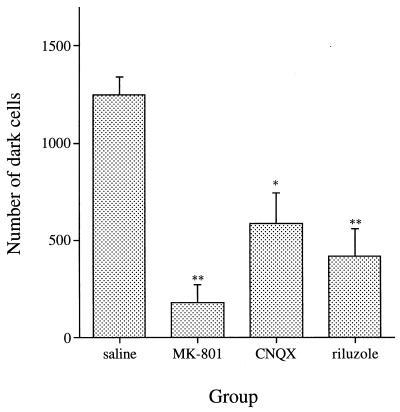
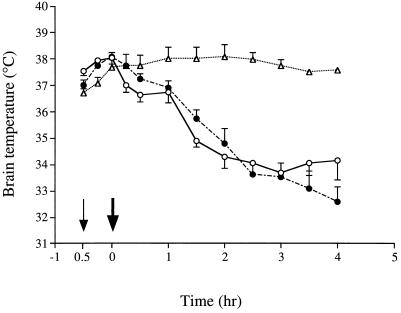
Figure 5 shows the effects of an inhibitor or antagonists of the glutamatergic system on the neurotoxicity of epsilon-toxin toward the hippocampus. Not only the glutamate receptor antagonists, MK-801 and CNQX, but also the glutamate release inhibitor, riluzole, greatly decreased the hippocampal damage caused by the toxin. These drugs have been reported to lower the brain temperature under certain conditions (4). To exclude the possibility that their protective effect may arise from their effect on temperature, the brain temperature was monitored after injection of the toxin, MK-801, and the toxin plus MK-801. MK-801 per se did not change the temperature under the conditions used. Although the toxin decreased the temperature, there was no significant difference between the groups injected with epsilon-toxin alone and MK-801 plus epsilon-toxin (Fig. 6). The use of the other drugs and toxin gave the same results (data not shown).
FIG. 5.
Effects of a glutamate release inhibitor and glutamate receptor antagonists on the neurotoxicity of epsilon-toxin toward pyramidal cells in the hippocampus. Rats were injected with riluzole (8 mg/kg, i.p.), MK-801 (3 mg/kg, i.p.), CNQX (100 nmol, lateral ventricle), or saline only (n = 8 per group) 30 min before 100 ng of epsilon-toxin per kg was injected i.v. The rats were sacrificed at 4 h p.i. of the toxin. The numbers of dark cells in HE-stained sections of the hippocampus were determined. Values represent means ± SEM. ∗, P <0.01; ∗∗, P < 0.001 (versus the saline group).
FIG. 6.
Effect of MK-801, a glutamate receptor antagonist, on the rat brain temperature. Saline or MK-801 (3 mg/kg, i.p.) was injected into three rat groups (n = 4 per group) at the time indicated by the thin arrow. Two groups were injected with 100 ng of epsilon-toxin per kg at the time indicated by the thick arrow. The brain temperature was monitored as described in Materials and Methods. Symbols: ○, saline- and epsilon-toxin-injected group; •, MK-801- and epsilon-toxin-injected group; ▵, MK-801-injected group. Values represent means ± SEM. There is no significant difference between the saline- and epsilon-toxin-injected group and the MK-801- and epsilon-toxin-injected group.
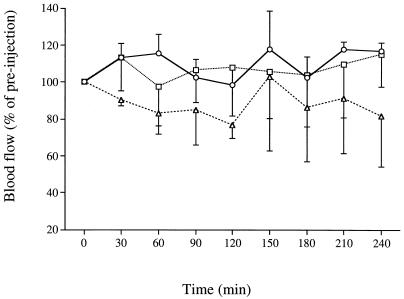
Hippocampal blood flow was monitored at 30-min intervals during the 4 h p.i. with 50 and 100 ng of epsilon-toxin per kg. The preinjection baseline of hippocampal blood flow did not decrease during this period, and no significant difference was found between the control group and the group given toxin at 50 ng/kg (Fig. 7). Hippocampal blood flow tended to decrease in the group given 100 ng/kg; however, there was no statistically significant difference between this group and the control group.
FIG. 7.
Effect of epsilon-toxin intoxication on hippocampal blood flow. The vehicle or epsilon-toxin was injected i.v. into rats at zero time. Hippocampal blood flow was monitored by LDF for 4 h p.i. Symbols: ○, group injected with vehicle alone (n = 4); □, group injected with 50 ng of epsilon-toxin per kg (n = 5); ▵, group injected with 100 ng of epsilon-toxin per kg (n = 5). Values represent means ± SEM. The results were analyzed by using the Kruskal-Wallis test followed by the Mann-Whitney U test. There is no significant difference between the toxin-injected and control groups.
DISCUSSION
This paper has presented evidence that epsilon-toxin intoxication causes both selective and extensive neurotoxicity toward the rat brain, depending on the dose of the toxin and the time after administration of the toxin. At 4 h p.i. with 100 ng of epsilon-toxin per kg, and also at 24 h p.i. with 50 ng of epsilon-toxin per kg, extensive necrosis was induced in the brain. This was consistent with the previous report by Finnie (10) that in mice given multiple sublethal doses of epsilon-toxin, lesions were found most commonly in the corpus striatum, cerebral cortex, vestibular area, corpus callosum, and corpus medullare cerebelli. In the mice receiving a single dose of toxin, however, the granular layer of the cerebellum was shown to be most vulnerable to toxin (10). In contrast, neuronal damage detectable in the rat brain shortly after injection of the toxin is confined to the cortex and hippocampus, with pyramidal cells in the CA1 and CA3 subfields in the hippocampus being highly sensitive to the toxin. The granular layer of the cerebellum is rather insensitive to epsilon-toxin in the rats. This discrepancy may arise from differences in sensitivities of the brain regions between the two animals.
Since the hippocampus is especially vulnerable in a variety of pathologic conditions, such as ischemia (37, 41) and epilepsy (18, 38), one may argue that the neurotoxicity toward the hippocampus is not due to a direct effect of epsilon-toxin on the pyramidal cells in the hippocampus. However, there was no ischemia, at least in the hippocampus, during 4 h p.i. with epsilon-toxin. The epsilon-toxin intoxication-associated seizures differed in terms of duration and frequency from those reported to cause hippocampal damage in the experimental epilepsy. These results exclude the possibility of secondary hippocampal damage due to ischemia or seizures.
Among the pyramidal cells in the hippocampus, those in the CA1 and CA3 subfields are highly sensitive to epsilon-toxin. Fibers terminating in the CA3 and CA1 subfields, mossy fibers and Schaffer’s collaterals, contain glutamate as a synaptic transmitter (8, 32). Epsilon-toxin intoxication reduced the Timm’s zinc staining intensity of the mossy fiber layers of the CA3 subfield. Zinc is stored in presynaptic vesicles together with glutamate and seems to regulate neurotransmission in the glutamatergic system (12, 39). Excessive stimulation of the glutamatergic system, which accompanies depletion of zinc stores, induces neuronal death in the hippocampus (12). Thus, it seems likely that epsilon-toxin stimulates the release of glutamate from presynaptic vesicles, leading to the neuronal damage to the CA3 and CA1 subfields in the hippocampus.
In contrast to the effect on zinc staining, epsilon-toxin intoxication did not alter the density or distribution of AChE-positive fibers, which correspond to cholinergic fibers (27). In addition, the prior administration of either a glutamate receptor antagonist or a glutamate release inhibitor reversed the hippocampal damage caused by epsilon-toxin. This supports our speculation that epsilon-toxin acts preferentially on neurons directly through the glutamatergic system. It may be possible that the toxin binds to the presynaptic sites of glutamatergic fibers and thereby induces excessive release of glutamate, which in turn results in postsynaptic dendritic damage and death of the pyramidal cells.
Nagahama and Sakurai reported that the administration of the toxin resulted in a significant decrease in the dopamine level in the mouse brain and that drugs inhibiting the release and receptors of dopamine lessened the lethal effect of epsilon-toxin (31). They used epsilon-toxin in amounts far exceeding the minimal lethal dose to examine its lethal toxicity. In the present study, we used the toxin in the sublethal-to-minimal lethal dose range to examine its selective neurotoxicity. Thus, it seems likely that the neurotoxicity of epsilon-toxin involves the stimulation of neurotransmitter release from the glutamatergic system at a low dose and from the dopaminergic system at a high dose. Since impairment of the blood-brain barrier competence would be necessary for epsilon-toxin to reach and spread throughout the brain (11, 34), the toxin seems to exhibit toxicity toward the endothelia of brain blood vessels. Although no appreciable brain edema was observed in this study, higher doses of epsilon-toxin would cause brain edema (7, 11, 34). Alternatively, the partially purified epsilon-toxin used in the previous studies might have contained other toxins, e.g., lambda-toxin, which increases vascular permeability (21), and the brain edema observed may be due to the synergistic effect of epsilon-toxin and other toxins. Further study is necessary to draw a conclusion regarding the pathogenesis of the brain edema.
Of the many cell lines so far examined, only the MDCK cell line is susceptible to epsilon-toxin (35), suggesting that a receptor specific for the toxin is present only on susceptible cells. MDCK cells require as much as 200 ng of epsilon-toxin per ml to be killed, being far less sensitive than the rat hippocampus. The rat hippocampus seems to be useful for studies on identification of the epsilon-toxin-specific receptor.
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministry of Education, Science and Culture of Japan.
We thank Shinichi Yamagami for excellent technical assistance.
REFERENCES
- 1.Auer R N, Olsson Y, Siesjö B K. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984;33:1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- 2.Book A A, Fischer I, Yu X J, Iannuzzelli P, Murphy E H. Altered expression of microtubule-associated proteins in cat trochlear motoneurons after peripheral and central lesions of the trochlear nerve. Exp Neurol. 1996;138:214–226. doi: 10.1006/exnr.1996.0060. [DOI] [PubMed] [Google Scholar]
- 3.Brierley J B. Cerebral hypoxia. In: Blackwood W, Corsellis J A N, editors. Greenfield’s neuropathology. 3rd ed. London, United Kingdom: Edward Arnold; 1976. pp. 43–85. [Google Scholar]
- 4.Buchan A, Pulsinelli W A. Hypothermia but not the N-methyl-d-aspartate antagonist, MK-801, attenuates neuronal damage in gerbils subjected to transient global ischemia. J Neurosci. 1990;10:311–316. doi: 10.1523/JNEUROSCI.10-01-00311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton A, Fraser G. Clostridium. In: Buxton A, Fraser G, editors. Animal microbiology. Vol. 1. Oxford, United Kingdom: Blackwell Scientific Publications; 1977. pp. 205–228. [Google Scholar]
- 6.Buxton D, Linklater K A, Dyson D A. Pulpy kidney disease and its diagnosis by histological examination. Vet Res. 1978;102:241. doi: 10.1136/vr.102.11.241. [DOI] [PubMed] [Google Scholar]
- 7.Buxton D, Morgan K T. Studies of lesions produced in the brains of colostrum-deprived lambs by Clostridium welchii (C. perfringens) type D toxin. J Comp Pathol. 1976;86:435–447. doi: 10.1016/0021-9975(76)90012-8. [DOI] [PubMed] [Google Scholar]
- 8.Crawford I L, Connor J D. Localization and release of glutamic acid in relation to hippocampal mossy fiber pathway. Nature. 1973;244:442–443. doi: 10.1038/244442a0. [DOI] [PubMed] [Google Scholar]
- 9.Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 10.Finnie J W. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1984;94:445–452. doi: 10.1016/0021-9975(84)90031-8. [DOI] [PubMed] [Google Scholar]
- 11.Finnie J W, Hajduk P. An immunohistochemical study of plasma albumin extravasation in the brain of mice after the administration of Clostridium perfringens type D epsilon toxin. Aust Vet J. 1992;69:261–262. doi: 10.1111/j.1751-0813.1992.tb09878.x. [DOI] [PubMed] [Google Scholar]
- 12.Frederickson C J, Hernandez M D, Goik S A, Morton J D, McGinty J F. Loss of zinc staining from hippocampal mossy fibers during kainic acid induced seizures: a histofluorescence study. Brain Res. 1988;446:383–386. doi: 10.1016/0006-8993(88)90899-2. [DOI] [PubMed] [Google Scholar]
- 13.Fujisawa M, Itano T, Miyamoto O, Masada T, Tokuda M, Matsui H, Nagao S, Hatase O. Mutual interaction between host and graft tissues in embryonic neural transplantation. Neuroreport. 1994;5:805–808. doi: 10.1097/00001756-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Gardner D E. Pathology of Clostridium welchii type D enterotoxemia. II. structural and ultrastructural alteration in the tissues of lambs and mice. J Comp Pathol. 1973;83:509–524. doi: 10.1016/0021-9975(73)90009-1. [DOI] [PubMed] [Google Scholar]
- 15.Gordon W S, Stewart J, Holman H H, Taylor A W. Blood changes and post-mortem findings following intravenous inoculation of sheep with culture filtrates of Cl. welchii, types A, C and D. J Pathol Bacteriol. 1940;59:251–269. [Google Scholar]
- 16.Henderson Z. Sprouting of cholinergic axons does not occur in the cerebral cortex after nucleus basalis lesions. Neuroscience. 1991;44:149–156. doi: 10.1016/0306-4522(91)90257-o. [DOI] [PubMed] [Google Scholar]
- 17.Honore T, Davies S N, Drejer J, Fletcher E J, Jacobsen P, Lodge D, Nielsen F E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 18.Hosokawa J, Itano T, Usuki T, Tokuda M, Matsui H, Janjua N A, Suwaki H, Okada Y, Negi T, Murakami T H, Konishi R, Hatase O. Morphological changes in the hippocampus in amygdaloid kindled mouse. Epilepsy Res. 1995;20:11–20. doi: 10.1016/0920-1211(94)00058-5. [DOI] [PubMed] [Google Scholar]
- 19.Hunter S E C, Clarke I N, Kelly D C, Titball R W. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect Immun. 1992;60:102–110. doi: 10.1128/iai.60.1.102-110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irving E A, McCulloch J, Dewar D. Intracortical perfusion of glutamate in vivo induces alterations of tau and microtubule-associated protein 2 immunoreactivity in the rat. Acta Neuropathol. 1996;92:186–196. doi: 10.1007/s004010050507. [DOI] [PubMed] [Google Scholar]
- 21.Jin F, Matsushita O, Katayama S, Jin S, Matsushita C, Minami J, Okabe A. Purification, characterization, and primary structure of Clostridium perfringens lambda-toxin, a thermolysin-like metalloprotease. Infect Immun. 1996;64:230–237. doi: 10.1128/iai.64.1.230-237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L, Murakami T H, Janjua N A, Itano T. Histochemical demonstration of heavy metals in mouse skin. Acta Histochem. 1995;97:383–388. doi: 10.1016/S0065-1281(11)80063-2. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa K, Matsumoto M, Niinobe M, Mikoshiba K, Hata R, Ueda H, Handa N, Fukunaga R, Isaka Y, Kimura K, Kamada T. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage—immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31:401–411. doi: 10.1016/0306-4522(89)90383-7. [DOI] [PubMed] [Google Scholar]
- 24.Koelle G B. The histochemical identification of acetylcholinesterase in cholinergic, adrenergic and sensory neurons. J Pharmacol Exp Ther. 1955;114:167–184. [PubMed] [Google Scholar]
- 25.Kurokawa Y, Tranmer B I. Interrupted arterial occlusion reduces ischemic damage in a focal cerebral ischemia model of rats. Neurosurgery. 1995;37:750–756. doi: 10.1227/00006123-199510000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Long J B, Gordon J, Bettencourt J A, Bolt S L. Laser-Doppler flowmetry measurements of subcortical blood flow changes after fluid percussion brain injury in rats. J Neurotrauma. 1996;13:149–162. doi: 10.1089/neu.1996.13.149. [DOI] [PubMed] [Google Scholar]
- 27.Mesulam M M, Geula C. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res. 1992;577:112–120. doi: 10.1016/0006-8993(92)90543-i. [DOI] [PubMed] [Google Scholar]
- 28.Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41:527–535. doi: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagahama M, Sakurai J. Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon. 1991;29:211–217. doi: 10.1016/0041-0101(91)90105-z. [DOI] [PubMed] [Google Scholar]
- 30.Nagahama M, Sakurai J. High-affinity binding of Clostridium perfringens epsilon-toxin to rat brain. Infect Immun. 1992;60:1237–1240. doi: 10.1128/iai.60.3.1237-1240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagahama M, Sakurai J. Effect of drugs acting on the central nervous system on the lethality in mice of Clostridium perfringens epsilon toxin. Toxicon. 1993;31:427–435. doi: 10.1016/0041-0101(93)90178-l. [DOI] [PubMed] [Google Scholar]
- 32.Nitsch C, Okada Y. Distribution of glutamate in layers of the rabbit hippocampal fields CA1, CA3, and the dentate area. J Neurosci Res. 1979;4:161–167. doi: 10.1002/jnr.490040302. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat in stereotaxic coordinates. New York, N.Y: Academic Press; 1982. [Google Scholar]
- 34.Payne D, Oyston E. The Clostridium perfringens ɛ-toxin. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 439–447. [Google Scholar]
- 35.Payne D W, Williamson E D, Havard H, Modi N, Brown J. Evaluation of a new cytotoxicity assay for Clostridium perfringens type D epsilon toxin. FEMS Microbiol Lett. 1994;116:161–167. doi: 10.1111/j.1574-6968.1994.tb06695.x. [DOI] [PubMed] [Google Scholar]
- 36.Petit L, Gibert M, Gillet D, Laurent-Winter C, Boquet P, Popoff M R. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J Bacteriol. 1997;179:6480–6487. doi: 10.1128/jb.179.20.6480-6487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulsinelli W A, Brierley J B, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 38.Sloviter R S. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- 39.Sloviter R S. A selective loss of hippocampal mossy fiber Timm stain accompanies granule cell seizure activity induced by perforant path stimulation. Brain Res. 1985;330:150–153. doi: 10.1016/0006-8993(85)90017-4. [DOI] [PubMed] [Google Scholar]
- 40.Songer J G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyoshima T, Yamagami S, Ahmed B Y, Miyamoto O, Itano T, Tokuda M, Matsui H, Hatase O. Expression of calbindin-D28K by reactive astrocytes in gerbil hippocampus after ischaemia. Neuroreport. 1996;7:2087–2091. doi: 10.1097/00001756-199609020-00006. [DOI] [PubMed] [Google Scholar]
- 42.Voll C L, Whishaw I Q, Auer R N. Postischemic insulin reduces spatial learning deficit following transient forebrain ischemia in rats. Stroke. 1989;20:646–651. doi: 10.1161/01.str.20.5.646. [DOI] [PubMed] [Google Scholar]
- 43.Wong E H, Kemp J A, Priestley T, Knight A R, Woodruff G N, Iversen L L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]