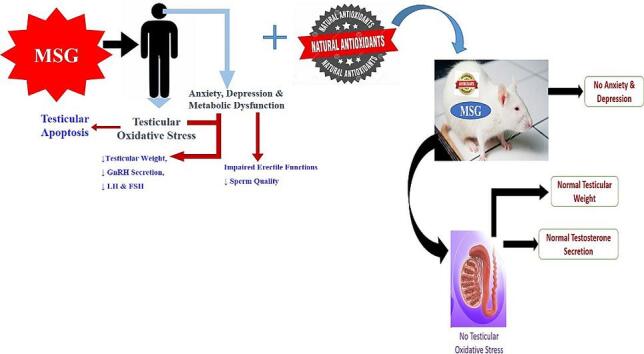
Graphical abstract
Keywords: Monosodium glutamate; Male reproduction; Sperm; Hormone, testes
Highlights
-
•
Monosodium glutamate (MSG) a food additive has multi-system effects.
-
•
MSG damages male reproductive accessory organs like prostate glands and epididymis.
-
•
MSG reduces serum concentration of testosterone, gonadotropin-releasing hormone and luteinizing hormone.
-
•
MSG alters sperm indices and male sexual behaviour.
-
•
Safety guidelines on MSG‘s tolerable limits in a biological system should be revisited.
Abstract
Monosodium glutamate (MSG) is one of the most extensively used flavour enhancers worldwide. Although it is widely regarded as a safe food additive with no recommended daily dosage, its over-consumption has been associated with notably pathophysiological events in various tissues and organs of the body. Previous studies have reported of the neuro- cardio- and hepato- toxic effects of its excessive exposure. Moreover, the food additive instigates metabolic dysfunction. It has been established that MSG damages male reproductive accessory organs like prostate glands and epididymis. In addition, it impairs serum enzymatic activities and serum levels of testosterone, gonadotropin-releasing hormone, luteinizing hormone and cholesterol. Reduced sperm count, sperm motility, sperm morphology, and sperm viability, imbalances in male reproductive hormones, alongside alteration in the histoarchitecture of the testes and other male reproductive tissues have also been connected with excessive exposure to MSG. Literature reports affirm the link between the over-consumption of MSG and reproductive organ weight and male sexual behaviour. This review article addresses the multi-systemic effects of exposure to MSG and the possible mechanism of action of the compound with a focus on the negative implications of the food additive on male reproductive functions and the possible role of natural antioxidants in male reproductive functions. carefully selected keywords were used during the literature search to gather credible and up-to-date information about the subject matter.
1. Introduction
Lifestyle changes, work pressure and socialization have increased the accessibility of processed food to the society and as a result, promote exposure to flavour enhancers, which enhances the palatability of processed food. One major additive that is commonly employed in the world is Monosodium glutamate (MSG). It is found not only in processed foods, but also in many spiced food products, disguised behind ingredient labels and listed under different names (Erb and Erb, 2003, Appaiah, 2010).
Monosodium glutamate is widely regarded as food additive that is safe for human consumption with no recommended daily dosage (Samuels, 1999). It is being abused in food due to its palatability characteristics and wide availability (Egbuonu et al., 2009). This flavour enhancer contains the sodium salt of L-glutamic acid (Hamza and Al-Harbi, 2014).
According to reports, the acceptable daily intake (ADI) for MSG set by the Joint FAO/WHO Expert Committee on Food Additives is 0.6 g per kilogram of body weight. This translates to 40 mg of MSG per kilogram of body weight per day (Roberts et al., 2018). MSG is added to various stock cubes in Africa, specifically Nigeria, to enhance the overall flavor and boost the impact, consistency, and complexity of the final preparation of such cubes (Kadir et al., 2011).
There are reports on the harmful effects of MSG on various biological functions (Farombi and Onyema, 2006, Husarova and Ostatnikova, 2013, Shimada et al., 2013), and more specifically on male reproductive system (Igwebuike et al., 2011, Rahimi Anbarkeh et al., 2019). Studies have shown that MSG damages male reproductive accessory organs like prostate glands and epididymis (Hanipah et al., 2018), decrease testosterone and epididymal sperm concentration in rats (Iamsaard et al., 2014), lower serum gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), testosterone and cholesterol concentrations (Ochiogu et al., 2015) and impairs serum ALT activities. Moreover, the compound causes testicular haemorrhage and apoptotic changes (Oforofuo et al., 1997). Exposure to MSG has also been associated with the etiology of an ovulatory sterility in females (Eweka and Om'iniabohs, 2011).
Several theories have been proposed on how the taste enhancer interferes with male reproductive function. Taste receptors are also expressed in tissues other than the tongue, including the digestive tract, liver, respiratory tract, ovaries, and testes (Damak et al., 2003, Li et al., 2005, Yang et al., 2021). Also, taste signaling factors including the taste receptor families 1, 2 and their downstream molecules, Gα and PLCβ2) are reportedly distributed in the testes and epididymis tissues and their functions evidently linked to spermatogenesis, sperm maturation, and fertilization, which are potential targets for regulating male reproduction (Mosinger et al., 2013, Luddi et al., 2019, Jiang et al., 2021, Liu et al., 2022). MSG-induced renal, hepatic, and testicular toxicity has been linked to increased oxidative stress (Hamza and Al-Harbi, 2014, Oforofuo et al., 1997), as the over-production of reactive oxygen molecules and hydrogen peroxide, which causes damage of the DNA and peroxidation of cell membranes, resulting in cell death (Rahimi Anbarkeh et al., 2019).
In this review, the authors address the multi-systemic effects and mechanism of action of MSG, focusing on the male reproductive system.
2.1. Physico-chemical properties of MSG
2.1.1. Chemistry of MSG
Monosodium glutamate (MSG) is the sodium salt of L-glutamic acid, with the IUPAC designation - sodium 2-aminopentanedioate, in which one sodium atom substitutes hydrogen atom in the carboxyl group (Stanska and Krzeski, 2016) (Fig. 1). The compound has a code number of 621 in both the International Numbering System (INS) and the European System for food additives (Mortensen et al., 2017). Monosodium glutamate is a colourless and odourless, crystalline substance that exists as a monohydrate. It has high water solubility (740 g/L); however, it is insoluble in organic solvents.
Fig. 1.
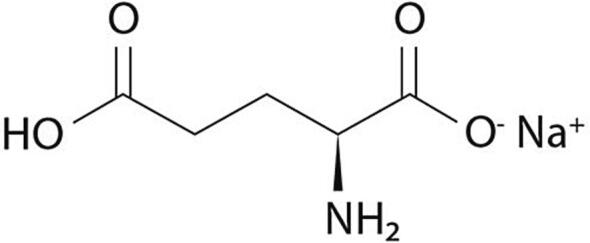
Chemical Structure of Monosodium glutamate.
The food additive, MSG, has a high melting point of 232 ℃, so it may be stored at room temperature for a long time without losing quality. The compound decomposes at a temperature above 350 °C, therefore, it maintains its stability during routine food processing (Mortensen et al., 2017).
Monosodium glutamate has a molar mass of 169.111 g/mol and a chemical formula of C5H8NO4Na and IUPAC name as sodium; 4-amino-5-hydroxy-5-oxopentanoate. Monosodium glutamate appears as white or off-white crystalline powder with a slight peptone-like odor with pH of 7.0. It is freely soluble in water and practically insoluble in ethanol or ether (Zanfirescu et al., 2019).
2.1.2. Monosodium glutamate in foods
The average dietary intake of MSG is about 0.3–1.0 g in European developing countries (Eweka, 2007). However, Asians consume 10 to 20 times the amount of MSG consumed by Europeans and Americans daily, which ranges from 1200 to 3000 mg (Brosnan et al., 2014). In Nigeria, there tends to be an excessive intake of MSG. This is because high MSG content in processed foods is sold without being labeled, therefore, and the compound is frequently advertised and regarded as a safe food additive that requires no specified daily intake limit. Major sources of MSG Fast food includes chips and snack foods; seasoning blends, frozen meals, and soups.
2.1.3. Pharmacology of monosodium glutamate: metabolism and pharmacokinetics
After consumption, MSG is absorbed from the gastrointestinal tract by an active transport system that is particular for amino acids. Under the action of several enzymes, the compound is taken by the cells and catabolized in the cytosol and mitochondria. Several metabolic end-products of MSG like lactate, glutathione, glutamine, alanine, and other amino acids (Burrin and Stoll, 2009) can be found in the stomach, intestines, and colon (Maluly et al., 2017). Asides catabolism, MSG produces alpha ketoglutarate, which enters the tricarboxylic acid cycle and releases energy (ATP) and carbon dioxide. The hepatic portal takes over Ingested glutamate that is not metabolized in the gastrointestinal tract is processed in the liver. The Krebs’s cycle converts the metabolites of the compound into energy or excretory products like urea for removal via urine (Burrin and Stoll, 2009). In the central nervous system, MSG activates glutamate receptors, causing the release of neurotransmitters that are important in both physiological and pathological processes (Chakraborty, 2018). It should be noted that there are no guidelines stating the upper limit of MSG in natural food in the literature.
2.2. Multi-systemic effects of MSG
Because of its popularity and effectiveness in boosting food palatability, the projected average daily intake (ADI) of MSG has been on the increase over the years (Sand, 2005). The associated side effects of the over-consumption of the food additive on various systems in a biological organism is widely reported in literature.
The consumption of MSG has been linked to changes in the morphology of heart tissue and cardiac rhythm (Insawang et al., 2012, Liu et al., 2013). MSG supplementation enhances oxidative stress in cardiac tissue, resulting in biochemical changes indicated by raising activities of lactate dehydrogenase, aspartate transaminase, and alanine transaminase (Kumar and Bhandari, 2013).
There are reports on the hepatotoxic effects of over-consumption of MSG, Eweka et al. (Eweka et al., 2011) reported on dilated central hepatic veins with lysed erythrocytes and deformed hepatic cells as a result of decreased liver cell membrane permeability following MSG administration in rats. Furthermore, metabolic abnormalities such as obesity, increased adiposity, hyperinsulinemia, elevated triglycerides, and LDL-cholesterol have been associated with exposure to MSG (Granholm et al., 2008) Supplementation with MSG resulted in an increase in oxidative stress markers in the hepatic tissue, which was linked to hepatic lipid peroxidation, reduced glutathione level, catalase and superoxide dismutase activities (Eweka and Om'iniabohs, 2011, Onyema et al., 2006, Nakanishi et al., 2008, Paul et al., 2012, El-Meghawry El-Kenawy et al., 2013).
Monosodium glutamate (MSG) administration has been linked to neurotoxicity and behavioural changes, such as increased aggression, decreased locomotor activity, and muscle weakness (Izumi et al., 2009, Campos-Sepúlveda et al., 2009, Rivera-Cervantes et al., 2015, Swamy et al., 2014). The aforementioned symptoms are associated with significant alterations in neuronal redox status and higher levels of oxidized lipid, concentration of nitrite, and lower concentration of antioxidants in the brain tissue (Rivera-Cervantes et al., 2015).
Elevated levels of acetylcholinesterase in the brain and serum have been reported post-administration of MSG (Onaolapo et al., 2016, Sadek et al., 2016). Moreover, the compound has been shown to precipitate a reduction in AMP-activated protein kinase activity and elevations in the levels of the apoptosis mediator - Fas ligand in the hippocampus and in the pro-apoptotic Bax protein (Sadek et al., 2016, Dief et al., 2014).
MSG administration in female Wistar rats was reported to cause alteration in the serum progesterone and esterogen level although no significant changes in the uterine morphology (Abdulghani et al., 2022). MSG also causes decreases the in the ovarian weight, and some changes in the histology of ovaries such as the congestion of blood vessels of the medulla, and increased atretic follicles were reported (Mondal et al., 2018, Agbadua et al., 2020). Mondal et al. (Mondal et al., 2017) reported that MSG suppresses the female reproductive function by impairing the functions of ovary and uterus. Also, MSG during pregnancy adversely influences fetal growth and skeletal development causing several biochemical and histological changes to the maternal and fetal liver and kidney tissues (Shosha et al., 2023).
3. Monosodium glutamate and the male reproductive system
Literature reports affirm the association between exposure to MSG and reproductive organ weight, reproductive hormones, sexual behaviour, sperm indices and reproductive capacity (Kayode et al., 2020, Haddad et al., 2021).
Scientific studies showed that the body weight of rats treated with MSG was significantly increased (Haddad et al., 2021, Abd El-Aziz et al., 2014) with mainly histological alterations including the lumina of the seminiferous tubules and interstitial tissues with exfoliation of spermatocytes and spermatids observed in rats (Nakanishi et al., 2008). The same study reported that many cells of the different types of spermatogenesis appeared necrotic with pyknotic nuclei with dilated congested blood vessels and vacuolar degeneration, thus establishing the deleterious effects of MSG on the testes (Bailey et al., 2004). These are further emphasized below.
3.1. Effect of monosodium glutamate on reproductive organ weight
Organ weight analysis is an important marker of detrimental effects of chemicals in toxicological investigations (Zenick et al., 1994). Studies have shown that changes in the weight of reproductive organs is a significant criterion for detecting changes in the levels of reproductive hormone (Iamsaard et al., 2014).
Significant decreases were detected in the weight of the epididymis, testosterone levels, and sperm concentration (Iamsaard et al., 2014). A decrease in testicular weight of Wistar rats has been reported following exposure to 4 mg/kg body weight of MSG administered intraperitoneally for 15 days (Iamsaard et al., 2014, Nayanatara et al., 2008) and subcutaneously for 75 days (Nosseir et al., 2012). Moreover, intraperitoneal administration of MSG at 2 mg/kg and 4 mg/kg for 65 days reduce testicular and epididymal weights (Fernandes et al., 2012). The weight loss in the reproductive organ weight was evident in the seminal vesicle in MSG-administered rats and rats treated with MSG exhibited partial testicular damage, characterized by sloughing of spermatogenic cells into the seminiferous tubular lumen, and their plasma testosterone levels. This is followed by decreased sperm concentration (Iamsaard et al., 2014).
In addition, the subcutaneous administration of MSG at 2 mg/g during the perinatal stage (second to the tenth day of life) in male Swiss albino mice increases the quantity of the pachytene phase of primary spermatocyte at day 75, relative to controls. In a similar study, administration of MSG (4 mg/g) at similar periods to newborn rats led to reduced pituitary glands and testes weights (Iamsaard et al., 2014).
Moreover, twelve weeks of oral exposure to MSG at 8 mg/kg decreased testicular weight of rats (Hamza and Al-Harbi, 2014). Reports also showed that the administration of MSG in Wistar rats at 3 mg/kg and 6 mg/kg for 30 days decreased the size and weight of the epididymis and seminal vesicle (Iamsaard et al., 2014).
Egbuonu et al. (Al-Husseini et al., 2022) reported that the ingestion of MSG might adversely alter the functional capacity of the prostate. Another recent study reported the toxic effect of MSG as it caused an elevation in the levels of NFκB in serum, testicular, and epididymis tissue fluid (Al-Husseini et al., 2022). Histological alternation in the reproductive organs was also reported to represent detachment and vacuolation of the seminal epithelium, degeneration of spermatogenesis edema in a lumen in testicular tissues (Al-Husseini et al., 2022). The study by Wang et al. (Wang et al., 2021) documented that MSG can cause reproductive toxicity to male mice by damaging GnRH neurons thus the hallmark of male reproductive toxicity. Meanwhile Rahimi et al. (Rahimi Anbarkeh et al., 2019) had reported that MSG-ingestion can lead to increase apoptotic changes in the germinal epithelial of the testicle.
3.2. Effect of monosodium glutamate on reproductive hormones
The hypothalamus is the principal higher coordinating centre for reproductive activities. It secretes GnRH, which stimulates the outflow of anterior pituitary hormones, viz; luteinizing and follicle-stimulating hormones, which controls testicular functions by acting on the Leydig’s and Sertoli’s cells respectively (Ekaluo et al., 2013, Liu and Veldhuis, 2014).
The administration of MSG at 4 mg/kg for 120 days in Wistar rats has been documented to reduce serum concentration of GnRH (Ochiogu et al., 2015), LH (Ochiogu et al., 2015, Nayanatara et al., 2008), FSH and testosterone (Nayanatara et al., 2008). Reports also showed that exposure of Sprague Dawley rats to orally administered MSG at 3 mg/kg, 4 mg/kg and 6 mg/kg for 30 and 48 days respectively, resulted in reduced serum testosterone levels (Iamsaard et al., 2014, Igwebuike et al., 2011).
Research affirms that GnRH is under the tight regulation of several neuropeptides (Skorupskaite et al., 2014) e.g. kisspeptin (Uenoyama et al., 2015), neurokinin-B (Krajewski et al., 2010), neuropeptide-Y (Tirassa et al., 1995), pro-opimelanocortin (POMC), galanin (Gabriel et al., 1988), vasoactive-intestinal ploypeptide (VIP) (Rojas-Castañeda et al., 2016, Yamakawa et al., 2019) and Agouti related protein (AgRP), which are negatively affected by MSG exposure.
The decreased secretion of GnRH following MSG exposure in rats has also been established to be associated with increased generation of reactive oxygen species in multiple organs and tissues including the testes (Hanipah et al., 2018). Impaired secretion of reproductive hormones at the hypothalamus and pituitary levels following MSG administration would compromise testosterone secretion and consequently negatively impact the process of spermatogenesis (Hamza and Al-Harbi, 2014, Pakarainen et al., 2005). It should however be noted that there are limited studies on the effects of MSG exposure on serum level of testosterone.
3.3. Effect of monosodium glutamate on sexual behaviour
MSG has neurotoxic effects and promotes a variety of neurobehavioral alterations, including anxiety and sadness (Narayanan et al., 2010). The compound is thought to affect the physiology of arousal, sensitization, and libido activities by causing neuroendocrine harm (Nemeroff et al., 1981).
3.4. Effect of monosodium glutamate on sperm indices
Fertility in men is evaluated solely on the quality of sperm produced from the testis. The World Health Organization has set standards for quality of semen that is necessary for reproduction. In both neonatal and adult animal studies, MSG has been shown to cause oligozoospermia (Lamperti and Blaha, 1976, França et al., 2006, Vinodini et al., 2010) and increase abnormality in sperm morphology (Onakewhor et al., 1998). Onakewhor et al. (Onakewhor et al., 1998) also submitted that MSG instigates substantial oligozoospermia and promotes aberrant sperm morphology in male Wistar rats in a dose-dependent manner. This is not surprising as glutamate receptors and transport proteins are widely released in the testicular tissue and sperm cells of mice, rats, and humans (Storto et al., 2001, Hu et al., 2004, Takarada, 2004).
França et al. (França et al., 2006) reported histological changes in the testes, as well as a decrease in spermatogenic cells in adult rats exposed to MSG. The presence of unsaturated fatty acids in the plasma membrane layer of the sperm cells and their low concentration of cytoplasmic antioxidants made spermatozoa susceptible to oxidative damage (Jones, 1979). This could be implicated in the documented increase in the death of sperm cells following MSG administration. Increased lipid peroxidation causes oxidative damage to sperm DNA, which affects membrane functions. This resulted in impaired sperm motility and in the growth of spermatozoa (Hamza and Al-Harbi, 2014, Aitken et al., 1989).
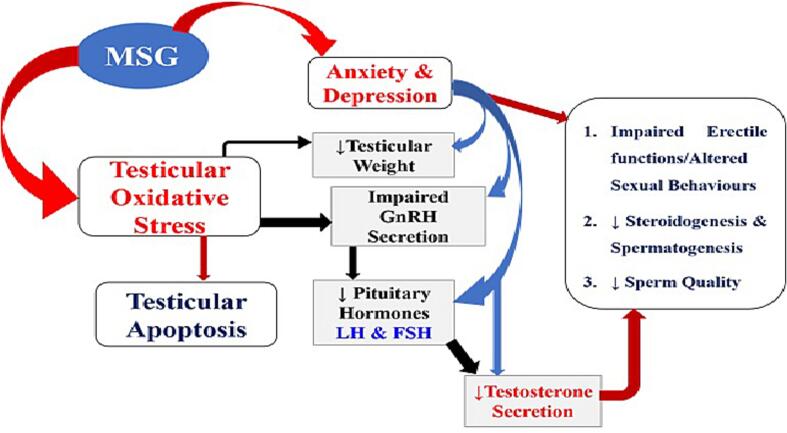
The susceptibility of hypothalamus to damages due to exposure to MSG is an indicator to the fact that it may impair the neural activation of the expression of reproductive hormones via the hypothalamic–pituitary–gonadal regulatory axis (Alalwani, 2014). Such impairment may consequently affect the reproductive ability of the affected animals. Administration of MSG to the animal models cause a reduction in the concentration of ascorbic acid in the testicular tissue and this could lead to oxidative damage in rat testes (Vinodini et al., 2010), and in different organs as reported by Moreno et al. (Moreno et al., 2005). An earlier study by Giovambattista et al. (Igwebuike et al., 2011) showed a significant decrease in the caudal epididymal sperm reserves of the rats that received MSG in comparison to the control rats. Fig. 2 shows the mechanism of MSG induced impairment of male reproductive functions.
Fig. 2.
Mechanism of MSG induced impairment of male reproductive functions.
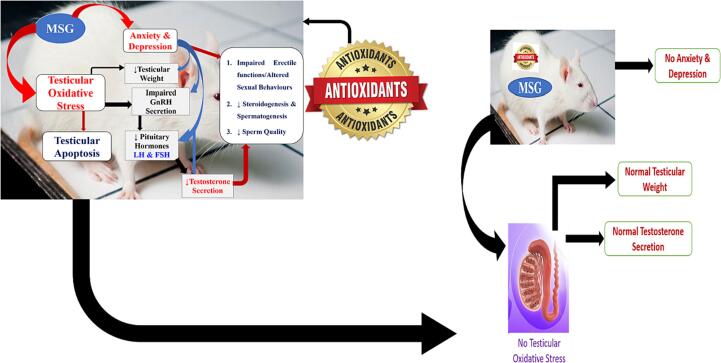
4. The protective role of natural antioxidants in MSG induced testicular toxicity
Antioxidants are substances whose presence in relatively low concentrations significantly inhibits oxidation. Owing to the continuous generation of partially reduced forms of oxygen by constitutive metabolic pathways of MSG in the reproductive tissue (Hamza and Al-Harbi, 2014), a number of protective antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GSH), glutathione-S-transferase (GST), and other non-enzymatic antioxidants, have been documented to deal with the toxic species (Jubaidi et al., 2019, Kayode et al., 2020). Since oxidation reactions produces free radicals, which start chain reactions that eventually damage the cells, antioxidants acts to terminate these chain reactions by simply removing the free radical intermediates and inhibit other oxidation reactions by being oxidized themselves (Rasheed et al., 2019).
Meanwhile, Antioxidants are abundant in fruits and vegetables, they are as well presents in other foods such as nuts, grains, and some meats, poultry, sweet potatoes, carrots, pumpkin, fish and mangoes (Xianquan et al., 2005). Antioxidants are majorly of two group viz: the primary or natural antioxidants and the secondary or synthetic antioxidants. The natural antioxidants are the chain breaking antioxidants which react with lipid radicals to convert them into more stable products. They are mainly phenolic in structures and include the antioxidant minerals such as selenium, copper and iron; antioxidant vitamins such as vitamin C, vitamin E, and vitamin B; then the phytochemicals such as the flavonoids. However, laboratory studies had significantly demonstrated the role of natural antioxidants of various classes in the prevention and management of MSG induced male reproductive toxicity (Kayode et al., 2020, Rani and Savalagimath, 2017, Hamza and Diab, 2020, Hajihasani et al., 2020).
4.1. Antioxidant minerals and vitamins effects on MSG-induced reproductive toxicity
Antioxidant minerals are the cofactor of antioxidants enzymes which their absence will definitely affect metabolism of many macromolecules such as carbohydrates. A prominent member in the category of antioxidant mineral is selenium. Selenium has a protective antioxidant effect on several tissues and laboratory studies had demonstrated that the administration of selenium diminished the effect of MSG which induced decrease in testosterone hormone levels and elevated oxidative stress markers. Selenium had a potent antioxidant effect and it is suggested to elevate the antioxidant enzymes significantly, thus decreasing the lipid peroxidation markers as often reported in MSG. Hence, selenium inhibit testicular injury by improving the testicular antioxidant (Hamza and Al-Harbi, 2014, Hamza and Diab, 2020).
Antioxidant vitamins are needed for most body metabolic functions. They include vitamin C, vitamin E, and vitamin B. Since MSG induces oxidative stress which leads to generation of free radicals, activation of proteases, phospholipases and endonucleases, transcriptional activation of apoptotic programs and genotoxicity (Kayode et al., 2020, Vinodini et al., 2010). Vitamin C also known as ascorbic acid had been extensively documented for it antioxidant protective role, and is considered the front line of defense against free radicals through its ROS scavenging, reduction of peroxides and repair of peroxidized biological membranes alongside its sequestration of iron. Ascorbic acid contributes to the redox mechanism also by salvaging the other antioxidants vitamin such as vitamin-E, urate, and β-carotene from their oxidized form while also quenching the free radicals present in the lipid membranes thus preventing the lipid peroxidation (El Kotb et al., 2020, van der Loo et al., 2003). Vitamin E as an antioxidant exhibit its protective action against lipid peroxidation by either reducing or preventing oxidative damage and by scavenging lipid peroxyl radicals (Zhang et al., 2015) Antioxidant vitamins are majorly prophylactic agent against MSG-induced reproductive toxicity (El Kotb et al., 2020).
4.2. Phytochemical antioxidant effect on MSG-induced reproductive toxicity
Antioxidant phytochemicals are found in many foods and medicinal plants, playing an important role in the prevention and treatment of chronic diseases that resulted from oxidative stress. They possess strong antioxidant and free radical scavenging abilities, as well as anti-inflammatory action, which are also the basis of other bioactivities and health benefits (Zhang et al., 2015). Examples of antioxidant phytochemicals includes tannins, flavones, triterpenoids, steroids, saponins, and alkaloids (Zhang et al., 2015). Polyphenols and carotenoids are the two main kinds of antioxidant phytochemicals and they include quercetin and flavonoids. Meanwhile, natural polyphenols are the most abundant antioxidants in human diets, their radical scavenging activities are related to substitution of hydroxyl groups in the aromatic rings of phenolics (Rokayya et al., 2014).
Several laboratory studies had reported that plants suggested to have high flavonoids and with high antioxidant properties decreases the reactive oxygen species reportedly generated from the consumption of MSG. A study had recently reported that the high antioxidant level and the active compound contained in some plants can improve the negative effect of MSG in male reproductive functions (Rahayu et al., 2021). Khaled et al. (Khaled et al., 2016) examine the protective effect of propolis on MSG-induced reproductive toxicity and suggested that propolis can be effective in the protection of MSG-induced reproductive toxicity. Mulyani (Mulyani, S., 2016) also in their study examined the effect of mung bean sprouts extract to the morphology and motility spermatozoa in mice exposed MSG reported that increase the percentage of morphology and motility of spermatozoa and attributed the fact that Mung bean sprouts are natural antioxidants as it reported to contains vitamins E and C and zinc (Mulyani, 2016). In another laboratory study quince leaf extract possess a protective effects being a natural antioxidant on the reproductive dysfunction induced by monosodium glutamate in rats (Kianifard et al., 2015), meanwhile, it has been demonstrated that flavonoid compounds from quince have strong antioxidant and immune-regulatory effects (Panche et al., 2016).
El sawy et al. (El-sawy et al., 2018) reported that the usage of camel milk as a therapy against MSG used in food industry is very indicative and protective more importantly improve the testicular dysfunctions attributed to MSG-toxicity (El-sawy et al., 2018). Ginger is a medicinal plant, medically used for its antioxidant protection activity and it has a beneficial effect on male reproductive functions had been documented to has protective effects against MSG on the rat testicular tissue (114). Fig. 3 represents the schematics of natural antioxidants in MSG induced reproductive dysfunctions.
Fig. 3.
Natural Antioxidants in MSG induced reproductive dysfunctions.
5. Conclusion
Monosodium glutamate negatively affects several indices of male reproductive functions by causing: oxidative damage to the reproductive organs, histomorphological alterations to testicular tissues, hormonal dysfunction, and ultimately reduced sperm quality. The safety guidelines on the tolerable limits of MSG in a biological system should be revisited and there should be adequate sensitization of the global populace on its potential toxic effects.
CRediT authorship contribution statement
David Tolulope OLUWOLE: Writing – review & editing. Oladipupo`Samuel EBIWONJUMI: Writing – review & editing. Lydia Oluwatoyin AJAYI: Writing – review & editing. Olubunmi Dupe ALABI: Writing – review & editing. Victor AMOS: Writing – review & editing. Grace AKANBI: Writing – review & editing. Wale Johnson ADEYEMI: Writing – review & editing. Ayodeji Folorunsho AJAYI: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Nil.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
David Tolulope OLUWOLE, Email: david.oluwole-t@cuab.edu.ng.
Ayodeji Folorunsho AJAYI, Email: aajayi22@lautech.edu.ng.
Data availability
Data will be made available on request.
References
- Abd El-Aziz G.S., El-Fark M.O., Hassan S.M., Badawoud M.H. Effects of prolonged oral intake of monosodium glutamate (MSG) on body weight and its correlation to stomach histopathological changes in male rats. Thai Veter. Med. 2014;44(2):201–208. [Google Scholar]
- Abdulghani M.A.M., Alshehade S.A., Kamran S., Alshawsh M.A. Effect of monosodium glutamate on serum sex hormones and uterine histology in female rats along with its molecular docking and in-silico toxicity. Heliyon. 2022;8(10):e10967. doi: 10.1016/j.heliyon.2022.e10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbadua O.G., Idusogie L.E., Chukwuebuka A.S., Nnamdi C.S., Sylvester S. Evaluating the protective and ameliorative potential of unripe palm kernel seeds on monosodium glutamate-induced uterine fibroids. Open Access Library Journal. 2020;7(6):1–11. [Google Scholar]
- Aitken R.J., Clarkson J.S., Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41(1):183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Alalwani A.D. Monosodium glutamate induced testicular lesions in rats (histological study) Middle East Fert. Soc. J. 2014;19(4):274–280. [Google Scholar]
- Al-Husseini A.M., Al-Waely L.A., Kazem A.A., Mashkoor N.R. IOP Conference Series: Earth and Environmental Science. 2022. Environmental effects of monosodium glutamate on (NF-κB) levels in the male reproductive system of rats; p. 1029. [Google Scholar]
- Appaiah K.M. Monosodium glutamate in foods and its biological effects. Ensuring Global Food Safety. 2010:217–226. [Google Scholar]
- Bailey S.A., Zidell R.H., Perry R.W. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004;32(4):448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- Brosnan J.T., Drewnowski A., Friedman M.I. Is there a relationship between dietary MSG and obesity in animals or humans? Amino Acids. 2014;46(9):2075–2078. doi: 10.1007/s00726-014-1771-6. [DOI] [PubMed] [Google Scholar]
- Burrin D.G., Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutrit. 2009;90(3):850S–856S. doi: 10.3945/ajcn.2009.27462Y. [DOI] [PubMed] [Google Scholar]
- Campos-Sepúlveda A.E., Martínez Enríquez M.E., Rodríguez Arellanes R., Peláez L.E., Rodríguez Amézquita A.L., Cadena Razo A. Neonatal monosodium glutamate administration increases aminooxyacetic acid (AOA) susceptibility effects in adult mice. Proc. West. Pharmacol. Soc. 2009;52:72–74. [PubMed] [Google Scholar]
- Chakraborty S.P. Patho-physiological and Toxicological Aspects of Monosodium Glutamate. Toxicol. Mech. Methods. 2018:1–35. doi: 10.1080/15376516.2018.1528649. [DOI] [PubMed] [Google Scholar]
- Damak S., Rong M., Yasumatsu K., Kokrashvili Z., Varadarajan V., Zou S., et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Dief A.E., Kamha E.S., Baraka A.M., Elshorbagy A.K. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: a potential role for cyclic AMP protein kinase. Neurotoxicology. 2014;42:76–82. doi: 10.1016/j.neuro.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Egbuonu A.C., Obidoa O., Ezeokonkwo C.A., Ezeanyika L.U., Ejikeme P.M. Hepatotoxic effects of low dose oral administration of monosodium glutamate in male albino rats. Afr. J. Biotechnol. 2009;8:3031–3035. [Google Scholar]
- Ekaluo U.B., Ikpeme E.V., Ibiang Y.B., Amaechina O.S. Attenuating role of vitamin C on sperm toxicity induced by monosodium glutamate in albino rats. J. Biol. Sci. 2013;13:298–301. [Google Scholar]
- El Kotb S.M., El-ghazouly D.E., Ameen O. The potential cytoprotective effect of Vitamin C and Vitamin E on monosodium glutamate-induced testicular toxicity in rats. Alexandria Journal of Medicine. 2020;56(1):134–147. [Google Scholar]
- El-Meghawry El-Kenawy A., Osman H.E., Daghestani M.H. The effect of vitamin C administration on monosodium glutamate induced liver injury. An experimental study. Experiment. Toxicol. Pathol. 2013;65(5):513–521. doi: 10.1016/j.etp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- El-sawy H., Soliman M.M., El-Shazly S.A., Ali H.A. Protective effects of camel milk and vitamin E against monosodium glutamate induced biochemical and testicular dysfunctions. Prog. Nutr. 2018;20:76–85. [Google Scholar]
- Erb J.E., Erb T.M. Paladins Press; 2003. The Slow Poisoning Of America. [Google Scholar]
- Eweka O. Histological studies of the effects of monosodium glutamate on the kidney of adult Wistar rats. Internet J Health. 2007;6:2. [Google Scholar]
- Eweka A., Igbigbi P., Ucheya R. Histochemical studies of the effects of monosodium glutamate on the liver of adult wistar rats. An. Med. Health Sci. Res. 2011;1(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Eweka A., Om'iniabohs F. Histological studies of the effects of monosodium glutamate on the ovaries of adult wistar rats. Ann. Med. Health Sci. Res. 2011;1(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Farombi E., Onyema O. Monosodium glutamateinduced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Hum. Exp. Toxicol. 2006;25(5):251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- Fernandes G.S., Arena A.C., Campos K.E., Volpato G.T., Anselmo-Franci J.A., Damasceno D.C., Kempinas W.G. Glutamate-induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 2012;10(1):105. doi: 10.1186/1477-7827-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França L.R., Suescun M.O., Miranda J.R., Giovambattista A., Perelló M., Spinedi E., Calandra R.S. Testis structure and function in a nongenetic hyperadipose rat model at prepubertal and adult ages. Endocrinology. 2006;147(3):1556–1563. doi: 10.1210/en.2005-0640. [DOI] [PubMed] [Google Scholar]
- Gabriel S.M., MacGarvey U.M., Koenig J.I., Swartz K.J., Martin J.B., Beal M.F. Characterization of galanin-like immunoreactivity in the rat brain: effects of neonatal glutamate treatment. Neurosci. Lett. 1988;87(1–2):114–121. doi: 10.1016/0304-3940(88)90155-3. [DOI] [PubMed] [Google Scholar]
- Granholm A.C., Bimonte-Nelson H.A., Moore A.B., Nelson M.E., Freeman L.R., Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. Journal of Alzheimers Disease. 2008;14(2):133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad M., Esmail R., Khazali H. Reporting The Effects of Exposure to Monosodium Glutamate on The Regulatory Peptides of The Hypothalamic-Pituitary-Gonadal Axis. International Journal of Fertility & Sterility. 2021;15(4):246–251. doi: 10.22074/IJFS.2021.522615.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajihasani M.M., Soheili V., Zirak M.R., Sahebkar A., Shakeri A. Natural products as safeguards against monosodium glutamate-induced toxicity. Iran. J. Basic Med. Sci. 2020;23(4):416–430. doi: 10.22038/IJBMS.2020.43060.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza R.Z., Al-Harbi M.S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Rep. 2014;1:1037–1045. doi: 10.1016/j.toxrep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza R.Z., Diab A.E.A. Testicular protective and antioxidant effects of selenium nanoparticles on Monosodium glutamate-induced testicular structure alterations in male mice. Toxicol. Rep. 2020;7:254–260. doi: 10.1016/j.toxrep.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanipah E.N., Yahya N.J., Ajik E.M., Yusoff N.A., Taib I.S. Monosodium glutamate induced oxidative stress in accessory reproductive organs of male Sprague-Dawley rats. JurnalSains Kesihatan Malaysia. 2018;16:67–73. [Google Scholar]
- Hu J.H., Yang N., Ma Y.H., Jiang J., Zhang J.F., Fei J., Guo L.H. Identification of Glutamate Receptors and Transporters in Mouse and Human Sperm. J. Androl. 2004;25(1):140–146. doi: 10.1002/j.1939-4640.2004.tb02769.x. [DOI] [PubMed] [Google Scholar]
- Husarova V., Ostatnikova D. Monosodium Glutamate Toxic Effects and Their Implications for Human Intake: A Review. JMED Research. 2013;2013:1–12. [Google Scholar]
- Iamsaard S., Sukhorum W., Samrid R., Yimdee J., Kanla P., Chaisiwamongkol K., Hipkaeo W., Fongmoon D., Kondo H. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Medica Academica. 2014;43(1):3–9. doi: 10.5644/ama2006-124.94. [DOI] [PubMed] [Google Scholar]
- Igwebuike U.M., Ochiogu I.S., Ihedinihu B.C., Ikokide J., Idika I.K. The effects of oral administration of monosodium glutamate (msg) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. VeterinarskiArhiv. 2011;81:525–534. [Google Scholar]
- Insawang T., Selmi C., Cha'on U., Pethlert S., Yongvanit P., Areejitranusorn P., Boonsiri P., Khampitak T., Tangrassameeprasert R., Pinitsoontorn C., Prasongwattana V., Gershwin M.E., Hammock B.D. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 2012;9(1):50. doi: 10.1186/1743-7075-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Yamamoto N., Matsuo T., Wakita S., Takeuchi H., Kume T., Katsuki H., Sawada H., Akaike A. Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. J. Neurochem. 2009;110(2):745–755. doi: 10.1111/j.1471-4159.2009.06178.x. [DOI] [PubMed] [Google Scholar]
- Jiang J., Liu S., Qi L., Wei Q., Shi F. Activation of ovarian taste receptors inhibits progesterone production potentially via NO/cGMP and apoptotic signaling. Endocrinology. 2021;162(3):bqaa240. doi: 10.1210/endocr/bqaa240. [DOI] [PubMed] [Google Scholar]
- Jones R.K. Migratory plant parasitic nematodes as pests of cereals. Ann. Appl. Biol. 1979;92(2):257–262. [Google Scholar]
- Jubaidi F.F., Mathialagan R.D., Noor M.M., Taib I.S., Budin S.B. Monosodium glutamate daily oral supplementation: study of its effects on male reproductive system on rat model. Syst. Biol. Reprod. Med. 2019;65(3):194–204. doi: 10.1080/19396368.2019.1573274. [DOI] [PubMed] [Google Scholar]
- Kadir R.E., Omotoso G.O., Balogun T.J., Oyewopo A.O. Effects of monosodium glutamate on semen quality and the cytoarchitecture of the testis of adult wistar rats. Internat. J. Biomed. Health Sci. 2011;7(1):39–46. [Google Scholar]
- Kayode O.T., Rotimi D.E., Kayode A.A.A., Olaolu T.D., Adeyemi O.S. Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics. 2020;8(1):7. doi: 10.3390/toxics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled F.A., Yousef M.I., Kamel K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Internat. J. Chem. Studies. 2016;4:04–09. doi: 10.1016/j.fct.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Kianifard D., Saiah G.V., Rezaee F. Study of the protective effects of quince (cydonia oblonga) leaf extract on fertility alterations and gonadal dysfunction induced by monosodium glutamate in adult male wistar rats. Roman. J. Diab. Nutrit. Metab. Dis. 2015;22:375–384. [Google Scholar]
- Krajewski S.J., Burke M.C., Anderson M.J., McMullen N.T., Rance N.E. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Bhandari U. Protective effect of Trigonella foenum-graecum Linn on monosodium glutamate-induced dyslipidemia and oxidative stress in rats. Indian Journal of Pharmacology. 2013;45(2):136–140. doi: 10.4103/0253-7613.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamperti A., Blaha G. The effects of neonatallyadministered monosodium glutamate on the reproductive system of adult hamsters. Biol Reprod. 1976;14:362–369. doi: 10.1095/biolreprod14.3.362. [DOI] [PubMed] [Google Scholar]
- Li X., Li W., Wang H., Cao J., Maehashi K., Huang L., et al. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet. 2005;1(1):27–35. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Gong T., Shi F., Xu H., Chen X. Taste receptors affect male reproduction by influencing steroid synthesis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.956981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.Y., Veldhuis J.D. The Hypothalamo-Pituitary Unit, Testis, and Male Accessory Organs. Yen & Jaffe’s Reproductive Endocrinology. 2014;272–286:e8. [Google Scholar]
- Liu Y., Zhou L., Xu H.F., Yan L., Ding F., Hao W., Cao J.M., Gao X. A preliminary experimental study on the cardiac toxicity of glutamate and the role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor in rats. Chin Med J (Engl) 2013;126(7):1323–1332. [PubMed] [Google Scholar]
- Luddi A., Governini L., Wilmskötter D., Gudermann T., Boekhoff I., Piomboni P. Taste Receptors: New Players in Sperm Biology. Int. J. Mol. Sci. 2019;20(4):967. doi: 10.3390/ijms20040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluly H., Arisseto-Bragotto A.P., Reyes F. Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects. Food Sci. Nutr. 2017;5(6):1039–1048. doi: 10.1002/fsn3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M., Sarkar K., Nath P.P., Paul G. Monosodium glutamate suppresses the female reproductive function by impairing the functions of ovary and uterus in rat. Environ. Toxicol. 2017;33(2):198–208. doi: 10.1002/tox.22508. [DOI] [PubMed] [Google Scholar]
- Mondal M., Sarkar K., Nath P.P., Khatun A., Pal S., Paul G. Monosodium glutamate impairs the contraction of uterine visceral smooth muscle ex vivo of rat through augmentation of acetylcholine and nitric oxide signaling pathways. Reprod. Biol. 2018;18(1):83–93. doi: 10.1016/j.repbio.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Moreno G., Perello M., Gaillardand R.C., Spine E. Orexin A stimulates hypothalamic-pituitary-adrenal (HPA) axis function, but not food intake in the absence of full hypothalamic NPY-ergic activity. Endocrine. 2005;26:99–100. doi: 10.1385/ENDO:26:2:099. [DOI] [PubMed] [Google Scholar]
- Mortensen A., Aguilar F., Crebelli R., Di Domenico A., Dusemund B., Lambré C. Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA J. 2017;15(7):e04910. doi: 10.2903/j.efsa.2017.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosinger B., Redding K.M., Parker M.R., Yevshayeva V., Yee K.K., Dyomina K., et al. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc. Natl. Acad. Sci. USA. 2013;110(30):12319–12324. doi: 10.1073/pnas.1302827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulyani, S. 2016. Effect of Mung Bean Sprouts Extract to the Morphology and Motility Spermatozoa in Mice Exposed Monosodium Glutamate.
- Nakanishi Y., Tsuneyama K., Fujimoto M., Salunga T.L., Nomoto K., An J.L., Takano Y., Iizuka S., Nagata M., Suzuki W., Shimada T., Aburada M., Nakano M., Selmi C., Gershwin M.E. Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. J. Autoimmun. 2008;30(1–2):42–50. doi: 10.1016/j.jaut.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Narayanan S.N., Kumar R.S., Paval J., Nayak S. Effect of ascorbic acid on the monosodium glutamate-induced neurobehavioral changes in periadolescent rats. Bratisel Lek Listy. 2010;111:247–252. [PubMed] [Google Scholar]
- Nayanatara A.K., Vinodini N.A., Damodar G., Ahemed B., Ramaswamy C.R., Shabarianth R.B., M. Role of ascorbic acid in monosodium glutamate mediated effect on testicular weight, sperm morphology and sperm countin rat testis. Journal of Chinese Clinical Medicine. 2008;3(1):1–5. [Google Scholar]
- Nemeroff C.B., Lamartiniere C.A., Mason G.A., Squibb R.E., Hong J.S., Bondy S.C. Marked reduction in gonadal steroid hormone levels in rats treated neonatally with monosodium L -glutamate: Further evidence for disruption of hypothalamic-pituitary-gonadal axis regulation. Neuroendocrinology. 1981;33:265–267. doi: 10.1159/000123243. [DOI] [PubMed] [Google Scholar]
- Nosseir N.S., Ali M.H.N., Ebaid H.M. A Histological and morphometric study of monosodium glutamate toxic effect on testicular structure and potentiality of recovery in adult albino rats. Res. J. Biol. 2012;2:66–78. [Google Scholar]
- Ochiogu I.S., Ogwu D., Uchendu C.N., Okoye C.N., Ihedioha J.I., Mbegbu E.C. Effects of monosodium-L-glutamate administration on serum levels of reproductive hormones and cholesterol, epididymal sperm reserves and testicular histomorphology of male albino rats. Acta veterinariaHungarica. 2015;63(1):125–139. doi: 10.1556/AVet.2015.011. [DOI] [PubMed] [Google Scholar]
- Oforofuo I.A.O., Onakewhor J.U.E., Idaewor P.E. The effect of chronic administration of MSG on the histology of the adult Wistar rat testes. Biosci. Res. Commun. 1997;9(2):30–56. [Google Scholar]
- Onakewhor J.U.E., Oforofuo I.A.O., Singh S.P. Chronic administration of Monosodium glutamate induces Oligozoospermia and glycogen administration in Wistar rat testes. Afr. J. Reproduct. Health. 1998;2(2):190–197. [Google Scholar]
- Onaolapo, O.J., Onaolapo, A.Y., Akanmu, M.A., Gbola, O., 2016. Evidence of alterations in brain structure and antioxidant status following 'low-dose' monosodium glutamate ingestion. Pathophysiology: the official journal of the International Society for Pathophysiology, 23(3), 147–156. [DOI] [PubMed]
- Onyema O.O., Farombi E.O., Emerole G.O., Ukoha A.I., Onyeze G.O. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J. Biochem. Biophys. 2006;43(1):20–24. [PubMed] [Google Scholar]
- Pakarainen T., Zhang F., Makela S., Poutanen M., Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- Panche A., Diwan A., Chandra S. Flavonoids: An overview. J. Nutrit. Sci. 2016;5:E47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M.V., Abhilash M., Varghese M.V., Alex M., Nair R.H. Protective effects of α-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol. Mech. Methods. 2012;22(8):625–630. doi: 10.3109/15376516.2012.714008. [DOI] [PubMed] [Google Scholar]
- Rahayu S., Annisa R., Anzila I., Christina Y.I., Soewondo A., Djati M.A., Ms, Marsilea crenata ethanol extract prevents monosodium glutamate adverse effects on the serum levels of reproductive hormones, sperm quality, and testis histology in male rats. Veterinary World. 2021;14(6):1529–1536. doi: 10.14202/vetworld.2021.1529-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi Anbarkeh F., Baradaran R., Ghandy N., Jalali M., Reza Nikravesh M., Soukhtanloo M. Effects of monosodium glutamate on apoptosis of germ cells in testicular tissue of adult rat: An experimental study. Internat. J. Reproduct. Biomed. 2019;17(4):261–270. doi: 10.18502/ijrm.v17i4.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani J., Savalagimath M.P. Effect of Dooshivishari Agada over MSG-induced reproductive toxicity w.s.r. ovary and follicle count. Ayu. 2017;38(1–2):88–92. doi: 10.4103/ayu.AYU_166_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed A., Azeez F.A., R. A review on natural antioxidants. Trad. Complement. Med. 2019 doi: 10.5772/intechopen.82636. [DOI] [Google Scholar]
- Rivera-Cervantes M.C., Castañeda-Arellano R., Castro-Torres R.D., Gudiño-Cabrera G., Feriay Velasco A.I., Camins A., Beas-Zárate C. P38 MAPK inhibition protects against glutamate neurotoxicity and modifies NMDA and AMPA receptor subunit expression. J. Mol. Neurosci. MN. 2015;55(3):596–608. doi: 10.1007/s12031-014-0398-0. [DOI] [PubMed] [Google Scholar]
- Roberts A., Lynch B., Rietjens I.M.C.M. Risk assessment paradigm for glutamate. Ann. Nutr. Metab. 2018;73(Suppl 5):53–64. doi: 10.1159/000494783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Castañeda J.C., Vigueras-Villaseñor R.M., Chávez-Saldaña M., Rojas P., Gutiérrez-Pérez O., Rojas C., Arteaga-Silva M. Neonatal exposure to monosodium glutamate induces morphological alterations in suprachiasmatic nucleus of adult rat. Int. J. Exp. Pathol. 2016;97(1):18–26. doi: 10.1111/iep.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokayya S., Li C.J., Zhao Y., Li Y., Sun C.H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014;14(11):6657–6662. doi: 10.7314/apjcp.2013.14.11.6657. [DOI] [PubMed] [Google Scholar]
- Sadek K., Abouzed T., Nasr S. Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Can. J. Physiol. Pharmacol. 2016;94(4):394–401. doi: 10.1139/cjpp-2015-0388. [DOI] [PubMed] [Google Scholar]
- Samuels A. The toxicity/safety of processed free glutamic acid (MSG): a study in suppression of information. Account. Res. 1999;6(4):259–310. doi: 10.1080/08989629908573933. [DOI] [PubMed] [Google Scholar]
- Sand J. A short history of MSG: good science, bad science and taste cultures. Gastronomica. 2005;5(4):38–49. [Google Scholar]
- Shimada A., Cairns B.E., Vad N., Ulriksen K., Pedersen A.M.L., Svensson P., Baad-Hansen L. Headache and mechanical sensitization of human pericranial muscles after repeated intake of monosodium glutamate (MSG) J. Headache Pain. 2013;14(2):1–9. doi: 10.1186/1129-2377-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shosha H.M., Ebaid H.M., Toraih E.A., Abdelrazek H.M.A., Elrayess R.A. Effect of monosodium glutamate on fetal development and progesterone level in pregnant Wistar Albino rats. Environ. Sci. Pollut. Res. Int. 2023;30(17):49779–49797. doi: 10.1007/s11356-023-25661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupskaite K., George J.T., Anderson R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update. 2014;20(4):485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanska K., Krzeski A. The umami taste: from discovery to clinical use. Otolaryngol. Pol. 2016;70(4):10–15. doi: 10.5604/00306657.1199991. [DOI] [PubMed] [Google Scholar]
- Storto M., Sallese M., Salvatore L., Poulet R., Condorelli D.F., Dell'Albani P., Marcello M.F., Romeo R., Piomboni P., Barone N., Nicoletti F., de Blasi A. Expression of metabotropic glutamate receptors in the rat and human testis. J. Endocrinol. 2001;170(1):71–78. doi: 10.1677/joe.0.1700071. [DOI] [PubMed] [Google Scholar]
- Swamy A.H., Patel N.L., Gadad P.C., Koti B.C., Patel U.M., Thippeswamy A.H., Manjula D.V. Neuroprotective activity of Pongamia pinnata in monosodium glutamate-induced neurotoxicity in rats. Indian J. Pharm. Sci. 2014;75(6):657–663. [PMC free article] [PubMed] [Google Scholar]
- Takarada T. Possible expression of functional glutamate transporters in the rat testis. J. Endocrinol. 2004;181(2):233–244. doi: 10.1677/joe.0.1810233. [DOI] [PubMed] [Google Scholar]
- Tirassa P., Lundeberg T., Stenfors C., Bracci-Laudiero L., Theodorsson E., Aloe L. Monosodium glutamate increases NGF and NPY concentrations in rat hypothalamus and pituitary. Neuroreport. 1995;6(18):2450–2452. doi: 10.1097/00001756-199512150-00003. [DOI] [PubMed] [Google Scholar]
- Uenoyama Y., Nakamura S., Hayakawa Y., Ikegami K., Watanabe Y., Deura C., Minabe S., Tomikawa J., Goto T., Ieda N., Inoue N., Sanbo M., Tamura C., Hirabayashi M., Maeda K.I., Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J. Neuroendocrinol. 2015;27(3):187–197. doi: 10.1111/jne.12257. [DOI] [PubMed] [Google Scholar]
- van der Loo B., Bachschmid M., Spitzer V., Brey L., Ullrich V., Lüscher T.F. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem. Biophys. Res. Commun. 2003;303(2):483–487. doi: 10.1016/s0006-291x(03)00360-7. [DOI] [PubMed] [Google Scholar]
- Vinodini N.A., Nayanatara A.K., Ramaswamy C., Anu V.R., Rekha D.K., Damadara G.K.M., Ahamed B., Shabarinath R.B. Study on evaluation of monosodium glutamate induced oxidative damage on renal tissue on adult wistar rats. J. Chin. Clin. Med. 2010;5(3):144–147. [Google Scholar]
- Vinodini N.A., Nayanatara A., Damodar G., Damodar B., Ramaswamy C.R., Shabarinath B., M.r., Effect of monosodium induced oxidative damage on rat testis. J. Clin. Med. 2010;3:370–373. [Google Scholar]
- Wang C.X., Zhang Y., Li Q.F., Sun H.L., Chong H.L., Jiang J.X., Li Q.C. The reproductive toxicity of monosodium glutamate by damaging GnRH neurons cannot be relieved spontaneously over time. Drug Des. Devel. Ther. 2021;15:3499–3508. doi: 10.2147/DDDT.S318223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianquan S., Shi J., Kakuda Y., Yueming J. Stability of lycopene during food processing and storage. J. Med. Food. 2005;8(4):413–422. doi: 10.1089/jmf.2005.8.413. [DOI] [PubMed] [Google Scholar]
- Yamakawa G.R., Weerawardhena H., Eyolfson E., Griep Y., Antle M.C., Mychasiuk R. Investigating the Role of the Hypothalamus in Outcomes to Repetitive Mild Traumatic Brain Injury: Neonatal Monosodium Glutamate Does Not Exacerbate Deficits. Neuroscience. 2019;413:264–278. doi: 10.1016/j.neuroscience.2019.06.022. [DOI] [PubMed] [Google Scholar]
- Yang L., Cui M., Liu B. Current progress in understanding the structure and function of sweet taste receptor. J. Mol. Neurosci. 2021;71(2):234–244. doi: 10.1007/s12031-020-01642-4. [DOI] [PubMed] [Google Scholar]
- Zanfirescu A., Ungurianu A., Tsatsakis A.M., Nițulescu G.M., Kouretas D., Veskoukis A., Tsoukalas D., Engin A.B., Aschner M., Margină D. A review of the alleged health hazards of monosodium glutamate. Compr. Rev. Food Sci. Food Saf. 2019;18(4):1111–1134. doi: 10.1111/1541-4337.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenick H., Clegg E.D., Perreault S.D., Klinefelter G.R., Gray L.E. In: Principles and Methods of Toxicology. 3rd ed. Hayes A.W., editor. Raven; New York: 1994. Assessment of male reproductive toxicity: a risk assessment approach; pp. 937–988. [Google Scholar]
- Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Li H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules (Basel, Switzerland) 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.