Abstract
Background
Diosgenin, an essential sapogenin steroid with significant biological implications, is composed of a hydrophilic sugar moiety intricately linked to a hydrophobic steroid aglycone. While the antiviral properties of diosgenin against numerous RNA viruses have been extensively documented, its potential in combating Human Immunodeficiency Virus infections remains unexplored.
Experimental procedure
This current investigation presents a comprehensive and systematic analysis of extracts derived from the leaves of Helicteres isora, which are notably enriched with diosgenin. Rigorous methodologies, including established chromatographic techniques and Fourier-transform infrared spectroscopy were employed for the characterization of the active diosgenin compound followed by molecular interaction analyses with the key HIV enzymes and mechanistic validation of HIV inhibition.
Key results
The inhibitory effects of extracted diosgenin on the replication of HIV-1 were demonstrated using a permissive cellular system, encompassing two distinct subtypes of HIV-1 strains. Computational analyses involving molecular interactions highlighted the substantial occupancy of critical active site pocket residues within the key HIV-1 proteins by diosgenin. Additionally, the mechanistic underpinnings of diosgenin activity in conjunction with standard controls were elucidated through specialized colorimetric assays, evaluating its impact on HIV-1 Reverse Transcriptase and Integrase enzymes.
Conclusions
To our current state of knowledge, this study represents the inaugural demonstration of the anti-HIV efficacy inherent to diosgenin found in the leaves of Helicteres isora, and can be taken further for drug design and development for the management of HIV infection.
Keywords: Helicteres isora, Diosgenin, Phytoconstituents, Anti-HIV activity, Preclinical, Drug discovery
Graphical abstract
Highlights
-
•
Diosgenin was extracted from the leaves of Helicteres isora Linn and subjected to comprehensive characterization.
-
•
Leaves extract of H. isora and characterized diosgenin exhibited anti-HIV activity against two different subtypes of HIV–1 strains.
-
•
Insilico and in vitro assays demonstrated inhibition of HIV-1 replication by targeting Integrase and Reverse Transcriptase.
1. Introduction
HIV infection has become a manageable infection with a wide range of antiretroviral agents (ARVs) that include chemical entities, biomolecules and therapeutic antibodies. The existing antiretrovirals are capable of controlling the pandemic, however, its complete eradication is still out of reach. By the end of 2022, at least 39 million people were living with HIV and 1.3 million were newly infected with the virus, whereas 6,30,000 individuals have died of HIV-related illness, worldwide [1]. Over the decades, it has been observed that long-term use of antiretroviral (ARV) medication causes hepatotoxicity, chronic immune activation, neurocognitive disorders, and ultimately lead to the emergence of drug-resistant viruses [2,3]. Together this has encouraged the constant mining of drug-like molecules by exploring chemical libraries, repurposing of already approved drugs and exploring traditional medicinal systems. More than 80 % of drug molecules were sourced from natural products or developed from phytochemicals prior to the introduction of high throughput screening [4].
Natural products can interact through the complex but diverse range of chemical structures and mechanisms of action. Further, evolution from biological systems makes them more adaptive and tolerable to the human body, compared to chemical pharmacophores. However, the therapeutic potential of natural products is dependent on their pharmacokinetic properties, bioavailability, and phytoconstituents which vary with plant growth conditions, harvesting methods and extraction techniques, influencing the therapeutic outcomes and side effects. Nevertheless, it should be noted that though herbal extracts or bioactive phytomolecules show promising activities in laboratory experiments, they often require further characterization, modification, optimization and clinical trials to develop them into an effective antiretroviral agent.
Recently, three Croton species have been used as herbal medicine in Africa and evaluated for cytotoxicity and HIV-1 replication inhibition potential. The leaves extract of Croton megalocarpus and aerial part extract of Croton dichogamus have demonstrated promising antiviral potential with low toxicity to healthy cells and high selectivity index [5]. Moreover, distinct Croton species, exemplified by Croton echinocarpus, demonstrated noteworthy anti-HIV activity by inhibiting reverse transcriptase [6]. Notably, alkaloids Corydine and Norisoboldine were extracted from Croton echinocarpus. Furthermore, Ermiasolides and crotocascarin ω, identified in Croton megalocarpus and Croton dichogamus, were observed to manifest anti-HIV activity, respectively [7,8].
Also, the coumarins of plant origin can act by several mechanisms, for example inhibition of HIV Reverse Transcriptase and Integrase, inhibition of cellular factors that regulate HIV-1 replication, and transmission of viral particles from infected macrophages to healthy cells. Additionally, some pyranocoumarins can target several HIV-1 replication steps, and even ensure resistance to HIV mutations [9]. It has also been demonstrated that Chinese herbal medicines boost the host immune system by increasing the CD4 cell count, hence concluded that ART medications if supplemented with herbal drugs, can delay the worsening of HIV infection [10]. The plants from the Malvaceae family have been shown to possess antiviral activity against several viruses [11,12]. Thus, it can be inferred that medicinal plants have the potential for the management of HIV/AIDS, but more studies are needed to reveal the rigorous efficacy and safety concerns by conducting clinical trials to explore the therapeutic impact of medicinal plants.
Helicteres isora, a botanical entity renowned in traditional medicinal practices, is notably recognized for its hepatoprotective attributes. Belonging to the Malvaceae family, Helicteres isora L (HI) is known as the Indian screw tree [13]. This tropical shrub, native to southeastern Asia, northern Australia, and cultivated across India, has various constituents—leaves, stem, bark, fruits, and seeds—historically harnessed within the traditional Indian System of Medicine for their curative potential against gastrointestinal maladies, dermal infections, scabies, lacerations, wounds, and snakebites, with recognized hepatoprotective attributes [14,15]. Hepatic impairment often stems from the deleterious effects of pharmaceutical agents, alcohol consumption, and viral infections. In-depth in vitro investigations on the impact of various plant parts of Helicteres isora have been conducted to assess their efficacy in mitigating liver damage [[16], [17], [18]], with the root and bark components of this botanical entity being previously documented for exhibiting hepatoprotective efficacy [17,19]. Emerging research underscores robust hepatoprotective capabilities within the aerial constituents of Helicteres isora [20]. Diosgenin, a prominent bioactive compound in Helicteres isora, has exhibited notable efficacy against RNA viruses, including Hepatitis C, as substantiated by studies conducted previously [21]. Given the commonality of both Hepatitis C virus and HIV as RNA viruses, an exploration into the potential anti-HIV activity of Helicteres isora and diosgenin was undertaken, with diosgenin being well-known for its medicinal values [22,23]. Helicteres isora has been identified among Thai medicinal plants demonstrating efficacy against SARS-CoV-2. Recent research revealed a substantial plaque reduction of approximately 99.49 % through the utilization of aerial components of Helicteres isora [24]. This empirical evidence underscores the potential antiviral properties inherent in Helicteres isora, suggesting its capability to mitigate the proliferation of SARS-CoV-2. Excessive alcohol, drugs, and HCV infection cause liver damage. Comprehensive in vitro inquiries have been undertaken to delineate the pharmacological efficacy of distinct anatomical constituents of Helicteres isora, focusing on their potential mitigating effects on hepatitis [[16], [17], [18]].
Rhizoma polygonati, a medicinal herbal food integral to traditional Chinese medicine, has been identified for its potential in treating COVID-19. Diosgenin, a principal bioactive constituent of this botanical, has emerged as a noteworthy candidate demonstrating interaction with key proteins involved in the viral infection process. Specifically, diosgenin has exhibited interactions with the 3C-like proteinase, angiotensin-converting enzyme 2 (ACE2), and RNA-dependent RNA polymerase, as elucidated earlier [25]. Diosgenin has demonstrated pronounced antiviral efficacy against the Hepatitis C Virus (HCV) by diminishing the phosphorylation levels of signal transducer and activator of transcription 3 [21].
Investigating the potential of Helicteres isora and diosgenin, the present study explores their prospective antiviral properties, with a particular emphasis on their efficacy against HIV. The therapeutic potential of Helicteres isora leaf aqueous extract (HI-AQ) and extracted diosgenin (ED) is assessed through preclinical in vitro analyses involving cellular safety and efficacy against HIV-1 infection. Additionally, the study investigates the mechanism of diosgenin with HIV-1 proteins using in silico tools to understand the cross talk between molecules. The evaluation extends to include colorimetric retroviral enzyme microtitre plate assays. Notably, this study reports the antiretroviral potential of diosgenin found in the leaves extract of Helicteres isora and its mode of action against HIV-1 infection through in vitro mechanistic assays.
2. Materials and methods
The study was collaboratively conducted between Sant Gadge Baba Amravati University (SGBAU), Amravati and ICMR-National AIDS Research Institute (ICMR-NARI), Pune. The preparation of aqueous extract, extraction of diosgenin from Helicteres isora leaves and chemical characterization were conducted at Sant Gadge Baba Amravati University (SGBAU), Amravati. ICMR-NARI, Pune has contributed by conducting cell-based assays against HIV-1 and evaluated the biological activities of HI-AQ, ED, and SD.
2.1. Collection of plant material
Leaves of Helicteres isora or commonly known as Indian screw tree, belonging to the Malvaceae family were collected from Chipoli, Melghat forest, Amravati district in the month of December 2021. The plant specimen was identified and authenticated by Botanist from the Botanical Survey of India (BSI), Pune. The specimen voucher was received from BSI with No.BSI/WRC/Iden.Cer./2022/0303220005831. The plant name has also been verified by accessing the data of MPNS at http://mpns.kew.org. Plant parts were thoroughly cleaned, and healthy leaves were recovered. These plant materials were shade dried and pulverized.
2.2. Aqueous percolation of Helicteres isora leaves extraction (HI-AQ)
A modified method following the earlier studies was employed to obtain HI leaves aqueous extract (HI/LA) wherein 10 g of powdered leaves extract was mixed with 100 mL of double distilled water in a conical flask plugged with cotton wool [26]. The mixture was stirred thoroughly and kept on a rotary shaker at 120 rpm for 24 h. The extract was filtered using muslin cloth and through Whatman filter paper 1 followed by centrifugation at 5000 rpm for 24 h. The filtrate was oven dried at 50 °C and the resultant residue was kept in a refrigerator for further use [26].
2.3. Extraction of diosgenin from HI leaves
A modified method of Sulakshana and Rani (2014) was employed for the extraction of diosgenin wherein 50 g of dried and powdered HI leaves were combined with sodium acetate solution (1.20 mg in 650 mL distilled water) [27]. The mixture was allowed to stand for 24 h and hydrolyzed with 570 mL of 5 % hydrochloric acid for nearly 14 h. The hydrolyzed mass was filtered and rinsed with water several times until it was acid-free. The residue was dried followed by extraction with n-hexane in the Soxhlet apparatus for 4 h. Hexane extract concentration produced a crude, light-yellow substance that was diosgenin. Further 95 % ethanol was added to the material to crystallize it and diosgenin in the form of colourless needles was obtained.
2.4. Characterization of diosgenin
Standard diosgenin (SD) was procured from Sigma Aldrich (USA). An aqueous extract of Helicteres isora, extracted diosgenin, and standard diosgenin were undertaken for thin layer chromatography on pre-coated silica gel plates with solvent combinations of N-hexane: ethyl acetate (7.5: 2.5). The plates were heated in an oven at 90 °C for three to 5 min to observe the spots after being sprayed with a solution of ethanol, sulfuric acid, glacial acetic acid, and anisaldehyde reagent (135:5:1:3.7) [28]. Waters High Performance Liquid Chromatography system equipped with Waters 1525 Binary HPLC Pump, Waters 2998 - Photodiode Array Detector (PDA) and Waters Autosampler was used for chromatographic determination of diosgenin on Waters C-18 column. Waters Breeze 2 Software system was used for analysis and data acquisition.
ED and SD were dissolved in acetonitrile and filtered via a 0.10 μm syringe filter before use. Samples were injected using a rheodyne injector fitted with a 20 μl fixed loop. The mobile phase reported by Deshpande and Bhalsing, (2014) was referred to wherein acetonitrile: water (90:10, v/v) was used as a solvent system with a flow rate of 0.7mL/min maintaining the ambient temperature [29]. The PDA detector was set at 273 nm to acquire the chromatogram.
2.5. Evaluation of anti-HIV activity by cell-based experiments
The newly developed herbal products HI-AQ and ED were screened for cytotoxicity and anti-HIV inhibitory potential by TZM-bl assay. A reference compound, standard diosgenin (Sigma Aldrich, MO, USA) were tested in each set of experiments for the comparative study. The methodology for these experiments was adopted from our earlier research work on this topic, with minor changes in the experimental setup [[30], [31], [32], [33], [34], [35]].
2.5.1. Cells
TZM-bl cells (genetically modified HeLa cell line) obtained from NIH-AIDS Research and Reference Reagent Program, NIH-ARRRP, USA) were used for these bioassays. These cells are highly permissive to HIV-1 infection because of their ability to express CD4 receptor, CXCR4 and CCR5 coreceptors. Further, the cells are genetically engineered to express the firefly luciferase gene (Luc) and Escherichia coli β galactosidase enzyme under the control of HIV-1 Tat. To maintain the cell cultures, complete growth medium Dulbecco's modified Eagle's medium- DMEM (GIBCO, USA) containing 10 % heat-inactivated fetal bovine serum (GIBCO, USA) supplemented with 25 mM HEPES buffer (GIBCO, USA), penicillin 50 U/mL and streptomycin 50 mg/mL (GIBCO, USA) was used. The Cultures were incubated at 37 °C in a humidified, 5 % CO2 atmosphere.
2.5.2. Viruses
Antiretroviral activity of herbal products was evaluated against two HIV-1 primary isolates, differing in their cell tropism and origin. The CCR5 tropic, HIV–1VB028 (Subtype C, Indian isolate) obtained from ICMR-NARI's virus repository and CXCR4 tropic, HIV-1UG070 (Subtype D, Ugandan isolate) obtained from NIH ARRRP were cultured in PHA-P activated (Sigma, USA) peripheral blood mononuclear cells (PBMC) derived from healthy donors and maintained in complete growth medium, RPMI 1640 supplemented with 10 % FBS and 10U/mL IL2. Viral growth in culture supernatants was quantified by detecting the P24 antigen using the ELISA technique (Advanced Bioscience Laboratories, USA). Antigen-positive, cell-free culture supernatants were collected by centrifugation, filtered, and stored in aliquots at −70 °C for further experiments. Moreover, for each virus stock, an infectious dose, TCID50 (50 % Tissue culture infectivity dose) was determined in the same cell line. Later, these pre-titrated virus stocks were used for antiviral assays.
2.6. Cell cytotoxicity
The cellular toxicity profile of the products under study was determined in cultured TZM-bl cells. Briefly, the cells were seeded in 96 well, flat bottom, microplates at a density of 104 cells/well and incubated overnight at 37 °C in a humidified 5 % CO2 atmosphere. The next day, the cells were fed with fresh growth media and double dilutions of HI-AQ, ED and SD were added onto the cells. Untreated cells in each experiment served as cell control (CC). The plate was incubated for 48hr in the same culture conditions followed by MTT assay. To evaluate drug toxicity and cell viability, the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide from Sigma, USA) was added at a final concentration of 0.5 mg/mL onto the treated and untreated cells. The microplates were further incubated for an additional 4 h at similar culture conditions, allowing MTT to react with mitochondrial dehydrogenase enzyme present only in live cells. This reaction was demonstrated by the formation of yellow formazan crystals, which were then solubilized into Dimethyl sulfoxide (DMSO). After an hour, the absorbance of the purple colour product was measured calorimetrically at 550/630 nm filter. Thus, the experiment revealed that the formation of formazan crystals was proportional to the viable cell count and indirectly measured the cellular toxicity and safety of herbal products, compared with cell control. The results were expressed as CC50 (The concentration of a product at which 50 % cells are viable) was calculated by non-linear regression curve analysis.
2.7. Cell-associated anti-HIV-1 assay
To assess the antiviral activity of products, the cell-associated virus was targeted. As described above pre-seeded, 96 well microplates with TZM-bl cells (104 cells/well) were used for cell-associated assay. Based on cytotoxicity results, subtoxic concentrations were selected for anti-HIV assays.
The pre-titrated HIV–1VB028 and HIV-1UG070 virus stocks were used for infecting pre-seeded TZM-bl cells. Two-hour post-infection, serial two-fold dilutions of subtoxic concentrations of products HI-AQ, ED and SD were overlaid onto the infected cells. Untreated cells (CC) and Virus-infected-untreated cells (VC) were included in each experiment. After 48 h of incubation, luciferase gene assay was performed by using Britelite plus reagent (PerkinElmer, USA), a substrate to detect luciferase gene product. Here, the activation of the luciferase gene, expressed in terms of relative luminescence units (RLUs) is a measure of viral replication. The RLUs were measured using an instrument Luminometer (Victor 3, PerkinElmer, USA) from Drug treated, CC and VC wells. Percent inhibition and EC50 value (concentration inhibiting 50 % of the virus) were calculated using LUC software (version 04.4) of the PerkinElmer Luminometer system. Additionally, for checking the reproducibility of results two independent assays were performed.
2.8. Protein structure retrieval and preparation for docking simulations
The crystal structures of HIV-1 proteins, such as HIV-1 Integrase (PDB: 1QS4, resolution 2.01 Å), HIV-1 Protease (PDB: 5KR0, resolution 1.8 Å), and HIV-1 Reverse Transcriptase (PDB: 3QO9, resolution 2.6 Å) were retrieved from the RCSB protein data bank (http://www.rscb.org). Each PDB was processed to obtain a single chain 3D structure by removal of other chains from the structure, hetatoms and previously docked or attached ligands using BIOVIA Discovery Studio Visualizer v21.1.0.20,298 (https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-discovery-studio/). Furthermore, single chain 3D structures were optimized under AutoDock v4.2 tools for molecular docking simulations studies [36]. The optimization process includes addition of polar hydrogens, the merging of non-polar hydrogens, missing of atoms and repairing of missing atoms if any and addition of Kollman charges to obtain an energy-minimized structure.
Preparation of Ligand for Molecular Docking Simulations: The 3D structure of diosgenin (PubChem CID: 99474), a phytomolecule found in HI was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) in SDF format. The 3D structure smile were converted into 3D coordinates using Avogadro [37]. Avogadro tool generates tautomeric, stereochemical, and ionization modifications, as well as energy minimization and flexible filtering. 3D coordinates of diosgenin under AutoDock v4.2 was subjected to addition of the Gasteiger charges and correct torsion bonds before docking simulations.
2.9. Molecular docking of phytomolecules against HIV-1 targets
These simulations provide crucial information about molecular interactions between the protein and the ligands of interest and render an opportunity to study their catalytic mechanism [38]. The HIV protein and ligand 3D structure files were fetched in AutoDockTools v4.2. Polar hydrogen(s) were added, non-polar(s) were merged and the Kollman charges were added to the protein, while Gasteiger charges were applied to the diosgenin for optimization of the ligand energy state. The Torsion tress was checked for choosing torsion settings in AutoDock v4.2. A grid box with a spacing of 0.375 Å was generated using AutoGrid 4 and centered on the active residues available in the binding pocket of HIV-1 protein to provide isolated environment. Diosgenin was subjected to interact with selected residues under isolated conditions. The Lamarckian Genetic algorithm with 10 iterations over 150 populations was used with default docking parameters. In silico molecular interactions and conformational analysis were visualized in the PMV tool available in AutoDockTools v1.5.7 and 2D plots were generated in BIOVIA Discovery Studio Visualizer v21.1.0.20,298.
2.10. Determination of HIV-1 RT inhibition activity of extracts
For the determination of the RT inhibitory activity of HI-AQ and ED a colorimetric RT assay kit (Roche, Mannheim, Germany) was used. Briefly, 0.2 ng of HIV-1 RT was added to wells in microtitre plates, followed by the addition products, standard HIV-1 RT inhibitor (Azidothymidine: AZT) and template. A well without the addition of HIV-1 RT was designated as ‘blank’. The set-up was incubated at 37 °C for 1 h. After the prescribed washing steps anti-digoxigenin peroxidase (anti-DIG-POD 200 U/mL) antibody was added to each well. The microtiter plate was then incubated at 37 °C for 1 h. After repeating the washing steps ABTS substrate solution was added to each well. The microtiter plates were kept in a shaker incubator (250 rpm) at 20 °C for 20 min. The appearance of green colour in the wells indicated the activity of RT. Absorbance readings were taken at 405 nm. The average of three experiments was considered for graphical analysis.
2.11. Determination of HIV-1 integrase (IN) enzyme inhibition
The efficiency of HI-AQ and ED on HIV-1 IN enzyme inhibition was tested using the Xpress Bio HIV-1 IN Assay Kit (Xpress Biotech International, USA). The ELISA method was followed exactly as directed by the manufacturer. Test products with three different concentrations were combined with 20 μl lysis buffer, pipetted into 200 μl aliquots in a microplate, and incubated for 1 h at 37 °C. After the prescribed washing steps each well was treated with detection antibody (100 μl) and incubated for 1 h at 37 °C. Repeating the washing steps, streptavidin-HRP conjugate (100 μl) was added, and plates were further incubated at room temperature for 30 min. After following the washing steps, the addition of substrate solution was done and the plates were incubated at room temperature for 30 min in a dark environment. The reaction was terminated by adding 100 μl stop solution and the absorbance was measured at 450 nm, using a microplate ELISA reader. Here, FDA approved HIV-1 Integrase inhibitor, Dolutegravir (DTG) was used as the standard inhibitor. The experiments were carried out in triplicate and the average value was used for graphical representation of the data.
3. Results
In the ongoing investigation, the aqueous extract and isolated diosgenin derived from the botanical species HI were subjected to comprehensive chemical characterization. Subsequently, a thorough biological assessment was conducted to evaluate their potential antiretroviral capabilities via experimentation involving cellular systems. Moreover, the discerned phytomolecule, diosgenin, was subjected to a meticulous analysis of its molecular interplays with pivotal proteins associated with HIV-1 through the utilization of in silico molecular interaction analysis.
3.1. Chemical characterization of diosgenin
The isolated diosgenin underwent comprehensive characterization through a multi-modal approach encompassing Thin Layer Chromatography (TLC), Fourier-transform infrared spectroscopy (FTIR), and High-Performance Liquid Chromatography (HPLC). A rigorous comparison was undertaken between the characterized and extracted diosgenin and the commercially acquired diosgenin or standard diosgenin procured from Sigma Aldrich, MO, USA.
The chromatographic technique was used for the identification and purification of herbal products. All products have shown the Rf value as 0.30 calculated by using the formula given below:
Evidently, the observed retention factor (RF) value of the extracted diosgenin (ED) coincided with that of the standard diosgenin (SD), thereby substantiating the inherent purity of the extracted diosgenin specimen (Fig. S1).
FTIR spectra were analyzed for the characterization of diosgenin in samples extracted from plants (Fig. 1A and B). The characteristic absorbance peak of ED almost overlapped with SD, which reveal the peak at 3476 cm−1 (Stretching vibration absorption peak of –OH), 2986 & 2862 cm−1 (Stretching vibration absorption peaks of –CH), 1709 cm−1 (stretching vibration absorption peak of C C), 1467 cm−1 (Bending vibration peak of C–H), 1185 cm−1 (Stretching vibration absorption peak of -CH3), 1067 cm−1 (Stretching vibration absorption peak of ether bond (C–O) in the five-membered ring) and 984 cm−1 (Stretching vibration absorption peak of ether bond (C–O) in six-membered ring. Thus, almost every important peak overlap with diosgenin standard spectrum.
Fig. 1.
FTIR spectra of (A) Extracted Diosgenin (ED) and (B) Standard Diosgenin (SD). The FTIR spectra of ED almost entirely overlapped with (SD) spectrum, indicating substantial similarity in their chemical composition. The spectra exhibited characteristic absorbance peaks at specific wavenumbers: 3476 cm-−1 (Stretching vibration absorption peak of –OH), 2986 & 2862 cm−1 (Stretching vibration absorption peaks of –CH), 1709 cm−1 (Stretching vibration absorption peak of C C), 1467 cm-−1 (Bending vibration peak of C–H), 1185 cm−1 (Stretching vibration absorption peak of -CH3), 1067 cm−1 (Stretching vibration absorption peak of ether bond (C–O) in the five-membered ring), and 984 cm−1 (Stretching vibration absorption peak of ether bond (C–O) in the six-membered ring).
In pursuit of product authentication and the acquisition of quantitative determinations, HPLC analysis was methodologically executed. This analytical modality is distinguished by its exceptional sensitivity, satisfactory linearity, and commendable reproducibility characteristics. Evident convergence was observed in the form of analogous peaks and retention times between ED and the SD. The detection wavelength of 273 nm was determined in a scan range of 210–400 nm. Sharp peaks were observed both in the case of extracted and standard diosgenin (Fig. 2A and B). The retention time on the 10-min scale for SD was 3.532 min and that of ED was 3.555 min.
Fig. 2.
Comparative Analysis of (A) ED and (B) SD peaks in HPLC Chromatograms. Characteristic peaks appear at the detection wavelength of 273 nm within a scan range of 210–400 nm. The retention time for SD was 3.532 min, while that for ED was 3.555 min on a 10-min scale. The remarkably close similarity in the observed peaks and retention times provides compelling evidence of a significant match between ED and SD in the HPLC analysis.
3.2. Cytotoxicity and anti-HIV activity of HI leaves extract and extracted diosgenin
In vitro viability testing of new drug candidates is an essential step in the process of drug discovery and clinical trials. Hence, initially we screened the HI-AQ, ED and SD for their effect on viability of TZM-bl cells using a quantitative MTT assay. The assay very well depicted the dose dependent cellular viability between the wide ranges of concentrations, 3.13–500 μg/mL for all of the three products. As expected, the pattern of increasing concentrations reduces the cell viability was clearly observed in this experiment. (Fig. 3A–C). Further, we calculated the CC50 values at 224.39 μg/mL, 109.97 μg/mL and 31.64 μg/mL for HI-AQ, ED and SD, respectively (Table 1 and Fig. 3D). Thus, the MTT assay represented encouraging safety profiles for both the extracts.
Fig. 3.
Determination of cytotoxic concentrations of Aqueous Extract, Extracted Diosgenin from the leaves of plant Helicteres isora and Standard Diosgenin. The graphical illustration of dose-dependent percent viability of (A) leaves extract (HI-AQ), (B) Extracted Diosgenin (ED) and (C) Standard Diosgenin (SD) on TZM-bl cells. (D) The mean CC50 values of Herbal products HI-AQ, ED, SD and Drug control, AZT from three independent assays.
Table 1.
Cell cytotoxicity and Anti-HIV activity of HI-AQ, ED and SD.
| Sl. No. | Product | CC50 (μg/ml) | Cell Associated Assay EC50 (μg/ml) |
|
|---|---|---|---|---|
| HIV-1 (R5 tropic) | HIV-1 (X4 tropic) | |||
| 1. | HI-AQ | 224.39 | 5.50 | 8.00 |
| 2. | ED | 109.97 | 7.84 | 6.05 |
| 3. | SD | 31.64 | 12.70 | 12.08 |
Values represent are average of three independent assays.
3.3. Anti-HIV-1 activities of aqueous extract and extracted diosgenin from HI
The antiretroviral activity of HI-AQ, ED and SD against two primary isolates of cell-associated HIV-1 was tested in TZM-bl cell line. Sub-cytotoxic concentrations with respect to CC50 values were selected for this screening. In this infection-inhibition assay, infected TZM-bl cells were exposed to a set of subtoxic concentrations (50–0.39 μg/mL) of these extracts, showed a clear dose dependent inhibition pattern against both the viral strains (Table 1, Fig. 4A and B). It was observed that even at lower dosages of extracts, both viral strains were efficiently inhibited. The results were expressed in terms of half the maximal effective concentration (EC50 values). Against cell-associated HIV–1VB028 EC50 values were calculated at 5.50 μg/mL, 7.84 μg/mL and 12.70 μg/mL for HI-AQ, ED and SD, respectively. Whereas, the products inhibited 50 % of the HIV-1UG070 virus at the concentrations of 8.0 μg/mL, 6.05 μg/mL and 12.08 μg/mL, in the same order (Fig. 4C).
Fig. 4.
Determination of inhibitory concentrations of Aqueous Extract (HI-AQ), Extracted Diosgenin (ED) from the leaves of plant Helicteres isora and Standard Diosgenin (SD). The graphical illustration of dose-dependent percentage inhibition against (A) CCR5 Tropic, HIV–1VB028 and against (B) CXCR4 Tropic HIV-1UG070 in TZM-bl cells. (C) The mean EC50 values of HI-AQ, ED, and SD from three independent assays.
3.4. Molecular docking simulations of phytomolecule diosgenin against HIV-1 proteins
The therapeutic efficacy inherent in phytomolecule sourced from natural origins has undergone thorough investigation and comprehensive documentation. Additionally, these phytomolecule, originating from natural reservoirs, exhibit significant promise in the arena of pharmaceutical innovation, particularly in the realm of drug development targeting HIV-1 proteins. Natural compounds, by virtue of their inherent properties, display a propensity for minimal or negligible manifestation of toxicological side effects, thereby positioning them as highly favorable contenders for advanced drug development endeavors. Within the context of HIV infection, the HIV-1 Integrase (HIV-1 IN), HIV-1 Protease (HIV-1 PR), and HIV-1 Reverse Transcriptase (HIV-1 RT) proteins have solidified their status as firmly established and promising targets for pharmaceutical intervention. In order to attain enhanced insights into the intricate molecular interplays between the aforementioned proteins and the bioactive phytomolecule diosgenin, extracted from HI and meticulously characterized via the diverse methodologies expounded in the diosgenin characterization section, a comprehensive investigative approach was employed.
Diosgenin 3D smile (PubChem CID: 99474) was obtained from the PubChem database and converted to a 3D structure using the Avogadro tool for molecular docking experiments. AutoDock v4.2 was used to dock diosgenin into the active site binding pocket of HIV-1 proteins and calculates the binding energy (Kcal/Mol) using where R is the universal gas constant ( and T is the temperature (298.15 K) of the docked structures to assess molecular interaction strength between the HIV-1 protein and the phytomolecule [33]. Diosgenin was successfully positioned into the binding pocket of HIV-1 proteins, where it contacted active site amino acids responsible for HIV-1 replication. The active site binding pocket residues of HIV-1 proteins were identified from literature [[39], [40], [41]], and the same site was used to dock known FDA-approved HIV-1 INT inhibitor cabotegravir, HIV-1 protease inhibitor Ritonavir and HIV-1 RT inhibitor Zidouvdine (https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines) as well as phytomolecule diosgenin. The lower binding energy scores reported in this manuscript signifies higher affinity for the ligand and hence, the best docking confirmation and were compared with FDA-approved HIV-1 inhibitor's binding energy scores as described previously [33]. After analyzing the molecular interactions of the HIV-1 IN, HIV-1 PR, and HIV-1 RT with the extracted diosgenin, it was found that diosgenin was able to effectively inhibit the activity of the enzymes (Table 2). These discerned outcomes bear significant implications for the prospective development of novel therapeutic interventions against HIV-1 infections.
Table 2.
Molecular interaction of herbal phytomolecule Diosgenin with HIV-1 Proteins.
| Sl. No. | HIV-1 Protein | Binding energy (Kcal/Mol) | Ligand efficiency | Inhibition Constant | Hydrogen bonding interaction | Hydrophobic interaction | Pi-alkyl bond interaction |
|---|---|---|---|---|---|---|---|
| 1 | HIV-1 Integrase (PDB: 1QS4) | −6.78 | 0.23 | 10.77 μM | Thr66, His67 and Gln148 | Asp64, Cys65, Asp116, Phe139, Ile151, Glu152, Asn155, Ly159 | |
| 2 | HIV-1 Reverse Transcriptase (PDB: 3QO9) | −10.57 | 0.35 | 18.0 nM | His235 | Pro95, Lys102, Gln182, Tyr188, Phe227, Asp227, and Pro230 | Leu 100, Tyr181, Trp 229, Leu 234, Tyr318 and a Pi-Sigma bond with Tyr183 |
| 3 | HIV-1 Protease (PDB: 5KR0) | −10.66 | 0.36 | 15.32 nM | Asp29, Asp30 | Gly48, Gly49, Ile50, Gly52, Phe53, Pro79, Thr80, Pro81 | Ala28, Val32, Ile47, Ile54, Ile84 |
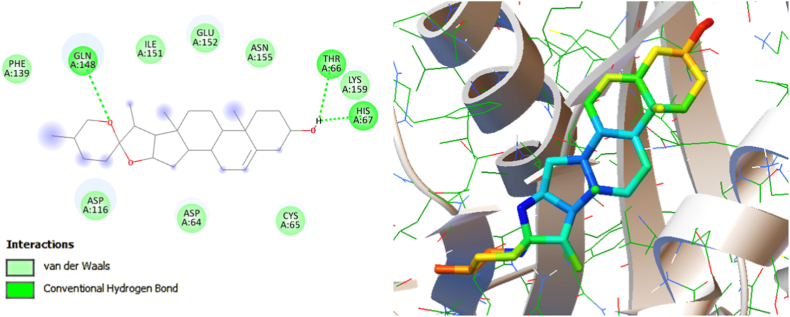
3.4.1. Molecular interaction between the HIV-1 integrase and diosgenin
The HIV-1 Integrase enzyme plays a pivotal role in orchestrating the integration of viral DNA into chromosomal DNA, thereby constituting a crucial phase within the replication trajectory of HIV-1 infection. In the ongoing investigation, the outcomes of molecular docking analysis divulged that the herbal phytomolecule diosgenin efficaciously forged the molecular interactions with the active site of HIV-1 Integrase, manifesting the formation of three distinct hydrogen bonds with residues Thr66, His67, and Gln148. This interaction is suggestive of diosgenin's potential to perturb and modulate the functional dynamics of HIV-1 Integrase, presenting a notable avenue for prospective antiretroviral intervention strategies. The low binding energy score of −6.78 Kcal/Mole indicates their strong occupancy of catalytic residues and hence, can obstruct HIV-1 Integrase activity. Additionally, the diosgenin phytomolecule rendered several hydrophobic interactions and van der Waals interactions with residues, such as Asp64, Cys65, Asp116, Phe139, Ile151, Glu152, Asn155, and Ly159 residues available in the active site binding pocket of HIV-1 Integrase (Fig. 5 and Table 2). These important residues are responsible for the catalytic activity of the HIV-1 Integrase. Hence, the occupancy of these residues by diosgenin phytomolecule may impede the replication of the HIV-1 Integrase. Collectively, the findings of this investigation yield substantial insights into the prospective utility of diosgenin as a potential inhibitor of HIV-1 Integrase, a proposition substantiated by the corroborating evidence derived from the concurrent cell-based assays conducted within this study.
Fig. 5.
Molecular interaction between the HIV-1 Integrase (PDB: 1QS4) and the natural phytomolecule Diosgenin (PubChem CID: 99474). Left panel (2D interaction plot) and right panel (3D map).
3.4.2. Molecular interaction between HIV-1 reverse transcriptase and diosgenin
HIV-1 Reverse Transcriptase plays a crucial role in HIV-1 replication. Diosgenin phytomolecule precisely established single hydrogen bonds with His235 residue, however, many hydrophobic interactions with Pro95, Lys102, Gln182, Tyr188, Phe227, Asp227, and Pro230 residues and Pi-alkyl bonds within the active binding pocket of HIV-1 Reverse Transcriptase (Fig. 6, Table 2). These findings suggest that the diosgenin found in Helicteres isora L. is a potential inhibitor of HIV-1 Reverse Transcriptase due to a high inhibition constant (Ki) value of 18.0 nM and association with important active site residues of the protein. Diosgenin could aid in the development of effective therapy for HIV infections.
Fig. 6.
Molecular interaction between the HIV-1 Reverse Transcriptase (PDB: 3QO9) and the natural phytomolecule Diosgenin (PubChem CID: 99474). Left panel (2D interaction plot) and right panel (3D map).
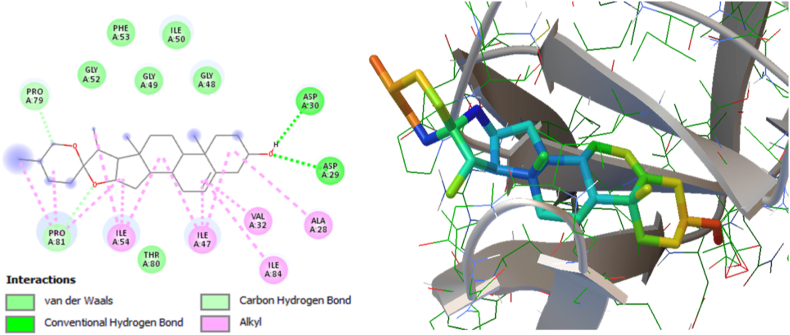
3.4.3. Molecular interaction between HIV-1 protease and diosgenin
The inhibition of HIV-1 Protease is a key focus in the development of HIV treatments, and recent studies have shown that phytomolecule may be effective inhibitors of this enzyme [[31], [32], [33]]. In these study, the in silico molecular docking simulations analysis revealed diosgenin as a lead phytomolecule that established two hydrogen bonds with Asp29 and Asp30 and many hydrophobic interactions with Gly48, Gly49, Ile50, Gly52, Phe53, Pro79, Thr80, Pro81 and Pi-alkyl contact with Ala28, Val32, Ile47, Ile54, Ile84 residues available in the active site binding pocket of HIV-1 Protease (Fig. 7 and Table 2). Interestingly, diosgenin showed the lowest binding energy (−10.66 Kcal/Mol) with an inhibition constant value of 15.52 nM and a ligand efficiency of 0.35 (Table 2). Overall, these findings suggest that diosgenin has occupied the active site residues in the binding pocket responsible HIV-1 Protease activity, hence, potentially hinders HIV-1 replication.
Fig. 7.
Molecular interaction between the HIV-1 Protease (PDB: 5KR0) and the natural phytomolecule Diosgenin (PubChem CID: 99474). Left panel (2D interaction plot) and right panel (3D map).
3.4.4. In silico prediction of ADMET properties of diosgenin
The Swiss-ADME cheminformatics platform renders valuable insights into the pharmacokinetics and drug-likeliness potential of pharmacophores. Diosgenin phytomolecule from HI was used for bioavailability RADAR analysis and oral bioavailability. Interestingly, diosgenin has high GI absorption and hence, can be metabolized and absorbed in the patient's body effectively. Furthermore, ADMET properties indicate that diosgenin is also a substrate of the P-glycoprotein transporter, which denotes that it will be transported across the cell membrane by the ABC transporter and will pass through the blood-brain barrier (Table 3 and Table 4). Moreover, the molecular interaction analysis of diosgenin with the HIV-1 proteins (i.e., Integrase, Protease, and Reverse Transcriptase) showed strong binding associations with the active pocket residues at the lowest binding energies (Table 2, Fig. 5, Fig. 6, Fig. 7) and hence, embarrassment of HIV protein replication can be expected.
Table 3.
In silico ADMET properties of Diosgenin.
| Phytomolecule | Druggability (Lipinski's Rule of Five) |
Pharmacokinetics properties |
||||
|---|---|---|---|---|---|---|
| GI Absorption |
BBB Permeate |
P-gp Substrate |
CYP | Log Kp (Skin permeation) | ||
| Diosgenin (PubChem CID: 99474) | Yes | High | Yes | No | No | −4.80 cm/s |
Table 4.
Structural properties of Diosgenin.
| Phytomolecule | Descriptors |
|||||
|---|---|---|---|---|---|---|
| Surface Area (Å2) | Molecular Weight (g/mol) | LogP | Rotatable Bonds | Acceptors | Donors | |
| Diosgenin (PubChem CID: 99474) | 38.69 | 414.62 | 4.94 | 1 | 3 | 1 |
3.5. Enzyme inhibition assays
Determination of mechanism of action of herbal extracts is the most important aspect of antiviral research. We aimed this testing in accordance with the results obtained from the biological assays and in silico docking studies. The cell associated assay has demonstrated the replication inhibition of HIV-1 in presence of extracted diosgenin. In support of these findings, the molecular interaction studies indicated the strong binding of diosgenin with HIV-1 proteins, namely the IN, RT and PR. Hence, for precise identification of mechanism of action the extracts were tested against commercially available Integrase (Xpress Bio) and Reverse Transcriptase (Roche) enzymes. However, the mechanistic validation of HIV-1 PR will be explored in the follow-up studies.
3.5.1. HIV-1 in inhibitory activity of HI-AQ and ED
The leaves extract HI-AQ and ED have shown dose dependent inhibitory patterns for Integrase enzyme in the ELISA-based assay. Different concentrations (200 - 50 μg/mL) of each extract, HI-AQ and ED, were tested against HIV-1 IN enzyme (Fig. 8A). The minimal concentration of HI-AQ (50 μg/mL) achieved 74.61 % inhibition of HIV-1 IN whereas the same concentration of ED inhibited the HIV-1 IN with 95.24 %. As expected, the standard Integrase inhibitor showed 100 % inhibition of HIV-1 IN. These results indicated that both the extracts might act as IN inhibitors, extracted diosgenin being more potent than the aqueous extract of HI leaves.
Fig. 8.
In vitro inhibition of HIV-1 Integrase and Reverse Transcriptase by Helicteres isora L., aqueous extract (HI-AQ) and Extracted Diosgenin (ED): (A) Integrase: Dose dependent Percentage inhibition of HI-AQ and ED tested against HIV-1 Integrase activity normalized to the kit control Azide (1.0 %). Known HIV-1 Integrase inhibitor Dolutegravir (5 nM) also used as the standard positive drug control. (B) Reverse transcriptase: Percentage inhibition of HIV-1 RTase enzyme activity in presence of the HI-AQ and ED extracts; as a positive control, AZT (0.49 μM), a known HIV-1 RT inhibitor, was utilized. The results shown as the means of at least three experimental replicates plus the standard deviations were calculated and represented as the error bar.
3.5.2. RT inhibitory activity of HI-AQ and ED
Similar to IN inhibition assay, the extracts HI-AQ and ED have also shown dose dependent (200 - 50 μg/mL) inhibitory activity for HIV-1 Reverse Transcriptase enzyme in the ELISA-based assay (Fig. 8B). The minimal concentration of HI-AQ (50 μg/mL) achieved 68.37 % inhibition of HIV-1 RT whereas at the same concentration ED has shown the RT inhibition at 71.72 %. These results indicated that both the extracts might act as RT inhibitors wherein extracted diosgenin exhibits superiority over the aqueous extract of HI.
4. Discussion
In an effort to manage HIV infection, medicinal plants have always been explored by the entire world. Natural resources are known to be the safe and reliable options to combat this infection. It has been reported that the botanicals like Rheum palmatum L., Rheum officinale, Trigonostem axyphophylloides, Vatica astrotricha, Vernonia amygdalina Hypoxias pelargonium, Sidoides hemerocallidea and Sutherlandia frutescens have been reported to possess anti-HIV activity [[42], [43], [44], [45]]. The alkaloids, flavonoids, polyphenols, terpenoids, tannins, proteins and coumarins are the key phytoconstituents isolated from these plants play the important role in HIV inhibitory activity. Although the exact mechanism of action is unknown, they have been identified as potential immunomodulators with minimal or no side effects [46]. The -alkaloids globospiramine, deoxyvobtusine, and vobtusine lactone from the medicinal plant, Voacanga globosa were explored for their inhibitory activity on TNF-α-induced viral replication in two latently HIV-infected cell lines, OM10.1 and J-Lat. It was found that induction of HIV replication in these cells elicited by TNF-α was blocked by globospiramine within the range of subtoxic concentrations [47]. It has been well reported that coumarin-based derivatives are useful scaffolds for the development of anti-HIV agents. They have the potential to inhibit different stages in the HIV replication cycle, inclusive of virus-host cell attachment, cell membrane fusion, integration, assembly and even the inhibition of the Reverse Transcriptase, Protease, and Integrase enzymes of HIV. Of these, a coumarin-based natural product, calanolide A, is known to be a potential anti-HIV agent [48]. Continuing the efforts, we envisioned the use of well-studied diosgenin, a steroidal sapogenin, for antiretroviral activity. Though the tubers of various Dioscorea species have been the potential source of diosgenin we explored the alternative sources of this compound considering the constraints in plant resources. HI has already been reported to be an important source of diosgenin besides containing a range of phytochemicals viz. alkaloids, flavonoids, triterpenoids, saponins and sugars [29].
Furthermore, it has been documented that the traditional dosage (510 mg/kg/day) of steroidal saponins wherein the main component is diosgenin have no significant toxicity to the experimental mice [49]. It was also documented that diosgenin derivatives had antithrombotic properties and cause less stomach mucosal injury [50]. Additionally, a study characterizing the ADME properties revealed that diosgenin has a modest inhibitory effect on cytochrome P450 enzymes (CYPs), confirming that diosgenin if fused with any other medicine would be safe and together, they would not cause cytotoxicity [51]. With this, we thought that these properties might become an added advantage to neutralize the adverse effects of present ART regimens and thus improve the quality of life of people living with HIV. The cell-based cytotoxicity experiments conducted under this study again confirmed that the extracted diosgenin is safe to the TZM-bl cells at ∼110 μg/mL (109.97 μg/mL) concentration compared to its chemical version (SD) which is more toxic to the cells (CC50 - 31.64 μg/mL). Solvent extraction often yields the compound of interest in high concentrations, accompanied by trace amounts of other entities. The interactions among these co-existing molecules may synergistically enhance anti-HIV efficacy while mitigating cytotoxic effects. To specifically assess diosgenin's role in inhibiting retroviral enzymes and its impact on anti-HIV activity, we used SD, which is the synthetic version of naturally occurring diosgenin and demonstrated a lower therapeutic index (TI) compared to the extracted diosgenin (ED) from Helicteres isora L. On the other hand, aqueous leaves extract was identified as the safest product compared to extracted and standard diosgenin. According to the recent pharmacological review on therapeutic perspectives of diosgenin, saponin fraction has a strong cytotoxic activity on cancer cells (HeLa and MOLT-4), attributed to its ability to increase reactive oxygen species production and caspases activity in the cells. This might have depicted the toxicity of TZM-bl cells, developed from HeLa cells [23].
To the best of our knowledge, we are the first to report antiretroviral potential of leaves extract of HI against two primary isolates of HIV-1. A cellular model based on reporter gene assay was used for evaluating these extracts that generated accurate, reliable and reproducible results. The bioassay showed that aqueous extract of HI has more potential to inhibit the HIV-1 replication compared to its ED and SD counterparts. However, it may be implicated to combining effect of other phytoconstituents present in crude aqueous extract viz. alkaloids, flavonoids, triterpenoids etc. This has encouraged a separate study based on medicinal, specifically the antiviral properties of other phytoconstituents in aqueous leaves extract of HI. The extracted diosgenin has shown the promising activity considering the cytotoxicity and anti-HIV activity profile, together. The selectivity index (SI) of ED was calculated as 14.02 and 18.17, respectively, for HIV–1VB028 and HIV-1UG070 strains, which is 5–7 times higher compared to the standard diosgenin, a reference compound used in each of the experiment. A comprehensive evaluation of its antiviral potential was conducted by testing three selected concentrations of HI-AQ and ED against two pivotal retroviral enzymes, Integrase and Reverse Transcriptase of HIV-1. Both HI-AQ and ED demonstrated notable inhibitory effects against the retroviral enzymes and exhibited promising activity against HIV.
In a recent study conducted on an immune booster, a mixture of 14 medicinal plants, it was observed that 25 μg/mL of product moderately inhibited the HIV-1 Integrase enzyme [52]. The active ingredients from the same herbal tonic have shown Integrase inhibition activities ranging between 17 and 21 %. In this study HI-AQ and ED exhibited Integrase inhibition activity at around 75–95 %, which has designated these extracts as potent Integrase inhibitors. On the other hand, HIV-1 RT enzyme inhibition activities of hexane extracts of Acorus calamus (Acoraceae family) and Artocarpus heterophyllus (Moraceae family) have been presented with the EC50 values at around 33 μg/mL and 35 μg/mL respectively which are less than 50 % of the activities exhibited by the two leaves extracts of HI tested here [44]. The Earlier studies on the biological activities of Heteropterys brachiate (Malpighiaceae) and Onopordum illyricum (Asteraceae) reported HIV-1 RT inhibitory concentrations higher than 1 mg/mL [42], whereas our study reports 68 % and 72 % RT inhibition at 50 μg/mL of HI-AQ and ED respectively. Yet it should be noted that, though the enzyme inhibition assays help in predicting the drug target they cannot replace the cell-based Time of Addition assay (ToA), which was an important gap in this study. Moreover, some unavoidable manpower and financial constraints in the study restricted our interest for screening these extracts against HIV-1 Protease enzyme inhibition and performing ToA that confirms the mechanism of action of a product in the cellular context. These assays would definitely be taken up in the next study based on the phytomolecules diosgenin. Together, the preliminary screening has demonstrated that both the extracts are promising candidates for further antiretroviral studies.
These findings represent the first-ever report of the potential application of HI-AQ and its diosgenin content as promising candidates worthy of further exploration in the quest for novel anti-HIV therapeutics. The study specifically pointed out that extracted diosgenin could be taken up further for antiretroviral drug designing and development after conducting confirmatory experiments on cellular and animal models. Even, it may be fabricated by certain functional groups or delivered through nanocarrier systems that may strengthen its safety in the human cells.
5. Conclusion
This preclinical study has identified diosgenin as potential herbal drug candidate against HIV-1 infection as summarized in the graphical abstract. In the present cellular model, both aqueous extract and extracted diosgenin from the leaves of HI were found safe for the cells. Also, the study highlighted their efficacy in suppression of two HIV-1 viral subtypes HIV–1VB028 (R5 tropic subtype C) and HIV-1UG070 (X4 tropic subtype D). Of the two extracts, though extracted diosgenin was found toxic compared to aqueous leaves extract of HI, it still holds the promise of upgradation based on pharmacological fabrication and further advancements in drug designing and delivery approaches. Surprisingly, the aqueous leaves extract (crude) has demonstrated good HIV inhibition capacity making it an encouraging outcome. Furthermore, in silico molecular docking simulations analysis revealed the potential of diosgenin for its strong associations with HIV-1 protein's active site pocket residues. The strong occupancy by diosgenin is expected due to low binding energies and good inhibition potential as reflected by the ligand efficiency and inhibition constant values. Given that, diosgenin extracted from HI may be considered as active pharmaceutical products and can be taken further for drug design and development for the management of HIV-1 infection.
Funding
Rajiv Gandhi Science and Technology Commission, Mumbai and ICMR-National AIDS Research Institute, Pune , funded this research vide grant number RGSTC/DPP-150/2018–3 (K.K.) NARI/SAC/Intra/2022–23/2266 (A.M.), respectively.
Institutional review board statement
No animal or human specimen was used in this study. However, the study was reviewed and the Institutional Ethics Committee sanctioned waiver approval vide the Protocol Number: NARI/EC/Approval/2022/662 dt. December 22, 2022.
Data availability statement
Data is contained within the results section of this article.
CRediT authorship contribution statement
Smita Rakshit: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft. Ashwini More: Data curation, Investigation, Methodology. Shraddha Gaikwad: Formal analysis, Investigation, Methodology, Validation, Writing – original draft. Chandrabhan Seniya: Investigation, Methodology, Writing – review & editing, Software. Aniket Gade: Formal analysis, Visualization. Vijaykumar Yogesh Muley: Formal analysis, Visualization. Anupam Mukherjee: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Kapil Kamble: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors are grateful to the Director, ICMR-National AIDS Research Institute (NARI), Pune for facilitating the anti-HIV studies at the Division of Virology and the Honorable Vice-Chancellor, Sant Gadge Baba Amravati University, Amravati for providing infrastructural facilities.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24350.
Contributor Information
Smita Rakshit, Email: smirakshit@gmail.com.
Ashwini More, Email: ashwini05.s@gmail.com.
Shraddha Gaikwad, Email: shraddhayg@gmail.com.
Chandrabhan Seniya, Email: chandrabhanseniya@vitbhopal.ac.in.
Aniket Gade, Email: aniketgade@gmail.com.
Vijaykumar Yogesh Muley, Email: vijay.muley@gmail.com.
Anupam Mukherjee, Email: amukherjee@nariindia.org.
Kapil Kamble, Email: kapilkamble@sgbau.ac.in.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
figs1.
References
- 1.World Health Organization, THE GLOBAL HEALTH OBSERVATORY . 2022. Explore a World of Health Data.https://www.who.int/data/gho/data/themes/hiv-aids [Google Scholar]
- 2.Kankara S.S., Ishaq Nuhu A., Abdullahi Bindawa K., Rabi’u Haruna M., Bello A., Babangida Abubakar I. Indigenous traditional knowledge of medicinal plants used for the management of HIV/AIDS opportunistic infections in Katsina State, Nigeria. Ethnobot. Res. Appl. 2022;23:1–17. [Google Scholar]
- 3.Singh J., Monika K., Kumar V., Sethi J. Medicinal plants with anti-HIV activity. Int J Nat Prod Sci. 2011;12:1–8. [Google Scholar]
- 4.Katiyar C., Gupta A., Kanjilal S., Katiyar S. Drug discovery from plant sources: an integrated approach. Ayu. 2012;33:10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terefe E.M., Okalebo F.A., Derese S., Muriuki J., Batiha G.E.-S. In vitro cytotoxicity and anti-HIV activity of crude extracts of Croton macrostachyus, Croton megalocarpus and Croton dichogamus. JEP (J. Environ. Psychol.) 2021;13:971–979. doi: 10.2147/JEP.S335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravanelli N., Santos K.P., Motta L.B., Lago J.H.G., Furlan C.M. Alkaloids from Croton echinocarpus baill.: anti-HIV potential. South Afr. J. Bot. 2016;102:153–156. doi: 10.1016/j.sajb.2015.06.011. [DOI] [Google Scholar]
- 7.Terefe E.M., Okalebo F.A., Derese S., Langat M.K., Mas-Claret E., Qureshi K.A., Jaremko M., Muriuki J. Anti-HIV Ermiasolides from Croton megalocarpus. Molecules. 2022;27:7040. doi: 10.3390/molecules27207040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terefe E.M., Okalebo F.A., Derese S., Muriuki J., Mas-Claret E., Langat M.K. Anti-HIV crotocascarin ω from Kenyan Croton dichogamus. Nat. Prod. Res. 2023;37:2809–2816. doi: 10.1080/14786419.2022.2134998. [DOI] [PubMed] [Google Scholar]
- 9.Sharapov A.D., Fatykhov R.F., Khalymbadzha I.A., Zyryanov G.V., Chupakhin O.N., Tsurkan M.V. Plant coumarins with anti-HIV activity: isolation and mechanisms of action. IJMS. 2023;24:2839. doi: 10.3390/ijms24032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Z., Zhang Y., Xie X., Wang J. Efficacy and safety of traditional Chinese herbal medicine combined with HAART in the treatment of HIV/AIDS: a protocol for systematic review and meta-analysis. Medicine. 2021;100 doi: 10.1097/MD.0000000000028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthanari S.K., Vanitha J., Ganesh M., Venkateshwaran K., Clercq D. Evaluation of antiviral and cytotoxic activities of methanolic extract of S. grandiflora (Fabaceae) flowers. Asian Pac. J. Trop. Biomed. 2012;2:S855–S858. doi: 10.1016/S2221-1691(12)60323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Shiekh R.A., Abdelmohsen U.R., Ashour H.M., Ashour R.M. Novel antiviral and antibacterial activities of Hibiscus schizopetalus. Antibiotics. 2020;9:756. doi: 10.3390/antibiotics9110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royal Botanic Gardens Kew, Helicteres isora L. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:321222-2 963.
- 14.Dhole P.A., Bhogaonkar P.Y., Chavhan V.N., Kshirsagar P.P. Some ethnomedicinal plants from Amravati district (M. S.) India. Int. J. Adv. Res. Biol. Sci. 2021;8:65–71. [Google Scholar]
- 15.Patil U.S., Kutemate O.G. A survey of some ethno-medicinal plants used by the tribes of Melghat in Amravati district, Maharashtra, India with reference to gastro-intestinal disorders. Asian J. Sci. Technol. 2017;8:6281–6282. [Google Scholar]
- 16.Tran T.L.G., Nhu L.P.Q., Ben T.T., Linh L.N.T., Dao V.Q., Oanh N.T.T., Thach B.D. Hepatoprotective activity of Helicteres isora ethanol extract against paracetamol-induced liver injury in mice. Biosci. Biotech. Res. Comm. 2021;14:1468–1472. doi: 10.21786/bbrc/14.4.15. [DOI] [Google Scholar]
- 17.Chitra M., Prema S. Hepatoprotective activity of Helicteres isora Linn. against CCl4 induced hepatic damage in rats. Hamdard Med. 2009;52:112–115. [Google Scholar]
- 18.Dhevi R., Gayathri K., Mohamed Shabi M., Subashini U., Dubey G., Victor Rajamanickam G., Chitra M. A preliminary biochemical screening of Helicteres isora L. stem bark in carbon tetrachloride induced toxicity in rats. Bulg. J. Vet. Med. 2008;11:235–242. [Google Scholar]
- 19.Fernandes D., De Assis E., Souza M., De Souza P., De Souza M., Helicteres L. Quim. Nova.; 2020. SPECIES (Malvaceae SENSU LATO) AS SOURCE of NEW DRUGS: A REVIEW. [DOI] [Google Scholar]
- 20.Giang T.T.L., Nhu L.P.Q., Ben T.T., Linh L.N.T., Dao V.Q., Oanh N.T.T., Thach B.D. Hepatoprotective activity of Helicteres isora ethanol extract against paracetamol-induced liver injury in mice. Biosci. Biotech. Res. Comm. 2021;14:1468–1472. doi: 10.21786/bbrc/14.4.15. [DOI] [Google Scholar]
- 21.Wang Y.-J., Pan K.-L., Hsieh T.-C., Chang T.-Y., Lin W.-H., Hsu J.T.-A. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J. Nat. Prod. 2011;74:580–584. doi: 10.1021/np100578u. [DOI] [PubMed] [Google Scholar]
- 22.Cong S., Tong Q., Peng Q., Shen T., Zhu X., Xu Y., Qi S. In vitro anti-bacterial activity of diosgenin on Porphyromonas gingivalis and Prevotella intermedia. Mol. Med. Rep. 2020;22:5392–5398. doi: 10.3892/mmr.2020.11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semwal P., Painuli S., Abu-Izneid T., Rauf A., Sharma A., Daştan S.D., Kumar M., Alshehri M.M., Taheri Y., Das R., Mitra S., Emran T.B., Sharifi-Rad J., Calina D., Cho W.C. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid. Med. Cell. Longev. 2022;(2022) doi: 10.1155/2022/1035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radapong S., Sahad T., Harnkit N., Suppajariyawat P., Okada P., Meechalad W., Sincharoenpokai P., Niumsakul S., Buaboa S., Ontong S., Ritchie K. Anti-SARS-CoV-2 activity screening of the selected Thai medicinal plants and potential host-target molecules. Bul Dep of Med. Sci. 2022;64:93–105. [Google Scholar]
- 25.Mu C., Sheng Y., Wang Q., Amin A., Li X., Xie Y. Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology: viral and cancer signaling mechanisms. J. Funct.Foods. 2021;77 doi: 10.1016/j.jff.2020.104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanda S.V., Kaneria M.J. Optimization of conditions for the extraction of antioxidants from leaves of syzygium cumini L. Using different solvents. Food Anal. Methods. 2012;5:332–338. doi: 10.1007/s12161-011-9242-0. [DOI] [Google Scholar]
- 27.Sulakshana G., Rani A.S. HPLC analysis of diosgenin in three species of Costus. Int. J. Pharma Sci. Res. 2014;5:747–749. [Google Scholar]
- 28.Indrayanto G., Utami W., Syahrani A. In: Medicinal and Aromatic Plants XI. Bajaj Y.P.S., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 1999. Costus speciosus (koenig) J.E. Smith: in vitro cultures, micropropagation, and the production of diosgenin and other phytosteroids; pp. 57–77. [DOI] [Google Scholar]
- 29.Deshpande H.A., Bhalsing S.R. Isolation and characterization of diosgenin from in vitro cultured tissues of Helicteres isora L. Physiol. Mol. Biol. Plants. 2014;20:89–94. doi: 10.1007/s12298-013-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaikwad S.Y., Phatak P., Mukherjee A. Cutting edge strategies for screening of novel anti-HIV drug candidates against HIV infection: a concise overview of cell based assays. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadaun P., Seniya C., Pal S.K., Kumar S., Kumar P., Nema V., Kulkarni S.S., Mukherjee A. Elucidation of antiviral and antioxidant potential of C-phycocyanin against HIV-1 infection through in silico and in vitro approaches. Antioxidants. 2022;11:1942. doi: 10.3390/antiox11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadaun P., shah P., Harshithkumar R., Said M.S., Bhoite S.P., Bokuri S., Ravindran S., Mishra N., Mukherjee A. Antiviral and ROS scavenging potential of Carica papaya Linn and Psidium guajava leaves extract against HIV-1 infection. BMC Compl. Med. Ther. 2023;23 doi: 10.1186/s12906-023-03916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jadaun P., Harshithkumar R., Gaikwad S.Y., Seniya C., Borse S., Gawai A.A., Chavan-Gautam P., Tillu G., Mukherjee A. Withania somnifera extracts induced attenuation of HIV-1: a mechanistic approach to restrict viral infection. Virol. J. 2023;20:173. doi: 10.1186/s12985-023-02130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Gupta S., Abadi L.F., Gaikwad S., Desai D., Bhutani K.K., Kulkarni S., Singh I.P. Synthesis and in–vitro anti–HIV–1 evaluation of novel pyrazolo[4,3–c]pyridin–4–one derivatives. Eur. J. Med. Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111714. [DOI] [PubMed] [Google Scholar]
- 35.Shah P., Naik D., Jariwala N., Bhadane D., Kumar S., Kulkarni S., Bhutani K.K., Singh I.P. Synthesis of C-2 and C-3 substituted quinolines and their evaluation as anti-HIV-1 agents. Bioorg. Chem. 2018;80:591–601. doi: 10.1016/j.bioorg.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock 4 and AutoDockTools 4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seniya C., Yadav A., Khan G.J., Sah N.K. In-silico studies show potent inhibition of HIV-1 reverse transcriptase activity by a herbal drug. IEEE ACM Trans. Comput. Biol. Bioinf. 2015;12:1355–1364. doi: 10.1109/TCBB.2015.2415771. [DOI] [PubMed] [Google Scholar]
- 39.Goldgur Y., Craigie R., Cohen G.H., Fujiwara T., Yoshinaga T., Fujishita T., Sugimoto H., Endo T., Murai H., Davies D.R. Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: a platform for antiviral drug design. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13040–13043. doi: 10.1073/pnas.96.23.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das K., Bauman J.D., Rim A.S., Dharia C., Clark A.D., Camarasa M.-J., Balzarini J., Arnold E. Crystal structure of tert-butyldimethylsilyl-spiroaminooxathioledioxide-thymine (TSAO-T) in complex with HIV-1 reverse transcriptase (RT) redefines the elastic limits of the non-nucleoside inhibitor-binding pocket. J. Med. Chem. 2011;54:2727–2737. doi: 10.1021/jm101536x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z., Huang X., Hu L., Pham L., Poole K.M., Tang Y., Mahon B.P., Tang W., Li K., Goldfarb N.E., Dunn B.M., McKenna R., Fanucci G.E. Effects of hinge-region natural polymorphisms on human immunodeficiency virus-type 1 protease structure, dynamics, and drug pressure evolution. J. Biol. Chem. 2016;291:22741–22756. doi: 10.1074/jbc.M116.747568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huerta-Reyes M., Sánchez-Vargas L.O., Villanueva-Amador G.S., Gaitán-Cepeda L.A. Anti-HIV and anti-candidal effects of methanolic extract from Heteropterys brachiata. IJERPH. 2021;18:7270. doi: 10.3390/ijerph18147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanna C., Rigano D., Cortis P., Corona A., Ballero M., Parolin C., Del Vecchio C., Chianese G., Saccon E., Formisano C., Tramontano E., Esposito F. Onopordum illyricum L., a Mediterranean plant, as a source of anti HIV-1 compounds. Plant Biosyst. - Int. J. Dealing with All Aspects of Plant Biol. 2018;152:1274–1281. doi: 10.1080/11263504.2018.1439118. [DOI] [Google Scholar]
- 44.Silprasit K., Seetaha S., Pongsanarakul P., Hannongbua S., Choowongkomon K. Anti-HIV-1 reverse transcriptase activities of hexane extracts from some Asian medicinal plants. J. Med. Plants Res. 2011;5:4194–4201. [Google Scholar]
- 45.Tshikalange T.E., Meyer J.J.M., Lall N., Muñoz E., Sancho R., Van de Venter M., Oosthuizen V. In vitro anti-HIV-1 properties of ethnobotanically selected South African plants used in the treatment of sexually transmitted diseases. J. Ethnopharmacol. 2008;119:478–481. doi: 10.1016/j.jep.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Laila U., Akram M., Shariati M.A., Hashmi A.M., Akhtar N., Tahir I.M., Ghauri A.O., Munir N., Riaz M., Akhter N., Shaheen G., Ullah Q., Zahid R., Ahmad S. Role of medicinal plants in HIV/AIDS therapy. Clin. Exp. Pharmacol. Physiol. 2019;46:1063–1073. doi: 10.1111/1440-1681.13151. [DOI] [PubMed] [Google Scholar]
- 47.de Jesus M.S.M., Macabeo A.P.G., Ramos J.D.A., de Leon V.N.O., Asamitsu K., Okamoto T. Voacanga globosa spirobisindole alkaloids exert antiviral activity in HIV latently infected cell lines by targeting the NF-kB cascade: in vitro and in silico investigations. Molecules. 2022;27:1078. doi: 10.3390/molecules27031078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z., Chen Q., Zhang Y., Liang C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia. 2021;150 doi: 10.1016/j.fitote.2021.104863. [DOI] [PubMed] [Google Scholar]
- 49.Qin Y., Wu X., Huang W., Gong G., Li D., He Y., Zhao Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J. Ethnopharmacol. 2009;126:543–550. doi: 10.1016/j.jep.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Zheng H., Wei Z., Xin G., Ji C., Wen L., Xia Q., Niu H., Huang W. Preventive effect of a novel diosgenin derivative on arterial and venous thrombosis in vivo. Bioorg. Med. Chem. Lett. 2016;26:3364–3369. doi: 10.1016/j.bmcl.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Manda V.K., Avula B., Ali Z., Wong Y.-H., Smillie T.J., Khan I.A., Khan S.I. Characterization of in vitro ADME properties of diosgenin and dioscin from Dioscorea villosa. Planta Med. 2013;79:1421–1428. doi: 10.1055/s-0033-1350699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotich W., Mas-Claret E., Sadgrove N., Guantai A., Padilla-González G.F., Langat M.K. HIV-1 integrase inhibitory effects of major compounds present in CareVidTM: an anti-HIV multi-herbal remedy. Life. 2022;12:417. doi: 10.3390/life12030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the results section of this article.