Abstract
Purpose
Accelerated partial breast irradiation (APBI) is an attractive treatment modality for eligible patients as it has been shown to result in similar local control and improved cosmetic outcomes compared with whole breast radiation therapy. The use of online adaptive radiation therapy (OART) for APBI is promising as it allows for a reduction of planning target volume margins because breast motion and lumpectomy cavity volume changes are accounted for in daily imaging. Here we present a retrospective, single-institution evaluation on the adequacy of kV-cone beam computed tomography (CBCT) OART for APBI treatments.
Methods and Materials
Nineteen patients (21 treatment sites) were treated to 30 Gy in 5 fractions between January of 2022 and May of 2023. Time between simulation and treatment, change in gross tumor (ie, lumpectomy cavity) volume, and differences in dose volume histogram metrics with adaption were analyzed. The Wilcoxon paired, nonparametric test was used to test for dose volume histogram metric differences between the scheduled plans (initial plans recalculated on daily CBCT anatomy) and delivered plans, either the scheduled or adapted plan, which was reoptimized using daily anatomy.
Results
Median (interquartile range) time from simulation to first treatment was 26 days (21-32 days). During this same time, median gross tumor volume reduction was 16.0% (7.3%-23.9%) relative to simulation volume. Adaptive treatments took 31.3 minutes (27.4-36.6 minutes) from start of CBCT to treatment session end. At treatment, the adaptive plan was selected for 86% (89/103) of evaluable fractions. In evaluating plan quality, 78% of delivered plans met all target, organs at risk, and conformity metrics evaluated, compared with 34% of scheduled plans.
Conclusions
Use of OART for stereotactic linac-based APBI allowed for safe, high-quality treatments in this cohort of 21 treatment courses. Although treatment delivery times were longer than traditional stereotactic body treatments, there were notable improvements in plan quality for APBI using OART.
Introduction
Accelerated partial breast irradiation (APBI) is a localized form of radiation therapy (RT) for early-stage breast cancer where only the lumpectomy cavity and surrounding tissue are treated.1 APBI has garnered interest within radiation oncology as it has been shown to have similar efficacy to whole breast irradiation in both ipsilateral breast tumor recurrence and toxicity,2 and allows for the radiation to be delivered in 1 to 2 weeks. Historically, APBI has been delivered with intraoperative radiation therapy,3 high-dose-rate brachytherapy,2,4 and linear-accelerator (linac) based 3-dimensional conformal radiation therapy or intensity modulated radiation therapy.5, 6, 7, 8 Both high-dose-rate brachytherapy and intraoperative radiation therapy allow for excellent localization because the treatment applicator is directly inserted into the lumpectomy cavity, and linac-based APBI relies on external localization. Additionally, because the treatment planning process for linac-based APBI can take weeks, the surgical bed may change in both volume and relative location between the postsurgery planning computed tomography (CT) image and treatment. The postoperative surgical bed has been observed to decrease in volume by as much 50% from time of simulation to start of treatment.9,10
Adaptive radiation therapy has been previously shown as a promising technique that allows for increased sparing of organs at risk (OAR) for several treatment sites.11,12 Online adaptive radiation therapy (OART) can be leveraged to further increase normal tissue sparing,13, 14, 15, 16 especially in hypofractionated treatment regimens.17,18 Through OART, clinical teams can modify treatments based on daily anatomic changes, further reducing setup uncertainty margins and increasing the potential for normal tissue sparing. OART presents additional benefits for APBI as the surgical bed can be delineated on daily imaging, allowing the dose to be optimized to the volume as seen during treatment delivery, as opposed to the volume observed at the time of planning CT acquisition. Considering the potential for significant changes in target volumes, OART could be considered the ideal treatment modality for patients receiving APBI.
In this work, we present our institution's initial experience treating early-stage breast cancer patients with 5-fraction APBI on the kV-cone beam CT (CBCT) guided Varian Ethos platform. Although APBI treatment with Ethos has been previously described,19 only select dose volume histogram (DVH) differences between scheduled (reference plans calculated on daily CBCT anatomy) and adapted plans were compared. Adding to the literature, our study includes a detailed summary of plan selection, daily target contouring, and planning and treatment timing. We also report dosimetric comparison between reference plans, scheduled plans, and delivered plans (either the scheduled or adapted plan, which was reoptimized on daily CBCT anatomy). Lastly, we provide a comparison of planning objective compliance with and without adaptive capabilities.
Methods and Materials
Online adaptive workflow
Our institutional workflow of simulation and OART has been published previously.20 Simulation and treatment planning was performed using our standard institutional guidelines for APBI. For a nonadaptive workflow, the initially approved treatment plan, from here on referred to as the “reference plan,” would be treated with daily image guidance. However, during CBCT-guided adaptive therapy, a synthetic CT scan was generated by associating the HUs from reference CT with the daily CBCT. Normal tissue and gross tumor volumes (GTVs) contours were automatically segmented via the onboard deformable image registration-based auto-contouring system, then edited as needed by the treating attending based on fiducial boundaries and comparison to the reference seroma contours.
After all structures were deemed satisfactory by the treating physician, the reference plan was then recalculated on the daily synthetic CT, referred to as the “scheduled” plan. Adaptive plans were generated by reoptimizing the reference plan, using the same optimization objectives as the reference plan, onto daily synthetic CT anatomy. Reference, scheduled, and adaptive plans were calculated using an independent HU calibration curve, which was identical to the simulation scanner calibration curve and validated as part of commissioning. The treating physician then selected either the scheduled plan or adaptive plan according to qualitative (ie, shape of the 50% isodose line) and quantitative (ie, planning target volume [PTV] V100%) plan characteristics; this finally selected plan is referred to as the “delivered” plan. Assuming the scheduled and adaptive plans both met all planning constraints, target coverage, ipsilateral breast dose, heart dose, and ipsilateral lung dose were considered among the most important factors when choosing the superior plan. Lastly, a position verification CBCT was performed and the Mobius3D-Adapt secondary calculation algorithm was used to verify correct plan MUs and perform gamma analysis. Synthetic CT structures were rigidly propagated from Ethos to Mobius and plans were required to meet a global gamma value >95% (using 3%/2 mm and a 10% threshold) before treatment, according to institutional guidelines.21
Patient cohort
Nineteen patients (21 targets due to 2 patients receiving bilateral treatment, 10 left-sided and 11 right-sided) with early-stage breast cancer received OART APBI treatment (30 Gy in 5 fractions) between January of 2022 and May of 2023 at our institution in this Institutional Review Board-approved (IRB-120703005) retrospective study. Adaptive treatment was not triggered by simulation anatomy, and all patients were treated adaptively during this time at our institution. Table 1 summarizes the key components of the cohort analyzed in this study, including age, laterality, target volumes, and tumor staging. Patients were scanned with 1-mm slice thickness and were immobilized using standard breast simulation setup. They received free-breathe and breath-hold scans (for left-breast treatments). The GTV was defined as the lumpectomy cavity including pertinent surgical clips. Clinical target volumes (CTV) were derived from the physician-contoured GTVs via isotropic 10 mm (n = 15), 8 mm (n = 1), 7 mm (n = 2), and 5 mm (n = 3) expansions. Smaller CTV margins were used by the treating physician when treating lower-risk disease or when the PTV to breast ratio was otherwise unacceptably high. PTVs were derived via isotropic 3 mm (n = 20) and 5 mm (n = 1) expansions of CTVs. The single 5 mm CTV to PTV expansion was deemed necessary due to extremely inferior and posterior right-sided target anatomy near the breast fold and liver. CTVs and PTVs were cropped out of the lungs, heart, chest wall, and skin (3 mm inward expansion of the body) to create a PTV_Eval structure.
Table 1.
Summary of the adaptive accelerated partial breast cohort used in this study
| Descriptor | Median (IQR) |
|---|---|
| Age (y) | 69 (59-74) |
| Target laterality | Left: 10, right: 11 |
| Simulation volume (cc) | GTV: 13.3 (8.2 – 24.7) CTV: 59.8 (52.9 – 87.8) PTV: 88.2 (79.2 – 123.6) |
| Tumor staging | Stage 0: TisN0: 2 Stage 1: T1aN0: 2 T1bN0: 9 T1cN0: 8 |
Abbreviations: GTV = gross tumor volume; CTV = clinical target volume; PTV = planning target volume.
Treatment planning
The Varian Ethos is a kVCBCT-guided, online-adaptive linear accelerator equipped with a 6MV flattening filter free beam, dual stacked and staggered MLC banks, and a maximum dose rate of 800 MU/min. Ethos TPS is designed to calculate dose using Acuros XB (v16.1.0), with dose-to-medium and a 2.5 mm dose grid. Ethos plans were optimized according to a planning objective template submitted for each patient. Although the exact optimization template used for individual patients varied case-by-case, our approach for APBI planning in Ethos has been previously described in detail.22 Patients were treated with intensity modulated radiation therapy using 7 (n = 3), 8 (n = 2), 10 (n = 3), and 12 fields (n = 1) or 2 partial VMAT arcs (n = 12). The Radiation Therapy Oncology Group (RTOG) conformity index (CI) and high dose spillage are defined in terms of prescription isodose volume (PIV) and treatment volume (TV) in equations 1 and 2.
| (1) |
| (2) |
Three left-sided targets were treated using breath-hold due to several factors including proximity of the target to the heart and prior RT; all other targets were treated free-breathe. Fiducials were used to aid in seroma boundary delineation23 in 18 of 21 treatment sites as it has been previously shown that they reduce interobserver variability for target delineation.24 Sufficient contrast between seromas and healthy tissue was present for the remaining 3 targets, thus fiducials were omitted. In the reference CT structure set, fiducials were assigned to titanium alloy and nearby artifact created by fiducials was assigned to water. The synthetic CT, where plans are calculated on, uses the Titanium and water HUs from the reference plan for dose calculation. IDENTIFY surface monitoring was used to verify initial surface position and track patient movement throughout all treatment fractions.25
Data analysis
All reference, scheduled, and adapted plans were exported to Eclipse retrospectively for this analysis. DVH metrics for reference, scheduled, and delivered plans (choice of either scheduled or adapted plans) were extracted via the Eclipse Scripting Application Programming Interface (version 16.1). The Mann-Whitney U unpaired, nonparametric test and the Wilcoxon paired, nonparametric test were used to compare reference (n = 21) versus delivered plans (n = 103) and scheduled (n = 103) versus delivered plans (n = 103), respectively. Statistical analyses were performed in the Python SciPy library without removal of outliers, using P < .05 for statistical significance.
Results
Treatment summary
Ninety-eight percent (103/105) of fractions’ plans were included in this analysis; plans for 2 fractions were unavailable for export within Ethos TPS. Eighty-six percent (89/103 fractions) were treated selecting the adaptive plan. An adaptive plan was chosen at least once for all patients. The adaptive plan was selected for all 5 fractions in 13 treatment courses. Five targets were treated with 4 adaptive fractions, and one target each was treated with 3, 2, and 1 adaptive fractions. The median (IQR) time from simulation to first adaptive treatment was 26 days (21-32 days). Individual adaptive treatments took a total of 31.3 minutes (27.3-36.6 minutes). Initial imaging, auto-contouring and contour editing, plan calculation and optimization, and plan selection required 20.5 minutes (16.8-26.4 minutes). Verification CBCT and treatment delivery took the remaining 10.3 minutes (7.4-12.9 minutes).
Adaptive target contouring
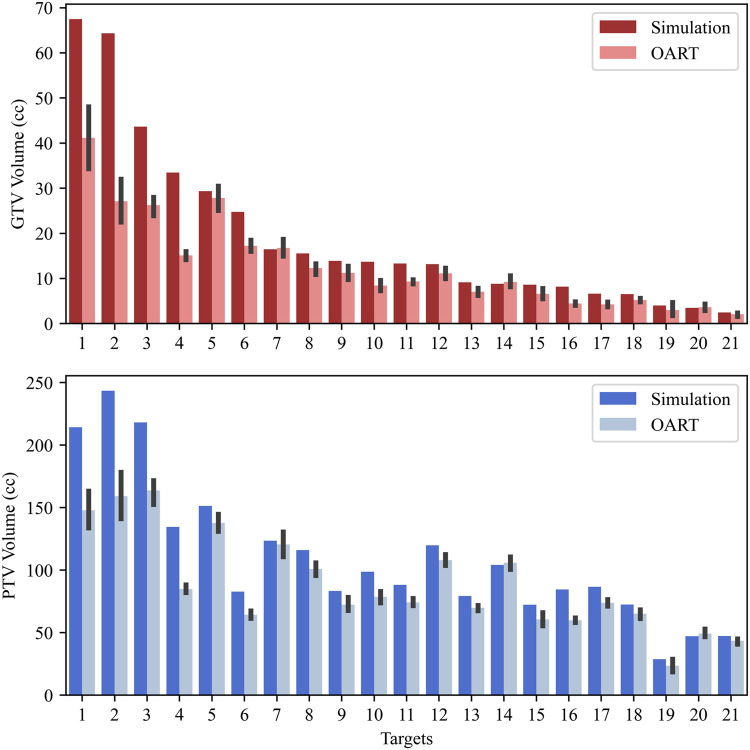
Barplots of the absolute GTV and PTV volumes during simulation and the mean OART values (± standard deviation) for every patient target are shown in Fig. 1. Of the 21 targets analyzed in this study, only 3 had increased GTV volumes at treatment compared with simulation, with percentage increases of 1.8% (0.31 cc), 4.6% (0.41 cc), and 4.6% (0.16 cc) for targets 7, 14, and 20, respectively. Only targets 14 and 20 had higher mean OART PTV volumes compared with simulation (1.6% and 4.3%, respectively). The coefficient of variation (CV, mean divided by standard deviation) was 18% ± 12% for GTVs compared with 8% ± 6% for PTVs. As an example, the CV for target 21 was 26.1% for GTV and 6.8% for PTV. Thus, even though there may be relatively large fluctuations in GTV volume, the effect of this variation on the final PTV is lessened due to volumetric expansion. The mean (standard deviation) GTV volume at simulation and each treatment fraction was as follows: simulation: 19.6 cc (11.4 cc); fraction 1: 13.1 cc (7.2 cc); fraction 2: 12.7 cc (7.1 cc); fraction 3: 12.8 cc (6.8 cc); fraction 4: 12.4 cc (7.0 cc); fraction 5: 13.3 cc (5.8 cc).
Figure 1.
Barplots of simulation volume versus mean contour volume drawn during online adaptive radiation therapy (OART) for gross tumor volumes (GTVs) and planning target volumes (PTVs) for every patient target. The black lines illustrate one SD around the mean.
Dosimetric comparison
Boxplots illustrating all reference, scheduled, and delivered data are shown in Fig. 2. There were no statistically significant differences observed between reference and delivered plan metrics for any planning objective (P > .05). However, delivered plans showed significant improvements compared with scheduled plans (P < .05) for all but the heart V1.5 Gy planning objective. Figure 2 highlights that the PTV V100%, Lung V9 Gy, RTOG CI, and high dose spillage scheduled metric distributions are inferior to the reference and delivered metric distributions, indicating that the reference dose distribution is not robust throughout the treatment course.
Figure 2.
Boxplots summarizing reference (Ref, n = 21), scheduled (Sched, n = 103), and delivered plans (Del, n = 103) for each planning goal. The white circles and dashed lines illustrate individual data points and planning goals, respectively. Only the left-sided heart V1.5 Gy constraint is illustrated for clarity. The Ref and Del plans were compared via the Mann-Whitney U unpaired, nonparametric test, and the Sched and Del plans were compared via the Wilcoxon paired, nonparametric test. Plots are annotated as follows: ns: p > 0.05; *: p ≤ 0.05.
Figure 3 displays the aggregate DVHs (median from all treatments) bound by the central 95% of data in addition to selected left- and right-sided patient median DVHs bound by minima and maxima. For the entire cohort, the median PTV DVH is similar for scheduled and delivered plans, but the lower bounds of delivered plans receive higher prescription coverage than scheduled plans. The lower bound, median, and upper bound of the delivered breast DVH is favorable to the scheduled DVH for almost all dose levels, although at times this difference is marginal. The upper bound of the delivered lung DVH is significantly lower than the scheduled DVH for dose levels <15 Gy. Delivered and scheduled heart DVHs are nearly indistinguishable across the entire population.
Figure 3.
Dose volume histograms (DVHs) of scheduled (dashed lines) and delivered (solid lines) plans for all patients (top) and select left- (middle) and right-sided patients (bottom). Planning objectives are illustrated by color-coded triangles tips. The median and central 95% of data are represented for all patient DVHs, whereas the median and minima/maxima are illustrated for individual patients. Only the left sided heart V1.5 Gy constraint is illustrated in the total patient DVHs for clarity. Abbreviation: PTV = planning target volume.
The median (range) prescription PTV coverage for the scheduled and delivered plans of the selected left-sided patient were 99.6% (97.4%-100%) and 97.1% (94.5%-97.9%), respectively; thus, even though the delivered plans received a marginally reduced V100% relative to scheduled plans and one delivered fraction was 0.5% below the PTV objective, the delivered target coverage was still considered clinically acceptable. However, the scheduled breast and lung DVHs were greater than the delivered breast and lung DVHs for nearly all dose levels, exhibiting a reduction in OAR dose with adaption. Heart doses were similar among scheduled and delivered plans. For the selected right-sided patient, scheduled and delivered plans resulted in similar PTV prescription coverage, but the scheduled breast and lung DVHs were again greater than the delivered breast and lung DVHs for all dose levels above approximately 3 Gy. Delivered plans resulted in marginally higher heart V1.5 Gy values but lower heart volume receiving above 2 Gy.
Planning objective compliance
Table 2 shows reference, scheduled, and delivered objective compliance for each planning goal, as well as the number (%) of each plan type meeting target and OAR objectives and all planning objectives (target/OAR objectives plus RTOG CI and high dose spillage). With the addition of adaption, delivered plans comprised an equal or lesser number of violations compared with scheduled plans for all planning metrics, resulting in a 10.7% increase in plans meeting all target and OAR objectives and a 43.7% increase in plans meeting all planning objectives. Delivered plans comprised an equal or lesser percentage of violations compared with reference plans for all target/OAR metrics, resulting in an 8.2% increase of plans meeting all target/OAR objectives; however, a marginal percentage increase in plans failing to meet RTOG CI and high dose spillage constraints was observed (1.0% and 1.9%, respectively). Scheduled and reference plans achieved target and OAR constraints at similar rates (71.4% vs 68.9%), but a large percentage of scheduled CI and high dose spillage metrics failed (37.9% and 43.7%, respectively), leading to a drastically reduced percentage of scheduled plans achieving all objectives compared with reference plans (34.0% vs 76.2%, respectively).
Table 2.
Summary of planning objective compliance for each plan type
| Plan type |
|||
|---|---|---|---|
| Planning objective failures | Reference | Scheduled | Delivered |
| PTV V100% ≥95% | 18 (85.7%) | 87 (84.5%) | 94 (91.3%) |
| Ipsilateral breast V30 Gy <20% | 21 (100.0%) | 103 (100.0%) | 103 (100.0%) |
| Ipsilateral breast V15 Gy <40% | 21 (100.0%) | 103 (100.0%) | 103 (100.0%) |
| Heart V1.5 Gy <5% (right) | 8 (72.7%) | 45 (75.0%) | 46 (76.7%) |
| Heart V1.5 Gy <40% (left) | 10 (100.0%) | 43 (100.0%) | 43 (100.0%) |
| Ipsilateral lung V9 Gy <10% | 20 (95.2%) | 99 (96.1%) | 103 (100.0%) |
| Skin D0.01 cc <39.5 Gy | 21 (100.0%) | 103 (100.0%) | 103 (100.0%) |
| Rib D0.01 cc <43.0 Gy | 21 (100.0%) | 103 (100.0%) | 103 (100.0%) |
| Plans meeting target/OAR objectives* | 15/21 (71.4%) | 71/103 (68.9%) | 82/103 (79.6%) |
| RTOG CI <1.30 | 21 (100.0%) | 64 (62.1%) | 102 (99.0%) |
| High dose spillage <15% | 21 (100.0%) | 58 (56.3%) | 101 (98.1%) |
| Plans meeting all objectives† | 15/21 (71.4%) | 35/103 (34.0%) | 80/103 (77.7%) |
Abbreviations: OAR = organ at risk; PTV = planning target volume; RTOG = Radiation Therapy Oncology Group.
The number (%) of plans with acceptable planning objectives for reference, scheduled, and delivered plans.
The number (%) of plans meeting target/OAR planning objectives and all planning objectives.
Discussion
In this study, we present our initial institutional experience treating APBI using OART. Adaptively generated plans were selected for treatment 86% of the time, with a median treatment time of 31 minutes (from setup to beam off). Delivered plans were not significantly different from reference plans for all objectives (P > .05) but resulted in DVH improvements (P < .05) for all planning objectives besides the heart V1.5 Gy compared with scheduled plans. Cohort DVHs for the delivered plans were moderately preferred to scheduled DVHs for the PTV, breast, and lungs, but considerable DVH improvements were realized with adaption for individual left- and right-sided patients. Target and OAR planning objectives were met for 76.2%, 81.6%, and 86.4% of reference, scheduled, and delivered plans, respectively. When including conformity measures with this stereotactic treatment, all planning objectives were achieved for 76.2%, 34.0%, and 77.7% of reference, scheduled, and delivered plans, respectively.
Although it was it was outside of the scope of this work, several groups have validated Ethos synthetic CT reliability. Kisling et al observed an average of 1.6% difference between phantom ion chamber measurements and synthetic CT doses, even when simulating weight losses and gains of up to 4 cm, which are more extreme than the differences expected in this study.26 Additionally, Nelissen et al observed <2% dose disparity between the synthetic CT and CBCT in high dose regions for palliative spine treatment.27 Although smaller calculation grids are preferred for SBRT plans, the Ethos platform (v1.1 MR3) offers a minimum optimization and dose calculation resolution of 2.5 mm at this time. Even though GTVs were often quite small, with a minimum volume of 1.1 cc, PTVs were significantly larger (GTV-to-PTV margin: 8-13 mm), with minimum and median values of 15.9 and 88.2 cc, respectively. Thus, only PTV coverage was evaluated in this work to exclude small volume calculation uncertainty. Although a smaller grid size is ideal, a 2.5 mm dose calculation grid is considered acceptable for these PTV sizes and is in line with professional guidelines.28
Three types of plans were analyzed in this work: reference plans, scheduled plans, and delivered plans. Reference plans represent the current RT standard of care, which assumes that the target coverage and OAR sparing achieved during initial planning is reproduced daily. The decrease in scheduled plan quality compared with reference plan quality challenges this assumption for external beam APBI, as delivering reference plans onto daily target and OAR anatomy resulted in decreased conformality and increased high dose spillage (P < .05, not annotated in Fig. 2). Given that the seroma shape, size, and location do vary, adaption offers a potential improvement on the standard of care for APBI. We further hypothesize that these findings may also occur in other treatment sites with significant target shrinkage or deformation, or mobile nearby healthy tissue with inherently high setup uncertainty.
The goal of this work was to illustrate the net improvement actualized via kV-CBCT guided OART. Thus, delivered plans were presented as opposed to adapted plans because scheduled plans were selected by the treating physician when adaption did not improve plan quality. Thus, there are a few cases where the scheduled plan is still comparable, even in the presence of tumor shrinkage or mobility.
The volume (cc) of the GTV was >110% of the size of the simulation GTV in 4 out of 103 OART treatment sessions. In 3 fractions, the physician covering the fraction was not the patient's primary radiation oncologist. Although the largest increase relative to simulation was 54.9%, this corresponds to an absolute increase of 2.2 cc for the GTV and a relative PTV increase of 16%. This highlights the importance of clear directives concerning target contouring and prescription dose coverage in the presence of uncertainty, as interobserver target variability can be present even when implanted fiducial markers are used to aid in target delineation.
Three primary sources of uncertainty arise from using fiducials for CBCT-based adaptive RT: contouring accuracy on the reference CT, magnitude of artifact on the daily CBCT, and the ability to accurately delineate GTVs on CBCT. First, if the fiducials and nearby artifact are not accurately assigned to Titanium and water on the simulation CT structure set, daily synthetic CTs will be systematically inaccurate as they are deformed from the simulation CT. Second, if artifact at daily adaptive treatment prohibits accurate contouring, the synthetically generated CT will not accurately reflect daily anatomy, and fiducials and healthy tissue may be deformed into a location where they are not physically present. Lastly, uncertainty in GTV delineation due to artifact leads to uncertainty in prescription target volume. Although the authors do not believe these effects to be very large, they are likely not negligible. The exact magnitude of these effects is speculative because, to our knowledge, these limitations have not been investigated for Ethos. Future studies are needed to quantify the effects of these uncertainties for this site and technique. Additionally, the expected uncertainties are <3 mm and thus accounted for in the CTV-PTV expansion. It is also worth noting that the treating radiation oncologist was present to define GTVs during daily adaptive process; it is part of our standard operating procedures to deliver schedule plans when there is large uncertainty in target definition, something that was not observed during delivery of the treatments used in this study.
We deduce that the overall delivered dose was less than the scheduled dose because the mean PTV volume was 18% smaller during OART compared with simulation. Figure 4 shows the GTV and PTV contours and the 100% reference/scheduled and adaptive plan prescription isodose lines for a representative right-sided patient. There is appreciable reduction in GTV and PTV size on daily CBCT compared with the reference plan; this led to a reduction in the 100% prescription isodose volume of each adapted fraction compared with the scheduled plan. The distances between the 2 fiducials visible on the adapted axial planes were on average 0.8 cm smaller than the distance between the same 2 fiducials on the reference scan, indicating a sizeable seroma volume shrinkage from time of simulation to treatment. For the 2 fiducials visible in the sagittal plane, the average adapted plan fiducial distance was 0.9 cm less than the reference plan. The range of distances between fiducials for the 5 adapted fractions in the axial and sagittal planes were 0.2 cm and 0.1 cm, respectively, indicating that most of the seroma shrinkage happened between simulation and the first treatment fraction. However, the PTV prescription coverage was greater for delivered plans than scheduled plans. This observation suggests that, not only target shrinkage, but also the target deformation and target mobility in patients with pendulous breasts on the day of treatment cannot be ignored, as an identically shaped but smaller target should still be entirely covered during scheduled treatment.
Figure 4.
Axial, coronal, and sagittal image slices of a select right-sided patient shown for the reference plan (Ref) and each delivered adaptive treatment fraction (Fx 1-Fx 5). Solid red and blue lines illustrate the gross tumor volume and planning target volume, respectively. Dotted green lines illustrate the reference 30 Gy isodose level for the Ref plan, translating to the scheduled 30 Gy isodose level for Fx 1-Fx 5. Dotted yellow lines illustrate the adaptive 30 Gy isodose level.
Although the prescription coverage planning goal was PTV V100% >95%, reference, scheduled, and adaptive plan target coverage was not normalized due to institutional protocol because the Ethos TPS was designed to achieve the highest possible coverage while limiting OAR doses, and the planning template was tuned without normalization.20 As a result, 3 of 21 reference plans and 9 of 103 delivered plans failed to achieve the planning goal of PTV V100% >95% (Table 2). To ensure that minimal satisfactory coverage was achieved, PTV V95% values were also analyzed: 100% of reference plans and 98% of delivered plans (101/103) met PTV V95% >95%. The 2 delivered fractions plans failing this objective belonged to a single outlier patient. The lowest delivered V95% for the remaining 20 patients was 98.7%. It should also be noted that many values PTV V100% values were greater than 98% because normalization was not used.
Figure 3 illustrates that OART benefits some patients more than others, likely due a combination of reasons including patient anatomy, target shrinkage, plan geometry, and optimization objectives. However, there is limited data to date that suggests why some patients benefit more than others. For this reason, OART is now the treatment standard for APBI in our clinic.
Although multiple studies covering MRI-based partial breast OART,29,30 only a single institutional series covering CBCT-based APBI using Ethos has been published to date.19 However, we observed significant differences between our methodologies and the findings of Montalvo et al. They focused solely on differences between adapted and scheduled plans for select DVH metrics differences, and did not discuss cohort or patient-specific DVHs or treatment compliance by plan type. Additionally, they observed significantly improved target coverage with adaption, but insignificant reduction in most OAR metrics. Contrarily, we observed significant improvements in target coverage and all OAR metrics besides the heart V1.5 Gy. As population-wide GTV volume changes were not reported by Montvalo et al, it is difficult to determine the cause of differences in these findings, suggesting that multi-institutional collaborative efforts are necessary to ensure uniform practice within the field of radiation oncology. One method to promote uniform practice across institutions within the Ethos system is through the generation of high-quality planning templates that can be shared across institutions.31
A limitation of this single-institutional work is the dependence of the validity of the presented results on target and OAR contouring accuracy from a single physician. In this study, the initially propagated GTV was adjusted in 85% (88/103) of cases. Although the extent of these adjustments was not captured, most changes were relatively minor. Improved onboard image quality could increase confidence in seroma/target contouring,32 but this remains to be investigated. Additionally, further studies are necessary to determine whether dosimetric improvement correlates to clinical outcomes, as there is not yet clinical data showing a benefit to kV-CBCT OART in this disease site.
Conclusion
We have successfully implemented an adaptive linac-based APBI program for early-stage breast cancer patients. The presented analysis demonstrates that OART provides dosimetric benefits as it allows to account for lumpectomy cavity volume changes between CT simulation and treatment delivery. Selection of adaptive treatment plans led to systematic decrease of most OAR DVH metrics compared with reference plans on daily anatomy. Clinical studies using OART for APBI are needed to evaluate clinical benefits compared with traditional (nonadaptive) 5 fraction linac-based APBI.
Disclosures
Richard A. Popple's institution, University of Alabama at Birmingham, has product evaluation agreements and research grants with Varian Medical Systems. He has a patent licensed by UAB Research Foundation to Varian Medical Systems. He has received honoraria for presentations on behalf of Varian Medical Systems. He has received a stipend to speak at Sun Nuclear meetings. Varian Medical Systems provides equipment to UAB as a part of a product evaluation agreement. Dennis N. Stanley and his institution, University of Alabama at Birmingham, have received payment and consulting fees from Varian Medical Systems.
Footnotes
Sources of support: This work had no specific funding.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;4:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya JS, Tobias JS, Baum M, et al. TARGeted Intraoperative radiotherapy (TARGIT): An innovative approach to partial-breast irradiation. Semin Radiat Oncol. 2005;15:84–91. doi: 10.1016/j.semradonc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Polgár C, Fodor J, Major T, Sulyok Z, Kásler M. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108:197–202. doi: 10.1016/j.radonc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51:451–463. doi: 10.1016/j.ejca.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Rahimi A, Thomas K, Spangler A, et al. Preliminary results of a phase 1 dose-escalation trial for early-stage breast cancer using 5-fraction stereotactic body radiation therapy for partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2017;98 doi: 10.1016/j.ijrobp.2017.01.020. 196-205 e2. [DOI] [PubMed] [Google Scholar]
- 7.Rahimi A, Morgan HE, Kim DW, et al. Cosmetic outcomes of a phase 1 dose escalation study of 5-fraction stereotactic partial breast irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2021;110:772–782. doi: 10.1016/j.ijrobp.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Veale C, Hablitz D, et al. Feasibility and short-term toxicity of a consecutively delivered five fraction stereotactic body radiation therapy regimen in early-stage breast cancer patients receiving partial breast irradiation. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.901312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon SH, Shin KH, Park S-Y, et al. Seroma change during magnetic resonance imaging-guided partial breast irradiation and its clinical implications. Radiat Oncol. 2017;12:103. doi: 10.1186/s13014-017-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sager O, Dincolglan F, Uysal B, et al. Evaluation of adaptive radiotherapy (ART) by use of replanning the tumor bed boost with repeated computed tomography (CT) simulation after whole breast irradiation (WBI) for breast cancer patients having clinically evident seroma. Jpn J Radiol. 2018;36:401–406. doi: 10.1007/s11604-018-0735-2. [DOI] [PubMed] [Google Scholar]
- 11.Yan D, Georg D. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 12.Sonke JJ, Aznar M, Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29:245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Foroudi F, Wong J, Kron T, et al. Online adaptive radiotherapy for muscle-invasive bladder cancer: Results of a pilot study. Int J Radiat Oncol Biol Phys. 2011;81:765–771. doi: 10.1016/j.ijrobp.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Moazzezi M, Rose B, Kisling K, et al. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J Appl Clin Med Phys. 2021;22:82–93. doi: 10.1002/acm2.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astrom LM, Behrens CP, Storm KS, et al. Online adaptive radiotherapy of anal cancer: Normal tissue sparing, target propagation methods, and first clinical experience. Radiother Oncol. 2022;176:92–98. doi: 10.1016/j.radonc.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Mao W, Riess J, Kim J, et al. Evaluation of auto-contouring and dose distributions for online adaptive radiation therapy of patients with locally advanced lung cancers. Pract Radiat Oncol. 2022;12:e329–e338. doi: 10.1016/j.prro.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Henke LE, Olsen JR, Contreras JA, et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol. 2019;4:201–209. doi: 10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiff JP, Price AT, Stowe HB, et al. Simulated computed tomography-guided stereotactic adaptive radiotherapy (CT-STAR) for the treatment of locally advanced pancreatic cancer. Radiother Oncol. 2022;175:144–151. doi: 10.1016/j.radonc.2022.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Montalvo SK, Kim N, Nwachukwu C, et al. On the feasibility of improved target coverage without compromising organs at risk using online adaptive stereotactic partial breast irradiation (A-SPBI) J Appl Clin Med Phys. 2023;24:e13813. doi: 10.1002/acm2.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley D.N., et al. A roadmap for implementation of kV-CBCT online adaptive radiation therapy and initial first year experiences. J Appl Clin Med Phys. 2023:e13961. doi: 10.1002/acm2.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X., Stanley D.N., Cardenas C.E., Harms J., Popple R.A. Do we need patient‐specific QA for adaptively generated plans? Retrospective evaluation of delivered online adaptive treatment plans on Varian Ethos. J Appl Clin Med Phys. 2023;24(2) doi: 10.1002/acm2.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogue J.A., et al. Leveraging intelligent optimization for automated, cardiac-sparing accelerated partial breast treatment planning. Front Oncol. 2023;13:1130119. doi: 10.3389/fonc.2023.1130119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coles CE, Wilson CB, Cumming J, et al. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: Audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol. 2009;35:578–582. doi: 10.1016/j.ejso.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Lowrey N, Koch CA, Purdie T, et al. Magnetic resonance imaging for breast tumor bed delineation: Computed tomography comparison and sequence variation. Adv Radiat Oncol. 2021;6 doi: 10.1016/j.adro.2021.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley D.N., et al. Evaluation and correlation of patient movement during online adaptive radiotherapy with CBCT and a surface imaging system. J Appl Clin Med phys. 2023 doi: 10.1002/acm2.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisling K, Keiper TD, Branco D, et al. Clinical commissioning of an adaptive radiotherapy platform: Results and recommendations. J Appl Clin Med Phys. 2022;23:e13801. doi: 10.1002/acm2.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelissen KJ, Versteijne E, Senan S, et al. Evaluation of a workflow for cone-beam CT-guided online adaptive palliative radiotherapy planned using diagnostic CT scans. J Appl Clin Med Phys. 2023;24:e13841. doi: 10.1002/acm2.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 29.Ng J, Pennell R, Formenti SC. The initial experience of MRI-guided precision prone breast irradiation with daily adaptive planning in treating early stage breast cancer patients. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuong MD, Clark MA, Henke LE, et al. Patterns of utilization and clinical adoption of 0.35 Tesla MR-guided radiation therapy in the United States: Understanding the transition to adaptive, ultra-hypofractionated treatments. Clin Transl Radiat Oncol. 2023;38:161–168. doi: 10.1016/j.ctro.2022.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogue JA, Cardenas CE, Harms J, et al. Benchmarking automated machine learning-enhanced planning with ethos against manual and knowledge-based planning for locally advanced lung cancer. Adv Radiat Oncol. 2023;8 doi: 10.1016/j.adro.2023.101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varian Receives FDA 510(k) clearance for Halcyon and ethos radiotherapy systems featuring hypersight imaging solution and announces first patient treatment. Accessed August 2, 2023. https://www.varian.com/about-varian/newsroom/press-releases/varian-receives-fda-510k-clearance-halcyon-and-ethos.