Abstract
The studies described herein were designed to evaluate the usefulness of the PCR in detecting persistent syphilitic infection. Three groups of animals were used: a nonimmune group infected with Treponema pallidum (NI/TP), a nonimmune group injected with heat-killed treponemes (NI/HKTP), and an immune and reinfected group (I/TP). All animals were inoculated with similar numbers of organisms distributed at 10 sites on the clipped back and in both testes. The persistence of the treponemes was examined by PCR and the rabbit infectivity test (RIT). The kinetic studies and statistical analysis of their results demonstrated that the rate of bacterial clearance from the NI/TP group was very low and incomplete at 4 months after infection. It was significantly different from those of both the NI/HKTP (P < 0.001) and I/TP (P < 0.05) groups. No statistically significant differences in treponemal elimination were found between the NI/HKTP and I/TP groups. PCR can detect the DNA of dead organisms, but the latter are eliminated by the host relatively quickly (15 to 30 days) as compared to elimination of live treponemes (>120 days). PCR results correlated well with RIT results. These data suggest that PCR-positive specimens obtained from an untreated patient(s) or collected weeks after treatment indicate persistent infection. They also show that the process of elimination of T. pallidum from primary sites of infection is prolonged and incomplete.
The paucity of practical and sensitive methods for detection of Treponema pallidum and the inability of the pathogen to be cultured in vitro have largely contributed to the poor understanding of the pathogenesis of syphilitic infection, its diagnosis, and treatment. In recent years, the introduction of the highly sensitive and specific PCR assay for detection of T. pallidum genomic DNA has been a welcome alternative to the impractical and frequently unavailable “gold standard” rabbit infectivity test (RIT). However, unlike the RIT, PCR cannot distinguish between living and dead organisms. Therefore, its reliability in determining active disease has been questioned not only for syphilis but for many other infectious diseases (16). The persistence of T. pallidum in various organs at different stages of infection, in untreated and treated individuals, has been evaluated extensively (4, 8, 13, 15, 21, 28, 29). However, only a few studies, using relatively insensitive methods and short experimental periods, have been done to determine specifically the kinetics of elimination of living treponemes from the primary site of inoculation in normal (1, 10, 24) or immune rabbits (5, 18). This information is important because the host’s early response to a pathogen determines the outcome of infection and disease progression (20).
The rationale and purpose of this study were to use PCR and RIT (i) to determine the rapidity with which normal rabbits eliminate virulent as opposed to dead treponemes from primary sites of infection, (ii) to compare this with the clearance of living organisms from T. pallidum-immune animals, and (iii) to determine whether a positive PCR can reliably be interpreted as detecting living organisms.
(This work was presented in part at the 96th General Meeting of the American Society for Microbiology, New Orleans, La. May 1996 [27].)
MATERIALS AND METHODS
Microorganisms.
T. pallidum subsp. pallidum was obtained from rabbit testes at the peak of orchitis. Heat-killed T. pallidum (HKTP) was prepared by heating T. pallidum at 56°C for 2 h. Each treponemal suspension was adjusted to a concentration of 4 × 107 organisms/ml in appropriate medium.
Animals and treatment.
Three groups of adult (3- to 4-month-old) male Nys (Flemish Giant) Venereal Disease Research Laboratory (VDRL) test-negative rabbits, free of mites and parasites, were used (Table 1). The first group consisted of eight nonimmune rabbits infected with a 0.1-ml suspension of T. pallidum (NI/TP group) at each of 10 marked sites on the clipped back and 1 ml per testis. The second group consisted of six nonimmune animals inoculated similarly to group I but with HKTP (NI/HKTP group). The third group included six T. pallidum-immune rabbits reinfected with T. pallidum (I/TP group). The animals of this group were first infected intratesticularly with 108 treponemes per testis. Six months later, three of the six animals were challenged intracutaneously at a single site with 2 × 106 treponemes to confirm resistance. Each immune rabbit received 40,000 U of penicillin G procaine per kg of body weight per day and 20 mg of probenecid per kg per day for a period of 10 days. Before and after antibiotic treatment, the testes from three animals were aspirated for examination of T. pallidum DNA. Two weeks later, these animals were challenged at 10 sites on the back and in both testes with the same size inoculum as that given to NI/TP animals. The length of the experimental period and the total number of samples examined by PCR for each group are shown in Table 1.
TABLE 1.
Groups of rabbits included in the study
| Group | No. of animals | Injected with: | Period of tissue collection (days)a | Total no. of samples examined by PCRb |
|---|---|---|---|---|
| NI/TP | 8 | T. pallidum | 1–120 | 217 |
| NI/HKTP | 6 | HKTP | 1–30 | 140 |
| I/TP | 6 | T. pallidumc | 1–50 | 164 |
Includes only the time of tissue collection after infection, injection, or reinfection.
Includes skin, testis, lymph node, spleen, heart, and brain specimens.
Immune and antibiotic-treated animals were reinfected with T. pallidum.
Specimen collection after primary inoculation and reinfection.
On a given day, a single animal from each group was sacrificed by an intravenous injection of 2 ml of Sleepaway (Fort Dodge Labs Inc., Fort Dodge, Iowa). The back of each animal was closely clipped and thoroughly disinfected. To avoid cross-contamination with treponemal DNA, individual sets of sterile blades and forceps were used to cut 10-mm2 pieces from each of the 10 injected skin sites and approximately six to eight slices from each testis. Specimens were immediately placed into individual sterile tubes containing 2× concentrated Tris-EDTA buffer and stored at −70°C until used. Small pieces of skin and testes from each animal were separately pooled, and extract from each pool was used for RIT. Selected specimens of skin and testes were collected for histology. Tissues from inguinal lymph nodes (ILN), spleen (SP), heart (HRT), and brain (BRN) were collected from four NI/TP animals sacrificed between 15 and 120 days after infection and from all I/TP rabbits sacrificed between 1 and 50 days after reinfection. Animal procedures were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center for Laboratories and Research.
Nested PCR and RIT.
Nested PCR and RIT have been fully described elsewhere (25, 26).
Statistical analysis.
A nonlinear least-squares regression analysis (2) was used to model the time-dependent local elimination of the spirochetes from the NI/TP, NI/HKTP, and I/TP groups. The F statistic was used to determine whether there was an injection site effect or a group-associated effect.
RESULTS
Course of infection.
In the NI/TP group, skin lesions developed by day 4; the lesions became indurated by day 10 and chancre-like (8 to 20 mm in diameter) with some degree of necrosis by day 15. The skin lesions subsided by day 30 and were healed by day 60. Inflammation of the testes started at approximately day 7, peaked at day 13, and subsided 7 to 10 days later. Some of the testes developed small (4- to 8-mm) syphilomas with a rubbery consistency which lasted for 1 to 3 weeks. The NI/HKTP animals did not show any cutaneous or testicular reaction to the inoculum.
All rabbits in the I/TP group responded uniformly to the primary intratesticular infection, with infiltration by day 6 and acute orchitis by day 10. They developed a high titer of VDRL test antibodies, which decreased within 5 months after infection. The immune status of the animals was confirmed by intradermal challenge with T. pallidum, and none of the three reinfected rabbits showed any cutaneous response for 3 weeks. Testis aspirates from three rabbits examined by PCR prior to antibiotic treatment were positive. Two weeks after treatment, all aspirates were negative.
PCR.
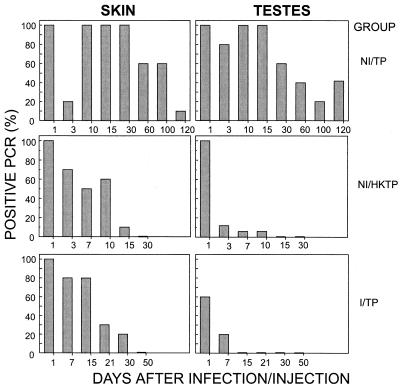
The individual results of PCR testing for the three groups of rabbits are shown in Fig. 1. The very slow pattern of treponemal elimination in the infected group (NI/TP) was remarkably different from the fast and continuous drop of treponemal DNA in the NI/HKTP and I/TP groups. While skin and testis samples from infected rabbits were still PCR positive at 4 months of infection, no treponemal DNA was detectable in the NI/HKTP or I/TP group after 7 to 30 days, depending on the site of inoculation. A temporary drop in treponemal DNA 3 days after primary infection in the NI/TP group was characteristic of the rapid dissemination process not encountered in the other groups.
FIG. 1.
PCR examination. Patterns of treponemal clearance in the NI/TP, NI/HKTP, and I/TP groups are shown for comparison.
Statistical evaluation.
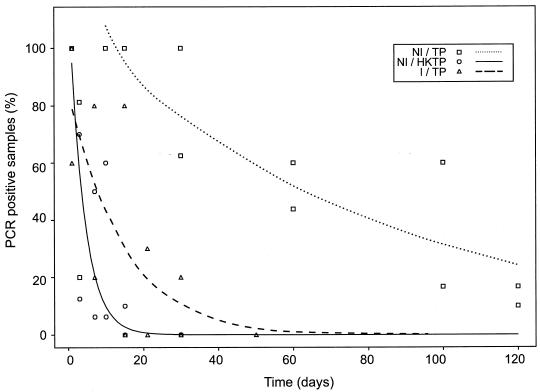
The analysis of the impact of group effect, regardless of site of inoculation, is shown in Fig. 2. The results showed statistically significant differences for rabbits inoculated with T. pallidum versus those inoculated with HKTP (P < 0.001). Significantly different results for nonimmune and immune rabbits injected with T. pallidum were also noted (P < 0.05). The difference between the I/TP and NI/HKTP groups was not significant.
FIG. 2.
Statistical analysis of group effect regardless of site of inoculation. The rate of T. pallidum DNA clearance in the NI/TP groups was significantly lower than those in the NI/HKTP (P < 0.001) and I/TP (P < 0.05) groups. There were no statistically significant differences between the rates of treponemal DNA elimination for the NI/HKTP and I/TP groups.
Correlation between PCR and RIT.
The correlation between PCR and RIT results obtained for the NI/TP and I/TP groups depended on the tissue examined (Table 2). The testis specimens provided an excellent correlation (100%) between the two methods. However, for skin specimens, RIT was less sensitive than PCR (rabbit 8 of NI/TP group; rabbits 17 and 18 of I/TP group [Table 2]). While 14 of 14 organ samples (ILN, SP, HRT, and BRN) collected from infected animals sacrificed between 15 and 120 days after infection were positive by PCR, all 24 similarly collected samples from six immune-reinfected rabbits were negative by PCR.
TABLE 2.
Sensitivity of PCR and RIT in detection of T. pallidum in tissues from nonimmune infected (NI/TP) and immune reinfected (I/TP) rabbits
| Rabbit group | Rabbit no. | Days after inf. or reinf.a | Test resultsb for tissues from:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin
|
Testes
|
ILN | SP | HRT | BRN | |||||
| PCR | RIT | PCR | RIT | |||||||
| NI/TP | 4 | 15 | ND | ND | ND | ND | + | + | + | + |
| 6 | 60 | 6/10 | + | 7/16 | + | + | + | ND | ND | |
| 7 | 100 | 6/10 | + | 6/16 | + | + | + | + | + | |
| 8 | 120 | 1/10 | − | 6/16 | + | + | + | + | + | |
| I/TP | 14 | 1 | 10/10 | + | 9/15 | + | − | − | − | − |
| 15 | 7 | 8/10 | + | 3/15 | + | − | − | − | − | |
| 16 | 15 | 9/10 | + | 0/16 | − | − | − | − | − | |
| 17 | 21 | 3/10 | − | 0/16 | − | − | − | − | − | |
| 18 | 30 | 2/10 | − | 0/16 | − | − | − | − | − | |
| 19 | 50 | 0/10 | − | 0/16 | − | − | − | − | − | |
inf., infection; reinf., reinfection.
+, positive; −, negative; ND, not determined. Values represent number of positive samples detected relative to the total number examined. ILN, SP, HRT, and BRN samples were tested by PCR alone.
Histopathological changes and presence of T. pallidum.
In nonimmune rabbits, the histopathological changes evoked by infection in the skin and testes resembled those previously reported (24). One day after infection, no major abnormalities were observed in either type of tissue, as evaluated by hematoxylin and eosin staining. Although both tissues were positive by PCR, no spirochetes could be visualized by silver staining. A mononuclear cell infiltration began after 5 days and reached its peak 15 days after infection. At this point, characteristic degeneration and necrosis of the epidermis with a dermal infiltrate of lymphocytes, plasma cells, and eosinophils were seen. In the testes on day 15, the tunica albuginea showed fibrous thickening and increased vascularity with focal and diffuse infiltration of lymphocytes and plasma cells. There was decreased spermatogenesis in some tubules but no marked atrophy. Steiner staining of skin and testis tissues showed numerous spirochetes in the epidermis-dermis junction and in the tunica albuginea, as well as in the intertubular connective tissue (data not shown).
One day after reinfection in the I/TP group, the skin showed a diffuse slight infiltration of subepithelially located lymphocytes and eosinophils. In the testes, a similar type of infiltration was seen in connective tissue septa separating the seminiferous tubules. At 15 days after reinfection, no gross abnormalities were found in the skin, whereas a mild infiltration of lymphocytes was observed in the intertubular connective tissue of the testes. A few focal lymphoid nodules adjacent to seminiferous tubules as well as focal areas of atrophy and aspermatogonia were noted (data not shown). Although skin and testis tissue sections were positive by PCR up to 30 (skin) and 7 (testes) days postreinfection, respectively (Table 2), they were all negative by silver staining.
DISCUSSION
Due to its extraordinary sensitivity and specificity, PCR is becoming an increasingly employed technology for the diagnosis of infectious diseases. For infections caused by organisms that are either not or only poorly cultivable, have late sequelae, cause late immune responses, or have prolonged seroreactivity postinfection, PCR may be of particular value for identifying the presence of suspected organisms. PCR has been utilized for diagnosis of infection with T. pallidum from multiple sites, including skin lesions, blood, cerebral spinal fluid, amniotic fluid, and various organs (7, 8, 13, 25, 26), as well as from paraffin-embedded tissue (8). However, the usefulness of PCR as a diagnostic modality of active infection has been questioned because of its inability to distinguish live from dead organisms (23).
The recent development of a reverse transcriptase PCR, targeting 16S rRNA, for detection of T. pallidum (3) is a welcome addition to existing procedures. However, reverse transcriptase PCR is a relatively new technique and its specificity must be rigorously examined to be compared to the PCR assay that has been employed for 11 years (12).
In the present study, using PCR in the rabbit syphilis model, we have addressed this issue by studying the kinetics of local elimination of living and dead organisms from nonimmune and immune animals. Dead-organism DNA could not be detected in the skin or testes by PCR after 15 or 10 days, respectively. Treponemal DNA elimination in immune rabbits occurred after 30 and 7 days in the skin and the testes, respectively. On the other hand, at 120 days, nonimmune rabbits infected with T. pallidum still had detectable treponemal DNA at both sites of infection and in many organs. In addition, preliminary data have demonstrated that chromosomal DNA isolated from T. pallidum and injected into the skin (multiple sites) and the testes of rabbits in concentrations from 10 fg to 100 ng (equivalent of 4 to 4 × 106 T. pallidum organisms) is eliminated, based on PCR assay, within 24 and 48 h from the skin and testes, respectively (26a). These results suggest that in most instances the presence of T. pallidum DNA as determined by PCR, reflects living organisms. The correlation between elimination of DNA and infectivity determined by RIT was excellent for testis samples and only slightly different for the skin (Table 2).
Elimination of treponemal DNA as determined by PCR occurred 2 weeks after reinfection in the antibiotic-treated group of immune animals. This timing is quite similar to the timing of the elimination of DNA of HKTP. However, because we did not specifically look at the kinetics of treponemal elimination in the cerebrospinal fluid of these rabbits, we cannot comment on the interpretation of PCR results for the central nervous system after antibiotic treatment. Nor can our results be compared to those using body fluids for PCR.
Whether the dead organisms detected by PCR were intact or were in the process of disintegration is not known. Similarly, in studies of infection with Borrelia burgdorferi, it is not clear whether whole spirochetes, membrane-associated extrachromosomal DNA, or soluble DNA is detected by PCR in various biological fluids such as cerebrospinal fluid, urine, and the milk of lactating women (11, 16, 17).
Our kinetic studies also shed light on the host immune response during the early phase of T. pallidum infection. We found an early, but temporary, drop in treponemal DNA at the site of inoculation (particularly the skin) in nonimmune rabbits infected with T. pallidum (Fig. 1). This seemed to be associated with the early systemic dissemination of treponemes, as previously reported for both rabbits and guinea pigs (5, 14, 27). This pattern was markedly different than that for immune rabbits, in which elimination of treponemal DNA was rapid and sustained (Fig. 1).
It is not certain exactly how treponemal DNA is eliminated in rabbits injected with killed T. pallidum because few mononuclear infiltrating cells were found at the site of infection (data not shown). In the immune group, a brisk immune response occurred; mononuclear cells were detected histologically by 24 h. However, the immune response appeared to be relatively inefficient in nonimmune rabbits, because recruitment of mononuclear infiltrating cells was delayed by more than 5 days and the mononuclear cell response peaked at 10 to 15 days and subsided thereafter.
The extremely slow removal of T. pallidum in nonimmune rabbits also corroborates the natural history studies of humans in which untreated patients developed secondary disseminated disease and up to 25% of patients relapsed within 1 year of the onset of latency (6). Thus, both clinical and laboratory studies indicate that the development of immunity to syphilis is a slow and incomplete process that is efficient in promoting latency and preventing reinfection but that may not eliminate the pathogen from the host (22). Our previous (24) and present findings with the rabbit model do not support the concept of an efficient immune clearance of treponemes from the primary site of infection as has been proposed by some investigators with the same animal model (1, 9, 10, 19). Differences in methodologies and reagents may account for the discrepancies in results and interpretation.
In conclusion, the data presented herein show that in syphilis, the elimination of the pathogen from the site of inoculation is a protracted and incomplete process. They also suggest that the presence of treponemal DNA in specimens obtained from untreated hosts or collected a month after treatment indicates the presence of living organisms.
ACKNOWLEDGMENTS
We thank Diana Decker for preparation of the histopathological samples, the Photography and Illustration Unit for their excellent work, Kathy Vindittie for preparation of the manuscript, and Felix Milgrom and Murray King for reading the manuscript and editorial comments.
This work was supported by Public Health Service grant A121833 from the National Institute of Allergy and Infectious Diseases.
Footnotes
This paper is dedicated to Mary Pangborn, the discoverer of cardiolipin at the Division of Laboratories and Research, New York State Department of Health, Albany, for her 90th birthday.
REFERENCES
- 1.Baker-Zander S A, Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am Assoc Pathol. 1980;101:387–414. [PMC free article] [PubMed] [Google Scholar]
- 2.Bates D M, Watts D G. Non-linear regression analysis and its applications. New York, N.Y: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- 3.Centurion-Lara A, Castro C, Shaffer J M, van Voorhis W C, Marra C M, Lukehart S A. Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. J Clin Microbiol. 1997;35:1348–1352. doi: 10.1128/jcm.35.6.1348-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collart P, Borel L J, Durel P. Significance of spiral organisms found after treatment in late human and experimental syphilis. Br J Vener Dis. 1964;40:81–89. doi: 10.1136/sti.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cumberland M C, Turner T B. The rate of multiplication of Treponema pallidum in normal and immune rabbits. Am J Syph Gonorrhea Vener Dis. 1949;33:201–212. [PubMed] [Google Scholar]
- 6.Gjestland, T. 1955. The Oslo study of untreated syphilis. An epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta Dermato-Venereol. 35(Suppl. 34):103–144. [DOI] [PubMed]
- 7.Grimprel E, Sanchez P J, Wendel G D, Burstain J M, McCracken G H, Jr, Radolf J D, Norgard M V. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera, and cerebrospinal fluid. J Clin Microbiol. 1991;29:1711–1718. doi: 10.1128/jcm.29.8.1711-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz H W, Valsamis M P, Wicher V, Abbruscato F, Larsen S A, Wormser G P, Wicher K. Cerebral syphilitic gumma confirmed by polymerase chain reaction in a man with human immunodeficiency virus infection. N Engl J Med. 1994;331:1488–1491. doi: 10.1056/NEJM199412013312204. [DOI] [PubMed] [Google Scholar]
- 9.Lukehart S A. Immunology and pathogenesis of syphilis. In: Quinn T, editor. Advances in host defense mechanisms. 8. Sexually transmitted diseases. New York, N.Y: Raven Press Ltd.; 1992. pp. 141–163. [Google Scholar]
- 10.Lukehart S A, Baker-Zander S A, Lloyd R M C, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of the cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980;124:461–467. [PubMed] [Google Scholar]
- 11.Maiwald M, Stockinger C, Hassler D, Von Knebel Doeberitz M, Sonntag H G. Evaluation of the detection of Borrelia burgdorferi DNA in urine samples by polymerase chain reaction. Infection. 1995;23:173–179. doi: 10.1007/BF01793860. [DOI] [PubMed] [Google Scholar]
- 12.Mullis K B, Faloona F, Scharf R, Saiki R K, Horn G T, Ehrlich H A. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Noordhoek G T, Wolters E C, De Jonge M E J, van Embden J D A. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol. 1991;29:1976–1984. doi: 10.1128/jcm.29.9.1976-1984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raiziss G W, Severac M. Rapidity with which Spirochaeta pallida invades the blood stream. Arch Dermatol Syphilol. 1937;35:1101–1105. [Google Scholar]
- 15.Ryan S J, Hardy P H, Hardy J M, Oppenheimer E H. Persistence of virulent Treponema pallidum despite penicillin therapy in congenital syphilis. Am J Ophthalmol. 1972;73:258–261. doi: 10.1016/0002-9394(72)90141-9. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt B L. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev. 1997;10:185–201. doi: 10.1128/cmr.10.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt B L, Aberer E, Stockenhuber C, Klade H, Breier F, Luger A. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis. Diagn Microbiol Infect Dis. 1995;21:121–128. doi: 10.1016/0732-8893(95)00027-8. [DOI] [PubMed] [Google Scholar]
- 18.Sell S, Salman J, Norris S J. Reinfection of chancre-immune rabbits with Treponema pallidum. Am J Pathol. 1985;118:248–255. [PMC free article] [PubMed] [Google Scholar]
- 19.Sell S, Hsu P-L. Delayed hypersensitivity, immune deviation, antigen processing and T cell subset selection in syphilis pathogenesis and vaccine design. Immunol Today. 1993;14:576–582. doi: 10.1016/0167-5699(93)90196-R. [DOI] [PubMed] [Google Scholar]
- 20.Sher A, Ahmed R. Immunity to infection. Editorial review. Regulation of innate and adaptive responses to infection: new avenues and angles. Curr Opin Immunol. 1995;7:471–473. [Google Scholar]
- 21.Smith J L, Israel C W, McCrory J A, Harner R E. Recovery of Treponema pallidum from aqueous humor removed at cataract surgery in man by passive transfer to rabbit testis. Am J Ophthalmol. 1967;65:242–247. doi: 10.1016/0002-9394(68)93595-2. [DOI] [PubMed] [Google Scholar]
- 22.Sparling P F. Natural history of syphilis. In: Holmes K K, Mardh P A, Sparling P F, Weisner P J, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill; 1984. pp. 298–305. [Google Scholar]
- 23.Tramont E C. Neurosyphilis in patients with human immunodeficiency virus infection. N Engl J Med. 1995;332:1169. . (Correspondence.) [PubMed] [Google Scholar]
- 24.Wicher K, Wicher V, Nakeeb S M, Dubiski S. Studies of rabbit testes infected with Treponema pallidum. I. Immunopathology. Br J Vener Dis. 1983;59:349–358. doi: 10.1136/sti.59.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicher K, Noordhoek G T, Abbruscato F, Wicher V. Detection of Treponema pallidum in early syphilis by DNA amplification. J Clin Microbiol. 1992;30:497–500. doi: 10.1128/jcm.30.2.497-500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicher K, Abbruscato F, Wicher V, Baughn R E, Noordhoek G T. Target organs of infection in guinea pigs with acquired and congenital syphilis. Infect Immun. 1996;64:3174–3179. doi: 10.1128/iai.64.8.3174-3179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Wicher, K. Unpublished data.
- 27.Wicher V, Abbruscato F, Wicher K. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Persistence of virulent and heat-killed Treponema pallidum in rabbit and guinea pig as determined by PCR, abstr. D-51; p. 250. [Google Scholar]
- 28.Yobs A R, Clark J W, Jr, Mothershed S E, Bullard J C, Artley C W. Further observations on the persistence of Treponema pallidum after treatment in rabbit and humans. Br J Vener Dis. 1968;44:116–130. doi: 10.1136/sti.44.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogeswari L, Chacko C W. Persistence of T. pallidum and its significance in penicillin-treated seropositive late syphilis. Br J Vener Dis. 1971;47:339–347. doi: 10.1136/sti.47.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]