Abstract
Eimeria tenella, an obligate intracellular apicomplexan parasite, is the major causative agent of chicken coccidiosis. Some epidermal growth factor (EGF)-like domain-containing proteins of other members of apicomplexan parasites have been reported to contribute to parasite survival. To date, however, EGF-like domain-containing proteins of E. tenella are not well studied. In this study, a gene fragment that encodes 4 EGF-like domains of E. tenella microneme protein 7 (EGF-EtMIC7) was amplified and expressed using an Escherichia coli expression system. Following generation of polyclonal antibodies that recognize recombinant EGF-EtMIC7 (rEGF-EtMIC7), the expression of EtMIC7 in sporozoites and merozoites was examined. Moreover, its roles in cellular regulation were investigated. The native EtMIC7 in E. tenella sporozoites and merozoites was detected by using Western blot and indirect immunofluorescence assays. rEGF-EtMIC7 could activate Akt, whereas blockade of EGF receptor (EGFR) failed to induce Akt phosphorylation. Compared with the control group, LMH cells treated with rEGF-EtMIC7 showed increased cell proliferation and expressed higher levels of B cell leukemia/lymphoma 2 (BCL-2). These findings contribute to the better understanding of parasite-host interactions at the molecular level during E. tenella infection.
Key words: Eimeria tenella, microneme protein 7, EGFR-Akt, cell proliferation, apoptosis
INTRODUCTION
Chicken coccidiosis, caused by obligate intracellular parasites that belong to the genus Eimeria, results in huge economic losses to the world's poultry industry (Morris and Gasser, 2006). Eimeria tenella, a highly pathogenic Eimeria species, is responsible for cecal coccidiosis (Morris and Gasser, 2006). E. tenella has proven to be able to activate epidermal growth factor receptor (EGFR) (Zhang et al., 2022), the ligands of which include epidermal growth factor (EGF) (Wee and Wang, 2017). Recently, EGF-like domains of E. tenella microneme protein 4 (MIC4) were found to be associated with EGFR activation, cell proliferation and apoptosis (Zhang et al., 2022). In addition, several EGF-like domain-containing MICs of E. tenella have been found by transcriptome sequencing analysis, such as MIC7 (XM_013374080.1) and MIC8 (XM_013376052.1) (Reid et al., 2014). EGF-like domains of MIC8 were reported to be involved in the adhesion of host cells (Zhao et al., 2021). To date, however, the biological significance of EGF-like domains of MIC7 remains unknown.
In this study, a gene fragment that encodes 4 EGF-like domains of E. tenella MIC7 (EGF-EtMIC7) was amplified from cDNA of E. tenella wild type Shandong strain (SD-01) and expressed in Escherichia coli. Following preparation of polyclonal antibodies that recognize recombinant EGF-EtMIC7 (rEGF-EtMIC7), the expression of EtMIC7 in E. tenella sporozoites and merozoites was examined by using Western blot and indirect immunofluorescence assays. Also, its roles in the regulation of Akt phosphorylation, B cell leukemia/lymphoma 2 (BCL-2) expression, and cell proliferation were determined.
MATERIALS AND METHODS
Parasites, Cell Lines, and Animals
The sporulated oocysts of E. tenella wild type Shandong strain (SD-01) (a generous gift from Professor Xiaomin Zhao, Shandong Agricultural University) were preserved in 2.5 percent potassium dichromate solution (Liu et al., 2022). Leghorn male hepatoma (LMH) cells (a chicken hepatoma cell line) were maintained in Dulbecco's modified eagle medium (DMEM), which was supplemented with 10% fetal bovine serum (FBS) and antibiotics. Cell culture was carried out at 37°C in a humidified incubator with 5% CO2. Female BALB/c mice (6–8 wk old) were used for generation of polyclonal antibodies. Hyline Brown cocks were inoculated with a dose of 1 × 104 sporulated oocysts of E. tenella wild type Shandong strain (SD-01) to provide sufficient parasites. Merozoites were collected from ceca at 120 h postinfection, and then purified using Percoll gradient. Unsporulated oocysts were recovered and purified from ceca at 7 days postinfection. Following sporulation, sporozoites were prepared by in vitro excystation. All experiments involving animals underwent review and approval by the Animal Ethics Committee of Shanxi Agricultural University.
Construction of the Recombinant Plasmid
The TRI Reagent (Sigma-Aldrich, St. Louis, MO) was used for total RNA isolation from E. tenella sporozoites according to the protocol suggested by the manufacturer. Then, first strand cDNA was synthesized by using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The gene fragment encoding the EGF-EtMIC7 (from aa53 to aa227) was amplified by PCR from the cDNA of E. tenella using the primers EGF-EtMIC7-F (5′-TTTGGATCCGATATAGACGAGTGCGCTCAGGGTCT-3′) and EGF-EtMIC7-R (5′-TTTGAATTCCTCGCATGTGTTGACTGTCCCG-3′). The PCR products were gel purified, and then ligated into a pET30a vector at the BamHI and EcoRI sites. The recombinant vector (designated pET30a-EGF-EtMIC7) was verified by restriction endonuclease digestion as well as sequence analysis (Sangon Biotech, Shanghai, China).
Recombinant Expression of EGF-EtMIC7 and Generation of Antiserum
The pET30a vector and pET30a-EGF-EtMIC7 were transformed into E. coli Transetta (DE3) (TransGen Biotech, Beijing, China), respectively. Following induction by isopropyl-β-d-thiogalactoside (IPTG), rEGF-EtMIC7 and pET-30a tag protein were purified using the His60 Ni Gravity Columns (Takara, Dalian, China), respectively. Then, endotoxin was removed by using Pierce High-Capacity Endotoxin Removal Resin (Thermo Fisher Scientific, Waltham, MA). The concentration was determined by using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer's specifications. Anti-rEGF-EtMIC7 serum was prepared from BALB/c mice as previously described (Liu et al., 2022).
Fluorescence Microscopy
The expression of EtMIC7 in sporozoites and merozoites was examined by using the immunofluorescence technique. Briefly, sporozoites and merozoites were separately smeared on each glass slide and fixed with 2% paraformaldehyde for 10 min at room temperature, and were permeabilized with 1% Triton X-100. Mouse anti-rEGF-EtMIC7 serum served as the primary antibody (dilution of 1:250), and FITC-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA) (dilution of 1:100) served as the secondary antibody. The fluorescence signal was examined by using an inverted fluorescence microscope (Nikon, Tokyo, Japan). The slides stained with the normal mouse serum as the primary antibody were used as control.
Cell-Based Assays
To determine whether rEGF-EtMIC7 could regulate Akt phosphorylation status, LMH cells were seeded in 6-well polystyrene plates at the rate of 5 × 105 cells/2 mL/well in DMEM with 10% FBS for 1 d at 37°C. Then, the medium was replaced with serum-free DMEM. After 12-h incubation, cells were incubated with rEGF-EtMIC7 or pET-30a tag protein for 15 min. To test for activity inhibition, LMH cells were incubated with or without the EGFR inhibitor (AG1478) for 1 h, and then stimulated with rEGF-EtMIC7 for 15 min.
To determine whether rEGF-EtMIC7 could regulate B cell leukemia/lymphoma 2 (BCL-2) expression, LMH cells were incubated with or without rEGF-EtMIC7 for 1 h. After treatments, cells were scraped off the plates and lysed in RIPA buffer (Beyotime, Shanghai, China) supplemented with protease and phosphatase inhibitors. After centrifugation at 4°C for 15 min, the resultant supernatant was boiled in protein loading buffer for 5 min, and then subjected to Western blot analysis.
The effects of rEGF-EtMIC7 on the proliferation of LMH cells were examined with Cell Counting Kit-8 (CCK-8) assay (Zhou et al., 2021). Briefly, LMH cells were cultured in DMEM with 10% FBS for 24 h at 37°C. Then, 100 µL of the diluted cell culture was added into each well of a 96-well culture plate (Corning Inc., Corning, NY) and cultured for 12 h. The cells were starved for 12 h in DMEM without FBS, and then treated with or without rEGF-EtMIC7 for 24 h. Finally, 10 µL of CCK-8 (Beyotime, Shanghai, China) was added to the cell cultures and incubated for 1 h. Absorbance was measured using a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA) at 450 nm.
Western Blot
Each protein sample was added into each channel of the SDS-PAGE gel, followed by being electrophoresed and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA). For Western blot analysis of native EtMIC7, protein extracts of sporozoites and merozoites were separately obtained by using RIPA lysis buffer (Beyotime, Shanghai, China), and mouse anti-rEGF-EtMIC7 serum (dilution of 1:1,000) was used as the primary antibody. The membrane probed with normal mouse serum was used as the control. Peroxidase-conjugated goat anti-mouse IgG (Abcam, Cambridge, UK) (dilution of 1:10,000) was used as a secondary antibody. To determine whether rEGF-EtMIC7 could activate Akt, the membranes were separately incubated with the primary antibodies purchased from Cell Signaling Technology (Danvers, MA) as follows: anti-phospho-Akt (Ser-473) antibody (#4060) (dilution of 1:2,000), anti-phospho-Akt (Thr-308) antibody (#9275) (dilution of 1:1,000), and anti-Akt antibody (#9272) (dilution of 1:1,000). To examine the level of BCL-2 protein expression, rabbit anti-beta-actin antibody (Servicebio, Wuhan, China) (dilution of 1:1,000) and rabbit anti-BCL-2 antibody (Proteintech, Chicago, IL) (dilution of 1:1,000) were used. After washing, the membranes were incubated with the appropriate secondary antibodies for 2 h. The bound antibody was examined with a chemiluminescence reagent (Thermo Fisher Scientific, Waltham, MA) or an enhanced HRP-DAB chromogenic substrate kit (TIANGEN, Beijing, China). The density of each band was quantified by Image Lab software (Bio-Rad, Hercules, CA).
Statistical Analysis
All data were presented as mean ± standard error of mean (SEM). The differences among groups were assessed using Student t test and 1-way analysis of variance (ANOVA), and statistical significance was defined at P < 0.05.
RESULTS AND DISCUSSION
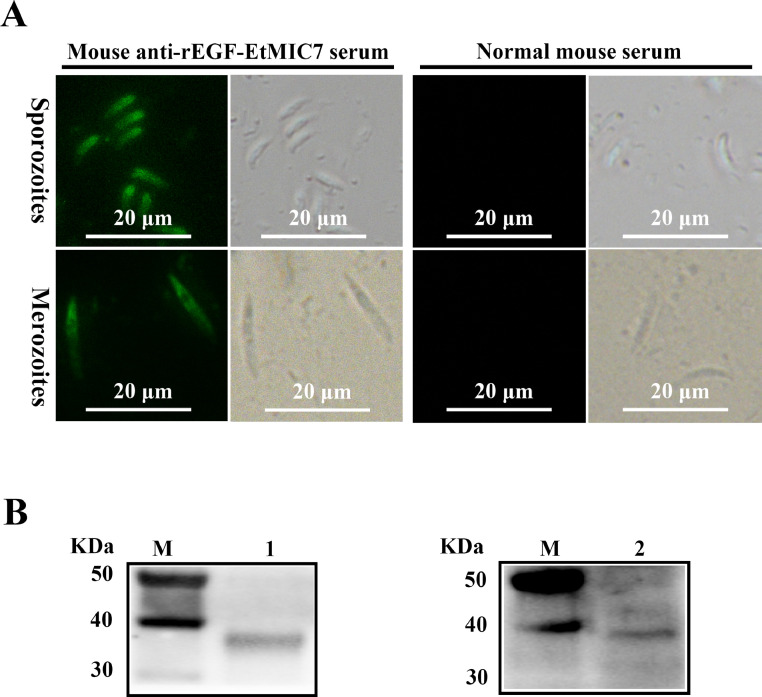
The protozoan parasite E. tenella undergoes a complex life cycle, including an endogenous phase in the intestine of chickens (Liu et al., 2023). The endogenous phase includes asexual reproduction, which is responsible for the majority of the intestinal damage (Morris and Gasser, 2006). Merozoites are the end products of each round of asexual multiplication, and both sporozoites and merozoites can initiate endogenous asexual replication (Liu et al., 2023). Indirect immunofluorescence analysis showed that sporozoites and merozoites were clearly labeled with mouse anti-rEGF-EtMIC7 serum, whereas no fluorescent signal was detected using normal mouse serum as the primary antibody (Figure 1A). Also, the expression of EtMIC7 in the 2 developmental stages of E. tenella was confirmed by using Western blot (Figure 1B). The presence of EtMIC7 in the 2 important stages indicated that it may be a research-worthy protein.
Figure 1.
Expression analysis of EtMIC7 in sporozoites and merozoites. (A) Indirect immunofluorescence analysis was performed using mouse anti-rEtMIC7 serum and normal mouse serum, respectively. (B) Western blot analysis of native EtMIC7 using mouse anti-rEtMIC7 serum as the primary antibody. Lane 1: protein extracts of sporozoites; lane 2: protein extracts of merozoites.
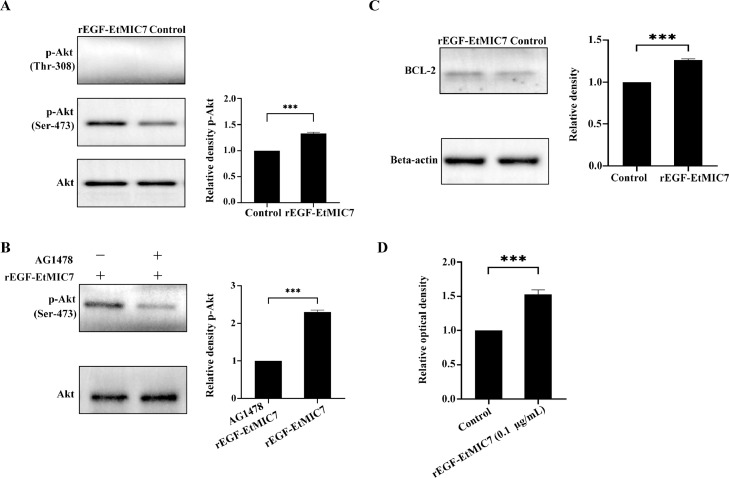
Given that EtMIC7 has several domains with homology to EGF and that the EGF of one species may show different effects on other species, the roles of EGF-EtMIC7 in the regulation of Akt phosphorylation in LMH cells were determined in the present study. Quantitative Western blot analysis showed that treatment with rEGF-EtMIC7 induced the phosphorylation of Akt at Ser-473 but not at Thr-308 (Figure 2A). Meanwhile, treatment of LMH cells with 20 µM AG1478 inhibited rEGF-EtMIC7-induced Akt phosphorylation (Figure 2B). Intriguingly, T. gondii MIC3 and MIC6 were also reported to be able to induce the phosphorylation of Akt at Ser-473 (Muniz-Feliciano et al., 2013).
Figure 2.
(A) The levels of Akt and its phosphorylated (Ser-473/Thr-308) form were examined by Western blot analysis. (B) AG1478 inhibited rEGF-EtMIC7-induced Akt phosphorylation in LMH cells. (C) Western blot analysis of BCL-2 expression in LMH cells with or without rEGF-EtMIC7 treatment. (D) The effects of rEGF-EtMIC7 on LMH cell proliferation. ***P < 0.001.
BCL-2 is an important downstream effector of EGFR/Akt signaling pathway (Alam et al., 2022). In the present study, the protein expression level of BCL-2 was determined. Compared with the control group, increased protein expression level of BCL-2 was observed in LMH cells treated with rEGF-EtMIC7 (Figure 2C). Given that BCL-2 is able to initiate an anti-apoptosis response (Alam et al., 2022), EtMIC7 may be involved in regulation of cell apoptosis through affecting EGFR/Akt signaling pathway during E. tenella infection.
Moreover, the effects of rEGF-EtMIC7 on LMH cell proliferation were further determined by Cell Counting Kit-8 (CCK-8) assay. As shown in Figure 2D, the LMH cell proliferation was significantly enhanced by the treatment of rEGF-EtMIC7 with a concentration of 0.1 µg/mL. The results showed that EGF-EtMIC7 is similar to chicken EGF, because both can promote the proliferation of the chicken cell lines (Zhou et al., 2021). In addition, Akt activation was reported to affect cell proliferation (Wee and Wang, 2017). This suggests that E. tenella may regulate host cell proliferation through EtMIC7.
ACKNOWLEDGMENTS
Project support was provided by the National Natural Science Foundation of China (Grant No. 31902298), the Shanxi Provincial Key Research and Development Program (Grant No. 2022ZDYF126), the Research Fund of Shanxi Province for Introduced High-level Leading Talents (Grant No. RFSXIHLT202101), the Special Research Fund of Shanxi Agricultural University for High-level Talents (Grant No. 2021XG001), and the Shanxi Province Innovation Project for Graduates Students (Grant No. 2022Y328).
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Alam M., Alam S., Shamsi A., Adnan M., Elasbali A.M., Al-Soud W.A., Alreshidi M., Hawsawi Y.M., Tippana A., Pasupuleti V.R., Hassan M.I. Bax/Bcl-2 cascade is regulated by the EGFR pathway: therapeutic targeting of non-small cell lung cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.869672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu X., Zhao X., Zhu X.Q., Suo X. Live attenuated anticoccidial vaccines for chickens. Trends Parasitol. 2023;39:1087–1099. doi: 10.1016/j.pt.2023.09.002. [DOI] [PubMed] [Google Scholar]
- Liu X., Mu B., Zheng W., Meng Y., Yu L., Gao W., Zhu X., Liu Q. Identification and protective efficacy of Eimeria tenella rhoptry kinase family protein 17. Animals (Basel) 2022;12:556. doi: 10.3390/ani12050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., Gasser R.B. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol. Adv. 2006;24:590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Muniz-Feliciano L., Van Grol J., Portillo J.A., Liew L., Liu B., Carlin C.R., Carruthers V.B., Matthews S., Subauste C.S. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A.J., Blake D.P., Ansari H.R., Billington K., Browne H.P., Bryant J., Dunn M., Hung S.S., Kawahara F., Miranda-Saavedra D., Malas T.B., Mourier T., Naghra H., Nair M., Otto T.D., Rawlings N.D., Rivailler P., Sanchez-Flores A., Sanders M., Subramaniam C., Tay Y.L., Woo Y., Wu X., Barrell B., Dear P.H., Doerig C., Gruber A., Ivens A.C., Parkinson J., Rajandream M.A., Shirley M.W., Wan K.L., Berriman M., Tomley F.M., Pain A. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014;24:1676–1685. doi: 10.1101/gr.168955.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.S., Zhao Y.J., Zhang Y., Xu T., Cui K.L., Duan B.T., Lv X.L., Zhang L., Xu Z.Y., Bai R., Zheng M.X. Role of EtMIC4 EGF-like in regulating the apoptosis of Eimeria tenella host cells via the EGFR pathway. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Ming S., Sun L., Wang B., Li H., Zhang X., Zhao X. Identification and characterization of Eimeria tenella microneme protein (EtMIC8) Microbiol. Spectr. 2021;9 doi: 10.1128/spectrum.00228-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Chen P., Shi S., Li X., Shi D., Zhou Z., Li Z., Xiao Y. Expression of Gallus epidermal growth factor (gEGF) with food-grade Lactococcus lactis expression system and its biological effects on broiler chickens. Biomolecules. 2021;11:103. doi: 10.3390/biom11010103. [DOI] [PMC free article] [PubMed] [Google Scholar]