Abstract
Background
Lichen planus is a chronic inflammatory disorder. Transcriptional coactivator with PDZ‐binding motif (TAZ/WWTR1) is an important downstream effector of the Hippo pathway which regulates organ size and tissue homeostasis. But little is known about the role of TAZ in lichen planus so far.
Objective
To explore the expression of TAZ in lichen planus and normal skin, and to discover the relationship between TAZ expression and the clinical characteristics of lichen planus patients.
Methods
The method of immunohistochemistry was performed to quantify the expression of TAZ in 262 patients with lichen planus and 90 control tissues. Western blot and quantitative real‐time reverse transcriptase‐PCR (qRT‐PCR) analysis were performed to examine and compare TAZ expression in 4 cases of fresh lichen planus lesions and normal skin tissues.
Results
TAZ was weakly expressed in the basal layers of the epidermis in normal skin tissues with a positive rate of 52.22% (47/90). But in lichen planus, TAZ was strongly expressed in almost the entire epidermis with a positive rate of 81.30% (213/262), and the difference between the two groups was statistically significant (p<0.05). Additionally, TAZ expression was significantly related to the location of the lichen planus, clinical phenotype, smoking, and alcohol preference (p<0.05). Western blot and qRT‐PCR showed that the expression of TAZ in protein and mRNA levels in four cases of lichen planus lesions was significantly higher than that in normal skin tissues.
Conclusion
TAZ may play a regulatory role in the occurrence and development of lichen planus, which might provide a new perspective for studying pathogenesis and theoretical treatment targets.
Keywords: lichen planus, pathogenesis, TAZ, therapy
1. INTRODUCTION
Lichen planus is a chronic lichenoid inflammatory disorder of the skin, mucous membranes, hair follicle and nails, which typically presents as polygon‐shaped, flat‐topped, itchy, violaceous popular with varying degrees of itching or pain. 1 The main pathological feature of lichen planus is the presence of rich inflammatory T cell infiltrations, which migrate in the upper part of the dermis, and are arranged in a band‐like pattern, so that the interface dermatitis with a lymphocytic band. 2 At present, its pathogenesis is not fully clarified. Although there are many clinical treatments, including biological agents, there is still a lack of specific therapy of lichen planus. Therefore, further research on the pathogenesis of lichen planus and providing suitable therapeutic targets are needed.
Transcriptional coactivator with PDZ‐binding motif (TAZ/WWTR1) is the main effector downstream of the Hippo pathway, which regulates organ size and tissue homeostasis. 3 Current studies have shown that TAZ is involved in the occurrence and development of a variety of neoplastic diseases. 4 In addition, TAZ is also related to the maintenance of inflammatory diseases. 5 , 6 , 7 , 8 However, its role and molecular mechanisms in lichen planus remain to be elucidated. Herein, we preliminarily explained the expression of TAZ in lichen planus, and analyzed the correlation between TAZ expression and the clinical features of lichen planus patients to explore the possible mechanism of TAZ in the occurrence and development of lichen planus.
2. METHODS AND MATERIALS
2.1. Patients and controls
All of the patient samples were obtained from the tissue bank of Department of Dermatology, the Second Affiliated Hospital of Xi'an Jiaotong University and all the patients were pathologically diagnosed with lichen planus. Two hundred sixty‐two patients were randomly selected for immunohistochemical staining. Among them, 158 were male and 104 were female, aging 7–82 years with the average age of (46.11 ± 16.70) years. Meanwhile, 90 cases of normal skin specimens were achieved from cosmetic surgery, including 44 males and 46 females, aging 23–79 years with the average age of (51.07 ± 13.73) years. The baseline information of the two groups was not significantly different and was comparable. Four cases of fresh tissues of lichen planus and normal skin tissues were obtained by biopsy under local lidocaine anesthesia. All specimens were obtained with informed consent, approved by the Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University and confirmed by pathological morphological examination.
2.2. Immunohistochemistry analysis
Immunohistochemical staining were performed using a standard staining procedure, 9 with primary antibody solution of TAZ (V386, Cell Signaling Technology, USA) (dilution 1:200). The staining results were evaluated under microscope by two independent pathologists and quantitated based on the following scoring system: (1) percentage of positive cells: ≤5% scored 0, 6%–25% scored 1, 26%–50% scored 2, 51%–75% scored 3, >75%, scored 4; (2) staining intensity: colorless scored 0, light yellow scored 1, yellowish brown scored 2, and chocolate brown scored 3. The overall score for each microscopic field was calculated by the product of the two scores. The average score from five fields was taken as the final score of the TAZ expression for each slide. Score 0 was considered as negative (‐); score 1–4 was considered as weakly positive (+); score 5–8 was considered as positive (++); score 9–12 was considered as strong positive (+++).
2.3. Quantitative real‐time reverse transcriptase‐PCR analysis
Total RNA was extracted from tissues using AG RNAex Pro Reagent (AG21101; Accurate Biology, China Sha, Hunan, China) according to the manufacturer's protocols. cDNA was synthesized using Evo M‐MLV RT Premix for qPCR (AG11706; Accurate Biology, China Sha, Hunan, China) and quantitative real‐time reverse transcriptase‐PCR was performed using SYBR Green Pro Taq HS Premix (AG11701; Accurate Biology, China Sha, Hunan, China). The primers used in this study are as follows: Human GAPDH: Forward: 5′‐ACCACAGTCCATGCCATCAC‐3′, Reverse: 5′‐TCCACCACCCTGTTGCTGTA‐3′; Human TAZ: Forward: 5′‐GGCTGGGAGATACCTTCAC‐3′, Reverse: 5′‐CTGAGTGGGGTGGTTCTGCT‐3′.
2.4. Western blot analysis
Four cases of fresh lichen planus lesion and normal skin tissues were collected and the total protein of tissue samples was lysed in cell lysis buffer (P0013; Beyotime) for western blot. Quantification of protein concentration was performed using BCA Protein Assay Kit (ZJ102, Shanghai Epizyme Biotechnology Co., Ltd.). The major antibodies used in western blot analysis are listed as follows: anti‐TAZ antibody TAZ (V386, Cell Signaling Technology, USA) (dilution 1:1000), anti‐GAPDH antibody (ab181602; Abcam, Cambridge, United Kingdom) (dilution 1:3000) and biotin‐labeled goat anti rabbit body IgG as secondary antibody (AB0101, Abways Technology, China). Western blot procedure was performed as previously described. 10
2.5. Statistical analysis
Statistical analysis was performed using the software SPSS23.0 (SPSS Inc, Chicago, IL). All data were presented as mean ± SEM. Chi‐square test and T‐test were used for statistical comparisons. The Spearman's rank test was used for correlation analyses. p<0.05 was considered statistically significant.
3. RESULTS
3.1. Expression of TAZ in lichen planus and normal skin tissues
Immunohistochemical staining was performed to evaluate the expression of TAZ in normal skin tissues and LP. As shown in Figure 1A,B, among 90 specimens of normal skin tissues, TAZ was weakly expressed within the basal layer of the epidermis, with a positive rate of 52.22% (47/90). However, TAZ was strongly expressed in almost the entire epidermis in lichen planus tissues, with a positive rate of 81.30% (213/262). The detailed expression of each group is shown in Table 1 and Figure 2. In conclusion, TAZ was upregulated in lichen planus tissues compared with normal skin tissues, and the difference between the two groups was statistically significant (p<0.001). Then, we detected the TAZ mRNA and protein levels in lichen planus and normal skin tissues, both of which were upregulated in lichen planus tissues compared with the levels in normal skin tissues (Figures 3 and 4A,B). Taken together, these results showed the upregulation of TAZ in lichen planus.
FIGURE 1.
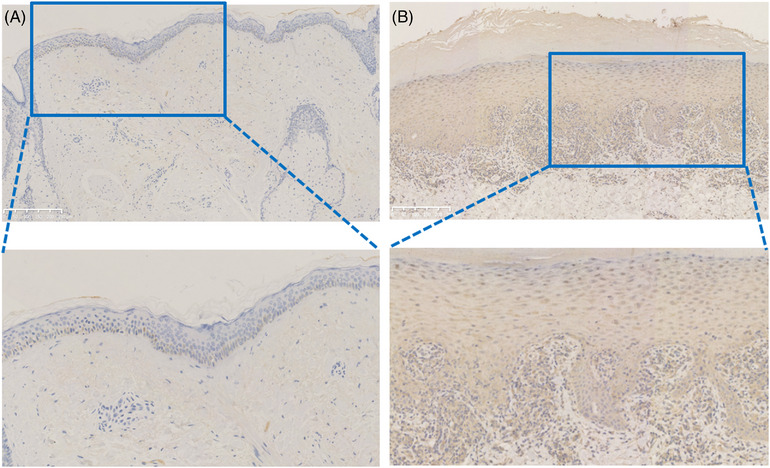
(A) Expression of TAZ in normal skin tissues (IHC staining, bar length = 250 μm), (B) Expression of TAZ in LP tissues (IHC staining, bar length = 250 μm).
TABLE 1.
Expression of TAZ in normal skin and LP.
| Groups | N | Expression grade | Positive rate (%) | |||
|---|---|---|---|---|---|---|
| – | + | ++ | +++ | |||
| Normal | 90 | 43 | 32 | 10 | 5 | 52 |
| LP | 262 | 49 | 73 | 97 | 43 | 81.30 *** |
p < 0.001.
FIGURE 2.
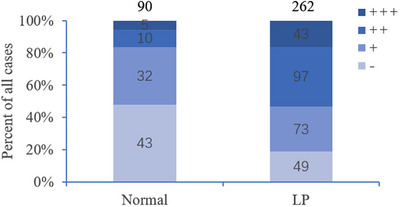
Semiquantitative analysis of TAZ Immunohistochemical staining in LP and normal skin tissues.
FIGURE 3.
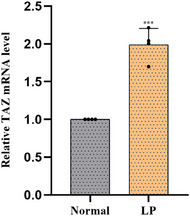
Quantitative real‐time reverse transcriptase‐PCR analysis of TAZ mRNA in LP and normal skin tissues.
FIGURE 4.
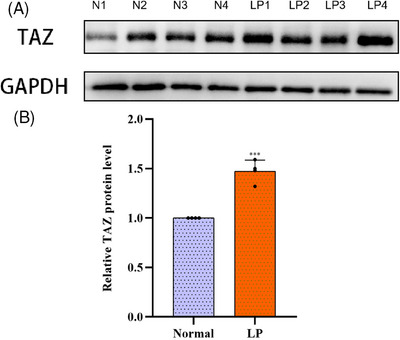
(A) Western blot analysis of TAZ protein levels in LP and normal skin tissues. (B) Semiquantitative analysis of TAZ protein levels in LP and normal skin tissues.
3.2. Relationship between the expression of TAZ and the clinical characteristics of lichen planus patients
We uncovered the correlation between TAZ expression and the clinical characteristics of lichen planus patients. TAZ expression was irrelevant to gender, age, duration, history of treatments, and clinical symptoms. Nevertheless, as shown in Table 2, TAZ expression was significantly associated with the location of disease, clinical phenotype, and smoking and alcoholic preference (p<0.05). The results showed that the expression of TAZ in lichen planus of skin (except for the vulva) and mucous was higher than that of lichen planus in the scalp, nail and vulva (p<0.05). Meanwhile, we observed that the expression of TAZ in erosive lichen planus and lichen planus with smoking and alcohol habits was significantly higher than that of nonerosive lichen planus and lichen planus patients without smoking and alcoholic preferences.
TABLE 2.
Correlation between TAZ expression and clinical characteristics of LP patients.
| Clinical features | TAZ Expression grade | Correlation coefficent | p | ||||
|---|---|---|---|---|---|---|---|
| – | + | ++ | +++ | ||||
| Gender | −0.094 | 0.129 | |||||
| Male | 24 | 45 | 61 | 28 | |||
| Female | 25 | 28 | 36 | 15 | |||
| Age(f) | −0.14 | 0.822 | |||||
| 0–29 | 9 | 14 | 20 | 3 | |||
| 30–60 | 28 | 43 | 60 | 33 | |||
| 60+ | 12 | 16 | 17 | 7 | |||
| Duration(m) | −0.025 | 0.684 | |||||
| ≤12 | 39 | 58 | 77 | 36 | |||
| >12 | 10 | 15 | 20 | 7 | |||
| Location | 0.242 | 0.000 *** | |||||
| Skin | 10 | 14 | 29 | 10 | |||
| Mucosa | 6 | 24 | 32 | 29 | |||
| Hair | 10 | 7 | 8 | 0 | |||
| Nail | 9 | 9 | 5 | 2 | |||
| Vulva | 14 | 19 | 23 | 2 | |||
| Clinical phenotype | 0.0581 | 0.000 *** | |||||
| Erosive | 42 | 35 | 12 | 3 | |||
| Non‐erosive | 7 | 38 | 85 | 40 | |||
| Smoking and alcohol habits | 0.125 | 0.043 * | |||||
| Yes | 18 | 38 | 53 | 25 | |||
| No | 31 | 35 | 44 | 18 | |||
| Treatment history | 0.027 | 0.667 | |||||
| Yes | 16 | 28 | 36 | 12 | |||
| No | 33 | 45 | 61 | 31 | |||
| Symptoms | 0.100 | 0.105 | |||||
| Yes | 15 | 26 | 49 | 16 | |||
| No | 34 | 47 | 48 | 27 | |||
P < 0.005; ***P < 0.01.
4. DISCUSSION
This study provided a comprehensive analysis that revealed the significant role of the TAZ in lichen planus (LP). Our research findings indicated that TAZ likely played a crucial role in the development of LP, aligning with findings from recent studies investigating TAZ's involvement in various skin conditions. Furthermore, notable variations in TAZ expression were observed across different subtypes of LP, notably with pronounced upregulation in the erosive form of the disease. This significant finding expands our understanding of TAZ's contribution to the pathogenesis of LP, offering a novel perspective.
LP, an autoimmune T‐cell‐mediated inflammatory disease, predominantly affects middle‐aged and older women. 11 As a chronic disorder, LP not only deteriorates mental and physical health but also imposes economic burdens on patients and families. Severe genital cases particularly affect the quality of life and sexual health. 12 Our current study found no significant gender difference in disease incidence, possibly due to a limited sample size and research scope. Future research could benefit from an expanded sample size.
The exact pathogenesis of LP remains unclear; however, numerous studies have demonstrated potential connections between its occurrence and various factors, including hepatitis B or C virus infection, abnormal proliferation, inflammation and apoptosis of keratinocytes, 13 , 14 stress factors, depression, anxiety, and dyslipidemia. 15 , 16 Previous reports suggest that the resolution of oral LP can be achieved by eliminating allergenic metals. 17 Furthermore, oral LP affecting the oral mucosa exhibits a higher prevalence among individuals who smoke or abuse alcohol. 18 Recent investigations have revealed the upregulation of TAZ in a broad range of human tumors, including breast cancer, liver cancer, malignant pleural mesothelioma, cervical cancer, esophageal cancer, and pancreatic cancer. 19 , 20 , 21 , 22 , 23 , 24 TAZ expression has also been linked to skin and mucosa tumors, such as head and neck squamous cell cancers, oral squamous cell carcinoma, and malignant melanoma. 25 , 26 , 27 Furthermore, TAZ has been implicated in inflammatory diseases. 6 , 7 Importantly, the Hippo pathway is a conserved evolutionary pathway that regulates various fundamental cellular processes, encompassing cell survival, proliferation, differentiation, and organ size. 28 , 29 As a downstream effector of the Hippo pathway, TAZ, a transcriptional coregulator possessing a PDZ‐binding motif, primarily associates with enhancer elements and employs TEAD as a DNA binding platform. 30 Originally identified as a 14‐3‐3‐binding molecule TAZ is subject to signal stimulation from extracellular signaling, the microenvironment, mechanotransduction, and microRNA biogenesis, thereby operating independently of the Hippo‐LATS cascade. 31 , 32 , 33 , 34 Interestingly, our findings align with prior research indicating a correlation between TAZ expression and lifestyle factors, such as smoking and alcohol abuse, in LP patients.
In this study, we identified the upregulation of TAZ in LP and observed higher expression of TAZ in mucosal LP, erosive LP, and LP patients with a history of smoking and alcohol consumption. While previous studies have indicated the rare progression of LP to cutaneous squamous cell carcinoma, our research findings suggested that erosive LP with increased TAZ expression was more prone to malignant transformation compared to non‐erosive LP. Hence, the expression of TAZ could serve as an important marker for the malignant progression of LP. In addition, our study revealed that mucosal LP posed greater treatment challenges compared to LP in other locations, accompanied by elevated TAZ expression. These findings imply the necessity of employing distinct treatment strategies for LP based on its location during clinical management. Numerous studies have demonstrated the association between TAZ and drug resistance in disease therapy. 35 However, further investigations are necessary to elucidate the precise mechanism underlying drug resistance.
In conclusion, TAZ may have a pivotal role in the pathogenesis of LP, making it a potentially valuable target for clinical treatment. Nonetheless, additional research is required to elucidate the specific mechanisms underlying the involvement of TAZ in the development of LP.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of The Second Affiliated Hospital of Xi'an JiaoTong University, and informed consent was obtained from all participants.
Wang N, Bai R, Cheng B, et al. TAZ acting as a potential pathogenic biomarker to promote the development of lichen planus. Skin Res Technol. 2024;30:e13597. 10.1111/srt.13597
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kusari A, Ahluwalia J. Lichen Planus. N Engl J Med. 2018;379:567. [DOI] [PubMed] [Google Scholar]
- 2. Solimani F, Forchhammer S, Schloegl A, Ghoreschi K, Meier K. Lichen planus—a clinical guide. J Dtsch Dermatol Ges. 2021;19:864‐882. [DOI] [PubMed] [Google Scholar]
- 3. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kong H, Han JJ, Kapilevich L, Zhang XA. Role of the Hippo pathway in autoimmune diseases. Exp Gerontol. 2023:112336. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Zheng Z, Caviglia JM, et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LaCanna R, Liccardo D, Zhang P, et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest. 2019;129:2107‐2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia X, He L, Yang Z. Recent advances in the role of Yes‐associated protein in dermatosis. Skin Res Technol. 2023;29:e13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinheiro C, Longatto‐Filho A, Scapulatempo C, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139‐146. [DOI] [PubMed] [Google Scholar]
- 10. Jia J, Li C, Luo S, et al. Yes‐associated protein contributes to the development of human cutaneous squamous cell carcinoma via activation of RAS. J Invest Dermatol. 2016;136:1267‐1277. [DOI] [PubMed] [Google Scholar]
- 11. Williams TR, Haider‐Shah H. Diffuse esophageal stricture secondary to esophageal lichen planus. Abdom Imaging. 2005;30:355‐357. [DOI] [PubMed] [Google Scholar]
- 12. Mauskar M. Erosive lichen planus. Obstet Gynecol Clin North Am. 2017;44:407‐420. [DOI] [PubMed] [Google Scholar]
- 13. Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723‐732. [DOI] [PubMed] [Google Scholar]
- 14. Alaizari NA, Al‐Maweri SA, Al‐Shamiri HM, Tarakji B, Shugaa‐Addin B. Hepatitis C virus infections in oral lichen planus: a systematic review and meta‐analysis. Aust Dent J. 2016;61:282‐287. [DOI] [PubMed] [Google Scholar]
- 15. Manolache L, Seceleanu‐Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437‐441. [DOI] [PubMed] [Google Scholar]
- 16. Tiwari S, Gupta S, Laeeq S, Mahdi AA. Anxiety and depression as risk factor for the development of oral lichen planus and its association with blood antioxidant level. 2017.
- 17. Nishizawa, A , Satoh, T , Yokozeki, H . Close association between metal allergy and nail lichen planus: detection of causative metals in nail lesions. J Eur Acad Dermatol Venereol. 2012;27:E231‐E234. [DOI] [PubMed] [Google Scholar]
- 18. Torrente‐Castells E, Figueiredo R, Berini‐Aytes L, Gay‐Escoda C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med Oral Patol Oral Cir Bucal. 2010;15:e685‐e690. [DOI] [PubMed] [Google Scholar]
- 19. Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell‐related traits on breast cancer cells. Cell. 2011;147:759‐772. [DOI] [PubMed] [Google Scholar]
- 20. Hayashi H, Higashi T, Yokoyama N, et al. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell‐like behaviors contributing to disease progression. Cancer Res. 2015;75:4985‐4997. [DOI] [PubMed] [Google Scholar]
- 21. Pulito C, Korita E, Sacconi A, et al. Dropwort‐induced metabolic reprogramming restrains YAP/TAZ/TEAD oncogenic axis in mesothelioma. J Exp Clin Cancer Res. 2019;38:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buglioni S, Vici P, Sergi D, et al. Analysis of the hippo transducers TAZ and YAP in cervical cancer and its microenvironment. Oncoimmunology. 2016;5:e1160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruber R, Panayiotou R, Nye E, Spencer‐Dene B, Stamp G, Behrens A. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up‐regulation of JAK‐STAT3 signaling. Gastroenterology. 2016;151:526‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou S, Liu S, Lin C, et al. TRIB3 confers radiotherapy resistance in esophageal squamous cell carcinoma by stabilizing TAZ. Oncogene. 2020;39:3710‐3725. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Li Z, Wu Y, et al. The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma. Cell Death Dis. 2019;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiemer S, Zhang L, Kartha V, Packer T, Varelas X. A YAP/TAZ‐regulated transcriptional signature associated with oral squamous cell carcinoma. FASEB J. 2015;13:957‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng Q, Guo P, Kang S, Zhao F. High expression of TAZ/YAP promotes the progression of malignant melanoma and affects the postoperative survival of patients. Pharmazie. 2018;73:662‐665. [DOI] [PubMed] [Google Scholar]
- 28. Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457‐467. [DOI] [PubMed] [Google Scholar]
- 30. Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co‐activator regulated by interactions with 14‐3‐3 and PDZ domain proteins. EMBO J. 2000;19:6778‐6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3‐kinase and phosphoinositide‐dependent kinase‐1. Proc Natl Acad Sci U S A. 2013;110:2569‐2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaulk SG, Lattanzi VJ, Hiemer SE, Fahlman RP, Varelas X. The Hippo pathway effectors TAZ/YAP regulate dicer expression and MicroRNA biogenesis through Let‐7. J Biol Chem. 2014;289(4):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wrighton KH. Mechanotransduction: YAP and TAZ feel the force. Nat Rev Mol Cell Biol. 2011;12:404. [DOI] [PubMed] [Google Scholar]
- 34. Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19:454‐464. [DOI] [PubMed] [Google Scholar]
- 35. Gao R, Kalathur RKR, Coto‐Llerena M, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13:e14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


