Abstract
Ehrlichia chaffeensis, an obligatory intracellular bacterium of monocytes or macrophages, is the etiologic agent of human monocytic ehrlichiosis. Our previous study showed that gamma interferon (IFN-γ) added prior to or at early stage of infection inhibited infection of human monocytes with E. chaffeensis; however, after 24 h of infection, IFN-γ had no antiehrlichial effect. To test whether ehrlichial infection disrupts Janus kinase (Jak) and signal transducer and activator of transcription (Stat) signaling induced by IFN-γ, tyrosine phosphorylation of Stat1, Jak1, and Jak2 in E. chaffeensis-infected THP-1 cells was examined by immunoprecipitation followed by immunoblot analysis. Viable E. chaffeensis organisms blocked tyrosine phosphorylation of Stat1, Jak1, and Jak2 in response to IFN-γ within 30 min of infection. Similar results were obtained with human peripheral blood monocytes infected with E. chaffeensis. Heat or proteinase K treatment but not periodate treatment of E. chaffeensis abrogated the inhibitory effect, suggesting that protein factor(s) of E. chaffeensis is responsible for the inhibition of IFN-γ-induced tyrosine phosphorylation. Preincubation of E. chaffeensis with the Fab fragment of dog anti-E. chaffeensis immunoglobulin G also abrogated the inhibitory effect. On the other hand, monodansylcadaverine, which does not block binding but blocks internalization of ehrlichiae into macrophages, did not have any influence on the tyrosine phosphorylation. These results indicate that ehrlichial binding to host cells is sufficient to inhibit Stat1 tyrosine phosphorylation induced by IFN-γ. Protein kinase A (PKA) activity in THP-1 cells increased approximately 25-fold within 30 min of infection with E. chaffeensis. In THP-1 cells pretreated with a PKA inhibitor, Rp isomer of adenosine 3′,5′-cyclic phosphorothioate, E. chaffeensis-induced inhibition of Stat1 tyrosine phosphorylation was partially abrogated. These results suggest that E. chaffeensis blocks IFN-γ-induced tyrosine phosphorylation of Jak and Stat through raising PKA activity in THP-1 cells, which may be an important survival mechanism of ehrlichiae within the host cell.
Ehrlichia chaffeensis is an obligatory intracellular bacterium that infects monocytes and macrophages (27) and is the etiologic agent of human monocytic ehrlichiosis in the United States (1, 11). Since the discovery of the disease in 1986, more than 400 cases of human ehrlichiosis have been reported in 30 states in the United States (12). A PCR assay detected E. chaffeensis DNA in the Lone Star tick Amblyomma americanum, implying that this tick is a vector of the disease (2). Gamma interferon (IFN-γ), a cytokine produced by activated T lymphocytes, is a major regulator of both the nonspecific and the specific immune response (13). It is the most potent lymphokine known for activating cells of the mononuclear phagocyte lineage. In these cells, IFN-γ induces or enhances numerous macrophage capabilities, such as tumoricidal activity, antigen presentation, phagocytosis, and cytokine production. It can also activate macrophages to destroy intracellular pathogens that have colonized within themselves. The signal transduction pathway through which IFN-γ stimulates gene transcription has recently been illuminated and has served as a model system for studies on activation of the Janus kinases (Jaks) and latent cytoplasmic transcription factors known as signal transducers and activators of transcription (Stats). Upon IFN-γ binding, the receptor dimerizes, allowing the associated Jaks to interact each other by tyrosine phosphorylation. Subsequently, the activated Jak1 and Jak2 directly phosphorylate the intracellular domains of the receptors on specific tyrosine residues. This phosphorylation allows the selective recruitment of Stat1 through a specific interaction between the Src homology 2 (SH2) domain of Stat1 and the phosphotyrosines of the receptor chains. This receptor-associated Stat1 is then rapidly phosphorylated by the activated Jaks. The phosphorylation of the Stat1 is followed by Stat1 dimerization, translocation to the nucleus, and activation of IFN-γ-responsive genes (6, 8).
The activation of macrophages and monocytes by IFN-γ is critical for the host defense against a variety of intracellular parasites such as Legionella pneumophila, Listeria monocytogenes, Leishmania major, or Toxoplasma gondii (5, 7, 14, 31). Ehrlichia risticii was also shown to be killed by mouse macrophages treated with IFN-γ through induction of cytoplasmic nitric oxide synthase (25). However, class II antigen upregulation in response to IFN-γ was blocked by E. risticii infection (20). Our recent study showed that IFN-γ inhibited infection of human monocytes with E. chaffeensis by inhibiting cytoplasmic iron availability (3). However, after 24 h of infection, IFN-γ did not show antiehrlichial effect. The result implied that E. chaffeensis infection might impair the signaling cascades stimulated by IFN-γ. In this study, therefore, we examined whether the infection of E. chaffeensis blocks the IFN-γ-induced Jak/Stat signal transduction pathway in human monocytes. The results demonstrate that binding of E. chaffeensis to THP-1 cells inhibits IFN-γ-induced tyrosine phosphorylation of Stat1, Jak1, and Jak2. The results also suggest that the elevation of protein kinase A (PKA) activity in host cells induced by E. chaffeensis infection serves as the mechanism by which E. chaffeensis blocks the IFN-γ-induced tyrosine phosphorylation of Jak and Stat in human monocytes.
MATERIALS AND METHODS
Cells.
The human THP-1 (acute monocytic leukemia) cell line was obtained from the American Type Culture Collection (Rockville, Md.) and was grown in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.) and 2 mM l-glutamine (GIBCO). Human peripheral blood monocytes were isolated from buffy coats from healthy donors (Ohio Red Cross, Columbus) as described previously (18), cultured for 1 week in RPMI 1640 medium, and then used for treatments.
E. chaffeensis.
E. chaffeensis, Arkansas isolate, was cultured in THP-1 cells in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine. When more than 90% of the cells were infected, as determined by examining the cells stained with Diff-Quik (Baxter Scientific Products, Obetz, Ohio), the infected cells were sonicated and centrifuged at 500 × g for 5 min. The supernatant was centrifuged at 10,000 × g for 10 min, and the pellet containing host cell-free E. chaffeensis organisms was used to infect THP-1 cells.
Treatment of cells.
THP-1 cells at 5 × 106 in 5 ml of RPMI 1640 medium were preincubated for 18 h with phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Company, St. Louis, Mo.) at 50 nM before treatment. Cells were briefly rinsed with prewarmed RPMI 1640 medium, stimulated with E. chaffeensis organisms derived from 5 × 107 infected cells, and treated with 1,000 U of recombinant human IFN-γ (GIBCO) per ml for 10 min. For time course experiment, PMA-treated cells were incubated with viable E. chaffeensis organisms for the indicated periods of time and stimulated with IFN-γ. Heat-killed ehrlichiae were prepared by boiling host cell-free E. chaffeensis for 10 min. Periodate-treated ehrlichiae were prepared by incubating host cell-free E. chaffeensis with 20 mM sodium periodate (Sigma) in 50 mM sodium acetate buffer (pH 4.5) for 1 h at room temperature in the dark followed by incubation with 50 mM sodium borohydride (Sigma) in sterile phosphate-buffered saline (PBS; 2.7 mM KCl, 1.8 mM KH2PO4, 137 mM NaCl, 10 mM NaH2PO4) for 30 min at room temperature. For proteinase K treatment, host cell-free E. chaffeensis organisms were incubated in 1 mg of proteinase K (GIBCO) per ml in distilled water at 60°C for 2 h. After incubation, 1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma) was added for 10 min, and then ehrlichiae were washed three times in RPMI 1640 medium. The lysate of E. chaffeensis was prepared by sonication of host cell-free organisms for 1 min. As a negative control, a single colony of Escherichia coli INVαF′ was cultured in Luria-Bertani medium for 16 h, washed twice in RPMI 1640 medium, and then used at 1.5 mg of protein per ml of medium. For the reversibility experiment, PMA-treated cells were stimulated with IFN-γ for 10 min and then incubated with viable E. chaffeensis organisms for 30 min. Fab fragment of normal dog antibody or dog anti-E. chaffeensis antibody was prepared as described previously (19). PMA-treated cells were incubated with viable E. chaffeensis organisms which had been preincubated with 0.2 mg of Fab fragment of normal antibody or anti-E. chaffeensis antibody at 37°C for 30 min and then stimulated with IFN-γ for 10 min. Monodansylcadaverine (250 μM; Sigma) or Rp isomer of adenosine 3′,5′-cyclic phosphothioate (Rp-cAMP[S]; 50 or 100 μM; Calbiochem-Novabiochem Corp., San Diego, Calif.) was added to PMA-treated cells 3 h prior to the addition of E. chaffeensis. After incubation with E. chaffeensis for 30 min, cells were stimulated with IFN-γ for 10 min. Human peripheral blood monocytes at 5 × 106 cells were treated with viable E. chaffeensis derived from 5 × 107 infected THP-1 cells for 30 min and then stimulated with IFN-γ for 10 min.
Immunoprecipitation and Western blot analysis.
After treatment, cells were rinsed with PBS (pH 7.4) and lysed with ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 0.15 M sodium chloride, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EGTA, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM PMSF, 100 mM microcystin, 20 μM leupeptin, 10 μg of aprotinin per ml, 2 μg of pepstatin A per ml). Protease inhibitors were obtained from Sigma. Lysates were centrifuged at 10,000 rpm for 10 min at 4°C. The supernatants containing 500 μg of protein in 500 μl were incubated for 16 to 18 h at 4°C with 4 μl of rabbit anti-human Stat1 antibody, rabbit anti-human Jak1 antiserum, rabbit anti-human Jak2 antiserum (Upstate Biotechnology Inc., Lake Placid, N.Y.), or normal rabbit serum. Protein G-Sepharose (50 μl of packed beads; Pharmacia Biotech Inc., Piscataway, N.J.) equilibrated in lysis buffer was then added to the mixture, and incubation was continued for 2 h at 4°C. After a brief centrifugation, Sepharose beads were washed three times with lysis buffer and then boiled in 50 μl of sodium dodecyl sulfate (SDS) sample buffer (17). The immunoprecipitated proteins (or total protein lysate above) were separated by SDS–7.5% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane by using a semidry electroblotting apparatus. Membranes were blocked with 3% nonfat dry milk in PBS containing 0.05% Tween 20 for 1 h at room temperature. The membranes were then incubated with antiphosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology Inc.) at 1 μg/ml in blocking solution at 4°C overnight and washed in PBS-Tween 20. The blots were then incubated with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Amersham Life Science Inc., Arlington Heights, Ill.) in blocking solution for 1 h and washed in PBS-Tween 20. The blots were developed by using an enhanced chemiluminescence kit (Amersham) as recommended by the manufacturer.
PKA assay.
THP-1 cells (107) were pretreated with 50 nM PMA for 18 h, washed with prewarmed RPMI 1640 medium, and treated with host cell-free E. chaffeensis organisms for the indicated times. Following treatment, cells were washed with PBS and resuspended in 0.5 ml of 20 mM Tris-HCl (pH 7.5)–5 mM EDTA–10 mM EGTA–0.3% β-mercaptoethanol–1 mM PMSF–10 mM benzamidine. After sonication for 30 s on ice, the cell suspension was centrifuged at 100,000 × g for 60 min at 4°C. The supernatants were collected, and 10 μg of protein of each lysate was assayed for PKA activity by using a protein kinase assay kit (Calbiochem-Novabiochem) according to the manufacturer’s protocol.
Flow cytometry of IFN-γ receptor expression.
THP-1 cells (2 × 106) pretreated with PMA were infected with E. chaffeensis for the indicated periods of time and detached by incubation in PBS–20 mM EDTA for 30 min at 37°C. After centrifugation, cells were resuspended in PBS–1 mM EDTA and incubated for 30 min at 4°C with 5 μg of mouse anti-human IFN-γ receptor monoclonal antibody (Genzyme Corporation, Cambridge, Mass.) or mouse IgG1 (Organon Teknika Corp., Durham, N.C.) as a negative control. The cells were washed twice by centrifugation in PBS–1 mM EDTA and incubated for 30 min at 4°C with a fluorescein isothiocyanate-conjugated goat anti-mouse IgG antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). The cells were washed, fixed in 1% paraformaldehyde solution, and analyzed with a Coulter EPICS Elite flow cytometer and Coulter Elite software (Coulter Corporation, Miami, Fla.).
RESULTS
IFN-γ induces tyrosine phosphorylation of Stat1, Jak1, and Jak2 in uninfected THP-1 cells.
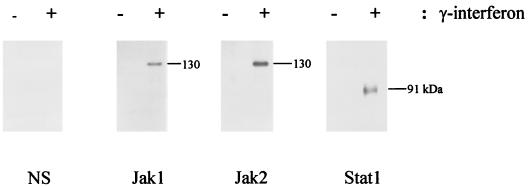
THP-1 cells are immature monocytes which grow in suspension (33). Once cells are treated with PMA for 18 h, THP-1 cells become adherent, stop dividing, and spread flat (32). Since THP-1 cells pretreated with PMA are more effectively infected with E. chaffeensis and respond better to IFN-γ than nontreated cells (3), we used PMA-pretreated THP-1 cells in this study. To verify that Stat1, Jak1, and Jak2 are tyrosine phosphorylated in uninfected THP-1 cells in response to IFN-γ, PMA-treated THP-1 cells were stimulated with 1,000 U of recombinant human IFN-γ per ml for 10 min. After immunoprecipitation of cell lysates with specific antibodies, Western blot analysis was performed with antiphosphotyrosine antibody. Figure 1 shows that IFN-γ induced tyrosine phosphorylation of Stat1, Jak1, and Jak2.
FIG. 1.
Tyrosine phosphorylation of Stat1, Jak1, and Jak2 during IFN-γ stimulation in THP-1 cells. THP-1 cells (5 × 106) were incubated with 50 nM PMA for 18 h, washed twice with prewarmed RPMI 1640 medium, and stimulated with or without IFN-γ (1,000 U/ml) for 10 min. Cells were washed twice with prewarmed PBS and lysed in ice-cold lysis buffer. Cell lysates were immunoprecipitated with antibodies specific for Stat1, Jak1, Jak2, or normal rabbit serum (NS) as described in Materials and Methods. The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis and subjected to Western blot analysis by using antiphosphotyrosine antibody. Stat1 (91 kDa), Jak1 (130 kDa), and Jak2 (130 kDa) are indicated. Data shown are from one of three independent experiments with similar results.
Time course of inhibition of tyrosine phosphorylation of Jak and Stat by E. chaffeensis.
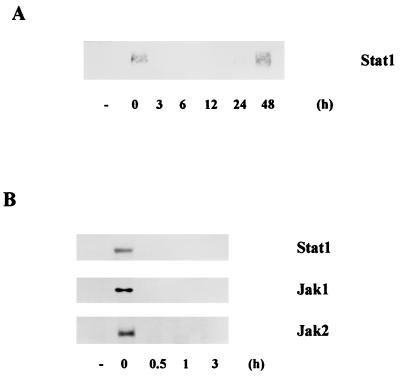
To examine whether the infection of E. chaffeensis inhibits tyrosine phosphorylation of IFN-γ signaling molecules and whether the inhibition is time dependent, PMA-pretreated THP-1 cells infected with E. chaffeensis for various period were stimulated with IFN-γ for 10 min and then cell lysates were subjected to immunoprecipitation followed by immunoblot analysis. As shown in Fig. 2A, IFN-γ-stimulated tyrosine phosphorylation of Stat1 was blocked in THP-1 cells within 3 h of E. chaffeensis infection but restored by 48 h of infection. Then we narrowed down the time periods that E. chaffeensis block tyrosine phosphorylation of Stat1. Figure 2B shows that E. chaffeensis block tyrosine phosphorylation of Stat1, Jak1, and Jak2 within 30 min of infection.
FIG. 2.
Time course of the effect of E. chaffeensis infection on tyrosine phosphorylation of Stat1, Jak1, and Jak2 in response to IFN-γ. THP-1 cells preincubated with PMA for 18 h were treated with host cell-free E. chaffeensis organisms for the indicated periods of time and stimulated with IFN-γ for 10 min. The cell lysates were immunoprecipitated with anti-Stat1, anti-Jak1, or anti-Jak2 antibody, and Western blot analysis was performed with antiphosphotyrosine antibody. Similar results were obtained in two experiments.
The suppression was not due to downregulation of surface IFN-γ receptors by E. chaffeensis. Flow cytometric analysis showed that the expression of IFN-γ receptors on THP-1 cells was not decreased but rather increased by E. chaffeensis infection (mean cell fluorescence: 0 h, 7.13; 0.5 h, 7.87; 1 h, 7.98; 3 h, 7.39; 6 h, 8.42; 12 h, 9.53; 24 h, 10.3; 48 h, 14.6).
Protein factors of intact E. chaffeensis organisms are required for the inhibition of the tyrosine phosphorylation.
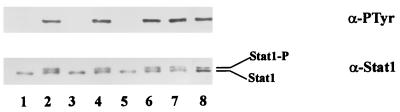
To examine which ehrlichial component is required for inhibition of IFN-γ signaling, we compared the effects of heat-killed, periodate-treated, or proteinase K-treated ehrlichiae to those of viable ehrlichiae. In cells treated with heat-killed or proteinase K-treated E. chaffeensis, Stat1 was tyrosine phosphorylated in response to IFN-γ (Fig. 3). Slowly migrating bands in anti-Stat1 blot are phosphorylated Stat1 as previously described (30). In contrast, in cells incubated with periodate-treated E. chaffeensis, tyrosine phosphorylation of Stat1 was inhibited. Mild periodate oxidation at acidic pH destroys carbohydrate without altering protein or lipid structure (35). These results show that protein factors but not carbohydrate of ehrlichial components are required for the inhibition of Stat1 tyrosine phosphorylation induced by IFN-γ. When cells were incubated with ehrlichial cell lysate prepared by sonication, tyrosine phosphorylation of Stat1 was detected. These results suggest that protein factors of intact E. chaffeensis organisms are required for the inhibition of Stat1 tyrosine phosphorylation induced by IFN-γ. Although E. coli is phagocytized by the monocyte-macrophage cell line THP-1 treated with PMA, the E. coli did not affect tyrosine phosphorylation of Stat1 in response to IFN-γ, indicating that Stat1 tyrosine phosphorylation is blocked specifically by E. chaffeensis.
FIG. 3.
Protein factors of E. chaffeensis are required for the inhibition of tyrosine phosphorylation of Stat1. THP-1 cells preincubated with PMA for 18 h were subjected to different treatment for 30 min followed by IFN-γ stimulation for 10 min. The cell lysates were immunoprecipitated with anti-Stat1 antibody and analyzed by immunoblotting. The upper panel represents the membrane probed with antiphosphotyrosine (α-PTyr), and the lower panel represents the membrane probed with anti-Stat1 (α-Stat1). Lanes: 1, untreated control cells without IFN-γ stimulation; 2 to 8, cells stimulated with IFN-γ; 2, untreated cells; 3, cells treated with viable E. chaffeensis; 4, cells treated with heat-killed E. chaffeensis; 5, cells treated with periodate-treated E. chaffeensis; 6, cells treated with proteinase K-treated E. chaffeensis; 7, cells treated with lysate of E. chaffeensis; 8, cells treated with E. coli. Stat1, nonphosphorylated Stat1; Stat1-P, phosphorylated Stat1 (20). Two separate experiments yielded similar results.
IFN-γ-induced tyrosine phosphorylation of Stat1 is not reversible by E. chaffeensis infection.
In previous experiments, when THP-1 cells were treated with IFN-γ prior to infection, E. chaffeensis was killed (3). Therefore, we examined whether Jak/Stat signaling is inhibited when E. chaffeensis is added after IFN-γ treatment. As shown in Fig. 4, E. chaffeensis did not affect the phosphorylated state of Stat1 induced by IFN-γ. The results suggest that once the signal transduction pathway is turned on by IFN-γ, it is not reversible by E. chaffeensis infection, which may be the reason why E. chaffeensis is killed by IFN-γ (3).
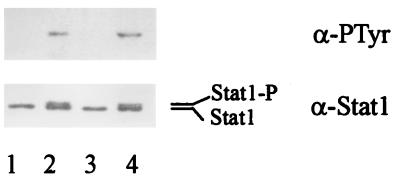
FIG. 4.
IFN-γ-induced tyrosine phosphorylation of Stat1 is irreversible by E. chaffeensis infection. THP-1 cells were pretreated with PMA for 18 h and then treated with E. chaffeensis for 30 min followed by IFN-γ stimulation for 10 min (lane 3). THP-1 cells were stimulated with IFN-γ for 10 min and then incubated with E. chaffeensis for 30 min (lane 4). Lanes: 1, untreated control cells without IFN-γ stimulation; 2, untreated cells stimulated with IFN-γ. Similar results were obtained in two experiments. For other details, see the legend to Fig. 3.
Ehrlichial binding to macrophages is sufficient for the inhibition of tyrosine phosphorylation of Stat1.
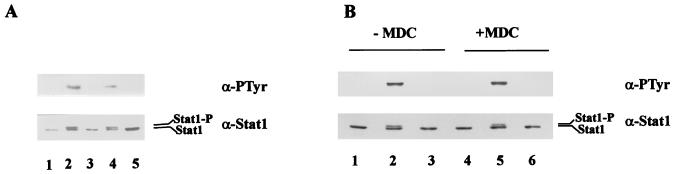
To determine whether ehrlichial binding is necessary for the inhibition of Jak/Stat signaling, we exposed THP-1 cells to E. chaffeensis preincubated with the Fab fragment of anti-E. chaffeensis antibody and examined tyrosine phosphorylation of Stat1. We have previously shown that the Fab fragment of antiehrlichial IgG blocks ehrlichial binding to the host cells (22). As shown in Fig. 5A, the inhibitory effect on tyrosine phosphorylation of Stat1 was abrogated when E. chaffeensis was preincubated with the Fab fragment of anti-E. chaffeensis antibody. The Fab fragment of normal IgG did not have an effect on tyrosine phosphorylation of Stat1 in response to IFN-γ. The results suggest that binding of E. chaffeensis to THP-1 cells is required for the inhibition of Jak/Stat signaling in response to IFN-γ. Next we tested whether internalization of E. chaffeensis is required for the inhibition of the Jak/Stat pathway induced by IFN-γ. THP-1 cells were incubated with E. chaffeensis in the absence or presence of a transglutaminase inhibitor, monodansylcadaverine, and tyrosine phosphorylation of Stat1 in response to IFN-γ was examined. We have previously shown that monodansylcadaverine inhibits ehrlichial internalization into host cells but does not affect ehrlichial attachment to host cells (21). Figure 5B shows that treatment with monodansylcadaverine did not affect E. chaffeensis-induced inhibition of Stat1 tyrosine phosphorylation. The results suggest that ehrlichial binding but not internalization is required for the inhibition of Jak/Stat signal transduction.
FIG. 5.
Effects of the Fab fragment of anti-E. chaffeensis antibody and monodansylcadaverine on E. chaffeensis-induced inhibition on Stat1 tyrosine phosphorylation. (A) THP-1 cells pretreated with PMA for 18 h were treated for 30 min with E. chaffeensis preincubated with the Fab fragment of anti-E. chaffeensis antibody or Fab fragment of normal dog IgG. After stimulation with IFN-γ for 10 min, cell lysates were immunoprecipitated with anti-Stat1 antibody and analyzed by immunoblotting. Lanes: 1, untreated control cells without IFN-γ stimulation; 2 to 5, cells stimulated with IFN-γ; 2, untreated cells; 3, cells treated with E. chaffeensis; 4, cells treated with E. chaffeensis preincubated with Fab fragment of anti-E. chaffeensis antibody; 5, cells treated with E. chaffeensis preincubated with Fab fragment of normal dog IgG. (B) THP-1 cells preincubated with PMA for 18 h were treated with E. chaffeensis for 30 min in the absence (lanes 1 to 3) or presence (lanes 4 to 6) of monodansylcadaverine (250 μM). Monodansylcadaverine was added 3 h prior to the addition of E. chaffeensis. The cells were then stimulated with IFN-γ for 10 min. Lanes: 1 and 4, untreated control cells; 2 and 5, untreated cells stimulated with IFN-γ; 3 and 6, cells treated with E. chaffeensis and stimulated with IFN-γ. Similar results were obtained in two independent experiments. For other details, see the legend to Fig. 3.
Elevated PKA activity in THP-1 cells infected with E. chaffeensis serves as a mechanism for inhibition of tyrosine phosphorylation.
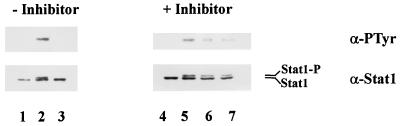
E. risticii infection causes a significant increase in cytoplasmic cAMP levels in the colonic mucosa of horses (28) and in mouse peritoneal macrophages (34). Recent studies suggest that 8-bromo-cAMP inhibits Stat1 activation in human mononuclear cells (16) and elevated cAMP inhibits IFN-β-induced tyrosine phosphorylation of Jaks and Stats in myeloma cell line U266 (10). With this background, we tested a possibility that PKA plays a role in the inhibition of Jak/Stat signaling by E. chaffeensis. First, we measured PKA activity in THP-1 cells infected with E. chaffeensis. As shown in Table 1, PKA activity in THP-1 cells was significantly increased within 30 min of E. chaffeensis infection, reached approximately 25-fold of the basal level, and decreased after 24 h. To further examine whether PKA activation is a mechanism by which E. chaffeensis blocks the Jak/Stat pathway, we examined tyrosine phosphorylation of Stat1 in the absence or presence of a PKA inhibitor, Rp-cAMP[S] (29). Figure 6 indicates that treatment of Rp-cAMP[S] did not affect IFN-γ-stimulated tyrosine phosphorylation of Stat1. However, in the presence of Rp-cAMP[S], the inhibitory effect of E. chaffeensis on Stat1 tyrosine phosphorylation was partially abrogated. The Ki value of Rp-cAMP[S] has been reported to be 11 μM (29). The effect of the inhibitor in our results appears to be partial and saturated at 50 μM. The results imply that E. chaffeensis may inhibit Jak/Stat signal transduction in response to IFN-γ through the elevated PKA activity in host cells.
TABLE 1.
PKA activity in THP-1 cells infected with E. chaffeensis
| Duration of infection | PKA activity (U/mg)a |
|---|---|
| 0 min | 1.67 ± 0.17 |
| 5 min | 2.54 ± 0.18 |
| 15 min | 32.29 ± 0.24 |
| 30 min | 41.71 ± 0.59 |
| 1 h | 41.10 ± 1.27 |
| 3 h | 41.11 ± 1.84 |
| 6 h | 40.81 ± 0.88 |
| 12 h | 40.33 ± 1.78 |
| 24 h | 21.90 ± 1.11 |
| 48 h | 9.42 ± 1.26 |
THP-1 cells pretreated with PMA were infected with E. chaffeensis for the indicated periods of time. Cell lysates were prepared and assayed for PKA activity as described in Materials and Methods. Results are expressed as the means ± standard deviations of three independent experiments. One unit is defined as the amount of enzyme that transfers 1 nmol of phosphate in 1 min from ATP to the synthetic PKA substrate Kemptide at 30°C, pH 7.5.
FIG. 6.
Effect of a PKA inhibitor on E. chaffeensis-induced inhibition of Stat1 tyrosine phosphorylation. THP-1 cells preincubated with PMA for 18 h were treated with E. chaffeensis for 30 min in the absence or presence of Rp-cAMP[S] (lanes 4 to 6, 50 μM; lane 7, 100 μM). Rp-cAMP[S] was added 3 h prior to the addition of E. chaffeensis. The cells were then stimulated with IFN-γ for 10 min. Lanes: 1 and 4, untreated control cells without IFN-γ stimulation; 2 and 5, untreated cells stimulated with IFN-γ; 3, 6, and 7, cells treated with E. chaffeensis and stimulated with IFN-γ. Similar data were obtained in two experiments. For other details, see the legend to Fig. 3.
Effects of E. chaffeensis infection on tyrosine phosphorylation of Jak and Stat in human peripheral blood monocytes.
When normal human peripheral blood monocytes were treated with E. chaffeensis for 30 min, tyrosine phosphorylation of Stat1, Jak1, and Jak2 in response to IFN-γ was impaired (Fig. 7), supporting the data obtained with the THP-1 cell line.
FIG. 7.
Effect of E. chaffeensis infection on tyrosine phosphorylation of Stat1, Jak1, and Jak2 in human peripheral blood monocytes. Human peripheral blood monocytes were treated with E. chaffeensis for 30 min followed by IFN-γ stimulation for 10 min. Cell lysates were immunoprecipitated with antibodies for Stat1, Jak1, or Jak2 and subjected to Western blot analysis. Lanes: 1, 4, and 7, untreated control cells without IFN-γ stimulation; 2, 5, and 8, untreated cells stimulated with IFN-γ; 3, 6, and 9, cells treated with E. chaffeensis and stimulated with IFN-γ. The data presented are from one of two independent experiments that gave similar results.
DISCUSSION
This report describes a mechanism by which an obligate intracellular bacterium exploits the physiologic signal transduction mechanism for its survival in the presence of active immune responses. This study explains our previous observation that E. chaffeensis was killed when THP-1 cells were treated with IFN-γ prior to infection but became resistant to IFN-γ once infection was established (15). The Jak/Stat pathway once induced was not inhibited by subsequent E. chaffeensis infection. However, when infection occurred first, the IFN-γ-induced Jak/Stat cascade was rapidly inhibited. The present study demonstrated that E. chaffeensis blocks the Jak/Stat pathway in both differentiated THP-1 cells and human peripheral blood monocytes. The inhibition by E. chaffeensis was rapid and occurred within 30 min of infection. The rapid inhibition suggests that E. chaffeensis exerts the inhibitory effect through posttranslational modification of signaling molecules but not through new synthesis of inhibitory factors. According to our results, binding of ehrlichiae is sufficient and does not require internalization of ehrlichiae into the host cells to disrupt the Jak/Stat pathway. Protein rather than carbohydrate of E. chaffeensis is required for this inhibition. Failure of inhibition of Stat1 tyrosine phosphorylation by ehrlichial cell lysate indicates that an intact organism or specific conformation of protein associated with the intact ehrlichial membrane may be required for the inhibition. In this context, several major proteins were identified in the outer membrane of E. chaffeensis (25). In agreement with the ehrlichial cell lysate study, our preliminary results showed that a 28-kDa recombinant major outer membrane protein did not inhibit Jak/Stat signaling. It is unlikely that the early protein synthesis by internalized ehrlichiae is involved in this inhibition, since ehrlichial binding alone without internalization can inhibit IFN-γ-induced signal transduction within 30 min of incubation. We previously reported that carbohydrate rather than protein of E. chaffeensis induces cytokine mRNA expression (18). Thus, independent signaling pathways are simultaneously induced with different ehrlichial components (protein and carbohydrate) upon interaction with host cells. A recent report describes attenuation of Jak/Stat signaling in a human monocyte cell line by Leishmania donovani, a eukaryotic intracellular parasite (23). In the case of L. donovani, the inhibition of Jak/Stat signaling in differentiated U937 cells started after 16 h of infection, suggesting that proliferation of leishmaniae or synthesis of an inhibitor of the signal transduction is required for the inhibition. Whether leishmanial internalization, proliferation, or some component of leishmaniae is required for this inhibition has not been examined. Although inhibition of IFN-γ-induced phosphorylation of Stat1 was reversed at 48 h postinfection, as we reported previously (4), at 48 h postinfection ehrlichiae remain refractory to IFN-γ treatment. The inhibitory mechanism of ehrlichiae by IFN-γ is limitation of availability of cytoplasmic iron (3), and we speculate that by 48 h postinfection ehrlichiae have accumulated sufficient amount of iron by upregulating the host transferrin receptor mRNA (4) and IFN-γ-induced downregulation of transferrin receptor no longer influences ehrlichial growth or survival.
Our present data provide the first evidence that PKA activity is rapidly and dramatically increased in differentiated THP-1 cells infected with E. chaffeensis and that incubation of cells with a PKA inhibitor results in partial abrogation of suppression of Stat1 phosphorylation by E. chaffeensis infection. The almost synchronized time course of IFN-γ responsiveness and cytoplasmic PKA activity further supports our hypothesis that an increase in PKA activity in response to infection is responsible for inhibition of the IFN-γ signal transduction pathway by E. chaffeensis infection. The restoration of responsiveness to IFN-γ by 48 h of infection appears to be also synchronized with the decreased cytoplasmic PKA activity toward the basal level by 48 h of infection. These results strongly suggest that E. chaffeensis modulates the Jak/Stat cascade through the activation of PKA. Recent findings showed that stimulation of the cAMP signaling pathway by 8-bromo-cAMP caused suppression of Stat1 DNA binding activity and downregulation of Stat1 protein and Stat1 mRNA levels in human mononuclear cells (16). In myeloma cell line U266, tyrosine phosphorylation of IFN-α/β receptor, Jak1, Tyk2, Stat1, and Stat2 and DNA binding activity of Stats were inhibited by an adenylate cyclase activator, forskolin (10). The report also demonstrated that PKA specifically associates with the cytoplasmic domain of the IFN-α/β receptor. However, PKA activity in these cells and reversibility of the inhibition with a PKA inhibitor were not examined.
Although the ehrlichial receptor for binding to the monocyte surface is not known, the rapid increase in PKA activity in monocytes exposed to E. chaffeensis suggests that E. chaffeensis could bind to a receptor directly or indirectly coupled to a regulatory unit of membrane adenylate cyclase of macrophages. Activation of the adenylate cyclase raises the cytoplasmic cAMP level, which in turn activates PKA (37). It is not clear why inhibition with Rp-cAMP[S] is partial. Since the inhibitor has different affinities for type I and type II PKA (33), it may not be able to inhibit all PKA involved in inhibition of the Jak/Stat pathway. Alternatively, E. chaffeensis induces an additional inhibitory signal which is independent of PKA activation. Additional possible inhibitory mechanisms are as follows. Our results indicated that surface expression of IFN-γ receptor was not downregulated by E. chaffeensis infection. Thus, binding of E. chaffeensis to macrophages may interfere with the dimerization of receptors since tyrosine phosphorylation of both Jaks and Stat was blocked. Alternatively, E. chaffeensis may transmit signals to activate tyrosine phosphatases within the host cells. Recent studies demonstrated that protein tyrosine phosphatases are important in limiting activation through the erythropoietin, interleukin-3, and type I IFN (IFN-α/β) receptor pathways by binding to and dephosphorylating Jaks and Stats involved in the respective pathways (9, 15, 38). The tyrosine kinase Tyk-2, which is physically associated with the type I IFN receptor, has been reported to form stable complexes with SH2-containing hematopoietic cell phosphatase in several hematopoietic cell lines. The hematopoietic cell phosphatase was shown to regulate tyrosine phosphorylation of the Tyk-2 kinase (36).
In summary, the present work demonstrates for the first time Jak/Stat inhibition by intracellular bacteria and describes a PKA-dependent mechanism for the inhibition. This may be one of the critical mechanisms that Ehrlichia spp. exploit to evade the host immune defense and survive within macrophages in the presence of IFN-γ. Our study also provides a valuable model for future studies on modulation of the Jak/Stat pathway by other intracellular parasites. Nevertheless, more research is needed to further clarify the detailed mechanisms which underlie the suppression of the Jak/Stat cascade by E. chaffeensis infection.
ACKNOWLEDGMENT
This research was supported by grant RO1AI33123 from the National Institutes of Health.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K E. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 3.Barnewall R E, Rikihisa Y. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin. Infect Immun. 1994;62:4804–4810. doi: 10.1128/iai.62.11.4804-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnewall R E, Rikihisa Y, Lee E H. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect Immun. 1997;65:1455–1461. doi: 10.1128/iai.65.4.1455-1461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj N, Nash T W, Horwitz M A. Interferon-γ-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986;137:2662–2669. [PubMed] [Google Scholar]
- 6.Briscoe J, Kohlhuber F, Muller M. JAKs and STATs branch out. Trends Cell Biol. 1996;6:336–340. doi: 10.1016/0962-8924(96)10028-3. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier N A, Schreiber R D. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 9.David M, Chen H E, Goelz S, Larner A C, Neel B G. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David M, Petricoin III E, Larner A C. Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. J Biol Chem. 1996;271:4585–4588. doi: 10.1074/jbc.271.9.4585. [DOI] [PubMed] [Google Scholar]
- 11.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 13.Farrar M A, Schreiber R D. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 14.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 15.Ivashkiv L B, Schmitt E M, Castro A. Inhibition of transcription factor Stat1 activity in mononuclear cell cultures and T cells by the cyclic AMP signaling pathway. J Immunol. 1996;157:1415–1421. [PubMed] [Google Scholar]
- 16.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lee E H, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–4219. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E H, Rikihisa Y. Anti-Ehrlichia chaffeensis antibody induces potent proinflammatory cytokine mRNA expression in human monocytes exposed to E. chaffeensis through sustained reduction of IκB-α and activation of NF-κB. Infect Immun. 1997;65:2898–2903. doi: 10.1128/iai.65.7.2890-2897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messick J B, Rikihisa Y. Suppression of I-Ad on P388D1 cells by Ehrlichia risticii infection in response to gamma interferon. Vet Immunol Immunopathol. 1992;32:225–241. doi: 10.1016/0165-2427(92)90048-u. [DOI] [PubMed] [Google Scholar]
- 21.Messick J B, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993;61:3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messick J B, Rikihisa Y. Inhibition of binding, entry, or intracellular proliferation of Ehrlichia risticii in P388D1 cells by anti-E. risticii serum, immunoglobulin G1 or Fab fragment. Infect Immun. 1994;62:3156–3161. doi: 10.1128/iai.62.8.3156-3161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandan D, Reiner N E. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat 1. Infect Immun. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J, Rikihisa Y. l-Arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992;60:3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reithmann C, Gierschik P, Jakobs K H. Stimulation and inhibition of adenylyl cyclase. Symp Soc Exp Biol. 1990;44:207–224. [PubMed] [Google Scholar]
- 27.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikihisa Y, Johnson G C, Wang Y, Reed S M, Fertel R, Cooke H J. Loss of absorptive capacity for sodium and chloride in the colon causes diarrhoea in Potomac horse fever. Res Vet Sci. 1992;52:353–362. doi: 10.1016/0034-5288(92)90037-3. [DOI] [PubMed] [Google Scholar]
- 29.Rothermel J D, Botelho L H P. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuai K, Schindler C, Prezioso V R, Darnell J E., Jr Activation of transcription by IFN-γ: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya S, Yamake M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of human monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1992;42:1530–1536. [PubMed] [Google Scholar]
- 34.Van Heeckeren A M, Rikihisa Y, Park J, Fertel R. Tumor necrosis factor alpha, interleukin-1α, interleukin-6, and prostaglandin E2 production in murine peritoneal macrophages infected with Ehrlichia risticii. Infect Immun. 1993;61:4333–4337. doi: 10.1128/iai.61.10.4333-4337.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 36.Yetter A, Uddin S, Krolewski J J, Jiao H, Yi T, Platanias L C. Association of the interferon-dependent tyrosine kinase Tyk-2 with the hematopoietic cell phosphatase. J Biol Chem. 1995;270:18179–18182. doi: 10.1074/jbc.270.31.18179. [DOI] [PubMed] [Google Scholar]
- 37.Yi T, Mui A L-F, Krystal G, Ihle J N. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor β chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokozaki H, Tortora G, Pepe S, Maronde E, Genieser H-G, Jastorff B, Cho-Chung Y S. Unhydrolyzable analogues of adenosine 3′:5′-monophosphate demonstrating growth inhibition and differentiation in human cancer cells. Cancer Res. 1992;52:2504–2508. [PubMed] [Google Scholar]