Greenspoon et al. (1) used of global population estimates of 392 mammal species to predict the global biomass of mammals. We caution against important limitations in their approach, which likely results in gross underestimations of biomass and its uncertainty.
The authors derive >97% of their estimates from the IUCN Red List (RL) database, which is particularly ill-suited for this scope since it is compiled using different standards than scientific ecological investigation and based on inconsistent approaches that are influenced by precautionary to evidentiary attitudes of different RL assessors (2). Because reliable population estimates are available only for relatively small areas (3), RL figures are generally obtained by summing up local estimates based on relatively scarce and biased data, including expert-based guesses with little supporting evidence (Table 1). Depending on the original purpose, RL figures might be over- or under-inflated (3, 4). Some RL’s reported population sizes are derived by applying an average density across an area, which may not align with the area used in Greenspoon et al. to estimate density, hence resulting in biased and unrealistic estimates when compared with field density estimates (Table 1).
Table 1.
Problems of data quality, collection, treatment, and reproducibility
| Category | Examples | Implications |
|---|---|---|
| Sum of inconsistently estimated population sizes. | Estimates for Odocoileus virginianus come from US wildlife state agencies using not transparently reported survey methods. Some states include both while-tailed and mule deers (e.g., Colorado). Canada population estimated by direct extrapolation from US numbers based on percent range area. Latin American population based on average density of 1 ind/km2. This figure yields a density estimate of 3.4 ind/km2 (5). Field estimates are 3× higher on average (6): M = 10.2 ind/km2; IQR = 2.177 to 20.5; N = 38. | Inconsistent estimates introduce biases in the population density calculation and model predictions. In this case, biomass is underestimated by −60% (2.7 vs. 8.2 Mt). |
| Population estimated from mean density, which divided by a different area leads to a different density estimate. | The RL estimates a total population size of ~104 K for the orangutan (Pongo pygmaeus) by multiplying a mean density of 0.67 ind/km2 by 155 K km2. Greenspoon et al. divided 104 K individuals by a habitat area of >82 K km2 obtaining an estimate of 1.26 ind/km2, almost twice the original density estimate. | Introduction of biases in the population density calculation and model predictions. |
| Density obtained by dividing the population size reported by the RL by area does not match the density reported in the same RL report. | The RL reports a total population of 323 to 955 for the Cozumel raccoon (Procyon pygmaeus), the midrange is 639 but Greenspoon et al. reported 465. The latter was divided by the habitat area resulting in 1.48 ind/km2. However, the RL reports that the species’ population density ranges between 12 and 112 ind/km2. | The mismatch suggests the approach taken is flawed and can lead to important deviations from known density estimates, which are propagated through the models and predictions. |
| Dividing population size by habitat area results in unrealistic population density estimates. | The mountain pigmy possum (Burramys parvus) is estimated to have a density of 2.69 ind/km2, field density estimates are substantially higher: M = 890 ind/km2, IQR = 510 to 1,335; N = 11 (6). The Tibetan antelope (Pantholops hodgsonii) is estimated to have a density of 1,437 ind/km2, field density estimates are M = 4.3 in/km2; IQR = 1.7 to 6.8; N = 11 (6). | Severe under or overestimation of mammal biomass. |
| Inconsistent use of RL reported populations, sometimes taken from the description, sometimes from the reported total, sometimes neither. | 12 M individuals for the Crab eater seal (Lobodon carcinophaga) in the SI Appendix, 10 M in Table 2, RL reports 4 to 8 M; 27.5 K for the Blue whale (Balaenoptera musculus), RL reports 10 to 25 K including the smaller pygmy blue whale and juveniles; 200 K for the Minke whale (Balaenoptera acutorostrata), RL reports 187 K; 35 K for the Bowhead whale (Balaena mysticetus), RL reports >25 K. 54 K for Hypogeomys antimena, RL reports 5,036 mature individuals. | Impossible to reproduce the results because of the inconsistent use of RL estimates. |
| Inconsistent inclusion of juveniles in total populations with use of adult body mass. | Methods do not report the juvenile/adult ratio used for marine mammals nor account for differences in body mass between juveniles and adults. Juveniles were added to the RL estimates of adults for Fin whale (Balaenoptera physalus), Sperm whale (Physeter macrocephalus), and Blue whale (B. musculus), but not to the other top 10 marine mammals calculations, even though RL clearly reports adult-only populations. | Excluding juveniles leads to biomass under-estimation, whereas using adult body mass for juveniles leads to over-estimation |
| Underestimation of uncertainty. | Uncertainty in the RL is expert-based and not meant to represent the real uncertainty around the total estimate. Population size of mature individuals of the African buffalo (Syncerus caffer) between 398 K and 401 K (<1% error). However, the RL clearly states that while some regional estimates are available through aerial counts, the total estimate is highly uncertain since estimates from many regions are absent or biased (7). | Uncertainty of the total population size based on many independent estimates is expected to be much higher than in individual estimates. This approach leads to severe underestimation of uncertainty of the global biomass prediction. |
M = Median, IQR = Interquartile range.
The RL dataset includes an overrepresentation of threatened species (60 vs. 27% of all mammals). The authors control for this bias by including RL categories as models’ predictors. However, no RL criteria relate to density, with ~80% of mammal species threatened due to small or decreasing ranges. Finally, model extrapolations are based on data heavily biased toward ungulates (40 vs. 4.7% of all mammals) while not controlling for phylogenetic relatedness but reassigning species to other taxonomic order for model predictions.
Consequently, predicted density estimates by Greenspoon et al. (1) deviate substantially from published estimates obtained in hundreds of field studies (6) (median absolute orders of magnitude deviation = 0.64, 95% = 0.06 to 2.5, N = 159, Fig. 1A) and are on average underestimated by threefolds (median orders of magnitude deviation = −0.47). This may have profound effects on global estimates (Fig. 1B), leading to a potential underestimation of biomass of ~5.5-fold difference (9.4 vs. 52 Mt for 159 species). Furthermore, we could not replicate the results of 7 of the top 10 marine mammals’ biomass due to inconsistent use of RL data (Table 1). The uncertainty computed by Greenspoon et al. (1) is based on several expert-based intervals and applied to all species, including marine mammals for which estimates of variability are available from literature and the RL but were not used (8). This results in a gross underestimation of the uncertainty around the global biomass prediction (Table 1).
Fig. 1.
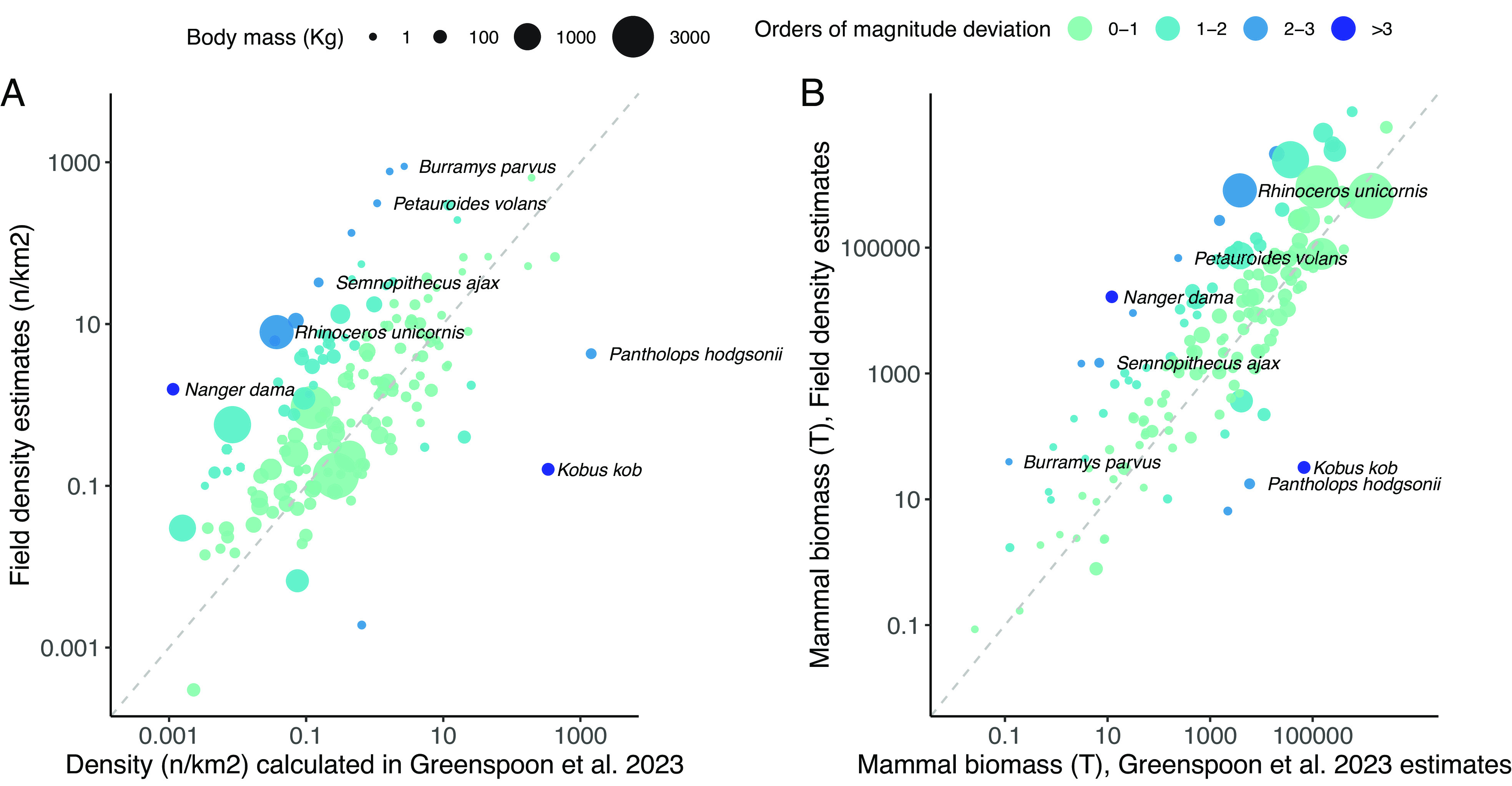
(A) Relationship between average empirical density estimates in Santini et al. (6) and those calculated by Greenspoon et al. (1) in log10 starting from total population size (N = 159). (B) Relationship between biomass estimates by Greenspoon et al. (1), and the biomass estimates derived from field densities (6) in log10, using equation 2 and Area of Habitat in Greenspoon et al. (1). Species labels represent some examples of high orders of magnitude deviations. This comparison follows the same assumption made in ref. 1 (i.e., species occupy all available habitat area), which requires further investigation.
While estimating biomass globally provides important insights, we call for a more careful consideration of data quality and consistency and a more robust reporting of uncertainty. We recommend fitting models on empirically derived field density estimates while controlling for phylogeny, environmental variables, and inconsistent sampling methods (e.g. refs. 6 and 9). Uncertainty should be derived directly from the statistical predictive error and, when possible, also from the underlying data. Alternatively, mechanistic eco-physiological models can be used to estimate global biomass following trait-based theory and validated with independent data (9).
Acknowledgments
We thank Moreno Di Marco, Luigi Boitani, and Joaquín Hortal for useful feedback on this letter. F.B. recognise the support from the OceanICU project funded by the European Union, Horizon Europe Funding Programme for research and innovation under grant agreement No.101083922.
Author contributions
L.S. and A.B.-L. designed research; L.S. performed research; L.S., F.B., and A.B.-L. analyzed data; and L.S., F.B., and A.B.-L. wrote the paper.
Competing interests
The authors declare no competing interest.
References
- 1.Greenspoon L., et al. , The global biomass of wild mammals. Proc. Natl. Acad. Sci. U.S.A. 120, e2204892120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IUCN, Guidelines for using the IUCN Red List Categories and Criteria. Version 15.1. Prepared by the standards and petitions subcommittee. (2022). https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf. Accessed 10 April 2023.
- 3.Gopalaswamy A. M., et al. , How “science” can facilitate the politicization of charismatic megafauna counts. Proc. Natl. Acad. Sci. U.S.A. 110, e2203244119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darimont C. T., Paquet P. C., Treves A., Artelle K. A., Chapron G., Political populations of large carnivores. Conserv. Biol. 32, 747–749 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Vercauteren K. C., et al. , Regulated commercial harvest to manage overabundant white-tailed deer: An idea to consider? Wildl. Soc. Bull. 35, 185–194 (2011). [Google Scholar]
- 6.Santini L., Benítez-López A., Dormann C. F., Huijbregts M. A., Population density estimates for terrestrial mammal species. Glob. Ecol. Biogeogr. 31, 978–994 (2022). [Google Scholar]
- 7.IUCN Ssc Antelope Specialist Group, Syncerus caffer, The IUCN Red List of Threatened Species 2019: e.T21251A50195031IUCN Red List Threat Species 2019. (2019). 10.2305/IUCN.UK.2019-1.RLTS.T21251A50195031.en. Accessed 20 April 2023. [DOI]
- 8.IUCN, The IUCN Red List of Threatened Species. Version 2023-1 (2022). https://www.iucnredlist.org. Accessed on 10 April 2023.
- 9.Berzaghi F., Zhu D., Alroy J., Ciais P., Trait-based mechanistic approach highlights global patterns and losses of herbivore biomass functional diversity. Funct. Ecol., in press (2023). [Google Scholar]


