Abstract
Cancer‐associated fibroblasts (CAFs), as important components of the tumor microenvironment, can regulate intercellular communication and tumor development by secreting extracellular vesicles (EVs). However, the role of CAF‐derived EVs in ovarian cancer has not been fully elucidated. Here, using an EV‐microRNA sequencing analysis, we reveal specific overexpression of microRNA (miR)‐296‐3p in activated CAF‐derived EVs, which can be transferred to tumor cells to regulate the malignant phenotypes of ovarian cancer cells. Moreover, overexpression of miR‐296‐3p significantly promotes the proliferation, migration, invasion, and drug resistance of ovarian cancer cells in vitro, as well as tumor growth in vivo, while its inhibition has the opposite effects. Further mechanistic studies reveal that miR‐296‐3p promotes ovarian cancer progression by directly targeting PTEN and SOCS6 and activating AKT and STAT3 signaling pathways. Importantly, increased expression of miR‐296‐3p encapsulated in plasma EVs is closely correlated with tumorigenesis and chemoresistance in patients with ovarian cancer. Our results highlight the cancer‐promoting role of CAF‐derived EVs carrying miR‐296‐3p in ovarian cancer progression for the first time, and suggest that miR‐296‐3p encapsulated in CAF‐derived EVs could be a diagnostic biomarker and therapeutic target for ovarian cancer.
Keywords: cancer progression, cancer‐associated fibroblast, extracellular vesicle, miR‐296‐3p, ovarian cancer
Abbreviations
- aCAF
activated CAF
- CAF
cancer‐associated fibroblast
- ecDNA
extrachromosomal DNA
- EV‐DP‐CM
EV‐depleted conditioned medium
- EV
extracellular vesicle
- miR
microRNA
- miRNA
microRNA
- MUT
mutant
- NF
normal fibroblast
- NTA
nanoparticle tracking analysis
- PTEN
phosphatase and tensin homolog
- qCAF
quiescent CAF
- SOCS6
suppressor of cytokine signaling 6
- STAT3
signal transducer and activator of transcription 3
- TEM
transmission electron microscope
1. INTRODUCTION
Ovarian cancer is the most lethal gynecological malignancy worldwide with high metastasis and poor prognosis rates. In 2020, approximately 314,000 new cases and up to 207,000 deaths were related to ovarian cancer worldwide, representing serious threats to women's health. 1 , 2
Cancer‐associated fibroblasts constitute a major component of the tumor microenvironment, and play pivotal roles in tumor proliferation, metastasis, drug resistance, and immunosuppression through cell‐to‐cell interactions or paracrine action. 3 , 4 As an important mediator of intercellular communication in the tumor microenvironment, secretion of EVs, including exosomes, microvesicles, and apoptotic bodies, is an important way for CAFs to regulate the biological behavior of cancer cells. 5 , 6 Extracellular vesicles are heterogeneous vesicles secreted by multiple cell types that carry a variety of biological molecules, including proteins, nucleic acids, and lipids. 7 Accumulating evidence indicates that CAF‐derived EVs play a key role in cancer occurrence and progression by communicating with cancer cells. 8 However, limited studies have focused on the effects of EVs carrying biological molecules from CAFs in ovarian cancer development.
As a type of noncoding RNA, miRNA can bind to the 3′‐UTR of target mRNAs to function as posttranscriptional regulators, inducing decay of mRNA and translational suppression. 9 Numerous studies have reported that miRNAs are stably encapsulated in EVs and transferred to recipient cells, thereby regulating intercellular communication. 10 , 11 Cancer‐associated fibroblast‐derived EVs carrying miRNAs play a vital role in regulating biological processes, including cell proliferation, metastasis, drug resistance, and angiogenesis. 12 For example, CAF‐derived exosomal miR‐181d‐5p has been shown to promote breast cancer proliferation, migration, invasion, and epithelial–mesenchymal transition by targeting CDX2. 13 Additionally, EVs carrying miR‐10a‐5p from CAFs have been shown to promote cervical cancer growth and angiogenesis through activation of the Hedgehog signaling pathway by inhibiting TBX5 expression. 14 Moreover, it has been reported that CAF‐derived EVs promoted ovarian cancer metastasis and drug resistance by delivering miR‐29c‐3p, miR‐21, or miR‐98‐5p. 15 , 16 , 17 As reported previously, miR‐296‐3p is dysregulated in various tumor types, where it can promote or suppress tumor development by regulating different downstream target genes. 18 , 19 , 20 , 21 However, the expression and specific mechanism of action of miR‐296‐3p in ovarian cancer have not yet been reported.
In this study, using the EV‐miRNA sequencing analysis, we reveal for the first time that miR‐296‐3p is specifically upregulated in activated CAF‐derived EVs and can be transferred to ovarian cancer cells. Increased expression of miR‐296‐3p promotes ovarian cancer progression by regulating the PTEN/AKT and SOCS6/STAT3 pathways. Clinically, miR‐296‐3p is significantly overexpressed in the plasma EVs of patients with ovarian cancer compared to those of healthy controls. Additionally, miR‐296‐3p is highly expressed in the plasma EVs of patients with drug‐resistant ovarian cancer compared to those of drug‐sensitive patients. Our findings indicate that CAF‐derived miR‐296‐3p promotes cancer progression through EVs, suggesting that EV‐encapsulated miR‐296‐3p could be an effective diagnostic biomarker and therapeutic target for ovarian cancer.
2. MATERIALS AND METHODS
2.1. Plasma samples
Plasma samples from healthy donors and patients with ovarian cancer were obtained from Sun Yat‐sen University Cancer Center according to the guidelines of the Institutional Ethics Committee. Plasma samples from 46 ovarian cancer patients were collected; none of these patients received any preoperative cancer treatment and all of them were histologically diagnosed with ovarian cancer after surgery. Plasma samples from 23 healthy donors were collected as controls. Moreover, plasma samples were collected from advanced ovarian cancer patients who were sensitive (n = 13) or resistant to paclitaxel‐based chemotherapy (n = 8). During chemotherapy, drug‐resistant patients experienced tumor recurrence and metastasis, while tumor lesions were reduced in drug‐sensitive patients with ovarian cancer. The plasma samples were stored at −80°C and used for experiments as indicated.
2.2. Cell culture
SKOV3, OVCAR‐5, OV‐90, ES‐2, and Caov3 cell lines were obtained from ATCC. HOSE 6‐3, HOSE 17‐1, HO‐8910, A2780, and HEK‐293FT cell lines were kindly provided by Professor Xin‐Yuan Guan (University of Hong Kong Medical Center). All cell lines were maintained in 5% CO2 at 37°C, and cultured in DMEM supplemented with 10% FBS.
2.3. Isolation and identification of EVs
Extracellular vesicles were isolated from conditioned medium by differential ultracentrifugation as described previously. 22 , 23 Briefly, cell supernatants were first centrifuged at 500 g and 12,000 g to remove cell debris, before being passed through a 0.22 μm filter and ultracentrifuged at 100,000 g for 70 min to collect EVs. The EVs were washed with sterilized PBS and purified by further ultracentrifugation at 100,000 g for 70 min at 4°C. Cancer‐associated fibroblast‐derived EVs (200 μg) were cocultured with ovarian cancer cells in the study.
Extracellular vesicles were isolated from plasma using Exosome Isolation Reagent for plasma or serum (RiboBio) according to the manufacturer's instructions. In brief, plasma samples were centrifuged at 2000 g for 20 min at room temperature to remove cell debris. Next, the supernatant was centrifuged at 10,000 g for 20 min at room temperature, before adding 1/3 volume of exosome isolation reagent for 30 min at 4°C. Finally, EVs were obtained after centrifugation at 15,000 g for 2 min at 4°C. Isolated EVs were determined by TEM, NTA, and western blot analysis, as previously described. 23
2.4. Xenograft tumors in nude mice
Four‐ to six‐week‐old female BALB/c nude mice were purchased from Hunan SJA Laboratory Animal Co., Ltd and fed in a specific pathogen‐free level animal laboratory at the School of Medicine, South China University of Technology. A total of 3 × 106 A2780 or SKOV3 ovarian cancer cells overexpressing miR‐296‐3p, anti‐miR‐296‐3p, or their respective controls were injected s.c. into mice (n = 7). Tumor growth was monitored periodically every 2 or 3 days, and the tumor volume was calculated according to the following formula: volume (mm3) = length × width2 × 1/2. All mice were killed and the tumor tissues were dissected, weighed, and photographed.
2.5. Statistical analysis
All statistical analyses were undertaken using GraphPad Prism 7.0 software and presented as the mean ± SD. One‐way ANOVA or Student's t‐test were used to statistically analyze all data. p < 0.05 was considered statistically significant, with the level of significance indicated as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Additional materials and methods are provided in Appendix S1. All uncropped full‐blots of western blotting have been provided in Appendix S2.
3. RESULTS
3.1. Activated CAF‐derived EVs promote the proliferation, migration, and invasion of ovarian cancer cells
Our previous study found that overexpression of SEI‐1 in the NIH3T3 mouse fibroblasts line promotes genomic instability, inducing the formation of ecDNA, an important marker of gene amplification. 24 , 25 We then repeated the experiments in the human ovarian cancer cell line A2780, and Sei‐1 and null‐vector‐transfected A2780 cell clone (A2780Sei‐1 and A2780Vec) were s.c. injected into nude mice. We found that the fibroblasts isolated from A2780Sei‐1 group showed a large amount of ecDNA during the passages in vivo, which was absent in the fibroblasts isolated from the A2780Vec group. Moreover, the fibroblasts isolated from A2780Sei‐1 group showed high expression of CAF‐associated markers, including FAP, FSP1, and PDGFR‐α/β, and significantly promoted ovarian cancer cell migration, invasion, and angiogenesis in vitro and tumor growth in vivo compared to those of the corresponding A2780Vec group. Therefore, the fibroblasts isolated from A2780Sei‐1 group were significantly activated and had the genomic instability, and we termed this subtype aCAFs; fibroblasts isolated from the A2780Vec group were named qCAFs (unpublished data).
As an important mediator of intercellular communication in the tumor microenvironment, CAF‐derived EVs promote tumor development by transporting proteins, noncoding RNAs, and lipids to recipient cells. 5 , 6 Extracellular vesicles were isolated from the conditioned medium of qCAFs and aCAFs by ultracentrifugation and characterized by TEM and western blot analysis. Transmission electron microscopy confirmed the double‐layer membrane structure and size of the isolated EVs (30–150 nm diameter) (Figure 1A). The western blot results revealed an abundance of CD63, TSG101, and HSP70 proteins in EVs, while the Golgi matrix protein GM130 was absent (Figure 1B), consistent with previous reports on EVs. 22 To determine whether CAF‐derived EVs could be internalized by ovarian cancer cells, PKH26‐labeled EVs were cocultured with A2780 and SKOV3 cells for 24 h. Red fluorescence was detected in ovarian cancer cells by immunofluorescence, indicating the effective uptake of CAF‐derived EVs (Figure 1C,D).
FIGURE 1.
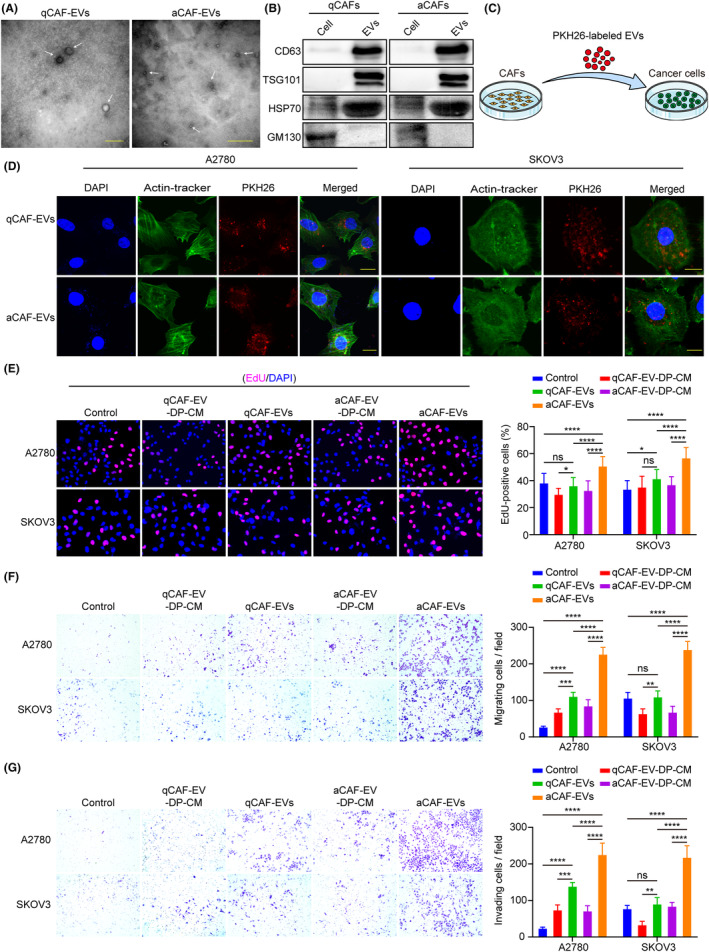
Activated cancer‐associated fibroblast (CAF)‐derived extracellular vesicles EVs promote the proliferation, migration, and invasion of ovarian cancer cells. (A) Transmission electron microscopy analysis of EVs isolated from quiescent CAFs (qCAFs) and activated CAFs (aCAFs); the white arrowhead signifies EVs (scale bar, 500 nm). (B) Western blot analysis of CD63, TSG101, HSP70, and GM130 protein expression in EVs and cell lysates. (C, D) Uptake of PKH26‐labeled EVs by A2780 and SKOV3 cells was detected by immunofluorescence (scale bar, 20 μm). (E–G) A2780 and SKOV3 cells were treated with PBS, qCAF‐derived EV‐depleted conditioned medium (qCAF‐EV‐DP‐CM), qCAF‐derived EVs (qCAF‐EVs), aCAF‐derived EV‐depleted conditioned medium (aCAF‐EV‐DP‐CM), and aCAF‐derived EVs (aCAF‐EVs). (E) Cell proliferation, (F) migration, and (G) invasion abilities were investigated by EdU, Transwell migration, and invasion assays, respectively. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, no significance.
To further elucidate the role of qCAF‐ and aCAF‐derived EVs in ovarian cancer development, PBS, qCAF‐EV‐DP‐CM, qCAF‐EVs, aCAF‐EV‐DP‐CM, or aCAF‐EVs were cocultured with A2780 and SKOV3 cells for 48 h, before conducting in vitro experiments. The results showed a significant increase in cell proliferation, migration, and invasion abilities after aCAF‐EV treatment compared with the qCAF‐EVs and control groups. However, the promoting effects were largely reduced in the aCAF‐EV‐DP‐CM group versus the aCAF‐EVs group, indicating a predominant role of EVs in the interaction between CAFs and tumor cells (Figure 1E–G). Taken together, these results suggest that aCAF‐derived EVs significantly promote the proliferation, migration, and invasion of ovarian cancer cells.
3.2. MicroRNA‐296‐3p is specifically upregulated in activated CAF‐derived EVs from ovarian cancer
In the tumor microenvironment, miRNAs are frequently encapsulated in EVs and are involved in intercellular communication. 26 Therefore, we next carried out miRNA sequencing to clarify the differentially expressed miRNAs in qCAF‐ and aCAF‐derived EVs. Data analysis revealed that among the differentially expressed miRNAs, the levels of miR‐296‐3p, miR‐31‐5p, miR‐147‐3p, and miR‐298‐5p were significantly upregulated in aCAF‐derived EVs, while the levels of miR‐433‐3p, miR‐134‐5p, miR‐434‐5p, and miR‐409‐3p expression were downregulated compared to qCAF‐derived EVs (Figure 2A, Table 1). To the best of our knowledge, no previous study has focused on the role of miR‐296‐3p in ovarian cancer development. Hence, miR‐296‐3p was selected for further investigation. The results of real‐time PCR showed that miR‐296‐3p expression was significantly increased in aCAFs and their secreted EVs compared to qCAFs, consistent with the sequencing data (Figure 2B). We further investigated the expression of miR‐296‐3p in ovarian cancer by biomedical database analysis. The data from the dbDEMC database showed a significant increase in miR‐296‐3p expression in ovarian cancer tissues compared to normal ovarian tissues (Figure 2C). Moreover, the miR‐296‐3p levels were tightly correlated with tumor stage according to UALCAN database analysis (Figure 2D). These data indicated that miR‐296‐3p might promote ovarian cancer progression.
FIGURE 2.
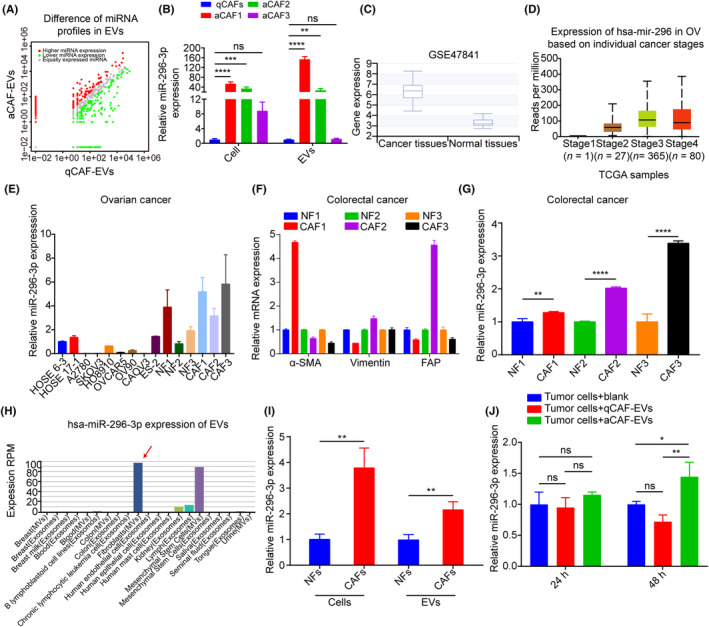
MicroRNA (miR)‐296‐3p is specifically upregulated in activated cancer‐associated fibroblast (CAF)‐derived extracellular vesicles (EVs) from ovarian cancer. (A) Volcano plot showing the differentially expressed miRNAs between quiescent CAF (qCAF)‐ and activated CAF (aCAF)‐derived EVs. (B) Real‐time PCR analysis of miR‐296‐3p expression in qCAFs, aCAFs, and their respective EVs. (C) dbDEMC database analysis of miR‐296‐3p levels in normal ovarian tissues and ovarian cancer tissues. (D) Bioinformatics analysis of miR‐296‐3p expression in ovarian cancer based on tumor stages using the UALCAN database. (E) Real‐time PCR showing the expression of miR‐296‐3p in normal ovarian epithelial cells (HOSE 6‐3 and HOSE 17‐1), ovarian cancer cells (A2780, SKOV3, HO8910, OVCAR5, OV90, CAOV3, and ES‐2), normal fibroblasts (NFs), and CAFs. (F) CAF markers, including α‐smooth muscle actin (α‐SMA), vimentin, and FAP, were determined in three pairs of NFs and CAFs in colorectal cancer by real‐time PCR. (G) Real‐time PCR showing the expression of miR‐296‐3p in three pairs of NFs and CAFs in colorectal cancer. (H) EVmiRNA database analysis of miR‐296‐3p expression in EVs from different sample sources. Red arrows indicate the miR‐296‐3p expression in EVs from fibroblasts. (I) Real‐time PCR showing the expression of miR‐296‐3p in NFs, CAFs, and their respective EVs in ovarian cancer. (J) Relative expression of miR‐296‐3p in tumor cells after incubation with blank or qCAF‐ or aCAF‐derived EVs for 24 or 48 h. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, no significance; RPM, reads per million; TCGA, The Cancer Genome Atlas.
TABLE 1.
Differentially expressed microRNAs (miR) between activated cancer‐associated fibroblast (aCAF)‐ and quiescent CAF (qCAF)‐derived extracellular vesicles
| MicroRNA | aCAFs vs. qCAFs Log2(fold change) | p value | Significance |
|---|---|---|---|
| mmu‐miR‐296‐3p | 6.7111 | 2.27569E‐13 | ** |
| mmu‐miR‐31‐5p | 6.5605 | 1.64385E‐14 | ** |
| mmu‐miR‐147‐3p | 6.2726 | 3.65581E‐10 | ** |
| mmu‐miR‐298‐5p | 5.8459 | 7.52646E‐12 | ** |
| mmu‐miR‐433‐3p | −12.253 | 8.033E‐21 | ** |
| mmu‐miR‐134‐5p | −9.4205 | 3.97211E‐20 | ** |
| mmu‐miR‐434‐3p | −9.2458 | 9.63394E‐17 | ** |
| mmu‐miR‐409‐3p | −9.2033 | 1.33072E‐18 | ** |
**p < 0.01.
To clarify the expression of miR‐296‐3p in clinical ovarian cancer CAFs, we isolated NFs and CAFs from normal ovarian tissues and ovarian cancer tissues, respectively 23 (Figure S1A–C). Importantly, we examined the expression of miR‐296‐3p in normal ovarian epithelial cells (HOSE 6‐3 and HOSE 17‐1), ovarian cancer cells (A2780, SKOV3, HO8910, OVCAR5, OV90, CAOV3, and ES‐2), NFs, and CAFs by real‐time PCR. The results indicated that miR‐296‐3p expression levels were significantly higher in fibroblasts, especially in CAFs compared to normal ovarian epithelial cells and ovarian cancer cells, indicating that miR‐296‐3p was specifically highly expressed in ovarian cancer CAFs (Figure 2E). Additionally, miR‐296‐3p levels were similarly increased in CAFs from colorectal cancer (Figures 2F,G and S1D).
To further investigate whether miR‐296‐3p is highly expressed in the EVs of CAFs, we analyzed the miR‐296‐3p expression in EVs from different sample sources using the EVmiRNA database. The results showed that the miR‐296‐3p levels were markedly elevated in the EVs of fibroblasts (Figure 2H). Then, we detected miR‐296‐3p expression in ovarian cancer NFs, CAFs, and their respective EVs, and the results showed that miR‐296‐3p was notably increased in CAFs and their secreted EVs (Figure 2I). We next incubated tumor cells with EVs isolated from qCAFs and aCAFs for 24 or 48 h, and found that aCAF‐derived EVs markedly increased expression of miR‐296‐3p in tumor cells following incubation for 48 h (Figure 2J). Based on these findings, we propose that miR‐296‐3p is specifically highly expressed in activated CAFs and transported to tumor cells through EVs, where it serves to regulate the progression of ovarian cancer.
3.3. MicroRNA‐296‐3p promotes ovarian cancer cell proliferation, migration, invasion, and drug resistance in vitro, and tumorigenesis in vivo
To further investigate the biological functions of miR‐296‐3p in ovarian cancer cells, A2780 and SKOV3 cells were transfected with miR‐296‐3p or anti‐miR‐296‐3p (Figure S2). Data from EdU and colony formation assays showed that miR‐296‐3p overexpression significantly promoted the proliferation and colony formation ability of tumor cells compared to the control group, while inhibition of miR‐296‐3p had the opposite effect (Figure 3A–D). Moreover, overexpression of miR‐296‐3p markedly increased the migration and invasion abilities of A2780 and SKOV3 cells, while inhibition of miR‐296‐3p decreased both functions by Transwell assays (Figure 3E–H). We also investigated whether the miR‐296‐3p levels confer drug resistance in ovarian cancer cells, and found that miR‐296‐3p overexpression enhanced paclitaxel resistance in cancer cells, thereby promoting cell viability and colony formation ability (Figure S3). These results suggest that CAF‐derived EVs carrying miR‐296‐3p promotes ovarian cancer cell proliferation, migration, invasion, and drug resistance in vitro.
FIGURE 3.
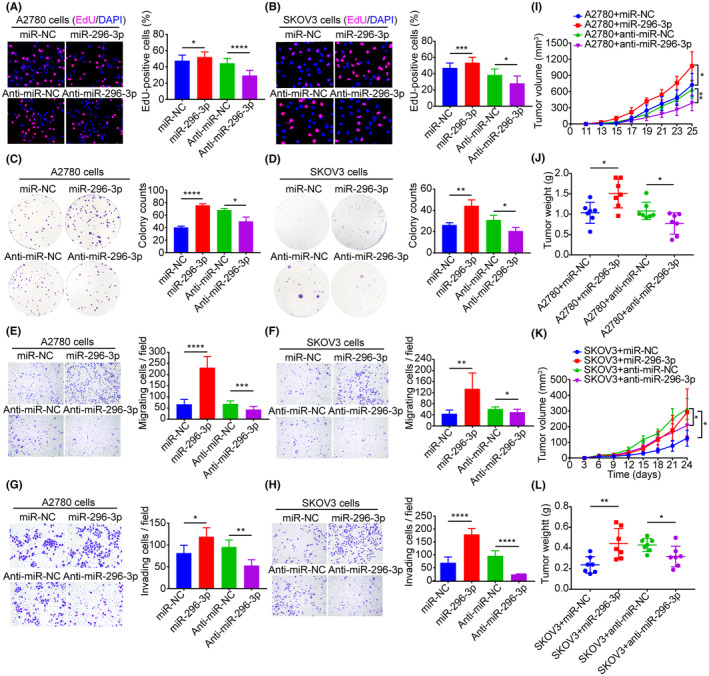
MicroRNA (miR)‐296‐3p promotes ovarian cancer development in vitro and in vivo. (A, B) Effects of miR‐296‐3p on the proliferation of A2780 and SKOV3 cells were detected by EdU assays. (C, D) Cell colony‐forming ability was measured using colony formation assay. (E, F) Effects of miR‐296‐3p on the migration of A2780 and SKOV3 cells were determined with Transwell migration assays. (G, H) Representative images from Transwell invasion assays of A2780 and SKOV3 cells. (I, J) A2780 cells transfected with miR‐296‐3p or anti‐miR‐296‐3p and their negative control were used to establish s.c. xenograft tumors (n = 7). Tumor growth curves and tumor weights are shown. (K, L) Effects of miR‐296‐3p on the tumor growth of SKOV3 cells in nude mice (n = 7). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
To evaluate the effect of miR‐296‐3p on ovarian cancer growth in vivo, we established mouse tumor models by s.c. inoculating miR‐296‐3p‐ or anti‐miR‐296‐3p‐transfected A2780 and SKOV3 cells. The results illustrated that overexpression of miR‐296‐3p promoted tumor growth in terms of tumor volume and weight compared to the control group. In contrast, the group with miR‐296‐3p inhibition showed a significant inhibition in the growth of xenografted tumors, with a smaller tumor size and lighter tumor weight (Figures 3I–L and S4A–D). These data indicate that miR‐296‐3p promotes tumor growth in vivo.
3.4. PTEN and SOCS6 are direct downstream target genes of miR‐296‐3p in ovarian cancer cells
Previous studies have shown that miRNAs can regulate tumor progression by binding to the 3′‐UTR region of target genes. 9 To explore the mechanism by which miR‐296‐3p promotes ovarian cancer development, we analyzed the potential target genes of miR‐296‐3p using the TargetScan database. The results showed that miR‐296‐3p was predicted to target the 3′‐UTR of PTEN and SOCS6 (Figure S5A). Additionally, PTEN and SOCS6 levels were decreased in ovarian cancer tissues compared to normal ovarian tissues by Gene Expression Profiling Interactive Analysis (GEPIA) (Figure S5B), and negatively correlated with miR‐296‐3p expression in ovarian cancer through cBioPortal database analysis (Figure S5C). These findings suggest that miR‐296‐3p could promote ovarian cancer progression through targeting PTEN and SOCS6.
Subsequently, we constructed a luciferase reporter plasmid containing WT or MUT binding sites in the 3′‐UTR of PTEN or SOCS6 (Figure 4A), and cotransfected them with miR‐NC or miR‐296‐3p into 293FT cells. After cotransfection with miR‐296‐3p, relative luciferase activities were significantly decreased in the WT group but not in the MUT group (Figure 4B), indicating that miR‐296‐3p can bind to the 3′‐UTR region of PTEN and SOCS6. Moreover, the PTEN and SOCS6 mRNA and protein levels were substantially decreased in A2780 and SKOV3 cells upon miR‐296‐3p overexpression, while suppression of miR‐296‐3p showed the opposite effect (Figure 4C,D). Additionally, the results of immunohistochemistry showed that PTEN and SOCS6 staining was much weaker in tumor tissues overexpressing miR‐296‐3p, but was enhanced following inhibition of miR‐296‐3p expression (Figure S4E,F). Taken together, these results strongly suggest that PTEN and SOCS6 are direct downstream target genes of miR‐296‐3p in ovarian cancer cells.
FIGURE 4.
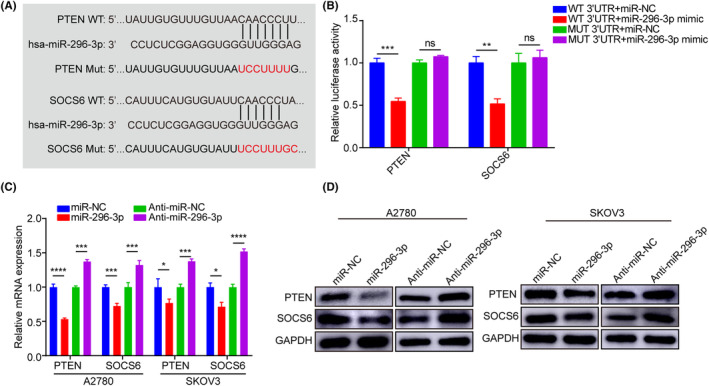
MicroRNA (miR)‐296‐3p directly targets phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling 6 (SOCS6). (A) Schematic of the constructed luciferase reporter plasmid constructed containing WT and mutant (Mut) binding sites in the 3′‐UTR of PTEN and SOCS6 mRNA. (B) Analysis of dual‐luciferase reporter assays revealed the interaction between miR‐296‐3p with PTEN and SOCS6. (C) Relative mRNAs expressions of PTEN and SOCS6 were analyzed by real‐time PCR in A2780 and SKOV3 cells. (D) Western blot analysis of PTEN and SOCS6 protein levels in A2780 and SKOV3 cells. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns, no significance.
3.5. MicroRNA‐296‐3p mediates the proliferation, migration, and invasion of ovarian cancer cells through downregulation of PTEN and SOCS6
To investigate the effects of the target genes PTEN and SOCS6 on the biological behavior of ovarian cancer cells, A2780 and SKOV3 cells were separately transfected with PTEN and SOCS6 plasmids. Successful overexpression of PTEN and SOCS6 was confirmed at both the mRNA and proteomic levels by real‐time PCR (Figure S6A,B) and western blot (Figure 5A) analysis. In vitro experiments showed that overexpression of PTEN or SOCS6 significantly decreased the proliferation, colony formation, migration, and invasion abilities of A2780 and SKOV3 cells (Figure 5B–E). Thus, PTEN and SOCS6 function as tumor suppressors in ovarian cancer, inhibiting cell proliferation, migration, and invasion.
FIGURE 5.
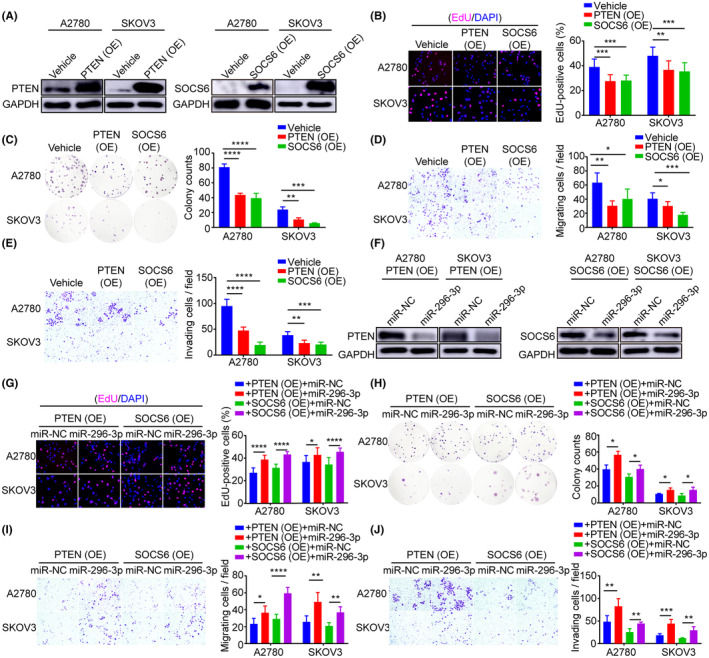
MicroRNA (miR)‐296‐3p mediates the proliferation, migration, and invasion of ovarian cancer cells in part through downregulation of phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling 6 (SOCS6). (A) Western blot analysis of PTEN and SOCS6 expression in A2780 and SKOV3 cells with PTEN or SOCS6 overexpression (OE). (B–E) Ectopic PTEN or SOCS6 expression in A2780 and SKOV3 cells. (B) Cell proliferation, (C) colony formation, (D) migration, and (E) invasion abilities were investigated by EdU, colony formation, Transwell migration, and invasion assays. (F) Western blot analysis of PTEN and SOCS6 levels in A2780 and SKOV3 cells cotransfected with miR‐296‐3p and PTEN or SOCS6. (G) Cell proliferation and (H) colony formation of A2780 and SKOV3 cells with PTEN or SOCS6 overexpression combined with or without miR‐296‐3p mimic. (I,J) Cell migration and invasion of A2780 and SKOV3 cells determined by Transwell assays. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. miR‐NC, mimic negative control.
Next, to determine whether miR‐296‐3p regulates the malignant phenotype of ovarian cancer cells by targeting PTEN and SOCS6, A2780 and SKOV3 cells were cotransfected with miR‐296‐3p and PTEN or SOCS6. The results showed that overexpression of miR‐296‐3p significantly decreased the mRNA (Figure S6C,D) and protein (Figure 5F) levels of PTEN and SOCS6 in A2780 and SKOV3 cells compared to the control group, which is consistent with our previous observations. Importantly, overexpression of miR‐296‐3p restored the proliferation, colony formation, migration, and invasion abilities of ovarian cancer cells, all of which were inhibited by ectopic expression of PTEN or SOCS6 (Figure 5G–J). The above results indicate that CAF‐derived EVs carrying miR‐296‐3p can promote cell proliferation, migration, and invasion of ovarian cancer by targeting PTEN and SOCS6.
3.6. MicroRNA‐296‐3p activates downstream AKT and STAT3 signaling pathways by targeting PTEN and SOCS6, respectively
Previous studies have shown that PTEN and SOCS proteins negatively regulate the AKT and STAT3 signaling pathways, respectively. 27 , 28 , 29 To further investigate whether miR‐296‐3p promotes ovarian cancer progression by targeting PTEN and SOCS6 to activate the downstream AKT and STAT3 signaling pathways, we transfected A2780 and SKOV3 cells with miR‐296‐3p mimic or inhibitor. The results showed increased protein levels of p‐AKT (S473), p‐AKT (T308), and p‐STAT3 in cells with miR‐296‐3p overexpression (Figure 6A), all of which were significantly decreased after inhibition of miR‐296‐3p expression (Figure 6B), indicating that miR‐296‐3p is involved in regulating the downstream AKT and STAT3 signaling pathways.
FIGURE 6.
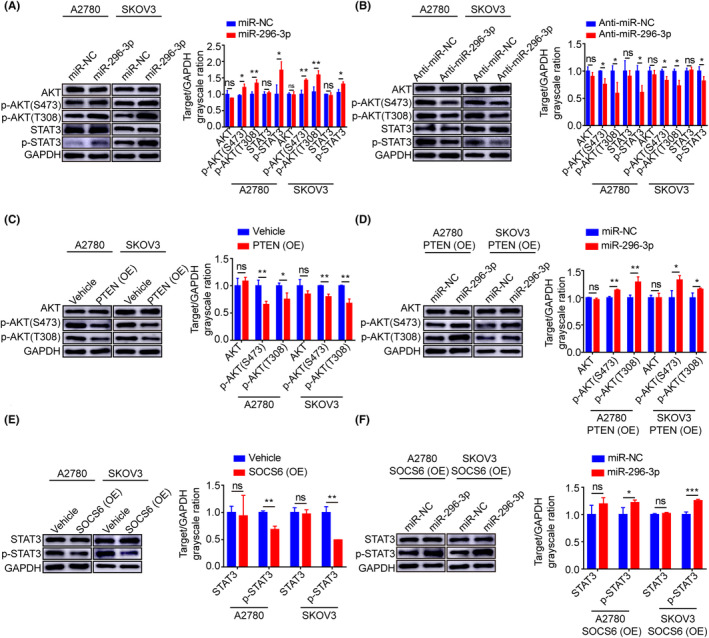
MicroRNA (miR)‐296‐3p activates downstream AKT and signal transducer and activator of transcription 3 (STAT3) signaling pathways by targeting phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling 6 (SOCS6), respectively. (A, B) Western blot analysis of the critical mediators of AKT and STAT3 signaling pathways, including AKT, p‐AKT (S473), p‐AKT (T308), STAT3, and p‐STAT3 in A2780 and SKOV3 cells transfected miR‐296‐3p or anti‐miR‐296‐3p. (C) Overexpression (OE) of PTEN significantly reduced the p‐AKT (S473) and p‐AKT (T308) protein levels in A2780 and SKOV3 cells. (D) Relative protein expressions of AKT, p‐AKT (S473), and p‐AKT (T308) were analyzed by western blot in A2780 and SKOV3 cells cotransfected with PTEN and miR‐NC or miR‐296‐3p. (E) Western blot results demonstrate STAT3 and p‐STAT3 expression in A2780 and SKOV3 cells with SOCS6 overexpression. (F) Relative protein levels of STAT3 and p‐STAT3 were analyzed by western blot in A2780 and SKOV3 cells cotransfected with SOCS6 and miR‐NC or miR‐296‐3p. Band intensity was assessed with ImageJ. *p < 0.05; **p < 0.01; ***p < 0.001. ns, no significance. miR‐NC, mimic negative control.
Next, we overexpressed PTEN in A2780 and SKOV3 cells, and found that high PTEN expression in tumor cells significantly inhibited the activation of downstream AKT signaling and decreased the levels of p‐AKT (S473) and p‐AKT (T308) (Figure 6C). Importantly, miR‐296‐3p restored the inhibitory effect of PTEN on the AKT signaling pathway and significantly increased the protein expression levels of p‐AKT (S473) and p‐AKT (T308) in A2780 and SKOV3 cells compared to the control group (Figure 6D). Similarly, we found that overexpression of SOCS6 significantly inhibited p‐STAT3 protein expression (Figure 6E), while the p‐STAT3 protein expression level was significantly increased in A2780 and SKOV3 cells cotransfected with SOCS6 and miR‐296‐3p compared with the corresponding control cells (Figure 6F). These results suggest that miR‐296‐3p specifically overexpressed by CAF‐derived EVs promotes the malignant phenotype of ovarian cancer cells through activation of the downstream AKT and STAT3 signaling pathways by targeting PTEN and SOCS6.
3.7. High miR‐296‐3p expression in plasma EVs predicts tumorigenesis and chemotherapy resistance in patients with ovarian cancer
We next analyzed the expression of miR‐296‐3p in the blood circulation using the dbDEMC database. The results revealed that the levels of miR‐296‐3p were significantly increased in the blood of patients with ovarian cancer compared to the control group (Figure 7A). Additionally, we collected plasma samples from healthy controls and patients with ovarian cancer, before purifying plasma EVs and validating them by TEM, NTA, and western blot analysis. The results showed that isolated EVs had a bilayer membrane structure with an approximate size of 100 nm (Figure 7B,C). Moreover, CD9, HSP70, Alix, and Annexin‐V proteins were found to be abundant in plasma EVs compared to cells (Figure 7D), consistent with the typical characteristics of EVs, implying successful purification of plasma EVs. Notably, miR‐296‐3p expression was significantly higher in plasma EVs from patients with ovarian cancer compared to the control group (Figure 7E). However, the association analysis found that overexpression of miR‐296‐3p had no significant differences in relation to patient age, tumor stage, or metastasis, which could be attributed to the small number of plasma samples (Table S1). Additionally, compared to plasma EVs from drug‐sensitive patients with ovarian cancer, plasma EVs from drug‐resistant patients with ovarian cancer showed increased expression of miR‐296‐3p (Figure 7F). Taken together, these findings suggest that miR‐296‐3p in plasma EVs is closely associated with tumorigenesis and chemoresistance in patients with ovarian cancer and could serve as a diagnostic biomarker.
FIGURE 7.
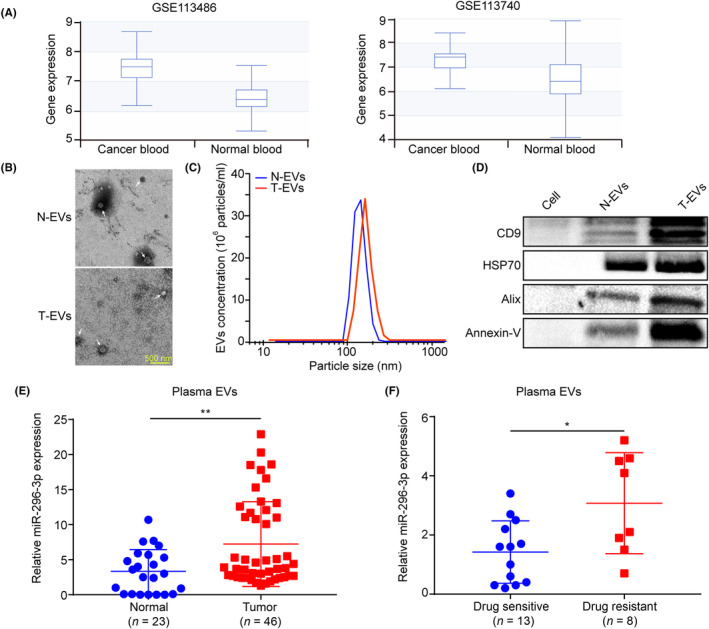
MicroRNA (miR)‐296‐3p in plasma extracellular vesicles (EVs) is closely associated with tumorigenesis and chemoresistance in patients with ovarian cancer. (A) miR‐296‐3p expression in the blood circulation of patients with ovarian cancer and healthy controls was analyzed using the dbDEMC database. (B) EVs isolated from the plasma of patients with ovarian cancer and healthy controls were observed by transmission electron microscope; white arrowhead signifies EVs (scale bar, 500 nm). (C) Nanoparticle tracking analysis of the isolated EVs. (D) Western blot analysis of EV marker protein expression, including CD9, HSP70, Alix, and Annexin‐V. (E) The miR‐296‐3p expression in plasma EVs of patients with ovarian cancer and healthy controls was detected using real‐time PCR. (F) miR‐296‐3p expression in plasma EVs of drug‐sensitive and drug‐resistant patients with ovarian cancer. *p < 0.05; **p < 0.01. N‐EV, normal control‐derived EVs; T‐EV, tumor patien‐derived EVs.
4. DISCUSSION
Cancer‐associated fibroblasts, as major components of the tumor microenvironment, regulate tumorigenesis, metastasis, drug resistance, metabolic reprogramming, and immunosuppression by interacting with tumor cells by secreting cytokines, chemokines, and EVs. 5 , 8 Extracellular vesicles are membrane‐encapsulated nanoparticles that contain multiple bioactive molecules, such as lipids, proteins, miRNA, and long noncoding RNA, which are involved in intercellular communication. 7 As the major RNA component of EVs, mounting evidence suggests that CAF‐derived EVs carrying miRNAs, such as miR‐21, miR‐500a‐5p, miR‐3188, and miR‐92a‐3p, are involved in the interaction of cancer cells with the tumor microenvironment and promote tumor progression. 16 , 30 , 31 , 32 However, the regulation of miRNAs encapsulated in CAF‐derived EVs in ovarian cancer progression is not yet fully understood.
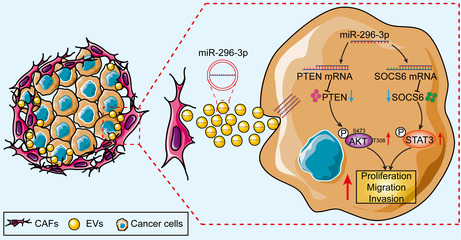
Previous studies have shown that miR‐296‐3p is dysregulated in various tumors, where it can promote or inhibit tumor progression by targeting different genes. 18 For example, miR‐296‐3p has been shown to be upregulated in prostate cancer and glioma and promoted tumor proliferation and metastasis by targeting ICAM‐1 and ICAT, respectively. 19 , 33 In contrast, miR‐296‐3p has also been reported to function as tumor suppressor and inhibited cell proliferation, metastasis, and drug resistance in lung cancer by targeting PRKCA. 34 However, the expression patterns, roles, and underlying molecular mechanisms of miR‐296‐3p in ovarian cancer have not been reported. In the present study, we determined for the first time that miR‐296‐3p is specifically encapsulated in ovarian cancer CAF‐derived EVs, and could be transferred to tumor cells, promoting cancer cell proliferation, migration, and invasion by regulating PTEN/AKT and SOCS6/STAT3 signaling pathways (Figure 8).
FIGURE 8.
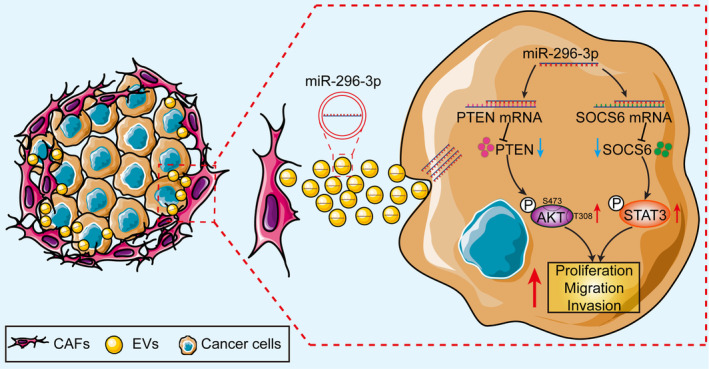
Schematic diagram illustrating the regulatory axis of cancer‐associated fibroblast (CAF)‐derived extracellular vesicles (EVs) carrying microRNA (miR)‐296‐3p involved in promoting ovarian cancer development. PTEN, phosphatase and tensin homolog; SOCS6, suppressor of cytokine signaling 6; STAT3, signal transducer and activator of transcription 3.
As a classical tumor suppressor, PTEN is involved in the regulation of multiple biological processes, including genomic stability, cell survival, migration, proliferation, and metabolism. 27 Increasing evidence has indicated that PTEN expression is downregulated in a variety of tumors, 35 , 36 , 37 and that inhibition of PTEN expression could significantly promote ovarian cancer growth and metastasis. 38 , 39 Consistently, we found that overexpression of PTEN significantly inhibited the proliferation, migration, and invasion of ovarian cancer cells and suppressed activation of the downstream AKT signaling pathway. Recently, it has been reported that CAF‐derived EVs carrying miRNAs, such as miR‐20a, miR‐181a, miR‐221, and miR‐148b‐3p, can be transferred to tumor cells and promote tumor development by targeting PTEN. 40 , 41 , 42 , 43 However, no previous studies have focused on the interaction of CAF‐derived EVs carrying miRNAs with PTEN in ovarian cancer. Here, we show for the first time that CAF‐secreted EVs encapsulating miR‐296‐3p could target PTEN to promote ovarian cancer progression and activate the downstream AKT signaling pathway, which were inhibited by ectopic expression of PTEN.
Accumulating evidence has shown that SOCS proteins are negative regulators of cytokine and growth factor signaling. 29 Indeed, SOCS6 has been shown to be significantly downregulated in multiple tumors, with decreased SOCS6 expression found to be closely associated with malignant phenotype and poor prognosis of tumors. 44 , 45 , 46 , 47 Moreover, it has been found that many miRNAs, including miR‐424‐5p, miR‐653‐5p, miR‐17‐5p, and miR‐301a, target SOCS6 and promote tumor progression by directly inhibiting SOCS6 expression in various cancer types. 48 , 49 , 50 , 51 However, the roles and specific molecular mechanisms of SOCS6 in ovarian cancer carcinogenesis and progression have not been investigated previously. Here, we reveal for the first time that overexpression of SOCS6 significantly inhibits the proliferation, migration, and invasion of ovarian cancer cells, indicating that SOCS6 can function as an ovarian cancer suppressor. Moreover, CAF‐derived EVs carrying miR‐296‐3p could target SOCS6 and inhibit its expression, thereby reversing the role of SOCS6 in inhibiting ovarian cancer progression. Studies have shown that compared to normal ovarian tissues, STAT3 signaling is aberrantly activated in ovarian cancer tissues, where it promotes tumor cell proliferation, invasion, angiogenesis, and chemotherapy resistance. 52 Notably, recent studies have shown that SOCS6 can act as a negative regulator of the STAT3 signaling pathway, promoting epidermal stem cell differentiation and regulating apoptosis of vascular endothelial cells by increasing the ubiquitination of JAK2 and inhibiting the activation of the JAK2/STAT3 pathway. 53 , 54 Moreover, some miRNAs, such as miR‐19, miR‐653‐5p, and miR‐548d‐3p, have been shown to promote tumorigenesis by targeting SOCS6 and reversing the inhibitory effect of SOCS6 on STAT3 signaling. 49 , 55 , 56 Interestingly, our study also revealed that overexpression of SOCS6 could significantly decrease the expression level of p‐STAT3, while miR‐296‐3p greatly increased the expression of p‐STAT3 by targeting SOCS6, suggesting that miR‐296‐3p promoted activation of the downstream STAT3 signaling pathway in ovarian cancer by targeting SOCS6.
Extracellular vesicles are widely present in biological fluids, including blood, urine, and saliva, and are often used in clinical research. Studies have shown that miRNAs encapsulated in circulating EVs are more stable than free miRNAs, suggesting that EVs carrying miRNAs have potential applications as tumor biomarkers. 57 , 58 , 59 Indeed, Matsumura et al. found that compared to healthy controls, exosomal miR‐19a‐3p was significantly highly expressed in the serum of patients with colorectal cancer and was tightly correlated with poor prognosis, suggesting that exosomal miR‐19a‐3p expression in the circulation could be used as a prognostic biomarker for such patients. 60 Additionally, Pan et al. revealed that miR‐21, miR‐100, miR‐200b, and miR‐320 were upregulated in the plasma EVs of patients with ovarian cancer compared to those of healthy controls, while miR‐16, miR‐93, miR‐126, and miR‐223 were downregulated, indicating that dysregulation of miRNA expression in plasma EVs could serve as an early diagnostic marker for patients with ovarian cancer. 61 Our study revealed for the first time that miR‐296‐3p can be used as a biomarker for the diagnosis of tumorigenesis and response to chemotherapy in patients with ovarian cancer. However, future studies should apply stricter inclusion criteria and a larger cohort to determine the true diagnostic efficiency of miR‐296‐3p.
In conclusion, our findings reveal that miR‐296‐3p is specifically highly expressed in CAF‐derived EVs and is involved in regulating the interaction of CAFs with ovarian cancer cells. Overexpression of miR‐296‐3p promotes the malignant phenotypes of ovarian cancer by inhibiting PTEN and SOCS6 expression and activating the downstream AKT and STAT3 signaling pathways. In addition, in vivo studies revealed the potential therapeutic value of miR‐296‐3p for inhibiting ovarian cancer growth. Moreover, miR‐296‐3p expression in plasma EVs could serve as an effective diagnostic biomarker for tumorigenesis and chemoresistance in patients with ovarian cancer.
AUTHOR CONTRIBUTIONS
Chunyu Zhang: Conceptualization; funding acquisition; project administration; supervision; writing – review and editing. Luyao Sun: Conceptualization; formal analysis; investigation; methodology; software; validation; writing – original draft. Miaola Ke: Data curation; formal analysis; investigation; resources; validation; visualization. Mengyuan Yin: Formal analysis; methodology; software; visualization. Ying Zeng: Methodology; software. Yutong Ji: Methodology; software. Yiming Hu: Methodology; software. Songbin Fu: Project administration; supervision.
FUNDING INFORMATION
This study was supported by the Science and Technology Program of Guangzhou, China (No. 2060206) and the National Natural Science Foundation of China (No. 81672572).
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: All procedures followed were in accordance with the ethical standards of the institutional review board of South China University.
Informed consent: All clinical samples used in this study were obtained from Sun Yat‐sen University Cancer Center, Guangzhou, China, and written informed consent was obtained from the patients and/or their immediate relatives using recognized guidelines.
Registry and registration no. of the study/trial: N/A.
Animal studies: All mouse experiments were performed according to our experimental protocols approved by the Guangzhou Committee for the Use and Care of Laboratory Animals, and by the Animal Ethics Committee of South China University of Technology University (approval number: 2019076).
Supporting information
Appendix S1.
Appendix S2.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Table S1.
ACKNOWLEDGMENTS
The authors would like to thank Professor Xin‐Yuan Guan (University of Hong Kong Medical Center) for his great assistance with the cell lines. The authors thank all the members listed in the article.
Sun L, Ke M, Yin M, et al. Extracellular vesicle‐encapsulated microRNA‐296‐3p from cancer‐associated fibroblasts promotes ovarian cancer development through regulation of the PTEN/AKT and SOCS6/STAT3 pathways. Cancer Sci. 2024;115:155‐169. doi: 10.1111/cas.16014
Luyao Sun and Miaola Ke contributed equally to this work.
DATA AVAILABILITY STATEMENT
Please contact the corresponding author for all data requests.
REFERENCES
- 1. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: An integrated review. Semin Oncol Nurs. 2019;35:151‐156. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 3. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer‐associated fibroblasts. Nat Rev Cancer. 2020;20:174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer‐associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated‐fibroblast‐derived exosomes in cancer progression. Mol Cancer. 2021;20:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shoucair I, Weber Mello F, Jabalee J, Maleki S, Garnis C. The role of cancer‐associated fibroblasts and extracellular vesicles in tumorigenesis. Int J Mol Sci. 2020;21:6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. [DOI] [PubMed] [Google Scholar]
- 8. He C, Wang L, Li L, Zhu G. Extracellular vesicle‐orchestrated crosstalk between cancer‐associated fibroblasts and tumors. Transl Oncol. 2021;14:101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460‐469. [DOI] [PubMed] [Google Scholar]
- 10. Groot M, Lee H. Sorting mechanisms for MicroRNAs into extracellular vesicles and their associated diseases. Cell. 2020;9:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munir J, Yoon JK, Ryu S. Therapeutic miRNA‐enriched extracellular vesicles: current approaches and future prospects. Cells. 2020;9: 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang F, Ning Z, Ma L, et al. Exosomal miRNAs and miRNA dysregulation in cancer‐associated fibroblasts. Mol Cancer. 2017;16:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Wei H, Wang J, Li L, Chen A, Li Z. MicroRNA‐181d‐5p‐containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic Acids. 2020;19:654‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X, Wang Y, Wang X, et al. Extracellular vesicles‐encapsulated microRNA‐10a‐5p shed from cancer‐associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via hedgehog signaling pathway. Cancer Gene Ther. 2021;28:529‐542. [DOI] [PubMed] [Google Scholar]
- 15. Han Q, Tan S, Gong L, et al. Omental cancer‐associated fibroblast‐derived exosomes with low microRNA‐29c‐3p promote ovarian cancer peritoneal metastasis. Cancer Sci. 2023;114:1929‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Au Yeung CL, Co NN, Tsuruga T, et al. Exosomal transfer of stroma‐derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer‐associated fibroblast‐derived exosomal microRNA‐98‐5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu L, Deng H, Hu J, Huang S, Xiong J, Deng J. The promising role of miR‐296 in human cancer. Pathol Res Pract. 2018;214:1915‐1922. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Du G, Fu H. miR‐296‐3p promotes the proliferation of glioblastoma cells by targeting ICAT. Mol Med Rep. 2020;21:2151‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bai Y, Liao H, Liu T, et al. MiR‐296‐3p regulates cell growth and multi‐drug resistance of human glioblastoma by targeting ether‐à‐go‐go (EAG1). Eur J Cancer. 2013;49:710‐724. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Chen R, Zhang Y. miR‐296‐3p targets APEX1 to suppress cell migration and invasion of non‐small‐cell lung cancer. Oncol Lett. 2019;18:2612‐2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 23. Sun L, Ke M, Wang X, et al. FAP(high) α‐SMA(low) cancer‐associated fibroblast‐derived SLPI protein encapsulated in extracellular vesicles promotes ovarian cancer development via activation of PI3K/AKT and downstream signaling pathways. Mol Carcinog. 2022;61:910‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tian X, Liu C, Wang X, et al. Sei‐1 promotes double minute chromosomes formation through activation of the PI3K/Akt/BRCA1‐abraxas pathway and induces double‐strand breaks in NIH‐3T3 fibroblasts. Cell Death Dis. 2018;9:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao Y, Liu J, You J, et al. Met promotes the formation of double minute chromosomes induced by sei‐1 in NIH‐3T3 murine fibroblasts. Oncotarget. 2016;7:56664‐56675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip rev RNA. 2017;8:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547‐562. [DOI] [PubMed] [Google Scholar]
- 28. Chalhoub N, Baker SJ. PTEN and the PI3‐kinase pathway in cancer. Annu Rev Pathol. 2009;4:127‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laudisi F, Cherubini F, Monteleone G, Stolfi C. STAT3 interactors as potential therapeutic targets for cancer treatment. Int J Mol Sci. 2018;19:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen B, Sang Y, Song X, et al. Exosomal miR‐500a‐5p derived from cancer‐associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932‐3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X, Qin X, Yan M, et al. Loss of exosomal miR‐3188 in cancer‐associated fibroblasts contributes to HNC progression. J Exp Clin Cancer Res. 2019;38:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu JL, Wang W, Lan XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial‐mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Chen Q, Yan J, et al. MiRNA‐296‐3p‐ICAM‐1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell‐resistant circulating tumour cells. Cell Death Dis. 2013;4:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu Q, Song X, Liu Z, et al. miRomics and proteomics reveal a miR‐296‐3p/PRKCA/FAK/Ras/c‐Myc feedback loop modulated by HDGF/DDX5/β‐catenin complex in lung adenocarcinoma. Clin Cancer Res. 2017;23:6336‐6350. [DOI] [PubMed] [Google Scholar]
- 35. Janaki Ramaiah M, Vaishnave S. BMI1 and PTEN are key determinants of breast cancer therapy: a plausible therapeutic target in breast cancer. Gene. 2018;678:302‐311. [DOI] [PubMed] [Google Scholar]
- 36. Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403‐414. [DOI] [PubMed] [Google Scholar]
- 37. Zhang L, Ma T, Brozick J, et al. Effects of Kras activation and Pten deletion alone or in combination on MUC1 biology and epithelial‐to‐mesenchymal transition in ovarian cancer. Oncogene. 2016;35:5010‐5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097‐2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao W, Han T, Li B, Ma Q, Yang P, Li H. miR‐552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi L, Zhu W, Huang Y, et al. Cancer‐associated fibroblast‐derived exosomal microRNA‐20a suppresses the PTEN/PI3K‐AKT pathway to promote the progression and chemoresistance of non‐small cell lung cancer. Clin Transl Med. 2022;12:e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pakravan K, Mossahebi‐Mohammadi M, Ghazimoradi MH, Cho WC, Sadeghizadeh M, Babashah S. Monocytes educated by cancer‐associated fibroblasts secrete exosomal miR‐181a to activate AKT signaling in breast cancer cells. J Transl Med. 2022;20:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richards KE, Xiao W, Hill R. On behalf of the Usc pancreas research T. Cancer‐associated fibroblasts confer gemcitabine resistance to pancreatic cancer cells through PTEN‐targeting miRNAs in exosomes. Cancers (Basel). 2022;14:2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shan G, Zhou X, Gu J, et al. Downregulated exosomal microRNA‐148b‐3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β‐catenin pathway and upregulating PTEN. Cell Oncol (Dordr). 2021;44:45‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoon S, Yi YS, Kim SS, Kim JH, Park WS, Nam SW. SOCS5 and SOCS6 have similar expression patterns in normal and cancer tissues. Tumour Biol. 2012;33:215‐221. [DOI] [PubMed] [Google Scholar]
- 45. Lai RH, Hsiao YW, Wang MJ, et al. SOCS6, down‐regulated in gastric cancer, inhibits cell proliferation and colony formation. Cancer Lett. 2010;288:75‐85. [DOI] [PubMed] [Google Scholar]
- 46. Qiu X, Zheng J, Guo X, et al. Reduced expression of SOCS2 and SOCS6 in hepatocellular carcinoma correlates with aggressive tumor progression and poor prognosis. Mol Cell Biochem. 2013;378:99‐106. [DOI] [PubMed] [Google Scholar]
- 47. Zhu JG, Dai QS, Han ZD, et al. Expression of SOCSs in human prostate cancer and their association in prognosis. Mol Cell Biochem. 2013;381:51‐59. [DOI] [PubMed] [Google Scholar]
- 48. Wu K, Hu G, He X, et al. MicroRNA‐424‐5p suppresses the expression of SOCS6 in pancreatic cancer. Pathol Oncol Res. 2013;19:739‐748. [DOI] [PubMed] [Google Scholar]
- 49. Li Z, Fan H, Chen W, et al. MicroRNA‐653‐5p promotes gastric cancer proliferation and metastasis by targeting the SOCS6‐STAT3 pathway. Front Mol Biosci. 2021;8:655580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Q, Luo G, Yang Z, et al. miR‐17‐5p promotes proliferation by targeting SOCS6 in gastric cancer cells. FEBS Lett. 2014;588:2055‐2062. [DOI] [PubMed] [Google Scholar]
- 51. Fang Y, Sun B, Xiang J, Chen Z. MiR‐301a promotes colorectal cancer cell growth and invasion by directly targeting SOCS6. Cell Physiol Biochem. 2015;35:227‐236. [DOI] [PubMed] [Google Scholar]
- 52. Liang R, Chen X, Chen L, et al. STAT3 signaling in ovarian cancer: a potential therapeutic target. J Cancer. 2020;11:837‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoshizumi T, Kubo A, Murata H, Shinonaga M, Kanno H. BC‐box motif in SOCS6 induces differentiation of epidermal stem cells into GABAnergic neurons. Int J Mol Sci. 2020;21:4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xiao F, Li L, Fu JS, Hu YX, Luo R. Regulation of the miR‐19b‐mediated SOCS6‐JAK2/STAT3 pathway by lncRNA MEG3 is involved in high glucose‐induced apoptosis in hRMECs. Biosci Rep. 2020;40:BSR20194370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun Z, Liu Q, Hong H, Zhang H, Zhang T. miR‐19 promotes osteosarcoma progression by targeting SOCS6. Biochem Biophys Res Commun. 2018;495:1363‐1369. [DOI] [PubMed] [Google Scholar]
- 56. Tan J, Xiang L, Xu G. LncRNA MEG3 suppresses migration and promotes apoptosis by sponging miR‐548d‐3p to modulate JAK‐STAT pathway in oral squamous cell carcinoma. IUBMB Life. 2019;71:882‐890. [DOI] [PubMed] [Google Scholar]
- 57. Köberle V, Pleli T, Schmithals C, et al. Differential stability of cell‐free circulating microRNAs: implications for their utilization as biomarkers. PloS One. 2013;8:e75184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell. 2023;186:1610‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paskeh MDA, Entezari M, Mirzaei S, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matsumura T, Sugimachi K, Iinuma H, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan C, Stevic I, Müller V, et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12:1935‐1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Table S1.
Data Availability Statement
Please contact the corresponding author for all data requests.


