Understanding how land conversion affects immunity against pathogens in wildlife can inform conservation decisions and our understanding of pathogen dynamics in host communities. We found evidence that Neotropical bats invested differently in cellular immunity over years of land conversion, suggesting that investment in immune defenses varies by species and diet.
Keywords: bacterial pathogens, cellular immunity, Chiroptera, ecoimmunology, land conversion
Abstract
Monitoring the health of wildlife populations is essential in the face of increased agricultural expansion and forest fragmentation. Loss of habitat and habitat degradation can negatively affect an animal’s physiological state, possibly resulting in immunosuppression and increased morbidity or mortality. We sought to determine how land conversion may differentially impact cellular immunity and infection risk in Neotropical bats species regularly infected with bloodborne pathogens, and to evaluate how effects may vary over time and by dietary habit. We studied common vampire bats (Desmodus rotundus), northern yellow-shouldered bats (Sturnira parvidens) and Mesoamerican mustached bats (Pteronotus mesoamericanus), representing the dietary habits of sanguivory, frugivory and insectivory respectively, in northern Belize. We compared estimated total white blood cell count, leukocyte differentials, neutrophil to lymphocyte ratio and infection status with two bloodborne bacterial pathogens (Bartonella spp. and hemoplasmas) of 118 bats captured in a broadleaf, secondary forest over three years (2017–2019). During this period, tree cover decreased by 14.5% while rangeland expanded by 14.3%, indicating increasing habitat loss and fragmentation. We found evidence for bat species-specific responses of cellular immunity between years, with neutrophil counts significantly decreasing in S. parvidens from 2017 to 2018, but marginally increasing in D. rotundus. However, the odds of infection with Bartonella spp. and hemoplasmas between 2017 and 2019 did not differ between bat species, contrary to our prediction that pathogen prevalence may increase with land conversion. We conclude that each bat species invested differently in cellular immunity in ways that changed over years of increasing habitat loss and fragmentation. We recommend further research on the interactions between land conversion, immunity and infection across dietary habits of Neotropical bats for informed management and conservation.
Introduction
Agriculture expansion fragments and degrades landscapes, reducing suitable habitat available to many terrestrial mammals (Laurance et al., 2014; Crooks et al., 2017). Tropical areas worldwide are experiencing rapid human population growth and associated agriculture expansion, which is particularly concerning for conservation since the tropics support about two thirds of global biodiversity (Bradshaw et al., 2009; Antonelli, 2022). Impacts of land conversion on animal physiology, and ultimately on fitness, depend on phenotypic plasticity and how habitat loss and fragmentation affect specific aspects of an animal’s ecology. Generalists may be less likely to be affected by land conversion than specialists (Ramiadantsoa et al., 2018), and some species may be epigenetically primed to take advantage of changing landscapes (Kilvitis et al., 2017). Species with specialized niches, including highly specific shelter or dietary needs, are more likely to be affected by the physiological challenges associated with habitat loss and fragmentation, resulting in population declines in such species (Kosydar et al., 2014). Conversely, some species may be less affected by or even benefit from land conversion because of increased access to human-provisioned food resources in the form of crops or livestock (Oro et al., 2013). The dietary habit of a species can thus impact how habitat changes associated with land conversion affect individual physiology (Hinam and Clair, 2008). Reduced physiological condition, as a result of habitat loss and fragmentation, can result in immunodeficiency and increased morbidity (Villafuerte et al., 1997; Johnstone et al., 2012; Seltmann et al., 2017). Understanding how land conversion differentially affects the ability of species to mount immune defenses against pathogens can inform conservation and land management decisions and contribute significantly to our understanding of pathogen dynamics in complex host communities.
Land use changes have been linked to changes in immune phenotype and glucocorticoid concentration in birds and mammals (Messina et al., 2018). In some species, land conversion may cause immunosuppression and immunomodulation, resulting in increased susceptibility to infections in wildlife (Aguirre and Tabor, 2008; Belasen et al., 2019). When an individual is experiencing more frequent or intense stressors (Davis et al., 2008) or is parasitized (Hernandez et al., 2018), plasma glucocorticoid levels can increase, often impairing the host immune response (Sapolsky et al., 2000; Coutinho and Chapman, 2011). Comparing estimated total white blood cell counts (TWBC) and differential white blood cell counts (DWBC) in mammals under different environmental conditions (i.e. in areas with differential levels of land conversion) is a low-cost and tractable means for determining how environmental changes impact individual investment in cellular immune defenses (Schneeberger et al., 2013; Becker et al., 2018b). For example, increased glucocorticoid levels are associated with increased TWBC, elevated neutrophil to lymphocyte ratio (NL ratio) and eosinopenia across vertebrates (Jain, 1986; Davis et al., 2008). Further, immunocompromised hosts often experience increased susceptibility to infection, and acute bacterial infection in particular can increase TWBC counts and cause neutrophilia, lymphopenia and monocytosis in vertebrates (Jain, 1986; Davis et al., 2004; Davis et al., 2008). Thus, documenting changes in the leukocyte profile and infection status of wildlife over time can help evaluate how land conversion impacts host cellular immunity and pathogen spread.
The Neotropics contain the greatest diversity of bats globally, with species that span almost all possible mammal dietary habits (Fenton, 1992; Mickleburgh et al., 2002). Neotropical bats are important members of tropical forest ecosystems because of their role in seed dispersal, insect predation and pollination (Kunz et al., 2011). Bats are also notable for their potential to spread virulent pathogens to humans, livestock and other species (Chan et al., 2013; Allocati et al., 2016). Humans that live in or near fragmented forests in the tropics have greater exposure to bat-borne pathogens due to their proximity to bat hosts at ecotone roost sites (Rulli et al., 2017). Because of their great ecological diversity, Neotropical bats are a valuable system for studying effects of land conversion on diverse ecological interactions.
In this study, we used Neotropical bats as a system to ask how decreasing forest cover and increasing rangeland resulting from conversion affect cellular immunity and infection status of diverse host species, with the goal of testing how dietary habit impacts the immune response to land conversion. Specifically, we studied common vampire bats (Desmodus rotundus), northern yellow-shouldered bats (Sturnira parvidens) and Mesoamerican mustached bats (Pteronotus mesoamericanus). These species represent the dietary habits of sanguivores, frugivores and insectivores, respectively (Bobrowiec et al., 2015; Ingala et al., 2021). All three bats are broadly distributed in Central America and are routinely found in landscapes affected by land conversion (Kraker-Castañeda et al., 2016; Herrera et al., 2018; Alpízar et al., 2019; Brändel et al., 2020); however, their response to forest loss and fragmentation likely differs owing to their different dietary habits. Across its range, Desmodus rotundus capitalizes on domestic animal prey (especially cattle) in rangeland dominated landscapes, and this human-provisioned food source is preferentially selected over wildlife prey (Voigt and Kelm, 2006; Bobrowiec et al., 2015; Ingala et al., 2019). Therefore, we predict that the cellular immunity of D. rotundus would not be negatively impacted by land conversion (or even could benefit from livestock prey; Becker et al., 2018b). Sturnira parvidens is a frugivore that specializes in fruit from early successional plants, which are most abundant in fragmented and disturbed forests (Galindo-Gonzalez et al., 2000; García-Morales et al., 2012; Kraker-Castañeda et al., 2016). Prior work has found that S. parvidens is one of the most abundant bat species in at least some fragmented forests (Ramírez-Lucho et al., 2017; Herrera et al., 2018). In contrast, Pteronotus mesoamericanus may be more vulnerable to increasing habitat loss and fragmentation, as these bats forage for insects using echolocation in dense vegetation within forest interiors (Alpízar et al., 2019; Núñez et al., 2019). Fragmentation resulting from land conversion could thus reduce access to forest interiors for bat foraging (Núñez et al., 2019), which could function as a stressor that impairs P. mesoamericanus immunity.
The likelihood of a bat being exposed to pathogens and the mode of pathogen exposure can also depend on the ecological niche of a species (Schneeberger et al., 2013). In this study, we focused on how land conversion affects the prevalence of Bartonella spp. and hemotropic Mycoplasma spp. (i.e. hemoplasmas), which are common bacterial pathogens in Neotropical bats (Ikeda et al., 2017; Becker et al., 2018a; Becker et al., 2018b; Becker et al., 2020; Volokhov et al., 2023). Bartonella spp. are intraerythrocytic and are vectored by hematophagous arthropods, including bat ectoparasites, and may also be transmitted through blood, saliva, or feces to a variety of hosts (Jacomo et al., 2002). In humans, Bartonella spp. can cause a variety of diseases, including cat-scratch disease, endocarditis and Carrion's disease (Jacomo et al., 2002). Hemoplasmas are parasites of erythrocytes and are thought to be transmitted by direct contact and possibly also vectored by hematophagous arthropods, including bat ectoparasites (Messick, 2004; Cohen et al., 2018). Hemoplasmas can cause hemolytic anemia, arthritis, pneumonia, conjunctivitis, infertility, and other acute to chronic diseases in humans and other mammals (Messick, 2004; Descloux et al., 2021; Millán et al., 2021). Due to the potential of these pathogens to cause zoonotic infections, which can be life-threatening in humans, understanding how the likelihoods of infection with Bartonella spp. and hemoplasmas in bats are affected by land conversion is important to forecast or prevent future pathogen spillover. Here, we compared the leukocyte profiles and infection status of D. rotundus, S. parvidens and P. mesoamericanus inhabiting northern Belize over a three-year period of decreasing forest cover. We hypothesized that increased habitat loss and fragmentation would impact the species’ cellular immunity and infection status differentially, according to their dietary habit, with P. mesoamericanus experiencing the greatest changes in cellular immunity (i.e. increased TWBC counts, monocytosis, neutrophilia and lymphopenia) and infection status (i.e. increased odds of infection) and D. rotundus experiencing only minor changes in cellular immunity and infection status, due to their foraging ecology. Evaluating the immunological impacts of land conversion on these bat species is important not only to inform bat conservation efforts, but also to predict how future habitat loss can influence pathogen spread in bats and potentially to humans.
Materials and Methods
Bat capture and sampling
As part of broader ecological, immunological and epidemiological studies of bats in Belize (Herrera et al., 2018; Becker et al., 2020; Becker et al., 2021a; Becker et al., 2022), we sampled D. rotundus, S. parvidens and P. mesoamericanus during April to May 2017–2019 within the Lamanai Archaeological Reserve (LAR) of Orange Walk District, Belize (N 17.76343, W 88.65292). The LAR is a broadleaf, secondary protected forest near the New River Lagoon, for which the surrounding matrix is experiencing increasing forest loss and fragmentation as land is converted to cropland and cattle pastures (Herrera et al., 2018; Ingala et al., 2019, Figure 1). As described previously, we used mist nets and harp traps to capture bats along flight paths and occasionally at the exits of roosts from 19:00 hours until 22:00 hours (Becker et al., 2020; Becker et al., 2021b). Bats were kept in clean cloth bags prior to processing and were identified and sexed based on morphology (e.g. Reid, 1997). Between 3 and 30 μl of blood were sampled, based on bat body mass, by lancing the propatagial vein with a sterile needle (23–30G) and collected in a heparinized capillary tube. Thin blood smears were prepared on glass slides and stained with Wright–Giemsa (Astral Diagnostics Inc., Hematology Stain Set Quick III), and remaining blood was stored on Whatman FTA cards at room temperature. All bats for this study were released following sampling. Field procedures were performed according to guidelines for the safe and humane handling of bats published by of the American Society of Mammalogists (Sikes et al., 2016) and were approved by the Institutional Animal Care and Use Committees of the University of Georgia (A2014 04-016-Y3-A5) and American Museum of Natural History (AMNHIACUC-20170403, AMNHIACUC-20180123, AMNHIACUC-20190129). Fieldwork and sampling were authorized by the Belize Forest Department under permits WL/2/1/17(16), WL/2/1/17(19), WL/2/1/18(16) and FD/WL/1/19(09).
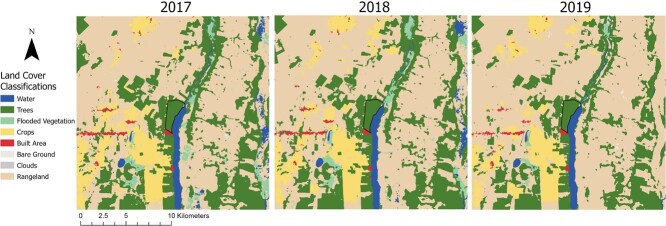
Figure 1.
Map of land cover change in northern Belize, within 10 km of the LAR, from 2017 to 2019. The 450-ha forest of the LAR is shown in black (Becker et al., 2020). Map and land use/land cover data were derived from the Sentinel-2 10 m land use/land cover time series of the world produced by Impact Observatory, Microsoft and Esri (Karra et al., 2021). This dataset is based on the data produced for the Dynamic World Project by National Geographic Society in partnership with Google and the World Resources Institute.
Land cover change quantification
Land cover data were derived from the Sentinel-2 10-m land use/land cover time series of the world produced by Impact Observatory, Microsoft and Esri (Karra et al., 2021) and imported into ArcGIS Pro (ESRI Inc., Redland, CA). Using the LAR as a central point, the resulting map was cropped to a 10 km extent, representing a conservative boundary of bat movement based on past estimates of Neotropical bat home ranges, including in this Belize study site (Fleming et al., 1972; Trajano, 1996; Fenton et al., 2000; Becker et al., 2021b). After masking the LAR, individual binary layers were created for each land cover type in the matrix on May 1 of each year of the study (2017, 2018 and 2019), where a value of 1 indicated pixels mapping to a single land cover classification and a value of 0 indicated pixels mapping to all other land cover classifications. As Sentinel-2 collects data every five days, May 1 was selected because it approximately marks the midpoint sampling period and because cloud-cover was minimal during each year. Total pixel counts for each land cover classification per year were plotted using the ggplot2 package in R (Wickham, 2016). The net change for each land cover classification was quantified by subtracting the 2019 binary layer from the 2017 binary layer. The number of pixels with a value of +1 and the number of pixels with a value of −1 in the resulting layer were each divided by the total number of pixels mapped to that land cover classification in 2017 to determine the percent gained and lost, respectively. The percent lost was subtracted by the percent gained to determine the net percent change.
White blood cell counts and statistical analyses
We estimated TWBC counts for each blood smear (n = 118) by averaging the number of leukocytes (neutrophils, lymphocytes, monocytes, eosinophils, basophils) under 10 random fields under 400X magnification (Schneeberger et al., 2013). DWBC counts were then estimated by identifying 100 leukocytes and recording the relative abundance of each type of white blood cell under 1000× (oil immersion) magnification. The absolute number of each white blood cell type was then determined by multiplying its relative abundance by the estimated TWBC count (Becker et al., 2021a), and we derived NL ratios. A subset of the hematology data from 2017 and 2018 were published previously (Cornelius Ruhs et al., 2021; Becker et al., 2021a).
We used generalized linear models (GLMs) to test how each of our cellular immunity measures (TWBC, absolute neutrophils, absolute lymphocytes, NL ratios, absolute monocytes, absolute eosinophils, and absolute basophils) varied among our three species across the three years. We fit separate GLMs with immunity predicted by year, bat species and their interaction (see Becker et al., 2020 for sex effects and other individual-level covariates), modeling each response with a Tweedie distribution (Dunn and Smyth, 2005). We used the mgcv package in R to fit Tweedie-distributed GLMs using maximum likelihood (Wood, 2006). We adjusted for the inflated false-discovery rate in post-hoc comparisons with the emmeans package (Benjamini and Hochberg, 1995).
Bartonella spp. and hemoplasma infection analyses
We expanded prior surveys of Bartonella spp. and hemoplasmas in Belize with analyses of paired blood samples from 2019 bats with blood smears (Volokhov et al., 2017; Becker et al., 2018a; Becker et al., 2020; Becker et al., 2021a; Volokhov et al., 2023). We extracted DNA from Whatman FTA cards using Qiagen QIAamp DNA Investigator Kits (Volokhov et al., 2017). We then used PCR and gel electrophoresis to determine the presence of Bartonella spp. (targeting the gltA gene) and hemoplasmas (targeting the 16S rRNA gene) with primers and procedures described previously (Volokhov et al., 2017; Becker et al., 2018a; Becker et al., 2020). The amplicons were directly sequenced by Sanger method and sequence analysis was performed for hemoplasmas to assess similarity to our previously established genotypes in Belize bats (see Supplemental Information; Becker et al., 2020).
Following our analyses of white blood cell data, we derived infection prevalence and 95% confidence intervals (Wilson interval) for each pathogen (and for co-infection) using the prevalence package. We then fit GLMs with a binomial distribution per pathogen, with infection status predicted by year, bat species and their interaction (see Becker et al., 2020 for effects of sex and other individual-level covariates on a larger sample size of Belize bat infection status). Because of low sample sizes for pathogen analyses in 2018, we limited this comparison to 2017 and 2019 (n = 89). To account for overall smaller sample sizes here, we used the brglm package to implement Firth’s bias reduction (Firth, 1993).
Results
Land cover change
The habitat matrix within 10 km of our study site was dominated by rangeland during every year of bat sampling, followed by trees and crops. Land cover change across years was characterized by an expansion of rangeland that coincided with a reduction of all other land cover classifications except for bare ground. Notably, tree cover was reduced by 14.5% from 2017 to 2019 while rangeland expanded by 14.3% (Figure 2).
Figure 2.
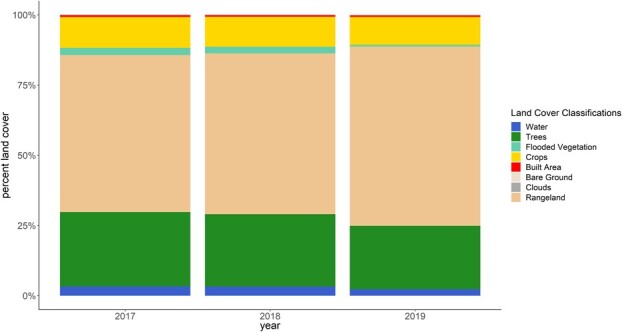
Change in land cover in the habitat matrix within 10 km of the LAR from 2017 to 2019.
Cellular immunity
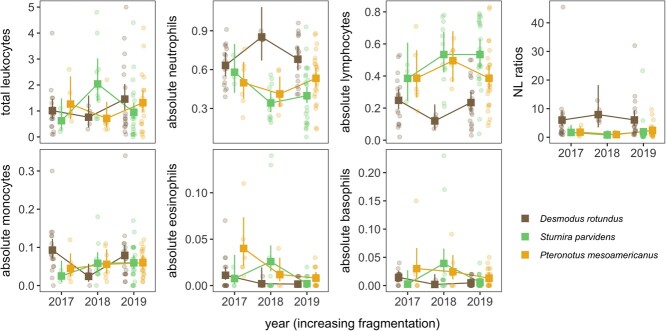
We estimated TWBC and DWBC counts from 42 D. rotundus, 40 S. parvidens and 36 P. mesoamericanus, in the LAR between 2017 and 2019. Our GLMs found generally strong support for species-specific responses of cellular immunity to year (species-by-year interaction: F4 = 1.16–3.91, p = 0.01–0.33; Table 1, Figure 3). For total leukocytes, the predicted means suggested TWBC of S. parvidens increased between 2017 and 2018 and decreased from 2018 to 2019 whereas TWBC of other species did not change over time, but these S. parvidens contrasts were not significant after adjusting for multiple comparisons (Supplementary Table S1 and Table 2). In contrast, although absolute neutrophil counts did not differ between species in 2017, temporal patterns varied among bats in subsequent years. Absolute neutrophil counts of S. parvidens declined from 2017 to 2018, whereas absolute neutrophil count marginally increased in D. rotundus between these same years (Supplementary Table S2 and Table 2). Absolute lymphocyte counts showed a weaker interactive effect of bat species and year (F4 = 2.04, p = 0.09), with S. parvidens and P. mesoamericanus generally having 1.8 and 2.3 times as many lymphocytes as D. rotundus (Supplementary Table S3 and Table 2). NL ratios did not vary by year (F4 = 1.16, p = 0.33), but both S. parvidens and P. mesoamericanus had lower NL ratios than D. rotundus (Supplementary Table S4 and Table 1). For absolute monocyte counts, predicted means suggested temporal variability in D. rotundus and little change for S. parvidens or P. mesoamericanus, but these contrasts were likewise not significant after adjusting for multiple comparisons (Supplementary Table S5 and Table 2). Absolute eosinophil counts demonstrated a significant species-by-year interaction, where these cells decreased from 2018 to 2019 in S. parvidens and marginally decreased in P. mesoamericanus between 2017 and 2018 (Supplementary Table S6 and Table 2). For absolute basophil counts, S. parvidens showed a marginal increase from 2017 to 2018 and a significant decrease from 2018 to 2019, while absolute basophil counts of other species did not change over time (Supplementary Table S7 and Table 2).
Table 1. Results of Tweedie-distributed GLMs predicting each cellular immunity measure by bat species, year and their interaction.
| Response | Predictor | F | p |
|---|---|---|---|
| Total leukocytes R2 = 0.05 | species | 0.86 | 0.43 |
| year | 1.78 | 0.17 | |
| species*year | 3.91 | <0.01 | |
| Absolute neutrophils R2 = 0.33 | species | 1.13 | 0.33 |
| year | 2.15 | 0.12 | |
| species*year | 3.34 | 0.01 | |
| Absolute lymphocytes R2 = 0.32 | species | 2.55 | 0.08 |
| year | 2.31 | 0.10 | |
| species*year | 2.04 | 0.09 | |
| NL ratios R2 = 0.08 | species | 5.38 | <0.01 |
| year | 0.18 | 0.84 | |
| species*year | 1.16 | 0.33 | |
| Absolute monocytes R2 = 0.05 | species | 4.95 | <0.01 |
| year | 4.26 | 0.02 | |
| species*year | 2.78 | 0.03 | |
| Absolute eosinophils R2 = 0.15 | species | 5.18 | <0.01 |
| year | 4.29 | 0.02 | |
| species*year | 2.81 | 0.03 | |
| Absolute basophils R2 = 0.06 | species | 2.37 | 0.10 |
| year | 3.06 | 0.05 | |
| species*year | 2.63 | 0.04 |
Figure 3.
Predicted means and 95% confidence intervals from Tweedie GLMs with interactive effects of bat species and year, for each cellular immunity measure. Raw data are overlaid and jittered.
Table 2. Post-hoc comparisons among years within each bat species for each cellular immunity measure. Results are subset from the full pairwise post-hoc analyses following adjustment for multiple comparisons (see Supplementary Tables S1–S7 for the complete list of comparisons).
| Response | Contrast | Ratio | SE | t | p |
|---|---|---|---|---|---|
| Total leukocytes | D. rotundus 2017/2018 | 1.33 | 0.56 | 0.68 | 0.67 |
| D. rotundus 2018/2019 | 0.52 | 0.22 | −1.57 | 0.36 | |
| S. parvidens 2017/2018 | 0.31 | 0.15 | −2.45 | 0.14 | |
| S. parvidens 2018/2019 | 2.15 | 0.56 | 2.94 | 0.12 | |
| P. mesoamericanus 2017/2018 | 1.77 | 0.80 | 1.27 | 0.39 | |
| P. mesoamericanus 2018/2019 | 0.54 | 0.20 | −1.7 | 0.33 | |
| Absolute neutrophils | D. rotundus 2017/2018 | 0.74 | 0.11 | −2.07 | 0.09 |
| D. rotundus 2018/2019 | 1.25 | 0.18 | 1.60 | 0.18 | |
| S. parvidens 2017/2018 | 1.69 | 0.34 | 2.60 | 0.03 | |
| S. parvidens 2018/2019 | 0.86 | 0.12 | −1.06 | 0.39 | |
| P. mesoamericanus 2017/2018 | 1.21 | 0.25 | 0.93 | 0.44 | |
| P. mesoamericanus 2018/2019 | 0.77 | 0.12 | −1.60 | 0.18 | |
| Absolute lymphocytes | D. rotundus 2017/2018 | 2.07 | 0.71 | 2.13 | 0.07 |
| D. rotundus 2018/2019 | 0.51 | 0.17 | −1.96 | 0.10 | |
| S. parvidens 2017/2018 | 0.72 | 0.19 | −1.25 | 0.28 | |
| S. parvidens 2018/2019 | 1.00 | 0.15 | 0.00 | 1.00 | |
| P. mesoamericanus 2017/2018 | 0.78 | 0.19 | −1.00 | 0.41 | |
| P. mesoamericanus 2018/2019 | 1.28 | 0.24 | 1.33 | 0.26 | |
| NL ratios | D. rotundus 2017/2018 | 2.07 | 0.71 | 2.13 | 0.07 |
| D. rotundus 2018/2019 | 0.51 | 0.17 | −1.96 | 0.10 | |
| S. parvidens 2017/2018 | 0.72 | 0.19 | −1.25 | 0.28 | |
| S. parvidens 2018/2019 | 1.00 | 0.15 | 0.00 | 1.00 | |
| P. mesoamericanus 2017/2018 | 0.72 | 0.16 | −1.44 | 0.23 | |
| P. mesoamericanus 2018/2019 | 1.28 | 0.24 | 1.33 | 0.26 | |
| Absolute monocytes | D. rotundus 2017/2018 | 3.87 | 1.79 | 2.91 | 0.14 |
| D. rotundus 2018/2019 | 0.30 | 0.14 | −2.56 | 0.14 | |
| S. parvidens 2017/2018 | 0.43 | 0.23 | −1.60 | 0.24 | |
| S. parvidens 2018/2019 | 0.98 | 0.25 | −0.08 | 0.98 | |
| P. mesoamericanus 2017/2018 | 0.81 | 0.34 | −0.50 | 0.77 | |
| P. mesoamericanus 2018/2019 | 0.93 | 0.29 | −0.23 | 0.96 | |
| Absolute eosinophils | D. rotundus 2017/2018 | 5.56 | 6.68 | 1.43 | 0.25 |
| D. rotundus 2018/2019 | 1.27 | 1.69 | 0.18 | 0.94 | |
| S. parvidens 2017/2018 | 0.29 | 0.23 | −1.54 | 0.23 | |
| S. parvidens 2018/2019 | 12.4 | 7.25 | 4.31 | <0.01 | |
| P. mesoamericanus 2017/2018 | 3.37 | 1.91 | 2.14 | 0.08 | |
| P. mesoamericanus 2018/2019 | 1.47 | 0.83 | 0.69 | 0.61 |
(Continued)
Bloodborne pathogen infections
For the 89 bats screened for bacterial infections in 2017 and 2019 (37 D. rotundus, 28 S. parvidens, 29 P. mesoamericanus), 69% were positive for Bartonella spp. (CI: 58–77%), 64% were positive for hemoplasmas (CI: 54–73%) and 45% had coinfections (CI: 35–55%). Our GLMs revealed no effects of species, year, or their interaction on the probability of infection for either Bartonella spp. or hemoplasmas (Table 3). Such results thereby suggest little shift in these potentially chronic infections over time on a per-species basis (Figure 4), although such conclusions may be limited by the smaller sample sizes here.
Table 3. Results of binomial GLMs predicting Bartonella spp. or hemoplasma infection status by bat species, year and their interaction. Reference levels are D. rotundus for bat species and 2017 for year.
| Response | Coefficient | OR | z | p |
|---|---|---|---|---|
| Bartonella spp. R2 = 0.09 | intercept | 3.22 | 2.11 | 0.03 |
| P. mesoamericanus | 1.14 | 0.11 | 0.91 | |
| S. parvidens | 2.79 | 0.58 | 0.56 | |
| 2019 | 0.64 | −0.59 | 0.55 | |
| P. mesoamericanus: 2019 | 1.19 | 0.13 | 0.90 | |
| S. parvidens: 2019 | 0.14 | −1.03 | 0.30 | |
| Hemoplasmas R2 = 0.06 | intercept | 1.00 | 0.00 | 1.00 |
| P. mesoamericanus | 1.80 | 0.60 | 0.55 | |
| S. parvidens | 1.00 | 0.00 | 1.00 | |
| 2019 | 1.35 | 0.46 | 0.65 | |
| P. mesoamericanus: 2019 | 0.74 | −0.26 | 0.80 | |
| S. parvidens: 2019 | 3.03 | 0.85 | 0.40 |
Figure 4.
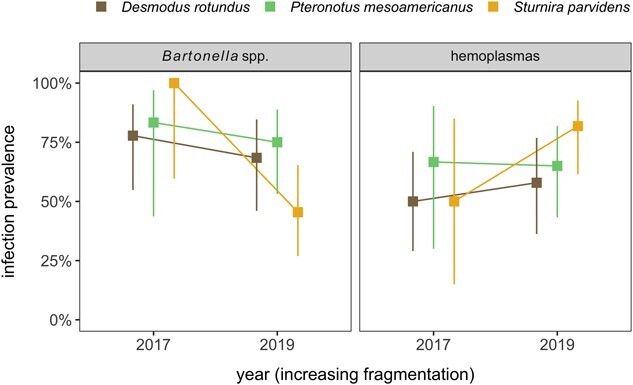
Prevalence of Bartonella spp. and hemoplasma infection alongside 95% confidence intervals (Wilson’s interval) stratified by bat species and year.
Sequencing of hemoplasma positives from 2019 updated a long-term dataset of the diversity of these pathogens in our study area (Volokhov et al., 2017; Becker et al., 2020; Volokhov et al., 2023; see Supplemental Materials for more information). We confirmed previously established genotypes in each of our three species (D. rotundus: VBG1, VBG2, VBG3; S. parvidens: SP1 groups A–C; P. mesoamericanus: PPM1). One S. parvidens had a novel genotype (SP2, GenBank accession OQ308927) showing 97% similarity to the APH3 genotype, observed only in Artibeus intermedius (Becker et al., 2020). P. mesoamericanus was also infected with the SP1 genotype and with new Pteronotus-specific genotype (PPM2, GenBank accession OQ308895; 97% similar to VBG1). Lastly, P. mesoamericanus was also infected by a novel non-hemotropic Mycoplasma, the M. moatsii–like genotype 4 (GenBank accession OQ308889). All 16S rRNA sequence data are available on GenBank through accession OQ308885-7, OQ308889, OQ308893, OQ308895-97, OQ308899-900, OQ308902-12, OQ308914-19, OQ308923-24, OQ308926-27, OQ533048, OQ546518-19, OQ546527-28, OQ546533, OQ546547, OQ546552, and OQ546555.
Discussion
By examining changes in leukocyte profiles and bloodborne pathogen (Bartonella spp. and hemoplasmas) prevalence in bat species representing distinct dietary habits over years of increasing habitat loss and fragmentation, we expanded our understanding of how anthropogenic habitat modification may differentially affect immune phenotype and infection prevalence of bats. Between 2017 and 2019, during a period of land conversion characterized by a reduction in tree cover and an expansion of rangeland, D. rotundus, S. parvidens and P. mesoamericanus exhibited differences in cellular immune defenses. These findings provide initial support for our prediction that hosts belonging to distinct dietary habits are differentially affected by a changing landscape over time and that these impacts manifest in changes to immune strategy. However, within this sample of bats, we found that Bartonella spp. and hemoplasma infection risk did not deviate among years or species, which is consistent with the findings that there were no significant changes in hemoplasma infection prevalence from 2017 to 2018 in D. rotundus (Becker et al., 2020). Interestingly, temporal and dietary differences in bat cellular immunity did not translate into variation in Bartonella spp. nor hemoplasma risk, at least not over the time scale of this study.
Hosts invest differently in their immune systems based on the varied costs associated with innate and adaptive immunity (Chaplin, 2010). Although investing more energy into adaptive immunity may be more energetically costly during development, innate immunity can also be costly to maintain later in life when an individual acquires a pathogen (Klasing, 2004; McDade et al., 2016). Among other factors, life history traits (Lochmiller and Deerenberg, 2000), lifespan (Previtali et al., 2012) and resource availability (Becker et al., 2018b) can influence immune investment. Thus, it is likely that species of bats with different ecological niches will show distinct immune investment strategies. For example, a study of the bat community at our study site previously found species-specific differences in the relationship between mercury and cellular immunity, wherein bats that rely on aquatic prey and bats in agricultural habitats had higher mercury levels and neutropenia as compared to bats of other ecological niches (Becker et al., 2021a). Therefore, the distinct ecological requirements of D. rotundus, S. parvidens and P. mesoamericanus may drive species-level variation in how these bats invest in immunity (and in turn how this is affected by land conversion over time).
Temporal patterns of immune investment, as measured by leukocyte profiles, varied among bat species, which could at least partially arise from differences in dietary habits. Because diet will likely impact the probability of acquiring a pathogen (Han et al., 2021) and can also directly shape immune phenotypes (Schneeberger et al., 2013), bat dietary habits will likely affect immune changes over time. Differences in dietary habits may explain why neutrophil counts declined in S. parvidens between 2017 and 2018, while neutrophil counts marginally increased in D. rotundus. If S. parvidens encounters fewer pathogens when feeding on contaminated fruit, it may be energetically favorable for them to invest less in innate immunity (i.e. decreased neutrophil count). Conversely, if D. rotundus encounters more pathogens when feeding on infected blood, it may be beneficial for them to increase absolute neutrophil counts to avoid mounting an energetically costly immune response during periods of increased stress, such as what might be caused by habitat degradation, roost disturbance or forest fragmentation (Read and Allen, 2000; Allen et al., 2008). However, NL ratios did not vary among years for any of the species, suggesting that it is unlikely that increased glucocorticoid levels play a major role in changing leukocyte counts over time. Similarly, dietary habits may also explain why eosinophil counts decreased in S. parvidens from 2018 to 2019, but did not change significantly in D. rotundus or P. mesoamericanus, in the event that frugivorous feeding behaviours are linked to ingesting the eggs or larvae of helminths, leading to variable helminth infections (which are often characterized by increased eosinophil counts; Huang and Appleton, 2016).
Table 2. Continued.
| Response | Contrast | Ratio | SE | t | p |
|---|---|---|---|---|---|
| Absolute basophils | D. rotundus 2017/2018 | 7.22 | 8.77 | 1.63 | 0.19 |
| D. rotundus 2018/2019 | 0.42 | 0.53 | −0.69 | 0.61 | |
| S. parvidens 2017/2018 | 0.06 | 0.08 | −2.22 | 0.10 | |
| S. parvidens 2018/2019 | 6.71 | 3.01 | 4.24 | <0.01 | |
| P. mesoamericanus 2017/2018 | 1.23 | 0.70 | 0.36 | 0.76 | |
| P. mesoamericanus 2018/2019 | 1.87 | 0.92 | 1.27 | 0.30 |
Other factors that may influence patterns observed in bat leukocyte profiles among species include sociality and roosting behaviour (Calisher et al., 2006; Mühldorfer et al., 2011). Both D. rotundus and P. mesoamericanus are highly social species that live in colonies of a few thousand individuals (Wilkinson, 1985; Wilkinson, 1986; Santana et al., 2011; Clement and Kanwal, 2012; Becker et al., 2020), while S. parvidens roost alone or in groups of up to 10 individuals (Fenton et al., 2000; Evelyn and Stiles, 2003). Highly social species, such as D. rotundus and P. mesoamericanus, may experience greater pathogen risk compared to less social species, such as S. parvidens (Stanko et al., 2002; Webber and Willis, 2016). Moreover, roosts that are permanent and protected from precipitation, such as caves, have increased infection risk compared to more ephemeral or less protected roosts, such as tree cavities (Patterson et al., 2007). Therefore, species, such as D. rotundus and P. mesoamericanus, which roost more commonly in caves, could have increased infection risk compared to bats that only inhabit caves rarely and only at night for brief periods (e.g. S. parvidens; Sapey, 2019). Variation in infection risk because of sociality, roosting behaviour and/or other factors is likely to affect individual investment in cellular immunity over time and contribute to the species-specific responses of cellular immunity we observed (Patterson et al., 2007; Allen et al., 2008; Schneeberger et al., 2013). The presence of other bacterial or protozoan infections may be responsible for neutrophilia and/or lymphopenia (Wyllie et al., 2004; Liew and Kubes, 2019); however, we cannot confirm this without data on other infectious agents.
Our findings suggest different species of bats experience changes in cellular immune defenses over time, some of which may be linked to habitat loss and fragmentation from land conversion. However, we did not find evidence for these changes being associated with increased risk of pathogen infection for the pathogens quantified. Absence of significant changes in infection may be attributed to similar rates of pathogen exposure over time and/or low levels of Bartonella spp. and hemoplasmas in the blood. Additionally, the ability to feed on diverse prey (D. rotundus) or select early-successional habitats for preferred fruit (S. parvidens) may buffer these bats from stressors associated with habitat loss and fragmentation. Although the matrix beyond the LAR is being converted to rangeland overtime (Herrera et al., 2018; Ingala et al., 2019, Figure. 1), our study site remains relatively large and contains mature trees and caves for roosting. If prey availability, caves and mature forests persist in the LAR, bats that rely on these dietary and shelter niches may be able to persist without experiencing increased pathogen exposure from habitat reduction.
Although our findings suggest that our select bat species invested differently in immune defenses and that temporal patterns of immune investment varied among species, further research is needed to establish the role of dietary habits in shaping such trends. We selected one representative species per dietary habit for this analysis, but replication is needed to better establish the role of foraging ecology relative to bat taxonomy and other species traits. For example, roosting behaviour, body size, sociality, and preferred habitat type are other factors that may influence how land use changes affect bats and their immunity (Schneeberger et al., 2013; Ávila-Gómez et al., 2015). Comparisons of other frugivorous and insectivorous species, across periods of land conversion would be highly informative, given the rarity of sanguivory among bats. Similarly, our single included insectivore (P. mesoamericanus) belongs to the sister family (Mormoopidae) of the other two species (members of Phyllostomidae); inclusion of insectivorous or nectarivorous and/or omnivorous phyllostomids would facilitate more comprehensive comparisons within a clade.
Due to the important ecological roles played by bats in Neotropical ecosystems (e.g. seed dispersal, pollination), understanding how land conversion impacts bat immunity is critical to conservation of both species and ecosystem function. Frugivorous bats, such as S. parvidens, play essential roles in forest regeneration in Neotropical ecosystems (Mello et al., 2008), while insectivorous bats, such as P. mesoamericanus, are important predators of insects (Alpízar et al., 2019; Núñez et al., 2019), including families that are considered agricultural pests (Ingala et al., 2021). Likewise, the preservation of mature trees (the preferred roosting habitat of S. parvidens), is essential for the conservation of S. parvidens (Galindo-Gonzalez et al., 2000; Evelyn and Stiles, 2003). When land conversion reduces access to mature trees, the physiological condition of these bats could suffer, and consequently, result in reduced immunocompetence. Similarly, P. mesoamericanus requires dense forest interiors to forage for insects, which makes them particularly vulnerable to the immunological stressors of major habitat loss and fragmentation (Alpízar et al., 2019; Núñez et al., 2019). In terms of conservation, while S. parvidens and P. mesoamericanus are of Least Concern status according to the IUCN (Solari, 2016; Solari, 2019), they are susceptible to extirpation in landscapes void of dense, mature forest. Thus, we suggest monitoring these species in areas experiencing increasing land conversion (Galindo-Gonzalez et al., 2000; Evelyn and Stiles, 2003; Hernández-Canchola and León-Paniagua, 2020). Lastly, understanding how habitat modification impacts the immunity of D. rotundus is important for monitoring rabies virus transmission and control strategies throughout the Neotropics (Becker et al., 2021b).
In sum, our study provides evidence that D. rotundus, S. parvidens and P. mesoamericanus invested differently in cellular immunity in ways that changed over time, suggesting that investment in immune defenses varies by species and dietary habit. Despite this, we found no evidence that Bartonella spp. or hemoplasma infection risk in these bats changed over time, although comparisons were limited by small sample sizes and the short time span of our study. Comparisons of infection prevalence of bats between habitat undergoing rapid land conversion and habitat remaining intact should be made to determine the role of habitat degradation in shaping infection risk. Studying the immunological effects of land use changes on Neotropical species is important for wildlife management, disease management and conservation. Continual monitoring of the bat species included in this study is recommended as their habitat becomes increasingly fragmented.
Supplementary Material
Acknowledgements
For assistance with field logistics, bat sampling and permits, we thank Mark Howells, Neil Duncan, and staff of the Lamanai Field Research Center, as well as many colleagues who helped net bats during 2017–2019 research in Belize. We also thank Eaqan Chaudhry and Konstantin Chumakov for laboratory support as well as two anonymous reviewers for their helpful feedback on this manuscript.
Contributor Information
Isabella K DeAnglis, Department of Environmental Biology, SUNY College of Environmental Science and Forestry, 1 Forestry Drive, Syracuse, NY, 13210, USA; Department of Biological Sciences, University of Arkansas, 1 University of Arkansas, Fayetteville, AR, 72701, USA.
Benjamin R Andrews, Department of Environmental Biology, SUNY College of Environmental Science and Forestry, 1 Forestry Drive, Syracuse, NY, 13210, USA.
Lauren R Lock, School of Biological Sciences, University of Oklahoma, 730 Van Vleet Oval, Norman, OK, 73019, USA.
Kristin E Dyer, School of Biological Sciences, University of Oklahoma, 730 Van Vleet Oval, Norman, OK, 73019, USA.
Anni Yang, Department of Geography and Environmental Sustainability, University of Oklahoma, 100 East Boyd St, Norman, OK, 73019, USA.
Dmitriy V Volokhov, Center for Biologics Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Avenue, Silver Spring, MD, 20993, USA.
M Brock Fenton, Department of Biology, University of Western Ontario, 1151 Richmond Street, London, Ontario, N6A 3K7, Canada.
Nancy B Simmons, Department of Mammalogy, Division of Vertebrate Zoology, American Museum of Natural History, 200 Central Park West, New York, NY, 10024, USA.
Cynthia J Downs, Department of Environmental Biology, SUNY College of Environmental Science and Forestry, 1 Forestry Drive, Syracuse, NY, 13210, USA.
Daniel J Becker, School of Biological Sciences, University of Oklahoma, 730 Van Vleet Oval, Norman, OK, 73019, USA.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: Daniel J. Becker, Cynthia J. Downs, Isabella K. DeAnglis, Benjamin R. Andrews, M. Brock Fenton, Nancy B. Simmons; field data collection: Daniel J. Becker, Nancy B. Simmons, M. Brock Fenton; other data collection: Daniel J. Becker, Dmitriy V. Volokhov, Cynthia J. Downs, Isabella K. DeAnglis, Benjamin R. Andrews, Lauren R. Lock, Kristin E. Dyer; analysis and interpretation of results: Isabella K. DeAnglis, Daniel J. Becker, Cynthia J. Downs, Dmitriy V. Volokhov, Lauren R. Lock, Anni Yang; draft manuscript preparation: Isabella K. DeAnglis, Daniel J. Becker, Cynthia J. Downs, Lauren R. Lock, Kristin E. Dyer, Anni Yang, Dmitriy V. Volokhov, Nancy B. Simmons, Benjamin R. Andrews. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
Funding
This work was supported by the National Science Foundation (IOS 1656551, DEB 1601052), ARCS Foundation, American Museum of Natural History (Theodore Roosevelt Memorial Fund, Taxonomic Mammalogy Fund), the SUNY-ESF Honors Program, National Geographic Society (NGS-55503R-19), and the Research Corporation for Science Advancement (RCSA). Financial support was also provided by the University of Oklahoma Libraries’ Open Access Fund. This work was conducted as part of Subaward No. 28365, part of a USDA Non-Assistance Cooperative Agreement with RCSA Federal Award No. 58–3022–0-005.
Data availability
All 16S rRNA sequence data are available on GenBank through accession OQ308885–7, OQ308889, OQ308893, OQ308895–97, OQ308899–900, OQ308902–12, OQ308914–19, OQ308923–24, OQ308926–27, OQ533048, OQ546518–19, OQ546527–28, OQ546533, OQ546547, OQ546552 and OQ546555.
Supplementary Material
Supplementary material is available at Conservation Physiology online.
References
- Aguirre AA, Tabor GM (2008) Global factors driving emerging infectious diseases. Ann N Y Acad Sci 1149: 1–3. 10.1196/annals.1428.052. [DOI] [PubMed] [Google Scholar]
- Allen LC, Turmelle AS, Mendonça MT, Navara KJ, Kunz TH, McCracken GF (2008) Roosting ecology and variation in adaptive and innate immune system function in the Brazilian free-tailed bat (Tadarida brasiliensis). J Comp Physiol 179: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocati N, Petrucci AG, Di Giovanni P, Masulli M, Di Ilio C, De Laurenzi V (2016) Bat–man disease transmission: zoonotic pathogens from wildlife reservoirs to human populations. Cell Death Discov 2: 16048. 10.1038/cddiscovery.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpízar P, Rodríguez-Herrera B, Jung K (2019) The effect of local land use on aerial insectivorous bats (Chiroptera) within the two dominating crop types in the northern-Caribbean lowlands of Costa Rica. PloS One 14: 1. 10.1371/journal.pone.0210364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli A (2022) The rise and fall of Neotropical biodiversity. Bot J Linn Soc 199: 8–24. [Google Scholar]
- Ávila-Gómez ES, Moreno CE, García-Morales R, Zuria I, Sánchez-Rojas G, Briones-Salas M (2015) Deforestation thresholds for Phyllostomid bat populations in tropical landscapes in the Huasteca region, Mexico. Trop Conserv Sci 8: 646–661. 10.1177/194008291500800305. [DOI] [Google Scholar]
- Becker DJ, Bergner LM, Bentz AB, Orton RJ, Altizer S, Streicker DG (2018a) Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. in vampire bats. PLoS Negl Trop Dis 12: 9. 10.1371/journal.pntd.0006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Broos A, Bergner LM, Meza DK, Simmons NB, Fenton MB, Altizer S, Streicker DG (2021b) Temporal patterns of vampire bat rabies and host connectivity in Belize. Transbound Emerg Dis 68: 870–879. 10.1111/tbed.13754. [DOI] [Google Scholar]
- Becker DJ, Czirják GÁ, Volokhov DV, Bentz AB, Carrera JE, Camus MS, Streicker DG (2018b) Livestock abundance predicts vampire bat demography, immune profiles and bacterial infection risk. Philos Trans R Soc Lond B Biol Sci 373: 20170089. 10.1098/rstb.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Lei GS, Janech MG, Bland AM, Fenton MB, Simmons NB, Relich RF, Neely BA (2022) Serum proteomics identifies immune pathways and candidate biomarkers of coronavirus infection in wild vampire bats. FrontVirol 2022: 20. [Google Scholar]
- Becker DJ, Speer KA, Brown AM, Fenton MB, Washburne AD, Altizer S, Streicker DG, Plowright RK, Chizhikov VE, Simmons NBet al. (2020) Ecological and evolutionary drivers of haemoplasma infection and bacterial genotype sharing in a Neotropical bat community. Mol Ecol 29: 1534–1549. 10.1111/mec.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Speer KA, Korstian JM, Volokhov DV, Droke HF, Brown AM, Baijnauth CL, Padgett-Stewart T, Broders HG, Plowright RKet al. (2021a) Disentangling interactions among mercury, immunity and infection in a Neotropical bat community. J Appl Ecol 58: 879–889. 10.1111/1365-2664.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasen AM, Bletz MC, Leite DD, Toledo LF, James TY (2019) Long-term habitat fragmentation is associated with reduced MHC IIB diversity and increased infections in amphibian hosts. Front Ecol Evol 6. 10.3389/fevo.2018.00236. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bobrowiec PE, Lemes MR, Gribel R (2015) Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. J Mammal 96: 54–63. [Google Scholar]
- Bradshaw CJ, Sodhi NS, Brook BW (2009) Tropical turmoil: a biodiversity tragedy in progress. Front Ecol Environ 7: 79–87. 10.1890/070193. [DOI] [Google Scholar]
- Brändel SD, Hiller T, Halczok TK, Kerth G, Page RA, Tschapka M (2020) Consequences of fragmentation for Neotropical bats: the importance of the matrix. Biol Conserv 252: 108792. 10.1016/j.biocon.2020.108792. [DOI] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19: 531–545. 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JFW, To KKW, Tse H, Jin DY, Yuen KY (2013) Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol 21: 544–555. 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD (2010) Overview of the immune response. J Allergy Clin Immunol 125: S3–S23. 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement MJ, Kanwal JS (2012) Simple syllabic calls accompany discrete behavior patterns in captive Pteronotus parnellii: an illustration of the motivation-structure hypothesis. Sci World J 2012: 1–15. 10.1100/2012/128695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Shemesh M, Garrido M, Messika I, Einav M, Khokhlova I, Tasker S, Hawlena H (2018) Haemoplasmas in wild rodents: routes of transmission and infection dynamics. Mol Ecol 27: 3714–3726. 10.1111/mec.14826. [DOI] [PubMed] [Google Scholar]
- Cornelius Ruhs E, Becker DJ, Oakey SJ, Ogunsina O, Fenton MB, Simmons NB, Martin LB, Downs CJ (2021) Body size affects immune cell proportions in birds and non-volant mammals, but not bats. J Exp Biol 1;224: jeb241109. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335: 2–13. 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks KR, Burdett CL, Theobald DM, King SR, Marco MD, Rondinini C, Boitani L (2017) Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc Natl Acad Sci 114: 7635–7640. 10.1073/pnas.1705769114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Cook KC, Altizer S (2004) Leukocyte profiles in wild house finches with and without Mycoplasmal conjunctivitis, a recently emerged bacterial disease. Ecohealth 1: 362–373. 10.1007/s10393-004-0134-2. [DOI] [Google Scholar]
- Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22: 760–772. 10.1111/j.1365-2435.2008.01467.x. [DOI] [Google Scholar]
- Descloux E, Mediannikov O, Gourinat AC, Colot J, Chauvet M, Mermoud I, Desoutter D, Cazorla C, Klement-Frutos E, Antonini Let al. (2021) Flying fox hemolytic fever, description of a new zoonosis caused by Candidatus mycoplasma haemohominis. Clin Infect Dis 73: e1445–e1453. 10.1093/cid/ciaa1648. [DOI] [PubMed] [Google Scholar]
- Dunn PK, Smyth GK (2005) Series evaluation of Tweedie exponential dispersion model densities. Stat Comput 15: 267–280. 10.1007/s11222-005-4070-y. [DOI] [Google Scholar]
- Evelyn MJ, Stiles DA (2003) Roosting requirements of two Frugivorous bats (Sturnira lilium and Arbiteus intermedius) in fragmented Neotropical Forest. Biotropica 35: 405–418. 10.1111/j.1744-7429.2003.tb00594.x. [DOI] [Google Scholar]
- Fenton MB (1992) Bats. Facts on file, New York [Google Scholar]
- Fenton MB, Vonhof MJ, Bouchard S, Gill SA, Johnston DS, Reid FA, Riskin DK, Standing KL, Taylor JR, Wagner R (2000) Roosts used by Sturnira lilium (Chiroptera: Phyllostomidae) in Belize. Biotropica 32: 729–733. 10.1111/j.1744-7429.2000.tb00521.x. [DOI] [Google Scholar]
- Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80: 27–38. 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- Fleming TH, Hooper ET, Wilson DE (1972) Three central American bat communities: structure, reproductive cycles, and movement patterns. J Ecol 53: 555–569. 10.2307/1934771. [DOI] [Google Scholar]
- Galindo-Gonzalez J, Guevara S, Sosa VJ (2000) Bat- and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conserv Biol 14: 1693–1703. 10.1111/j.1523-1739.2000.99072.x. [DOI] [PubMed] [Google Scholar]
- García-Morales R, Chapa-Vargas L, Galindo-González J, Badano EI (2012) Seed dispersal among three different vegetation communities in the Huasteca region, Mexico, analyzed from bat feces. Acta Chiropt 14: 357. 10.3161/150811012X661675. [DOI] [Google Scholar]
- Han BA, Castellanos AA, Schmidt JP, Fischhoff IR, Drake JM (2021) The ecology of zoonotic parasites in the Carnivora. Trends Parasitol 37: 1096–1110. 10.1016/j.pt.2021.08.006. [DOI] [PubMed] [Google Scholar]
- Hernandez SE, Strona ALS, Leiner NO, Suzán G, Romano MC (2018) Seasonal changes of faecal cortisol metabolite levels in Gracilinanus agilis (Didelphimorphia: Didelphidae) and its association to life histories variables and parasite loads. Conserv Physiol 6: 1. 10.1093/conphys/coy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Canchola G, León-Paniagua L (2020) Sturnira parvidens (Chiroptera: Phyllostomidae). Mamm Species 52: 57–70. 10.1093/mspecies/seaa005. [DOI] [PubMed] [Google Scholar]
- Herrera JP, Duncan N, Clare E, Fenton MB, Simmons N (2018) Disassembly of fragmented bat communities in Orange walk district, Belize. Acta Chiropterologica 20: 147–159. 10.3161/15081109ACC2018.20.1.011. [DOI] [Google Scholar]
- Hinam HL, Clair CCS (2008) High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of northern saw-whet owls. Biol Conserv 141: 524–535. 10.1016/j.biocon.2007.11.011. [DOI] [Google Scholar]
- Huang L, Appleton JA (2016) Eosinophils in Helminth infection: defenders and dupes. Trends Parasitol 32: 798–807. 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda P, Seki MC, Carrasco AO, Rudiak LV, Miranda JM, Gonçalves SM, Hoppe EG, Albuquerque AC, Teixeira MM, Passos CEet al. (2017) Evidence and molecular characterization of Bartonella spp. and hemoplasmas in neotropical bats in Brazil. Epidemiol Infect 145: 2038–2052. 10.1017/S0950268817000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingala MR, Becker DJ, Bak Holm J, Kristiansen K, Simmons NB (2019) Habitat fragmentation is associated with dietary shifts and microbiota variability in common vampire bats. Ecol Evol 9: 6508–6523. 10.1002/ece3.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingala MR, Simmons NB, Wultsch C, Krampis K, Provost KL, Perkins SL (2021) Molecular diet analysis of neotropical bats based on fecal DNA metabarcoding. Ecol Evol 11: 7474–7491. 10.1002/ece3.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomo V, Kelly PJ, Raoult D (2002) Natural history of Bartonella infections (an exception to Koch’s postulate). Clin Vaccine Immunol 9: 8–18. 10.1128/CDLI.9.1.8-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NC (1986) Schalm's Veterinary Hematology. Lea and Febiger, Philadelphia, PA [Google Scholar]
- Johnstone CP, Lill A, Reina RD (2012) Does habitat fragmentation cause stress in the agile antechinus? A haematological approach. J Comp Physiol 182: 139–155. 10.1007/s00360-011-0598-7. [DOI] [PubMed] [Google Scholar]
- Karra K, Kontgis C, Statman-Weil Z, Mazzariello JC, Mathis M, Brumby SP (2021) Global land use/land cover with sentinel 2 and deep learning. 2021 IEEE international geoscience and remote sensing symposium IGARSS 4704–4707. [Google Scholar]
- Kilvitis HJ, Hanson H, Schrey AW, Martin LB (2017) Epigenetic potential as a mechanism of phenotypic plasticity in vertebrate range expansions. Integr Comp Biol 57: 385–395. 10.1093/icb/icx082. [DOI] [PubMed] [Google Scholar]
- Klasing KC (2004) The costs of immunity. Acta Zool Sin 50: 961–969. [Google Scholar]
- Kosydar AJ, Conquest LL, Tewksbury JJ (2014) Can life histories predict the effects of habitat fragmentation? A meta - analysis with terrestrial mammals. Appl Ecol Environ Res 12: 505–521. 10.15666/aeer/1202_505521. [DOI] [Google Scholar]
- Kraker-Castañeda C, Cajas-Castillo JO, Lou S (2016) Opportunistic feeding by the little yellow- shouldered bat Sturnira lilium (Phyllostomidae, Stenodermatinae) in northern Guatemala: a comparative approach. Mammalia 80: 349–352. 10.1515/mammalia-2014-0139. [DOI] [Google Scholar]
- Kunz TH, Torrez EB, Bauer D, Lobova T, Fleming TH (2011) Ecosystem services provided by bats. Ann N Y Acad Sci 1223: 1–38. 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29: 107–116. 10.1016/j.tree.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Liew PX, Kubes P (2019) The Neutrophil’s role during health and disease. Physiol Rev 99: 1223–1248. 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88: 87–98. 10.1034/j.1600-0706.2000.880110.x. [DOI] [Google Scholar]
- McDade TW, Georgiev AV, Kuzawa CW (2016) Trade-offs between acquired and innate immune defenses in humans. Evol Med Public Health 1: 1–16. 10.1093/emph/eov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello MAR, Kalko EKV, Silva WR (2008) Movements of the bat Sturnira lilium and its role as a seed disperser of Solanaceae in the Brazilian Atlantic forest. J Trop Ecol 24: 225–228. 10.1017/S026646740800480X. [DOI] [Google Scholar]
- Messick JB (2004) Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet Clin Pathol 33: 2–13. 10.1111/j.1939-165X.2004.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Messina S, Edwards DP, Eens M, Costantini D (2018) Physiological and immunological responses of birds and mammals to forest degradation: a meta-analysis. Biol Conserv 224: 223–229. 10.1016/j.biocon.2018.06.002. [DOI] [Google Scholar]
- Mickleburgh SP, Hutson AM, Racey PA (2002) A review of the global conservation status of bats. Oryx 36: 18–34. [Google Scholar]
- Millán J, Di Cataldo S, Volokhov DV, Becker DJ (2021) Worldwide occurrence of haemoplasmas in wildlife: insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis 68: 3236–3256. 10.1111/tbed.13932. [DOI] [PubMed] [Google Scholar]
- Mühldorfer K, Speck S, Kurth A, Lesnik R, Freuling C, Müller T, Kramer-Schadt S, Wibbelt G (2011) Diseases and causes of death in European bats: dynamics in disease susceptibility and infection rates. PloS One 6: 12. 10.1371/journal.pone.0029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez SF, López-Baucells A, Rocha R, Farneda FZ, Bobrowiec PE, Palmeirim JM, Meyer CF (2019) Echolocation and stratum preference: key trait correlates of vulnerability of insectivorous bats to tropical Forest fragmentation. Front Ecol Evol 7. 10.3389/fevo.2019.00373. [DOI] [Google Scholar]
- Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A (2013) Ecological and evolutionary implications of food subsidies from humans. Ecol Lett 16: 1501–1514. 10.1111/ele.12187. [DOI] [PubMed] [Google Scholar]
- Patterson BD, Dick CW, Dittmar K (2007) Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). J Trop Ecol 23: 177–189. 10.1017/S0266467406003816. [DOI] [Google Scholar]
- Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB (2012) Relationship between pace of life and immune responses in wild rodents. Oikos 121: 1483–1492. 10.1111/j.1600-0706.2012.020215.x. [DOI] [Google Scholar]
- Ramiadantsoa T, Hanski I, Ovaskainen O (2018) Responses of generalist and specialist species to fragmented landscapes. Theor Popul Biol 124: 31–40. 10.1016/j.tpb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Ramírez-Lucho I, Coates RI, González-Christen A (2017) The understory bat community in a fragmented landscape in the lowlands of the los Tuxtlas, Veracruz, Mexico. Therya 8: 99–107. 10.12933/therya-17-463. [DOI] [Google Scholar]
- Read AF, Allen JE (2000) The economics of immunity. Science 290: 1104–1105. 10.1126/science.290.5494.1104. [DOI] [PubMed] [Google Scholar]
- Reid F (1997) A field guide to the mammals of central America and Southeast Mexico. Oxford University Press, New York, NY [Google Scholar]
- Rulli M, Santini M, Hayman D, D’Odorico P (2017) The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci Rep 7: 41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana SE, Dial TO, Eiting TP, Alfaro ME (2011) Roosting ecology and the evolution of pelage markings in bats. PloS One 6: 10. 10.1371/journal.pone.0025845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses?. Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Czirják GÁ, Voigt CC (2013) Measures of the constitutive immune system are linked to diet and roosting habits of Neotropical bats. PloS One 8: e54023. 10.1371/journal.pone.0054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann A, Czirják GÁ, Courtiol A, Bernard H, Struebig MJ, Voigt CC (2017) Habitat disturbance results in chronic stress and impaired health status in forest-dwelling paleotropical bats. Conserv Physiol 5: cox020. 10.1093/conphys/cox020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes RS & the Animal Care and Use Committee of the American Society of Mammalogists (2016) 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97: 663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari S (2016) Pteronotus mesoamericanus. The IUCN Red List of Threatened Species 2016 e.T88018392A88018395. [Google Scholar]
- Solari S (2019) Sturnira parvidens. The IUCN Red List of Threatened Species 2019 e.T88154376A88154380. [Google Scholar]
- Stanko M, Miklisová D, Gouy de Bellocq J, Morand S (2002) Mammal density and patterns of Ectoparasite species richness and abundance. Oecologia 131: 289–295. 10.1007/s00442-002-0889-5. [DOI] [PubMed] [Google Scholar]
- Trajano E (1996) Movements of cave bats in southeastern Brazil, with emphasis on the population ecology of the common vampire bat, Desmodus rotundus (Chiroptera). Biotropica 28: 121. 10.2307/2388777. [DOI] [Google Scholar]
- Villafuerte R, Litvaitis JA, Smith DF (1997) Physiological responses by lagomorphs to resource limitations imposed by habitat fragmentation: implications for condition-sensitive predation. Can J Zool 75: 148–151. 10.1139/z97-019. [DOI] [Google Scholar]
- Voigt CC, Kelm DH (2006) Host preference of the common vampire bat (Desmodus rotundus; Chiroptera) assessed by stable isotopes. J Mammal 87: 1–6. 10.1644/05-MAMM-F-276R1.1. [DOI] [Google Scholar]
- Volokhov DV, Becker DJ, Bergner LM, Camus MS, Orton RJ, Chizhikov VE, Altizer SM, Streicker DG (2017) Novel hemotropic mycoplasmas are widespread and genetically diverse in vampire bats. Epidemiol Infect 145: 3154–3167. 10.1017/S095026881700231X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokhov DV, Lock LR, Dyer KE, DeAnglis IK, Andrews BR, Simonis MC, Stockmaier S, Carter GG, Downs CJ, Fenton MBet al. (2023) Expanded diversity of hemoplasmas in rare and undersampled Neotropical bats. One Health 17: 100633. 10.1016/j.onehlt.2023.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber QMR, Willis CKR (2016) Sociality, parasites, and pathogens in bats. Sociality in Bats 105–139. [Google Scholar]
- Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York [Google Scholar]
- Wilkinson GS (1985) The social organization of the common vampire bat. Behav Ecol Sociobiol 17: 123–134. 10.1007/BF00299244. [DOI] [Google Scholar]
- Wilkinson GS (1986) Social grooming in the common vampire bat, Desmodus rotundus. Anim Behav 34: 1880–1889. 10.1016/S0003-3472(86)80274-3. [DOI] [PubMed] [Google Scholar]
- Wood SN (2006) Generalized additive models: an introduction with R. Chapman and hall/CRC, Boca Raton, FL [Google Scholar]
- Wyllie DH, Bowler ICJW, Peto TEA (2004) Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol 57: 950–955. 10.1136/jcp.2004.017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All 16S rRNA sequence data are available on GenBank through accession OQ308885–7, OQ308889, OQ308893, OQ308895–97, OQ308899–900, OQ308902–12, OQ308914–19, OQ308923–24, OQ308926–27, OQ533048, OQ546518–19, OQ546527–28, OQ546533, OQ546547, OQ546552 and OQ546555.