Abstract
Background:
Patients with ischemic cardiomyopathy are at risk from both myocardial ischemia and heart failure. Invasive testing is often used as first-line investigation and there is limited evidence whether stress testing can effectively provide risk stratification.
Objectives:
This study investigated the prognostic value of stress cardiovascular magnetic resonance (CMR) in patients with reduced left ventricular (LV) systolic function.
Methods:
In this substudy of a multicenter registry from 13 United States centers, we included patients with reduced LV ejection fraction (LVEF<50%), referred for stress CMR for suspected myocardial ischemia. Primary outcome included cardiovascular death and non-fatal myocardial infarction (MI). Secondary outcome was a composite of cardiovascular death, non-fatal MI, hospitalization for unstable angina or congestive heart failure (CHF), and unplanned late CABG.
Results:
Among 582 patients (age 62 ± 12 years, female 34%), 40% had prior history of CHF and median LVEF was 39% (IQR 28–45%). At median follow-up of 5.0 years, 97 patients experienced the primary outcome and 182 patients experienced the secondary outcome. Patients with no CMR evidence of ischemia or LGE experienced an annual primary outcome event rate of 1.1%. The presence of ischemia, LGE, or both were associated with higher event rates. In a multivariate model adjusted for clinical covariates, ischemia and LGE were independent predictors of primary (HR 2.63; 95% CI: 1.68–4.14; p<0.001 and HR 1.86; 95% CI: 1.05–3.29; p=0.03) and secondary (HR 2.14; 95% CI: 1.55–2.95; p<0.001 and HR 1.70; 95% CI: 1.16–2.49; p=0.007) outcomes. The addition of ischemia and LGE led to improved model discrimination for primary outcome (C-statistic from 0.715 to 0.765, p=0.02). The presence and extent of ischemia was associated with higher rates of utilization of downstream coronary angiography, revascularization, and cost of care spent on ischemia testing.
Conclusion:
Stress CMR was effective in risk stratifying patients with reduced LVEF.
Keywords: Stress cardiac MRI, cardiomyopathy, prognosis
In patients with acute coronary syndromes, left ventricular (LV) systolic function is a potent predictor of all-cause mortality (1,2). In patients suspected of having stable coronary artery disease, those with ischemic cardiomyopathy represent a distinct, high-risk subgroup (3,4), and remain challenging to risk stratify. Noninvasive imaging of patients with reduced LV function may be limited by thinned LV myocardial wall and multivessel disease with a propensity for balanced ischemia. In patients with heart failure and chest pain, the latest AHA/ACC guidelines continue to recommend a low threshold for the use of invasive angiography as a first-line test (5). Stress cardiac magnetic resonance imaging (CMR) has been shown to be an effective prognostic tool in many clinical subgroups of patients with suspected coronary artery disease (6–9). It also has demonstrated high diagnostic utility in patients with left main stem or equivalent coronary artery disease (10). However, whether stress CMR can adequately risk stratify patients with impaired LV systolic function remains unclear. We therefore conducted an analysis of patients with impaired LVEF referred for stress CMR for suspected myocardial ischemia, using a combined dataset from the multicenter Stress CMR Perfusion Imaging in the United States (SPINS) registry and a tertiary referral center.
Methods
SPINS Registry
The details behind the design, rationale, and infrastructure of the SPINS Registry have been previously described in detail (11,12). In brief, SPINS included 13 participating experienced CMR centers across the United States (7 university hospitals, 2 cardiovascular group practices, 2 multi-specialty practices, and 2 US government or military hospitals). Sites were required to have an active stress CMR program of at least 10 years duration and to contribute between 100–500 consecutive patients. Study related PHI-free data were entered into an encrypted web-based database (CMRCOOP.org).
Study population
The study cohort included patients presenting referred for a stress CMR from either the SPINS Registry or a single center registry. Between 2008–2013, SPINS enrolled consecutive, intermediate risk patients who 1) were 35–85 years of age at the time of the study, 2) underwent a vasodilator stress CMR for evaluation of chest pain, dyspnea, abnormal ECG, or other clinical presentation that raised a suspicion of myocardial ischemia as determined by the treating clinician, 3) had at least two of the following coronary risk factors: age >50 years for male or >60 years for female; diabetes mellitus; hypertension; hypercholesterolemia; family history of premature coronary artery disease (CAD) as defined by diagnosis in a first-degree male relative ≤55 years old or a female relative ≤65 years old; body mass index ≥30 kg/m2; peripheral vascular disease; and history of percutaneous coronary intervention (PCI) or myocardial infarction (MI). In this study, we included patients with evidence of reduced LV systolic function as defined by a left ventricular ejection fraction (LVEF) <50% measured on CMR. Exclusion criteria included history of coronary artery bypass surgery (CABG), recent MI within 30 days preceding the index CMR study, severe-grade valvular heart disease, previously known and documented non-ischemic cardiomyopathy with a LVEF <40%, infiltrative or hypertrophic cardiomyopathy, constrictive pericarditis, active pregnancy, competing medical illnesses with expected survival <2 years, and known inability to follow-up. In addition to SPINS, we included patients meeting the same inclusion and exclusion criteria from the Brigham and Women’s Hospital, Boston, who underwent stress CMR during the same years of 2008–2013. LVEF <50% was chosen as the criteria for reduced LV systolic function, as it represents a value which was >3 standard deviation below a normal population reference for both sexes (13,14). At each participating site, local institutional review board approval was obtained to conduct this clinical follow-up study with a waiver of written informed consent.
Stress CMR protocol and definition
CMR protocol consisted of, in order, stress perfusion (FLASH - fast low angle single-shot, EPI - echo-planar imaging, or SSFP - steady-state free precession), ventricular function (SSFP), late gadolinium enhancement (IR-GRE - inversion recovery prepared gradient-echo), and rest myocardial perfusion, and included use of scanners at both 1.5T and 3.0T, as well as make and model from all three major vendors. Vasodilator agents used included adenosine, regadenoson, or dipyridamole. The following CMR variables were collected: left ventricular volumes and dimensions, with papillary muscles and trabeculae included as LV cavity volume, segmental (presence or absence) stress perfusion according to the American Heart Association (AHA) 16-segmental model, and late gadolinium enhancement (LGE) according to the AHA 17-segmental model. A perfusion defect was present if there was a region of hypoenhancement densest in the endocardium with a transmural gradient across the wall thickness, which persisted beyond peak myocardial enhancement and conformed to a coronary distribution. An MI was present if there was finding of LGE in a coronary disease pattern in at least one myocardial segment. Inducible ischemia was defined as the presence of a perfusion defect during stress, in the absence of matching LGE in a segment (15). Peri-infarct ischemia was defined by any ischemic segment that immediately neighbors an LGE infarct segment either circumferentially or longitudinally. Mild, moderate, and severe defects were defined as the involvement of 1–2, and 3–5, and ≥6 segments, respectively. Mildly reduced LVEF was defined as 40–50%, whereas moderate-severely reduced LVEF was defined as <40%. Study image quality was rated on a 1–5 scale for cine, perfusion, and LGE sequences using the following criteria: 5 = excellent quality, no artefacts; 4 = good quality, mild artefacts; 3 = fair quality, moderate artefacts; 2 = poor quality, severe artefacts; 1 = non-diagnostic.
Clinical follow-up
Detailed follow-up of all subjects was mandated for at least 4 years following index stress CMR. Clinical outcomes were ascertained from electronic medical records and by direct patient contact via a standardized checklist questionnaire or scripted telephone conversation. End of follow-up data collection and locking of database occurred on May 25, 2018. Major clinical cardiovascular outcomes were in accordance to previously published recommendations (16). Primary outcome was defined as cardiovascular death and non-fatal MI. Only Type 1 or Type 2 event, according to the third universal definition were counted (17). Post-procedural MI after coronary revascularization was not included in the primary endpoint, given its limited association with downstream hard cardiac outcomes (18) and the possibility to create a bias for worsened outcomes in patients referred for revascularization. Secondary outcome was defined by a composite of cardiovascular death, non-fatal MI, hospitalization for unstable angina (worsening chest pain or anginal equivalent with evidence of myocardial ischemia by cardiac imaging or obstructive lesion on coronary angiography), hospitalization for congestive heart failure (CHF), and unplanned late CABG (performed more than 6 months after the index stress CMR). For either the primary outcome or MACE, only the first event was counted when multiple events occurred in a subject.
In addition, subsequent performance of all noninvasive tests for CAD (exercise stress testing, stress echocardiography, nuclear perfusion imaging, coronary computed tomographic angiography, repeat stress CMR), as well as invasive coronary angiography (XCA), and revascularization procedures was also collected, and their costs estimated. Cost of these downstream testing for myocardial ischemia was determined as previously described (12), based on published average national payment rates from the Medicare Hospital Outpatient Prospective Payment System, specific to the technical component of the most common Healthcare Common Procedure Coding System code and the year of the procedure. Costs due to complications of test procedures, subsequent hospitalization or revascularization were not collected.
Statistical analysis
For descriptive statistics, continuous variables were expressed as means ± standard deviation or median with interquartile range (IQR), for normal and skewed distributions respectively. Categorical variables were expressed as counts with percentages. Comparison between groups was performed with Student’s t-test or Wilcoxon rank sum test for continuous data and chi-squared test or Fisher’s exact test for categorical data. Event free survival was estimated by the Kaplan-Meier method and compared using a log-rank test. Univariate Cox regression models were used to estimate unadjusted hazard ratio of selected clinical and CMR covariates for primary and secondary outcomes. To determine the independent prognostic value of CMR parameters, we first constructed a multivariable Cox model for the primary outcome by inclusion of significant clinical covariates on univariable screen using a stepwise forward selection algorithm (p < 0.05 for model retention). We a priori forced LVEF into the model, due to its recognized prognostic importance. We then added presence or absence of ischemia and LGE to determine whether they each provided incremental prognostic value. The goodness-of-fit of each model (−2 log L) was calculated and compared using the likelihood-ratio test and discriminative capacity was determined according to the Harrell’s C statistic, at baseline and after addition of CMR-assessed ischemia and LGE.
In addition, to evaluate the ability of stress CMR to reclassify patients, we calculated net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (19), using pre-determined AHA/ACC guideline-based risk categories of <1%, 1–3%, and >3%/year event rates of cardiac death or AMI (20), to define low, moderate, and high-risk for the primary outcome. We tested for significant interaction between LVEF, as a continuous variable, and CMR-detected ischemia or LGE. Proportional hazards assumption was evaluated using visual inspection of the log-log survival curves and the Schoenfeld residuals test. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and a p < 0.05 was used to establish statistical significance.
Results
Baseline Patient Demographics and CMR characteristics
Of the 2,349 patients enrolled in the SPINS Registry, 403 met the LVEFv<50% cutoff to be included in this study. An additional 179 patients were identified from the clinical registry at Brigham and Women’s Hospital; thus, the overall study cohort included a total of 582 patients. Vasodilator stress CMR was well tolerated, with no occurrence of serious adverse events. Baseline demographic and clinical characteristics are summarized in Table 1, stratified by absence vs. presence of inducible ischemia or LGE. The mean age in the overall cohort was 62 ± 12 years with 34% female. Median number of cardiac risk factor was 3 (IQR 2–4) and slightly less than a quarter had prior MI and PCI. Forty percent of the cohort had history of CHF. Compared to patient without ischemia or LGE, those with ischemia or LGE were less likely to be female (29% vs. 45%, p=0.003) and had more cardiac risk factors (median number 4 (IQR 3–4) vs. 3 (IQR 2–4), p<0.001). They were also more likely to have prior PCI (31% vs. 6%, p<0.001), MI (40% vs. 3%, p<0.001), but not CHF (40% vs. 39%, p=0.60). Patients with ischemia or LGE were more likely to have chest pain as the initial symptom for test referral (41% vs. 29%, p<0.004) and were also more likely to have been on aspirin (74% vs. 44%, p<0.001), beta-blocker (76% vs. 59%, p<0.001), angiotensin-conversion enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) (70% vs. 57%, p=0.002), and statin (69% vs. 48%, p<0.001).
Table 1.
Demographics and Baseline Characteristics
| Overall (n=582) | No Ischemia or LGE (n=261) | Ischemia or LGE (n=321) | p-value | |
|---|---|---|---|---|
| Clinical Data | ||||
| Follow-up (years), median (IQR) | 5.0 (4.0–6.3) | 5.1 (4.2–6.3) | 4.9 (3.8–6.2) | 0.13 |
| Age (years), mean ± SD | 62 ± 12 | 61 ± 12 | 62 ± 12 | 0.22 |
| Female, n (%) | 197 (34) | 105 (45) | 92 (29) | 0.003 |
| BMI (kg/m2), mean ± SD | 30 ± 7 | 30 ± 7 | 30 ± 7 | 0.80 |
| Number of cardiac risk factors, median (IQR) | 3 (2–4) | 3 (2–3) | 4 (3–4) | <0.001 |
| Risk factors, n (%) | ||||
| Hypertension | 452 (78) | 184 (71) | 268 (83) | <0.001 |
| Hypercholesterolemia | 352 (60) | 140 (54) | 212 (66) | 0.002 |
| Diabetes mellitus | 176 (30) | 59 (23) | 117 (36) | <0.001 |
| Significant smoking (>10 packed-years) | 218 (38) | 89 (34) | 129 (40) | 0.13 |
| History of premature CAD in 1st degree relative | 165 (29) | 66 (26) | 99 (32) | 0.12 |
| CAD Consortium Score (Basic), median (IQR) | 34 (24–54) | 34 (17–44) | 44 (32–54) | <0.001 |
| History of PCI, n (%) | 114 (20) | 16 (6) | 98 (31) | <0.001 |
| History of MI, n (%) | 136 (24) | 9 (3) | 127 (40) | <0.001 |
| History of heart failure, n (%) | 234 (40) | 102 (39) | 132 (40) | 0.60 |
| Presenting Reasons, n (%) | ||||
| Chest pain | 206 (35) | 76 (29) | 130 (41) | 0.004 |
| Dyspnea | 236 (41) | 120 (46) | 116 (36) | 0.02 |
| Arrhythmias | 37 (6) | 21 (8) | 16 (5) | 0.13 |
| Abnormal ECG | 40 (7) | 17 (7) | 23 (7) | 0.76 |
| Other symptoms/reasons | 63 (11) | 27 (10) | 36 (11) | 0.74 |
| Medications | ||||
| Aspirin | 348 (60) | 115 (44) | 233 (74) | <0.001 |
| Beta-blocker | 397 (69) | 153 (59) | 244 (76) | <0.001 |
| Calcium channel blocker | 83 (14) | 43 (17) | 40 (13) | 0.19 |
| ACE-inhibitor or ARB | 371 (64) | 149 (57) | 222 (70) | 0.002 |
| Aldosterone receptor antagonist | 42 (7) | 20 (8) | 22 (7) | 0.74 |
| Statin | 343 (59) | 124 (48) | 219 (69) | <0.001 |
| Stress CMR | ||||
| Scanner field strength | ||||
| 1.5 Tesla, n (%) | 320 (55) | 149 (57) | 171 (53) | 0.34 |
| 3.0 Tesla, n (%) | 262 (45) | 112 (43) | 150 (47) | |
| CMR manufacturers | ||||
| Siemens, n (%) | 439 (76) | 194 (74) | 245 (77) | 0.13 |
| General Electric, n (%) | 98 (17) | 41 (16) | 57 (18) | |
| Phillips, n (%) | 44 (8) | 26 (10) | 18 (6) | |
| Quality of cine sequence | ||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.62 |
| Quality of perfusion sequence | ||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.31 |
| Quality of LGE sequence | ||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.56 |
| LV ejection fraction (%), median (IQR) | 39 (28–45) | 39 (29–45) | 39 (27–44) | 0.17 |
| LVEDVI, mL/m2, median (IQR) | 100 (79–125) | 99 (76–120) | 101 (81–129) | 0.06 |
| LVESVI, mL/m2, median (IQR) | 60 (44–84) | 58 (42–81) | 62 (46–87) | 0.07 |
| LVEF < 40%, n (%) | 308 (53) | 135 (52) | 173 (54) | 0.60 |
| Ischemia, n (%) | 175 (30) | 0 | 175 (55) | <0.001 |
| Ischemic segments (number), median (IQR) | 0 (0–1) | 0 | 1 (0–3) | <0.001 |
| LGE, n (%) | 277 (48) | 0 | 277 (86) | <0.001 |
| LGE segments (number), median (IQR) | 0 (0–5) | 0 | 4 (2–7) | <0.001 |
Baseline CMR characteristics are displayed in Table 1. Median study quality for all 3 key sequences was 5 (excellent) and did not differ between the two groups. The overall cohort had a median left ventricular end-diastolic volume index (LVEDVi) of 100 ml/m2 (IQR 79–125 ml/m2), median left ventricular end-systolic volume index (LVESVi) of 60 ml/m2 (IQR 44–84 ml/m2), and median LVEF of 39% (IQR 28–45%). Moderate or severe LV dysfunction was present in 53%. Thirty percent had ischemia on stress CMR and 48% had evidence of prior MI by LGE. Compared to patients without ischemia or LGE, those with ischemia or LGE had similar LV chamber size and LVEF.
Association of Stress CMR with Primary and Secondary Outcomes
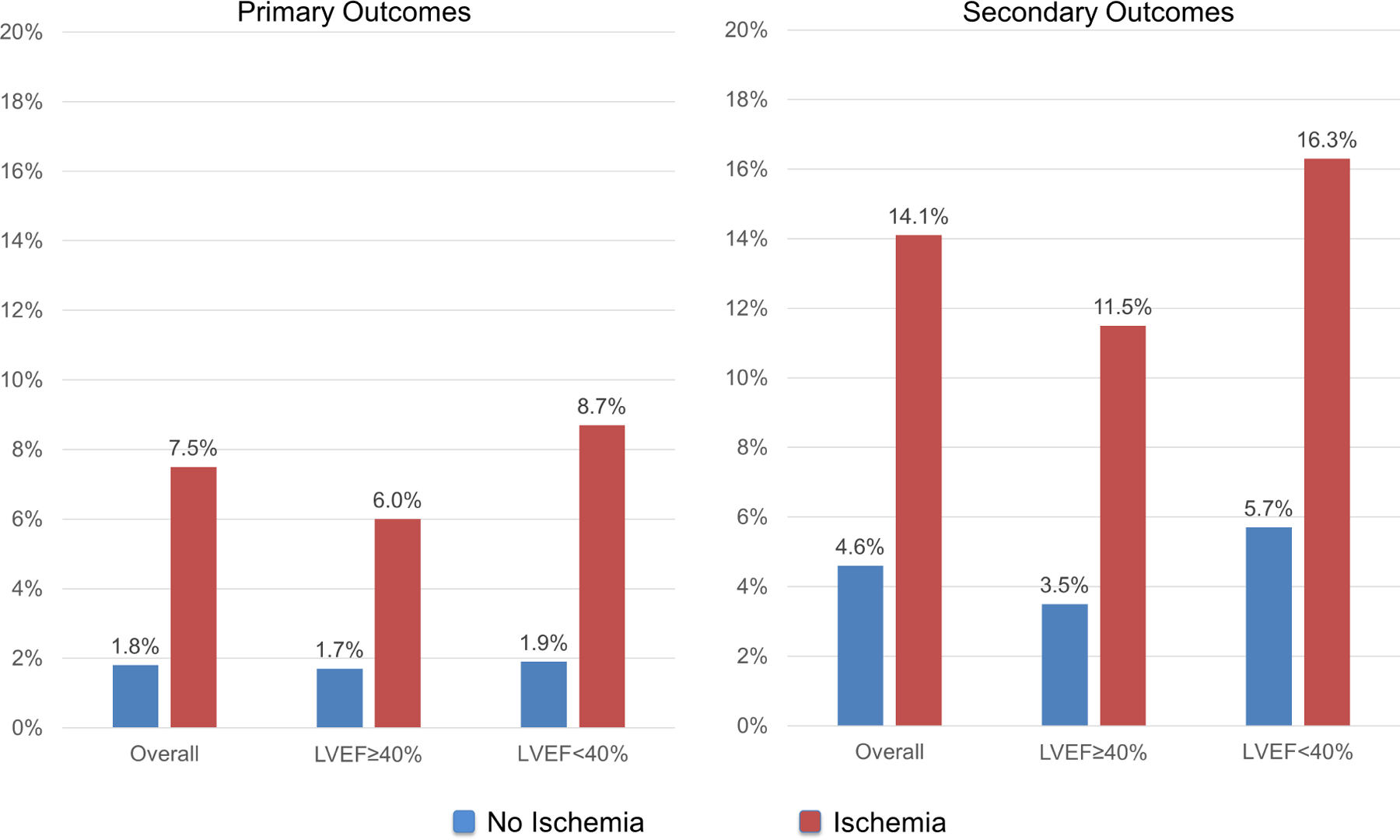
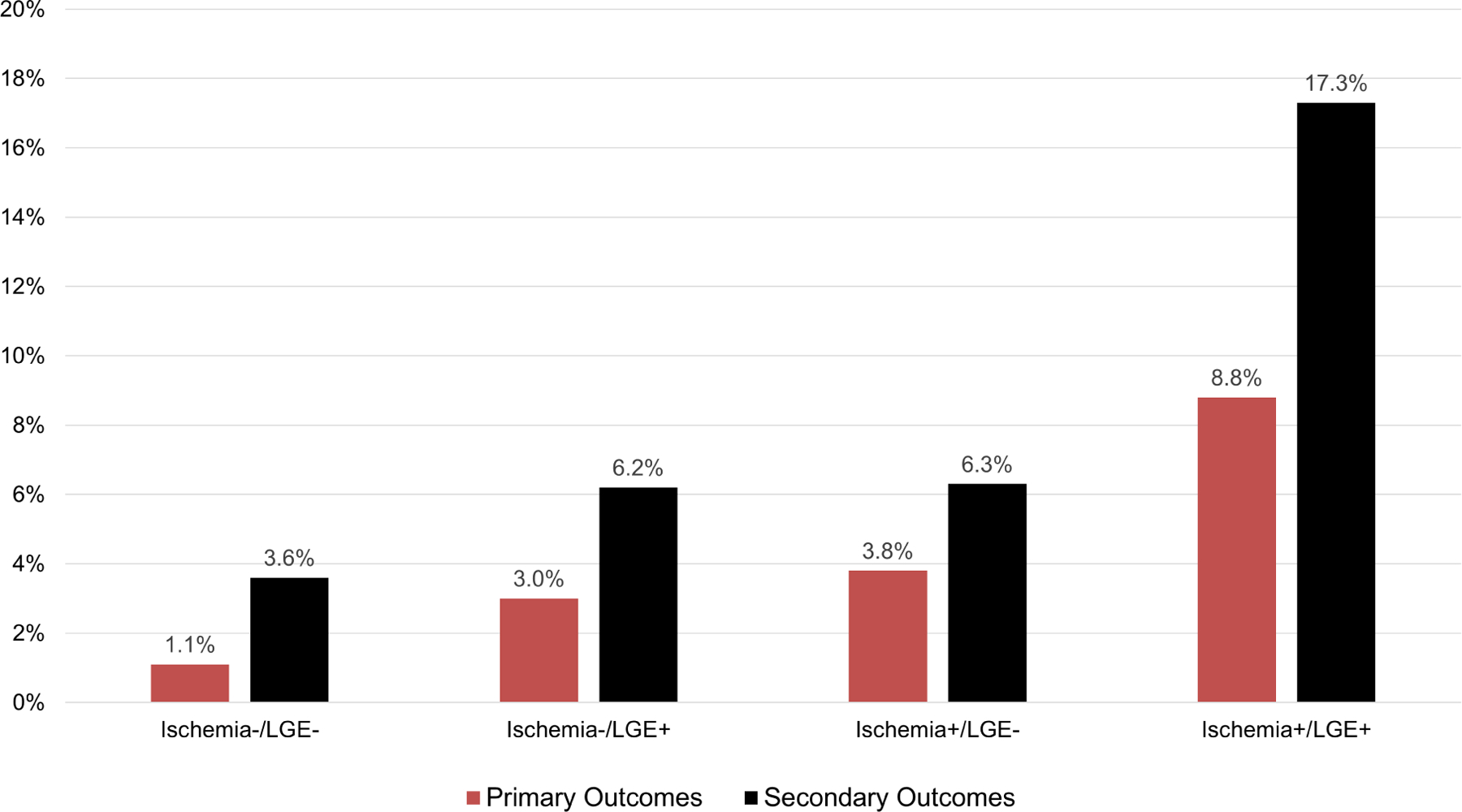
Successful follow-up of ≥ 4 years was achieved in 95% of the study cohort, with median duration of 5.0 years (IQR 4.0–6.3 years). During study follow-up, primary outcome occurred in 97 patients, whereas secondary outcome occurred in 182 patients. Annualized event rates, stratified by presence of ischemia and LVEF, are presented in Figure 1 for primary and secondary outcomes. For primary outcome, patients without ischemia and with LVEF ≥40% experienced an event rate of 1.7% per year, whereas those with ischemia and LVEF <40% experienced an event rate of 8.7% per year. Figure 2 provides annualized event rates, stratified by presence of ischemia and LGE. Patient without the presence of either experienced the lowest rate of primary (1.1% per year) or secondary (3.6% per year) outcomes. Event rate, according to CMR findings for individual components of the primary endpoint are shown in Supplemental Figure 1 and 2. Patient without the presence of either ischemia or LGE experienced an annualized cardiovascular mortality rate of 0.9% and non-fatal MI rate of 0.2%. In contrast, those with both ischemia and LGE had events rate of 5.8% and 3.8% for cardiovascular mortality and non-fatal MI, respectively.
Figure 1: Primary and secondary outcomes event rates.
Annualized rates of primary (left) and secondary (right) outcomes, stratified by presence vs. absence of ischemia and LVEF 40–50% vs. <40%.
Figure 2: Primary and secondary outcomes event rates.
Annualized rates of primary and secondary outcomes, stratified by presence vs. absence of ischemia and LGE.
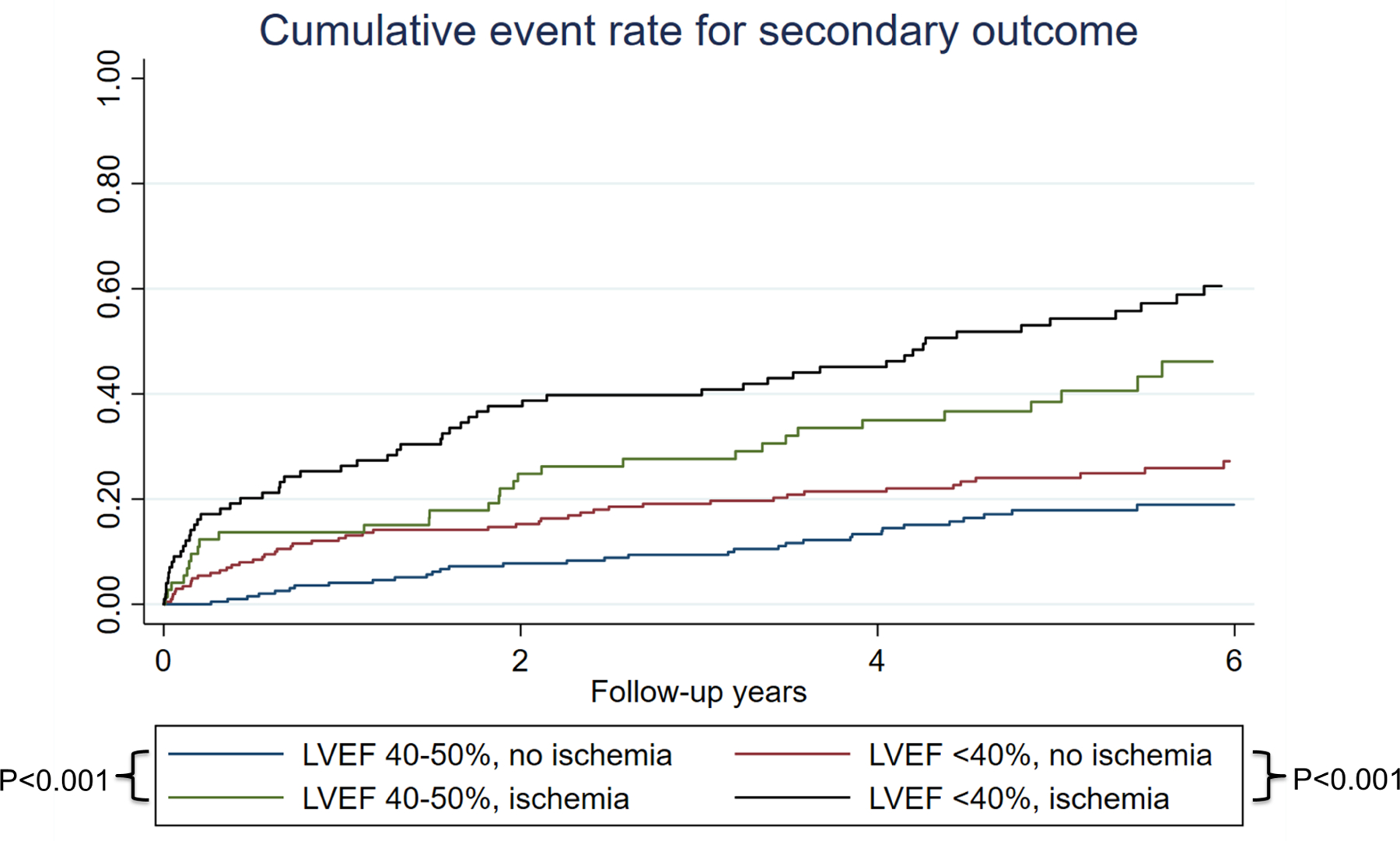
Univariable analysis of patient and CMR characteristics for association with primary and secondary outcomes are presented in Table 2. Age, male sex, history of diabetes, history of smoking, history of PCI, history of MI, history of CHF, LVEF, LVEDVi, LVESVi, presence and extent of ischemia, and presence and extent of LGE were all significantly associated with the primary outcome in univariable Cox models. Ischemia and LGE were also strongly associated with individual components of the primary outcome, namely cardiovascular death (HR 3.60; 95% CI: 2.23–5.80, p<0.001 and HR 3.20; 95% CI: 1.87–5.47, p<0.001, respectively) or non-fatal MI (HR 5.08; 95% CI: 2.54–10.2, p<0.001, and HR 7.11; 95% CI: 2.77–18.3, p<0.001, respectively). Out of the 175 patients with presence of ischemia, 125 (71%) had peri-infarct ischemia. Presence of peri-infarct ischemia and number of peri-infarct ischemia segments both demonstrated significant association with the primary outcome (cardiovascular death or MI) (HR 4.37; 95% CI: 2.92–6.53, and HR 1.28; 95% CI: 1.18–1.38, both p<0.001). However, when entered into a multivariable model, presence of peri-infarct ischemia was no longer associated with primary outcome once adjusted for presence of ischemia and presence of LGE (adjusted HR 1.15; 95%CI 0.49–2.69, P=0.74). Kaplan-Meier cumulative event rate for primary and secondary outcomes stratified by presence vs. absence of inducible ischemia and LVEF ≥40% vs. <40% are shown in Central Illustration and Figure 3. Patients with LVEF 40–50% and ischemia had higher cumulative events compared to those with LVEF<40% and no ischemia (p<0.001). There was no significant interaction between LVEF and CMR-detected ischemia or LGE. Visual inspection of the log-log survival curves and calculation of the Schoenfeld residuals showed that the proportionality assumption was not violated.
Table 2.
Univariable Cox Association of Clinical and Stress Cardiac Magnetic Resonance Indices with Outcomes
| Characteristics | Primary outcome | Secondary outcome | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Demographics | ||||||
| Age (per year) | 1.02 | 1.01–1.04 | 0.006 | 1.02 | 1.01–1.03 | 0.001 |
| Female | 0.58 | 0.36–0.94 | 0.03 | 0.76 | 0.55–1.05 | 0.10 |
| BMI (per 1 kg/m2) | 0.98 | 0.95–1.01 | 0.17 | 0.99 | 0.97–1.01 | 0.33 |
| Cardiac risk factors | ||||||
| Hypertension | 1.41 | 0.84–2.39 | 0.20 | 1.71 | 1.13–2.58 | 0.01 |
| Hypercholesterolemia | 1.10 | 0.73–1.67 | 0.64 | 0.91 | 0.68–1.24 | 0.56 |
| Diabetes mellitus | 2.22 | 1.48–3.32 | <0.001 | 1.87 | 1.39–2.53 | <0.001 |
| Smoking | 1.56 | 1.04–2.35 | 0.03 | 1.34 | 0.99–1.81 | 0.06 |
| Family history of CAD | 0.69 | 0.42–1.13 | 0.14 | 0.92 | 0.66–1.29 | 0.64 |
| History of PCI | 1.62 | 1.04–2.53 | 0.03 | 1.56 | 1.12–2.18 | 0.009 |
| History of MI | 2.62 | 1.74–3.93 | <0.001 | 1.94 | 1.42–2.65 | <0.001 |
| History of CHF | 1.90 | 1.27–2.84 | 0.002 | 1.59 | 1.18–2.14 | 0.002 |
| Stress CMR | ||||||
| LVEF (per +5% ∆) | 0.88 | 0.80–0.97 | 0.009 | 0.85 | 0.80–0.91 | <0.001 |
| LVEF<40% (vs. ≥40%) | 1.38 | 0.92–2.08 | 0.12 | 1.61 | 1.19–2.20 | 0.002 |
| LVEDVi (per +5 mL/m2 ∆) | 1.03 | 1.01–1.06 | 0.009 | 1.03 | 1.01–1.05 | 0.001 |
| LVESVi (per +5 mL/m2 ∆) | 1.03 | 1.01–1.06 | 0.01 | 1.04 | 1.02–1.06 | <0.001 |
| Ischemia | 4.02 | 2.66–6.07 | <0.001 | 3.00 | 2.23–4.04 | <0.001 |
| Extent of ischemia (per segment) | 1.14 | 1.09–1.19 | <0.001 | 1.12 | 1.08–1.16 | <0.001 |
| LGE | 3.64 | 2.28–5.83 | <0.001 | 2.63 | 1.91–3.62 | <0.001 |
| Extent of LGE (per segment) | 1.06 | 1.02–1.10 | 0.004 | 1.05 | 1.01–1.08 | 0.003 |
Central Illustration:
The left panel shows an example of a CMR study showing a stress perfusion defect (dark arrowheads) with its myocardial extent exceeding the 2 foci of subsegmental LGE (red arrows). This suggested ischemia from flow-limiting coronary stenosis in the right coronary artery. The right panel shows time-to-event curves of the study cohort for primary outcome, stratified by presence vs. absence of ischemia and according to LVEF 40–50% vs. <40%.
Figure 3: Cumulative incidence rate.
Time-to-event curves of the study cohort for secondary outcomes, stratified by presence vs. absence of ischemia and according to LVEF 40–50% vs. <40%.
Multivariate Associations, Model Discrimination and Risk Reclassification Improvement
For the primary outcome, age, sex, history of diabetes, history of MI, history of CHF, and LVEF were chosen by the forward selection algorithm to form the baseline clinical multivariable model (−2 log L: 1,081) (Table 3, Clinical Model). Adjusted to the effects of the covariates in the clinical model and to each other, presence of ischemia (HR 2.63, 95% CI 1.68–4.14, p<0.001) and LGE (HR 1.86, 95% CI 1.05–3.29, p=0.03) maintained significant association with the primary outcome. Presence of ischemia and presence of LGE independently improved this clinical model for primary outcome when they were separately added (−2 log L: 1,055 and 1,069, for ischemia and LGE, respectively, both p<0.001) or when both added (−2 log L: 1,051) to the model. The addition of ischemia and LGE to the clinical model for primary outcome also improved the model discrimination (Harrell’s C-statistic=0.715 to 0.765; p=0.02). Finally, the addition of ischemia and LGE resulted in an NRI of 0.21 (95% CI 0.10–0.32) and IDI of 0.069 (95% CI 0.041–0.096) (p<0.001 for both), across pre-determined AHA/ACC guideline-based risk categories.
Table 3.
Multivariable Analysis for Prediction of Primary and Secondary Outcome
| Primary Outcome | Secondary Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Model | Clinical Model + CMR | Clinical Model | Clinical Model + CMR | |||||
| Statistic | p-value | Statistic | p-value | Statistic | p-value | Statistic | p-value | |
| Harrell’s Cstatistic | 0.715 | -- | 0.765 | 0.02* | 0.678 | -- | 0.716 | 0.03* |
| Covariates | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Age | 1.03 (1.01–1.05) | 0.004 | 1.02 (1.01–1.04) | 0.01 | 1.02 (1.01–1.03) | 0.002 | 1.02 (1.00–1.03) | 0.008 |
| Female | 0.62 (0.38–1.00) | <0.05 | 0.72 (0.44–1.19) | 0.20 | 0.81 (0.58–1.12) | 0.21 | 0.93 (0.66–1.31) | 0.67 |
| Diabetes mellitus | 1.90 (1.25–2.88) | 0.003 | 1.56 (1.02–2.39) | 0.04 | 1.69 (1.24–2.29) | 0.001 | 1.40 (1.02–1.91) | 0.04 |
| History of MI | 2.27 (1.50–3.43) | <0.001 | 1.39 (0.88–2.19) | 0.16 | 1.68 (1.22–2.31) | 0.001 | 1.14 (0.81–1.61) | 0.45 |
| History of CHF | 1.76 (1.12–2.75) | 0.01 | 1.83 (1.1702.86) | 0.008 | 1.30 (0.94–1.81) | 0.12 | 1.31 (0.95–1.82) | 0.10 |
| LVEF (per +5% ∆) | 0.96 (0.86–1.06) | 0.39 | 1.00 (0.98–1.02) | 0.99 | 0.89 (0.83–0.97) | 0.005 | 0.92 (0.85–0.99) | 0.03 |
| Ischemia | -- | 2.63 (1.68–4.14) | <0.001 | -- | 2.14 (1.55–2.95) | <0.001 | ||
| LGE | -- | 1.86 (1.05–3.29) | 0.03 | -- | 1.70 (1.16–2.49) | 0.007 | ||
Compared to Clinical Model
Table 3 further displays the multivariate model for secondary outcome. Adjusted to the effects of the clinical covariates and to each other, presence of ischemia (HR 2.14, 95% CI 1.55–2.95, p<0.001) and LGE (HR 1.70, 95% CI 1.16–2.49, p=0.007) maintained significant association with the secondary outcome. Their addition improved baseline model goodness of fit (−2 log L: 2,048 to 2,010, p<0.001) and discrimination (Harrell’s C-statistic=0.678 to 0.716; p=0.03).
Downstream Testing, Revascularization, and Cost
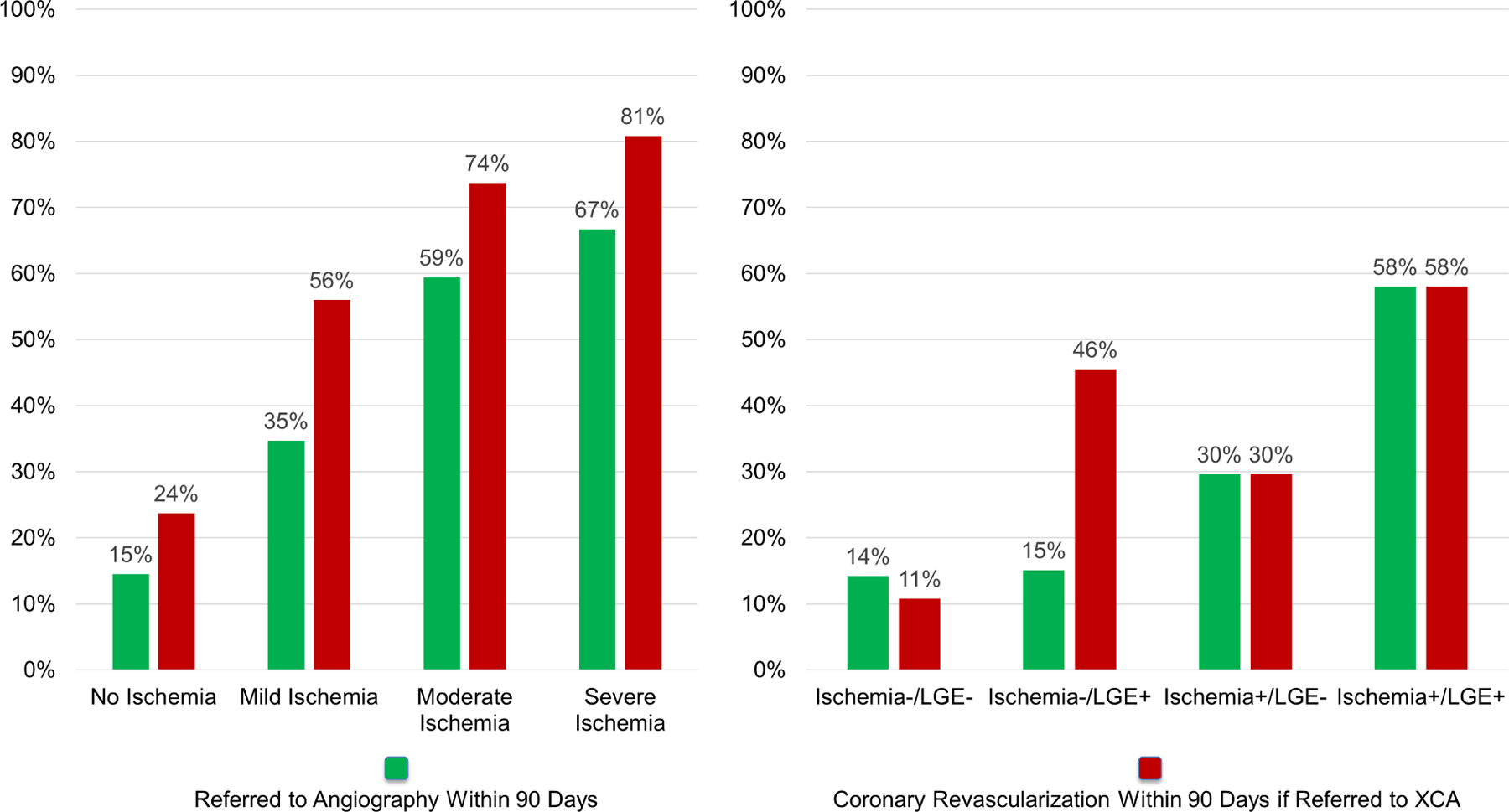
Referral rates to invasive coronary angiography and subsequent performance of revascularization procedures within the first year of CMR, stratified by presence and extent of ischemia are shown in Figure 4A and by presence or absence of ischemia and LGE is shown in Figure 4B. Both the presence and extent of myocardial ischemia was associated with incrementally higher probability of undergoing coronary angiography and revascularization procedures (p-trend <0.001 for all). Among patients without evidence of ischemia on their stress CMR, 59 (15%) underwent coronary angiography at 90 days per discretion of the caring physician, with 14/59 (24%) undergoing any type of coronary revascularization including CABG (6/59, 10%). Of these 14 patients, 10 were shown to have had prior infarct by LGE. All six patients who underwent CABG had prior infarct by LGE.
Figure 4: Invasive coronary angiography and revascularization at 90 days.
Referral to invasive angiography at 90 days, with corresponding percentage of patients undergoing revascularization, stratified by presence and extent of ischemia (4A) and presence or absence of ischemia and LGE (4B).
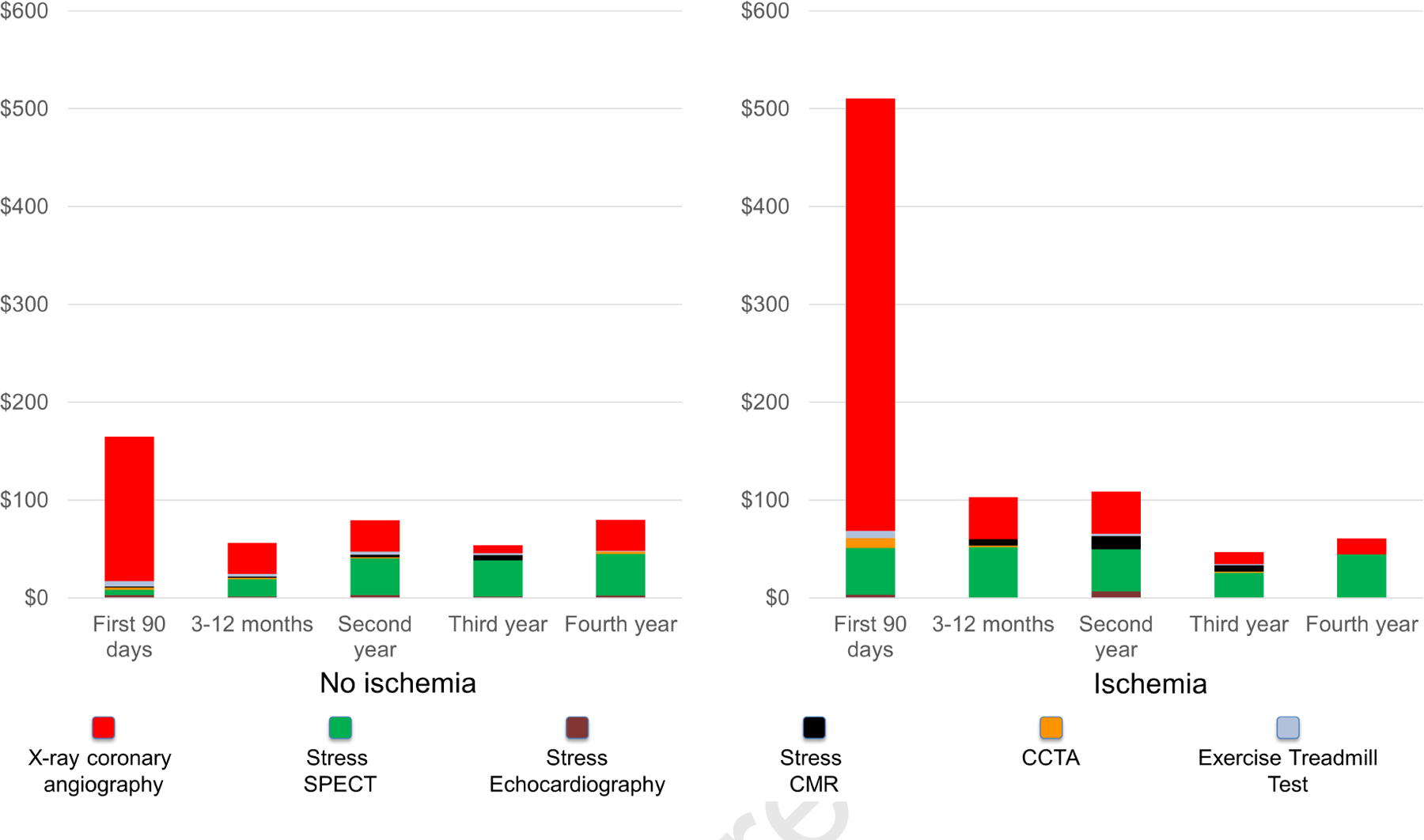
Figure 5 illustrates the average cost spent in cardiac tests according to follow-up periods. The difference was most marked during the first 90 days after CMR, where patients with ischemia incurred an approximately 3-fold higher costs than did those without ($510 vs. $165, p < 0.001), mostly driven by higher referral rates to coronary angiography. After the first 90 days, cost spent in cardiac tests was low (average $74 per year) across all years of follow-up for patients without ischemia. Whereas coronary angiography contributed the most to overall costs during the first year, SPECT contributed the most in later years.
Figure 5: Costs of Downstream Ischemia Testing at 4-year.
Costs of cardiac tests to detect myocardial ischemia incurred during follow-up, stratified by stress cardiac magnetic resonance imaging findings with breakdown by modality. Costs are displayed in U.S. dollars spent per patient.
Discussion
In this retrospective cohort of patients with reduced LVEF referred to stress CMR for suspicion of CAD, we observed that stress CMR-detected myocardial ischemia and LGE provided incremental value to a clinical model for hard cardiovascular outcomes. Furthermore, in this cohort with evidence of a cardiomyopathy, those with neither CMR ischemia nor infarct by LGE, constitute a low risk group with an annualized hard event rate of 1.1%.
Previous and contemporary observational studies (7,9), as well as randomized controlled trials (21–23) of stress CMR have mostly included LVEF with median or mean in the normal range. In a meta-analysis of 19 studies and 11,636 patients with known or suspected CAD undergoing stress CMR, Lipinski et al. reported an annualized hard outcome (cardiovascular death and non-fatal MI) rate of 4.9% and 0.8% for positive vs. negative ischemia and 4.6% and 1.4% for positive vs. negative LGE at median follow-up of 25 months (8). Mean LVEF for the included studies, however, ranged between 55% and 67%. Few studies have examined the prognostic impact of stress CMR in a population with impaired LVEF. Husser et al. reported on 391 patients with reduced LVEF (mean 39%) undergoing stress CMR (24). At median follow-up of 1.8 years, presence of perfusion defect, but not LGE was associated with major cardiovascular events. The study was, however, limited by its relatively short follow-up duration, and lack of adjustment for history of heart failure and LVEF. Our study significantly expands upon prior results and demonstrated that at a median follow-up of 5.0 years, independent of LVEF, CMR-detected myocardial ischemia and LGE provided incremental prognostic value to a clinical model for cardiovascular outcomes.
In patients with heart failure, and particularly in those with depressed LVEF, assessment of etiology is of paramount importance in determining prognosis and treatment. Studies have previously demonstrated that both the presence and extent of coronary disease are predictive of long-term mortality in patients with cardiomyopathy (25,26). Although pharmacological therapies remains the mainstay treatment for left ventricular dysfunction in either ischemic or non-ischemic cardiomyopathies (5,27), there are significant differences in interventional therapies, including options for revascularizations. There is also emerging evidence to support differential effectiveness of implantable defibrillator therapy for protection against sudden cardiac death (28).
Accurate detection of CAD in LV dysfunction, however, remains a challenge using traditional stress imaging modalities. Single photon emission computed tomography (SPECT) relies on the presence of regional wall motion abnormalities and reversible perfusion defects to detect CAD. In the presence of LV dysfunction, however, a significant portion of non-ischemic cardiomyopathies may already display baseline regional wall motion abnormalities (29,30). Regional perfusion abnormalities can also occur in non-ischemic dilated cardiomyopathies (31,32) and to further complicate matters, relative perfusion can remain normal in the presence of multivessel disease and “balanced ischemia” (33). For stress echocardiography, there have been relatively few studies which examined its performance in patients with reduced LVEF. The largest series, including 70 patients, reported a sensitivity and specificity of 83% and 71%, respectively (34). Due to its excellent spatial resolution, LGE imaging by CMR has the ability to accurate characterize the location and extent of myocardial scar. Previous studies examining patients with known obstructive CAD and reduced LVEF have detected ischemic pattern LGE in 80–100% of cases (35–37). Diagnostic accuracy of CMR to discern ischemic etiology in patients with new-onset heart failure was >95% and similar to coronary angiography (38). This is of particular significance, given the proportion of patients with prior infarct, but no clinical history of MI, and the prognostic importance of unrecognized MI (39). In our study, ischemic pattern LGE was present in 48% of the cohort, but a history of MI only in 24%.
A few studies with different imaging modalities have examined the prognostic value of non-invasive stress testing in patients with low LVEF. Majmudar et al. studied 510 consecutive patients referred for stress positron emission tomography (PET) with resting LVEF ≤45% (40). The presence of scar, but not ischemia was a univariable predictor of major adverse cardiovascular events. In a multivariable model including etiology of LV dysfunction, neither was a significant predictor of cardiovascular events. In a sub-study of the STITCH trial, which enrolled patients with ischemic heart failure with LVEF ≤35% and randomized them to CABG in addition to medical therapy, Panza et al. evaluated 399 patients who underwent stress testing (41). Approximately half underwent SPECT and the other half dobutamine stress echocardiography. Sixty four percent had evidence of ischemia and at a median follow-up of 56 months, the presence of ischemia did not predict all-cause mortality, cardiovascular mortality, or all-cause mortality plus cardiovascular hospitalization. Our results suggest that stress CMR provides incremental value above a clinical model in predicting long-term hard cardiovascular outcomes, and that this finding was independent of LVEF. A few key differences, however, exist between the current study and that reported by Panza et al., including differences in non-invasive modalities, population (suspected or known CAD vs. known CAD), LVEF (median 39% vs. mean 26%), and LV volumes.
We reported on downstream use of invasive angiography, revascularization, and cost of ischemic testing. Consistent with current guidelines (5) and clinical practice, the presence and extent of ischemia was a strong driver behind invasive investigation and revascularization therapy. Referral to angiography remained at the discretion of the treating physician. We observed that 35% of patients with mild, 59% with moderate and 67% with severe ischemia on stress CMR being referred to angiography. Many studies that have shown benefit of revascularization in ischemic cardiomyopathy (42) were published towards the towards the end of the eligibility period of SPINS (2008–2013), which may explain the relatively lower rate of invasive therapy in those with higher burden of ischemia. 15% of patients with no evidence of ischemia still underwent diagnostic coronary angiography at 90 days, which likely reflects clinical practice at the time of study performance and the clinical recognition of high-risk features. In patients without CMR-detected ischemia who underwent revascularization, the majority had prior infarct. Because this study was conducted before widespread adoption of fractional flow reserve (FFR) to guide revascularization, we do not know whether all these interventions would have been performed under current indications. From a cost of care of downstream ischemic testing, presence of ischemia was significant driver of resource utilization, particularly in the first 90 days, where it was associated with a 3-fold higher cost. The difference in cost was no longer significant after the second year of follow-up.
Study Limitations
A few limitations deserve mention. First, our participating sites consisted of experienced, high-volume CMR centers and therefore it is unclear whether the results can generalize to less experienced centers. Second, given the retrospective design and limited number of patients who underwent revascularization, our study is unable to assess for CMR guidance of medical therapy or coronary revascularization towards improvement of cardiovascular outcomes. Third, there was a limited number of patients with severe LV dysfunction (LVEF<30%) and hence our results may not be generalizable to this population or to those with end-stage ventricular remodeling. Fourth, LVEF determination was by CMR only so the prognostic value of ischemia and LGE, adjusted to LVEF, may be different than if a non-CMR based LVEF was used. Finally, we excluded patients with a prior history of CABG from our study; this will have to be addressed in a future study.
Conclusions
In summary, in this study of patients with impaired LVEF referred for clinical assessment for CAD, presence of ischemia and LGE on stress CMR was associated with worsened long-term cardiovascular prognosis. Presence of ischemia was increased downstream referral to coronary angiography and revascularization, and increased cost of care from subsequent ischemic testing.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE:
In patients with reduced LVEF, stress CMR perfusion imaging can identify those at lower risk of ischemic events and guide referral for subsequent coronary angiography.
TRANSLATIONAL OUTLOOK:
Future studies should compare the cost-effectiveness of a stress CMR-first strategy with other noninvasive and invasive modalities in the evaluation of patients with LV dysfunction suspected of having underlying ischemic heart disease.
Funding:
The SPINS Registry was funded by the SCMR, using a research grant jointly sponsored by Siemens Healthineers (Erlangen, Germany) and Bayer AG (Leverkusen, Germany). These sponsors to SCMR provided financial support for the study but did not play a role in study design, data collection, analysis, interpretation, or manuscript drafting.
Abbreviations:
- CMR
cardiac magnetic resonance imaging
- LGE
late gadolinium enhancement
- LVEF
left ventricular ejection fraction
- CAD
coronary artery disease
- MI
myocardial infarction
- CABG
coronary artery bypass graft surgery
- PCI
percutaneous coronary intervention
- CHF
congestive heart failure
Footnotes
Disclosures: Dr. Antiochos has received research funding from the Swiss National Science Foundation (grant P2LAP3_184037), the Novartis Foundation for Medical-Biological Research, the Bangerter-Rhyner Foundation and the SICPA Foundation. All other authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration information: http://www.clinicaltrials.gov. Unique identifier: NCT03192891.
REFERENCES
- 1.Burns RJ, Gibbons RJ, Yi Q et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002;39:30–6. [DOI] [PubMed] [Google Scholar]
- 2.Zaret BL, Wackers FJ, Terrin ML et al. Value of radionuclide rest and exercise left ventricular ejection fraction in assessing survival of patients after thrombolytic therapy for acute myocardial infarction: results of Thrombolysis in Myocardial Infarction (TIMI) phase II study. The TIMI Study Group. J Am Coll Cardiol 1995;26:73–9. [DOI] [PubMed] [Google Scholar]
- 3.Sharir T, Germano G, Kavanagh PB et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation 1999;100:1035–42. [DOI] [PubMed] [Google Scholar]
- 4.Lertsburapa K, Ahlberg AW, Bateman TM et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol 2008;15:745–53. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 6.Bodi V, Sanchis J, Lopez-Lereu MP et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. Journal of the American College of Cardiology 2007;50:1174–9. [DOI] [PubMed] [Google Scholar]
- 7.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation 2011;123:1509–18. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah R, Heydari B, Coelho-Filho O et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation 2013;128:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley JRJ, Kidambi A, Biglands JD et al. A comparison of cardiovascular magnetic resonance and single photon emission computed tomography (SPECT) perfusion imaging in left main stem or equivalent coronary artery disease: a CE-MARC substudy. J Cardiovasc Magn Reson 2017;19:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Cardiovascular Magnetic Resonance Registry I, Kwong RY, Petersen SE et al. The global cardiovascular magnetic resonance registry (GCMR) of the society for cardiovascular magnetic resonance (SCMR): its goals, rationale, data infrastructure, and current developments. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2017;19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwong RY, Ge Y, Steel K et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients With Chest Pain. J Am Coll Cardiol 2019;74:1741–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeon SB, Salton CJ, Gona P et al. Impact of age, sex, and indexation method on MR left ventricular reference values in the Framingham Heart Study offspring cohort. J Magn Reson Imaging 2015;41:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks KA, Tcheng JE, Bozkurt B et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015;132:302–61. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 18.Baker NC, Lipinski MJ, Escarcega RO et al. Definitions of periprocedural myocardial infarction as surrogates for catheterization laboratory quality or clinical trial end points. Am J Cardiol 2014;113:1326–30. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 20.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:2564–603. [DOI] [PubMed] [Google Scholar]
- 21.Schwitter J, Wacker CM, Wilke N et al. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson 2012;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagel E, Greenwood JP, McCann GP et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med 2019;380:2418–2428. [DOI] [PubMed] [Google Scholar]
- 24.Husser O, Monmeneu JV, Bonanad C et al. Prognostic value of myocardial ischemia and necrosis in depressed left ventricular function: a multicenter stress cardiac magnetic resonance registry. Rev Esp Cardiol (Engl Ed) 2014;67:693–700. [DOI] [PubMed] [Google Scholar]
- 25.Bart BA, Shaw LK, McCants CB Jr. et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. Journal of the American College of Cardiology 1997;30:1002–8. [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. Journal of the American College of Cardiology 2002;39:210–8. [DOI] [PubMed] [Google Scholar]
- 27.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 28.Kober L, Thune JJ, Nielsen JC et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 29.Wallis DE, O’Connell JB, Henkin RE, Costanzo-Nordin MR, Scanlon PJ. Segmental wall motion abnormalities in dilated cardiomyopathy: a common finding and good prognostic sign. J Am Coll Cardiol 1984;4:674–9. [DOI] [PubMed] [Google Scholar]
- 30.Juilliere Y, Marie PY, Danchin N et al. Radionuclide assessment of regional differences in left ventricular wall motion and myocardial perfusion in idiopathic dilated cardiomyopathy. Eur Heart J 1993;14:1163–9. [DOI] [PubMed] [Google Scholar]
- 31.Wu YW, Yen RF, Chieng PU, Huang PJ. Tl-201 myocardial SPECT in differentiation of ischemic from nonischemic dilated cardiomyopathy in patients with left ventricular dysfunction. J Nucl Cardiol 2003;10:369–74. [DOI] [PubMed] [Google Scholar]
- 32.Her SH, Yoon HJ, Lee JM et al. Adenosine Tc-99m tetrofosmin SPECT in differentiation of ischemic from nonischemic cardiomyopathy in patients with LV systolic dysfunction. Clin Nucl Med 2008;33:459–63. [DOI] [PubMed] [Google Scholar]
- 33.Burrell S, MacDonald A. Artifacts and pitfalls in myocardial perfusion imaging. J Nucl Med Technol 2006;34:193–211; quiz 212–4. [PubMed] [Google Scholar]
- 34.Sharp SM, Sawada SG, Segar DS et al. Dobutamine stress echocardiography: detection of coronary artery disease in patients with dilated cardiomyopathy. J Am Coll Cardiol 1994;24:934–9. [DOI] [PubMed] [Google Scholar]
- 35.Bello D, Shah DJ, Farah GM et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation 2003;108:1945–53. [DOI] [PubMed] [Google Scholar]
- 36.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol 2005;45:743–8. [DOI] [PubMed] [Google Scholar]
- 37.McCrohon JA, Moon JC, Prasad SK et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003;108:54–9. [DOI] [PubMed] [Google Scholar]
- 38.Assomull RG, Shakespeare C, Kalra PR et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation 2011;124:1351–60. [DOI] [PubMed] [Google Scholar]
- 39.Schelbert EB, Cao JJ, Sigurdsson S et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majmudar MD, Murthy VL, Shah RV et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging 2015;16:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panza JA, Holly TA, Asch FM et al. Inducible myocardial ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 2013;61:1860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff G, Dimitroulis D, Andreotti F et al. Survival Benefits of Invasive Versus Conservative Strategies in Heart Failure in Patients With Reduced Ejection Fraction and Coronary Artery Disease: A Meta-Analysis. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


