Abstract
Introduction
Fetal surgery for open spina bifida (OSB) requires comprehensive preoperative assessment using imaging for appropriate patient selection and to evaluate postoperative efficacy and complications. We explored patient access and conduct of fetal magnetic resonance imaging (MRI) for prenatal assessment of OSB patients eligible for fetal surgery. We compared imaging acquisition and reporting to the International Society of Ultrasound in Obstetrics and Gynecology MRI performance guidelines.
Material and methods
We surveyed access to fetal MRI for OSB in referring fetal medicine units (FMUs) in the UK and Ireland, and two NHS England specialist commissioned fetal surgery centers (FSCs) at University College London Hospital, and University Hospitals KU Leuven Belgium. To study MRI acquisition protocols, we retrospectively analyzed fetal MRI images before and after fetal surgery for OSB.
Results
MRI for fetal OSB was accessible with appropriate specialists available to supervise, perform, and report scans. The average time to arrange a fetal MRI appointment from request was 4 ± 3 days (range, 0–10), the average scan time available was 37 ± 16 min (range, 20–80 min), with 15 ± 11 min (range, 0–30 min) extra time to repeat sequences as required. Specific MRI acquisition protocols, and MRI reporting templates were available in only 32% and 18% of units, respectively. Satisfactory T2‐weighted (T2W) brain imaging acquired in three orthogonal planes was achieved preoperatively in all centers, and 6 weeks postoperatively in 96% of FSCs and 78% of referring FMUs. However, for T2W spine image acquisition referring FMUs were less able to provide three orthogonal planes presurgery (98% FSC vs. 50% FMU, p < 0.001), and 6 weeks post‐surgery (100% FSC vs. 48% FMU, p < 0.001). Other standard imaging recommendations such as T1‐weighted (T1W), gradient echo (GE) or echoplanar fetal brain and spine imaging in one or two orthogonal planes were more likely available in FSCs compared to FMUs pre‐ and post‐surgery (p < 0.001).
Conclusions
There was timely access to supervised MRI for OSB fetal surgery assessment. However, the provision of images of the fetal brain and spine in sufficient orthogonal planes, which are required for determining eligibility and to determine the reversal of hindbrain herniation after fetal surgery, were less frequently acquired. Our evidence suggests the need for specific guidance in relation to fetal MRI for OSB. We propose an example guidance for MRI acquisition and reporting.
Keywords: accessibility, fetal surgery, magnetic resonance imaging, open spina bifida, protocols, sequences
Fetal surgery for open spina bifida (OSB) is performed in selected patients. We explored ease of access, acquisition and reporting of fetal magnetic resonance imaging (MRI) in patients undergoing fetal surgery for OSB. Agreed criteria for fetal MRI in cases of OSB is a priority to ensure correct patient selection as well as to critically evaluate the efficacy of fetal surgery.
Abbreviations
- DTI
diffusion tensor imaging
- DWI
diffusion weighted imaging
- EPI
echoplanar images
- FLAIR
fluid attenuated inversion recovery
- FMU
fetal medicine unit
- FSC
fetal surgery centers
- GE
gradient echo
- ISUOG
International Society of Obstetrics and Gynecology
- MFM
maternal fetal medicine
- MRI
magnetic resonance imaging
- OSB
open spina bifida
- SRR
super resolution reconstruction
- SSFP
steady‐state free precession
- T1W
T1‐weighted
- T2W
T2‐weighted
Key message.
Agreed criteria for fetal magnetic resonance imaging (MRI) in cases of open spina bifida (OSB) is a priority to ensure correct patient selection as well as to critically evaluate the efficacy of fetal surgery. Guidance for fetal MRI acquisition and reporting are required to improve the evaluation of fetal OSB with enhanced communication between fetal surgery and referring units.
1. INTRODUCTION
Fetal open spina bifida (OSB) surgery is now offered in many countries based on level I evidence of improved postnatal outcome. 1 Before embarking on fetal surgery, robust imaging is essential to provide comprehensive preoperative evaluation and prognostication. 2 , 3 Whilst ultrasound remains the primary imaging modality for both first‐line screening and detailed assessment due to its low cost, real‐time capability and high spatial resolution, it is susceptible to variable image quality due to factors such as maternal habitus, fetal position, and reverberation artifacts from the calvarium. 4 , 5 , 6 , 7 , 8 Magnetic resonance imaging (MRI) is an important adjunct as it provides excellent soft tissue contrast and enhances visualization through multiplanar imaging with a large field‐of‐view. 3 Moreover, MRI is more likely to detect and characterize supratentorial anomalies (eg corpus callosum dysgenesis, heterotopia). 3 , 6 , 7 , 9 , 10 Individuals with these migrational disorders can have neurodevelopmental delay which is of prognostic significance. 3 , 11 Postoperative MRI permits detailed assessment of hindbrain herniation reversal, which has been proposed as a method to gauge fetal surgery response. 3 , 6 , 7 , 9 , 10 , 12 It also allows for evaluating ventricular growth, aqueduct integrity, and identifying intracranial hemorrhage post‐procedure which may be an exacerbating factor for hydrocephalus and predictive of ventriculoperitoneal shunt requirement. 9 , 13 , 14 , 15 , 16 , 17 , 18 MRI quality, however, varies due to factors such as operator experience, different MRI equipment, and accessibility. 19 , 20 When MRI imaging is suboptimal due to fetal motion artifacts, post‐acquisition research advances such as super resolution reconstruction (SRR) may mitigate these effects allowing for three‐dimensional segmentation, and volumetry. 21 , 22 In offering an OSB fetal surgery service it is important to promote MRI consistency and enhance fetal imaging expertise to benefit patient care. This is in line with work from other organizations such as the European Society of Pediatric Radiology which aim to improve perinatal MRI uniformity. 23 Our study objectives were to explore MRI capacity and acquisition for prenatal OSB assessment. We first undertook a survey assessing access, conduct and reporting of fetal MRI for OSB fetal surgery in the UK, Belgium and Ireland. Second, we evaluated MRI acquisition protocols in comparison with the International Society of Obstetrics and Gynecology (ISUOG) fetal MRI performance guidelines. 20
2. MATERIAL AND METHODS
2.1. Survey
We devised an electronic questionnaire (Table S1) sent to healthcare professionals in regional referring fetal medicine units (FMUs) in the UK and Ireland who had referred patients to the two NHS England commissioned Fetal Surgery Centers (FSCs) at University College London Hospital, London and University Hospitals KU Leuven Belgium. The questionnaire was collaboratively designed by maternal fetal medicine (MFM) specialists, neurosurgeons, pediatric radiologists, fetal neuroradiologists, MRI physicists, and fetal medical image analysis specialists in the FSCs. Respondents' professional role, hospital, and country of practice was collected. In the questionnaire, we described the clinical rationale for fetal MRI use in prenatal OSB closure, which includes diagnosis of intra‐ and extracranial anomalies that may not be detectable on ultrasound but are important to aid with selection of appropriate fetal surgery candidates. The NHS England fetal surgery protocol also requests NHS centers to perform an MRI evaluation approximately 6 weeks postoperatively to evaluate hindbrain herniation reversal and ventriculomegaly.
The questionnaire was distributed between April and November 2021 to regional referring FMUs across the UK and Ireland that had referred patients to the FSCs since December 2018. More than one answer could be selected for some questions, accompanied by a free text field to enter any additional information that was not listed. Responses were collected and analyzed using questionnaire survey software (Survey Monkey, Momentive Inc., San Mateo, California, USA, www.momentive.ai). We requested the respondent's views on MRI accessibility in their units, the interval between the request and the OSB fetal MRI appointment, the average duration of a second trimester fetal MRI slot, and how much extra time is available during imaging to repeat sequences due to fetal motion, if necessary. We also analyzed details (where questions could have multiple responses) on the professional roles and expertise of the individuals supervising, conducting, and reporting the fetal MRI scans of OSB patients, and assessed what clinical details and ultrasound imaging details were available to the radiologist prior to reporting of the MRI scan. Information on available MRI reporting templates, MRI fetal centile growth charts, and MRI acquisition protocols was also collected.
2.2. MRI acquisition and sequences
A consecutive sample of 50 OSB patients were retrospectively analyzed to infer MRI details on acquisition protocols, sequences and parameters. Images were obtained in patients meeting the management of myelomeningocele study criteria for fetal surgery. 1 , 24 MRI was performed as recommended by the NHS England commissioned service, at three (or more) time points for each NHS patient: before prenatal surgery, approximately 1 week after surgery which was always performed by the FSC, and 6 weeks after fetal surgery. The initial MRI scan was performed by the regional referring FMUs and/or the FSCs. The six‐week post fetal surgery MRI scan was performed in either the regional referring FMUs or the London FSC. For each time point, MRI acquisition parameters were collected including details of the sequences used and area of body scanned, field strength, slice thickness (mm), spacing between slices (mm), echo time (TE, ms), repetition time (TR, ms), pixel bandwidth (Hz), pixel spacing (mm), flip angle (FA, degrees), and specific absorption rate. Data on the standard, additional, and advanced MRI sequences, along with the number of orthogonal planes acquired for the fetal brain and body, were also analyzed and compared to the ISUOG MRI practice guidelines. 20 We furthermore objectively assessed the quality of the three orthogonal T2‐weighted 2D MRI stacks of the fetal brain through the application of post‐acquisition SRR of the fetal brains which was performed using the NiftyMIC algorithm. 21 SRR is an emerging research tool which depends on satisfactory original data quality acquired in at least three orthogonal T2‐weighted 2D MRI stacks of the fetal brain.
2.3. Statistical analyses
Automatic extraction of relevant MRI acquisition parameters from all stacks was performed using an automated Python script. Statistical analysis was performed with Excel (Microsoft 365) and SPSS Statistics (IBM Corp. Released 2020. IBM SPSS Statistics for Macintosh, version 27.0. Armonk, NY: IBM Corp). Summary statistical calculations with 95% confidence interval of the mean were performed on all survey data. Kruskal‐Wallis one‐way analysis of variance with correction for multiple comparisons was used to assess MRI sequences and plane acquisition between centers. Results are documented as test statistic (degree of freedom) and the p‐value. Statistical significance was set at <5%. Owing to the limited number of comments, for free text answers, a summary of the common theme was discussed without formal qualitative or quantitative analysis.
3. RESULTS
3.1. Fetal MRI survey respondents
The survey was sent to two FSCs and 27 regional referring FMUs which offered a fetal MRI service. A response rate of 76% was achieved (22 responses). Only one response was received per FMU and each of the two FSCs. Job roles included nine pediatric radiologists, seven pediatric and fetal neuroradiologists, five MFM specialists, and one senior radiographer (Figure 1).
FIGURE 1.
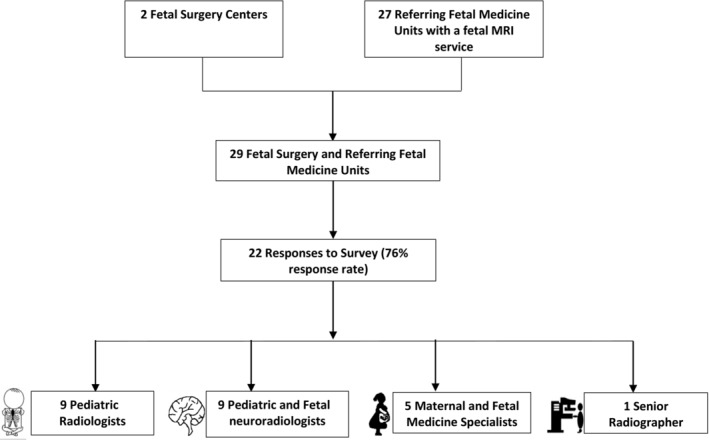
Flow chart of survey response and breakdown of respondent professional roles.
3.2. Fetal MRI accessibility
From the time of MR request, it reportedly took an average of 4 ± 3 days (range, 0–10 days) to arrange any fetal MRI appointment. The reported duration of a second trimester MRI slot was 37 ± 16 min (range, 20–80 min), with 15 ± 11 min (range, 0–30 min) extra time available to repeat sequences due to fetal motion if necessary.
3.3. Fetal MRI performance and reporting
The 22 respondents indicated that OSB fetal MRI acquisition was mainly supervised by pediatric neuroradiologists (9), pediatric radiologists (9) and fetal radiologists (6). Other professionals who supervised the fetal OSB MRI scans included adult neuroradiologists (3), senior radiographers (2), pediatric MRI radiographers (2), and senior MRI physicists (2). The majority of our respondents indicated that a senior radiographer (21) carried out the OSB fetal MRI scan (Figure 2).
FIGURE 2.
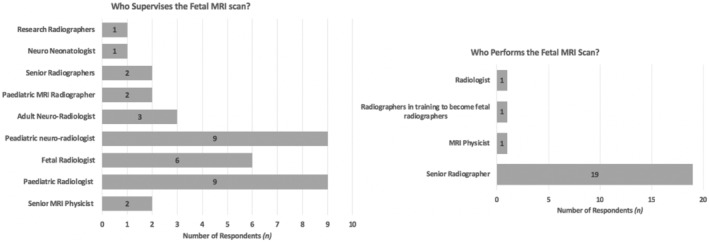
Results of the survey regarding the professional role of the individuals supervising the fetal open spina bifida (OSB) magnetic resonance imaging (MRI) scan (left), and the role of the individual preforming the fetal OSB MRI scan (right).
The fetal OSB MRI scans (n = 22 respondents) were reported by pediatric neuroradiologists (11), pediatric radiologists (9), and fetal radiologists (8). Radiologists reporting the MRIs (n = 22 respondents) had access to any available previous MRI images and reports (18), and detailed FMU ultrasound reports (19). Radiologists reported having limited access to the details of fetal surgery (4) and postoperative progress (6), Figure 3.
FIGURE 3.
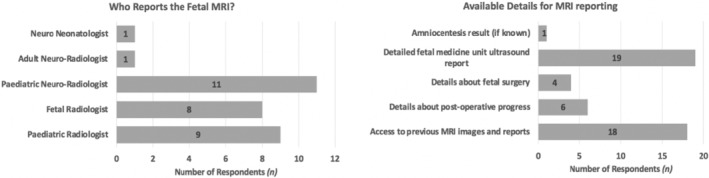
Results of the survey regarding the specialty of the radiologist reporting the fetal open spina bifida (OSB) magnetic resonance imaging (MRI) scan (left), and the details that are available for reporting the fetal OSB MRI scan (right).
We furthermore wished to gauge the availability of MRI acquisitional protocols and reporting templates for fetal OSB MRI scans. Only 32% of MRI units (which include both the FSCs) had specific MRI acquisition protocols and sequences available for fetal MRI OSB scans. Specific fetal OSB MRI reporting templates were available in 18% of MRI units whilst fetal centile growth charts for reporting were available in 55%.
3.4. Free text commentary
Respondents were also given the opportunity to leave any free text comments in the questionnaire. These are reported in Table 1, grouped by topic. The key themes are the need for guidance on optimal fetal OSB MRI acquisition and reporting, and improved communication between FSCs and referring FMUs.
TABLE 1.
Free text commentary.
| Topic | Comment |
|---|---|
| Fetal open spina bifida (OSB) magnetic resonance imaging (MRI) acquisition and reporting |
|
| Communication |
|
3.5. Observational MRI pilot study
We collected MRI data of 50 consecutive patients eligible for OSB surgery before (mean 23 + 3 ± 1 + 2 weeks + days [range 20 + 4–26 + 1]), approximately 1 week after (mean 26 + 3 ± 1 + 2 weeks + days [range 24 + 1–27 + 2]) and 6 weeks after fetal surgery (mean 32 + 3 ± 1 + 2 weeks + days [range 28 + 2–34 + 1]). One patient did not undergo any MRI due to claustrophobia, and one patient declined fetal surgery and opted for postnatal repair and were thus excluded. Some patients had more than one scan before and after surgery. Of the 178 scans available for analysis, 63 were performed presurgery at FSCs, and 16 at referring FMUs. Approximately 1 week after surgery 49 scans were performed at FSCs, and only one at an FMU. Six weeks after surgery 26 scans were performed at FSCs, and 23 scans in FMUs.
3.6. Standard MRI acquisition protocol and sequences
All MRI data acquisitions, and sequences were compared against the ISUOG practice guidelines for performance of fetal MRI which recommends good quality acquisitions in at least three orthogonal planes for the fetal brain and body using T2‐weighted (T2W) contrast, and one or two planes using T1‐weighted (T1W) and T2* gradient echo (GE) or echoplanar images (EPI). 20
3.7. T2W imaging
T2‐weighted (T2W) imaging in three orthogonal planes for the fetal brain was acquired in 100% of presurgery MRI scans. There was no difference in T2W fetal brain imaging acquisition between FSCs (96%) and referring FMUs (78%) 6 weeks post‐surgery (test statistic [1] = 3.6, p = 0.059). However, there was a difference in the acquisition of T2W spine imaging in three orthogonal planes between FSCs and referring FMUs presurgery, and 6 weeks post‐surgery (98% vs. 50% completion; test statistic [1] = 29.3, p < 0.001), and (100% vs. 48% completion; test statistic [1] = 17.6, p < 0.001), Figure 4.
FIGURE 4.
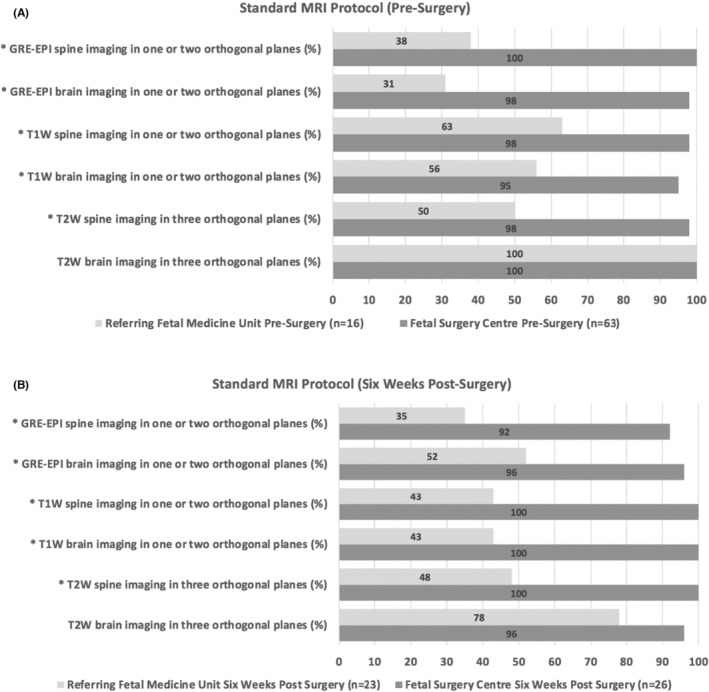
Standard magnetic resonance imaging (MRI) protocol compliance for scans (A) presurgery, and (B) 6 weeks post‐surgery. The y‐axis indicates the recommended International Society of Obstetrics and Gynecology practice guidelines for standard MRI sequences and plane acquisition. The x‐axis displays the percentage (%) of scans which meet the guidance. Dark gray bars indicate the fetal surgery centers, and light gray indicate the referring fetal medicine units. *Demonstrates a significant difference between groups p < 0.001. The standard MRI protocol compliance for T2W brain imaging in three orthogonal planes 6 weeks post‐surgery was insignificant (p‐value = 0.059).
3.8. T1W imaging
For T1W fetal brain imaging acquired in one or two orthogonal planes there was a significant difference in performance between FSCs and referring FMUs presurgery, and 6 weeks post‐surgery (95% vs. 56% completion; test statistic [1] = 17.3, p < 0.001), and (100% vs. 43% completion; test statistic [1] = 19.6, p < 0.001). Similarly, there was also a difference in performance of T1W fetal spine imaging acquired in one or two orthogonal planes between FSCs and referring FMUs presurgery, and 6 weeks post‐surgery (98% vs. 63% completion; test statistic [1] = 20.1, p < 0.001) and (100% vs. 43% completion; test statistic [1] 19.0, p < 0.001) Figure 4.
3.9. T2*W GE or EPI imaging
T2*W GE or EPI fetal brain image acquisition in one or two orthogonal planes was more often completed in FSCs vs referring FMUs presurgery, and 6 weeks post‐surgery (98% vs. 31% completion; test statistic [1] = 44.1, p < 0.001) and (96% vs. 52% completion; test statistic [1] = 12.5, p < 0.001). Likewise, T2*W GE or EPI fetal spine image acquisition was more likely to be completed at FSCs compared to referring FMU's presurgery, and 6 weeks post‐surgery (100% vs. 38% completion; test statistic [1] = 44.5, p < 0.001) and (92% vs. 35% completion; test statistic [1] = 17.4, p < 0.001), Figure 4. Almost all scans performed one‐week post‐surgery were done in FSCs; the compliance for each ISUOG standard MRI practice recommendation was ≥96% (Figure S2).
3.10. Additional MRI sequences
Some additional MRI sequences performed in both FSC and referring FMUs included dynamic steady‐state free precession (SSFP), diffusion weighted imaging (DWI), and diffusion tensor imaging (DTI) which are described as optional sequences in the ISUOG MRI practice guidance. Other optional additional sequences performed by FSCs include fluid attenuated inversion recovery (FLAIR). Further additional MRI sequences performed by referring FMUs included short tau inversion recovery, and modified liver acquisition with volume acceleration flexible MRI (LAVA FLEX) (Figures S3 and S4).
3.11. MRI acquisition parameters
Fetal MRI in FSCs and referring FMUs were all acquired at 1.5T, which is the most commonly used magnetic field strength providing acceptable resolution even in early second trimester scans. 8 , 20 All MRI acquisition parameters of standard and additional optional sequences for FSCs pre‐, one‐week and 6 weeks after fetal surgery are illustrated in Tables S2–S4.
3.12. Automated super‐resolution reconstruction
SRR of the fetal brain was not possible in 10 (16%) of presurgery FSC scans, compared to nine (56%) of referring FMU scans due to excessive fetal motion. For the scans performed one‐week post‐surgery, SRR of the fetal brain was not possible in four (8%) of FSC scans due to excessive fetal motion, and one scan (100%) from a referring FMU due to an acquisition of less than three orthogonal T2‐weighted 2D MRI stacks. SRR of the fetal brain was successful in all FSC scans performed 6‐weeks post‐surgery. In the referring FMUs, SRR of the fetal brain was not possible in 11 (48%) of the scans performed 6‐weeks post‐surgery. This was due to excessive fetal motion resulting in not enough artifact‐free data in six (26%) cases, and an acquisition of less than three orthogonal planes in five (22%) cases (Figure 5).
FIGURE 5.
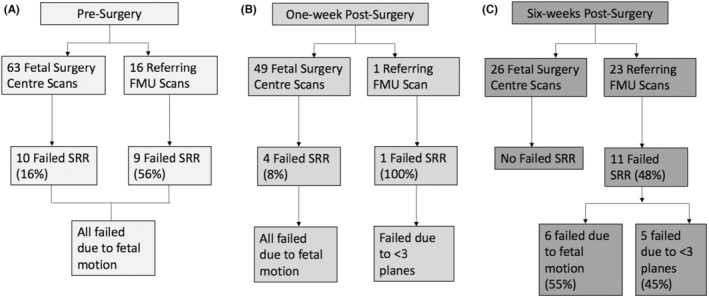
Flow chart of the success of post‐acquisition fetal brain super resolution reconstruction (SRR) magnetic resonance imaging for (A) presurgery, (B) one week post‐surgery scans, and (C) six weeks post‐surgery scans in fetal surgery centers (FSC) and referring fetal medicine units (FMU).
4. DISCUSSION
Our survey showed that there is MRI accessibility with appropriate time allocation allowing for protocol completion as well as extra time dedicated to sequence repetition in case of excessive fetal movement. MRI supervision, and performance was carried out by individuals with specific expertise and training which ensures appropriate choice of protocols, techniques, and adaptations based on unforeseen artifacts and fetal motion. MRI reporting was performed by specialists able to provide targeted information specific to fetal OSB, although 82% of units indicated they do not have a specific OSB reporting template. Table 2 shows a suggested targeted OSB reporting template which may be used in addition to the standard evaluation of the whole fetal and maternal anatomy (eg chest, abdomen, placenta, and cervix). Supporting information provided for MRI reporting consisted of mainly detailed MFM sonographic findings, and access to previous MR imaging and reports. However, communication about fetal surgery details, postoperative progress and access to genetic prenatal diagnostic results could be enhanced as this could aid reporting. Most FMUs (68%) indicated they did not have specific fetal OSB MRI acquisition protocols, which potentially contributed to the variability in sequences and plane acquisition observed. 20 Satisfactory T2W brain imaging acquired in three orthogonal planes was, however, almost always achieved which is essential for comprehensive brain imaging and interpretation. This highlights the potential of standardized OSB imaging for optimal service provision with dissemination of knowledge and skills between units. Furthermore, although brain SRR success was reduced, this was owing to challenges of small fetal size and increased fetal motion encountered in the early second trimester in the majority of cases. 28
TABLE 2.
Targeted reporting template for fetal open spina bifida.
| Anatomy | Description |
|---|---|
| Cranial biometric assessments | Measurements are performed according to gestational age and using fetal centile growth charts:
|
| Brain |
|
| Spine and cord |
|
| Musculoskeletal |
|
| Other |
|
Comprehensive imaging is required to select the optimal OSB candidate for fetal surgery and to properly assess postoperative efficacy and complications. This assessment should be performed in accordance with the ISUOG MRI practice guidance advising good quality acquisitions in three orthogonal planes (axial, coronal, and sagittal) using T2W information, and one or two orthogonal planes (preferably coronal and sagittal) using T1 and T2*‐weighted GE or EPI sequences for the fetal brain and body. These should be repeated if excessive fetal motion corrupts good quality image acquisition. 8 , 20 T2W information is the mainstay of fetal MRI, and the main two sequences used are single shot fast spin echo and SSFP. 8 In fetuses with OSB, single shot fast spin echo is more commonly used as it allows excellent depiction of the brain and fluid filled cavities, while SSFP allows better depiction of vessels and the fetal heart. 8 However with adjusted parameters SSFP may be used for fetal brain evaluation with equivalent diagnostic accuracy. 29 The most robust fetal T1W sequences are fast low angle shot or fast field echo sequences. In relation to fetal OSB they are helpful for identifying methaemoglobin in subacute hemorrhage. 20 , 30 T2*‐weighted sequences (with GE or EPI imaging) are extremely useful in detecting blood breakdown products such as deoxyhemoglobin suggesting acute hemorrhage, and hemosiderin representing an older bleed. 20 This is particularly important in fetal OSB with ventriculomegaly and hydrocephalus, as the detection of hemosiderin deposits in the ventricular lining is diagnostic of past hemorrhage. 8 , 31 , 32 T2*‐weighted GE or EPI acquisitions are also useful for fetal OSB as they visualize susceptibility artifacts to detect bony structures which can be used for fetal skeleton evaluation. 8 , 33 , 34 After performing the above standard sequences, optional additional imaging such as dynamic SSFP, DWI, and DTI can be performed if there is sufficient time remaining. 20 Dynamic SSFP evaluates lower limb movements in OSB while DWI characterizes brain lamination by providing quantifiable measurements of the constant random (Brownian) water molecule movement using apparent diffusion coefficient values. 8 , 35 , 36 , 37 , 38 , 39 This is also possible to visualize with the EPI‐FLAIR sequence. 40 DTI can provide more detailed microstructural information through markers sensitive to the degree of directional restriction of water molecule diffusion, which can be presented as fractional anisotropy maps or post‐processed to identify and reconstruct the brain connectivity by tractography. 41 , 42 , 43 , 44 , 45 Table 3 displays standard and optional imaging sequences for fetal OSB as per the ISUOG guidance which is important to allow diversity and research between units. Although predominantly a research tool, SRR has the potential for clinical adoption. Post‐acquisition SRR can be performed to mitigate fetal motion effects and allow for enhanced fetal brain assessment. This is particularly useful in fetal OSB where the initial MRI assessment is usually performed prior to 25 + 6 weeks (the upper gestational age limit for prenatal surgery) which poses challenges due to small fetal size. 3 , 21 , 22 SRR is dependent on good quality original imaging obtained with sufficient image stacks in all three planes, as evident from our study given the number of unsuccessful reconstructions, which further supports following the standard ISUOG MRI acquisition criteria. SRR may be used in conjunction with original imaging for diagnosis but further technical developments are still needed to support its full clinical translation. 21 , 22
TABLE 3.
Imaging sequences for fetal OSB MRI adapted from ISUOG guidance. 20
| Anatomy | Sequences | Open spina bifida (OSB) application |
|---|---|---|
| Fetal brain and body (standard protocol) | Three planes T2W single shot fast spin echo (SSFSE), or HASTE | Depiction of brain and fluid filled cavities |
| One or 2 planes T1‐weighted (T1W), fast low angle shot (FLASH) | Evaluation of subacute hemorrhage and T1 hyperintense tissues (eg myelin) | |
| One or 2 plane gradient echo (GE) or echo planar (EP) susceptibility imaging (eg T2*) | Evaluation of hemorrhage and fetal skeleton | |
| Fetal brain (optional) | Diffusion weighted imaging (DWI) | Evaluation of brain lamination |
| Diffusion tensor imaging (DTI) | Fractional anisotropy maps for structural brain development and tractography for evaluation of fibers | |
| Fetal body (optional) | Dynamic steady‐state free precession (SSFP) | Assessing lower limb movement |
| Thick slab imaging | Provide 3D imaging of fetal movement to assess limb movement |
FSCs have an established multidisciplinary team of specialists such as pediatric radiologists, neuroradiologists, pediatric neurologists, MFM specialists, and neurosurgeons who are familiar with this condition and the option of fetal surgery to provide optimal parental counseling. Our survey highlights the need for improved communication and sharing of MRI acquisition and reporting guidance between FSCs and referring FMUs. Possible future studies could additionally include a DELPHI international survey in order to achieve a wider consensual protocol for the conduct of MRI, acquisition and reporting which is targeted for OSB at all time points pre‐ and post‐surgery.
One strength of our study was that we received information from a range of healthcare professionals involved in fetal OSB MRI such as MFM specialists, radiologists, and radiographers. This is important as the clinical pathway from MRI performance to interpretation requires a multidisciplinary collaborative approach. Another strength of our study was reinforcing our survey results by analyzing a large number of longitudinal scans (178) to test MRI acquisition protocols against ISUOG standards. We furthermore objectively tested data quality by performing post‐acquisition SRR of the fetal brain. One limitation of our work was responder bias, as well as response rate which is a challenge encountered with any survey. Participant selection bias was another limitation which was reduced through contacting units via a pre‐made mailing list produced since commencement of the OSB fetal surgery service.
5. CONCLUSION
Our survey demonstrated that there is timely access to supervised MRI for assessment of patients with OSB for fetal surgery in our population. However, the provision of images in sufficient orthogonal planes, which are required to confirm eligibility and determine the efficacy of fetal surgery in the brain and spine, are less frequently acquired. There is a need for specific guidance on protocols for fetal MRI acquisition and image reporting to optimize diagnostic accuracy, parental counseling, and post‐natal management. We propose an example guidance for MRI acquisition and reporting.
AUTHOR CONTRIBUTIONS
NM designed the study, analyzed the data, wrote the first draft and corrected the final version of the manuscript. MA and DT provided advice on MRI acquisition content in the manuscript, and supervised all the MRI images acquired in KU Leuven, Belgium. DT and PDV provided advice on neurosurgical and neuroanatomical content in this manuscript. JD, ALD and AM supported and supervised this work since conception.
FUNDING INFORMATION
This study was supported by the Guided Instrumentation of Fetal Therapy and Surgery (GIFT‐Surg) project, funded by the Wellcome Trust (203148/Z/16/Z; 203145Z/16/Z; WT101957) and Engineering and Physical Sciences Research Council (EPSRC) (NS/A000049/1; NS/A000050/1; NS/A000027/1; EP/L016478/1). This grant included external peer review for scientific quality with a patient and public involvement panel. SO is the principal investigator on this grant, and ALD, JD, TV and AM are coinvestigators. LF is funded by the European Union's Horizon 2020 research and innovation program under the Marie‐Sklodowska‐Curie grant agreement TRABIT no. 765148. NM is funded with support of the Wellcome/EPSRC center for Interventional and Surgical Sciences (WEISS) (203145Z/16/Z). PDV is a senior clinical investigator of Research Fund Flanders (FWO 18B2322N). ALD is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Center. The funders had no direction in the study design, data collection, data analysis, manuscript preparation or publication decision.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
6. Ethics statement
Ethical approval was given for this retrospective observational study. All MRI data were analyzed under the study entitled “Guided Instrumentation for Fetal Therapy and Surgery (GIFT‐Surg): Fetal MRI to Improve Prenatal Diagnosis and Therapy for Fetal Abnormality” (Hampstead Research Ethics Committee, 15/LO/1488 on October 19, 2015 with ammendments approved on February 28, 2019 and August 16, 2022). Women provided written informed consent for fetal MRI research. All images were transferred with Caldicott Guardian approval from University College London Hospitals to collaborators at partner academic institutions (University College London, King's College London, and Katholieke Universiteit Leuven [KU Leuven]) via the secure GIFT‐cloud platform, which ensures complete de‐identification through XNAT technology. 25 No ethical approval was required for the survey.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The authors would like to thank Dr Fernando Perez Garcia for writing the Python script for the automatic extraction of relevant MRI acquisition parameters.
The GIFT‐Surg Imaging Working Group: David Atkinson, Center for Medical Imaging, University College London, London, UK; Foteini Emmanouella Bredaki, Women's Health Division, University College London Hospitals, London, UK; Joanna Chappell, School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, UK; Luc De Catte, Department of Obstetrics and Gynecology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium; Roland Devlieger, Department of Obstetrics and Gynecology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium; Michael Ebner, School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, UK; Lucas Fidon, School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, UK; Trevor Gaunt, Radiology Department, Great Ormond Street Hospital for Children, London, UK; Giles S. Kendall, Women's Health Division, University College London Hospitals, London, UK, Elizabeth Garrett Anderson Institute for Women's Health, University College London, UK; Sebastien Ourselin, School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, UK, Medical Physics and Biomedical Engineering, University College London, UK; Kelly Pegoretti Baruteau, Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, University College London Hospitals, UK; Adalina Sacco, Women's Health Division, University College London Hospitals, London, UK, Elizabeth Garrett Anderson Institute for Women's Health, University College London, UK; Magdalena Sokolska, Department of Medical Physics and Biomedical Engineering, University College London Hospitals, UK; Tom Vercauteren, School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, UK, Medical Physics and Biomedical Engineering, University College London, UK.
Collaborator contributions: Magdalena Sokolska, David Atkinson, Trevor Gaunt and Kelly Pegoretti Baruteau guided MRI acquisitions and protocols in UCLH, London, UK. Trevor Gaunt and Kelly Pegoretti Baruteau also reported the acquired MRI images. Luc De Catte performed and supervised the acquisition of ultrasound imaging. Roland Devlieger provided advice on fetal surgery content in this manuscript. Giles S. Kendall provided advice on neuro‐neonatal content. Foteini Emmanouella Bredaki and Adalina Sacco helped in case collection as fetal surgery coordinators. Michael Ebner developed and optimized the SRR algorithm. Lucas Fidon developed the automated atlas‐based segmentation methods. Joanna Chappell carried out technical analysis on reconstructed images. Tom Vercauteren and Sebastien Ourselin supervised the development and optimisation of the super resolution reconstruction algorithm.
Mufti N, Aertsen M, Thomson D, et al. Longitudinal MRI in the context of in utero surgery for open spina bifida: A descriptive study. Acta Obstet Gynecol Scand. 2024;103:322‐333. doi: 10.1111/aogs.14711
Contributor Information
Nada Mufti, Email: n.mufti@ucl.ac.uk.
the GIFT‐Surg Imaging Working Group:
David Atkinson, Foteini Emmanouella Bredaki, Joanna Chappell, Luc De Catte, Roland Devlieger, Michael Ebner, Lucas Fidon, Trevor Gaunt, Giles S. Kendall, Sebastien Ourselin, Kelly Pegoretti Baruteau, Adalina Sacco, Magdalena Sokolska, and Tom Vercauteren
REFERENCES
- 1. Adzick NS, Thom EA, Spong CY, et al. A randomised trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunpalin Y, Richter J, Mufti N, et al. Cranial findings detected by second trimester ultrasound in fetuses with myelomeningocele: a systematic review. BJOG. 2021;128:366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mufti N, Sacco A, Aertsen M, et al. What brain abnormalities can magnetic resonance imaging detect in foetal and early neonatal spina bifida: a systematic review. Neuroradiology. 2022;64:233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Araujo Junior E, Nakano ML, et al. Comparison between 2D ultrasonography and magnetic resonance imaging for assessing brain and spine parameters in fetuses with spina bifida. Arch Gynecol Obstet. 2013;287:845‐849. [DOI] [PubMed] [Google Scholar]
- 5. Saleem SN, Said AH, Abdel‐Raouf M, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected neural tube defects (NTDs): impact on diagnosis and management decision. Neuroradiology. 2009;51:761‐772. [DOI] [PubMed] [Google Scholar]
- 6. Levine D, Trop I, Mehta TS, Barnes PD. MR imaging appearance of fetal Cereberal ventricular morphology. Radiology. 2002;223:652‐660. [DOI] [PubMed] [Google Scholar]
- 7. Simon EM, Goldstein RB, Coakley FV, et al. Fast MR imaging of fetal CNS anomalies in utero. AJNR Am J Neuroradiol. 2000;21:1688‐1698. [PMC free article] [PubMed] [Google Scholar]
- 8. Aertsen M, Diogo MC, Dymarkowski S, Deprest J, Prayer D. Fetal MRI for dummies: what the fetal medicine specialist should know about acquisitions and sequences. Prenat Diagn. 2020;40:6‐17. [DOI] [PubMed] [Google Scholar]
- 9. Mangels KJ, Tulipan N, Tsao LY, Alarcon J, Bruner JP. Fetal MRI in the evaluation of intrauterine myelomeningocele. Pediatr Neurosurg. 2000;32:124‐131. [DOI] [PubMed] [Google Scholar]
- 10. Griffiths PD, Bradburn M, Campbell MJ, et al. Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet. 2017;389:538‐546. [DOI] [PubMed] [Google Scholar]
- 11. Hino‐Shishikura A, Niwa T, Aida N, Okabe T, Nagaoka T, Shibasaki J. Periventricular nodular heterotopia is related to severity of the hindbrain deformity in Chiari II malformation. Pediatr Radiol. 2012;42:1212‐1217. [DOI] [PubMed] [Google Scholar]
- 12. Sutton LNA, Bilaniuk LT, Johnson MP, Cromblehome TM, Flake AW. Improvement in hinbrain herniation demonstrate by serial fetal magnetic imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826‐1831. [DOI] [PubMed] [Google Scholar]
- 13. Rethmann C, Scheer I, Meuli M, Mazzone L, Moehrlen U, Kellenberger CJ. Evolution of posterior fossa and brain morphology after in utero repair of open neural tube defects assessed by MRI. Eur Radiol. 2017;27:4571‐4580. [DOI] [PubMed] [Google Scholar]
- 14. Aertsen M, Verduyckt J, De Keyzer F, et al. Reliability of MR imaging‐based posterior fossa and brain stem measurements in open spinal dysraphism in the era of fetal surgery. AJNR Am J Neuroradiol. 2019;40:191‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant RA, Heuer GG, Carrion GM, et al. Morphometric analysis of posterior fossa after in utero myelomeningocele repair. J Neurosurg Pediatr. 2011;7:362‐368. [DOI] [PubMed] [Google Scholar]
- 16. Didier RAM‐SJS, Oliver ER, DeBari SE, et al. Incidence and concordance of suspected intraventricular haemorrhage (IVH) on fetal us and MRI in open spinal dysraphism with postnatal follow‐up. Pediatr Radiol. 2019;49:1‐245.30937493 [Google Scholar]
- 17. Nasiadko CS, Meuli M, Moehrlen U, Ochsenbein N. Fetal brain morphology after in utero repair of open neural tube defects. Neuroradiology. Springer, Verlag. 2014;56(Suppl 1):104. [Google Scholar]
- 18. Nagaraj UD, Bierbrauer KS, Zhang B, Peiro JL, Kline‐Fath BM. Hindbrain herniation in Chiari II malformation on fetal and postnatal MRI. AJNR Am J Neuroradiol. 2017;38:1031‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malinger G, Lev D, Lerman‐Sagie T. Is fetal magnetic resonance imaging superior to neurosonography for detection of brain anomalies? Ultrasound Obstet Gynecol. 2002;20:317‐321. [DOI] [PubMed] [Google Scholar]
- 20. Prayer D, Malinger G, De Catte L, et al. ISUOG practice guidelines (updated): performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2023;61:278‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebner M, Wang G, Li W, et al. An automated framework for localization, segmentation and super‐resolution reconstruction of fetal brain MRI. Neuroimage. 2020;206:116324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davidson JR, Uus A, Matthew J, et al. Fetal body MRI and its application to fetal and neonatal treatment: an illustrative review. Lancet Child Adolesc Health. 2021;5:447‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitby E, Offiah AC, Shelmerdine SC, et al. Current state of perinatal postmortem magnetic resonance imaging: European Society of Paediatric Radiology questionnaire‐based survey and recommendations. Pediatr Radiol. 2021;51:792‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sacco A, Ushakov F, Thompson D, et al. Fetal surgery for open spina bifida. Obstet Gynaecol. 2019;21:271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doel T, Shakir DI, Pratt R, et al. GIFT‐cloud: a data sharing and collaboration platform for medical imaging research. Comput Methods Programs Biomed. 2017;139:181‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'addario VP, Del Bianco A, Di Naro E, Tartagni G, Miniello G, Serio G. The clivus–supraocciput angle: a useful measurement to evaluate the shape and size of the fetal posterior fossa and to diagnose Chiari II malformation. Ultrasound Obstet Gynecol. 2001;18:146‐149. [DOI] [PubMed] [Google Scholar]
- 27. Tsai T, Bookstein FL, Levey E, Kinsman SL. Chairi‐II malformation: a biometric analysis. Eur J Pediatr Surg. 2002;12:S12‐S18. [DOI] [PubMed] [Google Scholar]
- 28. Resta MGP, D'Addario V, Florio C, et al. Magnetic resonance imaging in pregnancy: study of fetal cerebral malformations. Ultrasound Obstet Gynecol. 1994;4:7‐20. [DOI] [PubMed] [Google Scholar]
- 29. Griffiths PD, Jarvis D, McQuillan H, Williams F, Paley M, Armitage P. MRI of the foetal brain using a rapid 3D steady‐state sequence. Br J Radiol. 2013;86:20130168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zizka J, Elias P, Hodik K, et al. Liver, meconium, haemorrhage: the value of T1‐weighted images in fetal MRI. Pediatr Radiol. 2006;36:792‐801. [DOI] [PubMed] [Google Scholar]
- 31. Putbrese B, Kennedy A. Findings and differential diagnosis of fetal intracranial haemorrhage and fetal ischaemic brain injury: what is the role of fetal MRI? Br J Radiol. 2017;90:20160253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanapo L, Whitehead MT, Bulas DI, et al. Intracranial hemorrhage: role of fetal MRI. Prenat Diagn. 2017;37:827‐836. [DOI] [PubMed] [Google Scholar]
- 33. Nemec SFNU, Brugger PC, Wadhawan I, Prayer D. Skeletal development on fetal magnetic resonance imaging. Top Mag Reson Imaging. 2011;22:101‐106. [DOI] [PubMed] [Google Scholar]
- 34. Robinson AJ, Blaser S, Vladimirov A, Drossman D, Chitayat D, Ryan G. Foetal “black bone” MRI: utility in assessment of the foetal spine. Br J Radiol. 2015;88:20140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bammer R. Basic principles of diffusion‐weighted imaging. Eur Jf Radiol. 2003;45:169‐184. [DOI] [PubMed] [Google Scholar]
- 36. Kim DH, Chung S, Vigneron DB, Barkovich AJ, Glenn OA. Diffusion‐weighted imaging of the fetal brain in vivo. Magn Reson Med. 2008;59:216‐220. [DOI] [PubMed] [Google Scholar]
- 37. Schneider MM, Berman JI, Baumer FM, et al. Normative apparent diffusion coefficient values in the developing fetal brain. AJNR Am J Neuroradiol. 2009;30:1799‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prayer D, Kasprian G, Krampl E, et al. MRI of normal fetal brain development. Eur J Radiol. 2006;57:199‐216. [DOI] [PubMed] [Google Scholar]
- 39. Kostovic I, Judas M, Rados M, Hrabac P. Laminar organisation of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536‐544. [DOI] [PubMed] [Google Scholar]
- 40. Diogo MC, Prayer D, Gruber GM, et al. Echo‐planar FLAIR sequence improves subplate visualization in fetal MRI of the brain. Radiology. 2019;292:159‐169. [DOI] [PubMed] [Google Scholar]
- 41. Woitek R, Prayer D, Weber M, et al. Fetal diffusion tensor quantification of brainstem pathology in Chiari II malformation. Eur Radiol. 2016;26:1274‐1283. [DOI] [PubMed] [Google Scholar]
- 42. Jakab A, Schwartz E, Kasprian G, et al. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci. 2014;8:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomason ME, Scheinost D, Manning JH, et al. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep. 2017;7:39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kasprian G, Brugger PC, Weber M, et al. In utero tractography of fetal white matter development. Neuroimage. 2008;43:213‐224. [DOI] [PubMed] [Google Scholar]
- 45. Mitter C, Prayer D, Brugger PC, Weber M, Kasprian G. In vivo tractography of fetal association fibers. PloS One. 2015;10:e0119536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


