Abstract
Background
Patients with atrial fibrillation (AF) and heart failure (HF) have a high risk of thromboembolism and other outcomes and anticoagulation is recommended.
Hypothesis
This study was aimed to explore the risk factors associated with HF worsening in patients with AF and HF taking rivaroxaban in Spain.
Methods
Multicenter, prospective, observational study that included adults with AF and chronic HF, receiving rivaroxaban ≥4 months before entering. HF worsening was defined as first hospitalization or emergency visit because of HF exacerbation.
Results
A total of 672 patients from 71 Spanish centers were recruited, of whom 658 (97.9%) were included in the safety analysis and 552 (82.1%) in the per protocol analysis. At baseline, mean age was 73.7 ± 10.9 years, 64.9% were male, CHA2DS2‐VASc was 4.1 ± 1.5, HAS‐BLED was 1.6 ± 0.9% and 51.3% had HF with preserved ejection fraction. After 24 months of follow‐up, 24.9% of patients developed HF worsening, 11.6% died, 2.9% had a thromboembolic event, 3.1% a major bleeding, 0.5% an intracranial bleeding and no patient had a fatal hemorrhage. Older age, the history of chronic obstructive pulmonary disease, the previous use of vitamin K antagonists, and restrictive or infiltrative cardiomyopathies, were independently associated with HF worsening. Only 6.9% of patients permanently discontinued rivaroxaban treatment.
Conclusions
Approximately one out of four patients with HF and AF treated with rivaroxaban developed a HF worsening episode after 2 years of follow‐up. The identification of those factors that increase the risk of HF worsening could be helpful in the comprehensive management of this population.
Keywords: anticoagulation, atrial fibrillation, direct oral anticoagulant, heart failure, rivaroxaban, worsening heart failure
Multicenter, prospective, observational study that included 672 adults from 71 Spanish centers with AF and chronic HF, receiving rivaroxaban. At baseline, mean age was 73.7 ± 10.9 years, 64.9% were male, CHA2DS2‐VASc was 4.1 ± 1.5, HAS‐BLED was 1.6 ± 0.9% and 51.3% had HF with preserved ejection fraction. After 24 months of follow‐up, 24.9% of patients developed HF worsening, 11.6% died, 2.9% had a thromboembolic event, 3.1% a major bleeding, 0.5% an intracranial bleeding and no patient had a fatal hemorrhage. Older age, the history of chronic obstructive pulmonary disease, the previous use of vitamin K antagonists, and restrictive or infiltrative cardiomyopathies, were independently associated with HF worsening. Only 6.9% of patients permanently discontinued rivaroxaban treatment.

1. INTRODUCTION
Heart failure (HF) is a major healthcare problem. 1 HF is associated with great morbidity and mortality. 2 Importantly, HF hospitalization rates are increasing over time. 3 In addition, it has been reported that one in six patients with HF with reduced ejection fraction may develop worsening HF within 18 months after HF diagnosis. 4 A recent Spanish study has shown that nearly 30% of patients are hospitalized for HF within 1 year after the diagnosis, with a mortality rate of 8% during hospitalization. 5 Therefore, worsening HF is a common condition, that can occur in different clinical settings (i.e., hospitalization, emergency department, outpatient). Identifying factors associated with worsening HF is warranted to reduce HF burden. 6
It has been estimated that over 35% of patients with atrial fibrillation (AF) are also diagnosed with HF. This is not surprising, since HF and AF share common risk factors and mechanisms. 7 , 8 The concomitance of both conditions markedly worsens the prognosis. 9 Patients with AF and HF have a high risk of thromboembolism, and anticoagulation is recommended. 10
Although in the last years some studies have analyzed the risk factors for HF worsening, most of them have investigated all‐cause mortality associated with clinical characteristics of HF patients, 11 , 12 , 13 only few studies have focused on variables associated with HF hospital admissions, 14 , 15 and in patients mainly taking vitamin K antagonists. 16 Therefore, it seems necessary to determine those factors predicting worsening HF in AF patients treated with anticoagulant agents different to vitamin K antagonists.
The ROCKET‐AF trial showed that rivaroxaban was at least as effective as warfarin for the prevention of stroke or systemic embolism in a high thromboembolic risk AF population, with a lower risk of fatal and intracranial bleedings. 17 A post hoc analysis showed that the relative efficacy and safety of rivaroxaban was independent of the presence of HF. 18
The primary objective of this study was to explore the risk factors associated with HF worsening (measured by hospitalizations and emergency visits because of HF exacerbations) in patients with AF and HF treated with rivaroxaban. In addition, the cut‐off values of quantitative variables associated with HF worsening, the clinical profile of this population and the persistence with rivaroxaban were also analyzed.
2. METHODS
This was a multicenter, prospective, observational, cohort study that included adult patients with a diagnosis of nonvalvular AF, 10 NYHA class I–IV chronic HF (regardless of ejection fraction), 2 receiving rivaroxaban at least 4 months before being enrolled. By contrast, patients participating in a research program which involved some intervention beyond clinical practice, with significant mitral stenosis or other heart valvular diseases that required specific treatment (prosthesis or valvuloplasty), or with severe cognitive impairment were excluded from the study. The study was approved by the research ethical committee of Parc de Salut Mar. All patients provided written informed consent, before being included.
Patients were consecutively recruited during a routine follow‐up visit between March 2018 and July 2019 in 71 participating centers from Spain (all patients who met the inclusion/exclusion criteria were asked to be included). As the study was based on the routine clinical practice of the management of HF/AF patients, no specific diagnostic or therapeutic action were required for participating. Data about clinical history of the patients were collected from the electronic health records of the patients and in addition the investigator could complement the information by interviewing the patient during the routine visit. Patients were followed‐up during 2 years (baseline, follow‐up visits 1–3, and end of observation), according to clinical practice.
At baseline, biodemographic data, AF data (time since AF diagnosis, type of AF, CHA2DS2‐VASc score, 19 HAS‐BLED score 20 ), HF data (time since HF diagnosis, NYHA functional class, type of HF, 2 etiology of HF), vital signs, cardiovascular risk factors, vascular disease, and other comorbidities, as well as concomitant treatments were recorded. In addition, the Barthel Test, 21 Frail scale, 22 and Charlson Index 23 were also calculated.
The information regarding treatment with rivaroxaban along the study, including the previous use of vitamin K antagonists, dosage, medication adherence, and any change during the follow‐up, was also collected. Laboratory parameters (hemoglobin, fasting glucose, glomerular filtration rate, BNP, and NT‐proBNP) were compared at baseline and at study end (24 months).
The variable for the primary objective was the first HF worsening, defined as first HF hospitalization or admission to emergency department due to a HF exacerbation. The factors potentially influencing the primary endpoint were analyzed and included baseline variables regarding demography, health behavior, vital signs, disease history, comorbidities, prior and concomitant treatments, and laboratory parameters. Additionally, the occurrence of death, thromboembolic events, major bleedings, 24 intracranial bleedings, and fatal hemorrhages were also determined. The event rates during the follow‐up according to the use of rivaroxaban before inclusion (<6 months vs. 6–12 months vs. ≥12 months and <1 year vs. ≥1 year) were calculated.
Three types of analysis population were defined: (1) Safety analysis set: all patients that had received antithrombotic treatment because of AF, with rivaroxaban since at least 4 months before entering the study. The safety analysis set was used for the description of the safety analysis; (2) full analysis set: all patients that had received antithrombotic treatment because of AF, with rivaroxaban since at least 4 months before entering the study and who had satisfied the inclusion/exclusion criteria defined in the study protocol. The full analysis set was used for the main analyses; (3) per protocol set: all patients that had received antithrombotic treatment because of AF, with rivaroxaban since at least 4 months before entering the study, who had satisfied the inclusion/exclusion criteria defined in the study protocol and that had had at least one postbaseline visit, except for premature terminations due to death or adverse events. The per protocol set was used for the baseline description and the analyses of the primary and secondary objectives.
2.1. Statistical analysis
For the descriptive analyses, absolute and relative frequency distributions were used for the qualitative variables, and measures of central tendency (mean) and dispersion (standard deviation) for quantitative variables. Categorical variables were compared with the χ 2 test or the Fisher exact test when appropriate. When two independent means were compared, the t student test was used. The evolution (study end‐baseline) of laboratory parameters were compared using the paired sample t test.
Kaplan–Meier curves were used to assess the time to the first clinical outcome (HF worsening episode, thromboembolic event, all‐cause death, and major bleeding) according to the previous use of rivaroxaban (<6 months vs. 6–12 months vs. ≥12 months and <1 year vs. ≥1 year) and the Log Rank (Mantel–Cox) was calculated for each comparison to determine the presence of statistical differences.
To explore the risk factors for first HF hospitalization/emergency visit (HF worsening episode), baseline variables, including demography, health behavior, vital signs, disease history, comorbidities, laboratory parameters, prior and concomitant treatments, and previous hospitalizations and admissions to the emergency department, were considered for inclusion in a Cox proportional hazard model. The Cox model was computed by considering only the first event (i.e., hospitalization/emergency visit) after the baseline visit. To assess the cut‐off values of quantitative variables associated with HF worsening, they were transformed into dichotomous variables according to the cut‐offs defined in the literature. 14 , 15 Initially, feasibility of the factors was explored using bivariate models. Then, those with a p < .15 were included in the multivariate models. Only the significant factors (p < .05) were finally considered to build the models. All analyses are performed with SAS® version 9.4 (SAS Institute, Inc.).
3. RESULTS
A total of 672 patients were recruited, of whom 658 (97.9%) patients were included in the safety analysis set, 598 (89.0%) in the full analysis set, and 552 (82.1%) in the per protocol set. Reasons for exclusion are summarized in Figure 1. At baseline, mean age was 73.7 ± 10.9 years, 64.9% were male, and 33.9% were considered as frail. With regard to AF, 53.9% of patients had permanent AF, mean CHA2DS2‐VASc was 4.1 ± 1.5 and HAS‐BLED 1.6 ± 0.9. With respect to HF, the majority of patients were on NYHA functional class II (58.7%) or III (23.2%), 51.3% had HF with preserved ejection fraction, and the most common etiologies of HF were hypertensive (28.6%), dilated (27.0%), and ischemic (22.8%). Comorbidities were common, 77.5% had arterial hypertension, 39.1% previous coronary artery disease, 37.3% diabetes and 32.4% chronic kidney disease. With regard to HF treatments, 85.5% were taking a renin angiotensin system inhibitor, 79.7% a beta blocker and 51.4% an aldosterone antagonist (Table 1).
Figure 1.
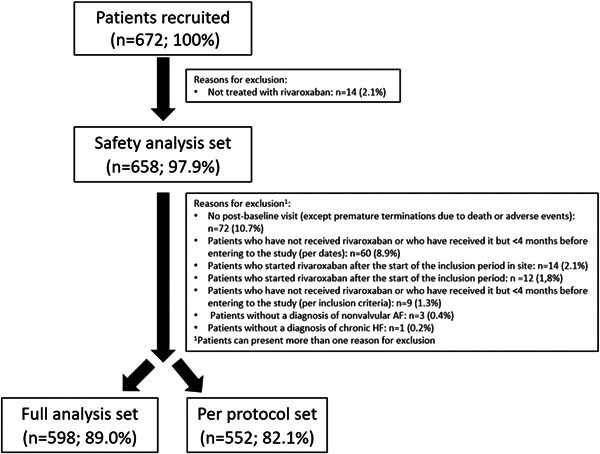
Flow chart of the study.
Table 1.
Baseline clinical characteristics of the study population, per protocol set (n = 552).
| Biodemographic data | |
|---|---|
| Age, years | 73.7 ± 10.9 |
| <65 years, n (%) | 118 (21.4) |
| ≥65–≤75 years, n (%) | 163 (29.5) |
| >75 years, n (%) | 271 (49.1) |
| Gender (male), n (%) | 358 (64.9) |
| Barthel test | 94.6 ± 12.4 |
| Frail scale | 1.5 ± 1.3 |
| Frail (score > 0), n (%) | 113 (33.9) |
| Charlson Index | 2.0 ± 1.1 |
| AF data | |
|---|---|
| Time since AF diagnosis, months | 58.8 ± 59.7 |
| Type of AF, n (%) | |
| Paroxysmal | 170 (31.1) |
| Persistent | 65 (11.9) |
| Long‐standing persistent | 17 (3.1) |
| Permanent | 295 (53.9) |
| Missing | 5 |
| CHA2DS2‐VASc score | 4.1 ± 1.5 |
| HAS‐BLED score | 1.6 ± 0.9 |
| HF data | |
|---|---|
| Time since HF diagnosis, months | 47.1 ± 50.2 |
| NYHA functional class, n (%) | |
| Class I | 96 (17.4) |
| Class II | 324 (58.7) |
| Class III | 128 (23.2) |
| Class IV | 4 (0.7) |
| Department where the patient was visited, n (%) | |
| Cardiology | 463 (83.9) |
| Internal medicine | 82 (14.9) |
| Other | 7 (1.2) |
| HF unit | 272 (49.3) |
| HF classification, n (%) | |
| HF with reduced ejection fraction | 173 (31.3) |
| HF with mildly reduced ejection fraction | 96 (17.4) |
| HF with preserved ejection fraction | 283 (51.3) |
| Etiology of HF, n (%) | |
| Hypertensive | 158 (28.6) |
| Dilated | 149 (27.0) |
| Ischemic | 126 (22.8) |
| Valvulopathy | 35 (6.3) |
| Hypertrophic | 18 (3.3) |
| Toxics | 10 (1.8) |
| Restrictive or infiltrative | 8 (1.5) |
| Missing | 48 (8.7) |
| Physical examination | |
|---|---|
| Body mass index (Kg/cm2) | 28.8 ± 5.3 |
| Heart rate (bpm) | 71.6 ± 14.7 |
| Systolic blood pressure (mmHg) | 125.2 ± 18.9 |
| Diastolic blood pressure (mmHg) | 72.8 ± 11.6 |
| Cardiovascular risk factors | |
|---|---|
| Arterial hypertension, n (%) | 428 (77.5) |
| Hyperlipidemia, n (%) | 302 (54.7) |
| Diabetes mellitus, n (%) | 206 (37.3) |
| Smoking, n (%) | |
| No | 492 (89.1) |
| Current smoker | 27 (45.0) |
| Recent ex‐smoker (<1 year) | 14 (23.3) |
| Former ex‐smoker (>1 year) | 19 (31.7) |
| Vascular disease | |
|---|---|
| Previous coronary artery disease, n (%) | 152 (39.1) |
| Previous cerebrovascular disease, n (%) | 69 (12.5) |
| Other comorbidities | |
|---|---|
| Chronic kidney disease, n (%) | 179 (32.4) |
| Chronic obstructive pulmonary disease | 110 (19.9) |
| Cancer | 59 (10.7) |
| Nonsevere dementia | 20 (3.6) |
| Liver dysfunction | 5 (0.9) |
| Other treatments | |
|---|---|
| Compliance with diet recommendations, n (%) | 474 (85.9) |
| Compliance with pharmacological treatments for HF, n (%) | 546 (98.9) |
| Diuretics, n (%) | 499 (90.6) |
| RAAS inhibitors, n (%) | 471 (85.5) |
| Angiotensin converting enzyme inhibitors, n (%) | 202 (36.7) |
| Angiotensin II receptor blockers, n (%) | 131 (23.8) |
| Sacubitril/valsartan, n (%) | 138 (25.0) |
| Beta blockers, n (%) | 439 (79.7) |
| Mineralocorticoid receptor antagonists, n (%) | 283 (51.4) |
| Digoxin, n (%) | 127 (23.0) |
| Ivabradine, n (%) | 17 (3.1) |
| Antiarrhythmics, n (%) | 108 (19.6) |
| Amiodarone | 81 (14.7) |
| Flecainide | 18 (3.3) |
| Dronedarone | 3 (0.5) |
| Propafenone | 3 (0.5) |
| Sotalol | 3 (0.5) |
| Antiplatelets, n (%) | 71 (12.9) |
| Implantable cardioverter‐defibrillator, n (%) | 77 (19.8) |
| Pacemaker, n (%) | 54 (13.9) |
| Resynchronization, n (%) | 40 (10.3) |
| Previous ablation, n (%) | 36 (9.2) |
Abbreviations: AF, atrial fibrillation; CrCl, creatinine clearance; HF, heart failure; INR, international normalized ratio; NYHA, New York Heart Association; RAAS, renin angiotensin system inhibitors; VKA, vitamin K antagonists.
Mean time from start of treatment to study entering was 25.5 ± 18.8 months (<6 months: 12.6%, 6–12 months: 21.1%, ≥12 months: 66.3%; <1 year: 33.7%, ≥1 year: 66.3% of the study population). 69.0% of patients were taking rivaroxaban 20 mg and the remaining 31.0% rivaroxaban 15 mg. After 2 years of follow‐up, only 6.9% permanently discontinued rivaroxaban treatment (excluding patients who died), mainly because of bleeding or severe worsening of renal function, 8.5% temporarily interrupted the treatment and in 13.0% of patients, the dose of rivaroxaban was modified. In the safety population (n = 658), only 3.0% of patients presented a serious adverse event related to rivaroxaban (Table 2).
Table 2.
Treatment with rivaroxaban during the study, per protocol set (n = 552).
| Time from start of treatment to study entering, months | 25.5 ± 18.8 |
| Previous VKA, n (%) | 248 (44.9) |
| Labile INR, n (%) | 183 (73.5) |
| Dose, n (%) | |
| 15 mg | 171 (31.0%) |
| 20 mg | 381 (69.0%) |
| Permanent discontinuation, n (%) | 38 (6.9) |
| Reasons for rivaroxaban withdrawal, n (%) | |
| Hemorrhagic events | 13 (34.2) |
| Advanced kidney disease | 11 (28.9) |
| Others | 14 (36.8) |
| Any change with rivaroxaban since the beginning of the study,a n (%) | 109 (19.8) |
| Dose adjustment, n (%) | 72 (13.0) |
| Temporary interruption, n (%) | 47 (8.5) |
Patient may present more than one option.
After 24 months of follow‐up, whereas hemoglobin and NT‐pro‐BNP values remained stable, there was a significant decrease of glomerular filtration rate and BNP levels (Table 3). With regard to outcomes, 11.6% of patients died during the follow‐up, 2.9% had a thromboembolic event, 3.1% a major bleeding, 0.5% an intracranial bleeding and no patient had a fatal hemorrhage. In addition, 24.9% of patients developed HF worsening (hospitalization of visit to the emergency department). No significant differences were observed in the event rates according to the use of rivaroxaban before inclusion: <6 months versus 6–12 months versus ≥12 months or <1 year versus ≥1 year (Supporting Information S1: Tables 1 and 2). Additionally, the Kaplan–Meier curves confirmed these results (Supporting Information S1: Figures 1–4).
Table 3.
Evolution of laboratory parameters, per protocol set (n = 552).
| Baseline | Study end (24 months) | p Value | |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.4 ± 1.9 | 13.5 ± 2.0 | .39 |
| Fasting glucose (mg/dL) | 115.2 ± 38.6 | 115.3 ± 36.2 | .96 |
| Glomerular filtration rate (mL/min per 1.73 m2) | 62.0 ± 20.2 | 58.5 ± 19.8 | .004 |
| BNP (pg/L) | 708.7 ± 1329.9 | 312.5 ± 288.7 | <.01 |
| NT‐proBNP (pg/L) | 2771.2 ± 3458.8 | 2527.1 ± 4800.6 | .33 |
Cut‐off values of quantitative variables associated with HF worsening were analyzed, and these included low diastolic blood pressure, renal dysfunction, and anemia (Table 4). In addition, the multivariate analysis showed that increasing age, the history of chronic obstructive pulmonary disease, the previous use of vitamin K antagonists, and restrictive or infiltrative cardiomyopathies were independently associated with HF worsening (Table 4). Low diastolic blood pressure, defined as <75 mmHg was reported in 54.3% of patients. Compared to patients with diastolic blood pressure ≥75 mmHg, those with <75 mmHg were older, more fragile, and had more prior coronary artery disease and chronic kidney disease, with a trend towards more HF with reduced ejection fraction (Supporting Information S1: Table 3).
Table 4.
Cutoff values of quantitative variables associated with HF worsening and independent factors associated with HF worsening.
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Cutoff values of quantitative variables associated with HF worsening | ||
| DBP < 75 vs. ≥75 mmHg | 1.67 (1.18.2.38) | .004 |
| eGFR <60 vs ≥60 mL/min/1.73 m2 | 1.67 (1.17.2.39) | .005 |
| Hemoglobin <13 vs. ≥13 g/dL | 1.61 (1.15.2.27) | .006 |
| Age <80 vs. ≥80 years | 0.52 (0.37.0.73) | .0001 |
| Age <75 vs. ≥75 years | 0.49 (0.35.0.70) | <.0001 |
| Independent factors associated with HF worsening | ||
| Age (years), per each unit of the variable | 1.03 (1.01–1.05) | .004 |
| COPD | 2.08 (1.36–3.17) | .001 |
| Previous use of VKA | 1.78 (1.21–2.63) | .004 |
| Etiology of HF diagnosis | ||
| Restrictive cardiomyopathy vs. others | 9.24 (2.21–38.62) | .002 |
| Infiltrative cardiomyopathy vs. others | 6.03 (2.17–16.76) | .001 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; VKA, vitamin K antagonists.
4. DISCUSSION
This study identified in a wide sample of patients with AF and HF treated with rivaroxaban, independent predictors of worsening HF episodes. Increasing age, previous chronic obstructive pulmonary disease, previous treatment with vitamin K antagonists and HF etiology. After 2 years of follow‐up, nearly 25% of patients developed a HF worsening episode, 12% of patients died, and thromboembolic and bleeding events were low.
In our study, patients were old, had many comorbidities, a high thromboembolic risk and approximately one‐third were considered as frail. In the subgroup of patients with HF included in the ROCKET‐AF trial, median age was 72 years, CHA2DS2‐VASc 5.1 and one‐third presented HF with reduced ejection fraction. 17 The GLORIA‐AF registry included newly diagnosed patients with AF and CHA2DS2‐VASc score ≥1. In this study, 24% of patients had HF, of whom 41% were ≥75‐year‐old, CHA2DS2‐VASc score was 3.9% and 38% had HF with reduced ejection fraction. 25 In summary, patients with HF and AF are usually elderly, with a significant burden of comorbidities, and share etiopathogenic and risk factors. 26 Therefore, our sample was representative of real‐life patients with AF and HF. On the other hand, our study provided relevant information of the whole spectrum of HF, regardless of ejection fraction or the etiology of HF, as this study lacked strict selection criteria and may capture the effects of rivaroxaban in the real‐world Spanish setting, supporting the generalizability of the results. This is important, as information regarding some phenotypes of HF, such as those with mildly reduced ejection fraction is very scarce. 27
Although other registries have analyzed the factors associated with the severity of HF and AF, patients were mainly treated with vitamin K antagonist, and these factors could change when other anticoagulants are taken. 28 , 29 In this study all patients were taking rivaroxaban to avoid possible bias when using different anticoagulants, facilitating the focus on the primary endpoint of the study, as this was a homogeneous population. One out of four patients developed a HF worsening episode, defined as first HF hospitalization or visit to the emergency department due to HF decompensation. Comorbidities such as chronic obstructive pulmonary disease were identified as risk factors for HF progression. In this context, it is necessary a comprehensive management of patients with AF and HF, focusing not only on the reduction of thromboembolic events with appropriate anticoagulation, but also on HF complications and comorbidities. 2 , 10 The previous use of vitamin K antagonists were also associated with worsening HF. One of the main limitations of these drugs is their great response variability, leading to frequent monitoring of anticoagulant activity and dose adjustments. This is even more marked in HF patients. 30 , 31 By contrast, rivaroxaban provides a consistent and stable anticoagulant effect, even in these patients, leading to a good safety profile and a protective effect. 18 Of note, event rates did nor differ according to the time of use of rivaroxaban before inclusion, emphasizing its safety in clinical practice.
Previous studies performed in HF population have also shown that chronic kidney disease is associated with HF hospitalization. 14 , 15 Although renal function decline is common among patients with AF, different studies have shown that compared with warfarin, rivaroxaban has a lower risk of renal function impairment. 32 , 33 , 34 In our study there was a modest reduction of renal function that could be explained by the high risk clinical profile (i.e., elderly patients with HF, AF, and many comorbidities). Although low diastolic blood pressure was associated with HF worsening, the worse clinical profile of these patients could explain at least in part this point.
Approximately 70% of patients were taking rivaroxaban 20 mg and 30% rivaroxaban 15 mg. Although it was not specifically analyzed, considering that around one‐third of patients had chronic kidney disease, these results suggest that in the majority of patients rivaroxaban was properly prescribed. A recent study has shown that in real‐life patients with AF, in less than 10% of patients rivaroxaban is underdosed. 35 The simplicity of rivaroxaban dosage may have contributed, as it only depends on renal function. 36 On the other hand, previous studies have also shown the high adherence and persistence with rivaroxaban, mainly related to the low risk of adverse events and its simplicity of use. 37 , 38 In our study, only 7% of patients permanently discontinued rivaroxaban.
In our study, after 2 years of follow‐up, nearly 12% of patients died and 3% had a thromboembolic event. Although in nonanticoagulated patients with AF the most important complication is stroke, in anticoagulated patients, mortality is mainly related to other causes different to cerebrovascular disease, 25 , 39 indicating the need for a comprehensive approach in the management of patients with HF and AF. Despite the risk of hemorrhage is increased in HF patients, 40 our study showed that rivaroxaban had a good safety profile in this population, with a low risk of major and intracranial hemorrhages in clinical practice.
This study has some limitations. First, a limitation of the study design might be the delay window between treatments start and study inclusion, at least 4 months after rivaroxaban treatment initiation. This constraint was introduced to prevent any interference on prescription behavior. Therefore, all events occurring during this period accounted for retrospective data, but not for the primary objective. Although this design may challenge the interpretation of the results and limit their scope, it is noteworthy that the purpose of our study was to assess the risk factors encountered in a follow‐up visit during rivaroxaban treatment, rather than those at the moment of treatment start. Second, spectrums of disease severity existed for comorbidities; however, for the purposes of modeling, these diseases were treated as binary events. Third, as patients included in this study were representative of the Spanish population with HF and AF taking rivaroxaban, the results can only be extended to patients with a similar clinical profile.
5. CONCLUSION
Approximately one out of four anticoagulated patients with HF and AF developed a HF worsening episode after 2 year of follow‐up. Increasing age, the history of chronic obstructive pulmonary disease, the previous use of vitamin K antagonists, and restrictive or infiltrative cardiomyopathies, were independently associated with HF worsening, suggesting that a comprehensive approach is required to reduce HF burden in this population.
AUTHOR CONTRIBUTIONS
All authors have contributed significantly to the work presented in this article, contributing to the conception, design, or acquisition of information, or to the analysis and interpretation of data. All the authors have participated in the drafting and/or revision of the manuscript and accept its publication.
CONFLICT OF INTEREST STATEMENT
The authors received honoraria from Bayer Hispania S.L. for their participation as researchers in the FARAONIC study sponsored by Bayer. Carles Rafols is an employee of Bayer Hispania S.L.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided by Content Ed Net, with funding from Bayer Hispania SL. The authors thanks to the centers and researchers participating in the study:
| Centre | Principal Researcher |
|---|---|
| Hospital del Mar | Nuria Farré López |
| Hospital Universitario Lucus Augusti | Margarita Regueiro Abel |
| Hospital Basurto | Ainara Lozano Bahamonde |
| Consulta Privada Dr. Torres | Francisco Torres Calvo |
| Complejo Hospitalario de Santiago | Rosa María Agra Bermejo |
| Clínica Cardiología Vera | Eduardo Sebastián López Sánchez |
| Consulta Cardiológica Ricardo Fajardo Molina | Ricardo Fajardo Molina |
| CHOU Ourense | Gloria López Barros |
| Hospital de Galdakao/Usansolo | Mª Angeles Eneriz |
| Hospital Universitario Ramón y Cajal | Susana del Prado |
| Complejo Hospitalario de Navarra | Ana Carmen Abecia Ozcariz |
| Consorci Sanitario de Terrassa | Joan Martinez Tur |
| Complejo Hospitalario de Ferrol (H. Arquitecto Marcide) | Manuel López Pérez |
| Hospital Regional de Málaga Carlos Haya | José María Pérez Ruiz |
| Hospital Virgen de la Victoria | Jose Manuel Garcia Pinilla |
| Hospital Universitari de Girona Doctor Josep Trueta | Julia Roure Fernandez |
| Hospital Rey Juan Carlos I de Móstoles | Elena Mejia Martinez |
| Hospital Rio Hortega de Valladolid | Mª del Mar de la Torre Carpente |
| Consulta Dr. Enrique Galve Basilio | Enrique Galve Basilio |
| Hospital Doce de Octubre | Daniel Ferreiro |
| Cardioempordà | Sara Darnés Soler |
| Hospital Clínico Universitario de Valladolid | Pedro Ángel de Santos Castro |
| Hospital Virgen de las Nieves | Silvia López‐Fernández |
| Hospital Puerto Real | Fco. Javier Camacho Jurado |
| Hospital Universitario San Cecilio | Jesús Gabriel Sanchez Ramos |
| Hospital La Paz | Isabel Antorrena |
| Hospital Universitario Donostia | Irene Rilo Miranda |
| Hospital Puerta del Mar | Daniel Bartolome Mateos |
| Hospital San Carlos | Francisco Manuel Brun Romero |
| Hospital Clínico Universitario de Salamanca | Elisabete Alzola Martinez |
| Complejo Asist. Univ. León | José Ignacio Iglesias Garriz |
| Hospital Costa de la Luz | María Rosario Perez Tristancho |
| Hospital de Burgos | Esther Sánchez Corral |
| Hospital Rio Carrión (Complejo Aistencial Universitario) | Jose Ignacio Cuende Melero |
| Hospital Comarcal Monforte de Lemos | Ricardo Izquierdo |
| Clínica Clivina | María Rosa Fernández Olmo |
| Complejo Asistencial de Soria (Hospital Santa Barbara) | Margarita Carrera Izquierdo |
| Fundación Hayge | Pere Álvarez García |
| Hospital Poniente | Juan A. Montes Romero |
| Hospital Universitario La Zarzuela (Sanitas) | Santiago de Dios |
| Hospital Virgen Macarena | Alejandro Recio Mayoral |
| Complejo Hospitalario de Pontevedra (Hospital de Montecelo) | Juan Carlos Rodríguez García |
| Hospital de Sierrallana | Pilar Ortiz Oficialdegui |
| Hospital Clínic i Provincial | Ana García Alvarez |
| Hospital Clínico Universitario Lozano Blesa | Juan Ignacio Perez Calvo |
| Hospital Miguel Servet | Ana Portoles Ocampo |
| Hospital Royo Vilanova | David Bierge Valero |
| Hospital Sanchinarro | Francisco Javier Parra |
| Hospital Monteprincipe | Francisco J. Rodriguez Rodrigo |
| Hospital Sant Pau | Sonia Mirabet Perez |
| Hospital Arrixaca | Domingo Pascual Figal |
| Hospital Morales Meseguer | Diego Miguel Giménez Cervantes |
| Hospital Moises Broggi | Roman Freixa Pamias |
| Hospital de Cruces | Ángel Sebastián Leza |
| Hospital de Bellvitge | Josep Comin Colet |
| Hospital Infanta Leonor de Madrid | David Vaqueriza Cubillo |
| Hospital Nuestra Señora de Sonsoles | Rosa Ana Lopez Jiménez |
| Hospital del Sagrat Cor | Martin Luis Descalzo |
| Hospital Sant Joan de Déu de Martorell | María Ysabel Saldarriaga Infante |
| Complejo Hospitalario Ruber Juan Bravo | María Carmen Gómez Rubín |
| Hospital Universitari Germans Trias i Pujol | Javier Santesmases Ejarque |
| Hospital de la Princesa | Berta Moyano |
| Hospital Universitari Vall d'Hebron | Teresa Soriano Sanchez |
| Hospital General San Jorge | Maria Teresa Villarroel Salcedo |
| Hospital Infanta Sofía | Diego Iglesias Del Valle |
| Hospital Virgen de la Luz | José Antonio Nieto Rodriguez |
| Centro Médico Lamar | Monzer Khanji Khatib |
| Clínica Nuestra Señora del Rosario | Maria Carmen Alonso Gutierrez |
| Hospital San Rafael | Gonzalo Peña Pérez |
| Hospital Povisa | Fernando Soto Loureiro |
Manito N, Cepeda‐Rodrigo JM, Farré N, et al. Factors associated with disease progression in patients with atrial fibrillation and heart failure anticoagulated with rivaroxaban. Clin Cardiol. 2024;47:24189. 10.1002/clc.24189
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Seferović PM, Vardas P, Jankowska EA, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021;23(6):906‐914. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599‐3726. [DOI] [PubMed] [Google Scholar]
- 3. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005‐2014): ARIC study community surveillance. Circulation. 2018;138(1):12‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. JACC. 2019;73(8):935‐944. [DOI] [PubMed] [Google Scholar]
- 5. Escobar C, Varela L, Palacios B, et al. Características clínicas, manejo y riesgo de complicaciones a un año en pacientes con insuficiencia cardíaca con y sin diabetes tipo 2 en España [Clinical characteristics, management, and one‐year risk of complications among patients with heart failure with and without type 2 diabetes in Spain]. Rev Clin Esp. 2022;222(4):195‐204. [DOI] [PubMed] [Google Scholar]
- 6. Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3(3):252‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC: Heart Failure. 2019;7(6):447‐456. [DOI] [PubMed] [Google Scholar]
- 8. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham heart study. Circulation. 2003;107(23):2920‐2925. [DOI] [PubMed] [Google Scholar]
- 9. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GYH. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660‐666. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. [DOI] [PubMed] [Google Scholar]
- 11. Pocock SJ, Ariti CA, McMurray JJV, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404‐1413. [DOI] [PubMed] [Google Scholar]
- 12. Senni M, Parrella P, De Maria R, et al. Predicting heart failure outcome from cardiac and comorbid conditions: the 3C‐HF score. Int J Cardiol. 2013;163(2):206‐211. [DOI] [PubMed] [Google Scholar]
- 13. Lopes RD, Pieper KS, Stevens SR, et al. Predicting outcomes over time in patients with heart failure, left ventricular systolic dysfunction, or both following acute myocardial infarction. J Am Heart Assoc. 2016;5(6):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Álvarez‐García J, Ferrero‐Gregori A, Puig T, et al. A simple validated method for predicting the risk of hospitalization for worsening of heart failure in ambulatory patients: the Redin‐SCORE. Eur J Heart Fail. 2015;17(8):818‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hummel SL, Ghalib HH, Ratz D, Koelling TM. Risk stratification for death and all‐cause hospitalization in heart failure clinic outpatients. Am Heart J. 2013;166(5):895‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pastori D, Farcomeni A, Poli D, et al. Cardiovascular risk stratification in patients with non‐valvular atrial fibrillation: the 2MACE score. Intern Emerg Med. 2016;11(2):199‐204. [DOI] [PubMed] [Google Scholar]
- 17. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. [DOI] [PubMed] [Google Scholar]
- 18. van Diepen S, Hellkamp AS, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ: Heart Fail. 2013;6(4):740‐747. [DOI] [PubMed] [Google Scholar]
- 19. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach. Chest. 2010;137(2):263‐272. [DOI] [PubMed] [Google Scholar]
- 20. Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation. Chest. 2010;138(5):1093‐1100. [DOI] [PubMed] [Google Scholar]
- 21. Yang CM, Wang YC, Lee CH, Chen MH, Hsieh CL. A comparison of test‐retest reliability and random measurement error of the Barthel Index and modified Barthel Index in patients with chronic stroke. Disabil Rehabil. 2022;44(10):2099‐2103. [DOI] [PubMed] [Google Scholar]
- 22. Aprahamian I, Cezar NOC, Izbicki R, et al. Screening for frailty with the FRAIL scale: a comparison with the phenotype criteria. J Am Med Dir Assoc. 2017;18(7):592‐596. [DOI] [PubMed] [Google Scholar]
- 23. Brusselaers N, Lagergren J. The charlson comorbidity index in registry‐based research. Methods Inf Med. 2017;56(5):401‐406. [DOI] [PubMed] [Google Scholar]
- 24. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemostasis. 2015;13(11):2119‐2126. [DOI] [PubMed] [Google Scholar]
- 25. Dubner SJ, Teutsch C, Huisman MV, et al. Characteristics and 2‐year outcomes of dabigatran treatment in patients with heart failure and atrial fibrillation: GLORIA‐AF. ESC Heart Fail. 2020;7(5):2679‐2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarullo FM, Schembri G, Nugara C, Sarullo S, Vitale G, Corrao S. Mutual relationship between heart failure and atrial fibrillation. Monaldi Arch Chest Dis. 2020;90(4). 10.4081/monaldi.2020.1264 [DOI] [PubMed] [Google Scholar]
- 27. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid‐range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Deursen VM, Urso R, Laroche C, et al. Co‐morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16(1):103‐111. [DOI] [PubMed] [Google Scholar]
- 29. Nabauer M, Oeff M, Gerth A, et al. Prognostic markers of all‐cause mortality in patients with atrial fibrillation: data from the prospective long‐term registry of the German atrial fibrillation NETwork (AFNET). EP Europace. 2021;23(12):1903‐1912. [DOI] [PubMed] [Google Scholar]
- 30. Sakai T, Motoki H, Fuchida A, et al. Comparison of prognostic impact of anticoagulants in heart failure patients with atrial fibrillation and renal dysfunction: direct oral anticoagulants versus vitamin K antagonists. Heart Vessels. 2022;37(7):1232‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santas E, Miñana G, Gummel J, et al. Razón internacional normalizada y mortalidad de los pacientes con insuficiencia cardiaca y fibrilación auricular tratados con antagonistas de la vitamina K [International normalized ratio and mortality risk in acute heart failure and nonvalvular atrial fibrillation patients receiving vitamin K antagonists]. Rev Esp Cardiol. 2019;72(8):616‐624. [DOI] [PubMed] [Google Scholar]
- 32. Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. JACC. 2017;70(21):2621‐2632. [DOI] [PubMed] [Google Scholar]
- 33. González Pérez A, Balabanova Y, Sáez ME, Brobert G, García Rodríguez LA. Renal decline in patients with non‐valvular atrial fibrillation treated with rivaroxaban or warfarin: a population‐based study from the United Kingdom. Int J Cardiol. 2022;352:165‐171. [DOI] [PubMed] [Google Scholar]
- 34. López‐Gálvez R, Rivera‐Caravaca JM, Anguita Sánchez M, et al. Use of rivaroxaban attenuates renal function impairment in patients with atrial fibrillation: insights of the EMIR study. Eur J Clin Invest. 2022;52(9):e13788. [DOI] [PubMed] [Google Scholar]
- 35. Fernández MS, Marín F, Rafols C, et al. Thromboembolic and bleeding events with rivaroxaban in clinical practice in Spain: impact of inappropriate doses (the EMIR study). J Comp Eff Res. 2021;10(7):583‐593. [DOI] [PubMed] [Google Scholar]
- 36. Barrios V, Escobar C. Rivaroxaban: a once‐daily anticoagulant for the prevention of thromboembolic complications. Expert Rev Cardiovasc Ther. 2013;11(2):129‐141. [DOI] [PubMed] [Google Scholar]
- 37. Coleman CI, Bunz TJ, Ashton V. Adherence and persistence to rivaroxaban in non‐valvular atrial fibrillation patients receiving 30‐ or 90‐day supply prescription fills. Curr Med Res Opin. 2022;38(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 38. McHorney CA, Ashton V, Laliberté F, et al. Adherence to rivaroxaban compared with other oral anticoagulant agents among patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23(9):980‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Apenteng PN, Virdone S, Hobbs FR, et al. Two‐year outcomes of UK patients newly diagnosed with atrial fibrillation: findings from the prospective observational cohort study GARFIELD‐AF. Br J Gen Pract. 2022;72(723):e693‐e701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mentias A, Briasoulis A, Shantha G, Alvarez P, Vaughan‐Sarrazin M. Impact of heart failure type on thromboembolic and bleeding risk in patients with atrial fibrillation on oral anticoagulation. Am J Cardiol. 2019;123(10):1649‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


