Abstract
Purpose
Tourniquets are commonly used intraoperatively in orthopaedic surgery to control bleeding and improve visibility in the surgical field. Recent evidence has thrown into question the routine use of tourniquets in the adult population resulting in a British Orthopaedic Association standard for intraoperative use. This systematic review evaluates the evidence on the practice, benefits, and risks of the intraoperative use of tourniquets for trauma and elective orthopaedic surgery in the paediatric population.
Methods
A prospectively registered systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PROSPERO: CRD42022359048). A search of MEDLINE, Embase, the Cochrane Library and a Grey literature search was performed from their earliest record to 23 March 2023. Studies reporting tourniquet data in paediatric patients undergoing orthopaedic surgery were included. Data extracted included demographics, involved limb, trauma versus elective use, tourniquet use as primary or secondary measure, and tourniquet parameters and complications.
Results
Thirty-nine studies were included. Tourniquet practices and information reporting varied considerably. Tourniquets were used uneventfully in the majority of patients with no specific benefits reported. Several physiological and biochemical changes as well as complications including nerve injury, compartment syndrome, skin burns, thrombosis, post-operative limb swelling, and pain were reported.
Conclusions
Tourniquets are routinely used in both trauma and elective paediatric orthopaedic surgery with no high-quality research affirming benefits. Severe complications associated with their use are rare but do occur. High-quality studies addressing their benefits, the exact indication in children, and the safest way to use them in this population are necessary.
Keywords: pediatrics, orthopedics, tourniquets, intraoperative care
Introduction
Tourniquets have traditionally been used in orthopaedic surgery to control bleeding and increase visibility in the intraoperative surgical field (1, 2, 3, 4). An increasing body of evidence has grown regarding the potential risks associated with their use. This is somewhat foreseeable given their mechanism of action: sustained circumferential pressure to occlude arterial flow resulting in reduced perfusion distal to the site of application. Laboratory research and studies in the adult population have shown that tourniquet use triggers changes in blood pressure and heart rate (5, 6), cerebral perfusion (7), coagulation (8, 9, 10), and core temperature (11). Local adverse effects include injury to muscle, blood vessels, nerves, and skin (2, 12, 13, 14, 15). Tourniquet use has also been associated with increased post-operative pain (16, 17, 18), longer length of hospital stay (16, 19), increased risk of wound infection and wound complications (19, 20), thromboembolic events (16, 18, 20), and compartment syndrome (16, 21). If not carefully sealed off, the accumulation of antiseptic surgical wash beneath the tourniquet during limb preparation can result in soft tissue damage and chemical burns (22).
Studies in the adult population have attempted to elucidate techniques for the safer use of tourniquets. Example of such methods include adjusting inflation pressures to the patient’s systolic blood pressure (SBP) and limb occlusion pressure (LOP) (23, 24, 25) as well as choosing wider, contoured cuffs (26, 27, 28). Omitting the use of tourniquets altogether has gained momentum. Studies in adult arthroplasty surgery suggest tourniquets provide limited benefit in reducing operative time but are associated with surgical adverse events and a higher risk of complications (16, 20, 29). It is estimated that avoiding their use in knee arthroplasty could prevent nearly 2000 serious adverse events per year in the UK alone (29). Similarly, wide-awake local anaesthesia no tourniquet (WALANT) surgery for adult upper limb orthopaedic operations has been increasing in popularity and has shown good clinical results with reduced risk and cost (30, 31, 32, 33).
The existing evidence on tourniquet use in lower limb fracture fixation surgery in adults suggest that using a tourniquet may cause patients harm with limited benefit (34, 35).
Despite the growth in evidence from studies in adults, there is a significant paucity of literature with respect to the paediatric population. The British Orthopaedic Association (BOA) published a standard in 2021 (BOAST) regarding the safe use of intraoperative tourniquets, stating that ‘tourniquets should only be used when clinically justified’ (36). With respect to paediatric patients specifically, the only specification in this document states that patients younger than 16 years old should have tourniquets applied at a pressure of 50 mm Hg above limb occlusion pressure or 50–100 mm Hg above systolic blood pressure. This systematic review analyses the available evidence on the intraoperative use of tourniquets in paediatric orthopaedic trauma and elective surgery, aiming to evaluate the practices used, the benefits and reported complications in this population.
Methods
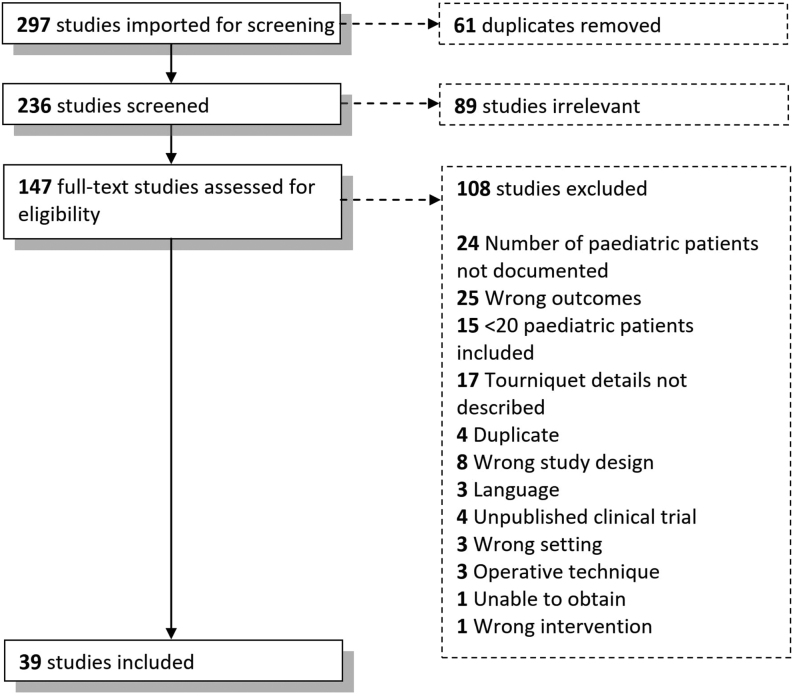
A systematic literature review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1). This was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO), registration number (CRD42022359048).
Figure 1.
PRISMA chart for the study collection process.
Search strategy and data extraction
The following electronic databases were searched from their earliest record to 23 March 2023: MEDLINE, Embase, and the Cochrane Library. A grey literature search was performed using referenced work in included studies and Google Scholar. Conference proceedings from the British Orthopaedic Association (BOA), European Paediatric Orthopaedic Society (EPOS), British Society for Children’s Orthopaedic Surgery (BSCOS), and European Federation of National Associations of Orthopaedics and Traumatology (EFORT) were also searched. The search terms may be found in Appendix 1 (see the section on supplementary materials given at the end of this article).
The search was not limited by journal type or level of evidence. The data were collected using Covidence as a systematic review management software tool (©Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia). Two reviewers (VP, CB) determined study eligibility and any conflicts were resolved by a senior author.
Study eligibility
All studies reporting tourniquet data in paediatric patients (defined as under 18 years old) undergoing orthopaedic surgery were screened. Studies with more than 20 paediatric patients published in English, French or with available translations were included. Where complications and adverse outcomes occurred, there was no lower limit on patient number and all papers were included. Studies including adult populations were included only when the paediatric data were separate and explicitly documented. The PRISMA for the study collection process is outlined in Fig. 1.
Bias assessment
Bias assessment was conducted separately by two authors (VP, CB). The RoB 2 bias assessment method was used for randomised studies (37). ROBINS-I was used for cohort studies, case–control studies and non-randomised experimental studies (38). The method outlined by Murad et al. was used for case series and retrospective reviews (39). The results of the bias assessment for each included study are shown in Table 1. The bias assessment is reported in Appendix 2.
Table 1.
Study characteristics and risk of bias assessment.
| Study | Study design | Tourniquet use* | Trauma/elective | Upper/lower limb | N | Bias |
|---|---|---|---|---|---|---|
| Reilly et al. (65) | RCT | Primary | Elective | Lower limb | 21 | Low |
| Tamai et al. (69) | RCS | Secondary | Both | Lower limb | 100 | High |
| Goodarzi et al. (47) | Experimental study | Primary | Elective | Lower limb | 30 | Low |
| Eidelman et al. (66) | Experimental study | Primary | Both | Both | 43 | High |
| Ida et al. (61) | Case report | Primary | Elective | Lower limb | 1 | High |
| Saw et al. (51) | Case report | Primary | Trauma | Lower limb | 1 | High |
| Goodarzi et al. (46) | Case–control study | Secondary | Not specified | Lower limb | 40 | Moderate |
| Bloch et al. (44) | Case–control study | Primary | Both | Lower limb | 47 | High |
| Ashraf et al. (68) | Case series | Secondary | Both | Lower limb | 1002 | Low |
| Lynn et al. (45) | Case series | Primary | Elective | Both | 15 | Low |
| Prakash et al. (73) | Case series | Secondary | Elective | Lower limb | 75 | Low |
| Tareco et al. (76) | Case series | Secondary | Elective | Lower limb | 30 | Low |
| Hanlon et al. (56) | Case series | Primary | Elective | Lower limb | 28 | Low |
| Tredwell et al. (55) | Case series | Primary | Elective | Both | 2 | Moderate |
| Lieberman et al. (64) | Case series | Primary | Not specified | Both | 29 | Moderate |
| Silver et al. (57) | Case series | Primary | Elective | Both | 8 | Moderate |
| Inan et al. (53) | Case series | Secondary | Elective | Lower limb | 28 | Moderate |
| Misra et al. (48) | Case series | Primary | Elective | Both | 2 | High |
| Hodgins et al. (58) | Case series | Primary | Elective | Lower limb | 2 | High |
| Dickinson et al. (59) | Case series | Primary | Elective | Upper limb | 3 | High |
| Kang et al. (67) | Case series | Primary | Elective | Upper limb | 113 | High |
| Murphy et al. (60) | Case series | Secondary | Elective | Lower limb | 2783 | High |
| Zampieri et al. (70) | Cohort study | Secondary | Elective | Lower Limb | 57 | High |
| Lindgren et al. (71) | Cohort study | Secondary | Trauma | Upper Limb | 24 | High |
| Hanna et al. (62) | Cohort study | Primary | Both | Lower limb | 204 | Low |
| Baghdadi et al. (77) | Cohort study | Secondary | Trauma | Upper limb | 204 | Low |
| Reinhardt et al. (78) | Cohort study | Secondary | Trauma | Upper limb | 31 | Moderate |
| Yalcinkaya et al. (54) | Cohort study | Secondary | Trauma | Upper limb | 45 | Moderate |
| Watts et al. (79) | Cohort study | Secondary | Trauma | Lower limb | 31 | Moderate |
| Cai et al. (80) | Cohort study | Secondary | Trauma | Upper limb | 32 | Moderate |
| Bloch et al. (43) | Cohort study | Primary | Elective | Lower limb | 56 | High |
| Jardaly et al. (52) | Cohort study | Secondary | Trauma | Lower limb | 95 | High |
| Oginni & Rufai (42) | Cohort study | Primary | Elective | Both | 39 | High |
| Ryan et al. (81) | RR | Secondary | Elective | Both | 197 | Moderate |
| Sullivan et al. (40) | RR | Secondary | Both | Both | 37 | Moderate |
| Vas et al. (63) | RR | Secondary | Elective | Lower limb | 160 | Moderate |
| Ross et al. (41) | RR | Primary | Not specified | Both | 49 | Moderate |
| Schrock et al. (49) | RR | Secondary | Elective | Lower limb | 42 | High |
| Slawski et al. (50) | RR | Secondary | Elective | Lower limb | 255 | High |
*Tourniquet use as a primary or secondary outcome.
RCT, randomised controlled trial; RCS, retrospective comparative study; RR, retrospective review.
Results
The initial literature search identified 297 articles. Thirty-nine studies met the eligibility criteria and were included for data extraction and analysis.
The majority of the studies reported on the general paediatric orthopaedic population. Three studies investigated tourniquet use in specific patient sub-populations: two were in osteogenesis imperfecta (40, 41) and one was in sickle cell disease (SCD) (42). Table 1 summarises data regarding study type, number of patients, limb involved, trauma versus elective use, tourniquet use as primary or secondary outcome, and risk of bias. Most of the studies (n = 22) reported tourniquet use in the lower limb with 17 studies reporting upper limb or both. Eight studies reported use in trauma cases and 22 in elective cases, three articles did not specify the setting, and the remaining papers were written with respect to both elective and trauma patients. Bias assessment showed 9 out of 39 studies were at low risk of bias, 13 out of 39 moderate, and 17 out of 39 at high risk (Table 1, Appendix 2).
Tourniquet practices and parameters are specified in Table 2. Most reported studies used pneumatic tourniquets and three used Esmarch bandage or similar technology.
Table 2.
Tourniquet variables.
| Study | Type of tourniquet | Mean (range) average tourniquet | |
|---|---|---|---|
| Time, minutes | Pressure, mm Hg | ||
| Kang et al. (67) | Other: ARGYLE Penrose tubing | (30–109) | (177–192) |
| Eidelman et al. (66) | Other: S-MART | 44 (15–75) | 227 (190–260) |
| 246 (160-342) | |||
| Oginni & Rufai (42) | Esmarch bandage | 31 (15–45) | – |
| Lynn et al. (45) | Pneumatic | 58 (31–94) | 248 (150–425) |
| 62 (2–26) | |||
| Ross et al. (41) | Pneumatic | 69 (10–120) | 200 (180–300) |
| Goodarzi et al. (47) | Pneumatic | 100 (50–140) | 217 (200–250) |
| Goodarzi et al. (46) | Pneumatic | 90.35 (55–132) | – |
| 90.05 (52–148) | |||
| Hodgins et al. (58) | Pneumatic | 85.5 (71–100) | 225 (200–250) |
| Hanlon et al. (56) | Pneumatic | 99 (45–160) | Not exceeding 250 |
| Reilly et al. (65) | Pneumatic | 91 | 300 |
| 89 | 151 (145.6–156.4) | ||
| Lieberman et al. (64) | Pneumatic | 54.5 | 175.8 (140–250) |
| Silver et al. (57) | Pneumatic | 54.5 | 283.3 (250–300) |
| Ida et al. (61) | Pneumatic | 23 | 250 |
| 27 | |||
| Saw et al. (51) | Pneumatic | 112 | 250 |
| Sullivan et al. (40) | Pneumatic | 80 | 250 |
| Tredwell et al. (55) | Pneumatic | 1 | 200 |
| Misra et al. (48) | Pneumatic | 60 | (125–185) |
| Bloch et al. (44) | Pneumatic | 103 (unilateral) | – |
| 115 (bilateral) | |||
| Hanna et al. (62) | Pneumatic | 75.91 (7–195) | – |
| Vas et al. (63) | Pneumatic | – | 150 |
| Bloch et al. (43) | Pneumatic | – | – |
| Dickinson et al. (59) | Pneumatic | – | – |
| Yalcinkaya et al. (54) | Pneumatic | – | – |
| Murphy et al. (60) | Not specified | 66 (23–127) | – |
| Slawski et al. (50) | Not specified | 91 (48–125) | – |
| Reinhardt et al. (78) | Not specified | 89.5 (54–126) | – |
| 35.6 (0–100) | |||
| Ashraf et al. (68) | Not specified | 78.4 (11–264) | – |
| Watts et al. (79) | Not specified | 76.1 (40–110) | – |
| 100.1 (60–135) | |||
| Tamai et al. (69) | Not specified | 104 (48–132) | – |
| Baghdadi et al. (77) | Not specified | 53.1 (15–120) | – |
| 55 (25–100) | |||
| Jardaly et al. (52) | Not specified | 50.6 (21–100) | – |
| Ryan et al. (82) | Not specified | 30.1 (1–118) | – |
| Tareco et al. (76) | Not specified | 81.5 | – |
| 58.4 | |||
| Cai et al. (80) | Not specified | 37.7 | – |
| 56.9 | |||
| Inan et al. (53) | Not specified | 48 | – |
| 60 | |||
| Prakash et al. (73) | Not specified | 26 | – |
| Schrock et al. (49) | Not specified | 57 | – |
| Zampieri et al. (70) | Not specified | 62 (median) | – |
| 90.5 (median) | |||
| Lindgren et al. (71) | Not specified | 46 | – |
| 92 | |||
Complications were uncommon but clinically significant, ranging from debilitating to life threatening. Table 3 summarises the reported complications.
Table 3.
Complications.
| Complication/ Study | Patients affected, n | Trend identified* |
|---|---|---|
| Alteration in physiological parameters† | ||
| Bloch et al (43) | Increased CBT | |
| Bloch et al. (44) | Increased CBT | |
| Lynn et al. (45) | Mixed respiratory and metabolic acidosis | |
| Goodarzi et al. (47) | Increased CBT, lactate, end-tidal CO2 | |
| Goodarzi et al. (46) | Increased BP, HR, CBT | |
| Misra et al. (48) | Increased BP and HR | |
| Nerve Injury | ||
| Schrock et al. (49) | 5 | |
| Slawski et al. (50) | 11 | |
| Saw et al. (51) | 1 | |
| Jardaly et al. (52) | 4 | |
| Yalcinkaya et al. (54) | 1 | |
| Inan et al. (53) | 5 | |
| Tredwell et al. (55) | 15 | |
| Compartment syndrome | ||
| Jardaly et al. (52) | 1 | |
| Skin burns | ||
| Hodgins et al. (58) | 2 | |
| Dickinson et al. (59) | 3 | |
| Thrombosis and Embolism | ||
| Murphy et al. (60) | 7 | |
| Ida et al. (61) | 1 | |
| Pain | ||
| Hanna et al. (62) | Increased pain and analgesia requirements | |
| Vas et al. (63) | 6 | |
| Limb swelling | ||
| Silver et al. (57) | Tourniquet use results in limb swelling |
*Trends are identified over the study population rather than specific individuals; †parameters such as core body temperature (CBT), heart rate (HR), blood pressure (BP) or serum lactate and pH.
Physiological effects
Four studies reported that tourniquet use in children triggers intraoperative physiological and biochemical changes. Bloch et al. 1992 and 1997 (43, 44) reported that use of a tourniquet caused an increase in core body temperature, which persisted until the time of tourniquet deflation and was more significant when bilateral lower limb application took place. The authors concluded this may be secondary to decreased effective heat loss from the skin of the limb distal to the tourniquet, thus trapping metabolic heat in the central compartment. They suggested this should be taken into consideration when warming is applied intraoperatively to avoid hyperthermia.
Lynn et al. reported an increase in serum lactate, potassium, base deficit, and PaCO2, as well as a decrease in blood pH in the systemic circulation upon tourniquet deflation. This created a mixed respiratory and metabolic acidosis independent of patient size (45). The metabolic acidosis generally took longer than 10 min to resolve. In this study, tourniquet pressure threshold was 275 mm Hg. Findings in keeping with Lynn et al. were also reported in two studies by Goodarzi et al. (46, 47), in which inflation pressures were set to 75 mm Hg above the patient’s SBP, in line with BOAST recommendations.
In these two studies, tourniquet use was associated with changes in blood pressure, pulse rate and temperature. Mean tourniquet time in the two studies was 100 min (range: 50–136) (47) and 90 min (range: 52–148) (46). Longer tourniquet time was associated with increased pulse rate, serum lactate levels, and end-tidal carbon dioxide (46, 47).
A case series by Misra et al. (48) looked at tourniquet pressures of 100 mm Hg and 50 mm Hg above SBP on two children for lower limb and upper limb tourniquets respectively. This is in accordance with the most recent BOAST guidelines (36). In both children, heart rate (HR) and blood pressure (BP) increased within 30 min of tourniquet inflation. Increasing the depth of anaesthesia had no effect, but when the tourniquet pressures were dropped to 50 mm Hg above SBP for the lower limb and 25 mm Hg above SBP for the upper limb, HR and BP returned to baseline.
Nerve injury
Seven studies reported nerve injury in paediatric patients undergoing orthopaedic surgery with the use of a tourniquet. In Schrock et al., five of 42 paediatric patients incurred peroneal nerve palsies associated with tourniquet use whilst undergoing tibial derotation osteotomies (49). Mean tourniquet time was 57 min, but tourniquet pressures were not documented. The recovery of the palsies was also not documented. In a similar study of tibial osteotomies by Slawski et al. (50), 11 cases of peroneal nerve injury were reported. All had recovered by 6 months post-operatively. Mean tourniquet time was 91 min (range: 48–125), similarly tourniquet pressures were not reported. Saw et al. reported a case of a 15-year-old who underwent arthroscopic surgery for a tibial eminence fracture (51). The patient developed common peroneal nerve palsy that resolved within 1 week. The tourniquet time and pressure were 112 min and 250 mm Hg, respectively. The authors of these studies believed the aetiology of these nerve injuries to be caused by the tourniquet. Schrock et al. also suggested that injury via intra-operative retraction or displacement of osteotomy fragments may also have played a role (49).
Jardaly et al. compared the use of closed reduction internal fixation (CRIF) to open reduction internal fixation (ORIF) for the surgical management of tibial tubercle fractures (52). A tourniquet was used for ORIF but not in the CRIF technique. The mean tourniquet time was 50.6 min (range: 21–100), but pressure data were not reported. In the ORIF group, three patients had numbness post-operatively, one had a peroneal nerve palsy and one had to undergo fasciotomy. Tourniquet time and pressures for these patients were not documented. Of note, 42% of patients in this study were obese and a considerably larger number of patients underwent ORIF than CRIF (81 and 17, respectively).
Inan et al. investigated the occurrence of neurological complications after supracondylar extension femoral osteotomy in 28 patients with cerebral palsy and fixed flexion deformity of the knee (53). Nineteen patients had bilateral procedures with 48 procedures performed in total, of which 40 used a tourniquet. Five patients had neurological complications. No association was found between tourniquet inflation time and neurological complication rate. In this study, the authors suggested that neurological complications were secondary to operative technique, especially inadequate femoral shortening.
Yalcinkaya et al. investigated the surgical management of unstable forearm fractures in 45 children. They attributed one case of radial nerve palsy with wrist drop to the use of tourniquet (54). A tourniquet with a pneumatic cuff was used, but tourniquet time and pressure were not mentioned. In a survey by Tredwell et al., of the 67 complications reported by 44 surgeons, 15 involved nerve injury (55).
Limb swelling and compartment syndrome
Jardaly et al. reported one case of post-operative compartment syndrome (52). Tourniquet time and pressure were not reported, but the maximal tourniquet inflation time in the study was 100 min. Hanlon et al. (54) measured compartment pressures in the central compartment of the foot after clubfoot surgery (56). In this study 16 of 39 operated feet had compartment pressures of a level that could trigger compartment syndrome with no correlation between compartment pressure recordings and tourniquet time.
Silver et al. found a 10% increase in limb volume after a pneumatic tourniquet was released. Swelling did not correlate with tourniquet pressure or time (57). The authors suggested that return of the exsanguinated blood and reactive hyperaemia secondary to ischaemia were responsible for this increase in limb volume. They recommend tourniquets are deflated prior to haemostasis and application of cylindrical bandaging to reduce the risk of compartment syndrome.
Skin burns
Two case series reported on chemical skin burns caused by pooling of antiseptic skin preparation underneath the tourniquet. Hodgins et al. reported two children who developed partial thickness chemical burns (58). Chlorhexidine gluconate 2% in 70% isopropyl alcohol was used for prepping the limbs. One case had a 10 × 15 cm deep partial-thickness burn on the posterior thigh that healed over the course of 4 weeks with silver-based dressings. The second case incurred a burn of 4 × 3 cm also on the posterior thigh, which again was managed conservatively. In the first and more severe case, no shut-off was used to isolate the tourniquet and the padding under the tourniquet had become soaked with antiseptic. Tourniquet pressures were 250 mm Hg and 200 mm Hg, and both children had tourniquets inflated for longer than one hour (100 min and 71 min). Dickinson et al. also reported on three children who incurred partial thickness chemical burns under tourniquets (59). Povidone–iodine with 70% alcohol content was used for prepping in this study. Tourniquet times and pressures, as well as the use of a shut-off were not documented. In the survey reported in Tredwell et al. the most reported complication was skin damage (21/67), whereas muscle injury accounted for eight of the 67 reported complications (55).
Thrombosis and embolism
Murphy et al. investigated the risk of thromboembolism after knee arthroscopy in 2,783 adolescents (60). Seven patients had symptomatic lower limb deep vein thromboses (DVT), which were subsequently confirmed on ultrasound. The average tourniquet time was 66 min (range: 23–127). In three of the seven patients, tourniquet time was longer than 60 min. One patient was subsequently diagnosed with factor V Leiden deficiency. There was no statistical analysis assessing the relationship between tourniquet time and venous thromboembolism (VTE) risk. Tourniquet data for the patients who did not have a VTE were not reported.
Ida et al. reported the case of a 5-year-old child who underwent reduction of hip dislocation and Achilles tendon lengthening to treat neurogenic hip dislocation and clubfoot (61). The child was reported to incur fat embolism syndrome triggered by the deflation of the tourniquet. The tourniquet had been applied at a pressure of 250 mm Hg for 23 min on the left lower limb and 27 min on the right lower limb.
Pain
Hanna et al. included 204 children who underwent lower limb orthopaedic surgery. Tourniquet use was associated with increased pain levels and post-operative analgesia requirements (62). Mean tourniquet time was 75.9 min (range: 7–195), but tourniquet pressures were not reported. This study also found that tourniquet use was associated with shorter operating time, reduced blood loss, and no increase in infection rates.
Vas et al. investigated the benefit of a three-in-one block in conjunction with a continuous sciatic block for intraoperative pain caused by the tourniquet in 160 children (63). They proposed that a sciatic block does not anaesthetise the area affected by the tourniquet entirely and this justified additional anaesthetic measures. The tourniquet was set to a predefined pressure of 150 mm Hg with no tourniquet times documented. The three-in-one block was mostly successful, but six children still had increased heart rates and upper body movement triggered by the painful stimulus of tourniquet inflation, which was managed with boluses of ketamine and propofol.
Sub-population studies
Sullivan et al. (40) and Ross et al. (41) investigated the incidence of fractures from tourniquet use in patients with osteogenesis imperfecta. Sullivan et al. included 37 patients (mean age = 10, s.d. = 4.8) with tourniquet pressure 250 mm Hg. Ross et al. included 49 patients (median age: 7.9, range: 0.2–17.7) and median tourniquet pressure was 200 mm Hg (range: 180–300). Neither study reported any iatrogenic fractures secondary to tourniquet use.
Oginni & Rufaiinvestigated the incidence of complications with the use of tourniquets for sequestration surgery in 18 children and one adult with SCD and 20 controls (42). Mean tourniquet time was 31 min in the SCD group and 33 min in the control group. Three cases of extremity swelling were reported in both the SCD and the control group.
Tourniquet technique
Two studies investigated the concept of LOP instead of standardised pressures or pressures determined based on SBP. In Lieberman et al. operations were successfully performed using 50 mm Hg above LOP. Mean tourniquet pressures of 173.4 mm Hg and 176.7 mm Hg were used in the upper and lower limb respectively, significantly lower pressures than the commonly used 250 mm Hg and 300 mm Hg (64).
The concept of LOP was also used by Reilly et al. who demonstrated significantly reduced inflation pressures could safely be used for arthroscopic knee surgery (65). In this randomised controlled study, paediatric patients undergoing primary arthroscopic elective anterior cruciate ligament (ACL) repair were randomised between a standardised pressure of 300 mm Hg and individualised LOP. Operative time was equivalent in the two groups, but average tourniquet pressure was 151 mm Hg in the LOP compared to 300 mm Hg in the standardised group. A wider contour cuff achieved effective blood flow occlusion at lower inflation pressures. Haemostasis was adequate in both the LOP and standardised inflation pressure groups, with no difference in quality of the surgical field on analysis of visual analogue scale scores. No complications were reported.
Eidelman et al. and Kang et al. explored different forms of elastic tourniquets. In Eidelman et al., 51 procedures in 43 patients were carried out using the S-MART tourniquet, which consists of an elastic ring, stockinet sleeve, and pull straps (66). Two sizes of S-MART tourniquet were used depending on limb circumference. The smaller system provided a pressure of 227 ± 37 mm Hg (mean ± s.d.) and the larger one 246 ± 86 mm Hg (mean ± s.d.). The authors reported good visibility in the surgical field without complications. In Kang et al. the ARGYLE elastic band tourniquet was used in young children needing upper limb surgery (67). This consisted of a 3.2 cm by 30 cm ARGYLE Penrose tubing. The tourniquet circumference was 70% of the arm circumference of the child and the tourniquet pressure range was 171–182 mm Hg. Visibility in the surgical field was reported as adequate and no complications reported.
When considering local impact of tourniquet use on skin, Tredwell et al. (55) explored the practices of paediatric orthopaedic surgeons in the USA and Canada. They looked at several aspects of tourniquet use including the degree of skin wrinkling caused by different models of tourniquets as well as the effect of the use of padding. When considering tourniquet pressure they found an equal share of surgeons select pressures based on either a standard pressure, a pressure based on age, extremity and size, or an inflation pressure based on SBP (either 100 mmHg above SBP, or doubling SBP). They found padding reduced both peak depth and number of wrinkles compared to using the cuffs without padding. The study reported one case where bilateral lower limb tourniquets were used, but the tourniquet on the primary leg was inadvertently left inflated when the contralateral leg was being operated on, with catastrophic consequence. The child later underwent limb amputation for the severe ischaemic injury suffered. The authors suggested tourniquets should be removed once they are no longer needed to prevent this type of error.
Infection
Ashraf et al. investigated complications from knee arthroscopy in children (68). A total of 1002 children were included. Mean age was 15.4 years (range: 4–17). A tourniquet was used in 643 surgeries with a mean tourniquet time of 78.4 min (range: 11–264). Tourniquet pressures were not reported. Longer tourniquet times (>114 min) were associated with increased risk of major complications (P < 0.001), including septic arthritis, wound complication requiring repeat closure, arthrofibrosis requiring manipulation under anaesthesia, and revision surgery.
No complications
Nine studies reported tourniquet use without any complications. Tourniquet use was defined; however, this was not the primary outcome of these studies. These studies investigated complications following peripheral nerve blocks (69), surgical fixations for recurrent patellar dislocation (70), and forearm re-fractures after surgical fixation (71).
Discussion
Tourniquets are commonly used in orthopaedic operations, with recognised benefits as well as risks. To guide safe practice, a new set of BOAST guidelines was published in October 2021 (36). This systematic review evaluated the available evidence regarding the use of tourniquets in orthopaedic surgery in the paediatric population.
Tourniquets were used uneventfully in the majority of patients, including patients with osteogenesis imperfecta (40, 41).
However, evidence of potential negative effects and complications of tourniquet use were found in the paediatric population reviewed. These include changes in physiological parameters such as blood pressure, heart rate, and core temperature, as well as biochemical changes triggering a state of mixed respiratory and metabolic acidosis (43, 44, 45, 46, 47). These changes can be explained by redistribution of blood flow, localised pressure damage and the state of limb ischaemia, resulting in skeletal muscle reverting to anaerobic respiration and lactate production (72). Apart from the suggestion that aggressive warming should be avoided in paediatric patients undergoing surgery with a tourniquet (43, 44), these studies do not discuss the clinical significance of the findings and whether they require a change in practice. Of note, in Bloch 1992, the patients in the control group underwent non-orthopaedic operations. It may be questioned whether specific aspects of orthopaedic surgery, such as drilling into bone, may trigger hyperthermia irrespective of tourniquet use. Furthermore, both studies by Bloch focused exclusively on lower limb surgery and did not report on tourniquet pressures. With regard to the serum changes detected in the study by Lynn et al. these occurred in the context of a tourniquet pressure threshold of 275 mm Hg, which is higher than recommended in the most recent BOAST guidelines (36). Higher tourniquet pressures may cause more significant tissue damage and cell breakdown underneath the tourniquet with release of intracellular contents and activation of the inflammatory response. One could argue this may have influenced the metabolic changes observed by Lynn et al. (45). However, the two studies by Goodarzi et al. (46, 47), which were performed in the context of inflation pressures of 75 mm Hg above the patient’s SBP, in line with BOAST recommendations, supported the findings by Lynn et al., providing evidence that these metabolic changes occur even when lower inflation pressures are used.
Seven studies reported nerve injury (49, 50, 51, 52, 53, 54, 55) and other anecdotal adverse events reported are increased risk of thrombotic events (60), fat embolism (61), compartment syndrome (52), and chemical skin burns (58, 59). Notably, in the study by Murphy et al. (60) only patients who were symptomatic for DVT were scanned so the incidence of DVT may have been underreported. Skin burns occur when there is pooling of disinfectant under the tourniquet. This is an example of a tourniquet associated adverse event that is entirely preventable through the correct application of a shut-off. Similar to the evidence from the adult population (16), tourniquet use is associated with increased post-operative pain and analgesia (62), which can prolong hospital stay and interfere with rehabilitation.
The study by Oginni & Rufai (42) investigated tourniquet use in patients with SCD. In the SCD group, one child developed mild jaundice post-operatively; however, this may also have been secondary to the stress of surgery rather than tourniquet use. The authors concluded that using a tourniquet in SCD carries risk, though they provided limited evidence for this.
BOAST guidelines state specific recommendations on tourniquet inflation time and pressures (36). This review found that tourniquet inflation times were generally better reported than inflation pressures. Details on tourniquet time were reported in 88% of the included studies, with only 41% providing information on the inflation pressures used (Table 1). Three studies reported a correlation between tourniquet time and potential risk: Ashraf et al. which identified a correlation between major complications and tourniquet times >114 min (68) and the two studies by Goodarzi et al. where longer tourniquet time was associated with increased pulse rate, serum lactate levels, and end-tidal carbon dioxide (46, 47).
In terms of inflation pressures, a variation in the settings used was identified: 50 mm Hg above LOP (64), or values above SBP of 75 mm Hg (45), 100 mm Hg (73), and 275 mm Hg (45). Misra et al. showed that HR and BP returned to baseline when pressures of 50 mm Hg above SBP for the lower limb and 25 mm Hg above SBP for the upper limb were used (48).
Tourniquet time is in most cases dependent on surgical time, which is affected by type and complexity of the operation being performed. Tourniquet inflation pressure, on the other hand, is often set independently at the beginning of the operation. Studies have shown that direct pressure, especially at the cuff edge interface, can cause damage to surrounding tissues and lead to muscle and nerve injury. Yates et al. showed that peripheral nerve injury secondary to tourniquet use is the consequence of both mechanical pressure and ischaemia, conduction abnormalities being disproportionately severe at the tourniquet border zones (74). Ochoa et al. demonstrated that pressure from the tourniquet could result in displacement of the nodes of Ranvier and axonal demyelination (75). Using LOP as a reference seems to result in lower pressures than the standard 250 and 300 mm Hg being used whilst providing adequate visibility of the surgical field (64, 65). The timing for measurement of LOP with regard to general anaesthesia and how changes in SBP may affect LOP intraoperatively are variables which have not been accounted for. Another consideration highlighted by Liebermann et al. is inadequate fitting of the tourniquet to the limbs of younger children which invites further consideration (64).
Despite the primary goals for the intraoperative use of tourniquets being to improve visibility of the surgical field and decreased blood loss, no study directly assessed quality of the surgical field with and without the use of a tourniquet and only one study reported on the difference in blood loss (62) which was not clinically significant. The routine use of tourniquet in both elective and fracture fixation is not evidence based, in terms of benefits as well as potential complications.
Conclusions
Although tourniquets continue to be commonly used in paediatric orthopaedic surgery, the evidence regarding the current practice is insufficient, as is the reporting of perceived benefit versus complications. There is a lack of high-quality studies investigating the use of tourniquets in paediatric orthopaedics but significant complications have anecdotally been reported and specific examples are easily preventable. Further research is required to elucidate their exact indication in children and the safest, most effective way to use them.
Recommendations
Based on the findings of this review, the authors make the following recommendations:
The necessity for tourniquet use needs to be assessed on a case-by-case basis.
When a tourniquet is used, documentation including specific parameters should be an integral part of the operation notes.
Tourniquets should be of the correct size, applied over a layer of padding and appropriately shut off. Pooling of disinfectant is to be avoided and, if occurs, should be promptly rectified to minimise the risk of burns.
The use of tourniquets in the context of bilateral limb surgery needs additional caution. Clinicians should consider the removal of the tourniquet from the limb once surgery on that side is completed to avoid the potentially catastrophic effects of a tourniquet that is inadvertently left inflated.
The anaesthetic team need to be mindful of the physiological responses to tourniquet use, including the risk of hyperthermia and acidosis.
When using a tourniquet, post-operative monitoring of pain, swelling, and signs of compartment syndrome should be adhered to.
There is a need for a large-scale high-quality study to clarify best practice and quantify the risks and benefits of tourniquet use in the paediatric population.
Supplementary Materials
ICMJE Conflict of Interest Statement
VP, CB, AB, CH and YG declare that they have no conflicts of interest concerning this article. AT receives payment for educational activities related to Smith and Nephew, Stryker, and Orthofix products. AT is a paid consultant of Stryker Trauma in relation to research and development. No payment or work is related to this project in any way, and there are no other conflicts of interest that could have inappropriately influenced this work.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Noordin S McEwen JA Kragh JF Eisen A & Masri BA. Surgical tourniquets in orthopaedics. Journal of Bone and Joint Surgery 2009912958–2967. ( 10.2106/JBJS.I.00634) [DOI] [PubMed] [Google Scholar]
- 2.Odinsson A & Finsen V. Tourniquet use and its complications in Norway. Journal of Bone and Joint Surgery 200688-B1090–1092. ( 10.1302/0301-620X.88B8.17668) [DOI] [PubMed] [Google Scholar]
- 3.Klenerman L. The tourniquet in surgery. Journal of Bone and Joint Surgery 196244-B937–943. ( 10.1302/0301-620X.44B4.937) [DOI] [PubMed] [Google Scholar]
- 4.Wakai A Winter DC Street JT & Redmond PH. Pneumatic tourniquets in extremity surgery. Journal of the American Academy of Orthopaedic Surgeons 20019345–351. ( 10.5435/00124635-200109000-00008) [DOI] [PubMed] [Google Scholar]
- 5.Kaufman RD & Walts LF. Tourniquet-induced hypertension. British Journal of Anaesthesia 198254333–336. ( 10.1093/bja/54.3.333) [DOI] [PubMed] [Google Scholar]
- 6.Crews JC & Sehlhorst CS. Response to maintenance of tourniquet inflation in a primate model. Regional Anesthesia 199116195–198. [PubMed] [Google Scholar]
- 7.Lam AM Slee T Hirst R Cooper JO Pavlin EG & Sundling N. Cerebral blood flow velocity following tourniquet release in humans. Canadian Journal of Anesthesia 199037(4 Pt 2) S29. [PubMed] [Google Scholar]
- 8.Klenerman L Chakrabarti R Mackie I Brozovic M & Stirling Y. Changes in haemostatic system after application of a tourniquet. Lancet 19771970–972. ( 10.1016/s0140-6736(7792276-0) [DOI] [PubMed] [Google Scholar]
- 9.Yoshida WB Thomazini IA Carvalho I Lastória S Curi PR & Maffei FH. Platelet activation following application of an Esmarch bandage and tourniquet in rabbits. Brazilian Journal of Medical and Biological Research 1989221091–1093. [PubMed] [Google Scholar]
- 10.Kohro S Yamakage M Arakawa J Kotaki M Omote T & Namiki A. Surgical/tourniquet pain accelerates blood coagulability but not fibrinolysis. British Journal of Anaesthesia 199880460–463. ( 10.1093/bja/80.4.460) [DOI] [PubMed] [Google Scholar]
- 11.Estebe JP le Naoures A Malledant Y & Ecoffey C. Use of a pneumatic tourniquet induces changes in central temperature. British Journal of Anaesthesia 199677786–788. ( 10.1093/bja/77.6.786) [DOI] [PubMed] [Google Scholar]
- 12.Liu D Graham D Gillies K & Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surgery and Related Research 201426207–213. ( 10.5792/ksrr.2014.26.4.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masri BA Eisen A Duncan CP & McEwen JA. Tourniquet-induced nerve compression injuries are caused by high pressure levels and gradients – a review of the evidence to guide safe surgical, pre-hospital and blood flow restriction usage. BMC Biomedical Engineering 202027. ( 10.1186/s42490-020-00041-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoen M Rotter R Gierer P Gradl G Strauss U Jonas L Mittlmeier T & Vollmar B. Ischemic preconditioning prevents skeletal muscle tissue injury, but not nerve lesion upon tourniquet-induced ischemia. Journal of Trauma 200763788–797. ( 10.1097/01.ta.0000240440.85673.fc) [DOI] [PubMed] [Google Scholar]
- 15.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovascular Surgery 200210620–630. ( 10.1016/s0967-2109(0200070-4) [DOI] [PubMed] [Google Scholar]
- 16.Ahmed I Chawla A Underwood M Price AJ Metcalfe A Hutchinson CE Warwick J Seers K Parsons H & Wall PDH. Time to reconsider the routine use of tourniquets in total knee arthroplasty surgery. Bone and Joint Journal 2021103–B830–839. ( 10.1302/0301-620X.103B.BJJ-2020-1926.R1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konrad G Markmiller M Lenich A Mayr E & R??ter A. Tourniquets may increase postoperative swelling and pain after internal fixation of ankle fractures. Clinical Orthopaedics and Related Research 2005433189–194. ( 10.1097/01.blo.0000151849.37260.0a) [DOI] [PubMed] [Google Scholar]
- 18.Xie J Yu H Wang F Jing J & Li J. A comparison of thrombosis in total knee arthroplasty with and without a tourniquet: a meta-analysis of randomized controlled trials. Journal of Orthopaedic Surgery and Research 202116408. ( 10.1186/s13018-021-02366-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magan AA Dunseath O Armonis P Fontalis A Kayani B & Haddad FS. Tourniquet use in total knee arthroplasty and the risk of infection: a meta-analysis of randomised controlled trials. Journal of Experimental Orthopaedics 2022962. ( 10.1186/s40634-022-00485-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W Li N Chen S Tan Y Al-Aidaros M & Chen L. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. Journal of Orthopaedic Surgery and Research 2014913. ( 10.1186/1749-799X-9-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirvensalo E Tuominen H Lapinsuo M & Heliö H. Compartment syndrome of the lower limb caused by a tourniquet: a report of two cases. Journal of Orthopaedic Trauma 19926469–472. ( 10.1097/00005131-199212000-00014) [DOI] [PubMed] [Google Scholar]
- 22.Yang JH Lim H Yoon JR & Jeong HI. Tourniquet associated chemical burn. Indian Journal of Orthopaedics 201246356–359. ( 10.4103/0019-5413.96366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L Ding CY Wang YL Wang ML Qian XH Huang L Xie XE & Ji HZ. Application effect of pneumatic tourniquet with individualized pressure setting in orthopaedic surgery of extremities: a meta-analysis. Journal of Advanced Nursing 2019753424–3433. ( 10.1111/jan.14196) [DOI] [PubMed] [Google Scholar]
- 24.Sun C Yang X Zhang X Ma Q Yu P Cai X & Zhou Y. Personalized tourniquet pressure may be a better choice than uniform tourniquet pressure during total knee arthroplasty: a PRISMA-compliant systematic review and meta-analysis of randomized-controlled trials. Medicine 2022101e28981. ( 10.1097/MD.0000000000028981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii Y Noguchi H & Takeda M. Higashihara T ichi. A new tourniquet system that determines pressures in synchrony with systolic blood pressure in knee surgery. Knee Surgery, Sports Traumatology, Arthroscopy 20091748–52. ( 10.1007/s00167-008-0640-9) [DOI] [PubMed] [Google Scholar]
- 26.Younger ASE McEwen JA & Inkpen K. Wide contoured thigh cuffs and automated limb occlusion measurement allow lower tourniquet pressures. Clinical Orthopaedics and Related Research 2004428286–293. ( 10.1097/01.blo.0000142625.82654.b3) [DOI] [PubMed] [Google Scholar]
- 27.Pedowitz RA Gershuni DH Botte MJ Kuiper S Rydevik BL & Hargens AR. The use of lower tourniquet inflation pressures in extremity surgery facilitated by curved and wide tourniquets and an integrated cuff inflation system. Clinical Orthopaedics and Related Research 1993287237–244. ( 10.1097/00003086-199302000-00038) [DOI] [PubMed] [Google Scholar]
- 28.Graham B Breault MJ McEwen JA & McGraw RW. Occlusion of arterial flow in the extremities at subsystolic pressures through the use of wide tourniquet cuffs. Clinical Orthopaedics and Related Research 1993286257–261. ( 10.1097/00003086-199301000-00038) [DOI] [PubMed] [Google Scholar]
- 29.Ahmed I Chawla A Underwood M Price AJ Metcalfe A Hutchinson C Warwick J Seers K Parsons H & Wall PD. Tourniquet use for knee replacement surgery. Cochrane Database of Systematic Reviews 202012CD012874. ( 10.1002/14651858.CD012874.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigney B Casey C McDonald C Pomeroy E & Cleary MS. Distal radius fracture fixation using WALANT versus general and regional anesthesia: a systematic review and meta-analysis. Surgeon 202321e13–e22. ( 10.1016/j.surge.2022.01.006) [DOI] [PubMed] [Google Scholar]
- 31.Rellán I Bronenberg Victorica P Kohan Fortuna Figueira v. SV Donndorff AG de Carli P & Boretto JG. What is the infection rate of carpal tunnel syndrome and trigger finger release performed under wide-awake anesthesia? Hand 202318198–202. ( 10.1177/1558944721994262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouveia K Harbour E Gazendam A & Bhandari M. Fixation of distal radius fractures under wide-awake local anesthesia: a systematic review. Hand 202215589447221109632. ( 10.1177/15589447221109632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahir M Chaudhry EA Zaffar Z Anwar K Mamoon MAH Ahmad M Jamali AR & Mehboob G. Fixation of distal radius fractures using wide-awake local anaesthesia with no tourniquet (WALANT) technique: a randomized control trial of a cost-effective and resource-friendly procedure. Bone and Joint Research 20209429–439. ( 10.1302/2046-3758.97.BJR-2019-0315.R1) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Farhan-Alanie MM Trompeter AJ Wall PDH & Costa ML. Tourniquet use in lower limb trauma and fracture surgery. Bone and Joint Journal 2021103–B809–812. ( 10.1302/0301-620X.103B5.BJJ-2020-2070.R1) [DOI] [PubMed] [Google Scholar]
- 35.Farhan-Alanie MM Dhaif F Trompeter A Underwood M Yeung J Parsons N Metcalfe A & Wall PDH. The risks associated with tourniquet use in lower limb trauma surgery: a systematic review and meta-analysis. European Journal of Orthopaedic Surgery and Traumatology 202131967–979. ( 10.1007/s00590-021-02957-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.British Orthopaedic Association & BOAST. The safe use of intraoperative tourniquets 2021. Available at: https://www.boa.ac.uk/resources/boast-the-safe-use-of-intraoperative-tourniquets.html [Google Scholar]
- 37.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019366l4898. ( 10.1136/bmj.l4898) [DOI] [PubMed] [Google Scholar]
- 38.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al.Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016355i4919. ( 10.1136/bmj.i4919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murad MH Sultan S Haffar S & Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine 20182360–63. ( 10.1136/bmjebm-2017-110853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan BT Margalit A Garg VS Njoku DB & Sponseller PD. Incidence of fractures from perioperative blood pressure cuff use, tourniquet use, and patient positioning in osteogenesis imperfecta. Journal of Pediatric Orthopedics 201939e68–e70. ( 10.1097/BPO.0000000000001105) [DOI] [PubMed] [Google Scholar]
- 41.Ross KE Gibian JT Crockett CJ & Martus JE. Perioperative considerations in osteogenesis imperfecta: a 20-year experience with the use of blood pressure cuffs, arterial lines, and tourniquets. Children 20207214. ( 10.3390/children7110214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oginni LM & Rufai MB. How safe is tourniquet use in sickle-cell disease? African Journal of Medicine and Medical Sciences 1996253–6. [PubMed] [Google Scholar]
- 43.Bloch EC. Hyperthermia resulting from tourniquet application in children. Annals of the Royal College of Surgeons of England 198668193–194. [PMC free article] [PubMed] [Google Scholar]
- 44.Bloch EC Ginsberg B Binner RA & Sessler DI. Limb tourniquets and central temperature in anesthetized children. Anesthesia and Analgesia 199274486–489. ( 10.1213/00000539-199204000-00002) [DOI] [PubMed] [Google Scholar]
- 45.Lynn AM Fischer T Brandford HG & Pendergrass TW. Systemic responses to tourniquet release in children. Anesthesia and Analgesia 198665865–872. ( 10.1213/00000539-198608000-00008) [DOI] [PubMed] [Google Scholar]
- 46.Goodarzi M Shier NH & Grogan DP. Does sympathetic blockade prevent the physiologic changes associated with tourniquet use in children? Journal of Pediatric Orthopedics 199717289–292. ( 10.1097/01241398-199705000-00004) [DOI] [PubMed] [Google Scholar]
- 47.Goodarzi M Shier NH & Ogden JA. Physiologic changes during tourniquet use in children. Journal of Pediatric Orthopedics 199212510–513. ( 10.1097/01241398-199207000-00018) [DOI] [PubMed] [Google Scholar]
- 48.Misra A Panda A & Sharma R. Tourniquet cuff pressures in pediatric patients: urgent need to device guidelines?. Paediatric Anaesthesia 201020369–370. ( 10.1111/j.1460-9592.2010.03270.x) [DOI] [PubMed] [Google Scholar]
- 49.Schrock RD. Peroneal nerve palsy following derotation osteotomies for tibial torsion. Clinical Orthopaedics and Related Research 196962172–177. ( 10.1097/00003086-196901000-00022) [DOI] [PubMed] [Google Scholar]
- 50.Slawski DP Schoenecker PL & Rich MM. Peroneal nerve injury as a complication of pediatric tibial osteotomies: a review of 255 osteotomies. Journal of Pediatric Orthopedics 199414166–172. ( 10.1097/01241398-199403000-00007) [DOI] [PubMed] [Google Scholar]
- 51.Saw KM & Hee HI. Tourniquet-induced common peroneal nerve injury in a pediatric patient after knee arthroscopy - raising the red flag. Clinical Case Reports 201751438–1440. ( 10.1002/ccr3.1060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jardaly A Conklin M Ashley P & Gilbert SR. Closed reduction in the treatment of tibial tubercle fractures. Injury 2021521336–1340. ( 10.1016/j.injury.2020.10.072) [DOI] [PubMed] [Google Scholar]
- 53.İnan M Sarikaya İA Yildirim E & Güven MF. Neurological complications after supracondylar femoral osteotomy in cerebral palsy. Journal of Pediatric Orthopedics 201535290–295. ( 10.1097/BPO.0000000000000264) [DOI] [PubMed] [Google Scholar]
- 54.Yalcinkaya M Doğan A Ozkaya U Sökücü S Uzümcügil O & Kabukçuoğlu Y. Clinical results of intramedullary nailing following closed or mini open reduction in pediatric unstable diaphyseal forearm fractures. Acta Orthopaedica et Traumatologica Turcica 2010447–13. ( 10.3944/AOTT.2010.2260) [DOI] [PubMed] [Google Scholar]
- 55.Tredwell SJ Wilmink M Inkpen K & McEwen JA. Pediatric tourniquets: analysis of cuff and limb interface, current practice, and guidelines for use. Journal of Pediatric Orthopedics 200121671–676. ( 10.1097/01241398-200109000-00023) [DOI] [PubMed] [Google Scholar]
- 56.Hanlon M Barnes M Lamb G & Nicol R. Central compartment pressure monitoring following clubfoot release. Journal of Pediatric Orthopedics 19961663–66. ( 10.1097/00004694-199601000-00013) [DOI] [PubMed] [Google Scholar]
- 57.Silver R de la Garza J Rang M & Koreska J. Limb swelling after release of a tourniquet. Clinical Orthopaedics and Related Research 198620686–89. ( 10.1097/00003086-198605000-00017) [DOI] [PubMed] [Google Scholar]
- 58.Hodgins J Wright J Howard A & Fish J. Chlorhexidine-gluconate-related burns under a tourniquet: a Report of Two Cases. JBJS Case Connector 20122e27. ( 10.2106/JBJS.CC.K.00072) [DOI] [PubMed] [Google Scholar]
- 59.Dickinson JC & Bailey BN. Chem ical burns beneath tourniquets. BMJ 19882971513–1513. ( 10.1136/bmj.297.6662.1513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy RF Heyworth B Kramer D Naqvi M Miller PE Yen YM Kocher MS & Shore BJ. Symptomatic venous thromboembolism after adolescent knee arthroscopy. Journal of Pediatric Orthopedics 201939125–129. ( 10.1097/BPO.0000000000000894) [DOI] [PubMed] [Google Scholar]
- 61.Ida M Matsunari Y & Kawaguchi M. Fat embolism syndrome in a child triggered by surgical tourniquet release: a case report. Paediatric Anaesthesia 201828371–372. ( 10.1111/pan.13337) [DOI] [PubMed] [Google Scholar]
- 62.Hanna RB Nies M Lang PJ & Halanski M. Effects of tourniquet use in paediatric lower leg surgery. Journal of Children’s Orthopaedics 202014466–472. ( 10.1302/1863-2548.14.200105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vas L. Continuous sciatic block for leg and foot surgery in 160 children. Paediatric Anaesthesia 200515971–978. ( 10.1111/j.1460-9592.2005.01620.x) [DOI] [PubMed] [Google Scholar]
- 64.Lieberman JR Staheli LT & Dales MC. Tourniquet pressures on pediatric patients: a clinical study. Orthopedics 1997201143–1147. ( 10.3928/0147-7447-19971201-08) [DOI] [PubMed] [Google Scholar]
- 65.Reilly CW McEwen JA Leveille L Perdios A & Mulpuri K. Minimizing tourniquet pressure in pediatric anterior cruciate ligament reconstructive surgery: a blinded, prospective randomized controlled trial. Journal of Pediatric Orthopedics 200929275–280. ( 10.1097/BPO.0b013e31819bcd14) [DOI] [PubMed] [Google Scholar]
- 66.Eidelman M Katzman A & Bialik V. A novel elastic exsanguination tourniquet as an alternative to the pneumatic cuff in pediatric orthopedic limb surgery. Journal of Pediatric Orthopedics 200615379–384. ( 10.1097/01202412-200609000-00014) [DOI] [PubMed] [Google Scholar]
- 67.Kang YC Arjandas M & Teoh LC. Revisiting the elastic band tourniquet in pediatric upper limb surgery. Techniques in Hand and Upper Extremity Surgery 20182243–45. ( 10.1097/BTH.0000000000000193) [DOI] [PubMed] [Google Scholar]
- 68.Ashraf A Luo TD Christophersen C Hunter LR Dahm DL & McIntosh AL. Acute and subacute complications of pediatric and adolescent knee arthroscopy. Arthroscopy 201430710–714. ( 10.1016/j.arthro.2014.02.028) [DOI] [PubMed] [Google Scholar]
- 69.Tamai RJ Sullivan BT & Lee RJ. Residual neurological symptoms after peripheral nerve blocks for pediatric knee surgery. Journal of Pediatric Orthopedics 201838e157–e161. ( 10.1097/BPO.0000000000001125) [DOI] [PubMed] [Google Scholar]
- 70.Zampieri A Girardin C Hocquet B Coursier R Fournier A Martin C Nectoux E & Canavese F. Patellar dislocation recurrence after pediatric MPFL reconstruction: bone tunnels and soft tissues versus suture anchors and interference screw. Orthopaedics and Traumatology, Surgery and Research 2022103515. ( 10.1016/j.otsr.2022.103515) [DOI] [PubMed] [Google Scholar]
- 71.Lindgren AM Sendek G Manhard CE Bastrom TP & Pennock AT. Subsequent forearm fractures following initial surgical fixation. Journal of Pediatric Orthopedics 202343e383–e388. ( 10.1097/BPO.0000000000002374) [DOI] [PubMed] [Google Scholar]
- 72.Korth U Merkel G Fernandez FF Jandewerth O Dogan G Koch T van Ackern K Weichel O & Klein J. Tourniquet-induced changes of energy metabolism in human skeletal muscle monitored by microdialysis. Anesthesiology 2000931407–1412. ( 10.1097/00000542-200012000-00011) [DOI] [PubMed] [Google Scholar]
- 73.Prakash J Boruah T Mehtani A Chand S & Lal H. Experience of supracondylar cheveron osteotomy for genu valgum in 115 adolescent knees. Journal of Clinical Orthopaedics and Trauma 20178285–292. ( 10.1016/j.jcot.2017.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yates SK Hurst LN & Brown WF. The pathogenesis of pneumatic tourniquet paralysis in man. Journal of Neurology, Neurosurgery, and Psychiatry 198144759–767. ( 10.1136/jnnp.44.9.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ochoa J Fowler TJ & Gilliatt RW. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. Journal of Anatomy 1972113433–455. [PMC free article] [PubMed] [Google Scholar]
- 76.Tareco J Sala DA Scher DM Lehman WB & Feldman DS. Percutaneous fixation in clubfoot surgery: a radiographic and gait study. Journal of Pediatric Orthopedics. Part B 200211139–142. ( 10.1097/00009957-200204000-00010) [DOI] [PubMed] [Google Scholar]
- 77.Baghdadi S Weltsch D Arkader A Harwood K & Lawrence JTR. Open reduction of medial epicondyle fractures in the pediatric population: supine versus prone position. Journal of Pediatric Orthopedics 202141273–278. ( 10.1097/BPO.0000000000001794) [DOI] [PubMed] [Google Scholar]
- 78.Reinhardt KR Feldman DS Green DW Sala DA Widmann RF & Scher DM. Comparison of intramedullary nailing to plating for both-bone forearm fractures in older children. Journal of Pediatric Orthopedics 200828403–409. ( 10.1097/BPO.0b013e31816d71f2) [DOI] [PubMed] [Google Scholar]
- 79.Watts CD Larson AN & Milbrandt TA. Open versus arthroscopic reduction for tibial eminence fracture fixation in children. Journal of Pediatric Orthopedics 201636437–439. ( 10.1097/BPO.0000000000000476) [DOI] [PubMed] [Google Scholar]
- 80.Cai L Wang J Du S Zhu S Wang T Lu D & Chen H. Comparison of hybrid fixation to dual plating for both-bone forearm fractures in older children. American Journal of Therapeutics 201623e1391–e1396. ( 10.1097/MJT.0000000000000227) [DOI] [PubMed] [Google Scholar]
- 81.Ryan C Dunleavy ML Burton A & Hennrikus W. Outcomes of hardware removal surgery for children. Orthopedics 202245e91–e95. ( 10.3928/01477447-20220105-06) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a