Abstract
Background
Patient decision aids are interventions designed to support people making health decisions. At a minimum, patient decision aids make the decision explicit, provide evidence‐based information about the options and associated benefits/harms, and help clarify personal values for features of options. This is an update of a Cochrane review that was first published in 2003 and last updated in 2017.
Objectives
To assess the effects of patient decision aids in adults considering treatment or screening decisions using an integrated knowledge translation approach.
Search methods
We conducted the updated search for the period of 2015 (last search date) to March 2022 in CENTRAL, MEDLINE, Embase, PsycINFO, EBSCO, and grey literature. The cumulative search covers database origins to March 2022.
Selection criteria
We included published randomized controlled trials comparing patient decision aids to usual care. Usual care was defined as general information, risk assessment, clinical practice guideline summaries for health consumers, placebo intervention (e.g. information on another topic), or no intervention.
Data collection and analysis
Two authors independently screened citations for inclusion, extracted intervention and outcome data, and assessed risk of bias using the Cochrane risk of bias tool. Primary outcomes, based on the International Patient Decision Aid Standards (IPDAS), were attributes related to the choice made (informed values‐based choice congruence) and the decision‐making process, such as knowledge, accurate risk perceptions, feeling informed, clear values, participation in decision‐making, and adverse events. Secondary outcomes were choice, confidence in decision‐making, adherence to the chosen option, preference‐linked health outcomes, and impact on the healthcare system (e.g. consultation length).
We pooled results using mean differences (MDs) and risk ratios (RRs) with 95% confidence intervals (CIs), applying a random‐effects model. We conducted a subgroup analysis of 105 studies that were included in the previous review version compared to those published since that update (n = 104 studies). We used Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to assess the certainty of the evidence.
Main results
This update added 104 new studies for a total of 209 studies involving 107,698 participants. The patient decision aids focused on 71 different decisions. The most common decisions were about cardiovascular treatments (n = 22 studies), cancer screening (n = 17 studies colorectal, 15 prostate, 12 breast), cancer treatments (e.g. 15 breast, 11 prostate), mental health treatments (n = 10 studies), and joint replacement surgery (n = 9 studies). When assessing risk of bias in the included studies, we rated two items as mostly unclear (selective reporting: 100 studies; blinding of participants/personnel: 161 studies), due to inadequate reporting. Of the 209 included studies, 34 had at least one item rated as high risk of bias.
There was moderate‐certainty evidence that patient decision aids probably increase the congruence between informed values and care choices compared to usual care (RR 1.75, 95% CI 1.44 to 2.13; 21 studies, 9377 participants).
Regarding attributes related to the decision‐making process and compared to usual care, there was high‐certainty evidence that patient decision aids result in improved participants' knowledge (MD 11.90/100, 95% CI 10.60 to 13.19; 107 studies, 25,492 participants), accuracy of risk perceptions (RR 1.94, 95% CI 1.61 to 2.34; 25 studies, 7796 participants), and decreased decisional conflict related to feeling uninformed (MD ‐10.02, 95% CI ‐12.31 to ‐7.74; 58 studies, 12,104 participants), indecision about personal values (MD ‐7.86, 95% CI ‐9.69 to ‐6.02; 55 studies, 11,880 participants), and proportion of people who were passive in decision‐making (clinician‐controlled) (RR 0.72, 95% CI 0.59 to 0.88; 21 studies, 4348 participants).
For adverse outcomes, there was high‐certainty evidence that there was no difference in decision regret between the patient decision aid and usual care groups (MD ‐1.23, 95% CI ‐3.05 to 0.59; 22 studies, 3707 participants).
Of note, there was no difference in the length of consultation when patient decision aids were used in preparation for the consultation (MD ‐2.97 minutes, 95% CI ‐7.84 to 1.90; 5 studies, 420 participants). When patient decision aids were used during the consultation with the clinician, the length of consultation was 1.5 minutes longer (MD 1.50 minutes, 95% CI 0.79 to 2.20; 8 studies, 2702 participants).
We found the same direction of effect when we compared results for patient decision aid studies reported in the previous update compared to studies conducted since 2015.
Authors' conclusions
Compared to usual care, across a wide variety of decisions, patient decision aids probably helped more adults reach informed values‐congruent choices. They led to large increases in knowledge, accurate risk perceptions, and an active role in decision‐making. Our updated review also found that patient decision aids increased patients’ feeling informed and clear about their personal values. There was no difference in decision regret between people using decision aids versus those receiving usual care. Further studies are needed to assess the impact of patient decision aids on adherence and downstream effects on cost and resource use.
Keywords: Humans, Decision Support Techniques, Psychotherapy, Referral and Consultation
Plain language summary
Patient decision aids to help people who are facing decisions about health treatment or screening
Review question
How effective/beneficial are patient decision aids for adults making decisions regarding health treatment or screening?
Key messages
‐ Patient decision aids are pamphlets or videos used in person or online. They clearly identify the healthcare decision to be made, provide information on options (benefits and harms), and help people clarify what is most important to them. Decision aids are designed to enhance and supplement consultation with the clinician, not replace it.
‐ Over 200 studies showed that patient decision aids helped adults be more involved in making health decisions by improving their knowledge and expectations of benefits and harms, and choosing an option that reflected what was most important to them.
‐ There were no unwanted effects for adults who used a patient decision aid.
What are patient decision aids?
Patient decision aids can help guide people making decisions when there is more than one option, including status quo (no change). They are pamphlets, videos, or web‐based resources that state the decision, describe the options, and help people think about which features of the options are most important to them (which features matter most). Usual care was defined as general information, risk assessment, clinical practice guideline summaries for health consumers, placebo intervention (e.g. information on another topic), or no intervention.
What did we want to find out?
We wanted to find out if patient decision aids used by patients who are facing health treatment or screening decisions are better than the usual care for choosing an option that reflects what is most important to them. We also wanted to find out if patient decision aids were associated with any unwanted effects.
What did we do?
We updated a previous Cochrane review that was first published in 2003 and then updated in 2017. Our search included studies that compared a patient decision aid with usual care in adults who were facing health decisions for themselves or a family member. Usual care may have been general patient information or nothing. We compared and summarized the results of the studies and rated our confidence in the certainty of the evidence.
What did we find?
We found 209 studies that involved 107,698 adults. The patient decision aids focused on 71 different decisions. The common decisions were about: surgery, screening (e.g. prostate cancer, colon cancer, prenatal), genetic testing, and long‐term medication treatments (e.g. insulin injections for diabetes, or statins for high cholesterol).
We are moderately confident that adults given patient decision aids were more likely to choose an option that reflected what features of the options were most important to them. Our confidence in the evidence is only moderate because the studies that provided results for our review represent only a small set of the studies evaluating patient decision aids. We are confident that when adults used patient decision aids, they had large increases in their knowledge, expectations of benefits and harms, and participation in making the decision. We are also confident that they felt better informed and were more clear about what mattered most to them. We are confident that patient decision aids did not cause any unwanted effects such as regret about the decision.
What are the limitations of the evidence?
Further research could strengthen the confidence in the evidence for choosing options that reflect which features of the options are most important to people.
How up‐to‐date is this evidence?
This review updates our previous review published in 2017. The evidence is up‐to‐date to March 2022.
Summary of findings
Summary of findings 1. Patient decision aids versus usual care for adults facing treatment or screening decisions.
| Patient decision aids compared with usual care for adults facing treatment or screening decisions | ||||||
|
Patient or population : adults considering treatment or screening decisions Settings : all settings Intervention : patient decision aid Comparison : usual care | ||||||
| Outcomes | Illustrative comparative benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed benefit | Corresponding benefit | |||||
| Usual care | Patient decision aid | |||||
|
Congruence between informed values and choice ‐ all studies Based on the proportion of participants who made a decision that aligned with what was most important to them. Assessed soon after exposure to the decision aid. |
295 per 1000c |
481 per 1000 The proportion of participants who made an informed values choice was probably higher. |
RR 1.75 (1.44 to 2.13) | 9377 (21 studies) |
⊕⊕⊕⊝ Moderatea,b,d | — |
|
Knowledge ‐ all studies Standardized on a scale from 0 (no knowledge) to 100 (perfect knowledge). Assessed soon after exposure to the decision aid. |
The mean knowledge score was 58.61% across control groups, ranging from 27.0% to 89.9%. | The mean knowledge score in the intervention groups was 11.90 higher (10.60 to 13.19 higher). | — | 25,492 (107 studies) | ⊕⊕⊕⊕ Higha,b | Higher scores indicate better knowledge. 82 out of 107 studies showed an improvement in knowledge. |
|
Accurate risk perceptions ‐ all studies Based on the accuracy of perceived outcome probabilities according to the percentage of individuals whose judgments corresponded to the scientific evidence about the chances of an outcome for similar people. Assessed soon after exposure to the decision aid. |
281 per 1000c |
532 per 1000 The proportion of participants who accurately perceived their risk was higher. |
RR 1.94 (1.61 to 2.34) | 7796 (25 studies) | ⊕⊕⊕⊕ Higha,b | — |
|
Decisional conflict: uninformed subscale ‐ all studies Standardized on a scale from 0 (informed) to 100 (uninformed). Assessed soon after exposure to the decision aid. |
The mean for the outcome 'feeling uninformed' ranged across control groups from 6.4% to 85.0%. Scores ≤ 25 are associated with following through on decisions. Scores > 38 are associated with delay in decision‐making. |
The mean feeling uninformed value in the intervention groups was 10.02 lower (12.31 to 7.74 lower). | — | 12,104 (58 studies) |
⊕⊕⊕⊕ Higha,b | Lower scores indicate feeling more informed. |
|
Decisional conflict: unclear about personal values subscale ‐ all studies Standardized on a scale from 0 (clear) to 100 (unclear). Assessed soon after exposure to the decision aid. |
The mean for the outcome 'feeling unclear about personal values' ranged across control groups from 4.28% to 56.9%. Scores ≤ 25 are associated with follow‐through with decisions. Scores > 38 are associated with delay in decision‐making. |
The mean feeling unclear value in the intervention groups was 7.86 lower (9.69 to 6.02 lower). | — | 11,880 (55 studies) |
⊕⊕⊕⊕ Higha,b | Lower scores indicate feeling clearer about values. |
|
Participation in decision‐making: clinician‐controlled decision‐making ‐ all studies Based on the proportion of participants who indicated a passive role in decision‐making where the decision was primarily made by the clinician. Assessed soon after consultation with the clinician. |
257 per 1000c |
188 per 1000 The proportion of participants who had a passive role in decision‐making (clinician‐controlled) was lower. |
RR 0.72 (0.59 to 0.88) | 4348 (21 studies) | ⊕⊕⊕⊕ Higha,b | Patient decision aids aim to increase patient involvement in making decisions; a lower proportion of clinician‐controlled decision‐making is better. |
|
Adverse events: decision regret ‐ all studies Standardized on a scale from 0 (no regret) to 100 (high regret). Assessed weeks to months after the decision is made. |
The mean regret score was 15.6% across control groups, ranging from 6.4% to 27.0%. | The mean regret score in the intervention groups was not different ‐1.23 (‐3.05 to 0.59). | — | 3707 (22 studies) |
⊕⊕⊕⊕ Higha,b | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI : confidence interval; RR : risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty : further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty : further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty : further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty : we are very uncertain about the estimate. | ||||||
a The vast majority of studies measuring this outcome were not at high risk of bias. b We did not downgrade for inconsistency (heterogeneity) given the generally consistent direction of effects across studies for the decision aid compared to usual care groups. c The data source for the assumed risk was the mean control event rate. d We downgraded for possible publication bias. See funnel plot in Figure 1 . It is unclear the extent to which there is publication bias for this primary outcome. Therefore, we used a cautious approach and downgraded the certainty of evidence. This outcome is more challenging to measure because it is a composite measure. Hence, it is more likely that it is not measured in most studies rather than not reported.
Background
Many health treatment and screening decisions have no single 'best' choice. Many, if not most, healthcare decisions are considered 'preference‐sensitive' because there is insufficient evidence about outcomes associated with specific options or there is a need to trade off known benefits and harms across options. Patient decision aids are interventions that can be used to present the evidence about known benefits, harms, and outcomes related to the options and have patients consider what is important to them (or what matters most to them) ( Brouwers 2010 ). Our original Cochrane review of patient decision aids was first published in 2003 ( O'Connor 2003 ); the most recent update, published in 2017, was the top most‐accessed active review for Cochrane Consumers and Communication and up to 2022 it has received the highest number of guideline citations overall ( Stacey 2017 ), with authors of clinical practice guidelines from around the world citing the 2017 review 94 times ( CDSR 2022 ). For example, this review provided the foundational evidence used in the shared decision‐making guideline from the UK National Institute for Health and Care Excellence (NICE) ( NICE 2021 ), which recommends using high‐quality patient decision aids. Since 2015, the US Centers for Medicare & Medicaid Services requires the use of patient decision aids for reimbursement of some health services.
Description of the condition
This review focuses on the use of patient decision aids compared to usual care for all healthcare conditions.
Description of the intervention
Patient decision aids are evidence‐based tools designed to help patients make specific and deliberate choices from among healthcare options; they are intended to supplement (rather than replace) clinicians' counseling about options. For this review, we are using the terms patients to refer to healthcare consumers, clients, and people in general making decisions for themselves or another close person, given that most patient decision aid studies are used in the healthcare system; we are using the term patient decision aids, given that decision aids are also used for decision support interventions for clinicians only. Patient decision aids meet the definition of complex interventions given the characteristics of their content and the way the content is presented ( Skivington 2021 ). According to the International Patient Decision Aid Standards (IPDAS) Collaboration ( Elwyn 2006 ; IPDAS 2005a ; Joseph‐Williams 2013 ; Stacey 2021 ), patient decision aids, at a minimum, include the following elements:
they explicitly state the decision that needs to be considered for the target population;
they provide evidence‐based, balanced information about a health condition, the options, associated benefits, harms; and
they help patients clarify, either implicitly or explicitly, the value they place on the benefits and harms of each option. To accomplish this, patient decision aids may describe the options in enough detail that patients can imagine what it is like to experience the physical, emotional, and social effects (to implicitly clarify values), or they may guide patients to consider which benefits and harms are most important to them using an explicit values clarification exercise.
Patient decision aids differ from health education materials. Whereas health education materials help patients to understand their diagnosis, treatment, and management in general terms, patient decision aids offer a process: they make the decision being considered explicit, providing a detailed, specific, and sometimes personalized focus on options and outcomes for the purpose of engaging patients in decision‐making. Given their broader perspective, health education materials are not focused on specific decision points or the decision‐making process; thus, they do not necessarily facilitate patients participating in decision‐making. Many patient decision aids are based on a decision‐making conceptual model or theoretical framework, where most health education materials are based on other conceptual models or theoretical frameworks, if used at all ( Durand 2008 ; Mulley 1995 ; O'Connor 1998b ; Rothert 1987 ).
In response to concerns about heterogeneity in the quality of patient decision aids, the IPDAS Collaboration developed the original IPDAS criteria for judging their quality based on evidence syntheses ( Elwyn 2006 ). The criteria address three domains of quality: clinical content, development process, and effectiveness. In 2013, an international team of researchers reached consensus on a shorter set of qualifying (n = 6), certifying (n = 6 for treatment, 10 for screening), and quality criteria (n = 28) ( Joseph‐Williams 2013 ). The IPDAS group updated the evidence on core IPDAS domains published in a series of papers ( Stacey 2021 ). The Washington State Health Care Authority launched the first patient decision aid certification program in 2016, based on the work of the IPDAS group ( Washington State Health Care Authority 2016 ). The IPDAS criteria are also used by the Norwegian Health Authority, the Center for Shared Decision Making in Denmark, and the Patient Decision Aid Research Group’s International A to Z Inventory of publicly available patient decision aids ( Dahl Steffensen 2022 ; Helsedirektoratet Norway 2017 ; Ottawa Hospital Research Institute 2023 ). Developers of patient decision aids are increasingly using the IPDAS framework to guide their development and evaluation processes.
How the intervention might work
Patient decision aids can be used before, during, or after a clinical encounter to facilitate patients becoming active, informed participants in making healthcare decisions. These decision support tools are typically process‐oriented; thus, they structure and support the decision‐making process with specific steps. Providing the patient decision aid before the consultation allows patients more time to digest the information and be ready to discuss the decision with the clinician, although this may not be feasible in some situations (e.g. antibiotics for upper respiratory infections). Patient decision aids can also facilitate shared decision‐making. Shared decision‐making is defined as a process through which clinicians and patients make informed healthcare choices together by using the best available evidence and incorporating patient’s informed preferences ( Légaré 2018 ; Makoul 2006 ). However, the way in which a clinician provides verbal information may strongly affect a patient's preferences ( Hibbard 1997 ), prompting the need for standardized, balanced information offered by patient decision aids. Patients who are more active in making decisions about their health have better health outcomes and healthcare experiences ( Hibbard 2013 ; Hughes 2018 ; Shay 2015 ). Also, patient decision aids are geared at helping patients grasp the probabilistic nature of evidence and, hence, help them navigate uncertainty, the hallmark of health evidence. In summary, patient decision aids may help clinicians and patients achieve a high‐quality decision‐making process, which will ultimately result in quality decisions, grounded in the patient's values and considering the potential trade‐offs between benefits and harms across different options.
Why it is important to do this review
As never before, choice amongst multiple options exists for patients who are facing health decisions. To make quality evidence‐ and values‐based decisions that are best suited for their circumstances, patients need access to the best available evidence about the possible options, opportunities to get them thinking about what is most important to them, and guidance to deliberate. Patient decision aids are designed to achieve this. Interest in patient decision aids has grown exponentially since the first Cochrane review on this topic was published in 2007. Given this growing interest, and their acknowledgment in over 90 clinical practice guidelines and in health policies internationally, there was a need to update this review. More specifically, we wanted to identify studies on new decisions or studies conducted in a broader range of countries and to strengthen the synthesized evidence in favor of patient decision aids for outcomes that do not yet have high‐certainty evidence, as per GRADE.
Results from previous reviews were used to inform clinical practice guidelines such as those from the National Institute for Health and Care Excellence (NICE) ( NICE 2021 ), Patient Experience in Adult NHS Services ( NCGC/NICE 2021 ), and Collaboration and Shared Decision‐Making Between Patients and Clinicians in Preventive Health Care Decisions and US Preventive Services Task Force ( Davidson 2022 ). Some groups have established strategies to collaboratively develop patient decision aids from clinical practice guidelines and evidence summaries to accelerate translation of best evidence to patients and increase the quality of decision‐making between clinicians and patients ( Alonso Coello 2022 ; NICE 2021 ; van der Weijden 2019 ).
Previous updates of this review have been used to conduct subgroup analyses focused on outcomes of anxiety ( Bekker 2003 ), adherence ( Trenaman 2016 ), values‐choice congruence ( Munro 2016 ), and quality of life ( Housten 2019 ; Rutherford 2019 ). Other subanalyses were about patients’ motivation for participation in a patient decision aid trial on patient decision aid efficacy ( Brown 2015 ), factors explaining the heterogeneity of effects on knowledge of outcome probabilities ( Gentles 2013 ), strategies for presenting overdiagnosis in cancer screening patient decision aids ( Housten 2019 ), and cancer‐related decisions ( McAlpine 2018 ).
Other systematic reviews were conducted on the use of patient decision aids as one type of intervention to facilitate shared decision‐making in clinical practice ( Coyne 2013 ; Duncan 2010 ; Elwyn 2013 ; Irish 2023 ; Légaré 2018 ; Mitropoulou 2022 ).
Objectives
To assess the effects of patient decision aids in adults considering treatment or screening decisions using an integrated knowledge translation approach.
Methods
Criteria for considering studies for this review
Types of studies
We included all published individual or cluster‐randomized controlled trials (RCT) evaluating patient decision aids. There were no restrictions on language or settings.
Types of participants
We included studies involving adults aged 18 years or older who were making health decisions about screening or treatment options for themselves, a child, or as a proxy for a significant other. We excluded studies in which adults were making hypothetical choices.
Types of interventions
We included studies that evaluated a patient decision aid. Patient decision aids were defined as an intervention designed to help patients make specific and deliberated choices among options (including the status quo), by, at a minimum, making the decision explicit, providing information on the options and outcomes (e.g. benefits/harms) relevant to a person’s health status, and implicit or explicit methods to clarify values. The patient decision aid also may have included: information on the disease/condition; costs associated with options; probabilities of outcomes tailored to personal health risk factors; an explicit values clarification exercise; information on others' experiences; personalized tailoring of information based on clinical characteristics; and guidance or coaching in the steps of making and communicating decisions with others.
We excluded studies if interventions focused on: decisions about lifestyle changes, social care, clinical trial entry, or general advance directives (e.g. do not resuscitate); education programs not geared to a specific decision; and interventions designed to promote adherence or elicit informed consent regarding a recommended option. Interventions focused on these decisions were excluded in the original review and subsequent updates continued to exclude them for consistency with the approved protocol ( O'Connor 2003 ). We also excluded studies when the relevant patient decision aid(s) were not adequately described in the article(s) or available from the authors, such that our team was not able to determine the aids’ characteristics and whether or not they met the minimum criteria to qualify as a patient decision aid.
Types of comparisons
We included studies that compared adults exposed to a patient decision aid to adults exposed to usual care. For the purpose of this review, usual care is defined as general information, risk assessment, clinical practice guideline summaries for health consumers, placebo intervention (e.g. information on another topic), or no intervention. We excluded studies that compared different formats or delivery methods of patient decision aids or compared two different types of patient decision aids (e.g. simpler versus more complicated) without also including a usual care comparison.
Types of outcome measures
We specified all primary and secondary outcomes in advance of the review ( Table 2 ).
1. Outcome measures.
| Outcome | How often is it measured* | How is it usually measured/examples | Ideal timing to collect | Rationale for timing |
|
Attributes of the choice made: Does the patient decision aid improve the match between the chosen option and the features that matter most to the informed patient? | ||||
| Informed values‐choice congruence | Less often: 35/209 studies |
Most often measured using the Multi‐Dimensional Measure of Informed Choice (MMIC) instrument, which comprises 3 dimensions: knowledge, attitude, and uptake ( Michie 2002 ). It can be measured other ways (e.g. “percent match” procedures by Sepucha et al (2007; 2008)). | We collected and reported data, however it is measured. We carefully reviewed how it was measured and standardized and pooled data if there was consistency across studies. | It is less often measured, so we included all timings. |
|
Attributes of the decision process: Does the decision aid help patients know the options and their features (knowledge and feeling informed), be clear about the features that matter most to them (clear values), improve communication with their clinician (patient‐clinician communication), become involved in their preferred ways (participation in decision‐making), be more prepared to make decisions, and more satisfied with the decision‐making process? | ||||
| Knowledge | Very often: 149/209 studies | Customized tests based on information contained in the decision aid. The proportion of accurate responses is transformed to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses). | Soon after exposure to the decision aid. | An outcome of the decision aid but knowledge decreases over time. |
| Accurate risk perceptions (i.e. perceived probabilities of outcomes) | Less often: 37/209 studies |
Based on the accuracy of perceived outcome probabilities according to the percentage of individuals whose judgments corresponded to the scientific evidence about the chances of an outcome for similar people. For studies that elicited risk perceptions using multiple items, we averaged the proportion of accurate risk perceptions. | Soon after exposure to the decision aid. | An outcome of the decision aid. |
| Decisional conflict subscale – feeling uninformed | Often: 75/209 studies |
Subscale of the original Decisional Conflict Scale ‐ 16 Items ( O'Connor 1995 ) | Soon after exposure to the decision aid. | An outcome of the decision aid. |
| Decisional conflict subscale – feeling unclear values | Often: 71/209 studies |
Subscale of the original Decisional Conflict Scale ‐ 16 Items ( O'Connor 1995 ) | Soon after exposure to the decision aid. | An outcome of the decision aid. |
| Patient‐clinician communication | Less often: 36/209 studies |
Most studies evaluated the extent of shared decision‐making communication by analyzing the audio or video recordings. Common instruments include the OPTION scale ( Elwyn 2005 ), the Shared Decision Making Questionnaire, patient (SDMQ9‐patient) and GP (SDM‐Q9‐doc) ( Kriston 2010 ), the CollaboRATE‐SDM ( Elwyn 2013b ), and the MAPPIN'SDM ( Kasper 2012 ). Other studies measured the proportion of patients who discussed the decision with the clinician. | It is usually measured during the consultation using audio or video recordings or soon after the consultation. There may be multiple measurements to extract. We extracted whether the outcome was patient‐reported, clinician‐reported, or observer‐reported. | As an outcome of the consultation. |
| Participation in decision‐making | Often: 42/209 studies |
Defined as clinician‐controlled decision‐making (passive role) or active patient involvement (patient‐controlled decision‐making and shared decision‐making). Common instruments include the Control Preferences Scale ( Degner 1992 ) and COMRADE ( Edwards 2003 ). Other studies may use similar researcher‐developed response statements to measure perceived involvement. | Soon after the consultation with the physician and whether it was actual or preferred participation. | As an outcome of the consultation. |
| Proportion undecided | Often: 46/209 studies |
Sometimes measured using the Stage of Decision‐making scale: "How far along are you with your decision?" ( O'Connor 2000 ). Other examples include: asking participants which option they were leaning toward ( Arterburn 2011 ) and reporting which option was chosen, including “undecided” ( Berry 2013 ). | We collected data in 2 subgroups: 1) Soon after exposure to the decision aid but prior to consultation. 2) Post‐consultation (or if decision aid was used during the consultation). |
An outcome of the decision aid and consultation. |
| Satisfaction with the decision‐making process | Rarely: 16/209 |
Sometimes measured using the Satisfaction with the Decision Making Process (SDMP), a 12‐item scale ( Barry 1997 ), or ‘‘How satisfied were you with this consultation?”, with response scale 0 to 10 ( Bozic 2013 ). | We collected and reported data however it was measured | It is rarely measured, so we included all timings. |
| Preparation for decision‐making | Rarely: 17/209 |
Preparation for Decision Making Scale (Bennett 2010b). | Soon after exposure to the decision aid. | An outcome of the decision aid. |
| Secondary outcomes | ||||
| Behavior | ||||
| Choice | Very often: 165/209 studies |
Choice is defined as the actual choice implemented. However, when studies did not report the actual choice, we used the patients' preferred option as a surrogate measure. | Usually measured post‐consultation. | Given we want actual choice, it needs to ideally be anytime after the consultation. |
| Confidence | Rarely: 27/209 studies |
Most often measured using the Decisional Self‐efficacy Scale ( O'Connor 2002 ). Sometimes referred to as “empowerment”. | We collected and reported data however it was measured. | It is rarely measured, so we included all timings. |
| Adherence (continuance/compliance) with chosen option | Rarely: 25/209 studies |
We grouped adherence according to adherence to the baseline choice and adherence to the treatment. It is usually measured a while after the decision has been made (e.g. 3 to 12 months post). | We collected and reported data however it was measured. | It is rarely measured, so we included all timings. |
| Health outcomes | ||||
| Preference‐linked health outcomes | Never: 0/209 studies |
The study needs to report health outcomes analyzed considering those the patient prefers to have versus those the patient prefers to avoid. | — | To our knowledge, it has never been measured. |
| Healthcare system effects | ||||
| Consultation length | Rarely: 23/209 studies |
Usually measured by analyzing recordings of the consultation. | — | — |
| Cost | Rarely: 8/209 studies |
Costs as related to the decision aid measured, using cost‐effectiveness analysis or total estimated costs. | We collected and reported data however it was measured | It is rarely measured, so we included all timings. |
| Healthcare resource use | Rarely: 7/209 studies |
Healthcare resource use as related to decision aid use, for example outcomes such as the scheduling of initial or repeat consultations, length of hospital stay, and hospital admissions. | We collected and reported data however it was measured. | It is rarely measured, so we included all timings. |
| Adverse events | ||||
| Decision regret | Less often: 30/209 studies |
Measured using the Decision Regret Scale ( Brehaut 2003 ), which measures "distress or remorse after a [health care] decision." | A while after the decision has been made (e.g. 6 to 24 months post decision). | A longer‐term outcome of the decision‐making process. |
| Emotional distress | Rarely: 5/209 studies |
Emotional distress is sometimes measured using the Impact of Events Scale ( Horowitz 1979 ). For example, “Trouble staying asleep (because of having to make the decision)?” | We collected and reported data however it was measured. | It is rarely measured, so we included all timings. |
*Based on the number of studies that measured the outcome in the current review: e.g. > 40 studies = often.
Primary outcomes
The outcome measures were mapped onto the International Patient Decision Aid Standards (IPDAS) criteria for evaluating the effectiveness of patient decision aids ( Elwyn 2006 ; IPDAS 2005b ; Sepucha 2013 ). The IPDAS criteria were attributes related to the choice and to the decision‐making process. For this update, there were enough studies reporting on attributes of the choice that knowledge and accurate risk perceptions were moved to process measures.
-
Attributes of the choice made:
Does the patient decision aid improve the match between the chosen option and the features that matter most to the informed patient (as demonstrated by informed values‐choice congruence)?
-
Attributes of the decision‐making process:
-
Does the patient decision aid help patients:
know the options and their features (knowledge, accurate risk perceptions, and feeling informed);
be clear about the features that matter most to them (clear values);
become involved in their preferred ways (participation in decision‐making);
adverse events;
improve communication with their clinician (patient‐clinician communication);
feel more satisfied with the decision‐making process; and
be more prepared to make decisions?
-
Secondary outcomes
Secondary outcomes were choice (the actual choice implemented; if not reported, the patients’ preferred option was used as a surrogate measure), confidence in decision‐making, adherence to the chosen option, preference‐linked health outcomes, and impact on the healthcare system (consultation length, costs, healthcare resource use).
Search methods for identification of studies
This is an update of a Cochrane review first published in 2003 ( O'Connor 2003 ), and last updated in 2017 ( Stacey 2017 ). For this update, the author team revised and streamlined the search strategies, based on their acquired knowledge of updated terms and practices. These revisions were achieved by testing altered terms against the search yield and with the use of 20 key and current references that were used to validate the strategy yields. We did this by checking that the references all appeared in the search results of the various databases searched. We also undertook forward citation checking of all 20 validation references. Our comprehensive search process included a range of electronic medical and social science databases, two clinical trial sites, forward citing of validation references, and grey literature sites known to the authors. New for this update was the use of the Cochrane RCT classifier to focus on identifying studies that were identified as RCTs and cluster‐RCTs.
Electronic searches
The cumulative search of electronic databases is as follows.
Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 3) in the Cochrane Library (searched to 11 March 2022).
MEDLINE Ovid (1966 to 11 March 2022).
Embase Ovid (1980 to 11 March 2022).
PsycINFO Ovid (1806 to 11 March 2022).
CINAHL Ovid (1982 to September 2008), then in EBSCO (to 11 March 2022).
We present the search strategies in Appendix 1 , Appendix 2 , and Appendix 3 .
Searching other resources
We searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing/unpublished studies. We also searched the reference lists of newly included studies, and of systematic reviews of patient decision aids or interventions to support shared decision‐making across various health conditions. We identified newly published studies from the trials in progress reported in the 2017 update ( Stacey 2017 ).
Data collection and analysis
We conducted this Cochrane review following the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2022 ). Using an integrated knowledge translation (KT) approach ( CIHR 2015 ), our team consisted of a study executive including a patient partner (DS, KBL, MS, RJV, ED, MC) that met every two weeks for decision‐making and a steering committee of an international group of researchers and knowledge users that were engaged in the entire systematic review process ( Bowen 2013 ). For each step of the review development process, we invited team members to participate to the degree they were able to, considering their interest and expertise ( Lewis 2023 ) (see Contributions of authors ).
For this current update, we focused data collection only on newly published studies and any secondary publications of the original studies included in the previous update ( Stacey 2017 ). The new data were analyzed together with the data from the previous update.
Selection of studies
Two independent authors (CB, MB, MC, KDS, ED, JF, AG, KBL, LPB, DS, RT, RJV) screened identified citations in Covidence ( Covidence 2022 ) using a two‐step screening process: (i) titles and abstracts; (ii) screening the full text of any citations identified as potentially relevant by at least one review author during the first step ( Figure 2 ). Any disagreements were discussed with the principal investigator (DS) and/or the executive committee (DS, KBL, MS, MC, ED, RJV, SK). Then study interventions (e.g. articles, patient decision aids if available) were screened by two independent review authors to ensure they met the minimal definition for a patient decision aid. We provided citation details and reported details of additional publications relevant to the included studies, so that each study (rather than an individual publication of the trial results) was the unit of interest. We described ongoing studies with available information. No review authors made eligibility decisions about their own studies in this update, nor in any previous versions of this review.
2.
PRISMA flow diagram
All articles excluded from step two were reported with reasons in Characteristics of excluded studies . One author screened all citations excluded using the RCT classifier to verify that it was not an RCT.
Data extraction and management
Two authors (LPB, JZ, MH) independently extracted data on the intervention, control, and outcomes, one of whom extracted data on all newly included trials (LPB). One author extracted data on the characteristics of the paper and Guidance for Reporting Involvement of Patients and Public (GRIPP2) ( Staniszewska 2017 ). One author (MC) compared findings and flagged inconsistencies to be resolved through discussion with the principal investigator (DS) and/or the executive committee (DS, KBL, MS, MC, ED, RJV). No review authors extracted data for their own studies in this update nor in any previous versions of this review.
One author (MC) entered all extracted data into Review Manager ( RevMan Web 2023 ). Results were audited by two authors (DS, KBL).
Assessment of risk of bias in included studies
Two authors (LPB, JZ, MH) independently appraised studies using the Cochrane tool for assessing risk of bias in randomized trials ( Higgins 2011 ), as we did for the previously published version ( Stacey 2017 ). We judged each item as conferring high, low, or unclear risk of bias as set out in the criteria provided by Higgins 2011 , and we provided a quote from the study report and a justification for our judgment for each item in the risk of bias table ( Characteristics of included studies ).
For the item on ‘other’ potential sources of bias, the assessment included: whether the same clinician provided consultation to both the intervention and usual care groups with measures taken post‐consultation, and potential sources of bias reported by the authors in the study limitations. For cluster‐RCTs, we considered other potential sources of bias when clustering was not accounted for in the analysis and if there was selective recruitment of cluster participants ( Higgins 2022 ). Studies were deemed to be at the highest risk of bias if any item on the risk of bias tool was scored at high risk.
We resolved inconsistencies by discussion with the principal investigator (DS) and, when necessary, with the executive team (DS, KBL, MS, MC, ED, RJV). No review authors appraised risk of bias for their own studies in this update, nor in any previous versions of this review.
Measures of treatment effect
For dichotomous outcomes, we analyzed data based on the number of events out of the total number of patients observed in the intervention and comparison groups. We used these data to calculate the risk ratio (RR) and 95% confidence interval (CI). For continuous measures, we analyzed data based on the reported means (or measure of central tendency), standard deviations (SD) (or dispersion measure), and number of patients assessed for both the intervention and comparison groups to calculate the mean difference (MD) and 95% CI.
The a priori comparison was patient decision aids versus usual care. For the 26 studies in which there were more than one intervention group, we extracted data from the two groups that provided the strongest contrast in intervention attributes (i.e. intensity) between the intervention and control groups. We pooled results across studies in cases where investigators used the same or similar outcome measures, and the effects were expected to be independent of the type of decision studied. For example, we expected patient decision aids to improve knowledge and create accurate perceptions of options, benefits, and harms; to reduce decisional conflict; and to enhance active participation in decision‐making. Therefore, we pooled data from included RCTs for these outcomes if trials used comparable measures. To facilitate pooling of data for some outcomes (e.g. knowledge, decisional conflict), we standardized the scores to range from 0 to 100 points. When analyzing the effects of patient decision aids on choices, we pooled outcomes on homogeneous subgroups of decisions (choice of major surgery over conservative options by surgery type; choice of screening versus no screening by test type; choice for starting diabetes medication).
Unit of analysis issues
Given that we included both RCTs and cluster‐RCTs, we assessed for unit of analysis errors. Where we found errors and sufficient information was available, we re‐analyzed the data using the appropriate unit of analysis by taking account of the reported intracluster correlation (ICC). As required, we obtained missing estimates of the ICC by contacting authors of included studies, or we imputed them using estimates from external sources. For five studies, it was not possible to obtain sufficient information to re‐analyze the data, and we reported these studies as being at high risk for ‘other’ bias based on these unit of analysis errors ( Kupke 2013 ; Lewis 2010 ; Perestelo‐Perez 2016 ; Saunier 2020 ; Stubenrouch 2022 ). For outcomes where these studies were included in the meta‐analysis, we conducted subanalysis without these studies identified as high risk of bias.
Dealing with missing data
Where possible, we conducted analyses on an intention‐to‐treat basis; otherwise, we analyzed data as reported. We reported on the levels of loss to follow‐up and assessed this as a source of potential bias.
Assessment of heterogeneity
If there was significant statistical heterogeneity according to the I 2 inconsistency index, we further examined the heterogeneity through visual assessment of forest plots.
For this update and in previous versions of the review, we grouped studies with the aim of assessing the effectiveness of patient decision aids across conditions. Given that patient decision aids are a well‐defined and clearly delineated type of intervention, we decided that this approach was defensible. On the basis of grouping studies across conditions, we anticipated that there would be a substantial degree of heterogeneity in our pooled effect estimates due to differences in the population, patient decision aid elements, comparators, and settings. However, we decided that we would consider the variability in the direction of effects rather than variability in the size of effects, as the major basis for our interpretation of heterogeneity.
In the 2009 update, we explored possible reasons for variability by conducting subgroup analysis when heterogeneity was present in pooled effect estimates ( O'Connor 2009b ). The post hoc analysis included the IPDAS effectiveness criteria to explore heterogeneity according to the following factors: the type of decision (treatment versus screening), the format of the patient decision aid (video/computer versus audio booklet/pamphlet), and the possibility of a ceiling effect based on usual care scores (resulting in the removal of studies with lower scores for knowledge and accurate risk perception and higher scores for decisional conflict using the subscales measuring levels of feeling uninformed and unclear values). We analyzed the effect of removing the biggest outlier(s) according to a visual inspection of forest plots. Given that these post hoc analyses did not alter the findings in the 2009 update, we have not re‐conducted these post hoc analyses in any subsequent update.
Assessment of reporting biases
If more than 10 studies were identified and included meta‐analysis, we explored publication bias using funnel plots and visual assessment of funnel plot asymmetry.
Data synthesis
We used RevMan Web 2023 to estimate a weighted intervention effect with 95% confidence intervals (CIs). For continuous measures, we used mean differences (MD); for dichotomous outcomes, we calculated pooled risk ratios (RRs). We analyzed all data with a random‐effects model because of the diverse nature of the studies being combined and then anticipated variability in the populations and interventions of the included studies.
Subgroup analysis and investigation of heterogeneity
For outcomes where meta‐analysis was possible, we conducted several subgroup analyses as follows: a) excluding studies rated as high risk of bias (see Sensitivity analysis ); b) studies published since 2015 (n = 104 studies) (i.e. new studies included in this update) versus studies published prior to 2015 (n = 105 studies); and c) for studies measuring informed values‐choice congruence using Multi‐Dimensional Measure of Informed Choice (MMIC) ( Michie 2002 ) (n = 13) versus studies that used other measures for calculating this outcome (n = 8). We pursued a subgroup analysis for newer versus older studies, given that there was a doubling of new studies added, the International Patient Decision Aid Standards Collaboration published minimal standards for patient decision aids in 2013 ( Joseph‐Williams 2013 ), which may have influenced the quality of patient decision aids being evaluated, and usual care may be improving with more clinical practice guidelines recommending use of patient decision aids ( CDSR 2022 ) and health policies recommending shared decision‐making in clinical practice ( Bravo 2022 ). For the subgroup analysis of studies using MMIC versus studies using other measures, given the different approaches for calculating informed values choice congruence ( Munro 2016 ), we were keen to know if those that used the most commonly used measure, MMIC, were the same or different from the other measures.
Sensitivity analysis
We performed post hoc sensitivity analyses to examine the effect of excluding studies that were at high risk of bias for any of the categories in the risk of bias assessment ( Higgins 2011 ).
Summary of findings and assessment of the certainty of the evidence
We prepared Table 1 to present the results for the major comparison based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions ( Schünemann 2022 ). We provided a source and rationale for each assumed risk cited in the table and used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to rank the certainty of the evidence for key primary outcomes (informed values‐choice congruence, knowledge, accurate risk perceptions, decisional conflict, participation in decision‐making, adverse events) on each of the following domains: risk of bias, inconsistency, imprecision, indirectness, and publication bias. Team members (DS, KBL, MS, MC, ED, RJV, LoT, JF) assessed the certainty of the evidence together using GRADEpro GDT in a meeting. We downgraded the evidence from high certainty by one level for serious study limitations (risk of bias), serious inconsistency, imprecision of effect estimates, indirectness of evidence, or potential publication bias . For our interpretation of heterogeneity, we considered the variability in the direction of effects rather than variability in the size of effects. Unlike drug trials where there is a standardized dose of a medication that is tested across trials in different people, patient decision aids are multi‐component complex interventions that have minimal elements to meet the definition but may include other elements (see descriptions in the Characteristics of included studies section). In addition, the comparator is usual care and there can be variability across studies in how the patient interacts with the clinical team (e.g. clinician only, interprofessional team). Hence, we were advised to focus on the variability in the direction of effects for our interpretation of heterogeneity. This decision meant that for those pooled effect estimates where the direction of effect was consistent across studies, we did not downgrade the GRADE rating for inconsistency, despite some variability in the size of effects across individual studies.
Results
Description of studies
This current version of our review updates our 2017 version ( Stacey 2017 ) with 104 newly included studies, bringing the total to 209 included studies that evaluated patient decision aids compared to usual care ( Figure 2 ; Characteristics of included studies ). Of the 104 new studies, only 10 (9.6%) reported patient involvement on the study research team. Few studies provided details on their involvement according to the GRIPP reporting guideline ( Staniszewska 2017 ). For example, one study reported providing training for patients on the team ( Durand 2021 ), two studies discussed the aim of patient involvement ( Hess 2016 ; LeBlanc 2015b ), three studies described the methods used to involve patients on the team ( Durand 2021 ; Hess 2016 ; Singh 2019 ), and one study reported results of involving patients on the team and discussed the extent to which patient involvement influenced the results ( Meier 2019 ).
Results of the search
In total, we identified 53,895 citations from the electronic database searches and 267 citations from other sources. Of these, we assessed 796 full‐text citations for eligibility (see Figure 2 ).
Included studies
The updated search yielded 104 new studies that met our inclusion criteria, leading to a total of 209 studies included in this update. The 209 studies, involving 107,698 patients, presented results from 19 countries (including nine new countries as indicated by *): USA (n = 106), Canada (n = 23), United Kingdom (n = 21), Australia (n = 17), the Netherlands (n = 10), Germany (n = 8), China (n = 7), Spain (n = 6), Denmark* (n = 2), Finland (n = 2), France* (n = 2), Japan* (n = 2), Greece* (n = 1), Italy* (n = 1), Malaysia* (n = 1), New Zealand* (n = 1), Sweden (n = 1), Switzerland* (n = 1), Turkey* (n = 1), and four studies that were conducted in two countries. We present study details below and in Characteristics of included studies .
Unit of randomization
One‐hundred and seventy‐five studies randomized individual patients and 34 studies randomized clusters. For 26 studies, the cluster effect was taken into account in the published outcome data, and the meta‐analysis used published results. Although Hamann 2006 did not account for the cluster effect in the published outcome data, the way this study was reported did not allow us to include it in the meta‐analysis, so we did not re‐analyze the data and report the study separately. For McAlister 2005 , meta‐analysis was done applying the design effect (based on the published ICC). For Fraenkel 2012 , the authors stated that adding a random effect for clinician clusters did not contribute to better‐fitting regression models, and we removed it from the analysis. Kupke 2013 , Lewis 2010 , Perestelo‐Perez 2016 , Saunier 2020 , and Stubenrouch 2022 did not account for clustering in their analyses.
Patient decision aids
The 209 included studies evaluated patient decision aids that were focused on 71 different decisions. The most common decisions were about cardiovascular treatment (n = 22 studies), cancer screening (n = 17 studies colorectal, 15 prostate, 12 breast), cancer treatment (e.g. 15 breast, 11 prostate), mental health (n = 10 studies), and joint replacement surgery (n = 9 studies). The most common new treatment decision topics are in obstetrics (n = 4 studies), cardiovascular disease (n = 2 studies), kidney disease (n = 4 studies), obstructive sleep apnea (n = 3 studies), lung cancer screening (n = 2 studies), and upper extremity conditions (n = 3 studies). There were no decision aids related to COVID‐19.
The patient decision aids used different formats, including 89 (43%) paper‐based, 70 (33%) web‐based or computer program, 33 (16%) including combinations of audio, video, web/computer‐based, and paper‐based, 15 (7%) video, and two (1%) scripts read aloud. Usual care consisted of various types of controls (e.g. usual care, general information, risk assessment, clinical practice guideline summaries for health consumers, placebo intervention (e.g. information on another non‐relevant topic such as use of seat belts), or no intervention). We noted the details of the usual care approach when reported (see Characteristics of included studies ).
According to the definition of a patient decision aid, all of the studies evaluated patient decision aids that included information about the options and outcomes and provided at least implicit clarification of values. Most patient decision aids included information on the clinical problem (92%) as well as outcome probabilities (88%). Fewer patient decision aids provided explicit methods to clarify values (67%), guidance in the steps of decision‐making (66%), and/or examples of others’ experiences (36%) (see Characteristics of included studies ).
Excluded studies
We excluded 451 studies upon close perusal of the full texts (see Characteristics of excluded studies ; Figure 2 ). The reasons for exclusion were: the study was not a randomized controlled trial (n = 73 studies); the decision was hypothetical, with patients not actually at a point of decision‐making (n = 30 studies); the intervention was not focused on making a choice (n = 25 studies); the intervention offered no decision support in the form of a patient decision aid (n = 166 studies) or did not provide enough information about the patient decision aid intervention (n = 15 studies); no comparison outcome data were provided (n = 3 studies); the study did not evaluate the patient decision aid (n = 11 studies); the study was a protocol (n = 1 study); the patient decision aid was about clinical trial entry (n = 2 studies), lifestyle choice (n = 4 studies), or advanced care planning (n = 18 studies); the study involved testing the presentation of the patient decision aid, but with no difference in the content of the patient decision aid between study groups (n = 9 studies); pediatric population (n = 2 studies); no outcomes of interest to this review (n = 12 studies); not a treatment or screening decision (n = 16 studies); or the study compared a detailed versus simple patient decision aid (n = 64 studies).
We also identified 128 ongoing studies through trial registration databases, personal contact, and published protocols in the electronic database searches (see Characteristics of ongoing studies ).
Risk of bias in included studies
Details on the ratings and rationale for risk of bias are in the Characteristics of included studies table and displayed in Figure 3 and Figure 4 .
3.

Risk of bias summary as percentages across all included studies.
4.

Risk of bias summary for each included study.
Allocation
When assessing risk bias for sequence generation, we rated all 209 studies as being at low (169 studies) or unclear risk of bias (40 studies). Allocation concealment methods prompted a rating of low in 125 studies, unclear in 82 studies, and high risk of bias in two studies ( Kupke 2013 ; Love 2016 ).
Blinding
We judged 204 studies to be at low (43 studies) or unclear risk (161 studies) of performance and detection bias for the blinding of participants and personnel, while five (2.4%) studies were at high risk of bias. High risk of bias was due to lack of blinding of clinicians to the status of patients randomized to the patient decision aid and alternative interventions ( Auvinen 2004 ; Cuypers 2018 ; Gagne 2017 ; Krist 2007 ; Man‐Son‐Hing 1999 ).
We rated the blinding of outcome assessment as leading to low risk in 192 studies or unclear risk in 15 studies, while two (0.96%) studies were at high risk of bias. High risk of bias was due to lack of blinding of assessors for observer‐reported outcomes ( Kunneman 2020 ; LeBlanc 2015b ).
Incomplete outcome data
For 200 studies, aspects related to incomplete outcome data conferred low (125 studies) or unclear risk of bias (75 studies). In nine (4.3%) studies ( Allen 2018 ; Case 2019 ; Chambers 2012 ; Crew 2022 ; Ickenroth 2016 ; Mott 2014 ; Roberto 2020 ; Tebb 2021 ; Wise 2019 ), there was high risk of bias due to high attrition rates (e.g. less than 90% of enrolled patients were included in the analysis) and significant differences in missing outcome data across groups ( Hartling 2012 ).
Selective reporting
We rated 208 studies as being at either low risk of bias (108 studies) because the protocol was registered publicly or at unclear risk of bias (100 studies) because we could not assess the extent or the impact of any reporting bias, while one study was at high risk of bias. The high risk of bias was because it was stated that knowledge was a primary outcome in the trial registry, but the study failed to report any results for this outcome ( Lin 2022 ).
Other potential sources of bias
Of the 209 studies, we rated 191 as being at low (n = 136) or unclear (n = 55) risk of other potential sources of bias. The other 18 (8.6%) studies discussed other potential risks of bias ( Aoki 2019 ; Bourmaud 2016 ; Brazell 2014 ; Clancy 1988 ; Cuypers 2018 ; Durand 2021 ; Hamann 2006 ; Knops 2014 ; Kupke 2013 ; LeBlanc 2015 ; Lewis 2010 ; Moin 2019 ; Perestelo‐Perez 2016 ; Reuland 2017 ; Saunier 2020 ; Schott 2021 ; Stubenrouch 2022 ; Tebb 2021 ). See Characteristics of included studies for details.
Effects of interventions
See: Table 1
1. Primary outcomes
Attributes of the choice made: does the patient decision aid improve the match between the chosen option and the features that matter most to the informed patient (informed values‐choice congruence)?
Of 209 studies, 35 (16.7%) measured congruence between the chosen option and the informed patients’ values with 21 studies pooled ( Analysis 1.1 ) and 14 not pooled ( Table 3 ). There was moderate certainty in the evidence, downgraded for possible publication bias ( Figure 1 ), that patient decision aids were probably more effective than usual care for selecting an option that was congruent with their informed values (RR 1.75, 95% CI 1.44 to 2.13; 21 studies) ( Analysis 1.1 ). The average proportion of patients selecting an option that was congruent with their informed values, by study arm, was 48.6 out of 100 patients in the patient decision aid group compared to 30.5 out of 100 patients in the usual care group. When the three studies assessed as high risk of bias were removed, the findings were similar (RR 1.96, 95% CI 1.54 to 2.50; 18 studies) ( Analysis 1.2 ). There were no differences between older and newer studies ( Analysis 1.3 ). A subanalysis of the 13 studies that used the Multi‐Dimensional Measure of Informed Choice (MMIC) ( Michie 2002 ) showed that patient decision aids were probably more effective than usual care for this outcome (RR 1.75, 95% CI 1.37 to 2.23) ( Analysis 1.4 ). A subanalysis of the eight studies that used different measures showed similar findings (RR 1.82, 95% CI 1.29 to 2.55) ( Analysis 1.5 ).
1.1. Analysis.

Comparison 1: Informed values‐choice congruence, Outcome 1: Informed values‐choice congruence ‐ all studies
2. Values congruent with chosen option.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Allen 2018 | Concordance between single‐item value score and patient‐reported treatment choice | 1 month after enrollment | 104 | 3.33 (SD 0.32) | 132 | 2.37 (SD 0.28) | P = 0.03 favoring the DA group |
| 6 months after enrollment | 104 | 3.65 (SD 0.39) | 132 | 3.12 (SD 0.33) | No difference, P = 0.32 | ||
| Arterburn 2011 | Percent match procedures described by Sepucha et al (2007; 2008). For values items were most predictive and used to specify logistic models to estimate predicted probability of selecting surgery > 0.5. | Post‐intervention | 75 | — | 77 | — | The intervention group experienced a more rapid early improvement in values concordance immediately after the intervention compared to control. |
| Berry 2013 | Concordant when men reported: a) sexual function influenced decision and they had radiation therapy; b) bowel function influenced decision and they had surgery; c) all effects influenced decision and they had surveillance | 6 months post‐intervention | 239 | — | 209 | — | No difference OR = 0.82, 95% CI 0.56 to 1.2 |
| Beulen 2016 | Value‐consistency prenatal test decision (attitudes were combined with prenatal test utilization to assess whether decision‐making regarding prenatal testing was value‐consistent) | Post‐intervention | 123 | 92.7% | 120 | 94.2% | P = 0.641 |
| Durand 2021 (in consult) | Decision Quality Instrument concordance subscale | Immediately post consultation | 60 | — | 220 | — | There was no effect of the intervention on the Decision Quality Instrument concordance subscale in comparison with usual care. |
| Frosch 2008a | Concordance between participant's preferences and values for potential outcomes related to the decision and the choice made | Within weeks | 155 | — | 151 | — | Men assigned to the decision aid who chose not to have a PSA test rated their concern about prostate cancer lower than did men who requested a PSA test. Men assigned to usual care provided similar ratings of concern about prostate cancer regardless of their PSA decision. There was no statistically significant difference between groups. |
| Legare 2008a | — | — | — | — | — | — | Women's valuing of non‐chemical aspects of natural health products was positively associated with their choice of nature health products, P = 0.006. No difference between groups. |
| Lerman 1997 | Association between values and choice | — | — | — | — | — | No difference; between‐group differences were not reported. |
| Lewis 2021 | Values‐choice concordance was analyzed descriptively because of the small sample size and insufficient outcome variability in actual/preferred choice | — | — | — | — | — | “Lower your chances of sudden cardiac arrest", 96.6%; “Peace of mind”, 90.0%; “Avoid risks”, 51.7%; “Allow a natural death", 51.7% |
| McGrath 2017 | Value congruence measured using a single item, ‘‘If you have already made your decision, to what degree have you made it based on what is important to you?’’ (response scale not reported) | 2 weeks post‐intervention | — | 2.76 (SD 0.63) | — | 2.77 (SD 0.43) | No difference P = 0.838 |
| McIlvennan 2018 (in consult) | Concordance between caregiver values for their loved one and stated caregiver treatment choice (1 to 10 scale) | 1 month post‐intervention | 53 | 3.63 (SE 0.43) | 89 | 2.79 (SE 0.34) | No difference P = 0.15 |
| 6 months post‐intervention | 50 | 4.27 (SE 0.44) | 78 | 3.05 (SE 0.35) | P = 0.045 | ||
| Perestelo‐Perez 2017 | Concordance between patients’ goals/concerns and their treatment intention using a "simple match" approach. | Immediately post‐intervention | 62 | 23 (37.1%) | 69 | 27 (39.1%) | No difference P = 0.811 |
| Perestelo‐Perez 2019 | Concordance between patients’ goals/concerns about the screening procedure and their intention to be screened as described by Sepucha 2014 | Immediately post‐intervention | — | — | — | — | Patients’ goals and concerns regarding the screening did not significantly predict their intention, and therefore the authors could not calculate a measure of concordance between the two constructs. |
| Vandemheen 2009 | Congruence between personal values and decision | 3 weeks | 70 | — | 70 | — | Patient choices were consistent with their values across both randomized groups. |
| Wallace 2021 | Congruence between personal values (1 to 10 scale from "not important" to "very important") and values‐trade off (1 to 10 scale from "Die quickly from any cause" to "Live as long as possible") | 1 month post‐intervention | 6 | 5 (83.3%) | 3 | 0 (0%) | P = 0.048 |
CI : confidence interval; DA : decision aid; OR : odds ratio; SD : standard deviation; SE : standard error
1.

Funnel plot of comparison: 3.1 Informed values‐choice congruence ‐ all studies
1.2. Analysis.

Comparison 1: Informed values‐choice congruence, Outcome 2: Informed values‐choice congruence ‐ without studies of high risk of bias
1.3. Analysis.

Comparison 1: Informed values‐choice congruence, Outcome 3: Informed values‐choice congruence ‐ old vs new studies
1.4. Analysis.

Comparison 1: Informed values‐choice congruence, Outcome 4: Informed values‐chose congruence ‐ using MMIC
1.5. Analysis.

Comparison 1: Informed values‐choice congruence, Outcome 5: Informed values‐chose congruence ‐ using non‐MMIC measures
Attributes of the decision process: does the patient decision aid help patients know the options and their features (knowledge and feeling informed), be clear about the features that matter most to them (clear values), become involved in their preferred ways (participation in decision‐making), improve communication with their clinician (patient‐clinician communication), feel more satisfied with the decision‐making process, and be more prepared to make decisions?
Knowledge
Of 209 studies, 149 (71.3%) assessed the effects of patient decision aids on knowledge with 107 studies pooled ( Analysis 2.1 ) and 42 studies not pooled ( Table 4 ). High‐certainty evidence indicated that patient decision aids were more effective than usual care on knowledge scores (mean difference (MD) 11.90 out of 100, 95% CI 10.60 to 13.19; 107 studies) ( Analysis 2.1 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 5 ). The average knowledge score by study arm was 70.9 out of 100 in the patient decision aid group compared to 58.6 out of 100 in the usual care group. When 12 studies assessed as high risk of bias were removed, the findings were similar (MD 12.13, 95% CI 10.74 to 13.52; 95 studies) ( Analysis 2.2 ). There was no difference between older and newer studies ( Analysis 2.3 ).
2.1. Analysis.

Comparison 2: Knowledge, Outcome 1: Knowledge ‐ all studies
3. Knowledge.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Bailey 2016 | 17 true or false questions. Change in knowledge from baseline. | 4 to 6 weeks after enrollment | 114 | 35 (SD 22.3) | 111 | 9.9 (22.2) | P < 0.0001 |
| Beulen 2016 | Sufficient knowledge: participants with a score of ≥ 12 | Post‐intervention | 131 | 88.5% | 130 | 70.8% | P < 0.001 |
| Bozic 2013 | Decision quality instrument, 19 items re knowledge (> 50%) | After 1st consultation with surgeon | 60 | 58.3% | 60 | 33.3% | P = 0.01 |
| Chen S 2021 | 15 items (true, false, or unsure) with points deducted for incorrect answers | Post‐intervention | 29 | 9.86 (SD not reported) | 30 | 9.77 (SD not reported) | P = 0.89 |
| Crew 2022 | 8 multiple choice items. Adequate knowledge defined as at least 50% correct responses. | 1 month post‐intervention | 120 | 58 (49%) | 133 | 36 (27%) | P < 0.001 |
| Evans 2010 | 12 true or false questions; scores ranging from ‐12 to 12 | Immediately post | 89 | 4.9 | 103 | 2.17 | P < 0.001 |
| Fagerlin 2011 | Insufficient (≤ 50% correct) | Immediately post | 383 | 31.8% | 102 | 93.1% | P < 0.001 |
| Sufficient | Immediately post | 383 | 61.9% | 102 | 6.9% | — | |
| Fraenkel 2012 | Open‐ended questions about medication options to reduce stroke ‐ knows medications | Post‐intervention | 66 | 61% | 62 | 31% | OR 3.5 (95% CI 1.6 to 7.7, P = 0.001) |
| Open‐ended questions about side effects of medications ‐ knows side effects | Post‐intervention | 53 | 49% | 46 | 37% | OR 1.9 (95% CI 0.9 to 4.0; P = 0.07) | |
| Fraenkel 2015 | Change in knowledge from baseline | 2 weeks post‐intervention | 60 | Median 1.0 (IQR ‐1.0 to 2.0) | 61 | Median 0 (IQR ‐2.0 to 1.0) | P = 0.007 |
| Fung 2021 | 23 true/false items; linearly transformed score range 0 (poor) to 100 (outstanding) | Post‐intervention | 36 | 75.1 (SD not reported) | 37 | 65.3 (SD not reported) | P = 0.04 |
| Gabel 2020b | Change in knowledge from baseline | 90 days post‐invitation | 863 | 0.44 (CI 0.33 to 0.54) | 860 | 0.34 (CI 0.24 to 0.45) | No difference (scale score differences: 0.09, 95% CI ‐0.05 to 0.24) |
| Gagne 2017 | 37 items with response options labeled true, false, and don’t know, with points deducted for incorrect answers (range ‐37 to +37) | 2 months post‐intervention | 26 | 25.1 (95% CI 23.1 to 27.0) | 25 | 26.0 (95% CI 24.0 to 28.0) | No difference between groups |
| Gokce 2019 | 10‐item questionnaire | Immediately post‐intervention | 58 | Median 8/10 (range 5 to 10) | 57 | Median 6/10 (range 3 to 10) | P = 0.045 |
| Adequate knowledge defined as 8 or more correct answers | Immediately post‐intervention | 58 | 43 (74.1%) | 57 | 32 (56.1%) | P = 0.04 | |
| Hamann 2006 | 7‐item multiple choice knowledge test (unable to standardize results) | On discharge (~ 1 month) | 49 | 15 (4.4 SD) | 58 | 10.9 (5.4 SD) | P = 0.01 |
| Heller 2008 | 12‐item multiple choice | Pre‐operatively | 66 | 14%* | 67 | 8%* | *Mean increase from baseline P = 0.02 |
| Ibrahim 2013 | Change in proportion answering 3 of 4 questions correctly | 1 month post‐intervention | 168 + 163 | — | 167 | — | Significant increase for patients who received the DA P < 0.05 |
| Ickenroth 2016 | 20 true or false questions with points deducted for incorrect answers (range ‐20 to +20). Diabetes/cholesterol. | Immediately post‐intervention | 224 217 |
10.5 (SD 3.56) 8.58 (SD 4.22) |
241 240 |
9.81 (3.71) 8.43 (4.11) |
P = 0.031 P = 0.682 |
| Sufficient knowledge (score of 10 or above). Diabetes/cholesterol. | 224 217 |
150 (67.0%) 102 (47.0%) |
241 240 |
129 (53.5%) 101 (42.1%) |
P = 0.003 P = 0.301 |
||
| Korteland 2017 | 5‐item questionnaire (proportion with all items correct) | Post‐intervention/pre‐operatively | 67 | 57 (85%) | 71 | 48 (68%) | P = 0.004 |
| Krishnamurti 2019 | 25‐item questionnaire (0 to 100; low to high) | 3 months post‐intervention | 23 | 52.90 (SD not reported) | 19 | 52.90 (SD not reported) | P = 0.12 |
| Kukafka 2022 | Change in knowledge from baseline | 1 month post‐intervention | 101 | 1.1 (SD 2.3) | 86 | 0.3 (SD 2.3) | P = 0.03 |
| Kunneman 2020 (in consultation) | 6‐item questionnaire (number of items correct) | Post‐intervention | 445 | ≤ 3: 24 (5.4%) 4: 76 (17.1%) 5: 207 (46.5%) 6: 138 (31.0%) |
433 | ≤3: 30 (6.9%) 4: 88 (20.3%) 5: 191 (44.1%) 6: 124 (28.6) |
No difference Effect (95% CI) 1.01 (1.0 to 1.02) |
|
LeBlanc 2015 (in consultation) |
13‐item questionnaire (median, IQR) total score | Immediately post | 32 | 7 (4.5 to 9.0) | 45 | 5.5 (2.5 to 8.0) | P = 0.11 |
| 9‐item knowledge based on decision aid | Immediately post | 32 | 6 (3.5 to 6.5) | 45 | 4 (2.0 to 8.0) | P = 0.01 | |
| LeBlanc 2015b (in consultation) | Tailored to information in the decision aid (0 = no correct, 100 = all correct) Mean (95% CI) | Immediately post | 137 | 58.1 (53.6 to 62.6) | 116 | 46.6 (42.6 to 50.5) | P < 0.001 |
| Generic (i.e. depression in general) | Immediately post | 137 | 72.5 (68.0, 77.0) | 116 | 72.4 (67.3 to 77.5) | P = 0.65 | |
| Legare 2008a | 10‐item yes/no/unsure general knowledge test about natural health products (not specific to outcomes of options) | Change scores from baseline to 2 weeks | 43 | 0.86 ± 1.77 P = 0.002 |
41 | 0.51 ± 1.47 P = 0.031 | No difference between groups (P = 0.162) |
|
Mann D 2010 (in consultation) |
14‐item survey | Immediately post | — | — | — | — | No difference in level of knowledge between groups |
| Mathers 2012 | Correctly answers question about best option to lower blood sugar | 6 months post‐intervention | 95 | 51.6% | 80 | 28.8% | P < 0.001 |
| Correctly answers question about best option to lower complications | 6 months post‐intervention | 95 | 31.0% | 80 | 29% | P = 0.90 | |
| Mathieu 2007 | 9‐item ‐ 4 concept questions and 5 numeric questions | — | 351 | — | 357 | — | Significantly higher mean increase for the intervention group (2.62) compared to the control group (0.68) from baseline, P < 0.001 |
| Miller 2005 | 8‐item survey | 2‐week, 2‐month, and 6‐month follow‐ups | — | — | — | — | Intervention type had no impact on general or specific knowledge |
| Nagle 2008 | Good level knowledge was scored higher than the mid‐point of the knowledge scale (greater than 4) | — | — | — | — | — | 88% (147/167) in DA group compared to 72% (123/171) in pamphlet group. OR 3.43 (95% CI 1.79 to 6.58). |
| Ozanne 2007 (in consultation) | Change in knowledge from baseline | Post‐test | 15 | 48% to 64% | 15 | 45% to 57% | Change in knowledge score was significant for decision aid (P = 0.01) but not control (P = 0.13) |
| Partin 2004 | 10‐item knowledge index score | 2 weeks | 308 | 7.44 | 290 | 6.9 | P = 0.001 |
| Perez‐Lacasta 2019 | 22‐item: 11 conceptual questions and 5 numerical questions | 2 to 4 weeks post DA | 203 | 13.3 (SD not reported) | 197 | 7.83 (SD not reported) | P < 0.001 |
| Reuland 2017 | 6‐item survey | Post‐consultation | 131 | 4.6 (SD not reported) | 131 | 2.8 (SD not reported) | P < 0.001 |
| Rubel 2010 | 24 items adapted from existing prostate cancer knowledge measures | Immediately post | 100 | — | 100 | — | The total mean standardized knowledge score was 84.38 (SD 12.38) |
| Schott 2021 (in consultation) | 4‐item survey: ordinal logistic mixed‐effect model (odds ratio and 95% confidence interval) | Immediately post | 33 | 3.88 (95% CI 1.39 to 10.78) | 33 | 1.0 (reference group) | P = 0.009 |
| Stubenrouch 2022 | Disease‐specific knowledge test (median and IQR) | Post‐intervention/consultation | 173 | Median 80.0 (IQR 60 to 91.7) | 138 | Median 66.7 (IQR 50 to 80) | P = 0.025 |
| Trevena 2008 | Adequate knowledge (positive score: understanding benefits/harms) | 1 month | 134 | 28/134 | 137 | 8/137 | P = 0.0001 |
| Watson 2006 | 12‐item true/false/don't know | Post‐test | 468 | 75% (range 0 to 100) | 522 | 25% (range 0 to 100) | P < 0.0001 |
| Weymiller 2007 (in consultation) | 14‐item ‐ 9 were addressed by decision aid; 5 were not | Immediately post | 52 | — | 46 | — | Mean difference between groups 2.4 (95% CI 1.5 to 3.3) P < 0.05 (when decision aid administered during the consultation only ‐ not if prior to the consultation) |
| Wise 2019 | 15‐item true/false/unsure questionnaire ‐ change in knowledge from baseline | 34 weeks gestation (2 to 3 months post‐intervention) | 146 | Increase of 2 points | 148 | Increase of 1.6 points | No difference P = 0.20 |
| Wyld 2021 (in consultation) | 8‐item ‐ disease‐specific knowledge test (median and IQR) | 6 weeks post‐intervention | 67 | Median 5/8 (IQR 45) | 58 | Median 3/8 (IQR 2 to 5) | P < 0.001 |
| Ye 2021 | Adequate knowledge n (%) using 12‐item questionnaire with 3 subscales | 2 weeks post‐intervention | 386 | 142 (36.8%) | 387 | 34 (8.79%) | P < 0.001 |
CI : confidence interval; DA : decision aid; IQR : interquartile range; OR : odds ratio; SD : standard deviation.
5.

Funnel plot of comparison: 1 Knowledge, outcome: 1.1 Knowledge ‐ all studies.
2.2. Analysis.

Comparison 2: Knowledge, Outcome 2: Knowledge ‐ studies without high risk of bias
2.3. Analysis.

Comparison 2: Knowledge, Outcome 3: Knowledge ‐ old vs new studies
Accurate risk perceptions (perceived probabilities of outcomes)
Of 209 studies, 37 (17.7%) examined the effects of patient decision aids on the accuracy of patients’ perceived probabilities of outcomes with 25 studies pooled ( Analysis 3.1 ) and 12 studies not pooled ( Table 5 ). There was high certainty in the evidence that patient decision aids were more effective than usual care for achieving accurate risk perceptions (risk ratio (RR) 1.94, 95% CI 1.61 to 2.34; 25 studies) ( Analysis 3.1 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 6 ). The average proportion by study arm was 53.2 out of 100 patients in the patient decision aid group who accurately interpreted risk compared to 28.1 out of 100 patients in the usual care group. When five studies assessed as high risk of bias were removed, the findings were similar (RR 1.99, 95% CI 1.60 to 2.48; 20 studies) ( Analysis 3.2 ). There was no difference between older and newer studies ( Analysis 3.3 ).
3.1. Analysis.

Comparison 3: Accurate risk perceptions, Outcome 1: Accurate risk perceptions ‐ all studies
4. Accurate risk perceptions.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Cox 2019 | Clinician‐surrogate concordance scale (“What percent chance do you think [the patient/your loved one] has of being alive 1 year from now if the current treatment plan is continued?”. Scores range from 0 to 100; higher values indicate greater discordance) | 3 days post‐intervention | 132 | 27.1 (95% CI 22.8 to 31.4) | 134 | 29.5 (95% CI 25.1 to 33.9) | P = 0.60 |
| Fraenkel 2012 | Accuracy of stroke risk (reported by taking the absolute value of the difference between the participant's risk as estimated by the DA and the estimate provided by the participant ‐ out of 100; lower score indicates more accurate estimation of risk) | Post‐intervention | 69 | 9.1 (SD 13.3) | 66 | 14.2 (SD 13) | P = 0.002 |
| Accuracy of bleeding risk (reported same as above) | Post‐intervention | 69 | 8.7 (SD 12.5) | 66 | 13.1 (SD 12.2) | P = 0.004 | |
| Hanson 2011 | Expectation of benefit index 11‐item score from 1 to 4 with lower score indicating better knowledge | Post (after reviewing DA) | 127 | 2.3 | 129 | 2.6 | P = 0.001 |
| Ibrahim 2013 | Hospital for Special Surgery Knee Expectations Survey 19‐item | 1 month post‐intervention | 168 +163 | — | 167 | — | No difference between groups P = 0.97 |
| Kuppermann 2014 | Correct estimate of amniocentesis miscarriage risk | 3 to 6 months post‐intervention | 357 | 263 (73.8%) | 353 | 208 (59.0%) | P < 0.001 |
| Correct estimate of Down syndrome risk | 3 to 6 months post‐intervention | 357 | 210 (58.7%) | 353 | 163 (46.1%) | P = 0.001 | |
| Mann E 2010 | 3 of 8 multiple choice items in the knowledge test (question 4, 5, 7) | 2 weeks post | — | — | — | — | Total knowledge reported only |
| Manne 2020 | Perceived risk of contralateral breast cancer after unilateral mastectomy and radiation (mean (SE)). Scale not reported. | 2 to 4 weeks after surgery | 46 | 6.44 (SE 1.97) | 47 | 5.10 (SE 1.98) | No difference |
| Perceived risk for chest wall recurrence after contralateral prophylactic mastectomy (mean (SE)). Scale not reported. | 2 to 4 weeks after surgery | 46 | 11.95 (SE 2.52) | 47 | 12.12 (SE 2.26) | No difference | |
| Mathieu 2010 | 5‐item numerical questions (max = 5) | Post | 113 | 3.02 | 189 | 2.45 | P < 0.001 |
| Miller 2005 | — | 2‐week, 2‐month, and 6‐month follow‐ups | — | — | — | — | Intervention type had no impact on risk perceptions |
| Oostendorp 2017 (in consultation) | Accuracy of chances of experiencing severe diarrhea (mean (SD) 0% to 100%) | 1 week post‐intervention | 68 | 30.9 (SD 22.1) | 40 | 34.9 (SD 22.1) | No difference P = 0.366 |
| Accuracy of chances of achieving partial or complete tumor response (mean (SD) 0% to 100%) | 1 week post‐intervention | 68 | 30.0 (SD 20.8) | 40 | 32.5 (SD 14.3) | No difference P = 0.463 |
|
| Schapira 2019 | Difference between perceived risk and risk determined by the National Cancer Institute Breast Cancer Risk Assessment Tool | 6 weeks post‐intervention | 54 | 3.3% (95% CI ‐2.7 to 9.3) | 59 | 9.3% (95% CI 2.3 to 16.3) | Both study arms overestimated lifetime breast cancer risk P = 0.2 |
| Smith 2010 | 8 numerical questions (max = 8) | — | 357 | 2.93 (SD 2.91) | 173 | 0.58 (SD 1.28) | P < 0.001 |
| Weymiller 2007 (in consultation) | — | Immediately | 52 | — | 46 | — | Difference between groups OR 22.4 (95% CI 5.9 to 85.8) when decision aid administered during the consultation only (not if prior to) OR 6.7 (95% CI 2.2 to 19.7) when the decision aid administered prior to or during the consultation |
CI : confidence interval; DA : decision aid; OR : odds ratio; SD : standard deviation; SE : standard error.
6.

Funnel plot of comparison 2.1 Accurate risk perceptions ‐ all studies
3.2. Analysis.

Comparison 3: Accurate risk perceptions, Outcome 2: Accurate risk perceptions ‐ studies without high risk of bias
3.3. Analysis.

Comparison 3: Accurate risk perceptions, Outcome 3: Accurate risk perceptions ‐ old vs new studies
Decisional conflict subscales – feeling uninformed and unclear values
Of 209 studies, 75 (35.9%) measured patients’ ‘feeling uninformed’ using the subscale of the Decisional Conflict Scale with 58 studies pooled ( Analysis 4.1 ) and 17 studies not pooled ( Table 6 ). There was high certainty in the evidence that patient decision aids were more effective than usual care in reducing patients’ degree of ‘feeling uninformed’ about options, benefits, and harms (MD ‐10.02 out of 100, 95% CI ‐12.31 to ‐7.74; 58 studies) ( Analysis 4.1 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 7 ). The average scores by study arm were 20.9 out of 100 in the patient decision aid group compared to 31.6 for the usual care group, with lower scores indicating feeling less uninformed. When seven studies assessed as high risk of bias were removed, the findings were similar (MD ‐11.18, 95% CI ‐13.82 to ‐8.54; 51 studies; Analysis 4.2 ). There was no difference between older and newer studies ( Analysis 4.3 ).
4.1. Analysis.

Comparison 4: Decisional conflict, Outcome 1: Decisional conflict ‐ uninformed ‐ all studies
5. Decisional Conflict Score.
| Study | Scale u sed | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Bailey 2016 | Decisional conflict scale ‐ change from baseline to 4 to 6 weeks post enrollment (standardized values) | Uninformed | 114 | Mean ‐29.9 SD 26.5 | 111 | Mean ‐8.4 (SD 27.4) | P < 0.0001 |
| Unclear values | 114 | Mean ‐27.1 SD 24.4 | 111 | Mean ‐8.9 (SD 22.1) | P < 0.0001 | ||
| Berry 2013 | Decisional conflict scale | Uninformed | — | — | — | — | No significant difference |
| Unclear values | — | ‐3.57 units | — | — | P = 0.002 | ||
| Chen S 2021 | Decisional conflict (1 to 5 scale) post‐intervention | Uninformed | 29 | 1.63 (SD not reported) | 30 | 1.63 (SD not reported) | No difference P = 0.99 |
| Unclear values | 29 | 1.54 (SD not reported) | 30 | 1.56 (SD not reported) | No difference P = 0.91 |
||
| Fraenkel 2012 | Decisional conflict subscales low‐literacy version ‐ immediately post | Informed | 69 | 13.0 | 66 | 24.8 | P = 0.01 |
| Values | 69 | 6.4 | 66 | 21.0 | P < 0.001 | ||
| Fraenkel 2015 | Decisional conflict scale ‐ change from baseline to 2 weeks post‐intervention | Uninformed | 60 | Median 16.7 (IQR 0 to 33.3) | 61 | Median 8.3 (IQR ‐8.3 to 25.0) | P = 0.04 |
| Unclear values | 60 | Median 16.7 (IQR 4.2 to 37.5) | 61 | Median 0 (IQR ‐16.7 to 16.7) | P = 0.001 | ||
| Frosch 2008a | Decisional conflict ‐ subscales only | Feeling uninformed | 155 | 23.37 | 151 | 29.68 | P < 0.05 |
| Feeling unclear values | 155 | 32.25 | 151 | 37.93 | P < 0.05 | ||
| Fung 2021 | Decisional conflict scale | Feeling uninformed ‐ post‐intervention | 36 | 18.3 (SD not reported) | 37 | 43 (SD not reported) | P < 0.001 |
| Feeling unclear values ‐ post‐intervention | 36 | 16.3 (SD not reported) | 37 | 31.9 (SD not reported) | P = 0.002 | ||
| Gagne 2017 | Decisional conflict subscale scores that underwent a natural log transformation | Feeling uninformed (2 months post) | 26 | 3.5 (95% CI 1.9 to 6.6) | 25 | 3.7 (95% CI 1.9 to 7.0) | No difference |
| Feeling unclear values (2 months post) | 26 | 3.8 (95% CI 1.9 to 7.4) | 25 | 4.8 (95% CI 2.4 to 9.5) | No difference | ||
| Gokce 2019 | Decisional conflict score ≤ 25 or > 25 | Feeling uninformed (immediately post‐intervention) | 58 | ≤ 25 48 (82.8%) > 25 10 (17.2%) |
57 | ≤ 25 37 (64.9%) > 25 20 (35.1%) |
P = 0.03 |
| Feeling unclear values (immediately post‐intervention) | 58 | ≤ 25 47 (81.1%) > 25 11 (18.9) |
57 | ≤ 25 40 (70.2%) > 25 17 (29.8%) |
No difference P = 0.17 |
||
| Karagiannis 2016 (in consult) | Decisional conflict subscale feeling uninformed with scale inverted (higher score = higher comfort) | Immediately post | 101 | 78.8 (95% CI 60.9 to 96.8) | 103 | 65.4 (95% CI 44.3 to 86.5) | No difference P = 0.19 |
| Korteland 2017 | Decisional conflict scale ‐ post‐intervention/pre‐operatively | Uninformed | 66 | Median 8 (range 0 to 100) | 70 | Median 17 (range 0 to 100) | P < 0.05 |
| Unclear values | 66 | Median 28 (range 0 to 72) | 70 | Median 27 (range 0 to 93) | No difference | ||
| Krishnamurti 2019 | Decisional conflict ‐ change from baseline to 3 months | Uninformed | — | ‐14.65 | — | 1.75 | P = 0.003 |
| Unclear values | — | ‐4.17 | — | ‐4.39 | No difference P = 0.97 |
||
| LeBlanc 2015 (in consult) | Informed subscale | Immediately post | 28 | 4.2 (95% CI 0 to 25) | 36 | 20.8 (95% CI 0 to 33.3) | P = 0.14 |
| Values subscale | Immediately post | 28 | 16.7 (95% CI 0 to 25) | 36 | 25.0 (95% CI 8.3 to 33.3) | P = 0.25 | |
| Mathieu 2010 | Based on approaches suggested by Marteau et al (informed choice) | Immediately after intervention | 91 | 71% | 110 | 64% | P = 0.24 |
| Perez‐Lacasta 2019 | Decisional conflict subscales low‐literacy version | Feeling uninformed (2 to 4 weeks post‐intervention) | 203 | 18.56 (SD not reported) | 197 | 28.26 (SD not reported) | P = 0.002 |
| Unclear values (2 to 4 weeks post‐intervention) | 203 | 14.16 (SD not reported) | 197 | 18.02 (SD not reported) | P = 0.157 | ||
| Singh 2019 | Decisional conflict subscales low literacy version ‐ change from baseline to immediately post intervention | Uninformed | 151 | 30.6 (SD 40.6) | 147 | 21.7 (SD 33.9) | P = 0.04 |
| Unclear values | 151 | 27.2 (SD 41.8) | 147 | 16.8 (SD 37.6) | P = 0.03 | ||
| Van Peperstraten 2010 | 15‐item questionnaire (1 to 5) ‐ informed (includes some items from DCS) | Post‐intervention, pre‐IVF | 124 | 77.5 | 128 | 87.5 | P = 0.001 |
| Weymiller 2007 (in consult) | Informed subscale | Administered during consultation | 52 | ‐17.3 (95% CI ‐22.6 to ‐12.0) | 46 | — | Mean difference indicates statistically significantly lower decisional conflict for decision aid compared to usual care. |
| Administered prior to consultation | 52 | ‐6.6 (95% CI ‐14.3 to ‐1.1) | 46 | — | |||
| Values subscale | Immediately post | 52 | ‐8.5 (95% CI ‐15.7 to ‐1.3) | 46 |
CI : confidence interval; DA : decision aid; DCS : Decisional Conflict Scale; IQR : interquartile range; IVF : in vitro fertilization; SD : standard deviation.
7.
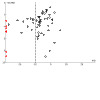
Funnel plot of comparison: 4.1 Decisional conflict: Uninformed ‐ all studies
4.2. Analysis.

Comparison 4: Decisional conflict, Outcome 2: Decisional conflict ‐ uninformed ‐ without studies having high risk of bias
4.3. Analysis.

Comparison 4: Decisional conflict, Outcome 3: Decisional conflict ‐ uninformed ‐ old vs new studies
Of 209 studies, 71 (34.0%) measured patients’ ‘feeling unclear about values’ using the subscale of the Decisional Conflict Scale with 55 studies pooled ( Analysis 4.4 ) and 16 studies not pooled ( Table 6 ). There was high certainty in the evidence that patient decision aids were more effective than usual care for reducing patients’ degree of ‘feeling unclear about values’ (MD ‐7.86 out of 100, 95% CI ‐9.69 to ‐6.02; 55 studies) ( Analysis 4.4 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 8 ). The average scores by study arm were 19.9 out of 100 in the patient decision aid group compared to 28.8 for the usual care group, with lower scores indicating feeling less unclear about values. When seven studies assessed as high risk of bias were removed, the findings were similar (MD ‐8.60, 95% CI ‐10.73 to ‐6.47; 48 studies) ( Analysis 4.5 ). There was no difference between older and newer studies ( Analysis 4.6 ).
4.4. Analysis.

Comparison 4: Decisional conflict, Outcome 4: Decisional conflict ‐ unclear values ‐ all studies
8.

4.5. Analysis.

Comparison 4: Decisional conflict, Outcome 5: Decisional conflict ‐ unclear values ‐ without studies having high risk of bias
4.6. Analysis.

Comparison 4: Decisional conflict, Outcome 6: Unclear values ‐ old vs new studies
Participation in decision‐making
Of 209 studies, 42 (20.1%) measured the effect of patient decision aids on patients’ perceived role in decision‐making with 25 studies pooled ( Analysis 5.1 ) and 17 studies not pooled ( Table 7 ). We conducted meta‐analyses using the groupings of the Control Preferences Scale ( Degner 1997 ).
5.1. Analysis.

Comparison 5: Participation in decision making, Outcome 1: Participation in decision‐making ‐ all studies
6. Participation in decision‐making.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Allen 2010 | Control preferences ‐ patients choosing active/collaborative decision‐making | Post‐intervention | 291 | 95% | 334 | 92% | No difference |
| Control preferences did not change | Post‐intervention | 291 | 92% | 334 | 87% | No difference | |
| Control preferences changed to passive | Post‐intervention | 291 | 3% | 334 | 5% | No difference | |
| Control preferences changed to active/collaborative | Post‐intervention | 291 | 3% | 334 | 7% | No difference | |
| Aoki 2019 (in consult) | COMRADE used to measure patients' perceived involvement in decisions | Post‐intervention | 32 | 88.0 median; 9 IQR | 53 | 76.0 median; 7 IQR | P < 0.001 |
| Cuypers 2018 | Problem‐Solving Decision‐Making Scale (perceived role) | Post‐consultation | 235 | 3.6 (SD 0.9) | 101 | 3.5 (SD 0.8) | No difference P = 0.5 |
| Fisher 2020 | Experienced preferred level of involvement in decisions (‘yes’ n (%)) | Post‐treatment decision 3 to 4 weeks Post‐intervention | 79 | 30 (38%) | 72 | 28 (39%) | No difference OR 0.96 (95% CI 0.50 to 1.86) |
| 3 months’ post‐decision follow‐up | 36 | 12 (33%) | 47 | 15 (32%) | No difference OR 1.07 (95% CI 0.45 to 2.55) |
||
| Fraenkel 2015 | COMRADE used to measure patients' perceived involvement in decisions | 2 weeks post‐intervention | 60 | Median 40.0 (IQR 26.5 to 43.0) | 61 | Median 35.0 (IQR 23.0 to 42.0) | No difference P = 0.1 |
| Hamann 2006 | COMRADE used to measure patients' perceived involvement in decisions | Post‐consultation | 49 | 79.5 (SD 18.6) 76.8 (SD 20.9) |
58 | 69.7 (SD 20.0) 73.5 (SD 19.3) |
Increased patient involvement in decision aid group post‐intervention compared to usual care at baseline. At discharge there was no difference between groups. |
| Hanson 2011 | Surrogates feeling somewhat or very involved in decision‐making | Post‐intervention | — | 83% | — | 77% | P = 0.18 |
| Kostick 2018 | Control Preferences Scale ‐ match in control preferences over time | 1 month post‐intervention | 27 | 48% | 31 | 52% | No difference P = 1.0 |
| Leighl 2011 | Achieved decision involvement | Post‐intervention | — | 32% | — | 35% | No difference |
| Loh 2007 (in consult) | Patients' perceived involvement in decision‐making | Post‐consultation | 191 | 26.3 pre 28.0 post | 96 | 24.5 pre 25.5 post |
Improved patient participation from baseline to post exposure to the decision aid (P = 0.010) and in comparison to the usual care group (P = 0.003), but there was no change in the control group for the pre‐post comparison |
| Oostendorp 2017 (In consultation) | Decision control (1 to 5) | 1 week post‐intervention | 68 | 4.2 (SD 0.7) | 40 | 4.3 (SD 0.6) | No difference |
| Decision control (1 to 5) | 8 weeks post‐intervention | 58 | 4.3 (SD 0.6) | 33 | 4.3 (SD 0.6) | No difference | |
| Problem‐Solving Decision‐Making Scale (perceived role) (1 to 5) | 1 week post‐intervention | 68 | 3.1 (SD 1.0) | 40 | 2.8 (SD 0.9) | No difference | |
| Problem‐Solving Decision‐Making Scale (perceived role) (1 to 5) | 8 weeks post‐intervention | 58 | 2.9 (SD 1.0) | 33 | 2.9 (SD 0.8) | No difference | |
| Perception of being offered a choice (yes/no) | 1 week post‐intervention | 68 | 45 (66%) | 40 | 26 (67%) | No difference | |
| Perception of being offered a choice (yes/no) | 8 weeks post‐intervention | 58 | 41 (71%) | 33 | 20 (61%) | No difference | |
| Perception of whether patient’s opinion mattered (yes/no) | 1 week post‐intervention | 68 | 51 (75%) | 40 | 30 (77%) | No difference | |
| Perception of whether patient’s opinion mattered (yes/no) | 8 weeks post‐intervention | 58 | 47 (81%) | 33 | 25 (76%) | No difference | |
| Politi 2020a | Decision process (DQI subscale 0 to 100) | Post‐consultation | 60 | 65.1 (SD 21.5) | 60 | 58.2 (SD 20.7) | No difference P = 0.06 |
| Rubel 2010 | Adapted from the Control Preferences Scale | Post‐intervention | — | — | — | — | The total mean scores were: 2.74 (SD 1.25) (N = 99) pre and 2.83 (SD 1.16) (N = 199) post; no statistically significant difference |
| Schonberg 2020 | Control Preferences Scale (merged Active Role and Collaborative Role) | Post‐consultation | 280 | 247 (88.1%) | 256 | 208 (81.2%) | P = 0.02 |
| Sheridan 2011 | Patient participation: 'Any' |
Immediately post | 79 | 79% | 78 | 51% | Absolute difference 28% (95% CI 9 to 45; P = 0.01) |
| 'None' | Immediately post | 79 | 21% | 78 | 49% | Absolute difference ‐28% (95% CI ‐45 to ‐9) | |
| Singh 2019 | Concordance between desired vs actual role using the Control Preferences Scale | Post‐consultation | 35 | 94% | 33 | 85% | No difference P = 0.25 |
| Van Peperstraten 2010 | Decision Evaluation scale (15 item questionnaire), Decision Control subscale | Post‐consultation | 124 | 85 | 128 | 87.5 | P = 0.33 |
CI : confidence interval; COMRADE : Combined Outcome Measure for Risk communication And treatment Decision making Effectiveness; DA : decision aid; DQI : Decision Quality Index; IQR : interquartile range; OR : odds ratio; SD : standard deviation.
Clinician‐controlled role in decision‐making
There was high certainty in the evidence that patient decision aids were more effective than usual care for reducing clinician‐controlled decision‐making (RR 0.72, 95% CI 0.59 to 0.88; 21 studies) ( Analysis 5.1 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 9 ). The average proportions for clinician‐controlled decision‐making by study arm were 18.8 out of 100 patients for the patient decision aid group compared to 25.7 out of 100 patients for the usual care group. When four studies assessed as high risk of bias were removed, the findings were similar (RR 0.81, 95% CI 0.66 to 0.98; 17 studies; Analysis 5.2 ). There was no difference between older and newer studies ( Analysis 5.3 ).
9.

5.2. Analysis.

Comparison 5: Participation in decision making, Outcome 2: Participation in decision‐making ‐ studies without high risk of bias
5.3. Analysis.

Comparison 5: Participation in decision making, Outcome 3: Participation in decision‐making ‐ clinician‐controlled ‐ old vs new studies
Patient‐controlled role in decision‐making
Patient decision aids were more effective than usual care for increasing patient‐controlled decision‐making (RR 1.22, 95% CI 1.05 to 1.43; 20 studies) ( Analysis 5.1 ). The average proportions for patient‐controlled decision‐making by study arm were 48.2 out of 100 patients for the patient decision aid group compared to 36.8 out of 100 patients for the usual care group. When five studies assessed as high risk of bias were removed, there was no difference between groups (RR 1.20, 95% CI 0.99 to 1.45; 15 studies) ( Analysis 5.2 ). There was no difference between older and newer studies ( Analysis 5.4 ).
5.4. Analysis.

Comparison 5: Participation in decision making, Outcome 4: Participation in decision‐making ‐ patient‐controlled ‐ old vs new studies
Shared role in decision‐making
There was no difference between patients in the patient decision aids compared to usual care groups on patients’ perception of achieving shared decision‐making with their clinician using the Collaborative role on the Control Preferences Scale (RR 0.98, 95% CI 0.88 to 1.09; 20 studies) ( Analysis 5.1 ). The average proportions for shared decision‐making by study arm were 38.3 out of 100 patients for the patient decision aid group compared to 41.4 out of 100 patients for the usual care group. When four studies assessed as high risk of bias were removed, the findings were similar (RR 0.96, 95% CI 0.83 to 1.10; 16 studies) ( Analysis 5.2 ). There was no difference between older and newer studies ( Analysis 5.5 ).
5.5. Analysis.

Comparison 5: Participation in decision making, Outcome 5: Participation in decision‐making ‐ shared decision‐making ‐ old vs new studies
Adverse events
Decision regret
Of 209 studies, 30 (14.4%) measured the effect of patient decision aids on decision regret, using the five‐item Decisional Regret scale ( Brehaut 2003 ) with 22 studies pooled ( Analysis 6.1 ) and eight not pooled ( Table 8 ). There was high certainty in the evidence that there was no increased decisional regret in patients exposed to patient decision aids as compared to those exposed to usual care (MD ‐1.23, 95% CI ‐3.05 to 0.59; 22 studies) ( Analysis 6.1 ). The funnel plot shows that these studies are at low risk for publication bias ( Figure 10 ). When five studies assessed as high risk of bias were removed, the findings were similar (MD ‐2.58, 95% CI ‐5.16 to ‐0.01; 17 studies) ( Analysis 6.2 ). There was no difference between older and newer studies ( Analysis 6.3 ).
6.1. Analysis.

Comparison 6: Decision regret, Outcome 1: Decision regret ‐ all studies
7. Adverse events.
| Author | Item |
N decision aid |
Proportion or mean (SD) |
N control |
Proportion or mean (SD) | Notes |
| Decision regret | ||||||
| Brown 2019 | Decision Regret Scale at 1 and 3 months post‐intervention | 16 | — | 21 | — | No difference ‐ authors say likely measured too soon |
| Hanson 2011 | 5‐item Decisional Regret Index | 126 | 11.9 | 127 | 14.3 | No difference P = 0.14 |
| Korteland 2017 | Decision Regret Scale 3 months postoperatively (proportion who experienced regret) | 71 | 30% | 67 | 36% | No difference P = 0.513 |
| Krishnamurti 2019 | Decision Regret Scale (out of 100) post decision | — | — | — | — | All had low levels of regret (n = 11) ranging from 0 to 25 |
| Legare 2011 | Proportion of patients with decisional regret | — | 7% | — | 9% | No difference P = 0.91 |
| Mathers 2012 | Decision Regret Scale at 6 months postintervention | 95 | 44.63 | 80 | 44.57 | No difference P = 0.872 |
| McLean 2020 | Decision Regret Scale (individual items) at 10 days postintervention. Proportion who who indicated "agreement" or "strong agreement". 'It was the right decision' 'I would make the same choice if I had to do it over again' 'The decision was a wise one' |
9 | 6 (66.67) 7 (77.78) 7 (77.78) |
5 | 5 (100.00) 4 (80.00) 3 (60.00) |
No difference P = 0.48 |
| Perez‐Lacasta 2019 | Anticipated regret (2 to 4 weeks post DA) Might later regret if do not screen
|
203 | 85 (41.9%) 68 (33.5%) 46 (22.7%) 4 (2%) |
197 | 90 (45.7%) 65 (33%) 37 (18.8%) 5 (2.5%) |
No difference P = 0.733 |
Might later regret if do screen
|
203 | 14 (6.9%) 49 (24.1%) 77 (37.9%) 63 (31%) |
197 | 21 (10.7%) 40 (20.3%) 65 (33%) 71 (36%) |
No difference P = 0.246 |
|
| Emotional distress | ||||||
| Cox 2019 | Post‐traumatic stress symptom inventory (range 10 to 70 points, with higher scores indicating greater distress)
|
409 161 154 |
26.6 (24.5 to 28.7) 24.8 (22.4 to 27.1) 24.5 (22.0 to 27.1) |
426 173 172 |
27.0 (24.8 to 29.3) 26.4 (24.1 to 28.6) 25.4 (23.0 to 27.7) |
No difference P = 0.91 P = 0.42 P = 0.83 |
| Lewis 2010 | Intrusive thoughts ‐ 3 items, 4‐point scale
|
210 | 139 (66.2%) 66 (31.4%) 5 (2.4%) |
231 | 157 (68.0%) 69 (29.9%) 5 (2.2%) |
No difference P = 0.92 |
| McCaffery 2010 | Intrusive thoughts ‐ measured using 1 item from the impact of events scale | 77 | 43% | 71 | 32% | No difference |
| McIlvennan 2018 | Perceived stress scale (0 to 40) | 50 | 12.7 (1.24) | 78 | 12.1 (1.00) | No difference P = 0.71 |
| Metcalfe 2017 | Impact of Event Scale
|
— | 24.6 (13.9) 9.3 (13.2) 17.7 (14.7) |
— | 26.8 (12.8) 25.2 (14.5) 22.4 (15.5) |
P = 0.33 P = 0.01 P = 0.05 |
DA : decision aid; SD : standard deviation
10.

6.2. Analysis.

Comparison 6: Decision regret, Outcome 2: Decision regret ‐ studies without high risk of bias
6.3. Analysis.

Comparison 6: Decision regret, Outcome 3: Decision regret ‐ old vs new studies
Emotional distress
Of 209 studies, five (2.4%) studies assessed the effect of patient decision aids on emotional distress, using various measures ( Table 8 ). In four studies, there was little to no difference between groups. In one study, women with a positive BRCA result reported significantly less cancer‐related distress at six months post‐patient decision aid compared to women receiving usual care (mean 9.3 (SD 13.2) versus 25.2 (SD 14.5), P = 0.01) ( Metcalfe 2017 ).
Proportion undecided
Of 209 studies, 46 (22.0%) measured the proportion of patients remaining undecided with 42 studies pooled ( Analysis 7.1 ) and four studies not pooled ( Table 9 ). A lower proportion of patients remained undecided after exposure to a patient decision aid (RR 0.68, 95% CI 0.58 to 0.80; 42 studies) ( Analysis 7.1 ). The average proportion by study arm was 16.7% undecided for the patient decision aid group compared to 24.8% for the usual care group. When five studies assessed as high risk of bias were removed, the findings were similar (RR 0.68, 95% CI 0.57 to 0.81; 37 studies) ( Analysis 7.2 ). There was no difference between older and newer studies ( Analysis 7.3 ).
7.1. Analysis.

Comparison 7: Proportion undecided, Outcome 1: Proportion undecided ‐ all studies
8. Proportion undecided.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Arterburn 2011 | Single item asking which option they were leaning towards. Proportion who were "unsure" | Immediately post‐intervention, 3 months post‐intervention | — | — | — | — | No difference |
| Kasper 2008 | Single item ‐ ranging from '0 = completely undecided' to '100 = made my decision' | — | — | — | — | — | No difference |
| Krishnamurti 2019 | Stage of Decision Making survey (10 multiple choice questions). Six‐point Likert scale ranging from, “I haven’t thought about the decision,” to “I have made my decision and am unlikely to change my mind.” | 3 and 6 months post‐intervention | — | — | — | — | No difference |
| Metcalfe 2017 | 15‐point scale: 1 = not leaning toward a breast cancer prevention option, 8 = unsure, and 15 = leaning toward a breast cancer prevention option. A total score of 6 to 10 was classified as undecided.
|
3 months post‐intervention | 72 | 19 (26.4%) 8 (11.3%) 15 (20.8%) |
69 | 15 (21.7%) 2 (2.9%) 15 (21.7%) |
P = 0.52 P = 0.05 P = 0.89 |
| Sawka 2012 | Answer "I don't know" to question "I favor taking adjuvant radioactive iodine" | Immediately post ‐ treatment preference | 37 | 10.8% | 37 | 21.6% | — |
| 6.3 months (mean) post ‐ actual decision | 37 | 13.5% | 37 | 8.1% | — | ||
| Answer "I don't know" to question "I favor not taking adjuvant radioactive iodine" | Immediately post ‐ treatment preference | 37 | 43.2% | 37 | 37.8% | — | |
| 6.3 months (mean) post ‐ actual decision | 37 | 40.5% | 37 | 51.4% | — |
DA : decision aid
7.2. Analysis.

Comparison 7: Proportion undecided, Outcome 2: Proportion undecided ‐ studies without high risk of bias
7.3. Analysis.

Comparison 7: Proportion undecided, Outcome 3: Proportion undecided ‐ old vs new studies
Patient‐clinician communication
Of 209 studies, 36 (17.2%) measured the effect of patient decision aids on patient‐clinician communication. Shared decision‐making was measured using the observer‐reported OPTION scale (n = 13), the patient‐reported CollaboRATE (n = 7), and the patient‐reported SDM‐Q‐9 (n = 5). Other measures of patient‐clinician communication included reporting that the decision topic was discussed with the clinician (n = 11) and/or other items (n = 8) ( Table 10 ). Analysis was conducted by instrument.
9. Patient‐clinician communication.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Berger‐Hoger 2019 | MAPPIN‐O dyad Total | Analysis of the consultation using video‐recordings | 36 | 2.29 (95% CI 1.77 to 2.81) | 28 | 0.42 (SD 0.51) (95% CI 0.0 to 0.88) | Significantly higher in the intervention arm P < 0.0001 |
| MAPPIN‐O patient Total | Analysis of the consultation using video‐recordings | 36 | 1.78 (95% CI 1.40 to 2.16) | 28 | 0.30 (95% CI 0.0 to 0.68) | No difference 1.48 (95% CI 1.00 to 1.95) |
|
| MAPPIN‐O professionals Total | Analysis of the consultation using video‐recordings | 36 | 2.23 (95% CI 1.79 to 2.67) | 28 | 0.32 (95% CI 0.0 to 0.68) | Significantly higher in the intervention arm 1.91 (95% CI 1.42 to 2.40) |
|
| MAPPIN‐Q physician | Physician reported immediately post consultation | 36 | 3.42 (95% CI 3.09 to 3.74) | 28 | 3.44 (95% CI 3.04 to 3.83) | No difference 0.02 (95% CI ‐0.47 to 0.43) |
|
| MAPPIN‐Q patient | Patient‐reported immediately post consultation | 36 | 3.87 (95% CI 3.78 to 3.96) | 28 | 3.82 (95% CI 3.68 to 3.96) | No difference 0.05 (95% CI ‐0.10 to 0.20) |
|
| Cox 2019 | Quality of communication questionnaire (range 0 to 110 points, with higher scores indicating better communication) | 3 days post‐intervention | 121 | 91.9 (95% CI 89.1 to 94.7) | 125 | 90.3 (95% CI 87.1 to 93.5) | No difference P = 0.149 |
| Coylewright 2016 (in consult) | OPTION Scale | Analysis of the consultation using video‐recordings | 34 | 21.3% | 20 | 16.0% | No difference P = 0.071 |
| Durand 2021 (in consult) | Decision Quality Instrument decision process subscale | Immediately post consultation | 66 | 80.9 (SD 17.7) | 257 | 65.3 (SD 30.1) | Significantly higher in the intervention arm P = 0.01 |
| CollaboRATE‐SDM (dichotomized grouping participants scoring 9 on all 3 items versus all others) | Patient‐reported immediately post consultation | 59 | 46 (78.0%) | 216 | 126 (58.3%) | No effect of the intervention | |
| Fraenkel 2012 | Discussed risk of stroke | Immediately post | 69 | 71% | 66 | 12% | P < 0.001 |
| Discussed risk of major bleeding | Immediately post | 69 | 69% | 66 | 20% | P < 0.001 | |
| Hanson 2011 | Discussed feeding with physician, nurse clinician, or physician's assistant | 3 months | 126 | 46% | 127 | 33% | P = 0.04 |
| Discussed feeding with other nursing home staff | 3 months | 126 | 64% | 127 | 71% | P = 0.42 | |
| Ibrahim 2013 | Discussed knee pain with primary care doctor | Patient‐reported within 1 year of intervention | 168 + 163 | 92% | 167 | 85% | P = 0.007 |
| Kostick 2018 | CollaboRATE‐SDM | Patient‐reported 1 month post‐intervention | 26 | 90.4 (SD 14.3) | 31 | 89.8 (SD 17.2) | No difference P = 0.94 |
| SDM‐9 | Patient‐reported 1 month post‐intervention | 25 | 87.5 (SD 12.8) | 31 | 85.2 (SD 15.0) | No difference P = 0.74 |
|
| Kunneman 2020 (in consult) | Quality of communication (The Consumer Assessment of Healthcare Providers and Systems Clinician and Group Survey with 3 subscales: Easy to understand, Listens carefully, Shows respect) | Patient‐reported immediately post consultation | 432 430 428 |
431 (99.8%) 428 (99.5%) 426 (99.5%) |
425 427 427 |
422 (99.3%) 427 (100%) 427 (100%) |
High in both groups |
| Lepore 2012 | Discussed PSA testing with physician post‐intervention | 8 months post‐intervention | 215 | 15.8% | 216 | 8.3% | P < 0.001 |
| Lewis 2018 | Discussed colorectal cancer screening | Patient‐reported immediately post consultation | 209 | 58.4% | 209 | 41.6% | P < 0.001 |
| Patient initiated screening discussion | Patient‐reported immediately post consultation | 120 | 61.7% | 87 | 41.4% | P = 0.004 | |
| Madden 2020 | Discussed contraception with provider | Patient‐reported immediately post consultation | 161 | 96.9% | 80 | 97.5% | No difference P = 0.79 |
| McGrath 2017 | Perceived ability to discuss concerns and values/preferences with the doctor (0 to 8 scale; not very able to very able) | Patient‐reported 2 weeks post‐intervention | 30 | 6.3 (2.04) | 37 | 6.95 (1.43) | No difference P = 0.20 |
| Meier 2019 (in consult) | SDM‐Q‐9 | Patient‐reported immediately post consultation | 51 | Median 88.89 (SE 1.84) | 48 | Median 90.74 (SE 1.92) | No difference P = 0.845 |
| Miller 2018 | Discussed screening with provider | Patient‐reported 1 day post consultation | 197 | 150 (76%) | 213 | 103 (48%) | P < 0.001 |
| Montori 2011 (in consult) | OPTION 100‐point scale | Analysis of the consultation using video‐recorded consultations | 38 | 49.8 | 32 | 27.3 | P < 0.001 |
| Politi 2020a | CollaboRATE (% with top score) | Patient‐reported immediately post consultation | 60 | 58.9% | 60 | 62.7% | P = 0.681 |
| Schonberg 2020 | Discussed mammography with doctor | Analysis of consultation notes 6 months post | 279 | 146 (52.3%) | 260 | 111 (42.7%) | No difference RR 1.16 (95% CI 0.95 to 1.42) |
| Schott 2021 (in consult) | CollaboRATE 3‐item: dichotomized as "every effort" and "not every effort" | Patient‐reported immediately post consult | 32 | 20 (62.5%) | 32 | 22 (68.75%) | No difference |
| Sheridan 2006 | Discussed CHD with doctor | Patient‐reported immediately post | 16/41 decision aid pre‐consultation with summary report to bring to consultation | — | 8/34 usual care | — | Absolute difference 16% (95% CI ‐4 to 37) |
| Plan to reduce CHD risk and discussed with doctor | Patient‐reported immediately post | 15/41 decision aid pre‐consultation with summary report to bring to consultation | — | 8/34 usual care | — | Absolute difference 13% (95% CI ‐7 to 34) | |
| Plan to reduce CHD risk and not discussed with doctor | Patient‐reported immediately post | 37/41 decision aid pre‐consultation with summary report to bring to consultation | — | 25/34 usual care | — | Absolute difference 16% (95% CI ‐1 to 33) | |
| Sheridan 2011 | Had CHD discussion with provider | Patient‐reported immediately post | 79 | 89% | 78 | 58% | Absolute difference 31% (95% CI 15 to 45; P < 0.001) |
| Patient‐raised discussion | Patient‐reported immediately post | 79 | 63% | 78 | 35% | Absolute difference 28% (95% CI 9 to 45; P = 0.02) | |
| Modified Healthcare Climate Questionnaire: 1. "My provider provided me with choices and options about lowering my chances of heart disease" | Patient‐reported immediately post | 79 | 91% | 78 | 76% | Absolute difference 15% (95% CI ‐0.1 to 31; P = 0.02) | |
| 2. "My provider understands how I see things with respect to lowering my chances of heart disease." | Patient‐reported immediately post | 79 | 95% | 78 | 86% | Absolute difference 9% (95% CI ‐7 to 25; P = 0.21) | |
| 3. "My provider conveyed confidence in my ability to make changes regarding lowering my chances of heart disease" | Patient‐reported immediately post | 79 | 88% | 78 | 77% | Absolute difference 11% (95% CI ‐5 to 27; P = 0.15) | |
| 4. "My provider encouraged me to ask questions" | Patient‐reported immediately post | 79 | 78% | 78 | 67% | Absolute difference 11% (95% CI ‐4% to 27%; P = 0.13) | |
| 5. "My provider listened to how I would like to do things" | Patient‐reported immediately post | 79 | 92% | 78 | 71% | Absolute difference 21% (CI 95% 6 to 37; P < 0.01) | |
| 6. "My provider tried to understanding how I see things before suggesting new ways to lower my chances of heart disease." | Patient‐reported immediately post | 79 | 84% | 78 | 69% | Absolute difference 15% (CI 95% ‐0.3 to 31; P = 0.05) | |
| Singh 2019 | Interpersonal Processes of Care short form ‐ 18 items | Patient‐reported 3 months post | — | 83.6 (SD 7.7) | — | 83.1 (SD 7.3) | No difference P = 0.50 |
| Active Patient Participation Coding Scheme (APPC) | Analysis of audio recordings | 8.1 (SD 7.2) | — | 9.2 (SD 7.3) | No difference P = 0.80 |
||
| Patient‐centered communication by the doctor using APPC | Analysis of audio recordings | — | 5.1 (SD 2.1) | — | 3.7 (SD 1.9) | No difference P = 0.06 |
|
| Smallwood 2017 | 4 yes/no items scored 0 to 4 (follow‐up discussion with a primary care physician, whether alternative treatment options were provided, discussed reasons for and against taking medication, and asked what they wanted to do regarding treatment) | Patient‐reported 3 months post, based on chart review | 29 | 3.19 (SD 1.2) | 21 | 2.91 (SD 1.3) | No difference P = 0.566 |
| Stubenrouch 2022 | SDM‐Q‐9 | Patient‐reported immediately post‐intervention/ consultation | 171 | Median 93.3% (IQR 82.2% to 100%) | 138 | 93.3% (IQR 79.4% to 100%) | No difference P = 0.71 |
| CollaboRATE | Patient‐reported immediately post‐intervention/ consultation | 171 | Median 83.3% (IQR 80.0% to 90.0%) | 137 | Median 86.7% (80.0% to 90.0%) | No difference P = 0.61 |
|
| SDM‐Q‐Doc | Clinician‐reported immediately post consultation | 175 | Median 80% (IQR 71.1 to 86.7%) | 143 | Median 73.3% (IQR 64.4 to 84.4%) | P = 0.002 | |
| Tebb 2021 | Discussed contraception with provider | Patient‐reported 48 hours post‐intervention | 320 | 285 (89.1%) | 436 | 301 (69.0%) | No difference |
| Weymiller 2007 (in consult) | OPTION Scale | Analysis of the consultation using video‐recorded consultations | 1/2 used decision aid prior to consultation and 1/2 used it during consultation | — | Usual care | — | Greater patient participation (MD 4.4, 95% CI 2.9 to 6.0) in decision aid compared to usual care group |
| Wyld 2021 (in consult) | CollaboRATE | Patient‐reported after decision‐making | 71 | Median 100 (IQR 96 to 100) | 77 | Median 100 (IQR 93 to 100) | No difference P = 0.729 |
CHD : coronary heart disease; CI : confidence interval; DA : decision aid; DCS : decisional conflict scale; ICC : intraclass correlation coefficient; IQR : interquartile range; MD : mean difference; OPTION scale : observing patient involvement scale; RR : risk ratio; SD : standard deviation
The analysis of eight studies that used the observer OPTION‐12 ( Elwyn 2005 ) showed that patient decision aids used during the consultation were more effective than usual care for improving patient‐clinician communication (MD 12.14 out of 100, 95% CI 8.12 to 16.16) ( Analysis 8.1 ). There were no differences between groups when patient‐clinician communication was measured using OPTION‐5 (MD 20.46, 95% CI ‐1.98 to 42.90; 2 studies) ( Analysis 8.1 ), CollaboRATE (MD 1.76, 95% CI ‐0.50 to 4.03; 2 studies) ( Analysis 8.1 ), or SDM‐Q‐9 (MD 1.38, 95% CI ‐2.50 to 5.25; 3 studies) ( Analysis 8.1 ).
8.1. Analysis.

Comparison 8: Patient‐clinician communication, Outcome 1: Patient‐clinician communication ‐ continuous measures (OPTION, CollaboRATE, SDM‐Q‐9)
A subanalysis of 11 studies that reported whether the decision topic was discussed with the clinician also showed that patient decision aids were more effective as compared to usual care for improving patient‐clinician communication (RR 1.42, 95% CI 1.19 to 1.70) ( Analysis 8.2 ). When studies assessed as high risk of bias were removed, the findings were similar across measures ( Analysis 8.3 ; Analysis 8.4 ).
8.2. Analysis.

Comparison 8: Patient‐clinician communication, Outcome 2: Discussed topic with provider
8.3. Analysis.

Comparison 8: Patient‐clinician communication, Outcome 3: Patient‐clinician communication ‐ continuous measures (OPTION, CollaboRATE, SDM‐Q‐9) ‐ studies without high risk of bias
8.4. Analysis.

Comparison 8: Patient‐clinician communication, Outcome 4: Discussed topic with provider ‐ studies without high risk of bias
Satisfaction with the decision‐making process
Of 209 total studies, 16 (7.7%) measured satisfaction with the decision‐making process with 12 studies pooled ( Analysis 9.1 ) and four studies not pooled ( Table 11 ). Patient decision aids were more effective than usual care for improving patient satisfaction with the decision‐making process (MD 3.33 out of 100, 95% CI 1.18 to 5.48; 12 studies) ( Analysis 9.1 ). The average scores by study arm were 79.4 out of 100 in the patient decision aid group compared to 76.4 for the usual care group. When four studies assessed as high risk of bias were removed, the findings were similar (MD 3.90, 95% CI 1.71 to 6.09; 8 studies) ( Analysis 9.2 ). There was no difference between older and newer studies ( Analysis 9.3 ).
9.1. Analysis.

Comparison 9: Satisfaction, Outcome 1: Satisfaction with the decision‐making process ‐ all studies
10. Satisfaction with the decision‐making process.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Satisfaction with the decision‐making process | |||||||
| Case 2019 | Satisfaction with decision‐making process (1 for strongly disagree to 5 for strongly agree) | Post consultation | 43 | — | 48 | — | High satisfaction with no difference by group P = 0.42 |
| Hess 2012 (in consult) | Satisfaction with decision process (0 for strongly agree to 5 for strongly disagree) | — | 101 | — | 103 | — | Patients in DA group reported greater satisfaction with the DM process (strongly agree, 61% DA vs 40% usual care) |
| Kunneman 2020 (in consult) | Satisfaction with the information‐sharing approach (proportion who would recommend to others) | Post consultation | 429 | 390 (90.9%) | 425 | 378 (88.9%) | No difference Effect size 1.0 (0.97 to 1.1) |
| Vodermaier 2009 | Satisfied with process | 1 week follow‐up | 53 | 42 | 56 | 50 | High satisfaction with no difference by group |
| Satisfaction with participating in decision‐making | |||||||
| Kennedy 2002 | Measured satisfaction with opportunities to participate in decision‐making using a single item | — | — | — | — | — | Compared to usual care, women who received the decision aid followed by nurse coaching were significantly more satisfied with the opportunities to participate in decision‐making (OR 1.5, 95% CI 1.1 to 2.0). |
| Satisfaction with the information provided | |||||||
| Cuypers 2018 | Satisfaction with Cancer Information Profile (SCIP) | Post consultation | 235 | 3.8 (SD 0.8) | 101 | 4.1 (0.6) | P = 0.04 |
| Hess 2016 (in consult) | Amount of information was just right | Post consultation | 441 | 416 (94%) | 438 | 401 (92%) | P = 0.133 |
| Information received was extremely clear | Post consultation | 440 | 335 (76%) | 438 | 296 (68%) | P = 0.011 | |
| Information received was extremely helpful | Post consultation | 441 | 320 (73%) | 438 | 303 (69%) | P = 0.506 | |
| Would recommend method to others | Post consultation | 440 | 387 (88%) | 437 | 349 (80%) | P = 0.004 | |
| Would use for other decisions | Post consultation | 440 | 346 (79%) | 437 | 335 (77%) | P = 0.813 | |
| Hess 2018 (in consult) | Amount of information | Post consultation | 476 | 455 (92%) | 469 | 441 (92%) | P = 0.29 |
| Clarity of information | Post consultation | 476 | 382 (78%) | 466 | 342 (72%) | P = 0.02 | |
| Helpfulness of the information | Post consultation | 478 | 377 (77%) | 468 | 344 (72%) | P = 0.05 | |
| Would recommend to others | Post consultation | 479 | 376 (76%) | 469 | 343 (72%) | P = 0.08 | |
| Would want to use for other decisions | Post consultation | 478 | 327 (66%) | 471 | 290 (61%) | P = 0.04 | |
| Kleiss 2021 | Understood all received information and felt adequately educated to make a decision | 2 weeks post consultation | 52 | 44 (84%) | 49 | 42 (86%) | P = 0.99 |
| LeBlanc 2015 (in consult) | Amount of information was just right | Post consultation | 29 | 25 (86%) | 37 | 34 (92%) | P = 0.69 |
| Information received was clear | Post consultation | 27 | 17 (63%) | 36 | 26 (72%) | P = 0.43 | |
| Information received was helpful | Post consultation | 28 | 21 (75%) | 34 | 23 (68%) | P = 0.53 | |
| Would recommend method to others | Post consultation | 28 | 24 (86%) | 35 | 27 (77%) | P = 0.52 | |
| LeBlanc 2015b (in consult) | Right amount of information given | Post consultation | 132 | 124 (92.5%) | 109 | 102 (91.9%) | P = 0.81 |
| Information given was extremely clear | Post consultation | 132 | 92 (68.7%) | 109 | 64 (58.7%) | P = 0.09 | |
| Information given was extremely helpful | Post consultation | 132 | 92 (69.2%) | 109 | 57 (52.8%) | P = 0.01 | |
| Strongly desire to receive information this way for other treatment decisions | Post consultation | 132 | 90 (68.2%) | 109 | 55 (50.5%) | P = 0.005 | |
| Strongly recommend the way information was shared to others | Post consultation | 132 | 104 (77.6%) | 109 | 65 (59.1%) | P = 0.002 | |
| Laupacis 2006 | Satisfaction with information received subscale 4‐item (0 to 100; low to high) | Average 10 days | 54 | 76 (15.5 SD) | 56 | 59 (23.3 SD) | P = 0.001 |
| McLean 2020 | Information about treatment options good or excellent | Post‐intervention | 16 | 16 (100%) | 15 | 10 (66.67) | P = 0.04 |
| Amount of information was just right | Post‐intervention | 16 | 13 (81.25%) | 15 | 11 (73.33) | P = 0.45 | |
| Information was useful when making a decision | Post‐intervention | 16 | 16 (100%) | 15 | 11 (73.33) | P = 0.05 | |
| Information made it easy to make a decision | Post‐intervention | 16 | 16 (100%) | 15 | 10 (66.67) | P = 0.04 | |
| Montori 2011 (in consult) | (7‐point scales) Participants' satisfaction with knowledge transfer
|
Post‐intervention | 49 | 6.6 6 6 6.1 6.4 |
46 | 6.3 6 5.8 5.8 6.2 |
P = 0.798 P = 0.296 P = 0.624 P = 0.248 P = 0.435 |
Clinicians' satisfaction with knowledge transfer
|
Post‐intervention | 39 | 5.8 6.1 5.9 |
33 | 5.2 4.9 4.8 |
P = 0.006 P < 0.001 P < 0.001 |
|
| Oakley 2006 | Satisfaction with information about medicines | 4 months post | 16 | 10.4 (SD 2.9) | 17 | 10.1 (SD 2.2) | No difference |
| Oostendorp 2017 (in consult) | Amount of information (1 to 7 from too little ‐ too much) | 1 week post 8 weeks post |
68 58 |
3.8 (0.7) 3.8 (0.5) |
40 33 |
4.0 (0.4) 3.9 (0.3) |
No difference |
| Undesired information (yes/no) | 1 week post | 68 | 6 (10%) | 40 | 7 (18%) | No difference P = 0.244 |
|
| Satisfaction with quality of information for severe adverse events, tumor response, and survival (1 to 6 from not satisfied ‐ very much satisfied) | 1 week post 8 weeks post |
68 58 |
— | 40 33 |
— | No difference for all items measured | |
| Balanced presentation (1 to 5 from clearly in favor of chemotherapy plus best supportive care to clearly in favor of best supportive care alone) | 1 week post | 68 | 2.7 (0.7) | 40 | 2.4 (1.1) | No difference 0.201 |
|
| Perez‐Lacasta 2019 | Length
|
2 to 4 weeks post‐intervention | 203 | 12.3% 82.8% 4.9% |
197 | 6.1% 83.2% 10.7% |
P = 0.008 |
Balance
|
2 to 4 weeks post‐intervention | 203 | 26.6% 16.7% 47.3% |
197 | 42.6% 14.2% 42.6% |
P < 0.001 | |
| Easy to understand (strongly agree/agree) | 2 to 4 weeks post‐intervention | 203 | 91% | 197 | 94% | P = 0.002 | |
| Helpful in decision‐making (strongly agree/agree) | 2 to 4 weeks post‐intervention | 203 | 76% | 197 | 86% | P = 0.076 | |
| Roberto 2020 | Amount of information (too much, too little, fair) | 7 to 10 days post‐intervention | 468 | 3.6% 4.1% 92.3% |
517 | 1.2% 6.0% 92.8% |
P = 0.01 |
| Clear information | 7 to 10 days post‐intervention | 469 | 92.5% | 517 | 91.3% | P = 0.47 | |
| Balanced information | 7 to 10 days post‐intervention | 469 | 36.9% | 517 | 33.7% | P = 0.37 | |
| Helped to decide | 7 to 10 days post‐intervention | 469 | 70.4% | 517 | 69.6% | P = 0.85 | |
| Recommend to others | 7 to 10 days post‐intervention | 469 | 96.8% | 517 | 98.1% | P = 0.21 | |
| van Dijk 2021 | Satisfaction with the given information (0 to 10 scale; low to high) | Post consultation | 66 | 8.6 (SD 1.1) | 65 | 7.6 (SD 1.8) | P = 0.00 |
| Varelas 2020 | Satisfaction with information provided (15 to 60 scale; low to high) | Post consultation | 13 | 56.8 (SD 4.2) | 13 | 47.9 (SD 8.2) | P = 0.0017 |
| Satisfaction with the clinician | |||||||
| Karagiannis 2016 (in consult) | Satisfaction with conversation with clinician
|
Post consultation | 101 | 66 (65.3%) 31 (30.7%) 4 (4.0%) 0 (0%) 0 (0%) |
103 | 58 (56.3%) 44 (42.7%) 1 (1.0%) 0 (0%) 0 (0%) |
No difference P = 0.54 |
| Kleiss 2021 | Satisfaction with the visit (11‐point scale; low to high) | 2 weeks post consultation | 52 | 9.2 (SD 1.4) | 49 | 8.8 (SD 1.7) | No difference P = 0.216 |
| Madden 2020 | Satisfaction with counseling from the provider and visit overall, 5‐point scale (1 to 5; low to high) | Post consultation | 161 | — | 80 | — | High satisfaction with no difference by group |
| Laupacis 2006 | Satisfaction with practitioner treatment during decision process subscale 4‐item (0 to 100; low to high) | Average 10 days | 54 | 69 (25.3 SD) | 56 | 54 (26.7 SD) | P = 0.004 |
| Miller 2005 | Satisfaction with cancer information service 1‐item (1 to 5; low to high) | 2 weeks | — | 4.37 (0.84 SD) | — | 4.38 (0.86 SD) | No difference |
| 6 months | — | 4.51 (0.75 SD) | — | 4.51 (0.64 SD) | No difference | ||
| van Dijk 2021 | Satisfaction with physician (0 to 10 scale; low to high) | Post consultation | 66 | 8.9 (SD 0.9) | 65 | 8.3 (SD 1.7) | P = 0.01 |
| Vodermaier 2009 |
|
1 week follow‐up | 53 | 49 (92.5%) 47 47 44 36 |
56 | 53 (94.6%) 50 51 45 36 |
High satisfaction with no difference by group |
CI : confidence interval; DA : decision aid; DM : decision‐making; OR : odds ratio; SD : standard deviation.
9.2. Analysis.

Comparison 9: Satisfaction, Outcome 2: Satisfaction with the decision‐making process ‐ studies without high risk of bias
9.3. Analysis.

Comparison 9: Satisfaction, Outcome 3: Satisfaction with the decision‐making process ‐ old vs new studies
Preparation for decision‐making
Of 209 studies, 16 (7.7%) measured patients’ preparation for decision‐making using the Preparation for Decision Making Scale ( Bennett 2010 ) with eight studies pooled ( Analysis 10.1 ) and eight not pooled ( Table 12 ). There was no difference in preparation for decision‐making by group (MD 6.63, 95% CI ‐3.09 to 16.35; 8 studies) ( Analysis 10.1 ). When one study assessed as high risk of bias was removed, patients exposed to patient decision aids felt more prepared for decision‐making than those receiving usual care (MD 9.24, 95% CI 4.78 to 13.71; 7 studies) ( Analysis 10.2 ). There was no difference between older and newer studies ( Analysis 10.3 ).
10.1. Analysis.

Comparison 10: Preparation for decision‐making, Outcome 1: Preparation for decision‐making ‐ all studies
11. Preparation for decision‐making.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Fraenkel 2007 | Preparation for Decision Making Scale | Pre‐consultation | 43 | 35 (median) | 40 | 20.5 (median) | P < 0.001 |
| Fung 2021 | Preparation for Decision Making Scale | Post‐intervention | 36 | 87.8 (SD not reported) | 37 | 66.2 (SD not reported) | P < 0.001 |
| Krishnamurti 2019 | Preparation for Decision Making Scale (difference in change in preparedness) | 3 months post‐intervention | 23 | — | 19 | — | No difference P = 0.16 |
| 6 months post‐intervention | 22 | — | 17 | — | P < 0.001 | ||
| Lewis 2018 | Prepared for individualized decision‐making (proportion having adequate knowledge (≥ 3 of 5 questions correct) and adequately clarified values (a score of ≤ 25 on the unclear values subscale, range 0 to 100)) | Post‐intervention; pre‐consultation | 212 | 67.6% | 210 | 31.9% | P < 0.001 |
| Manne 2020 | Preparation for Decision Making Scale | 2 to 4 weeks after surgery | 46 | 3.46 (0.61) | 47 | 3.42 (0.55) | No difference |
| McLean 2020 | Preparation for Decision Making Scale Helped you recognize that a decision needs to be made |
Post‐intervention | 16 | 11 (68.75) | 15 | 6 (40.00) | P < 0.01 |
| Prepared you to make a better decision | Post‐intervention | 16 | 14 (87.50) | 15 | 4 (26.67) | ||
| Helped you think about the pros and cons of each option | Post‐intervention | 16 | 14 (87.50) | 15 | 4 (26.67) | ||
| Helped you think about which pros and cons are most important | Post‐intervention | 16 | 13 (81.25) | 15 | 6 (40.00) | ||
| Helped you know that the decision depends on what matters most to you | Post‐intervention | 16 | 13 (81.25) | 15 | 8 (53.33) | ||
| Helped you organize your own thoughts about the decision | Post‐intervention | 16 | 9 (56.25) | 15 | 6 (40.00) | ||
| Helped you think about how involved you want to be in this decision | Post‐intervention | 16 | 11 (68.75) | 15 | 10 (66.67) | ||
| Helped you identify questions you want to ask your physician | Post‐intervention | 16 | 11 (68.75) | 15 | 9 (60.00) | ||
| Prepared you to talk to your physician about what matters most to you | Post‐intervention | 16 | 14 (87.50) | 15 | 10 (66.67) | ||
| Prepared you for a follow‐up visit with your physician | Post‐intervention | 16 | 13 (81.25) | 15 | 8 (53.33) | ||
| Stacey 2014a | Preparation for Decision Making Scale item (5‐point scale from: 1 not at all to 5 a great deal) 'Help recognize decision to be made' |
Post‐intervention; pre‐consultation | 66 | 4.12 (SD 1.21) | 64 | 3.78 (SD 1.25) | No difference |
| Preparation for Decision Making Scale item 'Help know decision depends on what matters most' |
Post‐intervention; pre‐consultation | 66 | 4.48 (SD 0.85) | 64 | 4.14 (SD 1.10) | No difference | |
| Preparation for Decision Making Scale item 'Help think about how involved you want to be in decision' |
Post‐intervention; pre‐consultation | 66 | 4.48 (SD 0.81) | 64 | 4.25 (SD 1.05) | No difference | |
| Preparation for Decision Making Scale item 'Prepare you to talk to your doctor about what matters most' |
Post‐intervention; pre‐consultation | 66 | 4.36 (SD 0.91) | 64 | 4.23 (SD 1.04) | No difference | |
| Stacey 2016 | Preparation for Decision Making Scale (4 of 10 items; 5‐point scale from: 1 not at all to 5 a great deal) Help recognize decision to be made |
2 weeks post‐intervention; pre‐consultation | 156 | 4.16 (SD 1.01) | 157 | 3.91 (1.17) | No difference 0.070 |
| Help know decision depends on what matters most | 2 weeks post‐intervention; pre‐consultation | 156 | 4.40 (SD 0.84) | 157 | 4.03 (1.14) | 0.003 | |
| Help think about how involved you want to be in decision | 2 weeks post‐intervention; pre‐consultation | 156 | 4.40 (SD 0.88) | 157 | 4.27 (1.05) | No difference 0.426 |
|
| Prepare to talk to your doctor about what matters most | 2 weeks post‐intervention; pre‐consultation | 156 | 4.47 (SD 0.68) | 157 | 4.10 (1.14) | 0.014 |
DA : decision aid; SD: standard deviation.
10.2. Analysis.

Comparison 10: Preparation for decision‐making, Outcome 2: Preparation for decision‐making studies without high risk of bias
10.3. Analysis.

Comparison 10: Preparation for decision‐making, Outcome 3: Preparation for decision‐making ‐ old vs new studies
2. Secondary outcomes
Choice
Of 209 studies, 165 (78.9%) studies measured the effect of patient decision aids on choice with 86 studies pooled ( Analysis 11.1 ; Analysis 11.2 ; Analysis 11.3 ; Analysis 11.4 ; Analysis 11.5 ; Analysis 11.6 ) and 79 studies not pooled ( Table 13 ; Table 14 ).
11.1. Analysis.

Comparison 11: Choice, Outcome 1: Choice: surgery over conservative option (subgroup by condition)
11.2. Analysis.

Comparison 11: Choice, Outcome 2: Choice for screening
11.3. Analysis.

Comparison 11: Choice, Outcome 3: Choice: diabetes medication (uptake of new medication)
11.4. Analysis.

Comparison 11: Choice, Outcome 4: Choice: surgery over conservative option ‐ studies without high risk of bias
11.5. Analysis.

Comparison 11: Choice, Outcome 5: Choice for screening ‐ studies without high risk of bias
11.6. Analysis.

Comparison 11: Choice, Outcome 6: Choice: diabetes medication (uptake of new medication) ‐ studies without high risk of bias
12. Choice.
| Study | Type of comparison | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Surgery ‐ elective major surgery | ||||||
| Ibrahim 2013 | Preference (knee replacement). Odds ratio of increased willingness at 1 month (5‐point scale; higher score indicates higher willingness) | 162 | OR 2.46 | 161 | OR 1.79 | No difference |
| Korteland 2017 | Actual choice (heart valve prosthesis type) ‐ mechanical vs biological | 67 | 23.9% | 71 | 21.1% | No difference |
| Luan 2016 | Preference (breast reconstruction) ‐ prosthetic vs autologous or combined | 8 | 2 (25%) | 8 | 1 (13%) | No difference |
| Surgery ‐ elective more minor surgery | ||||||
| Carroll 2017 | Actual choice (ICD replacement) | 41 | 24 | 41 | 24 | No difference |
| Lewis 2021 | Actual choice (ICD replacement)
|
14 13 12 |
0 4 8 |
15 15 14 |
0 7 10 |
No difference |
| Wallace 2021 | Actual choice (ICD implanted) | 15 | 6 | 6 | 4 | No difference |
| Hanson 2011 | Actual choice (feeding tube) | 127 | 1 | 129 | 3 | No difference |
| Love 2016 | Preference (skin cancer)
|
13 | 50.0% 33.3% 16.7% |
16 | 62.5% 25.0% 6.25% |
No difference |
| Wilkens 2019 | Preference (trapeziometacarpal arthritis) | 45 | 3 | 45 | 3 | No difference |
| Wong 2006 | Actual choice (abortion) | — | — | — | — | No difference |
| Ye 2021 | Preference (cataract surgery) ‐ definitely or likely | 386 | 87 | 387 | 132 | Intervention decreased preference for surgery. P < 0.001 |
| Screening ‐ breast cancer genetic testing | ||||||
| Miller 2005 | Preference | — | — | — | — | Intervention decreased intention for genetic testing in women at average risk; increased in women at high risk |
| Screening ‐ breast screening | ||||||
| Mathieu 2010 | Preference of women who were decided | 96 | 52% | 127 | 65% | P = 0.05 |
| Screening ‐ cardiac stress testing | ||||||
| Hess 2012 (in consult) | Actual choice | 101 | 58% | 100 | 77% | P < 0.001 |
| Screening ‐ colorectal cancer | ||||||
| Perestelo‐Perez 2019 | Preference
|
53 | 96.2% 94.3% |
52 | 86.5% 84.6% |
No difference between groups after correcting for attenuation |
| Screening ‐ cervical cancer | ||||||
| Elliott 2022 | Actual choice | 3080 | 35.8% | 4402 | 37.7% | No difference P = 0.55 |
| Screening ‐ diabetes | ||||||
| Marteau 2010 | Actual choice | 633 | 353 | 639 | 368 | P = 0.51 |
| Mann E 2010 | Preference | 273 | — | 134 | — | No difference |
| Screening ‐ lung cancer | ||||||
| Elliott 2022 | Actual choice | 459 | 20.2% | 781 | 23.6% | No difference P = 0.55 |
| Volk 2020 | Actual choice | 67 | 85.1% | 85 | 80.0% | No difference P = 0.60 |
| Screening ‐ prenatal | ||||||
| Bekker 2004 (in consult) | Actual choice | — | — | — | — | No difference |
| Nagle 2008 | Actual choice | — | — | — | — | No difference |
| Screening ‐ prostate cancer testing | ||||||
| Frosch 2008a | Actual choice | — | — | — | — | The experimental interventions led to significant reductions in requests for prostate‐specific antigen tests (~2 times greater decline). |
| Lepore 2012 | Actual choice 2 years postintervention |
215 | 62.7% | 216 | 66.7% | No difference Exp (B) = 0.829 CI 95% 0.564 to 1.218 |
| Williams 2013 | Actual choice | — | — | — | — | No difference (P > 0.3) |
| Lepore 2012 | Preference | 215 | 80.9% | 216 | 80.1% | No difference Exp (B) = 0.994 95% CI 0.614 to 1.610 |
| Diagnostic testing ‐ cardiac testing for chest pain | ||||||
| Hess 2016 | Actual choice | 451 | 38.1% | 447 | 45.6% | P = 0.013 |
| Diagnostic testing ‐ computerized tomography (CT) scan for brain injury | ||||||
| Hess 2018 | Actual choice | 493 | 22% | 478 | 24% | No difference |
| Diagnostic testing ‐ prenatal genetic testing | ||||||
| Kuppermann 2014 | Invasive diagnostic testing without screening test | 357 | 11 (3.0%) | 353 | 16 (4.6%) | P = 0.37 |
| Screening test followed by invasive diagnostic test | 357 | 10 (2.9%) | 353 | 27 (7.7%) | Not reported | |
| Medication ‐ antibiotics for upper respiratory infections | ||||||
| Legare 2011 (in consult) | Actual choice | 81 | 33 | 70 | 49 | P = 0.08 |
| Legare 2012 (in consult) | Actual choice | — | 27.2% | — | 52.2% | Absolute difference 25.0; RR 0.5 (95% CI 0.3 to 0.7) |
| Medication ‐ atrial fibrillation anti‐thrombosis ‐ uptake | ||||||
| Man‐Son‐Hing 1999 | Actual choice | — | — | — | — | 25% decrease in DA group, not statistically significant |
| McAlister 2005 | Actual choice | — | — | — | — | No difference |
| Schott 2021 | Actual choice | — | — | — | — | No difference |
| Thomson 2007 (in consult) | Actual choice | — | 93.8% | — | 25% | RR 0.27 (95% CI 0.11 to 0.63) |
| Medication ‐ autoimmune disease | ||||||
| Fraenkel 2015 | Actual choice (rheumatoid arthritis) |
60 | 73% | 61 | 72% | No difference |
| Singh 2019 | Preferred choice (lupus nephritis) |
151 | 72.9% | 147 | 59.9% | P = 0.01 |
| Medication ‐ breast cancer prevention | ||||||
| Crew 2022 | Actual choice | 115 | 3 | 131 | 5 | No difference |
| Fagerlin 2011 | Actual choice | 383 | 0.5% | 102 | 0% | No difference |
| Medication ‐ cardiovascular disease prevention | ||||||
| Bonner 2022 | 1 (strongly disagree) to 7 (strongly agree)
|
285 | 4.7 (1.2) 2.5 (1.4) 3.1 (1.6) |
290 | 4.5 (1.4) 2.5 (1.4) 3.2 (1.6) |
No difference No difference No difference |
| Sheridan 2011 | DA versus usual care. Any effective CHD risk reducing strategy | 79 | 63% | 78 | 42% | Absolute difference 21%, 95% CI 5 to 37 |
| Blood pressure medication, if hypertensive (n = 55) | — | 26% | — | 29% | Absolute difference ‐3%, 95% CI ‐30 to 25 | |
| Cholesterol medication, if abnormal cholesterol (n = 69) | — | 39% | — | 9% | Absolute difference 30%, 95% CI 14 to 46 | |
| Smoking cessation, if smoking (n = 21) | — | 80% | — | 50% | Absolute difference 30%, 95% CI ‐16 to 76 | |
| Aspirin, if CHD risk > 6% (n = 140) | — | 43% | — | 24% | Absolute difference 19%, 95% CI ‐1 to 39 | |
| Diet low in saturated fat | 79 | 29% | 78 | 40% | Absolute difference ‐11%, 95% CI ‐27 to 6 | |
| Regular exercise | 79 | 53% | 78 | 54% | Absolute difference ‐1%, 95% CI ‐17 to 16 | |
| Medication ‐ chemotherapy | ||||||
| Leighl 2011 | For advanced cancer | 107 | 77% | 100 | 71% | No difference |
| Oostendorp 2017 | For advanced cancer | 57 | 88% | 31 | 84% | No difference |
| Whelan 2003 (in consult) | For early breast cancer | — | — | — | — | No difference |
| Wyld 2021 | For adjuvant therapy | 526 | 69 | 547 | 99 | P = 0.013 |
| Medication ‐ diabetes management insulin | ||||||
| Mathers 2012 | Preference for insulin | 92 | 18.5% | 78 | 11.5% | P = 0.41 |
| Medication ‐ hypertension | ||||||
| Montgomery 2003 | Uptake | — | — | — | — | No difference |
| Medication ‐ menopausal symptom treatment | ||||||
| Murray 2001b | Uptake hormone therapy | — | — | — | — | 8% decrease in DA group, not statistically significant |
| Legare 2008a | Preference for natural health products | 41% | 41% | No difference | ||
| Medication ‐ multiple sclerosis immunotherapy | ||||||
| Kasper 2008 | Uptake | — | — | — | — | No difference |
| Medication ‐ osteoporosis | ||||||
| LeBlanc 2015 (in consult) | Preference Prescription during encounter |
29 29 |
12 (41%) 13 (41%) |
38 38 |
11 (29%) 12 (27%) |
P = 0.57 P = 0.2 |
| Montori 2011 (in consult) | Uptake | 52 | 44% | 48 | 40% | No difference |
| Smallwood 2017 | Uptake | 29 | 15.4% | 21 | 50.0% | No difference P = 0.111 |
| Medication ‐ pain | ||||||
| Omaki 2021 | Prescription during encounter
|
65 | 18% 37% 45% |
59 | 20% 34% 46% |
No difference P = 0.93 |
| Mental health treatment | ||||||
| Fisher 2020 | Uptake medication and/or psychoeducation | 77 | 61.0% | 71 | 67.6% | No difference |
| Hamann 2006 | Uptake prescribed medication Uptake psychoeducation |
— — |
— — |
— — |
— — |
No difference Higher uptake in DA group (P = 0.003) |
| Mott 2014 | Uptake of 9 psychoeducation sessions | 9 | 44% | 11 | 9% | All 4 decision aid participants received 9 or more sessions. 1 of 5 usual care received 9 or more sessions. |
| Perestelo‐Perez 2017 | Preference
|
64 | 5 15 42 |
79 | 7 13 49 |
No difference |
| Watts 2015 | Uptake evidence‐based treatment | 63 | 75% | 65 | 57% | P = 0.04 |
| Treatment ‐ dialysis | ||||||
| Subramanian 2019 | Preference
|
53 | 42.8% 36.5% 4.8% |
42 | 22.9% 31.4% 5.7% |
No difference after removing those who were undecided |
| Treatment ‐ obstructive sleep apnea | ||||||
| Bergeron 2018 | Actual choice
|
24 | 4.2% 20.8% 16.7% 58.3% |
26 | 11.6% 23.1% 19.2% 46.2% |
No difference P = 0.86 |
| Treatment ‐ skin disorder | ||||||
| McLean 2020 | Preference (hidradenitis suppurativa ‐ many treatment options e.g. none, topical, systemic, biological, laser, etc.) | 18 | — | 16 | — | No difference in preferred treatment options between groups |
CI : confidence interval; CHD : congenital heart disease; DA : decision aid; ICD : implantable cardioverter defibrillator; OR : odds ratio; RR : risk ratio.
13. Choice (continued).
| Study | Type of comparison | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Obstetrics ‐ birth control method | ||||||
| Langston 2010 | Preference | 114 | — | 108 | — | No difference in the methods chosen between groups; participants in the intervention group were not more likely to initiate the requested method immediately compared to those in the usual care group (OR 0.65, 95% CI 0.31 to 1.34) |
| Madden 2020 | Uptake | 161 | — | 80 | — | No difference in the methods chosen between groups |
| Stephenson 2020 | Uptake long‐acting reversible contraception | 349 | 30.4% | 364 | 31.0% | No difference |
| Tebb 2021 | Uptake non‐barrier contraception
|
257 295 |
63.0% 62.7% |
359 379 |
44.8% 43.8% |
Greater increase in uptake for DA group from baseline at 3 months (P = 0.04) and 6 months (P = 0.005) |
| Obstetric ‐ childbirth procedure | ||||||
| Chen S 2021 | Uptake ‐ vaginal birth after cesarean | 29 | 3 | 30 | 3 | No difference |
| Kuppermann 2020 | Uptake ‐ vaginal birth after cesarean | 727 | 231 | 732 | 233 | No difference |
| Montgomery 2007 | Uptake | — | — | — | — | No difference |
| Nassar 2007 | Uptake | — | — | — | — | No difference |
| Shorten 2005 | Preference | — | — | — | — | No difference |
| Wise 2019 | Attempted vaginal birth after cesarean | 146 | 56.9% | 148 | 60.8% | No difference |
| Obstetric ‐ embryo preservation | ||||||
| Ehrbar 2019 | Preference | 24 | 91.7% | 27 | 55.6% | P = 0.014 |
| Obstetric ‐ embryo transplant | ||||||
| Van Peperstraten 2010 | Uptake ‐ single embryo transfer | 152 | 43% | 156 | 32% | P = 0.05 |
| Obstetric ‐ rooming‐in | ||||||
| Wang 2021 | Actual choice
|
75 | 88.0% 10.7% 1.3% |
75 | 76.0% 8.7% 5.3% |
No difference P = 0.129 |
| Other ‐ organ transplant | ||||||
| Gordon 2017 | Kidney ‐ willingness to accept increased risk donor kidney (1 to 5 scale; lower scores reflect greater willingness) | 133 | 2.57 (95% CI 2.34 to 2.81) | 155 | 2.78 (95% CI 2.58 to 2.97) | No difference P = 0.22 |
| Patzer 2018 | Kidney ‐ living donor inquiry, placement on transplant waiting list, receipt of a living or deceased donor transplant | 226 | — | 217 | — | No difference P = 0.49 |
| Vandemheen 2009 | Lung transplant referral | — | — | — | — | No difference |
| Other ‐ pre‐operative blood transfusion | ||||||
| Laupacis 2006 | Uptake | — | — | — | — | No difference |
| Other ‐ pelvic organ prolapse treatment | ||||||
| Brazell 2014 | Uptake | — | — | — | — | No difference; P = 0.835 |
| Other ‐ thyroid cancer adjuvant radioactive iodine treatment | ||||||
| Sawka 2012 | Preferred treatment immediately post | 37 | 35.1% | 37 | 32.4% | — |
| Uptake at follow‐up (~ 6.3 months post) | 37 | 29.7% | 37 | 18.9% | No difference (Chi 2 = 1.18; df = 1; P = 0.28) | |
| Vaccines | ||||||
| Chambers 2012 | Uptake flu shot | 48 | 46% | 59 | 27% | No difference |
| Clancy 1988 | Uptake hepatitis B | — | — | — | — | Significant increase of 76% in the DA group |
| Lin 2020 | Uptake rotavirus vaccine | 90 | 79 | 90 | 64 | P = 0.01 |
| Saunier 2020 | Uptake flu shot | — | 38.7% (95% CI 36.5 to 40.9) | — | 31% (95% CI 28.7 to 33.3) | P < 0.005 |
| Shourie 2013 | Measles, mumps, rubella in infant | 48 | 48 (100%) | 71 | 70 (99%) | No difference |
CI : confidence interval; DA : decision aid; OR : odds ratio
Choice for major elective surgery
Of 209 studies, 38 (18.2%) studies focused on choices regarding major elective surgery, defined as typically requiring general anesthetic. The effects of patient decision aids on choosing surgery over a conservative option were variable depending on the surgery type ( Analysis 11.1 ). When two or more studies evaluated the same surgery type, fewer patients chose major elective surgery over conservative options when exposed to patient decision aids versus usual care during decision‐making for implantation of left ventricular assist device (RR 0.75, 95% CI 0.60 to 0.93; 3 studies) ( Analysis 11.1 ) or for undergoing coronary revascularization (RR 0.76, 95% CI 0.62 to 0.94; 2 studies) ( Analysis 11.1 ). The use of patient decision aids did not have an effect on choice for other surgery types (breast cancer, joint replacement, upper extremity conditions, prostate cancer, benign prostatic hyperplasia, abdominal aortic aneurysm, renal stone treatment, bariatric surgery, and menorrhagia) ( Analysis 11.1 ). Subanalysis without six studies rated as high risk of bias showed the same direction of effect (RR 0.91, 95% CI 0.86 to 0.97; 32 studies) ( Analysis 11.4 ).
Choice for prostate‐specific antigen screening
Of 209 studies, 13 (6.2%) studies focused on choice regarding prostate‐specific antigen (PSA) screening with 11 studies pooled ( Analysis 11.2 ) and two studies not pooled ( Table 13 ). Fewer patients chose PSA screening when exposed to patient decision aids as compared to usual care (RR 0.89, 95% CI 0.81 to 0.99; 11 studies) ( Analysis 11.2 ). Subanalysis without one study at high risk of bias showed the same direction of effect (RR 0.88, 95% CI 0.77 to 0.99; 10 studies) ( Analysis 11.5 ).
Choice for colorectal cancer screening
Of 209 studies, 18 (8.6%) studies reported preferences or uptake rates for colorectal cancer screening with 17 studies pooled and one not pooled ( Table 13 ). More patients chose colorectal cancer screening when exposed to patient decision aids as compared to usual care (RR 1.22, 95% CI 1.07 to 1.41; 17 studies) ( Analysis 11.2 ). Subanalysis without two studies at high risk of bias showed the same direction of effect (RR 1.17, 95% CI 1.02 to 1.35; 15 studies) ( Analysis 11.5 ).
Choice for cancer genetic screening
Of 209 studies, five (2.4%) studies reported preferences or uptake rates for breast cancer genetic screening, with four studies pooled and one not pooled ( Table 13 ). There was no difference in screening rates among patients who used a patient decision aid as compared to those who did not (RR 1.04, 95% CI 0.77 to 1.39, 4 studies) ( Analysis 11.2 ). None of the studies were rated as high risk of bias and all continued to be included in the subanalysis ( Analysis 11.5 ).
Choice for breast screening
Of 209 studies, eight (3.8%) studies reported preferences or uptake rates for breast cancer screening with seven studies pooled and one not pooled ( Table 13 ). Fewer patients chose mammography screening when exposed to a patient decision aid as compared to usual care (RR 0.97, 95% CI 0.94 to 0.99, 7 studies) ( Analysis 11.2 ). A subgroup analysis without two studies rated as high risk of bias showed no difference in patients who chose mammography screening (RR 0.94, 95% CI 0.89 to 1.00; 5 studies) ( Analysis 11.5 ).
Choice for prenatal screening
Of 209 studies, six (2.9%) studies reported preferences or uptake rates for prenatal screening with four studies pooled and two not pooled ( Table 13 ). There was no difference in screening rates among patients who used a patient decision aid as compared to those who did not (RR 1.03, 95% CI 0.95 to 1.10; 4 studies) ( Analysis 11.2 ). None of the studies were rated as high risk of bias and all continued to be included in the subanalysis ( Analysis 11.5 ).
Choice for diabetes treatment with new medications
Of 209 studies, seven (3.3%) studies reported preferences or uptake rates for starting new medications for diabetes with six studies pooled and one not pooled ( Table 13 ). There was no difference in preference or uptake rates for starting new anti‐diabetic medications among patients who used a patient decision aid as compared to those who did not (RR 2.43, 95% CI 0.64 to 9.17; 6 studies) ( Analysis 11.3 ). Subanalysis without two studies rated as high risk of bias showed increased preferences or uptake rates for starting new medications for diabetes (RR 1.65, 95% CI 1.06 to 2.56; 4 studies) ( Analysis 11.6 ).
Confidence in decision‐making
Of 209 studies, 27 (12.9%) studies measured the effect of patient decision aids on confidence in decision‐making using the Decisional Self‐efficacy Scale (n = 13), COMRADE (n = 2), or a range of other measures (see Table 15 ). A subanalysis of six studies that used the Decisional Self‐efficacy Scale ( O'Connor 2002 ) showed no difference between groups (MD 2.49 out of 100, 95% CI 0.03 to 4.95) ( Analysis 12.1 .1). A subanalysis of six studies that used other measures showed that patient decision aids were more effective than usual care for increasing patient confidence in decision‐making (MD 7.36, 95% CI 2.67 to 12.05; 6 studies) ( Analysis 12.1 .2). When studies assessed as high risk of bias were removed, the findings were similar ( Analysis 12.2 ). There was no difference between older and newer studies ( Analysis 12.3 ).
14. Confidence.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Aoki 2019 (in consult) | COMRADE confidence subscale | Post‐intervention | 32 | Median 41 (6 IQR) | 53 | Median 37 (7 IQR) | P = 0.005 |
| Arterburn 2011 | Decisional self‐efficacy | Changes from baseline | 75 | + 3.0 (95% CI 0.6 to 5.4) | 77 | + 2.8 (95% CI 0.9 to 4.8) | No difference P = 0.78 |
| Bailey 2016 | Decisional self‐efficacy scale (change from baseline to follow‐up) | 4 to 6 weeks after enrollment | 114 | 3.7 (SD 16.7) | 111 | ‐3.9 (SD 19.2) | P = 0.0018 |
| Chambers 2012 | Mean confidence with decision: scale from 1 (low confidence) to 5 (high confidence) | Post‐intervention | 48 | 4 | 59 | 3.6 | P = 0.02 |
| Fraenkel 2007 | Decisional self‐efficacy scale | Pre‐consultation | 43 | 32 (median) | 40 | 27 (median) | P = 0.001 |
| Fraenkel 2015 | COMRADE confidence subscale | 2 and 8 weeks post‐intervention | — | — | — | — | No difference |
| Gattellari 2003 | Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | 3 days post | 106 | — | 108 | — | P = 0.008; DA group more likely to agree that they could make an informed choice about PSA screening |
| Gattellari 2005 | Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | Immediately post | 131 | — | 136 | — | No difference |
| Hoffman 2017 | 12‐item Patient Self‐Advocacy Scale (Yes = 1, Unsure = 2, No = 3), summed, and divided by 12 for an average score, with lower scores indicating greater self‐advocacy | Immediately post‐intervention | 58 | 1.6 (SD 0.3) | 28 | 1.8 (SD 0.3) | P = 0.01 |
| Krishnamurti 2019 | Decisional self‐efficacy scale ‐ change from baseline | 3 months post‐intervention | 23 | — | 18 | — | Study reports higher change for DA group P = 0.05 |
| 6 months post‐intervention | 23 | — | 18 | — | P = 0.06 | ||
| Kukafka 2022 | Decisional self‐efficacy scale ‐ change from baseline | 1 month post‐intervention | 101 | 1.6 (10.6) | 86 | 0.4 (12.8) | No difference P = 0.52 |
| 6 months post‐intervention | 88 | 0.0 (12.0) | 75 | ‐0.6 (13.8) | No difference P = 0.89 |
||
| Lin 2020 (in consult) | Information provided can help me to have more confidence in deciding whether or not to let the baby receive the vaccine (1 to 5; strongly disagree to strongly agree) | Post‐intervention | 90 | 4.58 (SD 0.65) | 90 | 3.76 (SD 0.96) | P < 0.001 |
| Manne 2020 | Confidence in the decision made rated on a scale from 0 (not confident at all) to 10 (extremely confident) | 2 to 4 weeks after surgery | 46 | 9.1 (SE 0.35) | 47 | 8.5 (SE 0.33) | Small to moderate effect size (Cohen's d ‐0.30) |
| Confidence in the ability to manage worries and uncertainty (e.g. recurrence, future surveillance) | 2 to 4 weeks after surgery | 46 | 4 (SE 0.16) | 47 | 3.85 (SE 0.14) | Small effect size (Cohen's d ‐0.16) |
|
| McBride 2002 | Confidence with ability to understand outcomes of hormone therapy, make a decision, engage in discussion with practitioner, 3 items (0 to 10; low to high confidence) | 1 month post | 273 | 78% (18% SD) | 284 | 70% (19% SD) | P < 0.001 |
| 9 months post | 261 | 80% (17% SD) | 278 | 75% (20% SD) | P = 0.0004 | ||
| Meade 2015 | Decision Self‐efficacy (Arthritis self‐efficacy scale (ASES) Scores range from 1 to 10 with higher scores indicating higher levels of self‐efficacy) | Baseline | 78 | 5.43 (SD 1.87) | 66 | 5.74 (SD 2.00) | |
| 2 to 4 weeks post‐intervention | 78 | 5.81 (SD 1.92) | 66 | 5.56 (SD 2.03) | P = 0.030 | ||
| Miller 2018 | Self‐efficacy to complete CRC screening with a 1‐item validated instrument from Vernon et al 1997 (scale not reported) | Post‐intervention | 223 | 3.89 (0.84) | 227 | 3.64 (1.00) | P = 0.004 |
| Politi 2020a | Patient Activation Measure (PAM 13) ‐ 3 of 13 items:
|
Post‐intervention | 57 | 96.5% | 60 | 98.3% | P = 0.612 |
| Post‐intervention | 57 | 100% | 60 | 96.7% | P = 0.496 | ||
| Post‐intervention | 57 | 98.3% | 60 | 83.3% | P = 0.009 | ||
| Perez‐Lacasta 2019 | Confidence in the decision made: 3 questions with 5 response options ranging from 1 = very little to 5 = very much | 2 to 4 weeks post‐intervention | 203 | 4.23/5 (SD 0.83) | 197 | 4.2/5 (SD 0.86) | No difference P = 0.761 |
| Smith 2010 | 3 items adapted from the Decisional Self‐efficacy Scale | 2‐week follow‐up | 357 | 4.67 (0.54 SD) | 173 | 4.61 (0.62 SD) | No difference P = 0.26 |
| Tebb 2021 | Study specific 3‐items: "How confident are you that you can: (1) “talk to your doctor about what birth control method(s) to use?” (2) “use birth control correctly so you do not get pregnant?” and (3) “have the information you need to choose the most appropriate birth control method for you?”" (0 = not at all confident to 10 = completely confident; total score range = 0 to 30) | 48 hours post‐intervention | 320 | 25.2 (SD 5.1) | 437 | 23.0 (SD 6.4) | Greater increase from baseline for DA group but not controls |
| 3 months post‐intervention | 282 | 25.2 (4.9) | 379 | 23.4 (6.1) | No difference | ||
| 6 months post‐intervention | 292 | 26.1 (4.4) | 379 | 23.4 (6.0) | DA group reported greater increase from baseline (P = 0.01) | ||
| Ye 2021 | Decision Self‐efficacy Scale | 2 weeks post‐intervention | 371 | Mean 73.5 | 376 | Mean 72.4 | No difference P = 0.33 |
CI : confidence interval; CRC : colorectal cancer; DA : decision aid; IQR : interquartile range; PSA : prostate‐specific antigen; SD : standard deviation; SE : standard error.
12.1. Analysis.

Comparison 12: Confidence, Outcome 1: Confidence ‐ all studies
12.2. Analysis.

Comparison 12: Confidence, Outcome 2: Confidence ‐ studies without high risk of bias
12.3. Analysis.

Comparison 12: Confidence, Outcome 3: Confidence ‐ old vs new studies
Adherence to chosen option
Of 209 studies, 25 (12.0%) measured adherence to the chosen option using various approaches ( Table 16 ). There were mixed results with some positive (n = 5 studies) and/or no difference (n = 20 studies). None of the studies showed a negative effect on adherence.
15. Adherence with chosen option.
| Reference | Scale used | N decision aid | Mean (SD) Decision aid | N comparison | Mean (SD) Comparison | Notes |
| Aoki 2019 (in consult) | 3 months ‐ medication adherence patient‐reported subjectively with VAS (0 to 10) | 22 | Median 9.0 (2.7 IQR) | 22 | Median 9.1 (2.3 IQR) | P = 0.91 No difference in adherence to treatment |
| 6 months VAS (0 to 10) | 44 | Median 9.2 (4.9 IQR) | 44 | Median 8.9 (2.3 IQR) | P = 0.872 No difference in adherence to treatment |
|
| Bergeron 2018 | Post consultation ‐ proportion who contacted the physician to modify their treatment | 24 | 0 (0.0%) | 26 | 4 (15.4%) | Patient DA higher adherence to baseline choice P = 0.04 |
| Karagiannis 2016 (in consult) | No missed medicine in prior week (patient‐reported)
|
70 | 67 (95.7%) | 81 | 69 (85.2%) | P = 0.35 No difference in adherence to treatment |
|
80 | 75 (93.8%) | 63 | 55 (87.3) | P = 0.61 No difference in adherence to treatment |
|
| Langston 2010 | 3 months ‐ using a contraceptive method that was in the same effectiveness group as the method requested at enrolment, 'very effective', as chosen option ‐ e.g. if chose sterilization and ended up using an IUD counted as adhering | 48 | 85% | 52 | 77% | P = 0.28 No difference in adherence to baseline choice |
| 3 months ‐ using a contraceptive method that was in the same effectiveness group, 'effective', as chosen option | 41 | 68% | 31 | 68% | P = 0.96 No difference in adherence to baseline choice |
|
| LeBlanc 2015 (in consult) | Filled prescription (of those who were given prescriptions), n/N (%) | 29 | 10/13 (83%) (1 missing) | 38 | 4/12 (40%) (2 missing) | P = 0.07 No difference in adherence to baseline choice |
| % of days covered out of 180 (median, 95% CI) | 29 | 46.7% (95% CI 39.2 to 46.7) | 38 | 85% (95% CI 55.3 to 92.6) | P = 0.08 No difference in adherence to treatment |
|
| LeBlanc 2015b (in consult) | Filled prescription (of those who were given prescriptions), n/N (%) | 158 | 94/109 (86.2%) (4 missing) | 139 | 82/88 (93.2) (5 missing) | P = 0.19 No difference in adherence to baseline choice |
| Proportion of patients with a percentage of days covered > 80% (of filled prescription) | 158 | 96 (98.0%) | 139 | 85 (97.7%) | P = 0.25 No difference in adherence to treatment |
|
| Legare 2012 (in consult) | 2 weeks post ‐ single question asking if the patient maintained the decision made, n (%) | 163 | 143 (87.7%) | 165 | 150 (91.5%) | Absolute difference 3.8; RR 1.0 (95% CI 0.9 to 1.0) No difference in adherence to baseline choice |
| Lepore 2012 | Congruence between intention to test and verified PSA test ‐ 1 year | 244 | 55.3% | 246 | 58.1% | No difference in adherence to baseline choice (95% CI 0.62 to 1.28) |
| Congruence between intention to test and verified PSA test ‐ 2 year | 244 | 59.0% | 246 | 59.3% | No difference in adherence to baseline choice (95% CI 0.69 to 1.42) | |
| Loh 2007 (in consult) | 6 to 8 weeks ‐ patient‐reported ‐ 5‐point Likert scale on steadiness of following the treatment plan: 1 = very bad to 5 = very good | 191 | 4.3 (0.9) | 96 | 3.9 (1.0) | No difference in adherence to treatment P = 0.073 |
| 6 to 8 weeks ‐ physician‐reported ‐ 5‐point Likert scale steadiness of following the treatment plan: 1 = very bad to 5 = very good | 191 | 4.8 (0.6) | 96 | 4.3 (1.1) | No difference in adherence to treatment P = 0.56 |
|
| Mann D 2010 (in consult) | 3 months ‐ telephone administration of the 8‐item Morisky adherence scale (7 yes/no items and 1 item with 5‐point Likert scale to elicit behaviors such as skipping medicines when they have no symptoms) | — | — | — | — | No difference in adherence to treatment 70% reported good adherence to statins; no difference between groups |
| 6 months ‐ telephone administration of the 8‐item Morisky adherence scale (7 yes/no items and 1 item with 5‐point Likert scale to elicit behaviors such as skipping medicines when they have no symptoms) | — | — | — | — | No difference in adherence to treatment 80% reported good adherence to statins; no difference between groups |
|
| Man‐Son‐Hing 1999 | 6 months ‐ self‐reported. Measured % of participants taking therapy initially chosen. | 129 | 95.35% | 134 | 93.28% | No difference in adherence to baseline choice P = 0.44 |
| Mathers 2012 | 6 months ‐ self‐reported. Measured % of patients who did not change their initially chosen treatment. | 95 | 68.1% | 80 | 56.3% | Patient DA higher adherence to baseline choice P = 0.041 |
| Miller 2018 | 24 weeks ‐ completed ordered colonoscopy | 72 | 44 (61%) | 47 | 25 (53%) | No difference in adherence to baseline choice |
| 24 weeks ‐ completed ordered fecal test | 81 | 21 (26%) | 25 | 8 (32%) | No difference in adherence to baseline choice | |
| Montgomery 2003 | ~ 3 years ‐ self‐reported – 6‐item adherence questionnaire: from 'I take all my tablets at the same time of day' to 'I take hardly any of my tablets' | — | — | — | — | No difference in adherence to baseline choice or adherence to treatment |
| Montori 2011 (in consult) | 6 months ‐ percentage of participants that self‐reported currently taking medication who have not missed 1 dose within last week | 17 | 65% | 19 | 63% | No difference in adherence to treatment P = 0.92 |
| 6 months ‐ percentage of participants who opted to take bisphosphonates who took their medication on more than 80% of the days for which it was prescribed, based on pharmacy records | 23 | 100% | 19 | 74% | Patient DA higher adherence to baseline choice P = 0.009 |
|
| Mott 2014 | 4 months ‐ percentage of participants who engaged in psychotherapy sessions | 9 | 44% | 11 | 45% | — |
| 4 months ‐ number of participants who engaged in 9 or more psychotherapy sessions | 4 | 100% | 5 | 20% | Adherence to treatment | |
| Mullan 2009 (in consult) | 6 months ‐ pharmacy records ‐ days covered (range) | 48 | 97.5% (range 0 to 100) | 37 | 100 (range 73.9 to 100) | Higher adherence to treatment for usual care AMD ‐8.88 (‐13.6% to ‐4.14%) Statistically significant |
| 6 months ‐ self‐reported by telephone call – did not miss a dose in last week | 41 | 76% | 31 | 81% | No difference in adherence to treatment OR 0.74 (95% CI 0.24 to 2.32) |
|
| Oakley 2006 | 4 months ‐ extent to which the participants' behavior in taking medications coincides with the clinical prescription | 16 | 10.4% (32) (improvement from baseline) | 17 | 2% (26) (improvement from baseline) | No difference in adherence to treatment |
| Perestelo‐Perez 2016 (in consult) | 3 months ‐ sometimes forget to take medicine | 56 | 18 (32.1%) | 42 | 15 (35.7%) | No difference in adherence to treatment P = 0.963 |
| 3 months ‐ all pills taken in the last week | 55 | 51 (92.7%) | 42 | 36 (81%) | No difference in adherence to treatment P = 0.189 |
|
| Sheridan 2011 | 3 month ‐ adherence to treatment | |||||
| Any therapy promoted in decision aid | 76 | 45 (59%) | 73 | 25 (34%) | P < 0.01 DA group showed higher adherence to treatment |
|
| Any therapy promoted in decision aid + others (e.g. diet or physical activity) | 77 | 64 (83%) | 77 | 52 (68%) | P = 0.02 | |
| Aspirin | 32 | 30 (94%) | 19 | 11 (58%) | P < 0.01 | |
| Cholesterol medicine | 14 | 12 (86%) | 6 | 5 (83%) | The intervention had little effect blood pressure or cholesterol medication, however, the sample sizes for these estimates were small and underpowered | |
| Blood pressure medicine | 9 | 9 (100%) | 12 | 11 (92%) | ||
| Stop smoking | 8 | 25% | 5 | 20% | No effect on smoking, although subgroups were small and underpowered | |
| Stephenson 2020 | Use of long‐acting reversible contraception at 6 months if using at baseline | 97 | 57 (58.8%) | 104 | 73 (70.2%) | No difference in adherence to baseline choice P = 0.12 |
| Trevena 2008 | 1 month ‐ fecal occult blood test uptake | 134 | 5.2% | 137 | 6.6% | No difference in adherence to baseline choice P = 0.64 |
| Weymiller 2007 (in consult) | 3 months ‐ self‐reported – mailed surveys and telephone call to non‐respondents On adherence to statin use: missed 1 dose or more within the last week |
33 | 93.94% | 29 | 79.31% | No difference in adherence to baseline choice or treatment when analysis adjusted by sex, cardiovascular disease, and number of medications |
| Wilkens 2019 | Change of treatment defined as choosing a more invasive treatment (e.g. change to surgery after nonsurgical treatment) ‐ 6 weeks post enrolment | 45 | 0 (0%) | 45 | 3 (7%) | No difference in adherence to treatment P = 0.24 |
| 6 months post enrolment | 45 | 3 (7%) | 45 | 5 (11%) | No difference in adherence to treatment P = 0.71 |
|
| Wise 2019 | Adherence to baseline choice of delivery mode at 34 weeks gestation (2 to 3 months post‐intervention)
|
146 | 77.0% 81.3% |
148 | 85.2% 87.1% |
No difference in adherence to baseline choice P = 0.5 P = 0.4 |
AMD : absolute mean difference; CI : confidence interval; DA : decision aid; IQR : interquartile range; IUD : intrauterine device; OR : odds ratio; PSA : prostate‐specific antigen; RR : risk ratio; SD : standard deviation; VAS : visual analogue scale
Preference‐linked health outcomes
None of the 209 studies measured preference‐linked health outcomes – that is, whether the patients experienced the outcomes they preferred and avoided the outcomes they wanted to avoid.
Impact on healthcare system
Consultation length
Of 209 studies, 23 (11.0%) examined the effects of patient decision aids on consultation length with 13 studies pooled by timing of intervention and 10 studies not pooled ( Table 17 ). When used in preparation for consultation, there was little to no difference in consultation length for those exposed to a patient decision aid as compared to usual care (MD ‐2.97 minutes, 95% CI ‐7.84 to 1.90; 5 studies) ( Analysis 13.1 ). When the patient decision aid was used during the consultation, the consultation length was 1.50 minutes longer compared to usual care (MD 1.50 minutes, 95% CI 0.79 to 2.20; 8 studies) ( Analysis 13.1 ). More specifically, the consultation was 5.9 minutes longer when the added step of a decision analysis was used for prenatal diagnostic testing decision ( Bekker 2004 ). When studies assessed as high risk of bias were removed, the findings were similar ( Analysis 13.2 ). There was no difference between older and newer studies ( Analysis 13.3 ; Analysis 13.4 ).
16. Healthcare system effects.
| Study | Scale used | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Difference between groups | Notes |
| Consultation length | |||||||
| Aoki 2019 (in consultation) | Recording of initial consultation in minutes | 35 | Median 26 (5 IQR) | 53 | Median 24 (22 IQR) | + 2 minutes | No difference between groups P = 0.983 |
| Bekker 2004 (in consultation) | Consultation length using decision analysis in the consultation (minutes) | 50 | 32.2 (SD 13.0) | 56 | 26.3 (SD 11.5) | + 5.9 minutes | P = 0.01 (longer with decision aid) |
| Krist 2007 | Time spent discussing prostate cancer with practitioner post‐DA (minutes) ‐ patient‐reported | 196 | 5.3 | 75 | 5.2 | + 0.1 minutes | No difference between groups |
| Time spent discussing prostate cancer with practitioner post‐DA (minutes) ‐ physician‐reported | 196 | 3.8 | 75 | 4.2 | ‐0.4 minutes | No difference between groups, but physicians thought they spent less time than patients (P < 0.001) | |
| LeBlanc 2015 (in consultation) | Consultation length with practitioner using DA in consultation (median, range in minutes) | 29 | 11.5 (5.4 to 21.4) | 37 | 10.7 (2.5 to 54.9) | + 0.8 minutes (‐33.6 to 3.0) | — |
| Love 2016 | Duration of time for informed consent discussion with physician (minutes) | 13 | 5.5 site 1 1.4 site 2 |
16 | 4.9 site 1 6.0 site 2 |
0.6 minutes ‐4.6 minutes |
No difference overall |
| Stubenrouch 2022 | Duration of consultation (minutes:seconds) | 191 | Median 16:30 (IQR 11:15 to 22:17) | 151 | Median 12:30 (IQR 08:55 to 17:18) | +4 minutes | P < 0.001 |
| Thomson 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 8 | 44 (39 to 55) | 10 | 21 (19 to 26) | +23 minutes | P = 0.001 Compared computerized decision aid with standard gamble within the consultation to guideline‐driven consultation |
| Vodermaier 2009 | Consultation length with practitioner post‐DA | ||||||
| 5 to 10 min | 53 | 6 (11.3%) | 54 | 5 (9.3%) | — | P = 0.91 | |
| 10 to 15 min | 17 (32.1%) | 19 (35.2%) | — | ||||
| 15 to 25 min | 15 (28.3%) | 14 (25.9%) | — | ||||
| 25 to 35 min | 7 (13.2%) | 5 (9.3%) | — | ||||
| Above 35 min | 8 (15.1%) | 11 (20.4%) | — | ||||
| Whelan 2003 (in consultation) | Consultation length using DA in consultation (minutes) | 50 | 68.3 | 50 | 65.7 | + 2.6 minutes | P = 0.53 |
| Weymiller 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 52 | — | 46 | — | + 3.8 minutes in DA group | Not statistically significant 3.8 min (95% CI ‐2.9 to 10.5) |
| Cost | |||||||
| Hollinghurst 2010 ; Montgomery 2007 | Total costs in the UK for decision about mode of delivery post previous cesarean | 235 | GBP 2019 (SD 741) | 238 | GBP 2033 (SD 677) | — | No difference |
| Kennedy 2002 | Cost‐effectiveness in the UK for decision about benign heavy menstruation | 296 300 |
USD 2026 (DA alone) USD 1556 (DA plus nurse coaching |
298 | USD 2751 | — | Mean differences: DA versus usual care USD 461 (95% CI 236 to 696) DA plus coaching versus usual care USD 1184 (95% CI 684 to 2110) |
| Murray 2001a | Total costs excluding intervention in the UK for decision about treatment of benign enlarged prostate | 57 | GBP 310.3 (SD 602.0) | 48 | GBP 188.8 (SD 300.4) | — | Mean difference GBP 121.5 (95% CI −58.9 to 302.0) |
| Total costs including intervention (interactive video disk equipment) in the UK for decision about treatment of benign enlarged prostate | 57 | GBP 594.10 (SD 602) | 48 | GBP 188.8 (SD 300.4) | — | Mean difference GBP 405.4 (95% CI GBP 224.9 to GBP 585.8) P < 0.001 |
|
| Murray 2001b | Total costs excluding intervention in the UK for decision about hormone replacement therapy | 85 | GBP 90.5 | 84 | GBP 90.9 (SD 39.2) | — | No difference |
| Total costs including intervention (interactive video disk equipment) in the UK for decision about hormone replacement therapy | 85 | GBP 306.5 (SD 42.8) | 84 | GBP 90.9 (SD 39.2) | — | Mean difference GBP 215.5 (95% CI 203.1 to 228.0), P < 0.001 | |
| Shourie 2013 | National Health Service costs (GBP) | 42 | 35.06 (SD 6.4) | 62 | 44.26 (SD 5.25) | Incremental cost ‐9.20 | |
| Societal costs (GBP) | 42 | 42.23 (SD 8.07) | 62 | 48.85 (SD 6.29) | Incremental cost ‐6.62 | ||
| Cost‐effectiveness (GBP) | 42 | 72% chance of being cost‐effective | 62 | 8% chance of being cost‐effective | DA has higher chance of being cost‐effective | ||
| Stacey 2016 ; Trenaman 2017 | Mean per‐patient costs (2016 CAD), by database | 161 | CAD 21,965 | 163 | CAD 23,681 | — | Similar mean per‐patient costs (CAD ‐1716, 95% CI ‐5631 to 2198) |
| Cost‐effectiveness at 2 years | 167 | CAD 7530 (6876 to 8114) | 167 | CAD 8033 (7360 to 8557) | The decision aid arm provided greater quality‐adjusted life‐years per patient (0.05, 95% CI ‐0.04 to 0.13) at a lower cost (CAD ‐560, 95% CI ‐1358 to 426) than the usual care arm | ||
| Van Peperstraten 2010 | Mean total savings per couple in the Netherlands for decision about embryo transfer for in vitro fertilization | — | — | — | — | — | Mean total savings per couple in the intervention group were EUR 169.75 (USD 219.12) |
| Vuorma 2003 | Total estimated costs in Finland for treatment decision about heavy benign menstruation | 184 | EUR 2760 | 179 | EUR 3094 | — | P = 0.1 No difference between intervention and control |
| Healthcare resource use | |||||||
| Cox 2019 | Hospital length of stay (days) | 138 | 42.8 (SD 31.6) | 139 | 39.4 (SD 27.3) | +3.4 days | No difference P = 0.84 |
| Hess 2016 (in consultation) | Repeat emergency department visit | 447 | 39 (9.3%) | 451 | 52 (12.5%) | No difference P = 0.156 |
|
| Readmission to hospital | 447 | 19 (4.5%) | 451 | 20 (4.8%) | No difference P = 0.884 |
||
| Outpatient clinic visit | 447 | 259 (62.0%) | 451 | 266 (64.1%) | No difference P = 0.568 |
||
| Hess 2018 (in consultation) | Emergency department length of stay (minutes) | 493 | 176 (SD 135) | 478 | 199 (SD 162) | ‐23 minutes | P = 0.02 |
| Admitted to hospital | 493 | 9 (2%) | 478 | 9 (2%) | — | P = 0.94 | |
| Emergency department visit within 7 days of discharge | 493 | 10 (2%) | 478 | 18 (4%) | — | P = 0.15 | |
| Ibrahim 2013 | Attended orthopedic consult | 162 | ~57% | 161 | ~50% | — | No difference P = 0.56 |
| Legare 2012 (in consultation) | Repeat consultation for the same reason, n (%) | 163 | 37 (22.7%) | 165 | 25 (15.2%) | Absolute difference 7.5 | RR 1.3 (95% CI 0.7 to 2.3) |
| Shourie 2013 | Resource utilization (actual and intended contacts with the National Health Service) | 42 | — | 62 | — | — | No statistically significant differences between groups |
| Thomson 2007 (in consultation) | GP consultations postintervention | 51 | 39 (76.5%) | 54 | 32 (59.3%) | — | P = 0.35 |
| Hospital appointments postintervention | 51 | 29 (56.9%) | 54 | 10 (18.5%) | — | P = 0.06 | |
| Volk 2020 | Scheduled a consultation to discuss screening within 6 months | 238 | 150 (63.0%) | 238 | 158 (66.4%) | ‐3.4 (‐11.9 to 5.2) | P = 0.47 |
CI : confidence interval; DA : decision aid; GP : general practitioner; IQR : interquartile range; RR : risk ratio; SD : standard deviation; SE : standard error.
13.1. Analysis.

Comparison 13: Consultation length, Outcome 1: Consultation length ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation)
13.2. Analysis.

Comparison 13: Consultation length, Outcome 2: Consultation length ‐ subgroup by timing of intervention ‐ studies without high risk of bias
13.3. Analysis.

Comparison 13: Consultation length, Outcome 3: Consultation length ‐ old vs new studies (in consultation)
13.4. Analysis.

Comparison 13: Consultation length, Outcome 4: Consultation length ‐ old vs new studies (in preparation for consultation)
Cost
Of 209 studies, eight (3.8%) examined costs ( Table 17 ). Three studies reported on cost‐effectiveness analysis ( Kennedy 2002 ; Shourie 2013 ; Stacey 2016 / Trenaman 2017 ) and six evaluated the effect of patient decision aids compared to usual care on total healthcare costs ( Montgomery 2007 / Hollinghurst 2010 ; Murray 2001a ; Murray 2001b ; Stacey 2016 / Trenaman 2017 / Trenaman 2020 ; Van Peperstraten 2010 ; Vuorma 2003 ). For all three cost‐effectiveness analyses, the use of a patient decision aid appeared to be more cost‐effective compared to usual care. Effects of patient decision aids on total healthcare costs mostly showed little to no difference in three of the six studies ( Montgomery 2007 – birth options after Cesarian, Stacey 2016 – surgery for joint replacement, Vuorma 2003 – hysterectomy for benign heavy bleeding). Two studies that used an interactive computer program ( Murray 2001a ‐ benign prostate enlargement; Murray 2001b – hormone replacement therapy) had increased costs, but when the decision aid intervention costs (interactive video disk equipment) were removed, there was little to no difference. Only one study showed significant reduced costs for the decision aid group ( Van Peperstraten 2010 ‐ embryo transfer for in vitro fertilization).
Healthcare resource use
Of 209 studies, eight (3.8%) examined healthcare resource use as related to patient decision aid use, for example outcomes such as the scheduling of initial or repeat consultations, length of hospital stay, and hospital admissions ( Table 17 ). Studies reported little to no difference regarding healthcare resource use, except for Hess 2018 , which reported a reduced length of stay in the emergency department following exposure to the patient decision aid in the consultation (MD 23 minutes; P = 0.02).
3. Heterogeneity across studies
When comparing patient decision aids to usual care, there was statistically significant heterogeneity in the primary outcomes. It should be noted that the heterogeneity of the effect was not manifested in its direction but only in its size.
For the 2009 update ( O'Connor 2009b ), we explored the potential factors contributing to heterogeneity ( Table 18 ). Overall, regardless of the subgroup analyses conducted, scores for outcomes were similar to the overall effect, as indicated by overlapping confidence intervals.
17. Heterogeneity (based on 55 trials in search to 2006).
| Outcome | Overall effect | Treatment decision | Screening decision | Video/computer decision aid | Audio/pamphlet Decision aid | Base risk control | Removal of outliers* |
| Knowledge ‐ decision aid versus usual care | 15.2 (11.7 to 18.7) | 16.5 (11.9 to 21.2) | 13.1 (7.7 to 18.5) | 21.3 (16.3 to 26.2) | 11.9 (8.3 to 15.6) | 15.5 (11.3 to 19.8) | 17.3 (13.6 to 20.9) (* Bekker 2004 , Gattellari 2003 , Johnson 2006 ) |
| Accurate risk perceptions ‐ probabilities versus no probabilities | 1.6 (1.4 to 1.9) | 1.6 (1.4 to 1.9) | 1.6 (1.1 to 2.3) | No data | 1.6 (1.4 to 1.9) | 1.3 (1.2 to 1.5) (P = 0.3) | 1.5 (1.3 to 1.7) (* Gattellari 2003 ) |
| Uninformed subscale of the Decisional Conflict Scale ‐ decision aid versus usual care | ‐8.4 (‐11.9 to ‐4.8) | ‐9.4 (‐13.3 to ‐5.5) | ‐3.5 (‐12.9 to 5.8) | ‐12.6 (‐19.5 to ‐5.8) | ‐4.9 (‐7.6 to ‐2.3) (P = 0.06) | ‐5.4 (‐7.7 to ‐3.2) (P = 0.11) | ‐6.2 (‐8.4 to ‐4.1) (P = 0.06) (* Montgomery 2003 ) |
| Unclear values subscale of the Decisional Conflict Scale ‐ decision aid versus usual care | ‐6.3 (‐10.0 to ‐2.7) | ‐6.0 (‐9.8 to ‐2.3) | Insufficient data | ‐8.0 (‐15.1 to ‐1.0) | ‐4.5 (‐8.4 to ‐0.6) | ‐3.6 (‐6.8 to ‐0.5) | ‐4.0 (‐6.7 to ‐1.3) (* Montgomery 2003 ) |
Discussion
Summary of main results
In this updated review, we added 104 new studies for a total of 209 studies comparing patient decision aids to usual care on a broad range of treatment and screening decisions. Studies were conducted in 19 countries across four continents (Asia, Europe, North America, Australia/Oceania). There was moderate certainty of evidence that patient decision aids likely resulted in better congruence between participants’ informed values for features of options and the choice made. There was high certainty of evidence that patient decision aids compared to usual care resulted in large increases in knowledge, accurate risk perceptions, and participation in decision‐making. There was reduced decisional conflict for subscales of feeling uninformed and unclear values. Overall, these findings indicate higher certainty of evidence for these primary outcomes compared to the previous review ( Stacey 2017 ).
For secondary outcomes, there continues to be variation in the effect of patient decision aids on patients’ choosing particular options. The number of patients choosing to have major elective surgery decreases (with more patients in favor of conservative options), increases in colorectal cancer screening, and decreases in prostate cancer screening; other decisions showed no difference, or variable differences, with and without studies rated as high risk of bias. These variations may be due to some options being underused and others overused at baseline relative to choices patients would make if they were more fully informed, including increased awareness of alternative options and understanding of potential benefits and potential harms/adverse effects across options.
New for this update, we conducted meta‐analysis that revealed that patient decision aids improved satisfaction with the decision‐making process, increased confidence in decision‐making, increased preparation for decision‐making (after a high risk of bias study was removed), increased observer‐reported shared decision‐making, and consultations were no longer when patient decision aids were used in preparation for the consultation, and were only 1.5 minutes longer when they were used during the consultation. No studies demonstrated adverse effects in patients exposed to patient decision aids compared to usual care as indicated by no increased decision regret or emotional distress. There continues to be inadequate evidence on adherence to the chosen option, and healthcare system effects. No studies measured preference‐linked health outcomes. In this update, we conducted subanalyses of pooled data for patient decision aids by older studies published earlier than 2015 and newer studies published from 2015 onwards. We found no difference in outcomes based on publication dates.
Overall completeness and applicability of evidence
We used a highly sensitive search strategy to exhaustively identify as many papers as possible from all relevant databases and also used handsearching. Our search included studies published up until March 2022 and this update doubled the number of included studies since the last publication. It is important to note that patient decision aids are complex interventions minimally defined as including specific elements (e.g. explicit decision, information on options/benefits/harms, implicit or explicit values clarification) and although some articles reported studies of patient decision aids they did not always meet this minimal definition to be included in our review. Another difference across studies is the range of outcome measures used, with some more consistently used (e.g. Decisional Conflict Scale, Decision Regret Scale, OPTION instrument) and others being unique to individual studies (e.g. knowledge test, measure of informed values‐choice concordance). Finally, the decisions and clinical settings within which the decisions are made also varied across studies.
Our review showed that few included trials (< 10%) reported engagement of patient partners. It is possible that patient partners were included on their research team, and this simply was not reported. These findings were consistent with other patient‐oriented intervention trials reporting very poor engagement of patient partners ( Fergusson 2018 ). Patient decision aid trialists should consider including patients and other knowledge user partners on their teams (and report their engagement), as early and meaningful engagement of knowledge users can facilitate and accelerate research findings into clinical practice ( Bowen 2013 ; Gagnon 2009 ).
Despite these differences, patient decision aids improved many attributes of the decision and decision‐making process across a wide variety of populations, and decisions. The largest and most consistent benefits of patient decision aids, relative to usual care, are better knowledge of options and outcomes, more accurate perceptions of outcome probabilities, feeling more informed, and clearer values. These observations are clinically important because these outcomes are important for ensuring informed decision‐making and suggest that current 'usual care' may not be good enough for supporting patients in the process of making these complex, values‐sensitive decisions. Patients need to comprehend the options and their associated benefits and harms in order to consider and communicate to their clinicians the personal value they place on the benefits versus the harms of the options. Furthermore, uninformed decisions indicate that these patients are not providing informed consent for the chosen option (if they proceed with it). In fact, patient decision aids make an imperfect ‘informed consent’ process better ( Spatz 2016 ). With the recent rise of misinformation and its potential negative impact on patients ( Bachtiger 2021 ; Stacey 2023 ), patient decision aids are a valuable tool to counter this misinformation by providing balanced, evidence‐based health information on all relevant options. Patient decision aids can be of benefit to vulnerable patient populations (e.g. including those with limited health literacy, lower socioeconomic status, racial/ethnic minorities), who benefit from using patient decision aids that can lead to advanced health equity ( Durand 2014 ; Grabinski 2018 ; Turkson‐Ocran 2021 ).
Compared to usual care, patient decision aids improved patients’ perception of involvement in decision‐making and observer evidence of shared decision‐making. These observations continue to suggest that the IPDAS criterion of helping patients participate "in ways that they prefer" needs to be assessed after a patient has adequate information about what involvement means using interventions such as patient decision aids. Clinicians may mistakenly assume that patients’ passivity in decision‐making is because they believe that the best choice relies on the expertise of the clinician (which option is medically reasonable?) rather than patients’ recognition of their own preferences for the features and outcomes of options (which outcomes matter most to me?). Yet in fact, both perspectives are necessary for achieving a quality evidence‐based health decision.
This update included more studies reporting the length of consultation and showed no difference in consultation length when patient decision aids were used in the preparation for the consultation. Yet, consultations were 1.5 minutes longer when it was used in consultation. This increase may be explained by the learning curve associated with using a patient decision aid in the consultation during these effectiveness studies.
The effect of patient decision aids on patients’ choosing of particular options continues to be variable. There may be several reasons for the variable effect of patient decision aids on the outcome of choices. First, these findings reflect the nature of preference‐sensitive decisions and we should expect variability in patient choices overall. Second, not enough is known about baseline rates for optimal use of specific options for specific decisions. Third, for studies reporting the outcome ‘choices’ at baseline and post‐patient decision aid, some options may have been under‐used and others over‐used, relative to the choices individuals would make if they were more fully informed. Under these circumstances, one could expect to observe directional effects on choices once patients become better informed and more involved in decision‐making.
Unknown effects of patient decision aids
Research is required to establish ways of measuring preference‐linked health outcomes to better determine the effect of patient decision aids on quality of life. Given health outcomes are part of the quintuple aim (e.g. patient outcomes, patient experience, clinician experience, efficiency, equity) ( Nundy 2022 ), and that different options can lead to different impacts on quality of life, this is an important research priority.
Other outcomes that require further research include adherence and cost‐effectiveness. When examining adherence, it would be important to do so in the early phase, when presumably the issue is actually decisional in nature (e.g. filling the prescription, picking up the prescription, refilling the prescription) rather than a situation whereby patients with chronic conditions revisit their decisions that may involve choosing to change or remain with the status quo, or challenges with adding a new option to their daily routine that may require other interventions such as motivational interviewing. Our update found few new studies that reported on costs or cost‐effectiveness; these findings are consistent with a previous paper reporting on this outcome ( Trenaman 2014 ). Although there may be additional costs involved in delivering patient decision aids, a clinical practice guideline on the patient experience reports that any increase is small relative to the benefit to patients in terms of improved decision quality and improved decision‐making processes when effective patient decision aids are used ( NCGC/NICE 2012 ).
Quality of the evidence
The certainty of evidence for key outcomes in Table 1 according to GRADE ranged from moderate to high. Most studies were judged as having minimal risk of bias. When the subanalysis was conducted by removing the few studies rated as high risk of bias, the direction of effect was consistent for primary outcomes and for most secondary outcomes . For informed values‐choice congruence, the GRADE rating was downgraded for potential publication bias. It is unclear the extent to which there is publication bias for this one primary outcome. Therefore, we used a cautious approach and downgraded the certainty of evidence. In addition, given that this outcome is more challenging to measure, it is more likely that it is not measured in most studies rather than not reported.
Several of the outcomes demonstrated considerable levels of heterogeneity. This reflects differences across clinically diverse studies, interventions and comparators; therefore, the pooled effect size and confidence intervals should be interpreted as a range across conditions, which may not be applicable to a specific condition. In Gentles 2013 , three potential sources of heterogeneity were explored: type of control intervention, patient decision aid quality score using IPDAS, and participants' baseline accurate risk perception, and it was found that participants' baseline accurate risk perception was an important variable for explaining heterogeneity. For example, heterogeneity would be expected for the outcome of knowledge, given that the knowledge tests themselves were not standardized. However, we did not downgrade the certainty of evidence for inconsistency since there was a consistent direction of findings across studies.
Potential biases in the review process
We followed standard procedures for conducting the systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins 2022 ). Our update showed continued poor‐quality reporting of the patient decision aid intervention and comparators ( Lewis 2017 ). However, we tried to obtain copies of patient decision aids and comparators when possible and only excluded trials if it was not possible to determine if the intervention was a patient decision aid. Other potential biases in the review process are due to limitations associated with having inadequate power to investigate any differences associated with the type of comparator used in studies. Measures for some outcomes were diverse, which may have biased those review findings. Finally, we limited the extracted study data to only two comparison groups (e.g. most intensive intervention including a patient decision aid and usual care); therefore, we did not investigate the possibility of intermediate effects with less intensive patient decision aid interventions.
Agreements and disagreements with other studies or reviews
Our results confirm many of the observations reported in the previous update ( Stacey 2017 ), and in a comparative effectiveness review that focused on studies evaluating oncology‐specific patient decision aids ( Trikalinos 2014 ). There have also been several systematic reviews of patient decision aids for specific clinical areas ( Irish 2023 ; Lin 2009 ; O'Neill 2017 ; Scalia 2019 ). These other clinically specific reviews typically include a broader range of study designs and some include any intervention with the title of patient decision aid without verifying that the intervention met the IPDAS definition of a patient decision aid. This makes it difficult to compare our results to other reviews. Our findings for consultation length were consistent with a systematic review focused on the duration of medical consultations for shared decision‐making in 63 studies (e.g. RCTs, quasi‐experimental studies, cross‐sectional studies) ( van Veenendaal 2022 ). These authors concluded that applying shared decision‐making does not necessarily require longer consultations and suggested that multi‐level implementation approaches can mitigate the possibility of increased consultation lengths. Furthermore, with greater opportunity for training and adaptation of work processes, clinicians get used to using them, which may eventually result in reduced or neutral time ( van Veenendaal 2022 ). A recent large‐scale implementation study titled ‘Share to Care’ included multi‐level implementation interventions in 22 clinics (e.g. 80 patient decision aids, training of healthcare professionals, campaign to activate patient participation in decision‐making, decision coaching) reported that consultation time initially increased and then decreased as clinicians’ skills in shared decision‐making improved ( Geiger 2022 ).
Authors' conclusions
Implications for practice.
Over the last 20 years during which this review has been updated, there continue to be positive effects of patient decision aids on the quality of the decision and decision‐making process across a wide variety of decisions, indicating sufficient evidence for using them in clinical practice. Findings from this latest update demonstrate that patient decision aids lead to large increases in knowledge, accurate risk perceptions, and patient participation in decision‐making, enriching the evidence base for using them in clinical practice.
According to the results of this review update, patient decision aids satisfy four of five elements in the quintuple aim, including better patient experiences, better patient outcomes, reduced inequities, and improved clinician experiences. Further research is required to determine higher efficiency. As reported in this review version, there is moderate to high certainty of evidence that patient decision aids improve patient decision‐making outcomes and experiences. Observer evidence reported in this update demonstrates that patient decision aids facilitate shared decision‐making between patients and their clinicians and, as such, influence clinician experiences.
However, few patient decision aids were used in clinical practice as revealed in a survey of investigators of studies that were included in the previous review versions we conducted ( Stacey 2014b ; Stacey 2017 ; Stacey 2019 ). The most common barriers reported by study authors were lack of funding or infrastructure support, outdated patient decision aids, lack of a mechanism for delivery, clinicians disagreeing with their use, and lack of a post‐trial plan. Facilitators to patient decision aid use were online delivery, end users on the development team, endorsement by organizations or clinical practice guidelines (e.g. government, charities, professional organizations), clinician awareness, training for clinicians, integration in the process of care, and leadership support.
Implications for research.
Studies are needed to assess the impact of patient decision aids on adherence and downstream effects on cost and resource use. Although there is some evidence that patient decision aids can improve outcomes for patients with lower health literacy and reduce biases by race/ethnicity ( Durand 2014 ; Grabinski 2018 ), further research is required to reduce health inequalities, with a particular focus on equity‐deserving groups. National granting agencies now encourage researchers to use equity, diversity, inclusion, and social justice lenses. Having stronger data demonstrating that patient decision aids can be used to improve health equity or reduce inequities may be the evidence required to further support their use in clinical practice and healthcare systems.
Our update included new studies conducted in Denmark, France, Japan, Greece, Italy, Malaysia, New Zealand, Switzerland, and Turkey, but studies continue to be conducted across four continents. The update did not find any trials from resource‐limited countries, including those in Africa ( Gogovor 2022 ).
Research should also explore the influence of specific elements included in patient decision aids on outcomes. For example, determine if specific elements or their format minimize patients’ cognitive processing, improve outcomes for individuals with lower health literacy, or improve their use in practice.
Further research needs to be conducted to tease out the reasons for heterogeneity underlying these results, including variability in study quality, comparators, independent and combined elements within patient decision aids, patient decision aid format (e.g. video, internet, paper‐based booklets), decision type, and the clinical settings, health systems, and countries in which they are used.
What's new
| Date | Event | Description |
|---|---|---|
| 29 January 2024 | New citation required and conclusions have changed | New for this update is higher‐certainty evidence that patient decision aids improve all the primary outcomes compared to usual care. |
| 29 January 2024 | New search has been performed | We updated the search in March 2022 and added 104 new studies comparing decision aids to usual care. For this update, we conducted a subgroup analysis for studies published since 2015 (n = 104 studies) (i.e. new studies included in this update) versus studies published prior to 2015 (n = 105 studies). |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 6 April 2017 | New search has been performed | We updated the search in April 2015 and added 18 new studies comparing decision aids to usual care. For this update, we removed 28 studies that were focused on detailed versus simple decision aids. We also conducted a subanalysis of decision aids used within the consultation and those used in preparation for the consultation. |
| 6 April 2017 | New citation required and conclusions have changed | New for this update is growing evidence that decision aids may improve informed, values‐congruent choices and the subanalysis indicated improved knowledge and accurate risk perceptions when decision aids are used either within or in preparation for the consultation. |
| 5 December 2013 | New citation required and conclusions have changed | This update added 33 new studies for a total of 115 studies involving 34,444 participants. GRADE was used to summarize the quality of the evidence, and findings were reported using a summary of findings table. We excluded three previously included trials on the basis of their quasi‐randomized controlled trial (q‐RCT) design, identified using the more rigorous risk of bias assessment tool, as well as one other study that used the same decision aid content for both groups but varied the format used. Overall, the results are similar to the previous update, but this update indicates the quality of the evidence to support the reported outcomes (high‐quality evidence that decision aids compared to usual care improve people’s knowledge and reduce their decisional conflict related to feeling uninformed and unclear about their personal values; moderate‐quality evidence that decision aids compared to usual care stimulate people to take a more active role in decision‐making and improve accurate risk perceptions when probabilities are included; and low‐quality evidence that decision aids improve the congruence between the chosen option and their values). We added two new authors to the review, LT in Sydney and JW in Ottawa who helped co‐ordinate this update. |
| 30 June 2012 | New search has been performed | Search strategies were updated and new searches run in June 2012. |
| 18 January 2012 | Amended | Minor change to wording, Plain Language Summary. |
| 5 September 2011 | New search has been performed | An update of this review was conducted in 2010 and published in Issue 10, 2011 of The Cochrane Library . Citations were searched from 2006 to December 2009. |
| 5 September 2011 | New citation required but conclusions have not changed | This update added 31 new studies, and all 86 included studies were assessed for risk of bias. Overall, the results were consistent with the previous update. New in this update is the meta‐analysis of informed, values‐based choices for decision aids including explicit values‐clarification compared to those with no explicit values‐clarification. We have also conducted a post hoc analysis to evaluate the effect of risk of bias assessment ratings on outcomes. |
| 29 April 2009 | New search has been performed | See the 'History' items dated 29 April 2009 and 28 July 2006. |
| 29 April 2009 | New citation required and conclusions have changed | A substantially updated version of this review was published in Issue 1, 2009 of The Cochrane Library . The changes are outlined in the 'History' (date 28 July 2006). The updated review ought to have had a new citation to reflect the new authorship and substantial changes to the review and its conclusions; however, because of a technical error this new citation was not given to the updated review. The new citation for this review for Issue 3, 2009 ( O'Connor 2009b ) reflects the updated review contents as actually published from Issue 1, 2009 onwards. |
| 28 April 2009 | Amended | Corrected mislabeled table 'Summary of pooled outcomes'. |
| 17 July 2008 | Amended | Converted to new review format. |
| 28 July 2006 | New search has been performed | Changes for the 2006 update (first published in Issue 1, 2009 of The Cochrane Library) :
Findings from the 2006 update (*new to this update):
|
| 21 February 2003 | New search has been performed | For the 2002 update ( O'Connor 2003 ), the following changes were made:
|
Acknowledgements
We would like to acknowledge the contribution of Junqiang Zhao and Mandy Huang, graduate students at the University of Ottawa, who assisted with data extraction and risk of bias assessment. We thank Dean Fergusson at the Ottawa Hospital Research Institute who provided consultation on the interpretation of findings for determining GRADE ratings for the 2017 update; his guidance was applied for this update.
Editorial and peer reviewer contributions
Cochrane Consumers and Communication supported the authors in the development of this review update. Cochrane Consumers and Communication editors, and academic and consumer referees, provided advice regarding the original review methods. We would like to acknowledge the guidance and contributions of Rebecca Ryan, Anne Parkhill, and Louisa Walsh; in particular, we thank Anne Parkhill for conducting the updated searches, responding to peer review feedback, and for revising the search strategy used for this update.
The following people conducted the editorial process for this review update.
Sign‐off Editor (final editorial decision): Norio Watanabe, Department of Health Promotion and Human Behavior, Kyoto University School of Public Health, Japan;
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments, and supported the editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service;
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service;
Peer reviewers (provided comments and recommended an editorial decision): Amy C Barradell, Centre for Exercise and Rehabilitation Science, University Hospitals of Leicester NHS Trust; and Department of Respiratory Sciences, University of Leicester, UK (clinical/content review); Hector P Rodriguez, University of California, Berkeley (clinical/content review); Brian Duncan (consumer review); Clarinda Cerejo, Patient Expert and Patient Advocate (consumer review); Jennifer Hilgart, Cochrane Evidence Production & Methods Directorate (methods review); Joanne Platt, Cochrane Evidence Production & Methods Directorate (search review). One additional peer reviewer provided clinical/content peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Revised search strategies January 2015 to March 2022
CENTRAL via the Cochrane Library
#1 MeSH descriptor: [Decision Support Techniques] explode all trees
#2 MeSH descriptor: [Decision Making, Shared] explode all trees
#3 MeSH descriptor: [Consensus] explode all trees
#4 ((decision* NEXT (aid* or box* or support* or technolog* or interven*))):ti,ab,kw (Word variations have been searched)
#5 ((option NEXT grid*)):ti,ab,kw (Word variations have been searched)
#6 {OR #1‐#5} with Cochrane Library publication date Between Jan 2015 and March 2022, in Trials
MEDLINE via Ovid
1 choice behavior/
2 exp decision making/
3 exp decision support techniques/
4 educational technology/
5 decision*.tw.
6 (choice* or preference*).tw.
7 communication package*.tw.
8 or/1‐7
9 exp health education/
10 health knowledge attitudes practice/
11 informed consent.tw,hw.
12 patient.tw,hw.
13 consumer.tw,hw.
14 or/9‐13
15 8 and 14
16 ((patient* or consumer*) adj1 (decision* or choice* or preferenc* or participat*)).tw.
17 ((women or men) adj1 (decision* or choice* or preferenc* or participat*)).tw.
18 (parent* adj1 (decision* or choice* or preferenc* or participat*)).tw.
19 ((personal or interpersonal or individual) adj (decision* or choice* or preferenc* or participat*)).tw.
20 shared decision making.tw.
21 decision aid*.tw.
22 informed choice.tw.
23 or/16‐22
24 15 or 23
25 (decision* adj (aid* or box* or support* or technolog* or interven*)).ti,ab,kw.
26 (option adj3 grid*).ti,ab,kw.
27 25 or 26
28 randomized controlled trial.pt.
29 controlled clinical trial.pt.
30 randomized.ab.
31 placebo.ab.
32 drug therapy.fs.
33 randomly.ab.
34 trial.ab.
35 groups.ab.
36 or/28‐35
37 exp animals/ not humans.sh.
38 36 not 37
39 and/24,27,38
40 limit 39 to yr="2015 ‐Current"
Embase via Ovid
1 decision support system/
2 patient decision making/ or shared decision making/
3 decision aid/
4 "decision tree"/
5 decision making.hw,kw,tw. and informed consent.hw,kw.
6 ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).tw,kw.
7 (decision adj (board* or guide* or counseling)).tw,kw.
8 ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw,kw.
9 (computer* adj2 decision making).tw,kw.
10 interactive health communication*.tw,kw.
11 (interactive adj (internet or online or graphic* or booklet*)).tw,kw.
12 (interacti* adj4 tool*).tw,kw.
13 ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw,kw.
14 shared decision making.tw,kw.
15 (informed adj (choice* or decision*)).tw,kw.
16 adaptive conjoint analys#s.tw,kw.
17 or/1‐16
18 randomized controlled trial/
19 controlled clinical trial/
20 single blind procedure/ or double blind procedure/
21 crossover procedure/
22 random*.tw.
23 placebo*.tw.
24 ((singl* or doubl*) adj (blind* or mask*)).tw.
25 (crossover or cross over or factorial* or latin square).tw.
26 (assign* or allocat* or volunteer*).tw.
27 or/18‐26
28 nonhuman/ not (human/ and nonhuman/)
29 27 not 28
30 17 and 29
31 (decision* adj (aid* or box* or support* or technolog* or interven*)).ti,ab,kw.
32 (option adj3 grid*).ti,ab,kw.
33 or/31‐32
34 and/30,33
35 limit 34 to yr="2015 ‐Current"
PsycINFO via Ovid
1 decision support systems/ or exp Decision Making/
2 (decision making or choice behavior).mp. and (informed consent.sh. or (patient* or parent* or carer* or caregiver* or care giver*).mp.)
3 ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).ti,ab,id.
4 (decision adj (board* or guide* or counseling)).ti,ab,id.
5 ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).ti,ab,id.
6 computer assisted therapy/
7 (computer* adj2 decision making).ti,ab,id.
8 interactive health communication*.ti,ab,id.
9 (interactive adj (internet or online or graphic* or booklet*)).ti,ab,id.
10 (interacti* adj4 tool*).ti,ab,id.
11 ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).ti,ab,id.
12 shared decision making.ti,ab,id.
13 (informed adj (choice* or decision*)).ti,ab,id.
14 adaptive conjoint analys#s.ti,ab,id.
15 or/1‐14
16 random*.ti,ab,hw,id.
17 intervention.ti,ab,hw,id.
18 trial.ti,ab,hw,id.
19 placebo*.ti,ab,hw,id.
20 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
21 (cross over or crossover).ti,ab,hw,id.
22 latin square.ti,ab,hw,id.
23 (assign* or allocat* or volunteer*).ti,ab,hw,id.
24 treatment effectiveness evaluation/
25 mental health program evaluation/
26 exp experimental design/
27 or/16‐26
28 (decision* adj (aid* or box* or support* or technolog* or interven*)).ti,ab.
29 (option adj3 grid*).ti,ab.
30 or/28‐29
31 and/15,27,30
32. limit 31 to yr="2015 ‐Current"
CINAHL via EBSCO
| S17 | S10 AND S15 |
| S16 | S10 AND S15 |
| S15 | S13 OR S14 |
| S14 | (MH "Decision Support Techniques") OR (MH "Decision Making, Computer Assisted") OR (MH "Decision Making, Shared") OR (MH "Decision Making, Patient") OR (MH "Decision Making, Family") |
| S13 | S11 OR S12 |
| S12 | TX (decision* N (aid* or box* or support* or technolog* or interven*)) |
| S11 | TX (option N3 grid*) |
| S10 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 |
| S9 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) |
| S8 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) |
| S7 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) |
| S6 | MH Quantitative Studies |
| S5 | MH Placebos |
| S4 | MH Random Assignment |
| S3 | MH Clinical Trials+ |
| S2 | PT Clinical Trial |
| S1 | PT "randomi?ed controlled trial" |
Appendix 2. Revised search strategies January 2009 to April 2015
CENTRAL via the Cochrane Library
(decision‐support or decision‐aid):kw in Trials
decision‐tree:kw in Trials
patient‐decision‐making:kw
(decision‐making or choice‐behavior):ti,ab,kw and (informed‐consent:kw,ti or (patient or parent* or carer or caregiver or care‐giver):ti,ab,kw) in Trials
((decision or decid*) near/4 (support* or aid* or tool or instrument or technolog* or technique or system or program* or algorithm or process or method or intervention or material)):ti,ab,kw
(decision next (board or guide or counseling)):ti,ab,kw
((risk‐communication or risk‐assessment or risk‐information) near/4 (tool or method)):ti,ab,kw
(computer* near/2 decision‐making):ti,ab,kw
(interactive‐health‐communication or (interacti* near/4 tool)):ti,ab,kw
(interactive next (internet or online or graphic* or booklet)):ti,ab,kw
((interactiv* or evidence‐based) near/3 (risk‐information or risk‐communication or risk‐presentation or risk‐graphic*)):ti,ab,kw
shared‐decision‐making:ti,ab,kw
(informed next (choice or decision)):ti,ab,kw
adaptive‐conjoint‐analysis:ti,ab,kw
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14), from 2009 to 2015
(Last line restricted to “Trials”, and to date range 2009 to 2015)
MEDLINE Ovid
1. decision support techniques/
2. decision support systems clinical/
3. decision trees/
4. (decision making or choice behavior).mp. and informed consent.sh.
5. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).tw.
6. (decision adj (board* or guide* or counseling)).tw.
7. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw.
8. decision‐making computer assisted/
9. (computer* adj2 decision making).tw.
10. interactive health communication*.tw.
11. (interactive adj (internet or online or graphic* or booklet*)).tw.
12. (interacti* adj4 tool*).tw.
13. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw.
14. shared decision making.tw.
15. (informed adj (choice* or decision*)).tw.
16. adaptive conjoint analys#s.tw.
17. or/1‐16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. clinical trials as topic.sh.
23. randomly.ab.
24. trial.ti.
25. or/18‐24
26. exp animals/ not humans.sh.
27. 25 not 26
28. 17 and 27
29. limit 28 to yr="2009 ‐Current"
Embase Ovid
1. decision support system/
2. patient decision making/
3. decision aid/
4. "decision tree"/
5. decision making.hw,kw,tw. and informed consent.hw,kw.
6. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).tw,kw.
7. (decision adj (board* or guide* or counseling)).tw,kw.
8. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw,kw.
9. (computer* adj2 decision making).tw,kw.
10. interactive health communication*.tw,kw.
11. (interactive adj (internet or online or graphic* or booklet*)).tw,kw.
12. (interacti* adj4 tool*).tw,kw.
13. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw,kw.
14. shared decision making.tw,kw.
15. (informed adj (choice* or decision*)).tw,kw.
16. adaptive conjoint analys#s.tw,kw.
17. or/1‐16
18. randomized controlled trial/
19. controlled clinical trial/
20. single blind procedure/ or double blind procedure/
21. crossover procedure/
22. random*.tw.
23. placebo*.tw.
24. ((singl* or doubl*) adj (blind* or mask*)).tw.
25. (crossover or cross over or factorial* or latin square).tw.
26. (assign* or allocat* or volunteer*).tw.
27. or/18‐26
28. nonhuman/ not (human/ and nonhuman/)
29. 27 not 28
30. 17 and 29
31. 30 and 20012:2015.(sa_year).
32. limit 31 to exclude medline journals
PsycINFO Ovid
1. decision support systems/
2. (decision making or choice behavior).mp. and (informed consent.sh. or (patient* or parent* or carer* or caregiver* or care giver*).mp.)
3. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).ti,ab,id.
4. (decision adj (board* or guide* or counseling)).ti,ab,id.
5. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).ti,ab,id.
6. computer assisted therapy/
7. (computer* adj2 decision making).ti,ab,id.
8. interactive health communication*.ti,ab,id.
9. (interactive adj (internet or online or graphic* or booklet*)).ti,ab,id.
10. (interacti* adj4 tool*).ti,ab,id.
11. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).ti,ab,id.
12. shared decision making.ti,ab,id.
13. (informed adj (choice* or decision*)).ti,ab,id.
14. adaptive conjoint analys#s.ti,ab,id.
15. or/1‐14
16. random*.ti,ab,hw,id.
17. intervention.ti,ab,hw,id.
18. trial.ti,ab,hw,id.
19. placebo*.ti,ab,hw,id.
20. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
21. (cross over or crossover).ti,ab,hw,id.
22. latin square.ti,ab,hw,id.
23. (assign* or allocat* or volunteer*).ti,ab,hw,id.
24. treatment effectiveness evaluation/
25. mental health program evaluation/
26. exp experimental design/
27. or/16‐26
28. 15 and 27
29. limit 28 to yr="2009 ‐Current"
CINAHL (EBSCO)
| # | Query | Limiters/Expanders |
| S31 | S30 | Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase |
| S30 | S28 and S29 | Search modes ‐ Boolean/Phrase |
| S29 | EM 2009‐ | Search modes ‐ Boolean/Phrase |
| S28 | S17 and S27 | Search modes ‐ Boolean/Phrase |
| S27 | S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 | Search modes ‐ Boolean/Phrase |
| S26 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S25 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S24 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) | Search modes ‐ Boolean/Phrase |
| S23 | MH Quantitative Studies | Search modes ‐ Boolean/Phrase |
| S22 | MH Placebos | Search modes ‐ Boolean/Phrase |
| S21 | MH Random Assignment | Search modes ‐ Boolean/Phrase |
| S20 | MH Clinical Trials+ | Search modes ‐ Boolean/Phrase |
| S19 | PT Clinical Trial | Search modes ‐ Boolean/Phrase |
| S18 | PT "randomi?ed controlled trial" | Search modes ‐ Boolean/Phrase |
| S17 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 | Search modes ‐ Boolean/Phrase |
| S16 | "informed choice*" or "informed decision*" | Search modes ‐ Boolean/Phrase |
| S15 | "shared decision making" | Search modes ‐ Boolean/Phrase |
| S14 | "adaptive conjoint analys?s" | Search modes ‐ Boolean/Phrase |
| S13 | (interactive N2 "risk information") or (interactive N2 "risk communication") or (interactive N2 "risk presentation") or (interactive N2 "risk graphic*") | Search modes ‐ Boolean/Phrase |
| S12 | "interactive internet" or "interactive online" or "interactive graphic*" or "interactive booklet*" or (interacti* N3 tool*) | Search modes ‐ Boolean/Phrase |
| S11 | "interactive health communication*" | Search modes ‐ Boolean/Phrase |
| S10 | computer* N1 "decision making" | Search modes ‐ Boolean/Phrase |
| S9 | ("risk communication" N3 tool*) or ("risk communication" N3 method*) or ("risk information" N3 tool*) or ("risk information" N3 method*) or ("risk assessment" N3 tool*) or ("risk assessment" N3 method*) | Search modes ‐ Boolean/Phrase |
| S8 | "evidence based risk communication" or "evidence based risk information" | Search modes ‐ Boolean/Phrase |
| S7 | "decision board*" or "decision guide*" or "decision counseling" | Search modes ‐ Boolean/Phrase |
| S6 | (decision* N3 support*) or (decision* N3 aid*) or (decision* N3 tool*) or (decision* N3 instrument*) or (decision* N3 technolog*) or (decision* N3 technique*) or (decision* N3 system*) or (decision* N3 program*) or (decision* N3 algorithm*) or (decision* N3 process*) or (decision* N3 method*) or (decision* N3 intervention*) or (decision* N3 material*) | Search modes ‐ Boolean/Phrase |
| S5 | ("decision making" or "choice behavior") and MH consent | Search modes ‐ Boolean/Phrase |
| S4 | MH decision making, computer assisted | Search modes ‐ Boolean/Phrase |
| S3 | MH decision making, patient | Search modes ‐ Boolean/Phrase |
| S2 | MH decision support systems, clinical | Search modes ‐ Boolean/Phrase |
| S1 | MH decision support techniques+ | Search modes ‐ Boolean/Phrase |
Appendix 3. Search strategies to 2009
CENTRAL
CENTRAL in the Cochrane Library was searched using the MEDLINE search above in Ovid to the end of 2006; for the 2011 update, the CENTRAL search was conducted at www.thecochranelibrary.com to the end of 2009 using the following search strategy:
1. decision.tw,hw.
2. patient.tw,hw.
3. consumer.tw,sh.
4. 1 and (2 or 3)
5. shared decision making.tw.
6. decision aid$.tw.
7. informed choice.tw.
8. or/4‐7
9. clinical trial.pt.
10. randomized controlled trial.pt.
11. random$.tw.
12. or/9‐11
13. 8 and 12
MEDLINE Ovid (1966 to December 2009)
1. choice behavior/
2. decision making/
3. exp decision support techniques/
4. Educational Technology/
5. decision$.tw.
6. (choic$ or preference$).tw.
7. communication package.tw.
8. or/1‐7
9. exp health education/
10. Health Knowledge, Attitudes, Practice/
11. informed consent.tw,hw.
12. patient.tw,hw.
13. consumer.tw,hw.
14. or/9‐13
15. 8 and 14
16. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
17. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
18. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
19. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
20. shared decision making.tw.
21. decision aid$.tw.
22. informed choice.tw.
23. or/16‐22
24. 15 or 23
25. clinical trial.pt.
26. randomized controlled trial.pt.
27. random$.tw.
28. (double adj blind$).tw.
29. double‐blind method/
30. or/25‐29
31. 24 and 30
CINAHL Ovid (1982 to September 2008)
1. exp Decision Making/
2. information seeking behavior/
3. Help Seeking Behavior/
4. (choic$ or preference$).tw.
5. decision$.tw.
6. Educational Technology/
7. or/1‐6
8. exp Health Behavior/
9. consumer participation/
10. exp Health Education/
11. health knowledge/ or exp professional knowledge/
12. exp Consent/
13. informed consent.tw.
14. patient.tw,hw.
15. consumer.tw,sh.
16. or/8‐15
17. 7 and 16
18. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
19. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
20. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
21. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
22. shared decision making.tw.
23. decision aid$.tw.
24. informed choice.tw.
25. or/18‐24
26. 17 or 25
27. exp clinical trials/
28. Clinical trial.pt.
29. (clinic$ adj trial$1).tw.
30. random$.tw.
31. Random assignment/
32. placebo$.tw,sh.
33. Quantitative studies/
34. Allocat$ random$.tw.
35. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw.
36. or/27‐35
37. 26 and 36
Embase Ovid (1980 to December 2009)
1. decision making/
2. decision theory/
3. decision$.tw.
4. Educational Technology/
5. or/1‐4
6. exp health behavior/
7. exp Patient Attitude/
8. exp health education/
9. informed consent.tw,sh.
10. patient.tw,sh.
11. consumer.tw,sh.
12. or/6‐11
13. 5 and 12
14. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
15. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
16. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
17. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
18. shared decision making.tw.
19. decision aid$.tw.
20. informed choice.tw.
21. or/14‐20
22. 13 or 21
23. Controlled Study/
24. Randomized Controlled Trial/
25. Clinical Study/
26. Clinical Trial/
27. Major Clinical Study/
28. Prospective Study/
29. Multicenter Study/
30. Randomization/
31. Double Blind Procedure/
32. Single Blind Procedure/
33. Crossover Procedure/
34. Placebo.tw,sh.
35. random$.tw.
36. (double adj blind$).tw.
37. or/23‐36
38. 22 and 37
PsycINFO Ovid (1806 to December 2009)
1. decision$.tw.
2. (choic$ or preference$).tw.
3. exp decision making/
4. computer assisted instruction/
5. or/1‐4
6. exp health education/
7. exp health personnel attitudes/
8. informed consent.tw,sh.
9. patient.tw,hw.
10. consumer.tw,hw.
11. exp health behavior/
12. or/6‐11
13. 5 and 12
14. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
15. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
16. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
17. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
18. shared decision making.tw.
19. decision aid$.tw.
20. informed choice.tw.
21. or/14‐20
22. 13 or 21
23. random$.tw.
24. (double adj blind$).tw.
25. placebo$.tw,hw.
26. or/23‐25
27. 22 and 26
Data and analyses
Comparison 1. Informed values‐choice congruence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Informed values‐choice congruence ‐ all studies | 21 | 9377 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.44, 2.13] |
| 1.2 Informed values‐choice congruence ‐ without studies of high risk of bias | 18 | 7182 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.54, 2.50] |
| 1.3 Informed values‐choice congruence ‐ old vs new studies | 21 | 9377 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.44, 2.13] |
| 1.3.1 Older studies (2014 and earlier) | 10 | 4626 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.46, 2.91] |
| 1.3.2 Newer studies (2015‐2022) | 11 | 4751 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.22, 1.89] |
| 1.4 Informed values‐chose congruence ‐ using MMIC | 13 | 6030 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.37, 2.23] |
| 1.5 Informed values‐chose congruence ‐ using non‐MMIC measures | 8 | 3327 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.29, 2.55] |
Comparison 2. Knowledge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Knowledge ‐ all studies | 107 | 25492 | Mean Difference (IV, Random, 95% CI) | 11.90 [10.60, 13.19] |
| 2.2 Knowledge ‐ studies without high risk of bias | 95 | 23083 | Mean Difference (IV, Random, 95% CI) | 12.13 [10.74, 13.52] |
| 2.3 Knowledge ‐ old vs new studies | 107 | 25492 | Mean Difference (IV, Random, 95% CI) | 11.90 [10.60, 13.19] |
| 2.3.1 Older studies (2014 and earlier) | 51 | 13194 | Mean Difference (IV, Random, 95% CI) | 13.02 [11.08, 14.96] |
| 2.3.2 Newer studies (2015‐2022) | 56 | 12298 | Mean Difference (IV, Random, 95% CI) | 11.01 [8.75, 13.27] |
Comparison 3. Accurate risk perceptions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Accurate risk perceptions ‐ all studies | 25 | 7796 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.61, 2.34] |
| 3.2 Accurate risk perceptions ‐ studies without high risk of bias | 20 | 6152 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.60, 2.48] |
| 3.3 Accurate risk perceptions ‐ old vs new studies | 25 | 7796 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.61, 2.34] |
| 3.3.1 Older Studies (2014 and earlier) | 16 | 5019 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.62, 2.64] |
| 3.3.2 Newer studies (2015‐2022) | 9 | 2777 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.30, 2.39] |
Comparison 4. Decisional conflict.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Decisional conflict ‐ uninformed ‐ all studies | 58 | 12104 | Mean Difference (IV, Random, 95% CI) | ‐10.02 [‐12.31, ‐7.74] |
| 4.2 Decisional conflict ‐ uninformed ‐ without studies having high risk of bias | 51 | 9982 | Mean Difference (IV, Random, 95% CI) | ‐11.18 [‐13.82, ‐8.54] |
| 4.3 Decisional conflict ‐ uninformed ‐ old vs new studies | 58 | 12104 | Mean Difference (IV, Random, 95% CI) | ‐10.02 [‐12.31, ‐7.74] |
| 4.3.1 Older studies (2014 and earlier) | 26 | 5585 | Mean Difference (IV, Random, 95% CI) | ‐8.73 [‐11.57, ‐5.90] |
| 4.3.2 Newer studies (2015‐2022) | 32 | 6519 | Mean Difference (IV, Random, 95% CI) | ‐11.03 [‐14.58, ‐7.47] |
| 4.4 Decisional conflict ‐ unclear values ‐ all studies | 55 | 11880 | Mean Difference (IV, Random, 95% CI) | ‐7.86 [‐9.69, ‐6.02] |
| 4.5 Decisional conflict ‐ unclear values ‐ without studies having high risk of bias | 48 | 9758 | Mean Difference (IV, Random, 95% CI) | ‐8.60 [‐10.73, ‐6.47] |
| 4.6 Unclear values ‐ old vs new studies | 55 | 11880 | Mean Difference (IV, Random, 95% CI) | ‐7.86 [‐9.69, ‐6.02] |
| 4.6.1 Older studies (2014 and earlier) | 22 | 4946 | Mean Difference (IV, Random, 95% CI) | ‐7.74 [‐10.51, ‐4.96] |
| 4.6.2 Newer studies (2015‐2022) | 33 | 6934 | Mean Difference (IV, Random, 95% CI) | ‐8.03 [‐10.69, ‐5.38] |
Comparison 5. Participation in decision making.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Participation in decision‐making ‐ all studies | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Clinician‐controlled decision‐making | 21 | 4348 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.88] |
| 5.1.2 Patient‐controlled decision‐making | 20 | 3715 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.05, 1.43] |
| 5.1.3 Shared decision‐making | 20 | 3799 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 5.2 Participation in decision‐making ‐ studies without high risk of bias | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Clinician‐controlled decision‐making | 17 | 3249 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.98] |
| 5.2.2 Patient‐controlled decision‐making | 15 | 2433 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.99, 1.45] |
| 5.2.3 Shared decision‐making | 16 | 2700 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 5.3 Participation in decision‐making ‐ clinician‐controlled ‐ old vs new studies | 21 | 4348 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.88] |
| 5.3.1 Older studies (2014 and earlier) | 16 | 3180 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.55, 0.83] |
| 5.3.2 Newer studies (2015‐2022) | 5 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.29] |
| 5.4 Participation in decision‐making ‐ patient‐controlled ‐ old vs new studies | 20 | 3715 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.05, 1.43] |
| 5.4.1 Older studies (2014 and earlier) | 15 | 3009 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.05, 1.55] |
| 5.4.2 Newer studies (2015‐2022) | 5 | 706 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.77, 1.68] |
| 5.5 Participation in decision‐making ‐ shared decision‐making ‐ old vs new studies | 20 | 3799 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 5.5.1 Older studies (2014 and earlier) | 16 | 3196 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.11] |
| 5.5.2 Newer studies (2015‐2022) | 4 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.17] |
Comparison 6. Decision regret.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Decision regret ‐ all studies | 22 | 3707 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐3.05, 0.59] |
| 6.2 Decision regret ‐ studies without high risk of bias | 17 | 2640 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐5.16, ‐0.01] |
| 6.3 Decision regret ‐ old vs new studies | 22 | 3707 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐3.05, 0.59] |
| 6.3.1 Older studies (2014 and earlier) | 5 | 1469 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐2.98, 3.52] |
| 6.3.2 Newer studies (2015‐2022) | 17 | 2238 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐4.06, 0.49] |
Comparison 7. Proportion undecided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Proportion undecided ‐ all studies | 42 | 8548 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
| 7.2 Proportion undecided ‐ studies without high risk of bias | 37 | 7471 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.57, 0.81] |
| 7.3 Proportion undecided ‐ old vs new studies | 42 | 8548 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
| 7.3.1 Older studies (2014 and earlier) | 22 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.52, 0.77] |
| 7.3.2 Newer studies (2015‐2022) | 20 | 3207 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
Comparison 8. Patient‐clinician communication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Patient‐clinician communication ‐ continuous measures (OPTION, CollaboRATE, SDM‐Q‐9) | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 OPTION‐12 | 8 | 2391 | Mean Difference (IV, Random, 95% CI) | 12.14 [8.12, 16.16] |
| 8.1.2 OPTION‐5 | 2 | 665 | Mean Difference (IV, Random, 95% CI) | 20.46 [‐1.98, 42.90] |
| 8.1.3 Collaborate | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐0.50, 4.03] |
| 8.1.4 SDM‐Q‐9 | 3 | 1525 | Mean Difference (IV, Random, 95% CI) | 1.38 [‐2.50, 5.25] |
| 8.2 Discussed topic with provider | 11 | 3913 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.19, 1.70] |
| 8.3 Patient‐clinician communication ‐ continuous measures (OPTION, CollaboRATE, SDM‐Q‐9) ‐ studies without high risk of bias | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.3.1 OPTION‐12 | 3 | 825 | Mean Difference (IV, Random, 95% CI) | 17.01 [9.40, 24.61] |
| 8.3.2 OPTION‐5 | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.3.3 Collaborate | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐0.50, 4.03] |
| 8.3.4 SDM‐Q‐9 | 3 | 1525 | Mean Difference (IV, Random, 95% CI) | 1.38 [‐2.50, 5.25] |
| 8.4 Discussed topic with provider ‐ studies without high risk of bias | 10 | 3157 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.17, 1.84] |
Comparison 9. Satisfaction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Satisfaction with the decision‐making process ‐ all studies | 12 | 2066 | Mean Difference (IV, Random, 95% CI) | 3.33 [1.18, 5.48] |
| 9.2 Satisfaction with the decision‐making process ‐ studies without high risk of bias | 8 | 1394 | Mean Difference (IV, Random, 95% CI) | 3.90 [1.71, 6.09] |
| 9.3 Satisfaction with the decision‐making process ‐ old vs new studies | 12 | 2066 | Mean Difference (IV, Random, 95% CI) | 3.33 [1.18, 5.48] |
| 9.3.1 Older studies (2014 and earlier) | 9 | 1663 | Mean Difference (IV, Random, 95% CI) | 2.57 [0.12, 5.01] |
| 9.3.2 Newer studies (2015‐2022) | 3 | 403 | Mean Difference (IV, Random, 95% CI) | 5.90 [1.52, 10.27] |
Comparison 10. Preparation for decision‐making.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Preparation for decision‐making ‐ all studies | 8 | 1249 | Mean Difference (IV, Random, 95% CI) | 6.63 [‐3.09, 16.35] |
| 10.2 Preparation for decision‐making studies without high risk of bias | 7 | 913 | Mean Difference (IV, Random, 95% CI) | 9.24 [4.78, 13.71] |
| 10.3 Preparation for decision‐making ‐ old vs new studies | 8 | 1249 | Mean Difference (IV, Random, 95% CI) | 6.63 [‐3.09, 16.35] |
| 10.3.1 Older studies (2014 and earlier) | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 11.20 [2.85, 19.55] |
| 10.3.2 Newer studies (2015‐2022) | 7 | 1100 | Mean Difference (IV, Random, 95% CI) | 6.02 [‐4.77, 16.80] |
Comparison 11. Choice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Choice: surgery over conservative option (subgroup by condition) | 38 | 8467 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.83, 0.95] |
| 11.1.1 Breast cancer ‐ mastectomy vs lumpectomy | 8 | 1078 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 11.1.2 Breast cancer ‐ surgery vs endocrine therapy | 1 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.01] |
| 11.1.3 Breast cancer ‐ reconstruction | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.00] |
| 11.1.4 Breast cancer ‐ prophylactic mastectomy | 2 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.67, 3.12] |
| 11.1.5 Joint replacement | 8 | 2080 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.04] |
| 11.1.6 Upper extremity conditions | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.74] |
| 11.1.7 Prostate cancer | 4 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.09] |
| 11.1.8 Benign prostatic hyperplasia | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.16, 12.84] |
| 11.1.9 Left ventricular assist device (LVAD) | 3 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.93] |
| 11.1.10 Coronary revascularization | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.94] |
| 11.1.11 Abdominal aortic aneurysm | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.46] |
| 11.1.12 Renal stone treatment | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.09] |
| 11.1.13 Bariatric surgery | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.99] |
| 11.1.14 Menorrhagia | 3 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 11.2 Choice for screening | 42 | 46638 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.10] |
| 11.2.1 PSA screening | 11 | 4185 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.99] |
| 11.2.2 Colorectal cancer screening | 17 | 17510 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.41] |
| 11.2.3 Breast cancer genetic testing | 4 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.39] |
| 11.2.4 Breast cancer screening (mammography) | 7 | 22498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 0.99] |
| 11.2.5 Prenatal diagnostic testing | 4 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.10] |
| 11.3 Choice: diabetes medication (uptake of new medication) | 6 | 1960 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [0.64, 9.17] |
| 11.4 Choice: surgery over conservative option ‐ studies without high risk of bias | 32 | 7121 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 11.4.1 Breast cancer ‐ mastectomy vs lumpectomy | 6 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.16] |
| 11.4.2 Breast cancer ‐ surgery vs endocrine therapy | 1 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.01] |
| 11.4.3 Breast cancer ‐ reconstruction | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.00] |
| 11.4.4 Breast cancer ‐ prophylactic mastectomy | 2 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.67, 3.12] |
| 11.4.5 Joint replacement | 8 | 2080 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.04] |
| 11.4.6 Upper extremity conditions | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.74] |
| 11.4.7 Prostate cancer | 2 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.98] |
| 11.4.8 Benign prostatic hyperplasia | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.16, 12.84] |
| 11.4.9 Left ventricular assist device (LVAD) | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| 11.4.10 Coronary revascularization | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.94] |
| 11.4.11 Abdominal aortic aneurysm | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 11.4.12 Renal stone treatment | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.09] |
| 11.4.13 Bariatric surgery | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.99] |
| 11.4.14 Menorrhagia | 3 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 11.5 Choice for screening ‐ studies without high risk of bias | 37 | 28877 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.10] |
| 11.5.1 PSA screening | 10 | 3914 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 0.99] |
| 11.5.2 Colorectal cancer screening | 15 | 16812 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.35] |
| 11.5.3 Breast cancer genetic testing | 4 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.39] |
| 11.5.4 Breast cancer screening (mammography) | 5 | 5706 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 1.00] |
| 11.5.5 Prenatal diagnostic testing | 4 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.10] |
| 11.6 Choice: diabetes medication (uptake of new medication) ‐ studies without high risk of bias | 4 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.06, 2.56] |
Comparison 12. Confidence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Confidence ‐ all studies | 12 | 4681 | Mean Difference (IV, Random, 95% CI) | 5.28 [2.27, 8.29] |
| 12.1.1 Decision Self‐efficacy Scale | 6 | 2550 | Mean Difference (IV, Random, 95% CI) | 2.49 [0.03, 4.95] |
| 12.1.2 Study‐specific questionnaire | 6 | 2131 | Mean Difference (IV, Random, 95% CI) | 7.36 [2.67, 12.05] |
| 12.2 Confidence ‐ studies without high risk of bias | 10 | 3671 | Mean Difference (IV, Random, 95% CI) | 5.53 [1.95, 9.11] |
| 12.2.1 Decision Self‐efficacy Scale | 5 | 2297 | Mean Difference (IV, Random, 95% CI) | 3.15 [‐0.04, 6.34] |
| 12.2.2 Study‐specific questionnaire | 5 | 1374 | Mean Difference (IV, Random, 95% CI) | 7.44 [0.97, 13.91] |
| 12.3 Confidence ‐ old vs new studies | 12 | 4681 | Mean Difference (IV, Random, 95% CI) | 5.28 [2.27, 8.29] |
| 12.3.1 Decision Self‐efficacy Scale ‐ older studies (2014 and earlier) | 1 | 625 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐1.83, 9.83] |
| 12.3.2 Decision Self‐efficacy Scale ‐ newer studies (2015‐2022) | 5 | 1925 | Mean Difference (IV, Random, 95% CI) | 2.37 [‐0.42, 5.17] |
| 12.3.3 Study‐specific questionnaire ‐ older studies (2014 and earlier) | 1 | 557 | Mean Difference (IV, Random, 95% CI) | 8.00 [4.93, 11.07] |
| 12.3.4 Study‐specific questionnaire ‐ newer studies (2015‐2022) | 5 | 1574 | Mean Difference (IV, Random, 95% CI) | 7.27 [1.05, 13.49] |
Comparison 13. Consultation length.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Consultation length ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation) | 13 | 3122 | Mean Difference (IV, Random, 95% CI) | 0.73 [0.05, 1.41] |
| 13.1.1 In consultation | 8 | 2702 | Mean Difference (IV, Random, 95% CI) | 1.50 [0.79, 2.20] |
| 13.1.2 In preparation for consultation | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐7.84, 1.90] |
| 13.2 Consultation length ‐ subgroup by timing of intervention ‐ studies without high risk of bias | 10 | 1871 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.15, 1.59] |
| 13.2.1 In consultation | 5 | 1451 | Mean Difference (IV, Random, 95% CI) | 1.75 [1.00, 2.50] |
| 13.2.2 In preparation for consultation | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐7.84, 1.90] |
| 13.3 Consultation length ‐ old vs new studies (in consultation) | 8 | 2702 | Mean Difference (IV, Random, 95% CI) | 1.50 [0.79, 2.20] |
| 13.3.1 Older studies | 2 | 317 | Mean Difference (IV, Random, 95% CI) | 2.48 [‐0.13, 5.09] |
| 13.3.2 Newer studies | 6 | 2385 | Mean Difference (IV, Random, 95% CI) | 1.43 [0.70, 2.16] |
| 13.4 Consultation length ‐ old vs new studies (in preparation for consultation) | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐7.84, 1.90] |
| 13.4.1 Older studies | 1 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.57, 2.37] |
| 13.4.2 Newer studies | 4 | 297 | Mean Difference (IV, Random, 95% CI) | ‐3.78 [‐10.41, 2.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2010.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 398 + 414 men considering prostate cancer screening in the USA | |
| Interventions | DA: computer tailored program on clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step‐by‐step process for making the decision; interactive computer program: inherently guided the patient through the decision aid and decision‐making process), tailored printout given to patients to promote discussion with others (practitioner, significant others). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: no intervention |
|
| Outcomes | Primary outcomes: decisional status, knowledge, decision self‐efficacy, decisional consistency Secondary outcomes: desire for involvement in decision‐making, decisional conflict, preferred options Outcomes assessed pre‐ and postintervention |
|
| Notes | Source of funding: This study was funded by the Centers for Disease Control and Prevention (Grant 3U48DP000064‐01S1, SIP 21‐04 Community Intervention to Increase IDM for Prostate Cancer). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sites were blocked on size and percent of male employees and randomly assigned by computer‐generated random numbers to condition within blocks" (p 2173, Setting) |
| Allocation concealment (selection bias) | Unclear risk | The study does not address this criterion. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study does not address this criterion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes measured were not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and low rate of attrition that was consistent between groups. |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Intervention delivery: mention of money incentive to complete paperwork, but was judged to have no effect on outcomes measured (p 2175). Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Allen 2018.
| Study characteristics | ||
| Methods | Cluster, stepped‐wedge trial, randomized to decision aid plus coaching vs usual care | |
| Participants | 113 + 135 adults with end‐stage heart failure considering destination therapy left ventricular assist device placement from 6 mechanical circulatory support programs across the USA | |
| Interventions | DA: pamphlet and video used during consultation that included decision delivered by trained clinicians. DA includes information on the clinical problem, outcome probabilities, explicit values clarification, patient narratives, and guidance in communication. The DA is publicly available at https://patientdecisionaid.org/lvad/ Comparator: usual care consisting of the program’s current education |
|
| Outcomes | Primary outcomes: decision quality, knowledge, and values‐choice concordance Secondary outcomes: decision conflict, decision regret, control preferences, illness acceptance, perceived stress, depression, and quality of life Other outcomes reported: treatment preference, actual treatment received |
|
| Notes | Source of funding: This work was supported through a Patient‐Centered Outcomes Research Institute (PCORI) Program Award (CDR‐1310‐06998). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or Methodology Committee. This work was also supported in part by the National Heart, Lung and Blood Institute (1K23HL105896‐01, Allen), the Heart Failure Society of America (McIlvennan), the National Institute on Aging (1K23AG040696, Matlock), and REDCap database hosting through University of Colorado supported by NIH/NCRR Colorado CTSI (Grant Number UL1 TR001082). Conflicts of interest: Dr Allen reports consulting for Novartis, Boston Scientific, Janssen, Amgen, Duke Clinical Research Institute, and Grants from the Patient‐Centered Outcomes Research Institute, National Institutes of Health, National Heart, Lung, and Blood Institute, and the American Heart Association. Dr Patel reports consulting for Abbott and Medtronic. Dr Cleveland reports consulting for Abbott. Dr Matlock reports funding from the American College of Cardiology Foundation. No other disclosures are reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is a high rate of attrition and it is significantly different between groups. At 6 months loss to follow‐up is 42% intervention versus 33% control (P = 0.02706). Significantly lower enrolment in the control group: 228 randomized to control group but only 135 were enrolled in the full study (59%); 157 randomized to intervention but only 113 enrolled (72%) (P = 0.01015). Limitation section in paper does not indicate what effect this may have on the data, but only normalizes the dropout rates: "First, missing data were somewhat frequent and concentrated among the group of patients who did not undergo implantation of DT LVAD. Death was the most common cause of missing data, followed by withdrawal from the study, both of which are common in studies targeting patients with life threatening illness. Our missing data rates are comparable to similar study types". |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02344576) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. Outcomes related to the feasibility and acceptability of the intervention are reported elsewhere. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Aoki 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid + coaching vs usual care | |
| Participants | 35 (decision aid + coaching) versus 53 (usual care) undergraduate and postgraduate students aged 20 years and older who visited the outpatient services for first‐time diagnosis of major depressive episode, including depressive phase of bipolar disorder in Japan | |
| Interventions | DA: 3 decision aid booklets on depression, bipolar disorder, and medication treatment provided to patients during the initial consultation and prior to the decision coaching intervention and the decision‐making consultation. The DAs contained general information on depression or bipolar disorder and their treatment options for patients undergoing psychiatric treatment for the first time, outcome probabilities, implicit values clarification, FAQs, guidance in communication, and a summary at the end of what was presented in the booklet. The DAs are available as a supplementary appendix in the article. Comparator: usual care delivered during the initial consultation |
|
| Outcomes | Primary: patient‐perceived involvement in medical decisions (COMRADE) Secondary: satisfaction, consultation duration, sharing information with others, looking up information on options/treatments, persistence with treatment, severity of depressive symptoms, medication adherence |
|
| Notes | Source of funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Conflicts of interest: Author Koichiro Watanabe has received manuscript fees or speaker's honoraria from Astellas Pharma, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Otsuka Pharmaceutical, Pfizer, Shionogi, Sumitomo Dainippon Pharma, and Yoshitomi and has received research/grant support from Astellas Pharma, Daiichi Sankyo, Eisai, MSD, Mitsubishi Tanabe Pharma, Meiji Seika Pharma, Otsuka Pharmaceutical, Pfizer, Shionogi, and Sumitomo Dainippon Pharma and is a consultant of Eli Lilly, Otsuka Pharmaceutical, Pfizer, Sumitomo Dainippon Pharma, Taisho Toyama Pharmaceutical, and Takeda Pharmaceutical. Author Yoshikazu Takaesu has received speaker's honoraria from Eisai, Eli Lilly, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, and Yoshitomi Pharmaceutical and has received research/grant support from Eisai, Meiji Seika Pharma, and Otsuka Pharmaceutical. The other authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to one of two arms, following the restricted randomization and minimization method of item 8 in CONSORT 2010 (Moher 2012)" COMMENT: *Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Allocation concealment (selection bias) | Low risk | The randomization was conducted by a research assistant not directly involved in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians and nurses were not blinded because of the design of the study. Low risk because objective measures used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A research assistant blinded to group allocation collected data at baseline, after the decision‐making consultation, and at each visit during the 6‐month trial period. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for attrition clearly described (see Figure 1). Missing outcome data balanced across groups: intervention 20/35 (57%), control 32/53 (60%). However, it was unclear how the high rate of missing data influenced the results. |
| Selective reporting (reporting bias) | Low risk | Registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000009239) before the commencement of data collection. Outcomes reported were consistent with the study protocol. |
| Other bias | High risk | The numbers randomized to each arm were extremely disproportionate (35 intervention; 53 control). Recognized in limitations but no discussion on the influence on the results: "Fourth, a slight difference was observed between the samples in the two arms despite our calculation and estimation of an appropriate sample size. As a result, our trial might not have had an adequate sample size to detect a difference between the two arms." |
Arterburn 2011.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 75 + 77 participants considering bariatric surgery in the USA | |
| Interventions | DA: booklet + video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to discuss with clinician). The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care (general information pamphlets on clinical problem) |
|
| Outcomes | Primary outcomes: knowledge, values, values concordance Secondary outcomes: treatment preference, decisional conflict, decisional self‐efficacy, proportion undecided Primary outcomes assessed at baseline, postintervention and 3 months follow‐up; secondary outcomes assessed at baseline and postintervention |
|
| Notes | Source of funding: This work was funded by the Foundation for Informed Medical Decision Making Inc., Grant nos. 0077‐4 and 0094‐1. The sponsor did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Conflicts of interest: D.E.A., E.O.W., T.A.B., and K.R.S. have support from the Foundation for Informed Medical Decision Making for the submitted work. D.E.A. receives research funding and has received salary support as a medical editor for the not‐for profit (501[3]c) Foundation for Informed Medical Decision Making (http://www.fimdm.org), which develops content for patient education programs ‐ including the bariatric surgery program that is the subject of this study. K.R.S. has also received research and salary support from the foundation. The Foundation has an arrangement with a for‐profit company, Health Dialog, to coproduce and market these programs to health‐care organizations. D.E.A. and K.R.S. have no relationship with any company making products for the treatment of obesity. The authors’ spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and authors have no nonfinancial interests that may be relevant to the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[U]sed computer‐assisted, block randomisation process to ensure balanced allocation of participants" (p 1670, Participants and randomization) |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment and no mention of impact on study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[S]tudy was not blinded" (p 1670, Participants and randomization); no mention of impact on study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Measures: mentioned 4 choices for treatment preference (surgery, drug therapy, diet and/or exercise program and unsure) but only reported on surgery and unsure options (p 1671); minimal attrition that was consistent between groups. |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol or trial registration; all pre‐specified outcomes included. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Auvinen 2004.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 100 men newly diagnosed with prostate cancer in Finland | |
| Interventions | DA: pamphlet patient decision aid created for study on options' outcomes, outcome probability, guidance. The DA is available as an appendix in the development article (Auvinen 2001). Comparator: usual care by clinical guideline |
|
| Outcomes | Primary outcome: uptake of options Secondary outcome: participation in decision‐making Other outcomes (from Huang 2014): death (5 years), disease‐free survival (10 years), biochemical failure (serum PSA elevation) (5 years), biochemical failure‐free survival (5 years), disease progression (5 years), disease progression‐free survival (5 years) (data from 104 + 106 men) |
|
| Notes | Source of funding: The study was supported financially by the Finnish Cancer Institute, Academy of Finland, Cancer Society of Finland, Pirkanmaa Cancer Society and Pohjois‐Savo Cancer Fund. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Auvinen 2001, p 2: "randomized centrally, using software based on a random number generator"; no blocking used Auvinen 2004, (primary study), p 1: "randomized using a computer algorithm based on random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Auvinen 2001, p 2, Patients and Methods: randomized centrally at the Finnish Cancer Registry Auvinen 2004, (primary study), p 1: randomized centrally Comment: central allocation confers low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Auvinen 2001, p 3: "recognized carry‐over effect because same physician in charge for intervention and control groups, diminish contrast between groups, as these physicians were more motivated to inform patients than those physicians not participating" Auvinen 2004 (primary study): no blinding but primary outcome is choice of treatment for prostate, objectively recorded. However, unsure how physicians may have influenced decisions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but primary outcome is choice of treatment for prostate, objectively recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Auvinen 2001, p 3: flow chart "Imbalance in the numbers of patients between the arms within two hospitals. Not expected to affect the results in any way"; "some participants refused to give informed consent, health deterioration, not seen by urologist" (p 4) Auvinen 2004 (primary study), p 2: flow diagram and results; low attrition and consistent between groups |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry. Auvinen 2001, p 2: "The study protocol was approved by an ethical committee in each participating hospital" Auvinen 2004 (primary study), p 1: "The study protocol was approved by the institutional review board at each participating hospital" |
| Other bias | Low risk | Appears to be free of other potential biases. |
Bailey 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 114 + 111 adults with type II diabetes considering additional antihyperglycemic medication to a metformin‐containing regimen to improve glycemic control in the USA | |
| Interventions | DA: interactive online decision aid that includes information on the clinical problem, explicit values clarification, guidance in decision‐making (steps in decision‐making, worksheet), and summary that can be taken to the consultation. The DA is not publicly available; a copy was provided by the author (Alicia C. Shillington: alicia.shillington@epi‐q.com). Comparator: usual care (no intervention) |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: decision self‐efficacy, decisional conflict | |
| Notes | Source of funding: The trial and manuscript submission was funded by Janssen Scientific Affairs, LLC. Conflicts of interest: R Bailey is an employee of Janssen Scientific Affairs, LLC and shareholder of Johnson and Johnson. Michael Pfeifer is an employee and shareholder of Johnson and Johnson. Alicia Shillington is an employee and shareholder of EPI‐Q Inc. Qing Harshaw is an employee of EPI‐Q Inc. Jeffery VanWingen is in private practice and received compensation from Janssen Scientific Affairs for enrollment of subjects into this investigation. Nananda Col is a consultant to Janssen Scientific Affairs. Martha Funnell has served on advisory boards for Eli Lilly, Bristol‐Myers Squibb, AstraZeneca Diabetes, Novo Nordisk, Omada Health, Sanofi US, and is a consultant to Janssen Scientific Affairs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "All staff analyzing data were blinded to treatment group assignment. Referring clinicians were blinded to group assignment, unless they were incidentally unblinded by subjects during a clinical consultation subsequent to enrollment (e.g., subjects mentioning the PDA or its contents during an office visit)." "... subjects were not blinded to treatment assignment, and this may have impacted results due to expectations raised regarding PDA participation benefits. Unclear risk because the participants were not blinded: "subjects were not blinded to treatment assignment, and this may have impacted results due to expectations raised regarding PDA participation benefits" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All staff analyzing data were blinded to treatment group assignment. Referring clinicians were blinded to group assignment, unless they were incidentally unblinded by subjects during a clinical consultation subsequent to enrollment (e.g., subjects mentioning the PDA or its contents during an office visit)." Outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All subjects were followed for approximately 6 weeks after randomization except for 20 who were lost to follow‐up (PDA group, n = 15; usual care group, n = 5)." 15/114 (13.2%) lost in patient DA arm versus 5/111 (4.5%) usual care arm. Additionally, another 5 from the patient DA group were "non‐adherent with the PDA". Reasons for attrition are not reported. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol is available (NCT02110979). One of the secondary outcomes (decision self‐efficacy) was not pre‐specified. |
| Other bias | Unclear risk | One or more of the authors are industry employees. Industry funding is declared with no description of role in the study. |
Barry 1997.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 104 + 123 patients considering benign prostatic hyperplasia treatment in the USA | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinion. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care using general information on the clinical problem |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: uptake of option, satisfaction with DM process, satisfaction with decision, interest in DM, general health outcomes, condition‐specific health outcomes |
|
| Notes | Source of funding: This project was funded by Grant Nos. HS 06540 and 08397 from the Agency for Health Care Policy and Research. The development of the first edition of the SDP for BPH was funded by a grant from the John A. Hartford Foundation. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified by study site in concealed blocks of 10" (p 2) |
| Allocation concealment (selection bias) | Low risk | Study co‐ordinator opened serially numbered, opaque, sealed envelopes (p 2). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but phase 1 eliminated risk of contamination. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but phase 1 eliminated risk of outcome assessor interfering with decision. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient accrual and follow‐up reported; post‐randomization withdrawals could have biased the results (more in intervention group). However, they reported no evidence of a differential effect on the study group (p 3). |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry. |
| Other bias | Low risk | Appears to be free of other potential biases. |
Bekker 2004.
| Study characteristics | ||
| Methods | Randomized to detailed vs routine consultation | |
| Participants | 59 + 58 pregnant women who have received a maternal serum screening positive test result for Down syndrome in the UK | |
| Interventions | DA (in consult): decision analysis plus routine consultation on options' outcomes, clinical problem, outcome probability, values clarification, guidance/coaching. The DA is available as an appendix in the article. Comparator: routine consultation on options' outcomes, outcome probability |
|
| Outcomes | Primary outcome: anxiety Secondary outcomes: uptake of option, knowledge, decisional conflict, informed decision‐making, satisfaction with consultation, consultation length |
|
| Notes | Source of funding: "Thank you to the MRC for funding Dr Bekker’s studentship". Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Bekker 2003, p 2 ‐ section 2.3 Sample and Procedure: "randomly allocated... using previously numbered... envelopes" Bekker 2004 (primary study), p 3: "Participants were randomly allocated by previously numbered envelopes"; does not mention how sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Bekker 2003, p 2 ‐ section 2.3 Sample and Procedure: "Using previously numbered, sealed, opaque envelopes" Bekker 2004 (primary study), p 3: previously numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded, personnel not blinded. Same personnel did control and intervention. Tape‐recorded sessions to ensure no bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Bekker 2003 flow diagram indicates post‐randomization attrition with more attrition in decision aid group; no discussion on implications of attrition. Bekker 2004 (primary study), p 4: results/flow diagram; baseline characteristics not included |
| Selective reporting (reporting bias) | Unclear risk | Bekker 2003: the coding frame was developed from literature. Does not mention protocol. Bekker 2004 (primary study): no information provided about central trials registry. |
| Other bias | Unclear risk | Bekker 2003: does not directly address baseline characteristics of participants. Bekker 2004 (primary study): appears to be free of other potential biases. |
Berger‐Hoger 2019.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid + decision coaching + structured physician's consultation vs usual care | |
| Participants | 37 (decision aid + coaching + structured consultation) versus 30 (usual care) German women, aged 18 years or older, with primary histologically confirmed ductal carcinoma in situ facing primary treatment decisions | |
| Interventions | Paper‐based decision aid provided before nurse decision coaching and in preparation for consultation with the physician. The DA included clinical information, outcome probabilities, explicit values clarification, QR code to access more information, and guidance in decision‐making and communication. The DA is not publicly available; a copy was provided by the author (Birte Berger‐Höger; birte.berger‐hoeger@uni‐bremen.de). Comparator: usual care |
|
| Outcomes | Primary outcome: extent of informed shared decision‐making Secondary outcomes: patients' and healthcare professionals' perspectives of shared decision‐making, informed choice (knowledge, attitude, uptake), decisional conflict, duration of coaching sessions and physician encounters |
|
| Notes | Source of funding: The German Federal Ministry of Health funded the study within the National Cancer Action Plan (Grant No. NKP – 332 – 054). Conflicts of interest: All authors have completed the disclosure form and declare no support from any organization for the submitted work other than those listed above; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, and no relationships or activities that could appear to have influenced the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The statistician (BH) provided a computer‐generated allocation sequence. During study progress, allocation might have become predictable. Thus, we used a random permuted block design with block sizes of 4, 6 or 8 to randomize clusters." |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed. An independent external person prepared sealed opaque envelopes. After baseline assessment of the respective cluster and its professionals, two researchers (BBH, KL) opened the sealed opaque envelope and revealed the center’s allocation on site. Patients were recruited by the participating physicians (electronic supplementary material S2) and kept unaware of their allocation status. After the final physician encounter, they were asked to guess whether they had received standard care or the new counselling approach." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[P]erson prepared sealed opaque envelopes"... "After baseline assessment of the respective cluster and its professionals, two researchers (BBH, KL) opened the sealed opaque envelope and revealed the center’s allocation on site"... "Patients were recruited by the participating physicians (electronic supplementary material S2) and kept unaware of their allocation status. After the final physician encounter, they were asked to guess whether they had received standard care or the new counselling approach." Participants were blinded so low risk of bias for that item. Unclear if personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for outcomes of interest to this review that were objectively measured and not subject to interpretation (i.e. knowledge, decisional conflict). High risk for the outcome of patient‐clinician communication only: "The primary outcome was the extent of informed shared decision‐making assessed by the observer‐based instrument of the validated inventory Multifocal APProach to the sharing‘ IN Shared Decision‐Making (MAPPIN’SDM). It assesses the mutual shared decision‐making‐behavior of health professionals and patients based on video‐recordings." "Due to the structural inequality between intervention and control group, video raters could not be blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For 8 patients, missing values were imputed (5 patients with missing values in 1, 2 or 3 items, 3 patients with missing values in all 11 items). |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | Cluster analysis on an individual level was planned; however, there were unanticipated low cluster sizes that resulted in unstable intracluster correlation coefficient estimations. As a result, cluster analysis was used as this is more robust, given the limitations of their recruitment/clusters. Free of other potential biases: no evidence of selective recruitment of cluster participants. |
Bergeron 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 24 + 26 families of children with obstructive sleep apnea and without tonsillar hypertrophy in the USA | |
| Interventions | DA: paper‐based option grid decision aid used during consultation that included clinical information and outcome probabilities. The DA is not publicly available; a copy was provided by the author (Stacey Ishman; stacey.ishman@cchmc.org). Comparator: usual care |
|
| Outcomes | Decisional conflict, preferred option including undecided, communication (collaboRATE scale) Secondary article: implemented treatment (actual choice), treatment modified |
|
| Notes | Source of funding: no funding Conflicts of interest: The authors have no funding, financial relationships, or conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out using a random number generator at the time of presentation to the clinic |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (outcomes assessor): the person administering the decisional conflict measures was blinded to the method used for each patient. No mention of blinding participants. Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single blinded (outcomes assessor): the person administering the decisional conflict measures was blinded to the method used for each patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, no loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bernstein 1998.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 65 + 53 patients with coronary artery disease considering revascularization surgery in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinion. The DA was available from the Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care (no information provided) |
|
| Outcomes | Primary outcome: satisfaction with decision and decision‐making process Secondary outcomes: uptake of option, knowledge, satisfaction with care, general health outcomes, condition‐specific health outcomes |
|
| Notes | Source of funding: This research was supported in part by a grant from the University of Michigan Hospitals Small Grant Program. Kim Skarupski was supported by a postdoctoral Health Services Research and Development fellowship by the Department of Veterans Affairs. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by study site in blocks of 10" (p 3) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization performed by a study coordinator opening opaque, sealed envelopes at study headquarters" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither participants nor study staff were blinded to treatment assignment ‐ could lead to different satisfaction ratings based on knowing the treatment received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3); low attrition of eligible participants randomized and consistent between groups |
| Selective reporting (reporting bias) | Unclear risk | No information provided indicating trial was included in central trials registry. |
| Other bias | Low risk | Appears to be free of other potential biases. |
Berry 2013.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 266 + 228 men considering prostate cancer treatment in the USA | |
| Interventions | DA: interactive web‐based video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to ask doctor and automated summary). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcome: preferred/actual treatment choice (pre‐ and post‐DA), proportion undecided Other outcomes (Bosco 2012): choice concordance (6 months post‐DA). (Data from 239 + 209 men). |
|
| Notes | Source of funding: NIH, R01‐NR009692. The funder did not have a role in the manuscript. This material is the result of work supported with resources and use of facilities at the Charlie Norwood VA Medical Center, Augusta, GA, VA Puget Sound Healthcare System, Seattle, WA, and the South Texas Veterans Health Care System, San Antonio, TX, all of which approved the submission of the manuscript. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods section, second paragraph, p 3: "Participants were randomized automatically by the P3P application to study groups (1:1 using a simple randomization scheme with no blocking)" |
| Allocation concealment (selection bias) | Low risk | Methods section, p 3: "Participants were randomized automatically by the P3P application to study groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded and study does not address the effect on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear whether outcome assessors are blinded, but outcomes are not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis and low dropout (p 4). |
| Selective reporting (reporting bias) | Low risk | Protocol made available |
| Other bias | Unclear risk | Was a multicentre trial, which could have lead to contamination, protocol violation, and biased questionnaire completion. |
Berry 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid plus usual education vs usual education plus links to reputable websites | |
| Participants | 198 + 194 men with clinically localized prostate cancer and an upcoming consultation in the USA | |
| Interventions | DA: online interactive decision aid plus usual care. The DA included a preliminary questionnaire including a values clarification exercise to elicit patients concerns and information was tailored based on their personal profile, guidance in communication, and an automated summary report that could be printed. Each clinician of an intervention group patient received the 1‐page summary of patient‐reported information to cue the provider to symptom issues, concerns, and preferences. The DA is publicly available at https://www.p3p4me.org/users/login . Comparator: usual education plus links to reputable websites |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: decision regret, actual choice (reported for full sample only) |
|
| Notes | Source of funding: Supported by National Institutes of Health, National Institute for Nursing Research R01NR009692 and CTN NCT01844999. Conflicts of interest: Traci M. Blonquist, financial interest and/or other relationship with Pfizer, and Johnson and Johnson |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomized to the intervention or the UC group in permuted blocks of 4 as stratified by clinic site via an algorithm embedded in the software" |
| Allocation concealment (selection bias) | Low risk | Web‐based central allocation: "participants were randomized to the intervention or the UC group in permuted blocks of 4 as stratified by clinic site via an algorithm embedded in the software" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research assistants were not blinded to study group assignment but all patient‐reported outcome measures were self‐administered. Outcomes were objectively measured and not subject to interpretation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate, but balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT01844999). One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified. For the primary outcome of "Decisional conflict [ Time Frame: Change from baseline to 6‐months ]" only 1‐month data are reported. The primary outcome of "Preparation for decision making [ Time Frame: 1‐month after study entry ]" is not reported. |
| Other bias | Unclear risk | "we excluded the lowest accruing sites from the final analytical sample, which were mainly independent or nonnetworked practices" |
Beulen 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 157 + 157 pregnant women aged 18 years or older in the Netherlands | |
| Interventions | DA: online interactive decision aid (text and video) on clinical problem, outcome probabilities, explicit values clarification, guidance in decision making (systematic steps to go through), guidance in communication, and summary to take to consult. The DA is publicly available at https://www.keuzehulp.info/cz/pnt/intro/1 . Comparator: usual care using standard counseling and brochure |
|
| Outcomes | Primary outcome: informed decision‐making Secondary outcomes: knowledge, attitudes, prenatal test utilization, value‐consistency, decision conflict, decisional regret, and anxiety |
|
| Notes | Source of funding: Foundation for Prenatal Screening in the Nijmegen Region. Conflicts of interest: The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After obtaining informed consent, participants were allocated to the control or intervention group by a computer‐generated randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 90% of enrolled patients are included in the analysis: the 261 remaining women (130 randomised to the control group and 131 randomised to the intervention group) were included in the analysis: 261/314 (83%). However, missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available and therefore there is no way to verify whether the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bjorklund 2012.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 236 + 247 women less than 11 weeks pregnant considering Down syndrome screening in Sweden | |
| Interventions | DA: linear video on options' outcomes, clinical problem, outcome probabilities, others' opinion, and guidance (step‐by‐step process for making the decision). The DA is no longer available at vimeo.com/34600615/. Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), attitude (post‐DA), uptake of combined ultrasound and biochemical screening (post‐DA) Secondary outcomes: values congruent with chosen option (post‐DA) |
|
| Notes | Source of funding: This study was supported by grants from Sophiahemmet University College and from Södersjukhuset, Department of Obstetrics and Gynecology, Stockholm, Sweden. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The midwife allocated the participants randomly by sealed envelopes" (p 391) but does not state the actual sequence generation method. |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes, "prepared, sequentially coded and distributed to the maternity units by the research group" (p 391). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was not possible to blind neither [sic] the midwives nor the participants due to the characteristics of the intervention" (p 395). The study does not address the effects of this on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of why some participants' data were excluded in Tables 2, 3, and 4. |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Bonner 2022.
| Study characteristics | ||
| Methods | Randomized to standard DA vs literacy sensitive DA vs control | |
| Participants | 293 + 301 + 299 from a national sample of people aged 45 to 74 in Australia | |
| Interventions | DA: decision aid used independently that includes a risk calculator of having a heart attack, options to decrease their risk, probabilities of outcomes, implicit values clarification, guidance in decision‐making (step‐by‐step process), and lifestyle action plan with summary. An example of the DA is available as a supplementary appendix in the article. Comparator: risk calculator, information plus action plan |
|
| Outcomes | Primary outcome: lifestyle intentions Secondary outcomes: ability to recall their risk, credibility of the risk results, emotional response to risk results, decisional conflict |
|
| Notes | Source of funding: This study was funded by a Vanguard Grant from the National Heart Foundation of Australia (ID 102215). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate and balanced across groups. Analyzed control 135 + 155 = 290/299 (missing 3%); analyzed standard DA 148 + 137 = 285/293 (missing 3%). |
| Selective reporting (reporting bias) | Low risk | The study protocol is registered (ACTRN12620000806965) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "A limitation is that the web‐based panel sample may not be representative of the general population and may better reflect users of web‐based heart age tools than patients presenting to primary care for CVD risk assessment. The study was powered by moderate effect sizes and therefore may have lacked the power to detect more subtle differences; however, these findings will be useful for informing sample size calculations for future studies" |
Bourmaud 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual information | |
| Participants | 7885 + 7959 women who were invited to participate in a population‐based breast cancer screening program in France | |
| Interventions | DA: paper‐based leaflet that included information on breast cancer risk, probabilities of outcomes, implicit values clarification, and guidance in communication. The DA is publicly available as a supplementary file in the publication https://www.oncotarget.com/article/7332/text/ . Comparator: usual standard information |
|
| Outcomes | Primary outcome: women’s attendance rate for the breast cancer screening program Secondary outcome: delay between the invitation and the date of attendance for breast cancer screening |
|
| Notes | Source of funding: This study was supported by the French National Association against Cancer (Ligue National Contre le Cancer). Conflicts of interest: PSM declares a conflict of interest through her activity, being a practitioner involved in breast cancer screening promotion at a local level. All the others authors declare no financial support for the submitted work; no relationships that might have an interest in the submitted work in the previous three years; None of their spouses, partners, or children have financial relationships that may be relevant to the submitted work; and none have non‐financial interests that may be relevant to the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomly assigned in a 1:1 ratio via a computer‐generated, centralized randomization sequence, which was done with a block randomization of four, to the DECIDEO or usual invitation group. The randomization was balanced through stratification according to the following hierarchy: the department, the age according to 2 classes (above or below 65), and the number of invitations already received by the women (leading or not, to participation in national screening)" |
| Allocation concealment (selection bias) | Low risk | "Women were randomly assigned in a 1:1 ratio via a computer‐generated, centralized randomization sequence" (central allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT analysis, reasons for participants excluded from analysis, low attrition rate (< 2% to 3%) |
| Selective reporting (reporting bias) | Low risk | The trial was registered retrospectively (NCT02093039). "This study was later registered in clinicaltrial.gov on 03/19/2014". However, it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | "imbalance in the number of women excluded from the analysis due to screening attendance before reception of the invitation (115 vs 41 in the intervention and control groups, respectively)", "Some women were excluded because there was a delay between the invitation being sent by the cancer screening association and its reception by the women; during the delay some of the randomized women had already attended breast cancer screening since they did not need to take the invitation letter with them." The difference is significant (P < 0.00001). Acknowledged in the limitations but not discussed: "One last limitation concerns the imbalance in the number of women excluded from the analysis due to screening attendance before reception of the invitation". |
Bozic 2013.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 95 + 103 participants with hip and/or knee osteoarthritis considering hip/knee surgery in the USA | |
| Interventions | DA: DVD and booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, and guidance/coaching with health coach. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. The authors have a copy of the video and booklet that was evaluated in the study. Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: informed decision/knowledge (pre, immediately post, and 6 weeks follow‐up) Secondary outcomes: preferred treatment choice (pre and immediately post), patient and provider satisfaction (immediately post), length of consultation time |
|
| Notes | Trial registration: NCT01492257 Source of funding: This work was supported by a grant from the RobertWood Johnson Foundation (RWJF). Funds were used to pay for salaries, employee benefits, and other direct costs such as office operations, communications, meetings, travel, surveys, and contracts. The funding source did not play a role in the investigation. Conflicts of interest: One or more of the authors received payments or services, either directly or indirectly (i.e. via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty‐six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was blocked with use of random permuted blocks in groups of four, six, or eight to help ensure that the groups were balanced" (p 1634) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to either the intervention group or the control group with use of the sealed envelop method" (p 1634) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[S]urgeons were not blinded to the intervention" (p 1635). Knowing the allocation of participants, surgeons' favorable scoring could be due to greater investment in decision‐making. Insufficient information to make a judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 62% (123/198) retention rate therefore high attrition rate; however, the attrition was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Brazell 2014.
| Study characteristics | ||
| Methods | Randomized to DA + standard counselling vs usual care + standard counselling | |
| Participants | 53 + 51 women presenting for the management and treatment of pelvic organ prolapse in the USA | |
| Interventions | DA: paper‐based or web‐based DA on clinical problem, options' outcomes, outcome probabilities, patient stories and standard counseling. The DA developed by Healthwise is available at https://decisionaid.ohri.ca/Azsumm.php?ID=1228 . Comparator: standard counseling alone |
|
| Outcomes | Primary outcomes: decisional conflict (immediately post‐consultation) Secondary outcomes: choice (3 months after making decision), decisional regret (3 months after making decision) |
|
| Notes | Source of funding: The decision aid used for this study was developed by Healthwise and provided to the authors at no cost. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized 1:1 using a random numbers table in blocks of 6" (p 231) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to make judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to make judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition but balanced between groups: "39 randomized subjects were either missed by the research assistant at their new patient visit and thus did not receive a DCS questionnaire to complete or they canceled their appointments and did not reschedule a new one" (p 233). There was a 48% (50/104) attrition rate for decisional regret measures. |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | High risk | Risk of contamination due to same physicians in both groups. Also, outcomes measured after the patient DA and physician consultation. |
Brown 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid + coaching vs usual care | |
| Participants | 19 (decision aid + coaching) versus 22 (usual care) adults aged 70 years of age and older with advanced chronic kidney disease attending hospital‐based nephrology services considering renal replacement therapy in Australia | |
| Interventions | DA: paper‐based decision aid and audio‐recording that included clinical information, probabilities of outcomes, explicit values clarification, guidance in decision‐making (step‐by‐step process), guidance in communication, with summary worksheets and examples of how to complete them, and suggested publicly available resources. Decision coaching: 1 month after receiving the DA, a trained renal nurse used the DA to support patient in active, autonomous role in decision‐making in 1 or 2 sessions in person for 45 minutes at a public hospital renal program and then again 3 months later if a decision had not been made. The DA is not publicly available; a copy was provided by the author (Leanne Brown; leanne.brown2@health.qld.gov.au). Comparator: usual care (education) |
|
| Outcomes | Primary: decision regret, decisional conflict Secondary: knowledge, quality of life, participants’ and nurses’ perceptions of the usefulness of the patient DA (preparation for decision‐making) |
|
| Notes | Source of funding: National Health and Medical Research Council; Australian Centre for Health Service Innovation (AusHSI); Queensland Health Nursing and Midwifery Research Fellowship; Chronic Kidney Disease Centre of Research Excellence; Sunshine Coast Hospital and Health Service; Wide Bay Hospital and Health Service. Conflicts of interest: No conflict of interest has been declared by the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... through a computer‐generated program using block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | "Randomisation occurred once the eligibility of the participant was confirmed, consent provided and baseline data collected. Allocation of the participant to either intervention or standard care occurred through a computer‐generated program using block randomisation." Nurse not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; it is unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | To minimize bias, the outcome research assistant and the lead researcher were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few dropouts. All participants included. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes as reported in study registration (ACTRN 12614001090606). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Carlson 2019.
| Study characteristics | ||
| Methods | Randomized to DA + standard counseling vs standard counseling alone | |
| Participants | 105 + 92 women with a singleton gestation at less than 22 weeks scheduled to meet with a genetic counselor at 1 of 3 prenatal diagnosis clinics for a discussion of aneuploidy screening in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that describes clinical condition, outcome probabilities, and explicit values clarification. It is the same evaluated by Kupperman 2014 but modified to include new options. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care (genetic counseling) |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: decisional conflict, choice of testing, pursuit of invase testing |
|
| Notes | Source of funding: The project described was supported by the Clinical and Translational Science Award program of the Division of Research Resources, National Institutes of Health, through grant award no. 1UL1TR001111, by the UNC Center for Maternal and Infant Health, through the Cefalo‐Bowes Young Researcher Award, and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development BIRCWH K12 Grant HD001441 (N.L.V.) and K23 HD088742 (N.L.V.). Conflicts of interest: The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via a coin‐flip algorithm within the app, women were randomly assigned to group 1 (control group) or group 2 (decision aid group). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As it was not pragmatic for this study, randomization assignment was not blinded. Unclear if measurements could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As it was not pragmatic for this study, randomization assignment was not blinded. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, 100% included in analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02991729) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Carroll 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 41 + 41 new candidates for implantable cardio‐defibrillators in Canada | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, role in decision‐making, SURE test, guidance in decision‐making (5‐step guide), and guidance in communication. The DA is not publicly available; a copy was provided by the author (Sandra L. Carroll; carroll@mcmaster.ca). Comparator: usual care (general education after decision to accept the implantable cardio‐defibrillator is established) |
|
| Outcomes | Primary outcome: feasibility of conducting the RCT Secondary outcomes: knowledge, decisional conflict, SURE test, Preparation for Decision‐Making Scale |
|
| Notes | Source of funding: This study was funded by the Canadian Institutes of Health Research (CIHR)—operating grant #119449. Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization took place prior to electrophysiology specialist consultations using a centralized Internet randomization service (https://www.randomize.net). |
| Allocation concealment (selection bias) | Low risk | The use of https://www.randomize.net ensured that the allocation sequence was concealed from the research assistant. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the nature of the intervention, patients and the research assistant collecting data were not blinded to study group assignment. Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The data analyst was blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Case 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 50 + 49 new patients over the age of 18 years being evaluated for chest pain with no known history of coronary artery disease in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that included clinical information, outcome probabilities, explicit values clarification, individualized risk calculator, and patient testimonies. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Knowledge, decisional conflict, satisfaction with the decision‐making process, trust in physician, acceptability of DA, preparation for decision‐making (reported for DA group only) | |
| Notes | Source of funding: A research grant from the Lee and Juliet Folger Fund Foundation. Conflicts of interest: The authors have reported that they have no relationships relevant to the contents of this paper to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by a random number generator with assignment blinded in a sealed folder before enrolment. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by a random number generator with assignment blinded in a sealed folder before enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The treating health care provider was blinded to randomization, and patients were advised not to discuss randomization with the provider. "although health care providers were blinded to enrollment and randomization, some unintentional unblinding may have occurred because patients enrolled in the study may have had their study folder and iPad with them in the patient room in order to maximize their time with using the PDA and completing the questionnaires. This may have biased providers in their interaction with patients who they suspected were enrolled in the study than they otherwise would be." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow diagram, high attrition rate in the DA group 7/50 (14%) compared to the standard care group 1/49 (2%). The difference between groups is significant (P = 0.029042). |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | "selection bias was also present in our study due the fact that in general patients who are referred to outpatient clinic are overall a lower risk patient population and less likely to undergo a higher risk test such as invasive coronary angiography" |
Chabrera 2015.
| Study characteristics | ||
| Methods | Randomized to DA vs usual care | |
| Participants | 73 + 74 men recently diagnosed with prostate cancer considering treatment options in Spain | |
| Interventions | DA: 2‐part decision support booklet with clinical problem, options' outcomes, outcome probabilities, patient stories, explicit values clarification, and guidance. The DA is not publicly available; a copy was provided by the authors (cchabrera@tecnocampus.cat). Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, satisfaction with decision‐making process Secondary outcome: coping Outcomes assessed at 3 months postintervention |
|
| Notes | Source of funding: This project was supported by the Official Nursing College of Barcelona and the Badalona Against Cancer Foundation. Conflicts of interest: The authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[S]tudy participants were randomized into 1 of 2 arms using a computer‐generated random list with unequal blocks" (p E44) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to make a judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make a judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to make a judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced attrition in both groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided; trial not registered |
| Other bias | Unclear risk | Prostate cancer in Catalonia is common; however, only 147 were recruited for this trial (p E44). |
Chambers 2012.
| Study characteristics | ||
| Methods | Randomized to DA vs usual care | |
| Participants | 74 + 77 healthcare workers who did not receive the influenza vaccine considering receiving the vaccine in Canada | |
| Interventions | DA: web‐based DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance. The DA is available at https://decisionaid.ohri.ca/Azsumm.php?ID=1562 . Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: confidence in decision (post‐DA) Secondary outcomes: impact on immunization intent (post‐DA), proportion undecided |
|
| Notes | Source of funding: This trial is funded under a three‐year, Canadian Institutes of Health Research (CIHR), Institute of Population and Public Health, Team Grant: Pandemic Preparedness ‐ Influenza Biology, Vaccines, Ethics, Legal and Social Research Grant #90189, in partnership with the CIHR Pandemic Preparedness programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated using the randomization function in Excel 2002 (version 10.6856.6856 SP3)" (p 199) |
| Allocation concealment (selection bias) | Low risk | "The list was imported from Excel into a Microsoft SQL Server database. The online application would sequentially assign a random identification number and their decision aid status (seeing the decision aid or not) from the randomization list when users logged into the survey." (p 199) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported whether or not they were blinded during the course of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Questionnaire scores are objective and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65% completion rate in intervention arm and 77% completion rate in control arm: attrition could be different where the respondents and non‐respondents are different. |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Unclear risk | Figure 1 numbers for exclusion are not logical. |
Chen C 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid + decision coaching vs standard education material | |
| Participants | 67 + 63 patients aged ≥ 20 years with first episode of low back pain with diagnosis of low back pain, spinal stenosis, intervertebral disc disorders, spondylolisthesis, or other spondylosis in Taiwan | |
| Interventions | DA: paper‐based booklet used in conjunction with a decision coach in preparation for consultation with the physician that included clinical information, explicit values clarification, knowledge test, guidance in decision‐making (5‐step guide), guidance in communication (used with a decision coach), knowledge test and plan for subsequent steps. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: standard educational material |
|
| Outcomes | Primary outcome: decision self‐efficacy Secondary outcomes: participation in decision‐making (Control Preferences Scale), shared decision‐making (SDM‐Q9), decisional conflict, satisfaction with decision (SWD) |
|
| Notes | Source of funding: This study was supported by the Ministry of Science and Technology (grant number MOST‐107‐2314‐B‐038‐026‐MY3), Taipei Medical University– Shuang Ho Hospital, Ministry of Health and Welfare (grant numbers 107 TMU‐SHH‐17, 108TMU‐SHH‐23), and Taipei Medical University Hospital (109TMUH‐H‐01). Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Few details: randomization was performed by a research assistant with no knowledge of the trial. |
| Allocation concealment (selection bias) | Low risk | After completing a pretest questionnaire, each participant opened a sealed, opaque randomization envelope that informed them of their assignment to either the intervention group (decision coaching with DAs) or the comparison group (patient education by another health educator). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was an RCT with blinding of both patients and their physicians… The decision coaching and patient education interventions were independently conducted in an assessment room separate from the consultation room to keep the patients blinded and minimize treatment contamination between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis, small loss to follow‐up (1 per group) with justification |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03679494) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Chen S 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid + usual care vs usual care | |
| Participants | 33 + 32 women eligible for vaginal birth after cesarean in Taiwan | |
| Interventions | DA: paper‐based booklet used in preparation for consultation that includes clinical information, probabilities of outcomes, explicit values clarification exercise with examples, glossary of terms, list of resources, guidance in decision‐making (step‐by‐step process), and guidance in communication. The DA is not publicly available; a copy was provided by the author (Allison Shorten; ashorten@uow.edu.au). The English version of the DA is available for purchase at https://www.capersbookstore.com.au/product/birth-choices-vaginal-or-caesarean-birth/ . Comparator: education on "do's and don’ts" during pregnancy |
|
| Outcomes | Primary outcomes: decisional conflict, knowledge Secondary outcomes: birth mode preference, birth outcome, and satisfaction with decision |
|
| Notes | Source of funding: This study was funded by the Ministry of Science and Technology in Taiwan (MOST 106‐2314‐B‐255‐006). Conflicts of interest: Dr. Shorten is the author of the birth choices decision aid booklet. She does not have any financial interest in the distribution or sale of the booklet. The authors declare that they have no competing interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer permuted block randomization, participants were allocated to control group (usual care) and intervention group (usual care plus the decision aid). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and providers were blinded to allocation in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis; missing outcome data are balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | Small sample size (randomized 33 + 32; last follow‐up 29 + 30), did not attain statistical power, matched pair t‐tests used |
Clancy 1988.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 753 + 263 health physicians considering hepatitis B vaccine in the USA | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification (personal decision analysis), guidance/coaching. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care (no information provided) |
|
| Outcomes | Uptake of option | |
| Notes | Source of funding: not reported Conflicts of interest: Dr. Clancy was a Henry J. Kaiser Family Foundation Fellow in General Internal Medicine at the University of Pennsylvania when this study was conducted, and Drs. Cebul and Williams were Kaiser Faculty Scholars in General Internal Medicine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table; all incoming residents were assigned to Group 2 (non‐randomized residents identified as subgroup) (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or personnel. Did not report on how this may affect their findings. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding, but decisions for screening were retrieved from health records (objective data). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow chart not included. Insufficient information to make a judgment. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Potential selection bias ‐ non‐randomized residents were added to group 2 and therefore potential unbalanced distribution (p 287). Low response rate among those offered decision analysis. |
Cox 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no intervention) | |
| Participants | 138 (plus 210 surrogate decision‐makers) + 139 (plus 206 surrogate decision‐makers) patients aged 18 or older with no anticipation of death or liberation from mechanical ventilation within 24 hours, and ventilation for at least 10 days in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, explicit values clarification, example patient/family scenarios, individualized 1‐year prognosis estimate, preferred role in decision‐making, guidance in decision‐making (step‐by‐step process), guidance in communication, and 2‐page summary for discussion with family and clinician. The DA is not publicly available; a copy was provided by the author (Christopher E. Cox; christopher.cox@duke.edu). Comparator: control (no intervention) |
|
| Outcomes | Primary outcome: clinician‐surrogate concordance (a measure of both the alignment of prognostic expectations and the quality of information exchange among decisional participants) Secondary outcomes: knowledge, satisfaction with clinician communication, anxiety and depression, post‐traumatic stress symptom inventory, decisional conflict, patient perception of care centeredness, patient length of stay |
|
| Notes | Source of funding: Supported by grant R01 HL109823 from the National Institutes of Health. Conflicts of interest: Drs. White, Hough, Kahn, and Olsen and Mr. Jones report grants from the National Institutes of Health during the conduct of the study. Dr. Carson reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study and grants from Biomarck Pharmaceuticals outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18‐2335. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A password‐protected computerized system randomly assigned patients and their surrogates 1:1 to either intervention or control in blocks of 4, stratifying by site. |
| Allocation concealment (selection bias) | Low risk | A password‐protected computerized system randomly assigned patients and their surrogates 1:1 to either intervention or control in blocks of 4, stratifying by site (central allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding during outcome assessment after randomization was ensured by use of a second co‐ordinator at each site who was unaware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate for Interview 2 when outcomes of interest to the review were collected and missing data are balanced across groups. Reasons for attrition provided. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01751061) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "unmeasured physician‐level effects or contamination among clinicians could have biased results toward the null hypothesis" |
Coylewright 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 70 + 62 adults (aged ≥ 18 years) who were candidates for both optimal medical therapy and percutaneous coronary intervention for the treatment of stable coronary artery disease in the USA | |
| Interventions | DA: paper‐based decision aid used during consultation that includes outcome probabilities, guidance in decision‐making, and communication (used during consultation). The DA is publicly available at https://carethatfits.org/pci-choice/ . Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcome: measure of shared decision‐making using OPTION |
|
| Notes | Source of funding: This study was supported by the Mayo Clinic Kern Center for the Science of Healthcare Delivery. Conflicts of interest: Dr Hess’s institution has received funding from the Patient Centered Outcomes Research Institute for investigator initiated research (952,12‐11‐4435, and 0876‐SAEM). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization took place on a secure study website using a computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | The randomization took place on a secure study website using a computer‐generated allocation sequence, which randomized patients in a concealed fashion to decision aid versus usual care. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible for patients and involved clinicians. Unclear how lack of blinding influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label according to trial registry. Low risk for outcomes that were objectively measured and not subject to interpretation (knowledge, decisional conflict). High risk for one outcome subject to interpretation (patient‐clinician communication: analysis of video‐recordings using the OPTION scale). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: low attrition rates and similar across arms (< 10%); reasons for attrition recorded. Evaluable for analysis 65/70 DA and 59/62 usual care (P = 0.579774). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01771536) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Crew 2022.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard usual care vs standard usual care | |
| Participants | 142 + 148 women aged 35 to 75 years with a 5‐year invasive breast cancer risk ≥ 1.67% in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, patient scenarios, risk game, individualized breast cancer risk factors, guidance in decision‐making (list of steps), and summary in the action plan that can be printed and discussed with the clinician. The DA is not publicly available; a copy was provided by the author (Katherine D. Crew; kd59@cumc.columbia.edu). All clinicians had access to the Breast cancer risk NAVigation toolbox for providing them with their patients’ personalized risks and preferences prior to the clinical encounter. Comparator: usual care (education) |
|
| Outcomes | Primary outcome: choice uptake Secondary outcomes: perceived breast cancer risk, breast cancer worry, chemoprevention knowledge, self‐efficacy, decision conflict, informed choice |
|
| Notes | Source of funding: none Conflicts of interest: The authors declare no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized 1:1 and stratified by Hispanic ethnicity and menopausal status". The investigators describe the use of stratification (use of computer implied). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow diagram, missing data across groups is significantly different (complete data at 1 month were 120/148 for DA group and 133/142 for control group (P = 0.001327)). No justification for loss to follow‐up provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT03069742). Several outcomes of interest to the review were not pre‐specified (self‐efficacy, decisional conflict, informed choice). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Cuypers 2018.
| Study characteristics | ||
| Methods | Cluster‐randomized to online DA + usual care vs usual care (information + counseling) | |
| Participants | 235 + 101 patients newly diagnosed with localized prostate cancer in the Netherlands | |
| Interventions | DA: online decision aid that included information on the clinical problem, outcome probabilities, explicit values clarification, decision‐making guidance, guidance in communication, and a summary sheet to share with the urologist. The DA is not publicly available; access to the decision aid was provided by the author (Maarten Cuypers: maarten.cuypers@radboudumc.nl). Comparator: usual care that included information and counseling |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: patient involvement, knowledge, satisfaction with information, anxiety, depression. Decision regret measured 12 months later (Cuypers 2019). |
|
| Notes | Source of funding: This research is funded by CZ Fund, a Dutch not‐for‐profit health insurer (Grant 2013‐00070) and Delectus Foundation, a Dutch non‐profit foundation aimed to initiate and stimulate research into shared decision‐making. The funding agreements ensured the authors’ independence in designing, conducting, and analyzing the results. MdV obtained funding from CZ; PK is chairman of Delectus Foundation. Conflicts of interest: The authors declare that they have no further conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eighteen Dutch hospitals were randomized to the intervention or control arm." Sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although patients were unaware of randomization at hospital level and were not informed that the DA was the subject of this study, care providers were aware that the purpose of the study was to compare the DA to usual information routines. During counseling, the novelty of the DA might have been over‐emphasized...In the control arm, this could have led to modifications of existing information or counseling routines due to the increased attention for SDM from this study, or in the DA group, to the creating of too high expectations as care providers could have (over‐)emphasized the novelty of the DA. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Response rate at T1 (after treatment decision‐making) was 235/273 (86%) in the DA group and 101/111 (91%) in the control group (Cuypers 2019). At 6 months, 214 (78%) and 94 (85%); at 12 months, 208 (76%) and 85 (77%). Low recruitment in the control groups. |
| Selective reporting (reporting bias) | Low risk | Dutch Trial Register (NTR4554); health‐related quality of life and skills not reported, but they were secondary outcomes and not of interest to the current review. |
| Other bias | High risk | Usual information and counseling was not described to determine if it was also a patient decision aid. Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Davison 1997.
| Study characteristics | ||
| Methods | Randomized to decision aid + audio‐taped consultation vs usual care | |
| Participants | 30 + 30 men with prostate cancer considering treatment in Canada | |
| Interventions | DA: written + audiotape consultation of options' outcomes, clinical problem, outcome probability, others' opinion. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care (general information pamphlets on clinical problem) |
|
| Outcomes | Primary outcomes: role in decision‐making Secondary outcomes: anxiety, depression |
|
| Notes | Source of funding: Supported by a studentship from the National Cancer Institute of Canada with funds provided by the Canadian Cancer Society to the first author, and by an investigator award from the Medical Research Council of Canada and the National Health Research and Development Program to the second author. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The group to which subjects were assigned was predetermined by a block randomization procedure. This ensured there were an equal number of subjects in both groups for each physician." (p 5, Data collection) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned; group assignment predetermined by block randomization procedure (p 5) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding and whether outcomes could be affected by unblinded assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No flow diagram; p 12 explains why certain men did not listen to audiotape. All men approached by study investigator agreed to participate; only 1 man refused to complete the second set of questionnaires. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other sources of bias; similar baseline characteristics |
De Achaval 2012.
| Study characteristics | ||
| Methods | Randomized to detailed vs simple vs usual care | |
| Participants | 70 + 70 + 71 patients diagnosed with knee osteoarthritis considering treatment in the USA | |
| Interventions | Complex DA: video booklet + interactive joint analysis on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (list of questions) Comparator DA: video booklet on options' outcomes, clinical problem, outcome probabilities, others' opinion and guidance (list of questions) The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. The authors have a copy of the video and booklet that was evaluated in the study. Comparator: usual care receiving generic booklet |
|
| Outcomes | Decisional conflict (baseline and postintervention) | |
| Notes | Source of funding: Supported by the Agency for Healthcare Research and Quality through the Center for Education and Research on Therapeutics (grant U18‐HS016093). Dr. Fraenkel’s work was supported by an NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases K23 award (AR‐048826‐05). Dr. Suarez‐Almazor holds a K24 career award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR‐53593‐06) and is the Director of the Houston Center for Education and Research on Therapeutics funded by the Agency for Healthcare Research and Quality. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list with uneven blocks (p 231) |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, and opaque envelopes (p 231) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Likely not blinded, but low threat of bias in study (p 231) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were not blinded but outcome was objectively measured (p 231). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts; missing data effect size unlikely to have significant impact on study outcome |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Dolan 2002.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 50 + 47 average risk for colorectal cancer considering screening in the USA | |
| Interventions | DA: computer with analytic hierarchy process on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance/coaching. The DA is not publicly available. Comparator: usual care with information on options, clinical problem |
|
| Outcomes | Primary outcomes: uptake of option, decisional conflict Secondary outcomes: role in decision‐making |
|
| Notes | Source of funding: This project was supported by grant number R03 HS10728 from the Agency for Healthcare Research and Quality. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomization schedules were created using a computer random number generator" (p 2, Study interventions) |
| Allocation concealment (selection bias) | Low risk | Computer‐based (p 2, Study interventions) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding of participants. All patient interviews in both the experimental and control groups were done by the same investigator; unclear on how this could contribute to risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram ‐ low attrition |
| Selective reporting (reporting bias) | Unclear risk | Nothing specifically mentioned re study protocol. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Durand 2021.
| Study characteristics | ||
| Methods | Cluster‐randomized to text‐only decision aid (Option Grid) vs pictorial decision aid (picture Option Grid) vs usual care | |
| Participants | 66 (text DA) + 248 (pictorial DA) + 257 (usual care) women 18 years and older with a biopsy‐confirmed diagnosis of early‐stage breast cancer (stages I‐IIIA) eligible for breast‐conserving surgery and mastectomy in the USA | |
| Interventions | DA: option grid decision aid used during consultation that included clinical information, probabilities of outcomes, and implicit values clarification. The DAs are presented in Figure 1 of the article. Data were extracted for the text‐only decision aid. Comparator: usual care, which was variable by site (e.g. information sheets, posters, video clips, etc.) |
|
| Outcomes | Primary outcomes: decision quality (3 subscales: extent to which patients are informed about treatment options (knowledge score), receive surgery aligned with their preferences (concordance score), and are involved in decision‐making (decision process score)) Secondary outcomes: treatment choice, treatment intention, shared decision‐making (collaboRATE and Observer OPTION‐5), anxiety, quality of life, decision regret, co‐ordination of care |
|
| Notes | Source of funding: The research reported in this article was funded through an award from the Patient‐Centered Outcomes Research Institute (1511‐32875). The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672). Conflicts of interest: Glyn Elwyn and Marie‐Anne Durand have developed the Option Grid patient decision aids, which are licensed to EBSCO Health; they receive consulting income from EBSCO Health and may receive royalties in the future. A. James O’Malley reports grants from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Patient‐Centered Outcomes Research Institute. Mary C. Politi reports grants from Merck outside the submitted work. Catherine H. Saunders holds a copyright in the consideRATE suite of tools. Karen Sepucha received salary support from 2014 to 2018 as a member of the scientific advisory board for Healthwise, a not‐for‐profit foundation that develops and distributes patient education and decision support materials; she also reports grants from the Agency for Healthcare Research and Quality, the Patient‐Centered Outcomes Research Institute, and the Patrick and Catherine Weldon Donaghue Medical Research Foundation outside the submitted work. Richard J. Barth reports grants and other from CairnSurgical, Inc, and grants from the National Institutes of Health outside the submitted work; in addition, Barth has a patent licensed to Dartmouth College. The other authors made no disclosures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "To minimize contamination, we randomized surgeons to 1 of 3 arms nested within 4 cancer centers. We used balanced block randomization to account for the varying number of surgeons at each site." (use of computer implied) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (outcomes assessor) according to the study protocol. Surgeons and participants were not blinded and therefore it is unclear how this may have affected the surgeon's performance in delivering the intervention/comparator and influence on the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The statistical analyst was blinded to site and arm assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, high attrition rates but missing data have been imputed using appropriate methods. "At T3, the number of patients with missing data ranged from 89 (19.9%) for knowledge to 98 (21.9%) for decision process. Multiple imputation analyses suggested minimally different estimates when data were imputed for most outcomes in comparison with no imputation. We can thus be assured that current findings are very unlikely to be overturned by accounting for missing data via multiple imputation." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03136367) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | High risk | Surgeons were randomized, patients were unit of observation (not randomized). This led to imbalanced allocation to arms due to differences in the number of patients seen by each surgeon (66 patients allocated to Option Grid and 257 patients allocated to usual care). They planned to stratify patients according to socioeconomic status in statistical analyses, but they did not enforce balance with respect to socioeconomic status when enrolling participants. Acknowledged in limitations but not discussed: “Randomization at the surgeon level led to chance imbalance between arms, with surgeons in the Option Grid arms having lower volumes of eligible patients and different distributions of the patient characteristics. Attempts to modify these patterns were unsuccessful. And lower recruitment than planned” Potential conflicts of interest: "Glyn Elwyn and Marie‐Anne Durand have developed the Option Grid patient decision aids, which are licensed to EBSCO Health; they receive consulting income from EBSCO Health and may receive royalties in the future" Selective recruitment of cluster participants: "We randomized breast surgeons to accrue patients in 1 of 3 trial arms for 18 months. We recruited English‐, Spanish‐, and Mandarin Chinese‐speaking women (18 years old or older) with a biopsy‐confirmed diagnosis of early‐stage breast cancer (stages I‐IIIA) eligible for breast‐conserving surgery and mastectomy according to medical records and participating surgeons’ judgment." (High risk) Free of other potential biases: adjustment for clustering performed. |
Ehrbar 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid + counseling vs control (counseling alone) | |
| Participants | 40 + 39 female patients aged 18 to 40 scheduled to undergo cancer treatment that potentially endangered their fertility in Switzerland and Germany | |
| Interventions | DA: online decision aid provided post‐consultation that included clinical information, explicit values clarification, guidance in decision‐making (step‐by‐step process), guidance in communication, and visual summary showing average values for both the pros and cons that can be printed or downloaded. The DA is publicly available at https://www.fertionco.ch/de/home/ . Comparator: standard counseling (no further details provided) |
|
| Outcomes | Primary outcome: decisional conflict Secondary objectives: knowledge (subjective), attitude and willingness regarding fertility preservation, decisional regret, final decision, satisfaction with the DA (intervention group only) |
|
| Notes | Source of funding: The study was funded by a grant from the Swiss Cancer Research (KFS‐3584‐02‐2015). Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After having given written informed consent, study participants were assigned with a block randomization to either the control or the intervention group by the study coordinator." The investigators describe the use of stratification or permuted blocking (use of computer implied). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding according to the study protocol. It is unclear if participants' awareness of their group allocation may have biased their responses for subjective measures (e.g. decisional conflict). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of attrition but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02404883) and all the study’s pre‐specified primary and secondary outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | As seen in the sample size section, only 63.71% of the patients who were asked eventually participated in the study. The small sample of the final analysis needs to be considered and the interpretation of the results therefore needs to be treated with caution. |
Elliott 2022.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid + clinical decision support (CDS) vs CSD alone vs usual care | |
| Participants | 34 clinics randomized in the USA: 11 DA + CDS (7807 patients), 11 CDS alone (8818 patients), 12 usual care (10,974 patients). Eligible patients were 1) aged 21 to 74 years; 2) not pregnant, cognitively impaired, or in hospice care; and 3) not up‐to‐date for breast, cervical, colorectal, or lung cancer screening at an index visit at a randomized clinic. | |
| Interventions | DA: paper‐based decision aids used in consultation plus web‐based clinical decision support (CDS). The decision aids included clinical information, probabilities of outcomes, explicit values clarification, and guidance in decision‐making. Short form versions of the decision aids notified patients and providers of the patients’ eligibility for a cancer screening test, briefly presented benefits and risks, options to consider, and invited the patient to access during the clinic visit the full‐length shared decision‐making tool and discuss with their provider. The CDS intervention was a web‐based, electronic health record‐linked system that included cancer prevention algorithms and the CDS output provided personalized recommendations to both primary care providers and patients in high‐literacy (provider) and low‐literacy (lay person) printed and electronic formats. The DAs are available as a supplementary appendix in the article. Comparator: usual care (no details provided) Comparator: CDS alone (not extracted) |
|
| Outcomes | Primary outcome: a composite indicator of the proportion of patients overdue for breast, cervical, or colorectal cancer screening at index who were up to date on these 1 year later. Secondary outcomes: breast cancer screening, cervical cancer screening, colorectal cancer screening, lung cancer screening. |
|
| Notes | Source of funding: Financial support for this study was provided entirely by a grant from National Cancer Institute of the National Institutes of Health. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award No. R01CA193396. Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Cluster randomization of the 34 primary care clinics was chosen to minimize contamination. Re‐randomization balanced clinic attributes across 3 study groups on 2 primary factors: clinic urbanicity based on Rural‐Urban Commuting Area (RUCA) codes, and the percentage of women at each clinic up to date on breast cancer screening to address clinic attentiveness to routine cancer screening." Referenced: Morgan KL, Rubin DB. Rerandomization to improve covariate balance in experiments. Ann Stat. 2012;40(2): 1263–82. |
| Allocation concealment (selection bias) | Low risk | "The first concealed randomization scheme meeting balance criteria was selected and resulted in n = 11 CDS, n = 11 CDS+SDM, and n = 12 UC clinics." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate and similar across arms (less than 10%), reasons for attrition recorded |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02986230) and all the study’s pre‐specified primary and secondary outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Evans 2010.
| Study characteristics | ||
| Methods | Randomized to online decision aid vs paper decision aid vs questionnaire vs usual care | |
| Participants | 129 + 126 + 127 + 132 men considering PSA screening in Wales | |
| Interventions | DA: online program on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer program; summary). The DA is no longer available (at www.prosdex.com). The authors have screenshots of the website that was evaluated in the study. Comparator: paper version of online DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer program; summary) Comparator: received a questionnaire Comparator: received nothing |
|
| Outcomes | Primary outcomes: knowledge (post‐DA) Secondary outcomes: attitude (post‐DA), intention to undergo PSA testing (post‐DA), anxiety (post‐DA), uptake of PSA test (post‐DA), total decisional conflict |
|
| Notes | Source of funding: The study was funded by Cancer Research UK. The researchers are entirely independent from the funders (Grant number: C6475/A7490). Cardiff University agreed to act as sponsor for the above project, as required by the Research Governance Framework for Health and Social Care (Sponsorship reference: SPON 304‐06). The sponsor acted as employer of members of the research team. The sponsor and funder were not involved in the review and final approval of the manuscript. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[A] random sample of 100 men was selected from the list." "The process ensured individual level randomization" (p 4, Recruitment process) |
| Allocation concealment (selection bias) | Low risk | "[A]ffirmative consent forms from each practice were transferred to the research officer who allocated each participant with a number provided remotely by the trial statistician to ensure concealment" (p 4, Recruitment process) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study does not address this outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram indicating high attrition consistently across groups |
| Selective reporting (reporting bias) | Low risk | Registered as a trial |
| Other bias | Low risk | The study appears free of other sources of bias. |
Fagerlin 2011.
| Study characteristics | ||
| Methods | Decision aid vs delayed intervention vs control | |
| Participants | 382 + 159 + 100 women with an elevated 5‐year risk of breast cancer considering breast cancer prevention medication in the USA | |
| Interventions | DA: tailored DA on options' outcomes, clinical problem, outcome probabilities, and explicit values clarification. The DA is no longer available (http://www.cbdsm.org/files/downloads/ChemopreventionDecisionAid.pdf). The authors have a word file version of the content of the website that was evaluated in the study. Comparator 1: given DA after 3‐month follow‐up Comparator 2: given DA after all outcome measures were taken |
|
| Outcomes | Decisional conflict (post‐DA), behavioral intent (post‐DA), actual behavior (post‐DA), proportion undecided, perception of benefits (post‐DA), perception of risk (post‐DA) Other outcomes:
|
|
| Notes | Primary outcome was not specified Source of funding: Financial support for this study was provided by a grant from the National Institutes for Health (P50 CA101451). Conflicts of interest: Drs. Fagerlin and Smith were supported by MREP early career awards from the U.S. Department of Veterans Affairs. Dr. Zikmund‐Fisher is supported by a career development award from the American Cancer Society. Dr. Hayes received support from Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was provided by the author. |
| Allocation concealment (selection bias) | Low risk | Central and web‐based allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding ‐ using an online decision aid would have avoided control participants accessing the decision aid. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not report exclusions; inadequate reporting on participant flow through the study to determine risk for attrition bias or incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Fisher 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs active control | |
| Participants | 103 + 93 participants aged 18 and older with bipolar II disorder considering treatment options for maintaining mood stability/preventing relapse in Australia | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, patient examples, additional resources, guidance in decision‐making (7 steps), and guidance in communication. The DA is publicly available at http://www.bipolardecisionaid.com.au/ . Comparator: information (publicly available website: https://www.blackdoginstitute.org.au/resources-support/bipolar-disorder/treatment/ ) |
|
| Outcomes | Decisional conflict, concordance between preferred and actual levels of decision‐making involvement, preparedness for decision‐making, knowledge, decision regret, value‐based informed choice, and uptake of treatment options | |
| Notes | Source of funding: This research was funded by an Australian Rotary Health Mental Health for Young Australians Grant (2017–2019). The funding body had no role in the design and conduct of the study, analysis and interpretation of the data, and reporting of results. Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Upon completion of T0 measures, participants were randomly allocated (1:1) to either the control or intervention group, using a website‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In protocol: neither participants nor the trial researchers will be blinded to participants’ group assignment. It is unclear how lack of blinding influenced the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In protocol: Neither participants nor the trial researchers will be blinded to participants’ group assignment. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate (post‐treatment decision T1: 56/103 completers DA and 56/93 completers control (P = 0.408883)) (3‐month follow‐up (T2): 40/103 completers DA and 44/93 control (P = 0.231112)), but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12617000840381) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Fraenkel 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 47 + 40 patients with knee pain considering treatment options in the USA | |
| Interventions | DA: interactive computer tool options' outcomes, outcome probability, explicit values clarification. The DA is not available. Author said the DA was never fully developed; all information about the DA is included in the article. Comparator: usual care using the Arthritis Foundation information pamphlet |
|
| Outcomes | Decisional self‐efficacy, preparation for decision‐making | |
| Notes | Primary outcome was not specified Source of funding: Supported in part by a grant from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (P30AG21342). Dr. Fraenkel is supported by the K23 Award AR048826‐01 A1. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided; computer‐generated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but study does not report if it had an impact on the outcomes measured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk of attrition bias ‐ outcome data for all 40 controls and 44 of 47 intervention (p 3, Results). |
| Selective reporting (reporting bias) | Unclear risk | No information provided; no indication if trial was registered centrally. |
| Other bias | Low risk | Appears to be free of other potential biases. |
Fraenkel 2012.
| Study characteristics | ||
| Methods | Cluster‐randomized controlled trial of clinics to decision aid versus usual care | |
| Participants | 69 + 66 patients with nonvalvular atrial fibrillation considering anticoagulation with aspirin or warfarin in the USA | |
| Interventions | DA: computer‐based tool on options' outcomes, clinical problem, options' probabilities, guidance, explicit values clarification. The DA is not publicly available; a copy was provided by the author (terri.fried@yale.edu). Comparator: control arm (no further information provided) |
|
| Outcomes | Primary outcomes: feeling informed and having clear values (baseline, immediately post) Secondary outcomes: knowledge (baseline, immediately post), accuracy of risk (baseline, immediately post), anxiety (baseline, immediately post), worry (baseline, immediately post), rationale for preferred treatment (during the encounter ‐ DA group only), discussion of related outcomes (during the encounter as captured on audiotape), change in treatment plan (post intervention), anxiety, accurate risk expectations (stroke, bleeding) |
|
| Notes | Trial registration NCT00829478 Source of funding: The project described was supported by the Donaghue Foundation Practical Benefit Initiative DF #06‐205 and by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG21342 NIH/NIA). Conflicts of interest: Dr. Fried is supported by K24 AG28443. Dr. Street is supported in part by the Houston Health Services Research and Development Center of Excellence (HFP90‐020) at the Michael E. DeBakey VA Medical Center. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To avoid contamination, participants were randomized at the level of the firm so that all participants in one firm received the intervention, and all participants in the second firm were included in the control arm" (p 1435) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An interviewer blinded to the participant's group assignment reassessed the primary and secondary outcomes after participant's primary care visit" (p 1436) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not appear to be incomplete outcome data; flow diagram does not report participation beyond randomization. |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Fraenkel 2015.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 62 + 63 patients aged 18 and older with rheumatoid arthritis in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that included clinical information, probabilities of outcomes, explicit values clarification, and knowledge tests with feedback. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care (education and counseling) |
|
| Outcomes | Change in objective knowledge, subjective knowledge and values clarity, risk communication and confidence in decision using COMRADE, decision to escalate care, and actual escalation of care | |
| Notes | Source of funding: Supported by a Disease Targeted Innovative Research Grant from the Rheumatology Research Foundation and by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (through a grant from the National Institute on Aging [P30AG021342]). Dr. Fraenkel’s work was supported by grant AR‐060231‐01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Mr. Charpentier’s work was supported by grant SES‐1155924 from the National Science Foundation. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Random treatment assignments were placed in numbered, opaque envelopes. Participants were randomly assigned to the intervention or usual care control group in a 1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up data were collected over the telephone by trained, blinded interviewers using a standardized script at 2 and 8 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate and similar across arms (< 10%) |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01721200) and all of the study’s pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Frosch 2008a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs decision aid + chronic disease trajectory vs chronic disease trajectory vs usual care (Internet information) | |
| Participants | 155 + 152 + 153 + 151 men considering prostate cancer screening in the USA | |
| Interventions | DA: information on options' outcomes, clinical problem, outcome probabilities, others' opinions. The DA is not publicly available; screenshots were provided by the author. Comparator 1: information on options' outcomes, clinical problem, outcome probabilities, others' opinions, explicit values clarification (utilities for outcomes associated with prostate cancer) Comparator 2: explicit values clarification (utilities for outcomes associated with prostate cancer) Comparator 3: usual care using public information on prostate cancer screening on American Cancer Society and Centers for Disease Control and Prevention websites 2005‐2006 |
|
| Outcomes | Primary outcomes: knowledge, actual option, decisional conflict Secondary outcomes: concern about prostate cancer, treatment preference if prostate cancer diagnosed |
|
| Notes | Source of funding: This study was supported by cooperative agreement U57/CCU920678 from the Centers for Disease Control and Prevention. Dr Frosch also received support from the Robert Wood Johnson Foundation Health & Society Scholars Program. Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm randomly assigned participants to the 4 study groups. |
| Allocation concealment (selection bias) | Low risk | Revealed after signed consent and completed baseline measures. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Accessed a secure Internet site that hosted all study materials; participants had unlimited access to assigned intervention; unclear blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were measured via questionnaires and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis; imputed missing data for participants who did not complete follow‐up assessments; minimal attrition. |
| Selective reporting (reporting bias) | Unclear risk | No indication of published protocol |
| Other bias | Low risk | Appears to be free of other potential biases. |
Fung 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 36 + 37 participants aged ≥ 60 years with newly diagnosed obstructive sleep apnea in the USA | |
| Interventions | Web‐based decision aid + paper‐based workbook used in preparation for consultation with the physician that includes clinical information, outcome probabilities, explicit values clarification, individualized sleep test results, exercise for patient to identify long‐term health goals, other resources, patients narratives (hypothetical), guidance in decision‐making (used with in‐person support), and guidance in communication. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: general information about sleep |
|
| Outcomes | Decisional conflict, preparation for decision‐making, knowledge | |
| Notes | Source of funding: This study was funded by the National Institute on Aging of the National Institutes of Health (K23AG045937 to C.H.F., K23AG055668 to Y.S., K23AG049955 to J.D., National Center for Advancing Translational Science, UCLA CTSI Grant UL1TR001881), as well as the American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies (The Beeson Career Development in Aging Research Award Program to C.H.F.). R.D.H. received support from the University of California, Los Angeles Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under the National Institutes of Health National Institute on Aging Grant P30‐AG021684. J.L.M. received support from the National Heart, Lung and Blood Institute at the National Institutes of Health (K24HL143055). Conflicts of interest: The authors have no financial or nonfinancial interests that are relevant to the submitted manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients who met inclusion criteria were randomized within each study site to receive Decide2Rest vs control. At the university site, participants were randomized using simple randomization to one of two groups (decision aid with in‐person support; control with in‐person support) on the day of the intervention. The Research Electronic Data Capture randomization tool was used to allocate the participants to either the Decide2Rest or control program. At the VA site, participants were randomized to one of four groups (decision aid with in‐person support, decision aid with telephone support, control with in‐person support, or control with telephone support) on the day of the intervention using a block randomization (block size = 4). The randomization sequence was created using Stata 13.1 (StataCorp LLC, College Station, Texas). |
| Allocation concealment (selection bias) | Low risk | A set of opaque, sequentially numbered envelopes was prepared during the setup phase of the study by research team member without direct contact with research participants, and at the time of randomization; the envelopes were opened sequentially by a staff member without contact with the research participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Decisional measures were assessed in all randomized participants postintervention at a separate in‐person research visit that occurred after the sleep clinic appointment (typically, the postintervention assessment occurred on the day of the intervention). At the VA site, the assessor was blinded to study arm assignment, whereas at the university site, blinding was not possible because of staffing limitations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Decisional measures were assessed in all randomized participants postintervention at a separate in‐person research visit that occurred after the sleep clinic appointment (typically, the postintervention assessment occurred on the day of the intervention). At the VA site, the assessor was blinded to study arm assignment, whereas at the university site, blinding was not possible because of staffing limitations. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT, all participants included in analysis, no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Registered (NCT03138993). Outcomes in the article do not match outcomes identified in trial registry. In article, outcomes are: decisional conflict, preparation for decision‐making, knowledge. In trial registry, outcomes are recruitment rates, enrolment rates, length of time for completing intervention session. |
| Other bias | Unclear risk | The two recruitment sites have different study groups: (site 1: DA vs control; site 2: decision aid with in‐person support, decision aid with telephone support, control with in‐person support, or control with telephone support). |
Gabel 2020a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 830 + 849 Danish citizens aged 50 to 74 | |
| Interventions | DA: online decision aid that includes information about screening, explicit values clarification, and summary page with a "choice indicator" and users answers to the values clarification exercise. The DA is not publicly available; screenshots of the web pages of the DA were obtained from the author (Mette Bach Larsen: metbacla@rm.dk). Comparator: no intervention |
|
| Outcomes | Primary outcome: informed choice based on the following proxy measures: knowledge, attitudes, and screening uptake Secondary outcomes: colorectal screening induced worries, decisional conflict |
|
| Notes | Source of funding: The trial has been funded by grants from public and private foundations: The Danish Foundation TrygFonden; The Danish Cancer Society; The Health Research Fund of Central Denmark Region; Health, Aarhus University; The Private Foundation of the Family Spogárd, The Health Foundation, Denmark; Danish Cancer Research Foundation; The Private Foundation of Ringgaard‐Bohn, and the Danish Health Authority. Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocation will be performed in the ratio 1:1 and will use a computer‐generated algorithm for randomization, based on a simple randomization procedure. Randomization will be conducted based on the study participants’ record‐ID numbers." (as per published protocol Gabel 2018) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of attrition but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03253822) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Gabel 2020b.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 3571 + 3571 Danish citizens aged 53 to 74 | |
| Interventions | DA: online decision aid that includes information about screening, explicit values clarification, and summary page with a "choice indicator" and users answers to the values clarification exercise. The DA is not publicly available; screenshots of the web pages of the DA were obtained from the author (Mette Bach Larsen: metbacla@rm.dk). Comparator: no intervention |
|
| Outcomes | Primary outcomes: components of informed choice assessed using 3 dimensions (knowledge about the options to choose from, attitudes towards the options, and actual behavior) Secondary outcomes: decisional conflict and stated use of the decision aid. |
|
| Notes | Source of funding: The trial has been funded by grants from: TrygFonden; The Danish Cancer Society; The Health Research Fund of Central Denmark Region Health, Aarhus University; The Private Foundation of the Family Spogárd, The Health Foundation, Denmark; Danish Cancer Research Foundation; The Private Foundation of Ringgaard‐Bohn, and the Danish Health Authority. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: The authors have declared that no competing interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "respondents were simultaneously randomised into intervention and control groups in a 1:1 ratio using a computer‐generated algorithm for randomization based on a simple randomization procedure randomly assigning participant ID numbers to intervention or control group" |
| Allocation concealment (selection bias) | Low risk | "Respondents to the baseline questionnaire were simultaneously randomised into intervention or control group. Allocation was based on participants’ record‐ID numbers using a computergenerated algorithm for randomization based on a simple randomization procedure. The algorithm was generated by an administrator of the REDCap (Research Electronic Data Capture) software [32], which was otherwise not attached to the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of attrition but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03253822) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Gagne 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid + education vs education alone | |
| Participants | 26 + 25 participants was aged 18 to 65 with mild to severe asthma and prescribed inhaled corticosteroids, either alone or in combination with long‐acting β2‐agonists in Canada | |
| Interventions | Intervention: decision aid plus education. The decision aid included information on the clinical condition, explicit values clarification, and guidance in decision‐making (step‐by‐step process). The education session included guidance in communication (elicited patients' concerns by asking questions and providing feedback) and participants were also provided with an individualized written action plan. The DA is publicly available at https://cts-sct.ca/wp-content/uploads/2020/10/GagneBoulet_DA_Asthma_ICS_IPDASFinalVersion_Color_English_v2015-11-04.pdf . Comparator: education that included information on the clinical condition, treatments and side effects, guidance in communication, and an individualized written action plan |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: decisional conflict, appropriate use of pharmacotherapy (adherence), and asthma control |
|
| Notes | Source of funding: LPB (principal investigator) and FL (co‐investigator) received a grant from the Allergy, Genes and Environment Network for funding the research: http://allergen‐nce.ca/. Conflicts of interest: Potential conflicts of interest to disclose are: 1) the Knowledge Translation, Education and Prevention Chair in Respiratory and Cardiovascular Health is supported by unrestricted grants from AstraZeneca, and 2) the Chair on Adherence to Treatments was supported by unrestricted grants from AstraZeneca, Merck Canada, Sanofi Canada, Pfizer Canada and the Prends soin de toi program. M.G., F.L., and J.M. have no conflict of interest to declare. L.P.B. considers having no conflict of interest but wishes to declare what can be perceived as potential conflicts of interest. Advisory Boards: GlaxoSmithKline, Novartis. Conferences (honoraria): AstraZeneca, GlaxoSmithKline, Merck, Novartis. Sponsorship for investigator‐generated research: AstraZeneca, GlaxoSmithKline, Merck Frosst, Schering. Sponsorship for research funding for participating in multicenter studies: AllerGen, Altair, Amgen, Asmacure, AstraZeneca, Boehringer‐Ingelheim, Genentech, GlaxoSmithKline, Novartis, Ono Pharma, Pharmaxis, Schering, Wyeth. Support for the production of educational materials: AstraZeneca, GlaxoSmithKline, Merck Frosst, Boehringer‐Ingelheim, Novartis. Organizational: Chair of the Global Initiative for Asthma (GINA) Guidelines Dissemination and Implementation Committee, Knowledge Translation, Education and Prevention Chair in Respiratory and Cardiovascular Health, Member of the Executive Committee of Interasma (Global Asthma Organization). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study was designed as a prospective two‐month randomized controlled parallel group trial (allocation ratio 1:1). A statistician generated a random allocation sequence of block size of four using a computer software program." |
| Allocation concealment (selection bias) | Low risk | "The study coordinator enrolled participants. Educators assigned participants to interventions using sequentially numbered, opaque, sealed and equally weighted envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After assignment to interventions, only the study co‐ordinator, who assessed the outcomes, was blinded. "the educators who were responsible for provision of patient education in both groups were not blinded to the experimental intervention and may have been more motivated to support control participants in making decisions. This may have diminished the impact of our DA on decisional conflict as well as reduced the probability to detect between‐group differences" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After assignment to interventions, only the study co‐ordinator, who assessed the outcomes, was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT, all participants included in analysis, loss to follow‐up similar between arms 2/26 (7.7%) for DA and 2/25 (8%) for control. |
| Selective reporting (reporting bias) | Unclear risk | The study was registered retrospectively in 2015 (NCT02516449) after recruitment was completed in 2013, and therefore no way to verify whether the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Gattellari 2003.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 126 + 122 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification. The DA is available as an appendix in the article. Comparator: usual care using brief information on screening test and chances of false‐positive results |
|
| Outcomes | Preferred option, knowledge, decisional conflict, accurate risk perceptions, perceived ability to make an informed choice | |
| Notes | Primary outcome was not specified Source of funding: Melina Gattellari was supported by an Australian Postgraduate Award at the time this study was conducted. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐randomized code ‐ no further information (p 1) |
| Allocation concealment (selection bias) | Low risk | Pre‐randomized code unobtrusively marked on envelopes (p 1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Consenting men were blinded to allocation, but unclear if personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pre‐test characteristics included. Flow chart not included and reasons for attrition not mentioned; some attrition but balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Gattellari 2005.
| Study characteristics | ||
| Methods | Randomized to decision aid booklet vs decision aid video vs usual care | |
| Participants | 140 + 141 + 140 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification. The DA is available as an appendix in a previously published article ( Gattellari 2003 ). Comparator 1: video on clinical problem, outcome probability, others' opinion Comparator 2: usual care using brief information on screening test and chances of false‐positive results |
|
| Outcomes | Preferred option, knowledge, decisional conflict, perceived ability to make an informed choice | |
| Notes | Primary outcome was not specified Source of funding: At the time of the study, Melina Gattellari was supported by a Commonwealth Department of Education, Science and Training Australian Postgraduate Award (APA) and was a doctoral candidate at the School of Public Health, University of Sydney. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unique identification codes assigned to participants according to date and time enrolled into the interventional component of the study. Block randomization of identification codes then performed via computer software (p 2 ‐ 2.3.1). |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured as the interviewers, responsible for enrolling participants onto the trial, were blinded to the randomized study design while one of the authors (MG) was responsible for randomisation. Hence, it was not possible for either participants or interviewers to be aware of the randomisation sequence." (p 2 ‐ 2.3.1) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interviewers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At post‐test, it was not possible to blind the interviewers but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition that is consistent across groups (figure 1) |
| Selective reporting (reporting bias) | Unclear risk | "[S]uccess of study protocol" limitation to protocol: men not confronted with actual decision to undergo PSA screening; no indication that trial registered in central trials registry (p 13, paragraph 5) |
| Other bias | Low risk | "[H]igh follow‐up rate and allocation concealment; study not subjected to selection bias" (p 13, paragraph 5). Appears to be free of other sources of bias. |
Gokce 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 60 + 59 patients aged 18 to 75 years with symptomatic non‐lower pole renal stones < 20 mm in Turkey | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information and explicit values clarification. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Decision (choice), decisional conflict, knowledge | |
| Notes | Source of funding: not reported Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software was used to generate random allocation sequence. |
| Allocation concealment (selection bias) | Low risk | The patients were randomized to two study groups. Computer software was used to generate random allocation sequence. The random allocation sequence was placed in preset, numbered envelopes and a nurse opened the envelopes for each patient to perform randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis, low attrition rate (97% retention rate for both groups) |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Gordon 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid + education vs education alone | |
| Participants | 133 + 155 participants aged 21 and older who have never received a kidney from an increased risk donor in the USA | |
| Interventions | DA: web‐based decision aid used after routine education and physician consultation that includes clinical information, outcome probabilities, implicit values clarification, patient stories, and knowledge tests. The DA is publicly available at https://informme.cbits.northwestern.edu/system/ . Comparator: routine education |
|
| Outcomes | Knowledge, willingness to accept increased risk donor kidney transplant | |
| Notes | Source of funding: This publication was supported by the NINR/NLM (Grant No. R21NR013660 to EJ Gordon). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of NIH. Conflicts of interest: The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Staff then randomized participants, using 1:1 equal allocation, to receive either routine education only (control arm) or Inform Me after attending routine education (intervention arm), using a computer‐generated random number list, with individual numbers inserted into sequentially numbered, sealed envelopes concealed until study arm was assigned. Randomization was stratified by site. |
| Allocation concealment (selection bias) | Low risk | Using a computer‐generated random number list, with individual numbers inserted into sequentially numbered, sealed envelopes concealed until study arm was assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was single‐blinded; research team members assessing outcomes (EJG, MWS, MGI) were blinded to assignments to the intervention. Unclear how lack of blinding of participants influenced the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was single‐blinded; research team members assessing outcomes (EJG, MWS, MGI) were blinded to assignments to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT01859884). Decisional conflict was pre‐specified as an outcome measure but is not reported in the article. |
| Other bias | Unclear risk | "Although 1:1 randomization was implemented, we realized that, due to technical problems with Internet connectivity and concomitant concerns over potential data loss for the intervention arm, we needed to recruit more participants to ensure at least 100 participants per arm. We therefore generated an additional 100 random numbers, which were mostly used at the NMH site. The study stopped recruitment after reaching our initial target sample size, twelve numbers were not used (6 per site). We recovered most data and obtained a larger sample than our initial recruitment target." |
Green 2001.
| Study characteristics | ||
| Methods | Randomized to decision aid + counseling vs counseling alone vs usual care | |
| Participants | 29 + 14 women with a first degree relative with breast cancer interested in learning about genetic testing in the USA | |
| Interventions | DA: CD‐ROM plus counseling on options' outcomes, clinical problem, others' opinions, guidance/coaching. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: counseling Comparator: usual care |
|
| Outcomes | Primary outcome: preferred options Secondary outcome: knowledge |
|
| Notes | Source of funding: This publication was supported by grant number 1R03 CA 70638 from the National Cancer Institute (NCI), and grant number 1 R01 CA84770 from NCI and the National Human Genome Research Institute (NHGRI). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[B]lock randomization schedule to one of three groups in a 2:2:1 ratio" (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[G]enetic counsellor blinded to randomization until just prior to the session" (p 2), unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Values do not always add up to the number of participants due to missing data"; reasons not mentioned (p 4). "Participants' baseline knowledge was reflected in the control group's answers"; participants balanced in study groups |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Hamann 2006.
| Study characteristics | ||
| Methods | Cluster‐randomized trial of decision aid vs usual care | |
| Participants | 54 + 59 patients with schizophrenia considering treatment options (cluster‐RCT with 12 wards paired and randomized) in Germany | |
| Interventions | DA: 16‐page booklet on options' outcomes, outcome probabilities, explicit values clarification, coaching/guidance. The DA is not publicly available; a copy was provided by the author (in German). Comparator: usual care |
|
| Outcomes | Knowledge, participation in decision‐making (COMRADE ‐ doctor gave me a chance to decide which treatment I thought was best for me), uptake of psycho‐education, rehospitalization, adherence, satisfaction with care, severity of illness (baseline only), attitudes about drug use, decision‐making preference | |
| Notes | Primary outcome was not specified Source of funding: The trial was funded by the German Ministry of Health and Social Security (217‐43794‐5/9) within the funding project 'Der Patient als Partner im medizinischen Entscheidungsprozess'. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[O]ne member of each pair being randomly assigned to the control or to the interventional condition" (p 266). Sequence generation method was not stated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Clustering was not accounted for in the analysis. Free of other potential biases: no evidence of selective recruitment of cluster participants. |
Hanson 2011.
| Study characteristics | ||
| Methods | Cluster‐randomized trial of decision aid vs usual care | |
| Participants | 127 + 129 patients diagnosed with advanced dementia and eating problems considering long‐term feeding tube placement in the USA | |
| Interventions | DA: booklet or audio recording on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (steps in decision‐making, worksheet, summary). The DA is available at https://decisionaid.ohri.ca/AZsumm.php?ID=1652 . Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (3 months post‐DA) Secondary outcomes: surrogate knowledge, risk perceptions, frequency of communication with providers (3 months post‐DA), feeding treatment use (3, 6, and 9 months post‐DA), participation in decision‐making, satisfaction with the decision, decisional regret |
|
| Notes | Source of funding: The study was funded by National Institutes of Health (NIH), National Institute for Nursing Research Grant R01 NR009826. Dr. Mitchell is supported by NIH, National Institute on Aging Grant K24AG033640. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number generation (p 2010, Randomization) |
| Allocation concealment (selection bias) | Unclear risk | No description of method used to conceal allocation (p 2010, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Cluster randomization prevented double blinding and may have introduced bias due to site effects" (p 2014, Discussion); study authors unsure of effect on study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[B]ecause of cluster randomization, data collectors were not blinded to group assignment" (p 2010, Randomization); authors believe this has little impact on study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention group missing data for 1 participant; reason for omission not reported (table 1) No explanation for number of participants in each group (n = 127), given numbers vary from those in 'recruitment and retention' figure (table 4) |
| Selective reporting (reporting bias) | Low risk | Registered with clinicaltrials.gov, protocol on website |
| Other bias | Low risk | Appears to be free of other potential biases (adjustment for clustering performed/no evidence of selective recruitment of cluster participants) |
Heller 2008.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 66 + 67 breast cancer patients eligible for breast reconstruction in the USA | |
| Interventions | DA: interactive software program on options' outcomes, others' opinions. The DA is not publicly available; a copy was provided by the author (computer disc mailed). Comparator: standard patient education | |
| Outcomes | Knowledge, anxiety, satisfaction with treatment choice, satisfaction with decision‐making ability | |
| Notes | Primary outcome was not specified Source of funding: not reported Conflicts of interest: There are no conflicts of interest regarding the publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "upon study entry, the participants were randomized (computer generated) to one of two groups" (p 2) |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline anxiety and knowledge included in graphs. Participant numbers between study groups are balanced (p 3). Reasons for incomplete questionnaires and study withdrawals are mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No information provided re protocol |
| Other bias | Low risk | Appears to be free of other potential biases. |
Hess 2012.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 105 patients in the emergency department with primary symptoms of nontraumatic chest pain, being considered for admission to the emergency department observation unit for monitoring and cardiac stress testing within 24 hours in the USA | |
| Interventions | DA (in consultation): 1‐page printout on options' outcomes, clinical problem, and outcome probabilities. The DA is presented in Figure 1 of the article. Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge Secondary outcomes: risk perceptions, decisional conflict, actual choice, satisfaction with the decision‐making process, patient‐practitioner communication |
|
| Notes | Source of funding: The project was funded by an investigator‐initiated grant from the Foundation for Informed Medical Decision Making. The study sponsor did not have any involvement in the design and conduct of the study, data analysis, interpretation of the data, or manuscript preparation or approval. Conflicts of interest: The investigative team has not had and does not have any for‐profit‐seeking intentions for the Chest Pain Choice decision aid. Our decision aids are freely available at http://shareddecisions.mayoclinic.org. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" (p 253) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" (p 253) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel were blinded, but unclear if patients were blinded (p 253, Outcome measures). However, the primary outcome is unlikely to be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators assessing outcomes were blinded (p 253, Outcome measures). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some of the numbers of patients reported in the results did not match the flow chart. |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | Appears to be free of other biases. |
Hess 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 458 + 455 adults aged 18 and older presenting to the emergency department with a chief complaint of chest pain who were being considered by the treating clinician for admission to the observation unit for cardiac stress testing or coronary computed tomography angiography in the USA | |
| Interventions | DA: paper‐based decision aid used during consultation that includes clinical information, outcome probabilities, and implicit values clarification. The DA is available as a supplementary file in the development article and at https://www.youtube.com/watch?v=LgOagKX_-nA . Comparator: usual care |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: uncertainty, decisional conflict, patient trust in their clinician, DA acceptability, patient engagement in decision‐making, safety (major cardiac event) |
|
| Notes | Source of funding: Research reported in this publication was funded through a Patient‐Centered Outcomes Research Institute (PCORI) award (contract 952). The views presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its board of governors, or the methodology committee. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All involved researchers’ maintained independence from the funder of the study. Conflicts of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: JEH has research funding from Alere, Trinity, Siemens, and Roche and has consulted for Janssen. DBD has research funding from Siemens and Roche and has consulted for Janssen. All other authors have no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was concealed by an online password‐protected randomization algorithm (Medidata Balance; Medidata Solutions, New York City, NY). Patients were randomized 1:1 and dynamically stratified by age, sex, and site because of the known associations of age and sex with cardiovascular risk, potential unmeasured differences between sites, and the availability of these data at the time of enrollment. Clinicians were not randomized. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by an online password‐protected randomization algorithm. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients, study co‐ordinators, and treating clinicians were not masked to allocation. Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients, study co‐ordinators, and treating clinicians were not masked to allocation. All other investigators were blinded to allocation. Primary and most secondary outcomes were objectively measured and not subject to interpretation. Five trained raters independently viewed videos of the patient‐clinician discussion and assessed the degree to which clinicians engaged patients in the decision‐making process using the observing patient involvement (OPTION) scale, but looks like they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT analysis, 98% of participants included in analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01969240) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "We used two versions of the decision aid in the trial—one that included the option of coronary computed tomography angiography (CCTA) and one that included only cardiac stress testing. Although this introduced a degree of heterogeneity in the intervention, the trial was intentionally pragmatic in design, and contextual fit of the decision aid to facilitate clinician‐patient discussions relevant to the clinical settings enrolling patients in the trial was essential. We randomized at the patient level, increasing the risk of contamination between intervention and control groups." |
Hess 2018.
| Study characteristics | ||
| Methods | Cluster randomized to decision aid + risk assessment vs usual care | |
| Participants | 88 clinicians (493 patients) + 84 clinicians (478 patients) caring for children with minor head trauma younger than 18 years with non‐high risk factors for clinically important traumatic brain injury in the USA | |
| Interventions | DA: 1‐page decision aid used during consultation plus personalized risk estimates. The DA included clinical information, outcome probabilities, and explicit values clarification. The DA is publicly available at https://carethatfits.org/head-ct-choice-desicion-aid/ . Comparator: usual care |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: clinician engagement of parents in the decision‐making process (OPTION scale), decisional conflict, trust in physician, choice (utilization of CT scan), safety of decision aid |
|
| Notes | Source of funding: This study was funded from contract 12‐11‐4435 through a Patient‐Centered Outcomes Research Institute Award. Conflicts of interest: Drs Hess, Tzimenatos, Nigrovic, and Kuppermann reported grants from the Patient‐Centered Outcomes Research Institute during the conduct of the study. Dr Kharbanda reported grants from the Patient‐Centered Outcomes Research Institute during the conduct of the study and grants from the National Institutes of Health outside the submitted work. Dr Shah reported grants from the Patient‐Centered Outcomes Research Institute during the conduct of the study; and grants from the Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid Innovations, US Food and Drug Administration, and National Science Foundation outside the submitted work. Mr Inselman and Dr Herrin reported personal fees from the Mayo Clinic during the conduct of the study. Dr Kuppermann reported grants from the National Institutes of Health and the Health Resources and Services Administration outside the submitted work. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician at a centralized location performed randomization to conceal allocation. Clinicians were randomized in a 1 to 1 ratio. Randomization was stratified by site and whether their primary clinical training was in a pediatric specialty (pediatrics or pediatric emergency medicine) or another clinical specialty (general emergency medicine, family medicine, or internal medicine). We used dynamic allocation to balance randomization within strata defined by site and clinician specialty. |
| Allocation concealment (selection bias) | Low risk | A statistician at a centralized location performed randomization to conceal allocation. Clinicians randomized to the intervention were educated separate from the Grand Rounds, and were provided information included in the decision aid and shown a video demonstrating its use. Intervention clinicians were required not to share the decision aid with other clinicians in the trial, and this was monitored by study research co‐ordinators. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind (participant) according to trial registration. "The main limitations were lack of blinding." Unclear how lack of blinding of study personnel may have influenced study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind (participant) according to trial registration. "The main limitations were lack of blinding." Low risk for outcomes that were objectively measured (e.g. knowledge, decisional conflict, choice). High risk for observer‐reported subjective outcomes (e.g. patient‐clinician communication). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate and similar across arms, all patients who received DA/usual care were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02063087) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Hoffman 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs attention control (education on another topic) | |
| Participants | 59 + 30 African American patients aged 49 to 75 years old scheduled for visit and due for colorectal cancer screening in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that included clinical information, outcome probabilities, explicit values clarification, actor portrayal of real life situation, guidance in decision‐making, and guidance in communication. The DA is not publicly available; a copy was provided by the author (Robert J. Volk; bvolk@mdanderson.org). Comparator: attention control (video on hypertension) |
|
| Outcomes | Knowledge, attitudes toward and perceived social normative pressure, intention to be screened, decisional conflict, self‐advocacy, screening rate | |
| Notes | Source of funding: The project was supported by grants from the National Cancer Institute (R21CA132669) and The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. Dr. Ashley Housten was supported by the National Cancer Institute of the National Institute of Health under Award Number R25CA057730 (Principal Investigator: Shine Chang, PhD) and by Cancer Center Support Grant CA016672 (Principal Investigator: Ronald DePinho, MD). Dr. Suzanne K. Linder was supported the Agency for Healthcare Research and Quality under Award Number R24HS022134 and by the Cancer Prevention Research Institute of Texas under Award Number RP140020. Conflicts of interest: The authors made no disclosures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using computer‐generated permuted blocks in a 2:1 ratio (intervention/control). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interviewers and participants were blinded until baseline questionnaires were completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers and participants were blinded until baseline questionnaires were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis, justification for participants not included/loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT01492049). Only one outcome measure was identified in the trial registry (cancer screening rate); all other outcome measures reported in the article were not identified (e.g. knowledge, decisional conflict, attitudes). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ibrahim 2013.
| Study characteristics | ||
| Methods | Randomized to DA alone vs DA + motivational interviewing (MI) vs motivational interviewing alone vs attention control (education) | |
| Participants | 168 (DA alone) + 163 (DA + MI) + 165 (MI alone) + 167 (control) African American patients greater than age 55 with knee OA in the USA | |
| Interventions | DA: video decision aid that included information on the clinical condition, probabilities of outcomes of options, implicit values clarification, video clips of patient experiences, and guidance in decision‐making and guidance in communication. The DA is not publicly available; the authors have a copy of the video evaluated in previous studies (Bozic 2013, De Achaval 2012, and Stacey 2014a). Comparator: attention control (education on OA but not specific to joint replacement) |
|
| Outcomes | Primary outcome: changes in patient willingness to undergo knee replacement with knowledge and expectations as possible mediating factors. Secondary outcomes: whether the patient discussed knee pain with primary care doctor, received a referral to orthopedics, saw an orthopedic surgeon within 12 months of the intervention |
|
| Notes | Source of funding: This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Service (IIR 05‐234‐2, PI: Said A. Ibrahim). Dr. Ibrahim was also supported by a K24 Award (1K24AR055259‐01) from the National Institutes of Musculoskeletal and Skin Disorders. The views expressed here are those of the authors and do not represent those of the Department of Veterans Affairs or the United States Government. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a 2×2 factorial design, patients at each site were randomized to one of the 4 study arms… We used permuted block randomization at the level of the patient...computer generated random assignment" |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelope" (unclear whether envelopes were sequentially numbered, opaque) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinical and study staff and study participants were all blinded to assignment until after the baseline interview. The nature of the intervention meant that participants were not blind to the condition after participation in the intervention. Unclear how lack of blinding of participants may have influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and study staff and study participants were all blinded to assignment until after the baseline interview. The nature of the intervention meant that participants were not blind to the condition after participation in the intervention. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in analysis. "There were no losses to follow‐up except for one patient in the MI arm and one patient in DA/MI arm. Over the course of the study 93% of the subjects completed at least 2 of the 3 follow‐up interviews with no differences among the 4 intervention groups (p=0.62)." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT00324857) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ibrahim 2017.
| Study characteristics | ||
| Methods | Randomized to video decision aid vs educational booklet | |
| Participants | 168 + 168 participants who self‐identified as black, aged 50 years or older with chronic and frequent knee pain and radiographic evidence of osteoarthritis of the knee in the USA | |
| Interventions | DA: video decision aid that included information on the clinical condition, probabilities of outcomes of options, implicit values clarification, video clips of patient experiences, and guidance in communication. The DA is not publicly available; the authors have a copy of the video which was evaluated in previous studies (Bozic 2013, De Achaval 2012, and Stacey 2014a). Comparator: educational booklet |
|
| Outcomes | Primary outcomes: the recommendation for total knee replacement by an orthopedic surgeon at 6 months after the intervention, receipt of total knee replacement surgery at 12 months after the intervention | |
| Notes | Source of funding: This study was supported by grant 1R01AR059615‐0 from the National Institute of Arthritis and Musculoskeletal Skin Diseases, National Institutes of Health. Dr Ibrahim reports receiving Mid‐Career Development Award K24AR055259 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to one of the 2 study arms using a computer‐generated assignment." |
| Allocation concealment (selection bias) | Low risk | "The computer‐generated randomization result was sent to the study coordinator via email before the scheduled intervention session." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Owing to the nature of the intervention, participants could not have been blinded to the study arm to which they were randomized. The orthopedic surgeons were blinded to patient randomization. Research staff who were not involved in the intervention and were blinded to the study arm abstracted this information from the medical record." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Research staff who were not involved in the intervention and were blinded to the study arm abstracted this information from the medical record" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01851785) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ickenroth 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid versus information | |
| Participants | 1137 participants aged 18 and older in the Netherlands. Two participant groups: those with an intention to do a diagnostic diabetes self‐test (n = 569; 285 vs 284) and those with an intention to do a diagnostic cholesterol self‐test (n = 568; 284 vs 284); both groups were randomly assigned to web‐based DA (intervention) vs short, non‐interactive and non‐test specific information on self‐testing (control). | |
| Interventions | DA: online decision aid that included general information on self‐testing and personal risk factors for cardiovascular disease or developing diabetes, and an explicit values clarification exercise. The DA is no longer accessible according to the authors (Trudy van der Weijden: trudy.vanderweijden@maastrichtuniversity.nl). Comparator: general information |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: intention to take a test, attitude towards self‐testing, informed choice |
|
| Notes | Source of funding: The Netherlands Organisation for Health Research and Development (ZonMw Prevention) (grant number 50‐50101‐96‐406). Supplemental financial support has been provided by the Centraal Ziekenfonds health insurance company. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Within each group, randomisation over experimental conditions (and invitation to view either the decision aid or the control condition) will be performed by Flycatcher using SPSS" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded for randomization. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lower response rate in the DA group "Response in the cholesterol intervention group to Questionnaire 2 (immediately after being exposed to the DA) was lower than in the control group (control 84.5% and intervention 76.4%; P = 0.020)". There was no acknowledgment or discussion in the limitations section. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NTR3149) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | People on the panel are on the whole more highly educated compared with the general population in the Netherlands, and women are over‐represented. This may have led to an overestimation of the knowledge level. |
Jalil 2022.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard consultation vs control (standard consultation alone) | |
| Participants | 30 + 30 patients recently diagnosed with localized/early‐stage prostate cancer in Malaysia | |
| Interventions | DA: paper‐based booklet used after standard consultation in preparation for follow‐up visit to decide on treatment options. The DA included information on treatment options, benefits, harms, and an explicit values clarification exercise. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: standard consultation |
|
| Outcomes | Knowledge, decisional conflict, preparation for decision‐making | |
| Notes | Source of funding: Financial support for this study was provided entirely by a grant from University Putra Malaysia. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Conflicts of Interest: The authors declare that there is no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomly assigned in a 1:1 ratio to control (standard consultation only) and intervention group (standard consultation plus PDA) using SPSS generated method of block randomization [17]. The block randomizations were structured with randomly permutated block sized by week with a minimum blocked size of 2 × 2 for each treatment group. Each hospital has different block arrangement for the selection of patients into the control or intervention group" |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence of each center was generated and kept by one researcher who was not involved in patient recruitment and data collection. The trained recruiting nurses at each hospital will contact the researcher by phone to determine the allocation of patients (control vs intervention arm). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trained recruiting nurses at each hospital will contact the researcher by phone to determine the allocation of patients (control vs intervention arm). The urologists were blinded to the allocation of patients. Unclear if patients were blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate but balanced across groups: 27/30 DA group and 22/30 control (P = 0.095274) |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12614000668606) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Power calculation based on satisfaction for decision‐making. This outcome is not collected in the study. Low sample size (30 + 30). Parametric statistics used even if low sample size and study does not report on homogeneity of sample. |
Jibaja‐Weiss 2011.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 51 + 49 women diagnosed with breast cancer considering surgical treatment in the USA | |
| Interventions | DA: computer program on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step‐by‐step process for making the decision). The DA is no longer available (www.bcm.edu/patchworkoflife). The authors have a copy of the technical report. Comparator: usual care + breast cancer treatment educational materials normally provided to patients |
|
| Outcomes | Surgical treatment preference (post‐DA), breast cancer knowledge (pre, post‐DA, post‐DA and consult), satisfaction with surgical decision (post‐DA), satisfaction with decision‐making process (post‐DA), decisional conflict (pre, post‐DA, post‐DA and consult), proportion undecided | |
| Notes | Primary outcome was not specified Source of funding: This research study was supported by the U.S. Army Medical Research and Materiel Command, under DAMD17‐98‐1‐8022. Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients at each hospital were randomized using permuted blocks" (p 42, Methods section) |
| Allocation concealment (selection bias) | Unclear risk | Not addressed in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no way to know if the plots include all of the participants' data, since they do not specify the number of patients used to obtain these mean scores. |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Appears to be free of other potential biases. |
Johnson 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 32 + 35 patients considering endodontic treatment options in the USA | |
| Interventions | DA (in consultation): decision board on options' outcomes, clinical problem, outcome probability, guidance. The DA is presented in Figure 1 of the article. Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, satisfaction with the decision‐making process, anxiety | |
| Notes | Source of funding: This research was supported in part by a grant from the Wach Fund. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[F]our computerized random generation lists to assign to one of two groups" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Not for residents: computer‐generated randomization lists (1 for each resident) were prepared by the PI (p 3‐4); therefore, residents would have had pre‐generated lists. Unclear for patients: "allocation was concealed from patients" (p 3) but does not explain how. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned. Allocation was concealed from patients only (p 3). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 6); all 40 patients agreed to participate in the study, but only 32 questionnaires were usable; several residents did not understand the need to enter data on the envelope and place the matched questionnaire in it (p 5). |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Unclear risk | "[B]aseline data obtained because possible that clinicians training in the EndoDB would alter usual care discussions" (p 5). Mentions taking baseline characteristics, but not included in article. |
Karagiannis 2016.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs control | |
| Participants | 106 + 109 patients with type II diabetes for more than 1 year in Greece | |
| Interventions | DA: 7 cards presented during consultation that display the benefits and harms of commonly used antidiabetic medications, which include probabilities of outcomes. The DA is publicly available at https://diabetesdecisionaid.mayoclinic.org/app/diabetes?lang=EN&v=m Comparator: usual care |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: knowledge, patient‐clinician communication, patient and clinician satisfaction, adherence to medication, ease of using DA and incorporating it into practice |
|
| Notes | Source of funding: This study was funded by a European Foundation for the Study of Diabetes (EFSD) research programme in patient education supported by an educational grant from AstraZeneca/BMS in 2012. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible practices were matched based on level of care (primary or secondary) by the study statistician, and were randomly allocated within each pair, using a computer‐generated allocation sequence, to either the use of the Diabetes Medication Choice Decision Aid or to usual care. Since there was more than one pair per level, the statistician paired the sites without study team input. |
| Allocation concealment (selection bias) | Low risk | Eligible practices were matched based on level of care (primary or secondary) by the study statistician, and were randomly allocated within each pair, using a computer‐generated allocation sequence, to either the use of the Diabetes Medication Choice Decision Aid or to usual care. Since there was more than one pair per level, the statistician paired the sites without study team input. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Owing to the nature of the intervention, clinicians and patients were not blinded. It is unclear how lack of blinding influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate but balanced across groups. "... our analysis following the intention‐to‐treat principle, with the exception of medical adherence and clinical outcomes which were analysed based on completed data available" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01861756) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "the addition of a ninth practice in the intervention arm in a non‐random manner, after loss of one of the initially randomised practices." "we had asked study coordinators at each practice to keep a record of all patients invited to the study and of those who declined to participate. However, investigators involved did not adhere to the suggested practice (claiming it was impractical in their daily routine). Therefore, it is unknown how many patients were initially invited to each practice and how many of these declined participation." Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Kasper 2008.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 150 + 147 multiple sclerosis patients considering immunotherapy in Germany | |
| Interventions | DA: booklet and worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification (based on IPDAS). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: information material on immunotherapy (80 pages) |
|
| Outcomes | Primary outcomes: role in decision‐making Secondary outcomes: choice, feeling undecided, helpfulness with making a decision, attitudes toward immunotherapy, expectations of side effects realized at 6 months |
|
| Notes | Source of funding: This study was supported by German Ministry of Health and Social Services (grant no. GMQQ01019401). Conflicts of interest: CH has received financial support from Biogen Elan, Bayer‐Health Care, Serono and Teva, SK, JK, IM and MN have nothing to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[A]llocation using computer generated random numbers" (p 5) |
| Allocation concealment (selection bias) | Unclear risk | Randomization was carried out by concealed allocation, but method of concealment was not described (p 2, Assignment). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not told whether the information they received was standard information or the newly developed DA (p 3, Masking). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were not told whether the information they received was standard information or the newly developed DA (p 3, Masking). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of participants (p 2, Fig 1); baseline data/characteristics included |
| Selective reporting (reporting bias) | Low risk | "The protocol of this study has been published with the trial registration at http://controlled‐trials.com/ ISRCTN25267500" (p 2) |
| Other bias | Unclear risk | Difference in preferred interaction style between groups at baseline (P value 0.04) (p 5) |
Kennedy 2002.
| Study characteristics | ||
| Methods | Randomized to decision aid + coaching vs decision aid only vs usual care | |
| Participants | 215 + 206 + 204 women considering treatment for menorrhagia in the UK | |
| Interventions | DA: video + booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance/coaching
Coaching: ~ 20 minute coaching with explicit values clarification by a registered nurse prior to seeing physician. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcomes: general quality of life Secondary outcomes: uptake of option, satisfaction, menorrhagia severity, cost‐effectiveness |
|
| Notes | Source of funding: Our research was supported by a grant from the UK National Health Service (NHS) Research and Development Health Technology Assessment Programme. The Health Economics Research Group receives funding from the UK Department of Health. Dr Sculpher received a career scientist award in public health funded by the NHS Research and Development Programme. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated by computer and stratified by consultant and the age at which the woman left full‐time education (p 3). |
| Allocation concealment (selection bias) | Low risk | "Secure randomization ensured by using a central telephone randomization system" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Possibility of contamination bias; clinicians could have applied the experience gained from consultations with the intervention groups in their consultations with the control group (p 6). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if blinding used, but most outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 1 and Figure 1 flow diagram (p 4‐5) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free from other risks of bias. |
Khalifeh 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 26 + 25 women planning a pregnancy or ≤ 30 weeks pregnant at enrolment who had been offered to start or continue antidepressant treatment for depression by their clinician in the UK | |
| Interventions | DA: online decision aid that includes information on the condition, probabilities of outcomes, explicit values clarification, guidance in decision‐making (step‐by‐step process), guidance in communication, and an automated printable summary of the information reviewed on risks and benefits, the participant’s rating of their relative importance and the participant’s perception of external influences on their decision‐making process. The DA is not publicly available; access to the decision aid was provided by the author (Simone Vigod: simone.vigod@wchospital.ca). Comparator: online general information |
|
| Outcomes | Decisional conflict, knowledge of depression treatment options, depressive symptoms, anxiety symptoms, feasibility, acceptability | |
| Notes | Source of funding: This research was supported by an NIHR Research Professorship, the NIHR Clinical Research Network (CRN), and the Biomedical Research Nucleus data management and informatics facility at South London and Maudsley NHS Foundation Trust. The latter is funded by the (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, and a joint infrastructure grant from Guy's and St Thomas' Charity and the Maudsley Charity. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care. Emma Molyneaux, Louise M Howard, and Hind Khalifeh were supported by a National Institute for Health Research (NIHR) Research Professorship to LMH (reference number: NIHR‐RP‐R3‐12‐011). Simone Vigod is supported by a Canadian Institutes for Health Research New Investigator Award and the Shirley A Brown Memorial Chair in Women’s Mental Health (Women’s College Research Institute, Centre for Addiction and Mental Health, University of Toronto). Conflicts of interest: The authors declare that no competing interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised in a 1:1 ratio using a computer‐generated random allocation sequence that was activated at first login to the study website, with stratification by whether they were recruited from primary care, maternity care, or psychiatric settings" |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised in a 1:1 ratio using a computer‐generated random allocation sequence that was activated at first login to the study website, with stratification by whether they were recruited from primary care, maternity care, or psychiatric settings (central allocation)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Researchers were blind to group allocation at all data collection time points. Participants were most likely able to identify whether they had been randomised to the PDA, as this multistage interactive tool was clearly different from the single page resource sheet (control condition)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Researchers were blind to group allocation at all data collection time points" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; low and similar attrition at first follow‐up when the outcomes of interest to this review were measured (i.e. knowledge, decisional conflict). |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT02492009) and the secondary outcome of treatment decision is not reported. "Treatment Decision(s) [ Time Frame: (a) Baseline (pre‐randomization) and (b) 4 Weeks post‐randomization and (c) 12 weeks postpartum (for participants who enrolled while pregnant) OR 6 months post‐randomization (for women who enrolled while planning a pregnancy) ]" |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kleiss 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 76 + 71 patients aged 18 to 89 years, presenting for a first specialist visit for a specific upper‐extremity condition, for whom the choice was injection or surgery or other nonsurgical treatments, and for whom a DA was available in the USA | |
| Interventions | DAs: online decision aids used independent of consultation that include clinical information, outcome probabilities, explicit values clarification, knowledge quiz, guidance in decision‐making (5‐step guide), and summary of results that can be printed or downloaded to discuss with the doctor. The decision aids are publicly available at https://www.decisionaid.info . Comparator: control (no decision aid) |
|
| Outcomes | Pain self‐efficacy questionnaire, physical function, pain intensity, satisfaction with visit, understanding of received information, feeling adequately educated to make decision, choice, decision regret, satisfaction with information received | |
| Notes | Source of funding: not reported Conflicts of interest: D.R. has received or may receive payment or benefits from Skeletal Dynamics, Wright Medical for elbow implants, Deputy Editor for Clinical Orthopaedics and Related Research, Universities and Hospitals, Lawyers outside the submitted work. No benefits in any form have been received or will be received by the other authors related directly or indirectly to the subject of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to either the intervention (viewing a DA) or the control (not viewing a DA) group in a 1:1 ratio, using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for patients. Open‐label according to trial registry. Unclear how lack of blinding influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate (52/76 completers DA and 49/71 completers Control) but similar across arms (P = 0.938236); reasons for withdrawals not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03643978) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | In the flow diagram, the number of patients invited to participate is equal to the number randomized. No information on how many patients were approached and declined. |
Knops 2014.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 91 + 87 patients with asymptomatic abdominal aortic aneurysm considering elective surgery vs watchful waiting in the Netherlands | |
| Interventions | DA: interactive CD‐ROM on options' outcomes, clinical problem, outcome probabilities, explicit values clarification. The DA is no longer available (www.keuzehulp.info/amc/AAA/landing‐page). The authors have a PDF version of the DA content. Comparator: usual care with regular information |
|
| Outcomes | Primary outcomes: decisional conflict (baseline, 1, 4, and 10 months) Secondary outcomes: patient knowledge (baseline and 1 month), anxiety (baseline, 1, 4, and 10 months), satisfaction with conversation with the surgeon (baseline and 1 month), final treatment choice (10 months), aneurysm rupture (10 months), possible date of surgery (10 months), postoperative morbidity and mortality (10 months), physical quality of life (baseline, 1, 4, and 10 months) |
|
| Notes | Trial registration: NTR1524 Source of funding: none Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation ALEA v.2.2, NKI‐AVL, the Netherlands) was performed by the investigators" (p 2) |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomisation ALEA v.2.2, NKI‐AVL, the Netherlands) was performed by the investigators" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and investigators could not be blinded after group assignment, a factor which is inherent to the decision aid and the design of the study. Surgeons and nurses involved in the outpatient care of the participants were blinded to the patient's allocation group, although patients were not prohibited from sharing their allocation with them." (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measurement is not likely to be influenced by lack of blinding as all outcomes were measured objectively using validated scales and data retrieved from medical records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to have similar attrition between groups. The proportion of values missing varied from 2% to 9% per outcome measure. Missing values were completed by multiple imputation analysis. If one of the outcome measures had more than 25% missing values, that outcome measure for that patient was excluded from analysis. Therefore, missing data have been handled appropriately (p 3). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make judgment |
| Other bias | High risk | "Considerable number of patients could not be included, were not asked to participation, or declined to participate. Selection bias may have occured in patients that were not included" (p 6) "Both patients and surgeons were aware of the aim and subject of the study and could not be blinded to the allocation. It is possible that surgeons in the contributing centres offered more than average information to their patients" (p 6). Performance bias may have been introduced in terms of altered communication style. |
Korteland 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard care vs standard care alone | |
| Participants | 77 + 78 adult patients who were accepted for elective isolated or combined aortic valve replacement and mitral valve replacement from 5 Dutch hospitals in the Netherlands | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, explicit values clarification, knowledge quiz, guidance in decision‐making, guidance in communication, and summary of patients’ situation and preferences. The DA is not publicly available; access to the decision aid was provided by the author (Johanna J.M. Takkenberg; j.j.m.takkenberg@erasmusmc.nl) Comparator: usual care |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: knowledge, participation in decision making, anxiety and depression, quality of life, decision regret |
|
| Notes | Source of funding: Stichting Kwaliteitsgelden Medisch Specialisten Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated by an independent statistician using a random number generator. |
| Allocation concealment (selection bias) | Low risk | The randomization sequence was generated by an independent statistician using a random number generator. Allocations were placed in serially numbered, opaque, sealed envelopes by 2 independent research assistants. The investigators were unaware of the allocation sequence to ensure allocation concealment. They selected the next randomization envelope in sequence, and outcome was noted in a randomization and identification log. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded. Unclear how lack of blinding influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; low rate of attrition (loss to follow‐up 9% for control and 13% for intervention) and justifications provided not related to outcomes |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NTR4350) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Based on power calculation, sample size needed to detect an effect size of 0.35 on the DCS was 140 patients; 138 patients were included in analysis. Low recruitment 115/306. |
Kostick 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid vs education | |
| Participants | 52+53 inpatients aged 30 to 85 years old considering left ventricular assist device (LVAD) treatment for advanced heart failure in the USA | |
| Interventions | DA: paper and web‐based DA provided after formal evaluation of LVAD eligibility and before receiving standard education. The DA includes clinical information, probabilities of outcomes, explicit values clarification, patient narratives, knowledge test, guidance in decision‐making (step‐by‐step process), and guidance in communication. The DA is publicly available at www.lvaddecisionaid.com . Comparator: standard education |
|
| Outcomes | Primary outcome: knowledge. Secondary outcomes: Decision Conflict Scale, patient preparedness for decision‐making, satisfaction with decision‐making process, regret, shared decision‐making, alignment with patent’s decision‐making preferences, accurate alignment of patient expectations with outcomes, satisfaction with life, perceived quality of care, preferred treatment, whether or not patients had an advance directive, and acceptability of the DA |
|
| Notes | Source of funding: PCORI award (1306‐01769). Conflicts of interest: Dr Jerry Estep serves as a consultant and medical advisor for Abbott and Medtronic. Neither company was involved in the design or conduct of the study. None of the other authors report any potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a parallel design and 1:1 allocation ratio. we used an online statistical computing program (www.graphpad.com/quickcalcs) to generate our randomization schedule with the use of 1:1 “block” randomization within sites and allocation concealment during enrollment by LVAD coordinators" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was accomplished by LVAD coordinators who blindly followed a predetermined allocation plan but became aware of which arm patients were assigned to after administering baseline surveys. We used an online statistical computing program (www.graphpad.com/quickcalcs) to generate our randomization schedule with the use of 1:1 “block” randomization within sites and allocation concealment during enrollment by LVAD coordinators" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients were not aware of which arm they were randomized to (ie, were not made aware of the difference between standard versus DA‐guided education). Allocation concealment was accomplished by LVAD coordinators who blindly followed a pre‐determined allocation plan but became aware of which arm patients were assigned to after administering baseline surveys, when they were enlisted to provide either standard or DA‐guided education." LVAD co‐ordinators who delivered the interventions were aware of treatment allocation, therefore it is unclear how they may have influenced decisions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of attrition but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02248974) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Krishnamurti 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 60 + 60 individuals aged 8 to 80 years old with sickle cell disease considering therapeutic options or parent/legal guardian of patients (age < 18 years) who are directly involved in decision making regarding healthcare treatment in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, explicit values clarification, interactive components (e.g. voice clips, videos, patient testimonies), guidance in decision making (step‐by‐step process), guidance in communication, and summary comparing treatment options can be printed or saved. The DA is publicly available at http://sickleoptions.org/en_US/ . Comparator: usual care |
|
| Outcomes | Acceptability, knowledge, values, stage of decision‐making, preparation for decision‐making, decisional regret, self‐efficacy, decisional conflict. | |
| Notes | Source of funding: This project was supported by PCORI grant CE‐1304‐6859 (LK). Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate (at 3 months 24/60 DA and 19/60 UC completed questionnaires) but missing data are balanced across groups, no justification for attrition |
| Selective reporting (reporting bias) | Low risk | The trial protocol is available (NCT03224429 & NCT02326597) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Krist 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid booklet vs decision aid web‐based vs usual care | |
| Participants | 196 + 226 + 75 patients considering prostate cancer screening in the USA | |
| Interventions | DA: 4‐page pamphlet with options' outcomes, clinical problem, outcome probability Comparator: website with same information as paper‐based DA The DA is no longer available (http://www.familymedicine.vcu.edu/research/misc/psa/index.html). Comparator: usual care |
|
| Outcomes | Primary outcomes: role in decision‐making Secondary outcomes: knowledge, decisional conflict, time spent discussing screening, choice (PSA test ordered) |
|
| Notes | Source of funding: This work was funded by the American Academy of Family Physicians Foundation under the Joint Grant Awards Program. Conflicts of interest: Dr Krist is a faculty member, practicing physician, and partial owner of Fairfax Family Practice Residency, where the study was conducted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]oordinator referred to pre‐generated randomisation tables to inform the participant to which arm he was randomised" (p 2) |
| Allocation concealment (selection bias) | Low risk | At the time of enrolment, the allocation was concealed from the co‐ordinator (p 2). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians were not blinded ‐ could affect decision‐making process and uptake of screening. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p 3, Results; p 4, Flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Uneven groups but done intentionally; ratio of 1:3:3 but appears to be free of other potential biases. |
Kukafka 2022.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid + standard usual care + decision support tool for clinician vs standard usual care | |
| Participants | 102 + 88 adults aged 21 to 75 years with no personal history of breast or ovarian cancer, no previous genetic counseling or testing for hereditary breast and ovarian cancer syndrome, and meeting family history criteria for BRCA1/2 genetic testing based upon family history. Participants were enrolled by 67 clinicians (physician, nurse practitioner, physician assistant, or nurse‐midwife). | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification for taking breast cancer reducing pill, implicit values clarification for genetic testing, patient scenarios, risk game, individualized breast cancer risk factors, guidance in decision‐making (list of steps), and summary in the action plan that can be printed and discussed with clinician. Clinicians had access to the Breast cancer risk NAVigation toolbox for providing them with their patients’ personalized risks and preferences prior to the clinical encounter. The DA is not publicly available; a copy was provided by the author (Katherine D. Crew; kd59@cumc.columbia.edu). Comparator: usual care (education) |
|
| Outcomes | Primary outcome: uptake of screening within 6 months of enrolment Secondary outcomes: receipt of genetic counseling at 24 months, genetic testing at 6 months, knowledge, breast cancer worry, decision self‐efficacy, and decisional conflict |
|
| Notes | Source of funding: This work was funded by grant RSG‐17‐103‐01 from the American Cancer Society to Dr Kukafka. Conflicts of interest: Dr Terry reported receiving grants from the National Institutes of Health during the conduct of the study. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded. Unclear how lack of blinding influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high rate of attrition at 1 month (completers 82/102 DA group and 77/88 UC group) but missing data are balanced across groups (P = 0.186122). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03470402) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Kunneman 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 475 + 467 adults aged ≥ 18 years with a diagnosis of nonvalvular atrial fibrillation and at high risk of experiencing a thromboembolic event in the USA | |
| Interventions | DA: online decision aid that was used in consultation with the physician that included information on the clinical problem, a personalized risk calculator, outcome probabilities, implicit values clarification, and a summary sheet at the end of the consultation. The DA is publicly available at https://anticoagulationdecisionaid.mayoclinic.org/ . Comparator: usual care during consultation |
|
| Outcomes | Quality of shared decision‐making (quality of communication, knowledge, accuracy of patient estimates of their own stroke risk, decisional conflict, and satisfaction), duration of the encounter, and clinician involvement of patients in the SDM process | |
| Notes | Source of funding: The clinical trial was funded by grant RO1 HL131535‐01 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Conflicts of interest: Dr Kunneman reported receiving grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Ms Branda reported receiving grants from the National Heart, Lung, and Blood Institute during the conduct of the study. Dr Hargraves reported receiving grants from the National Institutes of Health during the conduct of the study. Ms Sivly reported receiving grants from Mayo Clinic during the conduct of the study and outside the submitted work. Dr Gorr reported receiving grants from the National Institutes of Health during the conduct of the study. Dr Burnett reported receiving grants and personal fees from the Mayo Clinic and the National Institutes of Health during the conduct of the study and personal fees from the Mayo Clinic outside the submitted work. Dr Jackson reported receiving grants from the Mayo Clinic during the conduct of the study and research funding from Amgen and the National Institutes of Health outside the submitted work. Dr Hess reported receiving grants from the Patient‐Centered Outcomes Research Institute outside the submitted work. Dr Linzer reported receiving grants from the National Institutes of Health during the conduct of the study and grants from the American College of Physicians, the American Medical Association, and the Institute for Healthcare Improvement outside the submitted work. Dr Brito reported being the medical director of the Shared Decision Making National Resource Center at the Mayo Clinic. Dr Montori reported receiving grants from the National Heart, Lung, and Blood Institute during the conduct of the study and serving as board chair of The Patient Revolution outside the submitted work. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization algorithm (generated within the Remote Data Capture [REDCap] software system; Vanderbilt University), which was built by the clinical trial statistician (M.E.B.), used a stratified block randomization with blocks of random size." |
| Allocation concealment (selection bias) | Low risk | "Encounters were randomized on a 1:1 ratio to either standard care or care that included use of the SDM tool, which allowed clinicians to participate in both study arms. The randomization algorithm (generated within the Remote Data Capture [REDCap] software system; Vanderbilt University), which was built by the clinical trial statistician (M.E.B.), used a stratified block randomization with blocks of random size. The clinical trial was stratified by medical center, cohort (start vs review), and stroke risk (CHA2DS2‐ VASc score of 1 for men and 2 for women vs >1 for men and >2 for women)." (Central allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were blinded, but personnel were not: "Except for patients, who will be informed that the trial will be testing different ways clinicians and patients with AF communicate about anticoagulation, all study personnel will be able to discern participant allocation." (Study protocol) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Bias may have affected the unblinded assessment of recorded encounters and the scoring of those encounters using the OPTION12 scale. Limitations acknowledged but not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT, > 90% of participants included in analysis, justifications for withdrawals reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02905032) and all of the studies original primary and secondary outcomes (submitted: 13 September 2016) have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Some authors received grants and personal fees from the Mayo Foundation, which is the developer of the DA. Selection bias could have been introduced when enrolled clinicians chose not to enroll an eligible patient encounter into the clinical trial (but this is true for any trial). |
Kupke 2013.
| Study characteristics | ||
| Methods | Cluster‐randomized trial of 2 groups of dental students to decision board group and non‐decision board group. Patients randomized to students in either group. | |
| Participants | 57 + 36 patients with defect in posterior tooth (class II defect) considering 6 treatment options, including no therapy in Germany | |
| Interventions | DA (in consultation): options' outcomes, outcome probabilities. The DA is presented in Figure 2 of the article. Comparator: usual care with discussion of the treatment options |
|
| Outcomes | Knowledge (costs/self‐payment, survival rate, characteristics, and treatment time) (postintervention); overall satisfaction with consultation (postintervention) | |
| Notes | Primary outcome not specified Source of funding: not reported Conflicts of interest: This study did not receive financial support from manufacturers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by a dice (selection of students and patient allocation) (p 20) |
| Allocation concealment (selection bias) | High risk | "The patients were assigned to the students according to common standards of the university independently and without knowing which group the student belonged to." (p 20) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were assigned to the students independently and without knowing which group the students belonged to" (p 20) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge if blinding of outcome assessment occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attribution in both groups; "missing answers were treated as incorrect answers, while illegible answers were treated as missing values" (p 22) |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol or trial registration. No way to ensure the outcomes they intended to measure are fully reported. |
| Other bias | High risk | Did not adjust for clustering in analysis. Free of other potential biases: no evidence of selective recruitment of cluster participants. |
Kuppermann 2014.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 375 + 369 11‐week pregnant women who had not yet undergone prenatal screening or diagnostic testing in the USA | |
| Interventions | DA: describes clinical condition, options, outcome probabilities, values clarification. The interactive web‐based decision aid is not publicly available. Access to a video version of the DA was provided by the authors. Comparator: usual care |
|
| Outcomes | Primary outcomes: invasive prenatal diagnostic testing (3 to 6 months) Secondary outcomes: testing strategy undergone (3 to 6 months), knowledge (3 to 6 months), accurate risk perception (procedure‐related miscarriage, Down Syndrome affected fetus) (3 to 6 months), decisional conflict (3 to 6 months), decisional regret (3 to 6 months) |
|
| Notes | Source of funding: This study was funded by grants from the National Institutes of Health (R01HD049686) and the March of Dimes Foundation (Social and Behavioral Sciences Research Grant 12‐FY09‐213). Conflicts of interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Kuppermann reports that she was the UCSF site primary investigator of a clinical study of cell‐free DNA testing funded by Ariosa Diagnostics and receipt of unrestricted research funding from Verinata Health and Natera. Dr Caughey reports serving as a medical advisor to Ariosa and Cellscape and receipt of stock options in both companies. Dr Norton reports that she was a site primary investigator and lead coprimary investigator of a clinical study of cell‐free DNA testing funded by Ariosa Diagnostics, and was site primary investigator of a clinical study of noninvasive prenatal testing funded by Cellscape; receipt of unrestricted research funding from Natera; and being an unpaid clinical advisor to Natera. No other disclosures are reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated random allocation sequence assigned participants to experimental groups within permuted blocks of random size, with a 1:1 allocation ratio, stratified by age, clinical site, parity, and interviewer" (p 1211) |
| Allocation concealment (selection bias) | Low risk | "The randomization code was not available to any study‐related personnel until data analysis was complete" (p 1211) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Different research associates facilitated baseline and follow‐up interviews and medical record review to ensure blinding to the randomization assignment" (p 1211) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Different research associates facilitated baseline and follow‐up interviews and medical record review to ensure blinding to the randomization assignment" (p 1211) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups. "[A]ll reported analyses were based on a modified intention‐to‐treat sample" (p 1211) |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | Low risk | Appears to be free of other sources of bias |
Kuppermann 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 742 + 743 women with 1 prior cesarean delivery in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes probabilities of outcomes, explicit values clarification, risk prediction calculator, guidance in decision making (4‐step guide), and summary to discuss with provider. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcome: delivery approach Secondary outcomes: vaginal birth, maternal outcomes, perinatal outcomes, neonatal outcomes, and decision quality (decisional conflict, knowledge, shared decision‐making, decision efficacy, and decision satisfaction) |
|
| Notes | Source of funding: This study was supported by grant R01 HD078748 (Dr Kuppermann) from the NIH. Conflicts of interest: Dr Kuppermann reported receiving grants from the National Institutes of Health (NIH), the Patient‐Centered Outcomes Research Institute, the March of Dimes, and the UCSF Preterm Birth Initiative funded by Mark and Lynne Benioff and the Bill & Melinda Gates Foundation. Dr Kaimal reported receiving grants from the NIH. Dr Gonzalez reported receiving grant funding from California Institute for Regenerative Medicine. Dr Altshuler reported receiving grants from the Society of Family Planning. Dr Bacchetti reported receiving grant funding from the NIH, the Bill and Melinda Gates Foundation, and amfAR, the Foundation for AIDS Research. Dr Grobman reported receiving grant funding from the NIH, the March of Dimes, and the Preeclampsia Foundation. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The computer‐generated allocation sequence used randomly permuted blocks of 8, 10, and 12, stratified by language and recruitment site. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not masked to the intervention. Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were not masked to the intervention, but primary and secondary outcomes were assessed by study staff unaware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis and similar between arms (99% included in both arms); justification for attrition reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lam 2013.
| Study characteristics | ||
| Methods | Randomized to decision aid or standard information booklet after initial consultation | |
| Participants | 138 + 138 women considering breast cancer surgery for early‐stage breast cancer in China | |
| Interventions | DA: take‐home booklet on clinical problem, options' outcomes, outcome probabilities, guidance, explicit values clarification. The DA is not publicly available; a copy was provided by the author (wwtlam@hku.hk) Comparator: standard information booklet |
|
| Outcomes | Primary outcomes: treatment decision‐making difficulties and decisional conflict scale at 1 week post consultation, knowledge at 1‐week postconsultation, decision regret at 1 month after surgery Secondary outcomes: postoperative psychological distress (anxiety and depression) at 1, 4, and 10 months after surgery, decision regret at 4 and 10 months after surgery, treatment decision |
|
| Notes | Source of funding: Supported by the Health and Health Services Research Fund (Grant No. 07080651), Food and Health Bureau, and Government of Hong Kong, Special Administrative Region, People’s Republic of China. Conflicts of interest: The author(s) indicated no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient assignment to treatment and control arms was performed using a prior computer‐generated random‐number sequence" (p 2880) |
| Allocation concealment (selection bias) | Low risk | "A serially labeled, opaque, sealed‐envelope method was used for block randomization" (p 2880) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Two research staff members ‐ one responsible for preintervention assessment and block allocation and the other for postintervention assessments ‐ ensured that the researcher performing follow‐up assessments was blinded regarding women's allocation status." "Blinding surgeons to allocation status proved impractical." (p 2880) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 1 research staff member was responsible for postintervention assessments to ensure that the researcher performing follow‐up assessments was blinded regarding women's allocation status (p 2880). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data; similar attrition in both groups |
| Selective reporting (reporting bias) | Low risk | Study protocol available online with published study |
| Other bias | Low risk | Does not appear to be subject to other sources of bias. |
Langston 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid + coaching vs usual care | |
| Participants | 114 + 108 women pregnant women in their first trimester considering use of contraceptives in the USA | |
| Interventions | DA: double‐sided flip chart on clinical problem, outcome probabilities, guidance (administered by a research assistant), coaching (structured, standardized, non‐directive contraceptive counseling) + usual care. The DA is available at https://www.who.int/publications/i/item/9241593229 . Comparator: usual care |
|
| Outcomes | Primary outcomes: proportion of participants choosing very effective contraceptive method (post‐DA and consult) Secondary outcomes: actual choice on day of procedure (post‐DA and consult), adherence of very effective and/or effective methods at 3 months and at 6 months (post‐DA and consult) |
|
| Notes | Source of funding: Financial support provided by a grant from an anonymous foundation. This foundation approved the study design. It did not have a direct role in the collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication. Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random‐number table, we determined the sequence for 1:1 allocation constrained by blocks of 10" (p 363, Methods ‐ study procedures) |
| Allocation concealment (selection bias) | Low risk | "Randomization assignments were sealed inside numbered, opaque envelopes" (p 363, Methods ‐ study procedures) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "No blinding of participants or coordinators was feasible due to the nature of the intervention. Physician‐providers did not know the participant's allocation group, did not discuss the study with patients, and were asked not to change their counselling" (p 363, Methods ‐ study procedures) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For "method initiation on the day of the procedure" it is only said that the "[p]articipants in the intervention group were not more likely to initiate the requested method immediately compared to those in the usual care group"; possible that the results contradicted the hypothesis and were excluded for this reason. |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol; not enough information to permit judgment |
| Other bias | Low risk | Appears to be free of other potential biases. |
Laupacis 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 60 + 60 patients undergoing elective open heart surgery considering pre‐operative autologous blood donation in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework). The DA is available at https://decisionaid.ohri.ca/docs/das/archive/Blood_Transfusion.pdf . Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: uptake of option, satisfaction with decision‐making process, satisfaction with decision, accurate risk perceptions |
|
| Notes | Source of funding: This study was supported by the Canadian Institutes for Health Research (Grant #MT‐15580). AL is a CIHR Senior Scientist and AO holds a Tier I, Canada Research Chair in Health Care Consumer Decision Support. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization envelopes were prepared centrally by a statistician" (p 2) |
| Allocation concealment (selection bias) | Low risk | "The envelopes were labeled with identification numbers and contained a card specifying the patient's group assignment. The envelopes were opened by the interviewer after completion of the baseline interview." (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results, p 4; fig 1, flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
LeBlanc 2015.
| Study characteristics | ||
| Methods | Randomized to decision aid vs individualized score only vs usual care | |
| Participants | 32 + 33 + 14 women over 50 years diagnosed with osteopenia or osteoporosis not taking biphosphonates or other prescription medication in the USA | |
| Interventions | DA (in consultation): clinical problem, individualized risk of condition, options' outcomes, guidance. The DA is presented in Figure 1 of the article. Comparator 1: individualized risk Comparator 2: usual care |
|
| Outcomes | Primary outcomes: knowledge (immediately post), decisional conflict (immediately post), participation in decision‐making process (immediately post), decision to start (immediately post), adherence (6 months), acceptability (timing not specified), satisfaction with the decision‐making process (not specified), quality of life (not specified), time (review of video consultation) Secondary outcome: decision quality (not reported) |
|
| Notes | Source of funding: This study was supported by a grant from the Foundation for Informed Medical Decision Making (Now the Informed Medical Decisions Foundation, http://www.informedmedicaldecisions.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: The authors have declared that no competing interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion" (p 5) |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion" (p 5) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and clinicians were aware of the overall objective, presented as improvement in communication between patients and clinicians during the clinical encounter, but remained blinded to the specific aims" (p 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After randomization, only data analysts remained blind to allocation" (p 5) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis; similar attrition in both groups |
| Selective reporting (reporting bias) | Unclear risk | Trial registered; checklists available for CONSORT and protocol. Sample size originally calculated based on adherence but re‐calculated for decisional conflict given inability to reach original target. |
| Other bias | High risk | "Possible contamination at the clinician level (i.e. clinician who, having used the decision aid with a prior patient, recreates elements of the decision aid with a subsequent patient allocated to receive FRAX alone or usual care) was monitored by a detailed review of the available video recorded encounters" (p 5) |
LeBlanc 2015b.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 159 + 139 patients with moderate to severe depression in the USA | |
| Interventions | DA: decision aid used during consultation formatted as laminated cards that presented information about each antidepressant and pros and cons in terms that matter to patients: weight change, sleep, libido, discontinuation, and cost. Patients could also access a video clip and storyboard demonstrating the basic use of the decision aid and a leaflet to take home. The DA is publicly available at https://carethatfits.org/depression-medication-choice/ . Comparator: usual care |
|
| Outcomes | Decision‐making quality as judged by patient knowledge and involvement in decision‐making, decisional conflict, satisfaction, encounter duration, medication adherence, and depression symptoms | |
| Notes | Source of funding: This study was funded by the Agency for Healthcare and Quality Research under the American Recovery and Reinvestment Act of 2009 (iADAPT‐1 grant R18 HS019214). Conflicts of interest: AL, VMM, NDS, MDW, KJY, MEB, JWI, SRD, EMH, ML, DHB, KMDW, MRM, and KKS report no potential conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "The lead study statistician therefore stratified practices by their history of accrual and the presence of the DIAMOND program and centrally randomized practices within these strata to either care with or without Depression Medication Choice." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Our study is at risk of bias. Lack of blinding of participants may have affected questionnaire responses" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Our study is at risk of bias. Lack of blinding of analysts, particularly those reviewing videos, may have biased video‐based outcomes" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate but similar across arms. "There was substantial loss to follow up (∼20%) for our primary endpoint, mainly due to logistical issues at the beginning of the study, where study coordinators were still adapting to the recruitment and follow up process. While these issues may increase the risk of bias in favor of the intervention, other limitations may bias it toward no difference" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01502891) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Legare 2008a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 45 + 45 women considering use of natural health products for managing menopausal symptoms in Canada | |
| Interventions | DA: booklet with worksheet on options' outcomes, clinical problem, explicit values clarification, guidance/coaching (Ottawa Decision Support Framework). The DA is not publicly available; a copy was obtained from the authors. Comparator: general information brochure on the clinical problem (did not address risks and benefits) |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge of natural health products in general (not specific option outcomes), preferred choice, values‐choice agreement, proportion undecided |
|
| Notes | Source of funding: This study was funded by the Canada Research Chair in Implementation of Shared Decision Making in Primary Care and the André et Lucie Chagnon Chair for an Integrated Approach to Health Promotion, Université Laval, Québec. Conflicts of interest: FL, DS, ST, AL and SD are involved in the development of PDAs in the area of women’s health. However, they receive no financial gains. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization scheme was carried out by a biostatistician using computer‐generated unequal blocks. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes containing 1 or the other documents (a PDA in the intervention group and a general information brochure in the control group) were prepared by another individual, external to the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigators were blinded but no mention of blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Figure 1 for flow diagram; reason for loss to follow‐up was described. |
| Selective reporting (reporting bias) | Low risk | Trial registration identifier is NCT00325923. |
| Other bias | Low risk | No statistically significant difference in women's characteristics between groups (Table 1) |
Legare 2011.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 245 + 214 patients with non‐emergent acute respiratory infections considering using antibiotics in Canada | |
| Interventions | DA (in consultation): pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance and coaching. The DA is available at https://www.decision.chaire.fmed.ulaval.ca/outil-en/601acf01dc64b246f77888c8 . Comparator: delayed intervention |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Source of funding: This study was funded by the Fonds de la Recherche en Santé du Québec. FL is Tier 2 Canada Research Chair in Implementation of Shared Decision Making in Primary Care. GG is Tier 1 Canada Research Chair in Health Behaviour. FL, ML, GG AOC and AL are members of Knowledge Translation Canada, a CIHR‐funded national research network. A O'Connor is a Tier 1 Canada Research Chair on Decision Support for Consumers. AL holds a Doctoral Scholarship from the Canadian Institute of Health Research. Conflicts of interest: The author(s) declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A biostatistician simultaneously randomised all FMGs and allocated them to groups using Internet‐based software" (p 99) |
| Allocation concealment (selection bias) | Low risk | "Using Internet‐based software" (p 99) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding of participants and personnel: only biostatistician was blinded (p 99) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biostatistician who assesses the outcomes is blinded, outcomes were objectively measured (p 99) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no missing data. |
| Selective reporting (reporting bias) | Low risk | No missing pre‐specified outcomes |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Legare 2012.
| Study characteristics | ||
| Methods | Cluster‐randomized controlled trial to decision aid vs usual care | |
| Participants | 239 + 210 adults and children with with a diagnosis of acute respiratory infection (e.g.,bronchitis, otitis media, pharyngitis, rhinosinusitis) in Canada | |
| Interventions | DA (in consultation): pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance and coaching (participating physicians also received training in the form of a 2‐hour online tutorial and a 2‐hour on‐site interactive workshop). The DA is available as a supplementary appendix in the article. Comparator: usual care |
|
| Outcomes | Primary outcome: use of antibiotics (immediately post consultation) Secondary outcomes: decisional conflict (immediately post), control preference scale (immediately post), quality of decision (immediately post), adherence to the decision (2 weeks post), repeat consultation (2 weeks post), decisional regret (2 weeks post), quality of life (2 weeks post) and intention to engage in SDM in future consultations regarding antibiotics for acute respiratory infections (2 weeks post) |
|
| Notes | Source of funding: This study was funded by a grant from the Conseil du médicament du Québec/Fonds de la recherche en santé du Québec. The funding organization had no role in the conception or design, conduct, analysis, interpretation or reporting of the study and no access to the data. None of the investigators received any financial compensation. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A biostatistician used internet‐based software to simultaneously randomize all 12 family practice teaching units to either the intervention group or control group. The teaching units were stratified according to rural or urban location" (p E728) |
| Allocation concealment (selection bias) | Low risk | "A biostatistician used internet‐based software to simultaneously randomize all 12 family practice teaching units to either the intervention group or control group. The teaching units were stratified according to rural or urban location" (p E728) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients with symptoms suggestive of an acute respiratory infection were initially recruited by a RA in the waiting room before consultation with a physician" (p E728) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The biostatistician was unaware of group allocation, the researchers and research assistants who recruited patients and collected data were not" and "Statistical analysis was performed by a statistician who was unaware of the teaching unit allocations" (p E729) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol registered and published |
| Other bias | Low risk | "To avoid contamination bias, access to the online tutorial was denied to providers in the control group during the trial" (p E728) Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Leighl 2011.
| Study characteristics | ||
| Methods | Randomized to DA + usual care vs usual care | |
| Participants | 107 + 100 patients diagnosed with metastatic CRC considering advanced chemotherapy in Australia and Canada | |
| Interventions | DA: booklet and audiotape on option' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance (steps in decision making + worksheet) Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), satisfaction with decision (post‐DA) Secondary outcomes: anxiety (pre and post‐DA), satisfaction with consultation (post‐DA), choice leaning (post‐DA), decisional conflict (post‐DA). achievement of their information preference (post‐DA), participation in decision‐making (post‐DA), acceptability (post‐DA), quality of life (post‐DA) |
|
| Notes | Source of funding: Supported by the Cancer Council New South Wales (N.B.L., P.N.B., M.H.N.T.) and an American Society of Clinical Oncology Career Development Award (N.B.L.). Conflicts of interest: The author(s) indicated no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized lists (p 2078, Study design) |
| Allocation concealment (selection bias) | Low risk | Code concealed in sealed envelopes until time of random assignment (p 2078, Study design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients not blinded and subjective outcomes may be affected by knowing their assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes are not subjected to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31% dropout rate, but similar losses across all groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Lepore 2012.
| Study characteristics | ||
| Methods | Randomized to decision support intervention (decision coaching by telephone + educational pamphlet) vs control | |
| Participants | 244 + 246 African American men aged 45 to 70 in the USA | |
| Interventions | DA: condition‐specific educational pamphlet on prostate cancer screening and tailored telephone education on options' outcomes, explicit values clarification, others' opinions, and guidance (decision coaching). The DA is not publicly available; a copy was provided by the author (slepore@temple.edu). Comparator: attention control (education on fruit and vegetable consumption) |
|
| Outcomes | Primary outcomes: knowledge (pretest and post‐test at 8 months post‐randomization), decisional conflict (post‐test), physician visit to discuss testing (post‐test), adherence as congruence between testing intentions and behaviors (post‐test) Secondary outcomes: testing intention (post‐test), benefit‐to‐risk ratio of testing (post‐test), PSA screening (post‐test), anxiety (pretest and post‐test) |
|
| Notes | Trial registration NCT01415375 Source of funding: This research was supported by grant R01 CA104223 from the National Cancer Institute of the National Institutes of Health. Conflicts of interest: The authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The principal investigator used a computer‐generated randomization schedule to randomize the participant." (p 322) |
| Allocation concealment (selection bias) | Unclear risk | "The principal investigator used a computer‐generated randomization schedule to randomize the participant and emailed the randomization assignment to the interventionist." (p 322) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Interventionists were not blind to condition. We can assume that patients were blinded as the study design was a telephone call for both intervention and control groups (p 322). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collectors were blind to condition but the interventionists were not" (p 322). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data |
| Selective reporting (reporting bias) | Low risk | Appears to have reported on all pre‐specified outcomes (protocol). |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Lerman 1997.
| Study characteristics | ||
| Methods | Randomized to decision aid vs waiting list control | |
| Participants | 122 + 114 + 164 women considering BRCA1 gene testing in the USA | |
| Interventions | DA: education and counselling on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: no intervention |
|
| Outcomes | Primary outcome: preferred option Secondary outcomes: knowledge, accurate risk perceptions, perceived personal risk/benefits/limitations, agreement between values and choice |
|
| Notes | Source of funding: Supported by Public Health Service grants (RO1MH/HG54435) from the National Institutes of Mental Health and the National Center for Human Genome Research, National Institutes of Health Department of Health and Human Services. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 440 women, 400 completed 1‐month follow‐up interviews; no reasons provided; baseline data/characteristics included (p 2). |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Lewis 2010.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 211 + 232 patients considering colorectal cancer screening in the USA | |
| Interventions | DA: web‐based, DVD and VHS videotape formats + stage targeted brochures (and booster kit if patients had not been screened) on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encouraged patients to communicate with their practitioners by asking questions and sharing preferences; summary). The DA is no longer available (decisionsupport.unc.edu/CHOICE6/). The authors have screenshots of the website that was evaluated in the study. Comparator: usual care using Aetna annual reminders to obtain CRC screening |
|
| Outcomes | Knowledge of the age at which screening should begin (post‐DA), completion of colorectal cancer screening (pre, post‐DA), intrusive thoughts (pre, post‐DA), interest in CRC screening (pre, post‐DA), intent to ask provider about screening (pre, post‐DA), readiness to be screened (pre, post‐DA), perceived risk of colon cancer (pre, post‐DA), general beliefs about colon cancer (pre, post‐DA), fears about colorectal cancer screening (pre, post‐DA), perceptions about whether participants had enough information (post‐DA), whether participants had enough information about specific screening tests (post‐DA), willingness to pay for screening tests (post), desire to participate in medical decision (post) Practice level measures: assess CRC screening practices (pre, post‐DA), referrals (pre, post‐DA), quality improvement initiatives |
|
| Notes | Primary outcome was not specified Source of funding: This study was supported by grant number PH000018 from the Centers for Disease Control and Prevention. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done using matched pairs and a blocking procedure." (p 2, Practice recruitment and randomization section) |
| Allocation concealment (selection bias) | Unclear risk | "Thus, purposive assignment to treatment group was used, resulting in a hybrid randomisation" (p 3, Practice recruitment and randomization section). There is no mention of the effect of this purposive assignment on the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As mentioned above, staff used purposive assignment and were therefore not blinded, but there is no mention of the effect on the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study did not address this outcome, but outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | High risk | Unadjusted cluster analysis Free of other potential biases: no evidence of selective recruitment of cluster participants. |
Lewis 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control | |
| Participants | 212 + 212 primary care patients 70 to 84 years of age with an upcoming appointment in the USA | |
| Interventions | DA: paper‐based decision aid that includes information on the clinical problem, outcome probabilities, explicit values clarification, guidance in communication, and personalized summary to bring to consultation. The DA is publicly available at https://eprognosis.ucsf.edu/decision_aids/Colon_Male_75-79.pdf . Comparator: education on an unrelated topic (safe driving) |
|
| Outcomes | Primary outcome: a composite measure of appropriate screening behavior 6 months following the index visit Secondary outcome: screening intent immediately after the index visit Intermediate outcomes: preparedness for individualized decision‐making (knowledge, unclear values), communication, proportion undecided, preparation for decision‐making, screening preference |
|
| Notes | Source of funding: Agency for Healthcare Research and Quality (AHRQ) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned through a centralized computer process to the decision support intervention or attention control condition. Because our primary outcome was a combined outcome across 3 health states, we wanted to ensure adequate numbers in each health state. Therefore, we assigned participants to the intervention or control arm using permuted blocks stratified by health state" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation was concealed from the RAs through the use of opaque, sealed envelopes." The use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Allocation was concealed from the RAs through the use of opaque, sealed envelopes. Thus, the RAs, who adminstered surveys and collected data, were blinded to the patients' assignment. Patients were also blinded to their assignment, as they did not know whether they were in the intervention or control group. Providers, however, may have been aware of patients' assignment because patients in the intervention arm brought a paper cue into the provider visit." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The RAs, who administered surveys and collected data, were blinded to the patients’ assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in analysis. Screening intent 210/212 (99%) DA and 211/212 (99%) control. Screening behavior 208/212 (98%) DA and 204/212 (96%) control. Reasons for attrition are documented and balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The trial protocol is available (NCT01575990) and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Lewis 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid plus coaching vs usual care | |
| Participants | 15 + 15 patients ≥ 18 years approaching implantable cardioverter‐defibrillator (ICD) depletion, who are deciding whether to have ICD replacement | |
| Interventions | DA + decision coaching: paper‐based decision aid that includes information on the clinical problem, outcome probabilities, explicit values clarification, self‐reflection questions, knowledge test, SURE test, frequently asked questions, guidance in decision‐making (step‐by‐step process for making the decision), guidance in communication, and summary at the end to identify needs to make a choice. Decision coaching was delivered by a trained nurse research assistant whose role was to make the decision explicit (i.e. accept vs decline), describe the options, clarify values, elicit the patient's preferred treatment option, and screen for decisional conflict. The DA is not publicly available; a copy was provided by the author (Krystina Lewis: Krystina.Lewis@uottawa.ca). Comparator: usual care consisted of a 1‐page educational leaflet describing the logistics of the ICD replacement procedure |
|
| Outcomes | Primary outcomes: feasibility measures (rates of recruitment, intervention use, and completeness of data collection) Secondary outcomes: preliminary effectiveness outcomes (knowledge, decisional conflict, preferred choice, actual choice, perception of involvement in decision‐making, values about ICD replacement, the Medical Outcomes Trust Short Form, acceptability and usability of decision support, survival) |
|
| Notes | Source of funding: This study was supported by a Canadian Council of Cardiovascular Nurses Research Grant. KBL's doctoral studies were supported by a Canadian Institutes of Health Research fellowship. Conflicts of interest: The authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned 1:1 to the intervention or usual care by the research assistant. A clinician researcher not otherwise involved in the study prepared a randomization schedule (http://www.randomization.com/). The sequence was generated using a permuted block design with randomly varying blocks of 4 to 8." |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed using sequentially numbered, opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To blind patients to group allocation, they were informed that “the study was looking at a new way to support patients facing ICD battery replacement, compared to the current way we do it.” Device clinic staff and the research assistant were not blinded owing to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate, reasons for attrition recorded |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02668900) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | "... there is potential for selection bias as patients who required pacing or who were eligible for an upgrade to cardiac resynchronization therapy did not meet inclusion criteria. In addition, a small proportion had their ICD previously replaced." |
Lin 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 144 + 138 families whose babies were around 1 month old and going for routine vaccination in Taiwan | |
| Interventions | DA: paper‐based decision aid used during consultation that included clinical information, probabilities of outcomes, explicit values clarification, level of understanding test, guidance in decision‐making (used in consultation), and guidance in communication (prompted to discuss concerns with doctor). The DA is available as a supplementary appendix in the article. Comparator: usual care |
|
| Outcomes | Decisional conflict, decision‐making difficulties, and rotavirus vaccine knowledge (perceived), and the overall rotavirus vaccination rate | |
| Notes | Source of funding: This work was supported by a research grant from Shuang Ho Hospital, Taipei Medical University (grant no.: 108HHC‐03). The sponsoring organization was not involved in the study design, data analysis, or interpretation of results. Conflicts of interest: Dr. Sheng‐Chieh Lin has received research grants from Shuang Ho Hospital, Taipei Medical University. All authors including Dr. Sheng‐Chieh Lin have no conflicts of interest or financial ties to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomly divided into control (non‐SDM) and experimental (SDM) groups using computer‐generated assignment by the outpatient clinic nurse" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The randomization result was given to the physician. Owing to the nature of the intervention, participants could not be blinded to the study arm to which they were randomized. The questionnaires were collected after the vaccination for their babies, and were anonymous to reduce stress on the respondents. Nurses who were blinded to randomization asked and noted down the response. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor‐blind: "Nurses who are blinded with randomization asked and noted down the response." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of attrition but missing data are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03804489) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | The SURE test was translated, resulting in more items (7). Selection bias: "Second, our study was performed in an urban area, so the effects of SDM in rural areas still need investigation. Third, we excluded infants whose families differed at 1 and 2 months, so we cannot assess the influence of SDM on these families" |
Lin 2022.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 97 women newly diagnosed with stage 1‐3 breast cancer or ductal carcinoma in situ who required breast tumor resection in Taiwan | |
| Interventions | DA: paper‐based decision aid used during consultation that includes clinical information, outcome probabilities, explicit values clarification, knowledge test, and guidance in communication. The DA is available as a supplementary appendix in the article. Comparator: usual care |
|
| Outcomes | Decisional conflict, decisional regret, psychological distress | |
| Notes | Source of funding and conflicts of interest: All authors have no conflict of interest or financial ties to disclose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly divided into the standard and PDA groups through computer‐generated assignment by a nurse in an ambulatory care clinic. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | During preoperative hospitalization 1 day before surgery, the effect of the PDA was investigated by an outcome assessor, who was a research assistant in Shared Decision Making Resource Center of Shuang Ho Hospital and blinded to participants’ group allocation. During their follow‐up visit 1 month after surgery, patients’ decisional regret and postoperative psychological distress were examined by the same outcome assessor who was blinded to the allocation. Blinding of patients was not reported in the article. Unclear how lack of blinding of participants may have influenced the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | During preoperative hospitalization 1 day before surgery, the effect of the PDA was investigated by an outcome assessor, who was a research assistant in Shared Decision Making Resource Center of Shuang Ho Hospital and blinded to participants’ group allocation. During their follow‐up visit 1 month after surgery, patients’ decisional regret and postoperative psychological distress were examined by the same outcome assessor who was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rates: 76/103 DA group and 75/97 usual care group analyzed, however missing data are balanced across groups (P = 0.561491). Justifications for attrition provided. |
| Selective reporting (reporting bias) | High risk | The study protocol is available (NCT03105076). Knowledge was included as a primary outcome in the trial registry, but there is no mention of this outcome in the article. The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Other bias | Unclear risk | Knowledge and satisfaction with decision were used to calculate statistical power for study but did not collect/report on knowledge and satisfaction in study. |
Loh 2007.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 263 + 142 patients with physician diagnosed depression (cluster‐RCT with 30 general practitioners randomized) in Germany | |
| Interventions | DA (in consultation): options' outcomes, clinical problem, explicit values clarification, guidance/coaching. The DA is not publicly available; a copy was provided by the author (in German). Comparator: usual care |
|
| Outcomes | Participation in decision‐making, adherence, satisfaction with clinical care, depression severity, consultation length | |
| Notes | Primary outcome was not specified Source of funding: The study was funded by the German Ministry of Health (BMGS Grant 217‐43794‐5/6 www.shared‐decision‐making.org). In continuation the German Ministry of Health also supported a project concerning the methodological tasks of the research consortium (BMGS Grant 217‐43794‐5/11) and a project to transfer shared decision‐making in medical education (BMGS Grant 217‐43794‐5/12, www.shared‐decision‐making.org). Celia E. Wills is a past recipient of a U.S. National Institute of Mental Health Mentored Clinical Scientist Career Development (K08) Award (MH01721; 2000‐2005) on depression treatment decision‐making of primary care patients. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]wo‐thirds of the general practitioners were randomly assigned to the intervention group by drawing blinded lots under the supervision of the principal investigator and two researchers" (p 3) |
| Allocation concealment (selection bias) | Low risk | Drawing blinded lots (p 3 ‐ 2.1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding; not enough information provided to assess whether this contributes to bias in outcomes not measured by using a scale (e.g. consultation time was documented in minutes by the physicians following each consultation). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Further results resting on the baseline phase of this trial were already presented elsewhere" (p 5, fig); "unequal distribution of physicians was due to possibility of higher dropout rate in intervention group because of additional time and effort" (p 3). |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Appears to be free of other potential biases (p 5‐6, details patient and physician baseline characteristics). Statistically significant differences were controlled for in outcome analyses. Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Love 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid + verbal discussion vs standard verbal discussion alone (control) | |
| Participants | 16 + 16 patients 18 years and older with an untreated, biopsy‐proven, primary basal cell carcinoma in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that includes clinical information, outcome probabilities, and implicit values clarification. The DA is available as a supplementary appendix in the article. Comparator: usual care |
|
| Outcomes | Knowledge, patient and physician satisfaction, length of time for informed consent, treatment preference | |
| Notes | Source of funding: none Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in 1:1 random sequence at each site |
| Allocation concealment (selection bias) | High risk | "Preprepared study packets were labeled in order (by E. M. L.), with even numbers assigned to control group and odd numbers assigned to video group. Study personnel were aware of patient cohort at the time of consent (but not at the time of recruitment). Patients were informed of study group after consent." (Alternation or rotation used) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study personnel were aware of patient cohort at the time of consent (but not at the time of recruitment); patients were informed of study group after consent; treating physicians were blinded at 1 site and not at the other site. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treating physicians at Emory University were blinded to patient study group; Atlanta VAMC treating physicians were not blinded because of workflow constraints." Low risk for outcomes objectively measured (i.e. knowledge). Unclear risk for informed consent time. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis in DA group (13/16 completed final knowledge test vs 16/16 for control) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT02158650). Treatment preference (secondary outcome of interest to this review) was not pre‐specified. |
| Other bias | Unclear risk | Small sample size (16 + 16), mean and SD but small sample size and nothing about heterogeneity (median and range may be more appropriate) |
Luan 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (standard information) | |
| Participants | 8 + 8 new adult breast reconstruction patients of one plastic surgeon undergoing reconstruction for mastectomy indicated for breast cancer in the USA | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, and guidance in communication. The DA is not publicly available; a copy was provided by the author (Anna Luan; aluan@stanford.edu). Comparator: usual care (standard information) |
|
| Outcomes | Decisional conflict, health‐related quality of life, decision regret, anxiety and depressive symptoms, utilization of any other sources of information regarding breast reconstruction, perceptions of desired level of involved, chosen option | |
| Notes | Source of funding: none declared Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A weekly block randomization structure was used. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram not included; insufficient reporting of attrition/exclusions (i.e. number enrolled/randomized not reported, no reasons for missing data provided) |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | "... our small sample size limits the power of our study", "distribution of surveys in the clinic setting may introduce bias into our results, as it is plausible that patients may consciously or subconsciously alter their responses" |
Madden 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control | |
| Participants | 167 + 86 women at risk for unintended pregnancy who planned to discuss reversible contraception at their scheduled appointment in the USA | |
| Interventions | DA: tablet‐based decision aid used in preparation for consultation that included clinical information, probabilities of outcomes, explicit values clarification, algorithm that identified the 3 contraceptive methods most concordant with the women’s preferences, guidance in decision‐making (algorithm), and printed summary of tailored information. The DA is not publicly available and we were unable to obtain a copy from the authors. The pros and cons of options were presented using information that is available on bedsider.org:
https://www.bedsider.org/birth-control . Comparator: control (tablet‐based education on reproductive health) with a non‐tailored handout describing recommendations for reproductive health care such as screening for cervical cancer and sexually transmitted infections |
|
| Outcomes | Primary outcome: change in decisional conflict Secondary outcomes: choice of contraceptive method and satisfaction with the healthcare visit; also reports communication (discussed with provider) |
|
| Notes | Source of funding: This research was supported in part by: (1) the Society of Family Planning (SFP, grant numbers SFP3‐1, SFP5‐8) and (2) the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) (grant number K23HD070979). The funders had no role in the identification, design, conduct, and reporting on this analysis. The content is solely the responsibility of the authors and does not necessarily represent the official view of NICHD. Conflicts of interest: Dr. Madden serves on a data safety monitoring board for phase 4 safety studies of Bayer contraceptive products. Dr. Peipert receives research funding from Bayer Healthcare Pharmaceuticals, CooperSurgical/TEVA, and Merck & Co, Inc. and serves on an advisory board for CooperSurgical Pharmaceuticals. Dr. Politi receives research funding from Merck & Co. The other authors do not have any potential conflicts of interest to report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The computer programmer who was not involved in recruitment used a random number generator to create the randomization scheme. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from the research team as the tablet computer implemented the random allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the differences in the 2 printouts provided to the study groups, which participants were encouraged to share with their healthcare provider, blinding of the participants and healthcare providers was not possible. Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis and similar attrition rate between arms (96% included for DA, 93% included for control), justification for attrition reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT01479985) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | One or more of the authors are industry employees: Dr. Madden serves on a data safety monitoring board for phase 4 safety studies of Bayer contraceptive products. Dr. Peipert receives research funding from Bayer Healthcare Pharmaceuticals, CooperSurgical/TEVA, and Merck & Co, Inc. and serves on an advisory board for CooperSurgical Pharmaceuticals. Dr. Politi receives research funding from Merck & Co. |
Man‐Son‐Hing 1999.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 139 + 148 patients on atrial fibrillation trial considering continuing on aspirin vs change to warfarin in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework). The DA is no longer available (decisionaid.ohri.ca/decaids‐archive.html). Comparator: usual care |
|
| Outcomes | Primary outcomes: uptake of options, adherence Secondary outcomes: help with making a decision, knowledge, accurate risk perceptions, decisional conflict, satisfaction with decision‐making process, role in decision‐making |
|
| Notes | Source of funding: This study was supported by grant R01 NS 242224 from the National Institute of Neurological Disorders and Stroke, Bethesda, Md. Original development of the audiobooklet decision aids fro patients with atrial fibrillation was supported in part by DuPont Pharmaceuticals. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme (p 2) |
| Allocation concealment (selection bias) | Low risk | Administered from a central location (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear blinding however, "contamination, physicians may have provided DA information to patients receiving usual care" (p 7) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | P 4, fig 2 flow chart. Reasons for attrition not mentioned. Baseline data not included. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential risks of bias |
Mann D 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 80 + 70 participants diagnosed with diabetes considering the use of statins to reduce coronary risk in the USA | |
| Interventions | DA (in consultation): healthcare provider led discussion using developed tool (Statin Choice) on options' outcomes, outcome probabilities, guidance (step‐by‐step process for making the decision; administered by the physician in the consultation). The website is no longer available (mayoresearch.mayo.edu/mayo/research/ker_unit/form.cfm). The authors have a PDF copy of the DA. Comparator: usual primary care visit + pamphlet |
|
| Outcomes | Knowledge (postconsult and post‐DA), decisional conflict (postconsult and post‐DA), risk estimation (postconsult and post‐DA), beliefs (postconsult and post‐DA), adherence (3 and 6 months postconsult and post‐DA) | |
| Notes | Primary outcome was not specified Source of funding: not reported Conflicts of interest: The authors have no relevant conflict of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized, but there is no mention of method used (p 138, Methods section). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline data were provided. |
| Selective reporting (reporting bias) | Unclear risk | Only reports on improvement (i.e. decisional conflict scale); does not present outcome data to fullest (no numerical data on knowledge results between groups, only describes in words). |
| Other bias | Unclear risk | "We did not adjust the clustering of effects given that few participants received care by the same clinicians" (p 139, Analysis section). No mention of the magnitude of change of data due to this choice. |
Mann E 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 278 + 139 participants considering diabetes screening in the UK | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities and explicit values clarification. The DA is available as a supplementary appendix in the article. Comparator: usual care using screening invitation on clinical problem |
|
| Outcomes | Primary outcomes: preferred option (post‐DA) Secondary outcomes: whether invitation type impacts on intention (post‐DA), impact on knowledge (post‐DA), impact on attitude (post‐DA), risk perception |
|
| Notes | Source of funding: not reported Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Invitation taken from the top of a randomly ordered pile (either standard or one of two versions of an informed decision choice invitation). The materials were ordered in a way that the invitation type was hidden until the recruitment process was completed" (p 2‐3, Methods, Participants section). Unclear how invitation type was hidden. |
| Allocation concealment (selection bias) | Low risk | "Invitation taken from the top of a randomly ordered pile; materials were ordered in a way that the invitation type was hidden until the recruitment process was completed" (p 2‐3, Methods, Participants section). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interviewers were not aware of the direction of anticipated effect of materials, and materials were dummy‐coded so that no sense of intervention or control would have been communicated to interviewers or participants (p 3, Methods, Participants section). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not address this outcome, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol; insufficient information to permit judgment |
| Other bias | Unclear risk | "Present sample was … not necessarily representative of the highest risk individuals in this age group"; "£5 incentive might have also added a selection bias"; "Lack of anonymity with verbally delivered questionnaire might encourage socially desirable responding" (p 6, Discussion section) |
Manne 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 47 + 46 women 18 years of age or older with a diagnosis of Stage 0‐3A breast cancer without hereditary breast cancer who were considering contralateral prophylactic mastectomy in the USA | |
| Interventions | DA: interactive, web‐based decision aid used independent of consultation that included clinical information, explicit values clarification, patient experiences, patient photograph examples, glossary, knowledge test, coping strategies, and summary of key points that can be printed and emailed to participant. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcome: knowledge, preparation for decision‐making, decisional conflict Secondary outcomes: self‐efficacy to manage worry, worry, motivations, risk for contralateral breast cancer, risk for chest wall recurrence after mastectomy, DA evaluation, DA user interface |
|
| Notes | Source of funding: This study was funded by an NIH R21 grant (CA187643) to Sharon Manne and Laurie Kirstein. Conflicts of Interest: The authors declare that they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Few details: after the consent and survey were received, participants were randomized to B‐Sure or usual care. Both sites followed the same randomization procedures. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis (44/47 (93%) in usual care, 39/46 (85%) DA group but balanced across groups P = 0.169126), no justification for dropouts provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Marteau 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 633 + 639 patients considering diabetes screening in England | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities, and explicit values clarification. The DA is available as a supplementary appendix in a previous article ( Mann E 2010 ). Comparator: usual care using screening invitation on clinical problem |
|
| Outcomes | Primary outcome: attendance for screening (post‐DA and consult) Secondary outcomes: intention to make changes to lifestyle (post‐DA and consult), satisfaction with decisions made among attenders (post‐DA and consult) |
|
| Notes | Source of funding: This trial was funded by the Wellcome Trust (grant No 076838 “Didactic versus informed choice invitations: balancing public health benefits and individual choice” principal investigator TMM). The funding body had no role in study design, data collection, analysis, interpretation, or writing of the report. Conflicts of interest: All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). SG has received honorariums from Eli Lilly, GlaxoSmithKline, Merck, Sharp & Dohme, Colgate Palmolive, Unilever, the University of Western Ontario, and the National Health Service for undertaking lectures at educational meetings not directly related to the topic of this paper. His second class rail travel costs for attending Department of Health meetings concerning the NHS health check were reimbursed by the Department of Health. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[G]enerated simultaneously in a batch by random numbers using Excel spreadsheet software, stratifying by number of participants in household" (p 2, Randomization section) |
| Allocation concealment (selection bias) | Low risk | "Randomisation … was undertaken by the study statistician from a central site" (p 2, Randomization section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel were blinded and it appears that patients were unaware which arm they were in (members of the same household received the same intervention) (p 2, Randomization section). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and trial staff taking measurements and entering data were unaware of the study arm to which participants had been assigned (p 2, Randomization section). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published protocol (p 2, Methods) |
| Other bias | Low risk | Appears to be free of other potential biases. |
Mathers 2012.
| Study characteristics | ||
| Methods | Cluster‐randomized controlled trial of 49 general practices in the UK to decision aid, healthcare professional training workshop and use of PDA in consultation, or usual care | |
| Participants | 95 + 80 participants with type 2 diabetes considering adding or changing to insulin therapy | |
| Interventions | DA: booklet about clinical problem, treatment options, options' outcomes, outcome probabilities, explicit values clarification, structured guidance. The DA is not publicly available; a copy was provided by the author (C.Ng@sheffield.ac.uk). Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (immediately postintervention), glycemic control (glycosylated hemoglobin, HbA1c) at 6 months Secondary outcomes: knowledge (immediately post), realistic expectations (immediately post), preference option (immediately post), proportion undecided (immediately post), participation in decision‐making (immediately post), regret (6 months), adherence with chosen option (6 months) |
|
| Notes | Trial registration: ISRCTN14842077 Source of funding: Funded by National Insitute for Health Research, Research for Patient Benefit. NM, CJN, MC, BC, IB and AB have support from the University of Sheffield for the submitted work. Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All eligible and willing practices were randomly allocated by a computer" (p 3) |
| Allocation concealment (selection bias) | Low risk | "A statistician generated the random allocation sequence while a secretary who was not involved in the research study assigned participants to either the intervention or control groups" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Blinding of the intervention and assessment of the process measures were not feasible in view of the nature of the intervention studied" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Blinding of the intervention and assessment of the process measures were not feasible in view of the nature of the intervention studied" (p 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | Unclear risk | Cannot make a judgment with information provided regarding cessation of recruitment at 175 (yet 320 required to allow detection of 0.5% difference in HbA1c). Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Mathieu 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid versus usual care | |
| Participants | 367 + 367 women aged 70 to 71 years and considering a subsequent screening mammography in Australia | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance with worksheet (Ottawa Decision Support Framework). The DA is not publicly available; a copy was provided by the author. Comparator: BreastScreen NSW brochure ‐ includes information for women 70 + but no numeric information about the outcomes of screening |
|
| Outcomes | Primary outcomes: actual decision, informed choice Secondary outcomes: knowledge (includes 5 questions about risk perceptions), anxiety, decisional conflict, breast cancer worry, preference/intention, attitudes about screening, relationship between objective and perceived risk of breast cancer |
|
| Notes | Source of funding: This study was supported by grant 211205 from the National Health and Medical Research Council of Australia. Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program, which assigned allocations in accordance with a simple randomization schedule (p 2, Methods) |
| Allocation concealment (selection bias) | Low risk | Randomized by interview staff who accessed a previously concealed computer program (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers (at follow‐up) were blinded; outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1 flow diagram (p 2) |
| Selective reporting (reporting bias) | Low risk | "The trial was registered with the Australian Clinical Trials Registry and the Clinical Trials Registration System" (p 5) |
| Other bias | Low risk | Appears to be free of other potential biases. |
Mathieu 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 189 + 223 women considering mammography screening in Australia | |
| Interventions | DA: Internet program + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (worksheet with questions relevant to decision‐making process; one or more questions that asked patients to clarify their preferences; summary). The DA is no longer available (http://www.psych.usyd.edu.au/cemped/com_decision_aids.shtml). The Internet‐based decision aid was based on a previously developed and evaluated paper‐based decision aid ( Mathieu 2007 ), modified to provide age‐appropriate data. Comparator: delayed intervention |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), risk perception Secondary outcomes: intention (post‐DA), values (post‐DA), informed choice (post‐DA), proportion undecided |
|
| Notes | Source of funding: This study was supported by grant 211205 from the National Health and Medical Research Council of Australia. The funding source had no role in the design or conduct of the study, the collection, analysis, or interpretation of the data or the preparation of the manuscript. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer generated simple randomization schedule" (p 66, Randomization and baseline questions section) |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomization was conducted in a concealed manner" (p 66). Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes mentioned in Outcome measures section were reported in the results section (p 68, Table 2; information for intention as well as anxiety and acceptability can be found in text format in the secondary outcomes section on p 67‐8). |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
McAlister 2005.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 219 + 215 patients considering antithrombotic therapy for nonvalvular atrial fibrillation (cluster‐RCT with 102 primary care practices randomized) in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework). The DA is no longer available (https://decisionaid.ohri.ca/decaids‐archive.html). Comparator: usual care |
|
| Outcomes | Primary outcomes: uptake of (appropriate) option Secondary outcomes: knowledge, decisional conflict, accurate risk perceptions |
|
| Notes | Source of funding: The DAAFI Trial was funded by the Canadian Stroke Network, the Alberta Heritage Foundation for Medical Research (AHFMR), and the University Hospital Foundation, Edmonton. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]luster randomization at level of primary care practice to minimize contamination; randomization was done centrally to preserve allocation concealment using a computer generated sequence" (p 2) |
| Allocation concealment (selection bias) | Low risk | Randomization was done centrally to preserve allocation concealment (p 2, Methods). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded, but not sure whether the lack of blinding would affect the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results and Fig 1 ‐ flow diagram (p 3) |
| Selective reporting (reporting bias) | Low risk | DAAFI trial protocol, including copies of the various questionnaires employed, has been published (p 1, Methods). |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
McBride 2002.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 289 + 292 perimenopausal women considering hormone replacement therapy in the USA | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, values clarification, others' opinions, guidance/coaching. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: delayed intervention |
|
| Outcomes | Primary outcome: accurate risk perceptions Secondary outcomes: satisfaction with decision, confidence with knowledge, and making/discussing decision |
|
| Notes | Source of funding: This work was supported by a grant from the National Cancer Institute (PO1‐CA‐72099‐05). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided; Bastian 2002, no information provided ‐ Study design is described elsewhere (p 4) |
| Allocation concealment (selection bias) | Unclear risk | No information provided; Bastian 2002, no information provided ‐ Study design is described elsewhere (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data are available for 520 (90%) of the women (p 2). Reasons why not mentioned (Bastian 2002, p 5, Results; p 6, Baseline characteristics/data included). |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Appears to be free of other potential biases; Bastian 2002, p 8 ‐ Eligible participants were willing to consider HRT and this may have favored recruitment of women with higher SES and those who had prior experience with HRT. |
McCaffery 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid + informed choice vs HPV testing vs repeat smear | |
| Participants | 104 + 104 + 106 women screened as HPV indeterminate considering HPV testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (worksheet). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator 1: no decision support, received immediate HPV testing Comparator 2: no decision support, received a repeat cervical smear at 6 months |
|
| Outcomes | Primary outcomes: quality of life (post‐DA) Secondary outcomes: waiting time anxiety (post‐DA), perceived risk (post‐DA), perceived seriousness of cancer (post‐DA), worriedness (post‐DA), intrusive thoughts (post‐DA), satisfaction with care (post‐DA), anxiety (post‐DA), distress and concerns (post‐DA), self‐esteem (post‐DA), effect on sexual behavior (post‐DA), help‐seeking behavior (post‐DA), knowledge (post‐DA) |
|
| Notes | Source of funding: This work was supported by an Australian National Health and Medical Research Council (NHMRC) Grant 402764 to the Screening and Test Evaluation Program. KM is supported by a NHMRC Career Development Award 402836. The NHMRC has played no role in the writing of this paper. Conflicts of interest: KM has received a speaker’s fee from CSL (producers of the HPV quadrivalent vaccine Gardasil) and a consultancy fee from GlaxoSmithKline (producers of the bivalent HPV vaccine, Cevarix). EW has received honoraria and research funding from GSK and CSL for her research in the area of HPV vaccination. All other authors have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised centrally by the research team within each clinic in blocks of three" (p 2, Design) |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised centrally by the research team within each clinic in blocks of three" (p 2, Design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and staff were unblinded, but objective outcomes were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes are on questionnaires; not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 3: sensitivity analysis was done to include most of the patients. |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Appears to be free of other sources of bias. |
McGrath 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 38 + 41 women with epilepsy of childbearing age (18 to 45) in Australia | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, patient narratives, guidance in decision‐making (5‐step process), and guidance in communication. The DA is available as a supplementary appendix in the article. Comparator: control (no intervention) |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, decisional self‐efficacy Secondary outcomes: certainty, patient‐practitioner communication, depression, anxiety |
|
| Notes | Source of funding: none Conflicts of interest: none of the authors has any conflict of interest to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to either receive the DA or the control group using a 1:1 allocation schedule. Random numbers corresponding to intervention vs control group were generated through www.randomizer.com. |
| Allocation concealment (selection bias) | Low risk | Random numbers corresponding to intervention vs control group were generated through www.randomizer.com. These numbers were linked in advance to participant identification numbers and concealed until allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate in the intervention arm 8/38 (21%) compared to the control arm 4/41 (9.7%), however the difference in missing data is not significant (P = 0.162203). Reasons for attrition are provided. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12613001082796). Low risk for all outcomes except for patient‐clinician communication and values congruence with chosen option, which were not included as an outcomes in the trial registry (high risk). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
McIlvennan 2018.
| Study characteristics | ||
| Methods | Cluster‐randomized, stepped‐wedge trial, decision aid vs usual care | |
| Participants | 71 + 111 informal caregivers of patients with end‐stage heart failure eligible for a destination therapy left ventricular assist device implantation in the USA | |
| Interventions | DA: booklet and video decision aid integrated with consultation that includes clinical information, outcome probabilities, explicit values clarification, patient narratives, guidance in communication, and summary page that can be shared in consultation. The DA is publicly available at https://patientdecisionaid.org/lvad/ . Comparator: usual care (education) |
|
| Outcomes | Primary outcome: decision quality (informed values‐choice congruence) Secondary outcomes: decision, decisional conflict, decision regret, perceived stress, preparedness for caregiving, satisfaction with care, depression, acceptability of the educational materials |
|
| Notes | Source of funding: This work was supported through a Patient‐Centered Outcomes Research Institute (PCORI) Program Award (CDR‐1310‐06998). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or Methodology Committee. This work was also supported in part by the National Heart, Lung and Blood Institute (1K23HL105896‐01, Allen), the Heart Failure Society of America (McIlvennan), the National Institute on Aging (1K23AG040696, Matlock), and REDCap database hosting through University of Colorado supported by NIH/NCRR Colorado CTSI (Grant Number UL1 TR001082). Conflicts of interest: Dr. Blue has received personal fees from Abbott and Medtronic. Dr. Patel has received personal fees from Abbott and Medtronic. Dr. Allen has received personal fees from ACI Clinical, Janssen, Cytokinetics, Novartis, Boston Scientific, Amgen, and Duke Clinical Research Institute. All other authors have reported that they have no relationships relevant to the contents of this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition; however, missing data for 1‐ and 6‐month outcomes is balanced across groups (1 month 53/71 completers DA group 89/111 completers control group (P = 0.379326); 6 months 50/71 completers DA group 78/111 completers control group (P = 0.9825)). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02344576) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | One or more of the authors received industry funding with no description of role in the study: "Dr. Blue has received personal fees from Abbott and Medtronic. Dr. Patel has received personal fees from Abbott and Medtronic. Dr. Allen has received personal fees from ACI Clinical, Janssen, Cytokinetics, Novartis, Boston Scientific, Amgen, and Duke Clinical Research Institute." Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
McLean 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid versus information | |
| Participants | 21 + 19 participants aged > 16 years with a diagnosis of hidradenitis suppurative diagnosis in Canada and the USA | |
| Interventions | DA: online decision aid that included information about the condition, explicit values clarification, guidance in decision‐making (step‐by‐step process), and printable summary of results. The DA is publicly available at https://www.informed-decisions.org/hidradenitispda.php . Comparator: website that included information about the condition, treatment options, and guidance in communication (e.g. basic questions to ask your doctor) |
|
| Outcomes | Primary outcomes: difference in knowledge and decisional conflict as well as preparation for decision‐making Secondary outcomes: resource acceptability, decisional conflict, and decision regret |
|
| Notes | Source of funding: Supported by an Advancing Science Through PfizereInvestigator Research Exchange (ASPIRE) grant (Dr Dellavalle). Conflicts of interest: Dr Sisic and Authors McLean and McBride received salaries from Windsor Clinical Research Inc. Dr Dellavalle is a member of Cochrane Council, received other independent peer‐reviewed grants from Pfizer, and has been a medical consultant for Altus Labs and ParaPRO. Dr Tan is the president of Windsor Clinical Research Inc. He has been a speaker and consultant fordor received honoraria, grants, and research support from Almirall, Bausch, Boots/Walgreens, Botanix, Cipher, Galderma, Incyte, L’Oreal, Novartis, Pfizer, Promius, SUN, and UCB. Author Samardzic has no conflicts of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1 randomization. Hidradenitis suppurativa patient decision aid and Mayo groups were randomly labeled as group A or B by one researcher (O.M.) via a coin toss, and allocation was concealed from another researcher (D.M.). D.M. performed simple block randomization using http://www.randomization.com with a fixed block size of 2 (group A or B) to generate a random sequence" |
| Allocation concealment (selection bias) | Low risk | "Hidradenitis suppurativa patient decision aid and Mayo groups were randomly labeled as group A or B by one researcher (O.M.) via a coin toss, and allocation was concealed from another researcher (D.M.). D.M. performed simple block randomization using http://www.randomization.com with a fixed block size of 2 (group A or B) to generate a random sequence that was concealed from O.M., and informed O.M. of participants’ allocation group. O.M. was responsible for enrollment and administration of surveys." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Content sources were deidentified, and both the hidradenitis suppurativa patient decision aid and Mayo were hosted on an independent website to ensure blinding. Only O.M. was aware of participants’ allocated group. outcomes were objectively measured and not subject to interpretation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Content sources were deidentified, and both the hidradenitis suppurativa patient decision aid and Mayo were hosted on an independent website to ensure blinding. Only O.M. was aware of participants’ allocated group. outcomes were objectively measured and not subject to interpretation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% of participants included in analysis (71.4% decision aid and 68.4% control but higher loss to follow‐up expected for online survey study), similar loss to follow‐up between arms and provided justification. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | Small sample size (21 versus 19) |
Meade 2015.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 107 + 81 women aged within their childbearing and rearing years that had been clinically diagnosed with rheumatoid arthritis and currently under the care of a rheumatologist, and contemplating having children or more children in Australia | |
| Interventions | DA: online printable decision aid that includes information on the clinical problem, outcome probabilities, explicit values clarification, patient narratives, checklists, information resources, guidance in decision‐making (step‐by‐step process for making the decision, checklist to identify decisional needs), and guidance in communication. The DA is publicly available at https://www.westernsydney.edu.au/__data/assets/pdf_file/0007/1541527/RAandmotherhooddecisiontool_PDF_DA.pdf . Comparator: no intervention |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: anxiety, depression, perceived self‐efficacy |
|
| Notes | Source of funding: This work was supported by the Australian Research Council, [grant number LP0989906] titled ‘Motherhood choices: a decision aid for women with Rheumatoid Arthritis’ in partnership with Arthritis NSW. Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As women provided consent, a member of the research team randomly allocated them to either the DA or control group, using the Bernoulli function in Excel. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, "A total of 188 women consented to participate in the study. Forty‐four (28 out of 107 DA; 16 out of 81 Control) participants did not complete pre or post questionnaire and after a number of efforts to contact them, were assumed to have withdrawn from the study." High attrition rate for intervention arm (27%) vs control (19%), but difference is not significant (P = 0.168634). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12615000523505) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "As a consequence of the random allocation, the control and intervention groups were not balanced for parity or gravity" |
Meier 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 51 + 48 parents considering adenotonsillectomy for their children, under 6 years of age, and presenting with sleep‐disordered breathing in the USA | |
| Interventions | DA: paper‐based decision aid used during consultation that includes clinical information, outcome probabilities, and explicit values clarification. The DA is presented in Figure 1 of the article. Comparator: usual care |
|
| Outcomes | Decisional conflict, shared decision‐making, patient‐clinician communication (OPTION scale) | |
| Notes | Source of funding: This study was supported by a Triological Society Career Development Award (J.D.M.). Conflicts of interest: The authors have no financial relationships, or conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization with opaque envelopes containing assignment to either receive the DA prototype during the visit (study group) or undergo the usual surgical consultation (control group). |
| Allocation concealment (selection bias) | Low risk | Simple randomization with opaque envelopes containing assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reports that parents were blinded, however participants were told in advance of randomization that they were either going to have consultation only or use a tool + consultation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Low risk of bias for decisional conflict and Shared Decision‐Making Questionnaire–Parent Version (SDM‐Q‐9). High risk for one outcome subjective to interpretation (video‐recordings coded with OPTION). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram not included; no reasons for missing data; no information about how many patients were initially invited for intervention and control groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Metcalfe 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 76 + 74 women with a BRCA mutation with no previous diagnosis of cancer recruited from 4 clinics in Canada and an online support network in the USA | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that included clinical information, outcome probabilities, explicit values clarification, guidance in decision‐making (step‐by‐step), and guidance in communication. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care (genetic counseling) |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: cancer‐related distress, knowledge, choice predisposition (undecided) |
|
| Notes | Source of funding: not reported Conflicts of interest: The authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "If all eligibility criteria were met, then the women were randomized centrally with a secure Web‐based randomization service (http://www.randomized.net)" |
| Allocation concealment (selection bias) | Low risk | The women were randomized centrally with a secure web‐based randomization service (http://www.randomize.net). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded; personnel were blinded. Unclear how lack of blinding may have influenced the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A research assistant blinded to group allocation telephoned all study participants at 3, 6, and 12 months post‐randomization to determine trial outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No flow diagram, low attrition rate (response rates were 94% at 3 months, 94% at 6 months, and 93% at 12 months). There was no difference in the response rate by group allocation. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Miller 2005.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 279 women considering BRCA1‐BRCA2 gene testing in the USA | |
| Interventions | DA: educational intervention on options' outcomes, personal family cancer history; clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: provision of general information about cancer risk |
|
| Outcomes | Preferred option, knowledge, perceived risk, satisfaction | |
| Notes | Primary outcome was not specified Source of funding: This research was supported in part by the Department of Defense DAMD17‐98‐1‐8306, DAMD17‐01‐1‐0238, and DAMD17‐02‐1‐0382 grants, the Fox Chase Cancer Center’s Behavioral Research Core Facility (P30CA06927), and NIH grant R01HG01766. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomized by the CATI system" (p 4) after self‐initiated telephone contact |
| Allocation concealment (selection bias) | Low risk | "[C]omputerized assisted telephone interview system (CATI)" (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not addressed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons stated for initial dropout of study participants (p 8). Patients contacted offered reasons for dropping out. Study protocol allowed patients to be reached up to 13 times at follow‐up, but still could not be reached. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Miller 2011.
| Study characteristics | ||
| Methods | Decision aid vs attention placebo | |
| Participants | 132 + 132 participants considering colon cancer screening in the USA | |
| Interventions | DA: computer‐based web program on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encourages patient‐practitioner communication, summary). The DA is no longer available (intmedweb.wakehealth.edu/choice/choice.html) Comparator: computer‐based web program on prescription drug refills and safety |
|
| Outcomes | Primary outcomes: receipt of CRC screening (post‐DA) Secondary outcomes: ability to state a preference, change in readiness to receive screening (pre and post‐DA), CRC test ordering (post‐DA), proportion undecided |
|
| Notes | Source of funding: This study was funded by a Cancer Control Career Development Award (DPM) from the American Cancer Society (CCCDA‐05‐162‐01). Conflicts of interest: MPP was supported by a National Cancer Institute Established Investigator Award (K05 CA129166). No other financial disclosures were reported by the authors of this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐randomized, stratified by literacy level (p 609, Methods) |
| Allocation concealment (selection bias) | Unclear risk | Study does not address this domain |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Health care providers were not notified of patients' enrolment in the study at any time (p 609, Methods). RAs that administered post‐DA questionnaire were not blinded but believed to be a low risk of bias (p 613, Discussion). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[C]linical outcome assessors were [blinded]" (p 613, Discussion) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol on ClinicalTrials.gov |
| Other bias | Unclear risk | USD 10 gift card for participation could affect the participant pool. |
Miller 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control | |
| Participants | 223 + 227 individuals aged 50 to 74 years who were scheduled to see a primary care provider and due for colorectal cancer screening in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, implicit values clarification, and patient narratives. The DA is not publicly available; a copy was provided by the author (David P. Miller Jr.; dmiller@wakehealth.edu). Comparator: attention control (education on another topic) |
|
| Outcomes | Primary outcome: chart‐verified completion of CRC screening within 24 weeks Secondary outcomes: ability to state a screening preference, intention to receive screening, screening discussions, and orders for screening tests |
|
| Notes | Source of funding: The study received funding and support from the National Cancer Institute (R01CA178941), the Wake Forest Clinical and Translational Science Institute study coordinator pool (UL1TR001420), and the shared resources provided by the Wake Forest Comprehensive Cancer Center (CCSG P30CA012197). No funding organization played a role study conduct, manuscript preparation, or decision to submit for publication. Conflicts of interest: Dr. Miller reports grants from the National Cancer Institute during the conduct of the study. Drs. Miller, Weaver, and Troyer report grants from the National Institutes of Health during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17‐2315. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study program randomly assigned participants, stratified by site, to either the mPATHCRC or Control Program with equal probability using variably sized permuted block randomization with random block sizes of 2 or 4. |
| Allocation concealment (selection bias) | Low risk | The random allocation sequences were generated by the study statistician using nQuery Advisor 7.0 and stored on the iPads used at each site in files accessible only by the study programmer. Two iPads were used at the largest clinic, each with its own allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study interviewers and outcome assessors were blinded to participant allocation. Not reported for patients but outcomes of interest were objectively measured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study interviewers and outcome assessors were blinded to participant allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; all participants randomized were included in analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02088333) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "Selection bias could also have affected our results because recruited patients had to agree to arrive at the clinic early to enroll. In addition, the postprogram survey assessing preferences may have triggered some patients to discuss CRC screening with their providers." |
Moin 2019.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid + shared decision‐making intervention vs usual care (using propensity score matched controls) | |
| Participants | 351 + 1028 overweight/obese patients with prediabetes in the USA | |
| Interventions | DA: web‐based interactive decision aid used during consultation that included clinical information, probabilities of outcomes, explicit values clarification, patient narratives, quiz section, guidance in decision‐making (step‐by‐step process), guidance in communication, and printed copy of a summary report with their decision and plan at the end of the visit. The DA is publicly available at https://decisionaid.ohri.ca/AZsumm.php?ID=1654 . Comparator: usual care (no details provided) |
|
| Outcomes | Primary outcome: diabetes prevention program (≥ 9 sessions attended) and/or metformin uptake at 4‐month follow‐up Secondary outcome: weight change at 12 months |
|
| Notes | Source of funding: National Institute of Diabetes and Digestive and Kidney Diseases (R18 grant number DK105464). Conflicts of interest: Dr. Duru is on the Healthwise scientific board. None of the other authors disclosed any potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We stratified 20 primary care clinics by clinic size and mean patient age, randomizing 10 clinics to the SDM intervention and 10 to usual care (we launched in 16 clinics [8 intervention and 8 control] and subsequently added the last 4)." The investigators describe the use of stratification or permuted blocking (use of computer implied). |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram for intervention clinics only |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02384109) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | High risk | Concerns regarding the ethical implications of usual care participants not consenting to participate. Actual choice for the usual care group subject to bias (results extremely low compared to the DA group). Article reported that: "Because it was not feasible to collect informed consent from matched controls, DPP suppliers could not share DPP participation data from controls. Therefore, we conducted natural language queries of all EMR progress notes between 2015 and 2018 to capture participation in DPP or any other structured weight loss program". Selection bias: "this trial was conducted at UCLA Health where pharmacists were integrated in a large network of primary care clinics, which may limit generalizability...". Confirmation bias: "the intervention patients who chose to participate may have been more motivated than others to lower their diabetes risk." Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Montgomery 2003.
| Study characteristics | ||
| Methods | Randomized to decision aid + decision analysis vs decision analysis vs decision aid vs usual care | |
| Participants | 51 + 52 + 55 + 59 newly diagnosed hypertensive patients considering drug therapy for blood pressure in the UK | |
| Interventions | DA: decision analysis plus information video and leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: decision analysis on options' outcomes, outcome probability, explicit values clarification Comparator: video and leaflet on options' outcomes, clinical problem Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: uptake of option, knowledge, anxiety |
|
| Notes | Source of funding: The Medical Research Council provided funding for the study and support for Dr Montgomery with a Training Fellowship in Health Services Research (G106/912). Professor Fahey was supported by a National Health Service Primary Care Career Scientist Award at the time of the research. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation schedule was computer‐generated by an individual not involved in the study (p 2) |
| Allocation concealment (selection bias) | Low risk | "[A]llocation was concealed to the author in advance by the nature of the minimization procedure" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded ‐ unclear if this would introduce bias to the outcome assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 5) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Montgomery 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid with values clarification vs decision aid without values clarification vs usual care | |
| Participants | 245 + 250 + 247 women with previous cesarean section in the UK | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, explicit values clarification. The DA is no longer available (http://www.computing.dundee.ac.uk/acstaff/cjones/diamond/Information.html). Comparator: options' outcomes, clinical problem, outcome probability Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: choice, anxiety, knowledge, satisfaction with decision, costs ( Hollinghurst 2010 ) |
|
| Notes | Source of funding: BUPA Foundation. AAM was part supported by a postdoctoral fellowship from the UK Department of Health National Coordinating Centre for Research Capacity Development. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked by using randomly permuted and selected blocks of sizes 6, 9, 12, and 15 generated by computer (p 2 Methods, Randomization) |
| Allocation concealment (selection bias) | Low risk | 1 member of the study team generated the randomization sequence by computer, and another member of staff with no other involvement in the trial performed the allocation (p 2 Methods, Randomization). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow of women through the study |
| Selective reporting (reporting bias) | Low risk | Trials registry ISRCTN84367722 |
| Other bias | Low risk | Recruited more than planned to account for lost data (p 4, Sample size); baseline characteristics were balanced. |
Montori 2011.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care + booklet | |
| Participants | 52 + 48 women with low bone mass or osteoporosis considering taking bisphosphonates in the USA | |
| Interventions | DA (in consultation): worksheet on options' outcomes, clinical problem, outcome probabilities, guidance (administered by physician). The DA is no longer available (shareddecisions.mayoclinic.org/decision‐aids‐for‐diabetes/other‐decision‐aids/). The authors have a PDF copy of the DA. Comparator: usual care + general information booklet on osteoporosis |
|
| Outcomes | Patient knowledge (post‐DA), satisfaction with knowledge transfer (post‐DA), decisional conflict (post‐DA), patient‐clinician communication (OPTION), trust with physician (during intervention), clinician's perception of decision quality (post‐DA), clinician's satisfaction with knowledge transfer (post‐DA), uptake (post‐DA), adherence (post‐DA), fidelity (post‐DA), contamination (post‐DA), risk perception | |
| Notes | Primary outcome was not specified Source of funding: The trial was funded by the Mayo Clinic Foundation for Medical Education and Research. The funding source had no role in the design, conduct, or decision to publish results of this trial. Conflicts of interest: The authors of this article disclose no financial conflicts of interest pertinent to this trial. In particular, the decision aid described in this article is in the public domain and can be obtained from the authors without charge. The authors, their relatives, or other associates have not initiated any business to profit from this decision aid (or any other decision aid they have developed and studied) or the dissemination of the results of this trial, beyond the usual benefits of academic recognition. The authors or any member of the team who participated in the development or evaluation of the decision aid have not received financial support from pharmaceutical companies that market bisphosphonates or their competitors. The KER UNIT, a laboratory within the Mayo Clinic where the study was conceived, run, and analyzed, and this report was prepared, had explicit rules in place before, during, and at the time of writing this note against receiving any funding from for‐profit pharmaceutical or device manufacturers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated allocation" (p 551, Randomization) |
| Allocation concealment (selection bias) | Low risk | Patients randomized "in a concealed fashion (using a secure study website)" (p 551, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of participants being blinded to their allocation; only mention of data collectors and analysts blinding (p 551, Randomization). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After randomization, data collectors and data analysts were blind to allocation" (p 551, Randomization); outcomes were not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | "The protocol for this trial has been reported in full" (p 550, Design) |
| Other bias | Unclear risk | Appears to be free of other potential biases. |
Montoya 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid plus standard care vs standard care alone | |
| Participants | 15 + 15 women 18 years and older referred for initial evaluation of primary symptomatic pelvic organ prolapse in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that included clinical information, implicit values clarification, and guidance in communication. The DA is not publicly available; a copy was provided by the author (T. Ignacio Montoya; teodoro.montoya@ttuhsc.edu) Comparator: usual care |
|
| Outcomes | Knowledge, satisfaction with initial treatment decision, decisional conflict | |
| Notes | Source of funding: Supported by Texas Tech University Health Sciences Center El Paso institutional seed grant. Conflicts of interest: The authors have declared they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization technique was performed using blocks of 6 (use of computer implied). |
| Allocation concealment (selection bias) | Low risk | Group assignments were predetermined and concealed in sealed envelopes, which were sequentially opened at the time of each participant’s inclusion into the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Providers were not blinded to the group allocation of the patients, potentially introducing bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; one participant from each group was lost to follow‐up (Fig. 1). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02850835) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Morgan 2000.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 120 + 120 patients with ischemic heart disease considering revascularization surgery in Canada | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinions. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care |
|
| Outcomes | Primary outcome: satisfaction with the decision‐making process Secondary outcomes: uptake of option, knowledge |
|
| Notes | Source of funding: This research was funded in part by the Ontario Ministry of Health and the Heart and Stroke Foundation of Ontario (Grant NA3039). Dr. Llewellyn‐Thomas is a National Health Scholar supported by the National Health Research & Development Program of Health Canada. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Morgan 1997, p 29: all randomization enrolment was performed by telephone at which time the participant was assigned. Morgan 2000 (primary study), p 2, Methods, Patient Population: "Only the statistician was privy to the two randomisation schedules and blocking factor used" |
| Allocation concealment (selection bias) | Low risk | Morgan 1997, p 29: only the statistician was privy to the two randomization schedules and blocking factor Morgan 2000, (primary study), p 2, Methods, Patient Population: "only the statistician was privy to the two randomisation schedules and blocking factor used. All randomization enrolment was performed by telephone" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[D]ue to nature of trial, neither patients or investigators were blinded to the study" ‐ may introduce bias to subjective outcomes such as satisfaction. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Morgan 1997, p 39, Patient accrual and follow‐up: baseline characteristics included Morgan 2000 (primary study): 78% completed follow‐up (90 of 120 in the intervention; 97 of 120 in the control). Reasons for attrition were provided. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Unclear risk | Morgan 1997, p 56: significant number of patients were lost to follow‐up (25%); Morgan 2000 (primary study): baseline data imbalance (high school grad, income, no. of diseased arteries). Dropout group reported lower incomes; may have affected results. (Discussion par. 6) "Selection bias was minimized by enrolling available consecutive patients" |
Mott 2014.
| Study characteristics | ||
| Methods | Randomized to shared decision‐making process with DA versus usual care | |
| Participants | 13 +14 military veterans in USA diagnosed with PTSD and had served in Iraq or Afghanistan | |
| Interventions | DA: booklet on clinical problem, options' outcomes, structured guidance. The DA is not publicly available; a copy was provided by the author (juliette.mott@va.gov). Comparator: usual care |
|
| Outcomes | Satisfaction with SDM qualitatively (postintervention), perceived advantages and disadvantages of SDM qualitative (postintervention), treatment preferences (4 months), adherence using treatment engagement (4 months) | |
| Notes | Not reported as registered in trials database; no primary outcome reported Source of funding: This research was supported by the Office of Academic Affiliations VA Advanced Fellowship Program in Mental Illness Research and Treatment, the Department of Veterans Affairs South Central Mental Illness Research Education and Clinical Center (MIRECC), and the VA HSR&D Houston Center of Excellence (HFP90‐020). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to SDM or UC using a computer‐generated randomization sequence" (p 146) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization envelopes were prepared by the study statistician to ensure that study staff remained masked to randomization sequence" (p 146) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff not blinded but because outcomes were taken from medical records. "At 4‐month follow‐up, study staff reviewed participants' medical records to extract information on treatment preferences and engagement. Medical‐record reviews were conducted by a single rater trained in use of the dataextraction form. A second rater, masked to initial ratings, reextracted data from 20% of patients" (p 146). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27 participants were consented and enrolled, yet only 20 (usual care = 11; SDM = 9) completed the study (p 146‐7). Only 5 participants in the SDM arm completed the exit interview. No mention of missing data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all expected outcomes are reported on. |
| Other bias | Low risk | Does not appear to be any other sources of bias. |
Mullan 2009.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 48 + 37 patients with type 2 diabetes considering treatment options (cluster‐RCT with 40 clinicians randomized) in the USA | |
| Interventions | DA (in consultation): decision cards with information on options, outcomes, outcome probability, explicit values clarification. The DA is presented in Figure 1 of the article. Compare: 12‐page pamphlet on oral anti‐hyperglycemic medications |
|
| Outcomes | Knowledge, decisional conflict, participation in decision‐making, acceptability of the information, change in medication, adherence, HbA1C levels, trust in physician, OPTION to analyze audio‐taped encounters | |
| Notes | Primary outcome was not specified Source of funding: The American Diabetes Association, through its competitive peer‐reviewed granting process, funded this study. Novo Nordisk, a maker of insulin, subsidized the American Diabetes Association granting program but did not have direct contact with the investigators and did not play any role in the awarding of the grant to the research team. Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were blinded, the clinicians were not, but each session was recorded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for attrition not included |
| Selective reporting (reporting bias) | Low risk | Trial registration no. at clinicaltrials.gov reported |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Murphy 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 21 + 56 men with prostatectomy in the UK | |
| Interventions | DA: web‐based decision aid that includes initial step to help customize grid of recommended products and implicit methods to clarify values. The DA is publicly available at https://www.continenceproductadvisor.org . Comparator: usual care (supplied with incontinence pads and advised to buy more as needed) |
|
| Outcomes | Decisional conflict | |
| Notes | Source of funding: This work was funded by the Movember Foundation in partnership with Prostate Cancer UK as part of True NTH programme. Conflicts of interest: No conflicts of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "After providing consent, men were randomised by the research nurse (using sealed brown paper envelopes to conceal allocation)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "it was not possible to blind participants as to the intervention". Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Twenty‐one were randomly assigned to Arm A (usual care) and n = 56 to Arm B or C (which included giving patients additional chosen products). After catheter removal, 27 men (five from Arm A and 22 from Arm B or C) did not have urinary leakage and therefore did not need to use the CP‐PDA”. No flow diagram; participants excluded after randomization because did not have urinary leakage. High rate of attrition: 22/56 (39%) DA and 5/21 (24%) usual care, but difference across groups is not significantly different (P = 0.204978). |
| Selective reporting (reporting bias) | Unclear risk | Registered (NIHR CPMS 31077) but unable to retrieve record. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Murray 2001a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 57 + 55 men considering treatment for benign prostatic hypertrophy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options, outcomes, clinical problem, outcome probability, others' opinions. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care |
|
| Outcomes | Primary outcomes: uptake of option, prostate symptoms, costs, anxiety Secondary outcomes: decisional conflict, role in decision‐making, general health status, utility |
|
| Notes | Source of funding: NHS national research and development programme, the BUPA Foundation, and the Kings's Fund. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomisation schedule, stratified according to recruitment centre, was generated by computer" (p 4) |
| Allocation concealment (selection bias) | Low risk | "Allocation were sealed in opaque numbered envelopes, opened by the study nurse" (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded but not sure how this would introduce bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 5); baseline data/characteristics included and balanced |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Murray 2001b.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 102 + 102 women considering hormone replacement therapy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options outcomes, clinical problem, outcome probability, other's opinion. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care |
|
| Outcomes | Primary outcomes: preferred option Secondary outcomes: help with making a decision, decisional conflict, role in decision‐making, anxiety, menopausal symptoms, costs, utility, general health status |
|
| Notes | Source of funding: BUPA Foundation and the King's Fund Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomisation schedule, stratified according to recruitment centre, was generated by computer" (p 3 Methods, Randomization) |
| Allocation concealment (selection bias) | Low risk | "Allocations were sealed in opaque numbered envelopes, opened by the study nurse after collection of the baseline data" (p 3 Methods, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See page 3 figure for progress of patients through trial |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not mentioned |
| Other bias | Low risk | Similar baseline characteristics; appears to be free of other potential biases. Educational achievement was higher in control group. Quote "Subsequent analysis showed that educational level not related to use of HRT nor was there an interaction between educational attainment and the intervention". |
Nagle 2008.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 167 + 172 women in early pregnancy considering genetic testing (26 + 29 general physicians) (cluster‐RCT with 60 general practitioners randomized) in Australia | |
| Interventions | DA: 24‐page booklet and worksheet on options, benefits and risks, test limitations, outcomes; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework). The DA is available at https://www.mcri.edu.au/images/documents/migrate/prenatal-screening-decision-aid.pdf . Comparator: standard pamphlet on prenatal testing |
|
| Outcomes | Primary outcomes: informed choice, decisional conflict Secondary outcomes: anxiety, depression, attitudes toward pregnancy, acceptability of the intervention, choice |
|
| Notes | Source of funding: This project was funded by the National Health and Medical Research Council (Project Grant 237124). J.H. and B.M. are both supported by Career Development Awards (ID 350989 and 216741, respectively). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (p 3) |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers by an independent statistician; allocation concealment was achieved (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Due to the nature of the intervention, it was not possible to blind women, GP's or researchers" (p 3); unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were not blinded, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results, p 4; Fig 1 ‐ flow diagram, p 5 |
| Selective reporting (reporting bias) | Low risk | Trial Registration ‐ The ADEPT trial was registered in the UK with Current Controlled Trials [ISRCTN22532458] and with the Australian Clinical Trials Registry (No: 012606000234516) (p 4). |
| Other bias | Low risk | Appears to be free of other potential biases (p 8); selection bias but was adjusted for in analysis. Free of other potential biases: adjustment for clustering performed. |
Nassar 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 102 + 98 women diagnosed with a breech presentation from 34 weeks gestation considering external cephalic version in Australia | |
| Interventions | DA: 24‐page booklet, 30‐minute audio‐CD and worksheet; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework). The DA is no longer available (http://sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php). Comparator: usual care counseling and information on the management of breech presentation |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, anxiety, satisfaction with the decision Secondary outcomes: preferred role in decision‐making, preferred choice |
|
| Notes | Source of funding: This study was supported by an Australian National Health and Medical Research Council project grant (211051). Natasha Nassar is funded by an Australian National Health and Medical Research Council Public Health Postgraduate Research Scholarship. Christine Roberts is funded by an Australian National Health and Medical Research Council Public Health Practitioner Fellowship. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomly generated using computer and stratified by parity and center using random variable block sizes" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[P]articipants were randomized by telephoning a remote, central location" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Womens were not blinded ‐ unclear if this would introduce bias to the outcome assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up because of onset of labor or incomplete data forms (p 3). Baseline characteristics are included and equal. Minimum of 84 participants in each study group achieved; p 4 ‐ flow diagram. |
| Selective reporting (reporting bias) | Low risk | ISRCTN14570598 |
| Other bias | Low risk | "Maternal characteristics and baseline measures of cognitive and affective outcomes were comparable between groups" (p 3 Results, Table 1) "Blinding clinicians and employment of a research midwife to interact with women" (p 6) |
Oakley 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 16 + 17 postmenopausal women with osteoporosis considering treatment options to prevent further bone loss in the UK | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework). The DA is no longer available (decisionaid.ohri.ca/decaids‐archive.html). The authors have a PDF copy. Comparator: usual care |
|
| Outcomes | Satisfaction with information, decisional conflict (intervention group only), improvement in adherence | |
| Notes | Primary outcome was not specified Source of funding: Unrestricted educational grants to support this work were provided by Eli Lilly & Co Ltd, Merck Sharp & Dohme Ltd, and Starakan. The Medicines Partnership provided practical support and funded production of the decision aid. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Group allocation was done by a third party, unconnected to the study and blinded to the identity of the patients (p 1). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding; some outcomes were assessed by open‐ended questions; do not know whether this contributes to risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample characteristics not included; baseline satisfaction score included. "No evaluation was carried out to determine the reasons for non‐participation" (p 2) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No baseline characteristics (p 2). Only 16 patients in the intervention group and 17 in the control group; small sample size. |
Omaki 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs attention matched health risk assessment | |
| Participants | 65 + 59 individuals aged 18 and older visiting the emergency department for an injury or pain‐related complaint in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that included clinical information, explicit values clarification, guidance in decision‐making (step‐by‐step process), guidance in communication, tailored summary based on the patient’s risk assessment and self‐identified priorities that is emailed to the patient, and other elements (e.g. SURE test, knowledge test, feedback on doctor visit). *Note: the DA was previously illustrated viahttps://myhealthychoices.nursing.jhu.edubut is no longer available. Comparator: attention matched health risk assessment |
|
| Outcomes | Comfort level with pain medication options, knowledge, decisional conflict, and shared decision‐making. Actual choice also reported. | |
| Notes | Source of funding: This work was supported by a grant from the National Center for Injury Control and Prevention, Centers for Disease Control and Prevention (grant number 1R49CE002466). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated roster in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate but missing data similar across arms (P = 0.942989) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT03012087) and one or more outcomes relevant to the review were not pre‐specified (decisional conflict, shared decision‐making). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Oostendorp 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 83 + 45 patients considering second‐line palliative chemotherapy for advanced breast or colorectal cancer in the Netherlands | |
| Interventions | DA: decision aid used during consultation with the nurse that included clinical information, outcome probabilities, explicit values clarification, guidance in communication, and a summary of all the information provided. A booklet with information tailored to the patient’s desire was available to take home. The DA is available as a supplementary appendix in the article. Comparator: usual care (information about the treatment choice from their oncologist) |
|
| Outcomes | Primary outcome: well‐being (anxiety, depression, general health, cancer worries, health‐related quality of life) Secondary outcomes: coping (including perceived participation, perceived involvement), information‐related measures (e.g. amount of information, satisfaction with quality of information, balanced presentation), knowledge (objective and subjective), risk perception (objective and subjective), decision‐related measures (decision satisfaction‐uncertainty, decision control, weighing pros and cons, treatment choice, strength of treatment preference), and treatment attitudes |
|
| Notes | Source of funding: This work was supported by the Dutch Cancer Society (grant number KUN 2006‐3465). Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Unequal randomisation (using a 1:2 ratio) was used because the sample size of the control group was based on the current evaluation of the DAs, while the sample size of the intervention group was based on more detailed analyses of patients' desire for information. Randomisation lists were computer generated per hospital and tumour type, using a block size of 3." |
| Allocation concealment (selection bias) | Low risk | "When a patient included in the study experienced disease progression and was offered second‐line chemotherapy, randomisation was performed. A nurse would open a sealed envelope to find out whether the patient would either:…oncologists were not aware of the allocation prior to randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Another limitation inherent to the nature of DAs is that complete blinding was not possible." In protocol: "Blinding of the medical oncologists and the patients is not feasible in this type of research, because patients may want to discuss the information from the decision aid with their oncologist. However, patients are blinded to the intervention in that they are not aware of the exact content of the decision aid; they are only informed that a new method of information giving is investigated. Nevertheless, oncologists were not aware of the allocation prior to randomisation." It is unclear how the knowledge of which group patients were allocated to influenced the oncologists and nurses in their delivery of the intervention and control. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Substantial proportion of patients withdrew from the study; however, difference is not significant across groups: 1‐week missing data 15/83 (18%) in the DA group and 5/45 (11%) in the control P = 0.300373; 8‐week missing data 25/83 (30%) in the DA group and 12/45 (27%) in the control (P = 0.680666). Reasons for loss to follow‐up not reported. |
| Selective reporting (reporting bias) | Low risk | Netherlands Trial Registry (NTR): NTR1113; protocol published; provides in appendix changes from published study and protocol |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Osaka 2017.
| Study characteristics | ||
| Methods | Randomized to DA with patient narratives vs DA without patient narratives vs standard information booklet (provided to all arms) | |
| Participants | 70 + 70 + 70 women newly diagnosed with early‐stage breast cancer in Japan | |
| Interventions | DA: paper‐based decision aid that included clinical information, probabilities of outcomes, explicit values clarification, patient narratives, guidance in decision‐making (step‐by‐step process), and guidance in communication. The decision aid is publicly available at https://www.healthliteracy.jp/decisionaid/decision/breast-surgery.html . Comparator: standard information booklet about the condition and options |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: satisfaction with decision‐making, anxiety |
|
| Notes | Source of funding: This work was supported by the Japan Society for the Promotion of Science Grants‐in‐Aid for Scientific Research (KAKENHI) Grant Number JP25670928. Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomly assigned to one of two intervention groups or a control group using a prior computer‐generated random‐number sequence. Block randomization was performed with a randomly selected block of six." |
| Allocation concealment (selection bias) | Low risk | "Allocation was performed in a different order in each block to ensure the investigators were blinded. A serially labeled opaque sealed‐envelope method was used for block randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was impossible to blind the participants to whether they had been allocated to an intervention or a control group; however, participants in the intervention groups were blinded to the difference between the two intervention groups. Health care professionals were blinded to the groups to which participants had been allocated." It is unclear how knowledge of their allocation could have influenced participant's responses to subjective measures (e.g. satisfaction with decision‐making). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram. High rate of attrition post‐intervention but balanced across groups: DA with narratives 13/70 (18.6%); control 16/70 (22.9%). Primary and secondary variables with > 25% missing responses or patients lost to follow‐up were managed by the last observation carried forward imputation method. |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | Exclusion criteria include: "answered questionnaire inconsistently". Potential selection bias: "Our subjects were more highly educated and younger than those in previous studies of Japanese women with breast cancer. However, their staging was similar to that of population‐based breast cancer data in Japan. Our single‐center setting in a metropolitan area may have introduced selection bias." |
Ozanne 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard counseling vs usual care (standard counseling) | |
| Participants | 15 + 15 women considering breast cancer prevention in the USA | |
| Interventions | DA (in consultation): interactive computer decision aid on options outcomes, outcome probability. The DA is not publicly available. A demo copy was obtained from the author. Comparator: standard counseling |
|
| Outcomes | Primary outcomes: consultation length Secondary outcomes: knowledge, decisional conflict, satisfaction with the decision, acceptability of the decision aid, physician satisfaction with the consultation |
|
| Notes | Source of funding: Pell Family Foundation Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomized evenly between groups; no information provided about generation (p 149) |
| Allocation concealment (selection bias) | Unclear risk | No information provided (p 149) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Demographic data included; reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol |
| Other bias | Unclear risk | Small sample size; does not say how many physicians participated in study; mentions that there were observed changes in physician behavior (based on doing both intervention and control). |
Partin 2004.
| Study characteristics | ||
| Methods | Randomized to decision aid with others' opinions vs decision aid without others' opinions vs usual care | |
| Participants | 384 + 384 + 384 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinions. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator 1: pamphlet on options' outcomes, clinical problem, outcome probability Comparator 2: usual care |
|
| Outcomes | Primary outcomes: knowledge Secondary outcomes: preferred option, help with making a decision, decisional conflict |
|
| Notes | Source of funding: Funded by VA Health Services Research and Development Service grant #IIR 99 277–1 to the Center for Chronic Disease Outcomes Research, Veterans Affairs Medical Center, Minneapolis, Minn. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated algorithm (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "[P]roviders were blinded to the fact that their patients were participating in a trial", "coordinator did not have direct contact with subjects" (p 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[F]ollow‐up interviewers blinded, statisticians were not". Outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 2); reasons for attrition mentioned and participants balanced across study groups. Sample characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Appears to be free of other potential biases. |
Patzer 2018.
| Study characteristics | ||
| Methods | Randomized to decision aid + education (standard of care) vs education alone (standard of care) | |
| Participants | 238 + 232 patients 18 to 70 years with end‐stage renal disease in the USA | |
| Interventions | DA: mobile and web‐based application decision aid used during consultation that includes information on the clinical condition, probabilities of outcomes, implicit values clarification, individualized risk prediction tool, and risk report that can be printed. The DA is publicly available at https://ichoosekidney.emory.edu/ . Comparator: education |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: access to transplant, decisional conflict, patient treatment preferences, provider opinions |
|
| Notes | Source of funding: Norman S. Coplon Satellite Healthcare Foundation Conflicts of interest: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... research assistants obtained informed consent and randomized patients 1:1 with a random number generator application via iPad" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Given the nature of the intervention, neither patients nor providers were blinded to the study group assignment." "Additional limitations included the inability to blind patients to the intervention, which could have confounded study results, and the inability to examine long‐term effects of iChoose Kidney use on patient transplant knowledge." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low attrition rate and similar across arms (less than 10%), reasons for attrition recorded |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT02235571) and the secondary outcome of decisional conflict was not prespecified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Perestelo‐Perez 2016.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 15 physicians (86 patients) + 14 physicians (82 patients) of patients aged 18 and older with type 2 diabetes in Spain | |
| Interventions | DA: online decision aid used during consultation that includes clinical information, probabilities of outcomes, implicit values clarification, an individualized risk prediction tool, and summary report. The DA is publicly available at https://statindecisionaid.mayoclinic.org/ . Comparator: usual care (no details provided) |
|
| Outcomes | Knowledge about statins, perception of cardiovascular risk, decisional conflict, satisfaction with the decision‐making process, taking statins at 3 months, adherence at 3 months, consultation time, anxiety, diabetes‐related stress | |
| Notes | Source of funding: This study was supported by the Spanish Ministry of Health, Social Services and Equality (grant number: EC10‐005). Conflicts of interest: The authors have no conflict of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Physicians who consented to participate were randomized to intervention or usual care by means of a computer‐generated list." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label according to trial protocol. Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram with high attrition rate of 22% for both groups. Reasons for loss to follow‐up not provided. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (EudraCT: 2010‐023912‐14) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | High risk | Clustering was not accounted for in the analysis. Selective recruitment of cluster participants: physicians were encouraged to recruit at least 13 patients each (high risk). |
Perestelo‐Perez 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs no intervention | |
| Participants | 68 + 79 adults 18 years and older with a major depressive disorder in Spain | |
| Interventions | DA: web‐based decision aid that was reviewed in the company of a researcher that included clinical information, probabilities of outcomes, explicit values clarification, guidance in decision‐making (8‐step process), and summary of preferences, concerns, and decision certainty that can be used to ask questions to healthcare professionals. The DA is publicly available at https://pydesalud.com/depresion/ . Comparator: no intervention |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: knowledge, treatment intention, decisional control preferences, concordance between patients’ goals/concerns |
|
| Notes | Source of funding: Canary Islands Agency for Research, Innovation, and Society of Information, Grant/Award Number: ProID20100251 Conflicts of interest: The authors have no conflict of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A simple randomization schedule (ratio 1:1) to intervention (web‐based DA) or control group (usual care) was performed by an independent researcher, by means of computer software. |
| Allocation concealment (selection bias) | Low risk | Both physicians and the researcher who informed and recruited the patients were unaware of patients’ allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not explicitly reported: a second limitation is that blinding of participants is difficult with these interventions, and therefore, a “novelty” or “attention” effect cannot be ruled out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Statistical analyses were performed by a researcher blinded to participants’ allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, none lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registered with the European Union Clinical Trials Register (EudraCT: 2012‐001673‐9), unable to locate registration. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Perestelo‐Perez 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no decision aid) | |
| Participants | 53 + 54 adults aged 50 to 69 with no previous colorectal cancer screening in Spain | |
| Interventions | DA: web‐based decision aid that was reviewed in the company of a researcher that included clinical information, probabilities of outcomes, explicit values clarification, guidance in decision‐making (8‐step process), and summary document including content explored and participant responses regarding their preferences. The DA is publicly available at https://pydesalud.com/cancer-colorrectal/ . Comparator: no decision aid |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: knowledge, intention to undergo screening, congruence between values and intention to be screened |
|
| Notes | Source of funding: This study was supported by the Spanish Ministry of Economy, Industry and Competitiveness (Carlos III Institute, Spain) (Grant number: PI12/00509). Funders have had no role in the study design, the collection, analysis, and interpretation of data, the writing of the article or the decision to submit it for publication. Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based simple randomization was performed by a statistician not involved in the study. |
| Allocation concealment (selection bias) | Low risk | Computer‐based simple randomization was performed by a statistician not involved in the study, and the researcher who recruited participants and established an appointment by phone was blinded to allocation (used a centralized off‐site computer allocation process). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "the researcher who recruited participants and established an appointment by phone was blinded to allocation". There is no mention of blinding the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up, ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered, ISRCTN98108615; outcome identified matches outcomes in main article. However, unclear bias from retrospective registration. |
| Other bias | Unclear risk | The absence of intervention in the control group may introduce a “novelty effect” in favor of the DA…Regarding external validity, a selection bias could be present since they recruited participants in primary care centers, who might not be completely representative of the population targeted for CRC screening. |
Perez‐Lacasta 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (standard leaflet) | |
| Participants | 260+264 woman aged 49 to 50 that in 2 to 4 months were going to be invited to participate in a breast cancer screening program for the first time in Spain | |
| Interventions | DA: paper‐based leaflet that included clinical information, implicit values clarification, and probabilities of outcomes. The DA is available as a supplementary appendix in the article. Comparator: standard leaflet that recommended accepting the invitation to participate in the breast cancer screening program |
|
| Outcomes | Primary outcome: informed choice (knowledge and intentions consistent with attitudes) Secondary outcomes: attitudes towards breast screening, intentions about breast screening, decisional conflict, confidence, anxiety, anticipated regret, temporal orientation, perceived risk, participated in screening program, opinions about the DA and control leaflets |
|
| Notes | Source of funding: “Women participation in decisions and strategies on early detection of breast cancer” (PI14/00113) from the Instituto de Salud Carlos III and cofunded by Fondo Europeo de Desarrollo Regional (FEDER)“Una manera de hacer Europa.” Anna Pons received a grant for PhD students from the Lleida Biomedical Research Institute. Conflicts of interest: The authors have declared that no competing interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study was designed as a parallel two‐stage randomised 1:1 controlled trial (RCT). In the first stage, elementary territorial units of the healthcare system named Basic Health Areas (BHAs) were stratified by socioeconomic level [14] and 40 of them were selected and randomised to intervention or control using computer‐generated blocks of size two. In the second stage, random samples of 30 to 50 women within each BHA were obtained." |
| Allocation concealment (selection bias) | Low risk | "The random allocation sequence was generated by a statistician with no contact with the participants (MR)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported in the main article. In the published protocol: "It will not be possible to blind the intervention to the interviewers and participants". Study does not report on how the results could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high attrition rate but balanced across groups: 57/260 (22%) in the decision aid group and 67/264 (25%) in the control group (P = 0.352074). |
| Selective reporting (reporting bias) | Low risk | The trial protocol is available (NCT03046004) and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | "only 56% of women in the initially selected sample could be reached and only around 38% of those invited completed the study. Thus, recruitment or dropout biases may limit, to some extent, the generalisation of our results to the target screening population" |
Pignone 2000.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 125 + 124 adults considering colon cancer screening in the USA | |
| Interventions | DA: video of options' outcomes, clinical problem, others' opinion. The DA is no longer available (http://www.med.unc.edu/medicine/edusrc/colon.htm). Comparator: video on car safety |
|
| Outcomes | Primary outcome: uptake of options | |
| Notes | Source of funding: By the National Cancer Institute, Robert Wood Johnson Foundation Clinical Scholars Program, and University of North Carolina–Lineberger Comprehensive Cancer Center. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputerized random number generator" (p 2, Methods, Group assignment) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization was performed centrally and was not balanced among centers. Assignments were placed in sealed, opaque, sequentially numbered envelopes and were distributed to the three sites" (p 2, Methods, Group assignment) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The providers and staff were not blinded to intervention status", "3 to 6 months after, different RA blinded to participant intervention examined clinic records" (p 2) Does not mention whether patients were blinded; unclear if lack of blinding contributed to potential risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A different research assistant who was blinded to participants' intervention status examined participants' clinic records in a standardized and validated manner to determine whether colon cancer screening tests were actually completed within 3 months of the index visit. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because of an administrative error, 18 controls did not complete the second and third questionnaires (p 4). |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not mentioned. |
| Other bias | Low risk | Baseline characteristics similar; appear to be no other potential sources of biases. Minimized bias from repeated measurements by administering the same questionnaires to the intervention and control participants. |
Politi 2020a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 60 + 60 women aged 18+, English‐speaking with stages 0–III breast cancer, who were considering a referral or were referred to 1 of 4 plastic/reconstructive surgeons in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, explicit values clarification, patient narratives, guidance in decision‐making (step‐by‐step process), guidance in communication, and summary that includes risks, personal values, and things to think about to discuss with the doctor. The DA is not publicly available; a copy was provided by the author (Mary C. Politi; mpoliti@wustl.edu). Comparator: information pamphlet |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, decision process quality Secondary outcomes: treatment preferences and preference concordance, quality of life, patient activation, shared decision‐making, treatment received, implementation outcomes (time spent using DA, consultation time, usability of DA) |
|
| Notes | Source of funding: This work was supported by Siteman Cancer Center through a Siteman Investment Program Pre‐R01 Award, funded by the Cancer Frontier Fund through the foundation for Barnes‐Jewish Hospital and Siteman Cancer Center, to Drs TMM and MCP. Conflicts of interest: MCP has a research contract (2017–2019) from Merck & Co. on a topic unrelated to the content of this article. MAO has grant funding from Pfizer, Merck, and Sanofi Pasteur, and is a consultant for Pfizer on topics unrelated to the content of this article. TMM is a consultant for, received advisory board remuneration, and received investigator‐initiated grant funding from Allergan Medical and RTI Surgical, on topics unrelated to the content of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized via computer random number generator, block size of 4, to 1 of 2 study conditions. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (all randomized included in analysis as per figure), "The percentage of missing data on items in analyses ranged from 1% to 6.7%. Missing data were considered missing at random and excluded, except missing BREAST‐Q data which were imputed according to guidelines" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03346161) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Protheroe 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 60 + 56 women considering treatment options for menorrhagia in the UK | |
| Interventions | DA: interactive computerized DA on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance. The computerized decision aid and Clinical Guidance Tree is no longer in existence; author sent chapter in thesis. Comparator: information leaflet |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge, anxiety, condition‐specific health outcomes, treatment preference, undecided |
|
| Notes | Source of funding: Financial support for this study was provided entirely by a grant from the Medical Research Council to Dr Protheroe with a Training Fellowship in Health Services Research G106/1048. The funding agreement ensured the author’s independence in designing the study, interpreting the data, and writing and publishing the report. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization, stratified by practice and minimized according to age (p 2, Methods) |
| Allocation concealment (selection bias) | Unclear risk | Random allocation was concealed from the individual who was making judgments of eligibility, but the method of concealment was not stated (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 6 flow diagram (p 5); baseline data/characteristics included and balanced (p 4) |
| Selective reporting (reporting bias) | Low risk | ISRCTN72253427 |
| Other bias | Low risk | Appears to be free of other potential biases. |
Reuland 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid + navigation vs control | |
| Participants | 133 + 132 participants aged 50 to 75 with average colorectal cancer risk in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that included clinical information, explicit values clarification, patient testimonies, and a 1‐page summary about the decision. Navigators met participants immediately after their clinician encounter and assisted in carrying out the screening plan. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: attention control (education on another topic) |
|
| Outcomes | Primary outcome: completion of screening test Secondary outcomes: knowledge, self‐efficacy (data not reported), intention to be screened |
|
| Notes | Source of funding: This study was funded by the American Cancer Society (grant RSG‐13‐165‐01–CPPB). Dr Brenner was supported by the Agency for Healthcare Research Quality’s National Research Service Award (grant No. 5‐T32HSHS000032). Dr Weaver was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant No. 1UL1TR001111‐01). Pilot work for this study was funded by University of New Mexico Clinical and Translational Science Center (grant No. 8UL1TR000041) and the North Carolina Translational and Clinical Sciences Institute at the University of North Carolina (grant No. 1UL1TR001111) and the UNC Lineberger Comprehensive Cancer Center. This study was supported in part by a grant from NIH (DK056350) to the University of North Carolina Nutrition Obesity Research Center OR from NCI (P30‐CA16086) to the Lineberger Comprehensive Cancer Center. Conflicts of interest: Dr Pignone is a member of the US Preventive Services Task Force. The views presented herein are not necessarily those of the Task Force. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized 1:1 to intervention or control groups using sequentially numbered, opaque, sealed envelopes generated by the study biostatistician (use of computer implied). |
| Allocation concealment (selection bias) | Low risk | Participants were randomized 1:1 to intervention or control groups using sequentially numbered, opaque, sealed envelopes generated by the study biostatistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "... research assistant conducting the enrollment and index visit data collection will also be the patient navigator, and therefore it is not feasible to blind the research assistant to treatment assignment after randomization occurs. However, a separate, blinded member of the research team will determine the primary study outcome of CRC screening test completion (based on medical record review at six months). In addition, the study biostatistician will program the primary models for addressing each of the aims using dummy treatment assignments and will remain blinded to actual treatment assignments until the models, along with any related assumptions, have been assessed and finalized." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "... research assistant conducting the enrollment and index visit data collection will also be the patient navigator, and therefore it is not feasible to blind the research assistant to treatment assignment after randomization occurs. However, a separate, blinded member of the research team will determine the primary study outcome of CRC screening test completion (based on medical record review at six months). In addition, the study biostatistician will program the primary models for addressing each of the aims using dummy treatment assignments and will remain blinded to actual treatment assignments until the models, along with any related assumptions, have been assessed and finalized." Also, outcomes objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, all included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Registered (NCT02054598) and protocol published. Outcomes across article, registry, and protocol are similar except for self‐efficacy not being reported in main article or intermediate outcome analysis. |
| Other bias | High risk | There is a mismatch of randomized participants between the main article (DA 133, control 132) vs intermediate outcomes analysis paper (DA 134, control 133). |
Rivero‐Santana 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 97 + 96 adults with knee OA who are candidates for total knee replacement in Spain | |
| Interventions | DA: online decision aid used in preparation for consultation that includes clinical information, probabilities of options, explicit values clarification, knowledge test, and a summary of patient's responses and comparison tables that is automatically generated and sent to her/his email. The DA is not publicly available; a copy was provided by the author (A. Rivero‐Santana; amado.riverosantana@sescs.es) Comparator: usual care (no details provided) |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: knowledge, satisfaction with the decision‐making process, treatment preference, having undergone surgery at 6 months follow up, decision regret |
|
| Notes | Source of funding: This work was funded by the Instituto de Salud Carlos III, Ministry of Health, Spain (grant number PI15/01264). The funding source had no role in the design, execution, analyses, interpretation of the data, or the decision to publish the results. Conflicts of interest: Authors have no competing interests to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients with knee OA were randomized to intervention (i.e. reviewing the patient DA accompanied by a researcher) or usual care (ratio 1:1). Computer‐based simple randomization, stratified by recruitment setting (hospital/primary care), was performed centrally by a statistician not involved in the study. |
| Allocation concealment (selection bias) | Low risk | Computer‐based simple randomization, stratified by recruitment setting (hospital/primary care), was performed centrally by a statistician not involved in the study. Patients’ allocation to intervention (patient DA) or usual care was concealed by means of sealed envelopes, which were open only after patients signed informed consent. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the nature of the intervention, researchers and patients could not be blinded. The researchers who assessed 6‐month outcomes by telephone were also non‐blinded. The impossibility of blinding patients and researchers to the intervention introduces an inherent risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in analysis; loss to follow‐up similar between arms; justifications provided for loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03254771). "Another modification of the protocol was carried out, after the beginning of the trial: the follow up was increased as much as possible within the time limits of the project (from 3 to 6 months), in order to assess TKR rates, not included previously as an outcome measure." |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Roberto 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (standard brochure) | |
| Participants | 1073 + 1046 women aged > 45 with no history of breast cancer in Italy | |
| Interventions | DA: web‐based decision aid that includes clinical information, probablilities of options, explicit values clarification with a summary of answers that can be printed. The DA is publicly available at: https://www.donnainformata-mammografia.it/en/ . Comparator: web‐based brochure that includes clinical information, probabilities about repeat or more in‐depth exams and false positives |
|
| Outcomes | Primary outcome: informed choice (knowledge and consistent attitude and intention) Secondary outcomes: participation rate, satisfaction with information, decisional conflict, time spent on the platform, DA acceptability |
|
| Notes | Source of funding: This project won a competitive grant of Italian Association for Cancer Research IG2015‐17274. Conflicts of interest: A.R., C. Colombo and P. Mosconi report grants from Italian Association for Cancer Research, competitive grant no. IG2015–17274, during the conduct of the study; G.C., R.S. and E.P. report grants from Mario Negri IRCCS Institute, during the conduct of the study; L.G. reports grants from Mario Negri IRCCS Institute and Gisma (Italian group that organised mammography screening) during the conduct of the study; P. Mantellini and M.V. report grants from Gisma (Italian group that organised mammography screening) during the conduct of the study. Authors not named here have disclosed no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study protocol: the random allocation will be on a 1:1 basis, provided by a computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not enough details (from protocol): "Women of this age in each screening center, will receive an invitation letter to the trial with a personal code number for registering on the platform. All code numbers will be extracted and transferred from the screening centers to the platform." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow diagram; a high rate of attrition and numbers are not balanced across groups: 472/1073 in the DA group and 529/1046 in the control group analyzed (P = 0.002401). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03097653) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Potential selection bias: "Participating women had a high level of education, which limits the generalisability of findings, in agreement with other studies. Most of the participants had already had a mammography before the invitation to the organised screening programme. This suggests that many had already received information that could have fostered the attitude and intention reported in this study. Finally, in order to participate, women had to have basic information technology skills. It is likely that technical developments will offer more user‐friendly tools for sharing information, increasing users’ knowledge and facilitating decision‐making in complex healthcare areas, such as mammography screening." |
Rubel 2010.
| Study characteristics | ||
| Methods | Randomized to pretest + decision aid + post‐test vs decision aid + post‐test vs pretest + posttest vs posttest | |
| Participants | 50 + 50 + 50 + 50 men considering prostate cancer screening in the USA | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + pretest and post‐test. The DA is no longer available; a copy was obtained from the authors. Comparator: booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + post‐test Comparator: pretest + post‐test Comparator: post‐test |
|
| Outcomes | Knowledge (pre, post‐DA), decisional anxiety (post‐DA), decisional conflict (post‐DA), participation in decision‐making (pre, post‐DA), schema for PSA testing (pre, post‐DA), perception of quality and interpretation of recommendation (post‐DA) | |
| Notes | Primary outcome was not specified Source of funding: This study was funded by the Centers for Disease Control and Prevention (CDC) Contract No. 200‐2002‐00574, Task 18. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronically generated random number sequence (p 309, Study design section) |
| Allocation concealment (selection bias) | Low risk | They were given sealed, sequentially numbered packets (p 309, Study design section). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding, but the outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol followed CONSORT checklist (p 310, Study design section). |
| Other bias | Low risk | Appears to be free of other potential biases. |
Ruffin 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 87 + 87 community‐dwelling adults not previously screened for CRC in the USA | |
| Interventions | DA: interactive website with information on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion, guidance. The DA is no longer available (colorectalweb.org). Comparator: non‐interactive website with information on clinical problem |
|
| Outcomes | Primary outcome: uptake of option | |
| Notes | Source of funding: Michigan Department of Community Health and the National Cancer Institute provided funding for this research. Dr. Ruffin's participation was also made possible by support from the National Cancer Institute (K24‐CA80846‐010). Dr. Fetters' participation was also made possible in part by the generous support of the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A block randomisation process programmed by the study computer support staff and verified by a statistician was used including two strata, race and gender" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators, data collectors, data entry, and data analyst were all blinded to study arm assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Saunier 2020.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs control | |
| Participants | 41 + 42 hospital departments including 3547 hospital healthcare workers in France | |
| Interventions | DA: paper‐based leaflet that included clinical information, outcome probabilities, explicit values clarification, knowledge test, SURE test, and guidance in decision‐making (4‐step process). The DA is not publicly available; a copy was provided by the author (Amandine Gagneux‐Brunon; amandine.gagneux‐brunon@chu‐st‐etienne.fr). Comparator: control (no details provided) |
|
| Outcomes | Vaccine coverage Decisional conflict and knowledge assessed in DA group only |
|
| Notes | Source of funding: This work was supported by a grant dedicated to research on vaccine of the group ‘‘Prevention vaccination” of la Société de Pathologie Infectieuse de Langue Française (SPILF). Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The block randomization was centralized and stratified on the number of HCWs in the departments, and on the vaccine coverage during the 2017–2018 Flu season". The investigators describe the use of stratification or permuted blocking (use of computer implied). |
| Allocation concealment (selection bias) | Low risk | "The block randomization was centralized and stratified on the number of HCWs in the departments, and on the vaccine coverage during the 2017–2018 Flu season" (Central allocation) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No flow diagram; difficult to understand flow of participants in both study groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | High risk | No information about procedures for the control group. Clustering was not accounted for in the analysis. Selective recruitment of cluster participants is not addressed. There does not seem to be any formal process for recruiting participants: "One thousand leaflets were distributed in all the departments included in the intervention group". Unclear how many in each group actually received intervention or control. |
Sawka 2012.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 37 + 37 individuals with early‐stage papillary thyroid cancer in Canada | |
| Interventions | DA: web‐based decision aid with clinical problem, options' outcomes, outcome probabilities, guidance, printout summary. The DA is not publicly available; a copy was obtained from the authors. Comparator: usual care (consultation with a specialized head and neck surgeon, and with 1 or more medical specialist) |
|
| Outcomes | Primary outcomes: knowledge (baseline and immediately post intervention) Secondary outcomes: decisional conflict, undecided, treatment decision (baseline, immediately post intervention, 6 to 12 months), individual primarily responsible for the treatment decision (6 to 12 months) |
|
| Notes | Trial registration: NCT01083550 Source of funding: Supported by a grant from the Ontario Ministry of Health and Long‐term Care (Alternate Funding Plan Innovation Fund) and by New Investigator Grant No. CNI‐80701 from the Canadian Institutes of Health Research (A.M.S.). A.M.S. holds a Chair in Health Services Research from Cancer Care Ontario, funded by the Ontario Ministry of Health and Long‐term Care. S.S. holds a Tier 1 Canada Research Chair. Conflicts of interest: The author(s) indicated no potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central computerized randomization in a 1:1 ratio was performed at a patient level by using variable block sizes of 2 and 4 (allocation designed by a study statistician)" (p 2908) |
| Allocation concealment (selection bias) | Low risk | "Before the random assignment/testing visit, neither the participant, study staff, investigators, nor treating physicians were aware of the allocation, because it had not yet been assigned" (p 2908) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "There was no blinding of participants, study staff, or treating physicians after random assignment was completed" (p 2908), but it is unlikely that the outcomes are affected by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "There was no blinding of participants, study staff, or treating physicians after random assignment was completed. However, the statistician was blinded to the allocation of groups at the time of data analysis." (p 2908) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Authors state the trial is registered, but no link to trial number. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Schapira 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid + risk assessment vs usual care | |
| Participants | 104 + 103 women aged 39 to 48 with no prior mammogram in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, probabilities of outcomes, individual risk estimates, explicit values clarification, exemplars of other women considering screening, guidance in decision‐making (8‐step guide), guidance in communication, and an interactive summary sheet that could be printed or emailed and sent to a mobile device. The link to the DA is no longer functional and we were unable to obtain a copy from the authors. Comparator: risk assessment + usual care |
|
| Outcomes | Primary outcomes: strength of association between breast cancer risk and mammography uptake at 12 months, knowledge, and decisional conflict. Secondary outcomes: breast cancer worry, anticipated regret, accuracy of risk perception, and breast cancer screening intentions. |
|
| Notes | Source of funding: Financial support for this study was provided the National Cancer Institute–funded consortium, Population‐based Research Optimizing Screening through Personalized Regimens (PROSPR) U54CA 163313. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Conflicts of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Randomization occurred by concealed assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants that were conducting the chart review to assess outcomes were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram; < 90% included in analysis but balanced across groups (DA 54/104 (53%) included, control 59/103 (58%) included (P = 0.438807)), no justification for attrition |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Schonberg 2020.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs control | |
| Participants | 283 + 262 women aged 75 to 89 years scheduled for a routine or physical examination with their primary care provider in the USA | |
| Interventions | DA: paper‐based decision aid that includes clinical information, probabilities of outcomes, explicit values clarification, and a health questionnaire to assess individualized benefits for having a mammogram. The DA is available as a supplementary appendix in the article. Comparator: attention placebo control (pamphlet on home safety) |
|
| Outcomes | Primary outcome: receipt of mammography screening Secondary outcomes: knowledge, decisional conflict, preferred decision‐making role, discussion of mammography with primary care provider, changes in screening intentions. |
|
| Notes | Source of funding: This research was supported by the NIH/NCI (R01CA181357) (Dr Schonberg). Dr Marcantonio was supported by a Midcareer Investigator Award in Patient‐Oriented Research from the National Institute on Aging (K24 AG035075). Conflicts of interest: Dr Schonberg reported receiving grants from the National Cancer Institute (NCI) and receiving royalties for reviewing an UpToDate page on geriatric health maintenance. Drs Wee, Marcantonio, and Davis reported receiving grants from the National Institutes of Health (NIH). No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization assignments were determined using a permuted block randomization scheme with randomly varying block sizes. |
| Allocation concealment (selection bias) | Low risk | Randomization assignments were determined using a permuted block randomization scheme with randomly varying block sizes and were placed in sequentially numbered, sealed envelopes by the statistician (R.B.D.), stratified by site and panel size. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "After the first patient participating for each PCP, RAs were not blinded to patient randomization assignment; however, RAs attempted to recruit all eligible patients". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants were not blinded to patient randomization assignment, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; > 90% of participants included in analysis; provide justifications for loss to follow‐up, similar rate between arms |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02198690) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Schott 2021.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 33 + 33 adults aged ≥ 18 years with atrial fibrillation and elevated stroke risk in the USA | |
| Interventions | DA: web‐based decision aid used during consultation that includes a personalized risk calculator, clinical information, outcome probabilities, implicit values clarification, guidance in decision‐making (step‐by‐step process), and printable summary of results. *Note: the DA was previously illustrated viahttps://www.healthdecision.org/tool#/tool/afibbut is no longer available. Comparator: usual care |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: decisional conflict, value concordance, shared decision‐making, trust in clinician, time spent on each DA page |
|
| Notes | Source of funding: This research was supported by the Cardiovascular Fellowship Award from the Dartmouth‐Hitchcock Heart and Vascular Center. Conflicts of interest: Dr Coylewright reports honoraria and research funding from Edwards LifeSciences and Boston Scientific, and honoraria from W.L. Gore. The other authors report no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator was accessed online for assignments by study personnel. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to allocation, yet those in the control arm did not have access to the decision aid; research staff were not blinded to allocation. Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible with the study design. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram; > 90% included in analysis; justification for participants not included/loss to follow‐up; missing data balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | High risk | "Clinicians involved in the usual care arm confirmed they did not use pDAs in their practice." Might contaminate control group if clinicians are aware of intervention being conducted (unclear risk). Selective recruitment of cluster participants: "Final patient selection was based on whether the clinician was planning a real‐world discussion of the treatment options surrounding stroke prevention" (high risk). Free of other potential biases: adjustment for clustering performed. |
Schroy 2011.
| Study characteristics | ||
| Methods | Randomized to detailed vs simple decision aid vs control | |
| Participants | 223 + 212 + 231 average‐risk patients considering CRC screening in the USA | |
| Interventions | Detailed DA: CRC risk assessment + web‐based interactive audiovisual DA on options' outcomes, clinical problem, outcome probabilities, others' opinion, and guidance. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator 1: web‐based decision aid only Comparator 2: usual care using pamphlet |
|
| Outcomes | Knowledge (pre and post‐DA), satisfaction with decision‐making process (pre and post‐DA), preferred choice (pre and post‐DA) | |
| Notes | Primary outcome was not specified Source of funding: Agency for Healthcare Research and Quality grant R01HS013912 (PCS) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization process |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Providers were not blinded, subjective outcomes such as satisfaction with decision‐making process could have been affected; unclear if participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors not blinded, but outcome measures not believed to be influenced by it. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data appear to be missing. |
| Selective reporting (reporting bias) | Unclear risk | No mention of examination of selective outcome reporting or study protocol. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Schwalm 2012.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 76 + 74 patients undergoing coronary angiography in Canada | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, and guidance. The DA is no longer available (http://www.phri.ca/workfiles/studies/presentations/PtDA Vascular Access 23‐May.2012.pdf). The authors have a copy of the DA on file. Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge, risk perception, value congruent with chosen option |
|
| Notes | Source of funding: Support for this study provided by (1) McMaster University, Department of Medicine, Internal Career Research Award; and (2) McMaster University, Department of Medicine, Division of Cardiology, AFP research competition grant. Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number generator (p 261, Study design) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes (p 261, Study design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and physicians were not blinded to the allocation (p 261, Study design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if DCS score assessed by unblinded individuals, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not seem to have incomplete data. |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | Appeared to be free of other biases. |
Schwartz 2001.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 181 + 190 Ashkenazi Jewish women considering genetic testing in the USA | |
| Interventions | DA: 16‐page booklet on genetic testing with options' outcomes, clinical problem. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: general information on breast cancer, Understanding Breast Changes: A Health Guide for all Women, published by the National Cancer Institute |
|
| Outcomes | Primary outcome: preferred option Secondary outcomes: knowledge, accurate risk perceptions |
|
| Notes | Source of funding: Supported by grants P30 CAS1008‐07 and KO7 CA65597 from the National Cancer Institute. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (p 3) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High retention rate, baseline data and reasons for lost to follow‐up were provided (p 2, Participants section). |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Schwartz 2009a.
| Study characteristics | ||
| Methods | Randomized to decision aid + genetic counseling vs genetic counseling alone | |
| Participants | 100 + 114 women considering prophylactic mastectomy for being BRCA1/2 mutation carriers in the USA | |
| Interventions | DA: CD‐ROM on options' outcomes, clinical problem, risk communication with individually tailored risk graphs, explicit values clarification, others' opinion; guidance/counseling ‐ genetic counseling as usual care (Ottawa Decision Support Framework). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: genetic counseling on benefits and risks of testing, clinical problem (risk assessment, cancer risks associated with mutations, process of testing and interpretation of results) plus written letter outlining all guidelines and recommendations |
|
| Outcomes | Primary outcomes: decisional conflict, satisfaction with decision, actual choice (risk reduction mastectomy) Secondary outcomes: remaining undecided |
|
| Notes | Source of funding: National Cancer Institute Grant RO1 CA01846. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized via computer‐generated random number in a 1:1 ratio (p 3, Procedure) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig. 1 ‐ flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other sources of bias (p 8), "when variable for not watching DA cd was considered in multivariate models, the results did not change substantively (data not shown)". |
Sheridan 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care (list of risk factors) | |
| Participants | 49 + 38 adults with no history of cardiovascular disease in the USA | |
| Interventions | DA: computerized decision aid on options' outcomes, outcome probabilities. The DA is no longer available (www.med‐decisions.com/cvtool/). Comparator: list of CHD risk factors to present to doctor |
|
| Outcomes | Patient‐practitioner communication (e.g. discussion with doctor, specific plan to reduce risk discussed with doctor) | |
| Notes | Primary outcome was not specified Source of funding: Our work was funded by the Department of Medicine at the University of North Carolina, who had no role in the design, conduct, or interpretation of the study. Conflicts of interest: Dr. Sheridan and Dr. Pignone have received consulting and licensing fees from Bayer, Inc. Dr. Simpson has received honoraria and consulting fees from Merck, Pfizer, and Galaxo Smith Kline and has received honoraria and grant funding from Schering Plough. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputerized random number generator" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[S]ealed in security envelopes" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded but the doctors who saw both groups were not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcome was patient‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results (p 5); flow diagram (p 10); baseline characteristics/data included |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov NCT00315978 |
| Other bias | Low risk | Appears to have no other potential risk of bias. |
Sheridan 2011.
| Study characteristics | ||
| Methods | Randomized to decision aid + tailored messages vs usual care | |
| Participants | 81 + 79 patients with moderate or high risk for CHD considering CHD prevention strategies in the USA | |
| Interventions | DA: web‐based decision aid on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, and guidance. The DA is no longer available (www.med‐decisions.com/h2hv3/). Comparator: usual care using computer program |
|
| Outcomes | Preferred choice (post‐DA), adherence Other outcomes (Sheridan 2014): patient‐provider communication (post‐DA), patient participation (post‐DA), patient's perceptions of discussions and the healthcare visit (post‐DA), preferred choice (baseline and post‐DA) (data from 81 +79 patients) |
|
| Notes | Primary outcome was not specified Source of funding: The research reported in this publication was supported in part by a grant from the American Heart Association (grant number 0530164N), the National Heart Lung and Blood Institute (grant number 1 K23 HL074375), and the National Cancer Institute (grant number K05 CA129166). Conflicts of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised by study staff who accessed an online randomised schedule" (p 2). Sequence generation method not stated. |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised by study staff who accessed an online randomised schedule" (p 2). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded and physicians unblinded, but objective outcomes are not likely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes deemed objective, therefore lack of blinding did not influence assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be no missing data. |
| Selective reporting (reporting bias) | Low risk | Protocol made available |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Shorten 2005.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 85 + 84 pregnant women who have experienced previous cesarean section considering birthing options in Australia | |
| Interventions | DA: decision aid booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (Ottawa Decision Support Framework). The DA is available from the author (ashorten@uow.edu.au) or www.capersbookstore.com.au/product.asp?id=301. Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: preferred option, help with making a decision |
|
| Notes | Source of funding: This project is supported by an MBF Research Grant, Sydney, The University of Wollongong New Researcher Grant Scheme, Wollongong, and NSW Midwives Association Research Scholarship, Sydney, New South Wales, Australia. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomized generation (p 3, Procedure) |
| Allocation concealment (selection bias) | Low risk | "[O]paque envelopes containing a random allocation for each participant code number" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants/midwives/doctors were blinded to patients' allocation. However, women who used the decision aid as specified and in a process of consultation with their midwife or doctor would have negated the blinding of their clinicians, and perhaps of the women themselves. For the intervention group, this may have affected the level and type of information exchanged between them and their caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 women were lost to follow‐up from the intervention group and 18 from the control group (no reasons listed) (p 4, Results). |
| Selective reporting (reporting bias) | Low risk | Reference to published protocol |
| Other bias | Low risk | Appears to be free of other potential biases. |
Shourie 2013.
| Study characteristics | ||
| Methods | Cluster‐randomized controlled trial of GP practices to web‐based MMR DA + usual care, MMR leaflet + usual care, versus usual care | |
| Participants | 50 + 93 + 77 parents of children facing their first dose MMR vaccination in the UK | |
| Interventions | Web‐based DA: clinical problem, options' outcomes, explicit values clarification, guidance. The DA is no longer available (www.leedsmmr.co.uk). MMR leaflet: Health Scotland leaflet, 'MMR: your questions answered' Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (baseline and 2 weeks postintervention) Secondary outcomes: choice uptake of first dose MMR (when child was 15 months), knowledge (baseline and 2 weeks; results not provided), MMR immunization cognitions (baseline and 2 weeks post; results not provided), immunization trade‐off beliefs (baseline and 2 weeks post; results not provided), anxiety (baseline and 2 weeks post; results not provided), use of the intervention (baseline and 2 weeks post) Follow‐up article (Tubeuf 2014): cost‐effectiveness |
|
| Notes | Trial registration: UK Clinical Research Network ‐ UKCRN ID 4811 Source of funding: The study was funded by the National Institute for HealthResearch, Research for Patient Benefit Programme (ref. PB‐PG‐0107‐12048). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation using a computer‐generated random list allocated GP practices on a 1:1:1 basis" (p 3) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher who had no contact with participants generated the allocation sequence and assigned the GP practices to their allocated arm" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "On receipt of the completed baseline questionnaire and consent form, the appropriate intervention was delivered. At this point the researchers and participants were no longer blind to allocation" (p 3). We do not know if receiving the intervention had an effect on the ultimate decision that was made. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data assessment does not depend on the assessor. It is an objective questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered. Primary outcome reported as stated. Secondary outcomes are not reported (p 3). |
| Other bias | Unclear risk | Difference in allocation to groups (50 + 93 + 77). Unclear what effect this difference had on the results. Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Singh 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 153 + 148 women aged 18 and older having a lupus nephritis flare and considering change or initiation of an immunosuppressive medication (current flare) or who had a prior lupus nephritis flare and were at risk for a future lupus nephritis flare (at risk for nephritis flare) in the UK | |
| Interventions | DA: online decision aid used in preparation for consultation that includes clinical information, outcome probabilities, implicit values clarification, frequently asked questions, and guidance in communication. The DA is not publicly available; a copy was provided by the author (Jasvinder A. Singh; Jasvinder.md@gmail.com). Comparator: information pamphlet |
|
| Outcomes | Primary outcome: decisional conflict, informed value‐concordant choice Secondary outcomes: preferred role in decision‐making, patient‐physician communication and care processes, patient participation, acceptability of the intervention |
|
| Notes | Source of funding: This study was funded by the Patient Centered Outcomes Research Institute (https:// www.pcori.org/), contract number PCORI CE‐1304‐6631, to JAS. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: JAS has received research grants from Takeda and Savient Pharmaceuticals and consultant fees from Savient, Takeda, Regeneron, Merz, Iroko, Bioiberica, Crealta/Horizon, Fidia, and Allergan Pharmaceuticals and WebMD, UBM LLC, Medscape, and the American College of Rheumatology. JAS served as the principal investigator for an investigator‐initiated study funded by Horizon Pharmaceuticals through a grant to DINORA, Inc., a 501 (c)(3) entity. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology, and receives arms‐length funding from 36 companies; a member of the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC); Chair of the ACR Meet‐the‐Professor, Workshop and Study Group Subcommittee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta‐analysis. MD serves on an Independent Data Monitoring Committee for Biogen, Genentech, and Janssen Pharmaceuticals and as a consultant to Abbvie, Kezar, and AstraZeneca. KLW reports grants and personal fees from Pfizer, grants and personal fees from BMS, personal fees from Abbvie, grants and personal fees from UCB, personal fees from Lilly, personal fees from Galapagos, and personal fees from GSK, outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients with lupus nephritis were randomized in a 1:1 ratio to the provision of the decision aid or the ACR lupus paper pamphlet. After obtaining written informed consent, we randomized participants using a computer‐generated randomization process based upon a permuted variable block design, stratified by study site and language (English versus Spanish), and designed by a biostatistician" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All outcomes were patient‐assessed and patient‐reported, and neither patients nor assessors were blinded. Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were patient‐assessed and patient‐reported, and neither patients nor assessors were blinded. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, ITT, > 90% of participants included in the analysis with justification for the ones not included; missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02319525) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Smallwood 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid vs attention control (education on another topic) | |
| Participants | 29 + 21 women aged ≥ 55 years of age with osteopenia or osteoporosis in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that included clinical information, personalized risk calculator, explicit values clarification, and 2 printouts at the end of the decision aid that contained extensive information about treatments and a personalized summary of risk information and values. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: attention control (education on another topic) |
|
| Outcomes | Primary outcomes: decision quality (preparation for decision‐making scale and the decisional conflict scale), feasibility. Secondary outcomes: treatment decisions, patient‐reported shared decision‐making. | |
| Notes | Source of funding: This study was funded by the Clinical and Translational Science Institute of Southeast Wisconsin (project number 5,520,204). Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two predetermined block randomization schedules for osteoporosis and osteopenia were created using a computer random number generator and maintained electronically. |
| Allocation concealment (selection bias) | Low risk | The study co‐ordinator was responsible for randomization and blinded to allocation until after consent was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither patients nor physicians could be adequately blinded to their treatment arm. Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, all participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | Small sample size (29 + 21) and parametric tests used, no mention of sample homogeneity. This study was underpowered for treatment decisions, limiting the power to detect differences between groups, which may have prevented statistically significant results like shared decision making at 3 months and durability of results for decisional conflict...sample of patients included some with prior treatment experience or FRAX scores that did not reach guideline recommendations. |
Smith 2010.
| Study characteristics | ||
| Methods | Randomized to detailed vs simple decision aid vs usual care | |
| Participants | 196 + 188 + 188 socioeconomically disadvantaged participants diagnosed with average or slightly above average risk of bowel cancer considering bowel cancer screening in Australia | |
| Interventions | DA: booklet + DVD + worksheet + question prompt list on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary). The DA is no longer available (sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php). The authors have a PDF version. Comparator: booklet + DVD + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary) Comparator: usual care using standard information booklet |
|
| Outcomes | Primary outcomes: values congruent with chosen option (post‐DA), participation in decision‐making (pre, post‐DA) Secondary outcomes: knowledge (pre, post‐DA), attitude, actual choice (post‐DA), decisional conflict (post‐DA), decision satisfaction (post‐DA), confidence in decision‐making (post‐DA), general anxiety (post‐DA), worry about developing bowel cancer (pre, post‐DA), risk perception Other outcomes (Smith 2014): screening participation (357 + 173 participants) |
|
| Notes | Source of funding: This work was supported by a grant from the National Health and Medical Research Council of Australia (No 457381). The funder had no role in the design or conduct of the study, in the collection, analysis and interpretation of data, or in the preparation or approval of the manuscript. Conflicts of interest: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that all authors had: no financial support for the submitted work from anyone other than their employer; no financial relationships with commercial entities that might have an interest in the submitted work; and no non‐financial interests that may be relevant to the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants who verbally consented to take part were then randomised to one of the three groups using random permutated blocks of size 6 and 9 for each sex stratum" (p 3, Participants and recruitment section) |
| Allocation concealment (selection bias) | Low risk | Central allocation; "interviewers responsible for recruiting participants were not aware of the randomization sequence or allocation and therefore did not know which intervention respondents would receive" (p 3, Participants and recruitment section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "It was not possible for the reviewers to be blinded to the group allocation. However, all questions used standardised wording with pre‐coded responses and were asked within a supervised environment, where interviewer performances were regularly monitored to ensure scripts were read as written" (p 3, Outcome measures section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[A]nalyses were by intention to treat and carried out blinded to intervention" (p 5, Statistical analysis section); outcomes measured were not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Explanation for the missing data reported at base of tables. |
| Selective reporting (reporting bias) | Low risk | Study protocol available (ClinicalTrials.gov NCT00765869 and Australian New Zealand Clinical Trials Registry 12608000011381) |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Stacey 2014a.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 71 + 71 adults diagnosed with knee osteoarthritis considering joint replacement in Canada | |
| Interventions | DA: DVD + booklet + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (1 page summary for the surgeon). The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. The authors have a copy of the video and booklet that was evaluated in the study. Comparator: usual care |
|
| Outcomes | Primary outcomes: feasibility (including recruitment, data collection), preliminary effectiveness Secondary outcomes: knowledge (post‐DA, pre‐surgeon consult), informed values‐congruent with chosen option (post‐DA, pre‐surgeon consult), uptake of chosen option at 1 year; decisional conflict (SURE test), preparation for decision‐making (4 items), wait times |
|
| Notes | Trial registration: NCT00743951 Source of funding: The study was funded using D Stacey’s research start‐up funds from the University of Ottawa, in Ottawa, Canada. The PtDAs were provided free of charge by the Informed Medical Decisions Foundation. Conflicts of interest: The authors (DS, GH, PT, IT, LB, MPP, AC, MT) declare that they have no competing interests. GFD is a paid consultant for Stryker Corporation advising on total and partial knee replacement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation schedule was computer‐generated centrally by a statistician using a permuted block design with randomly varying block lengths of 4, 6, or 8." (p 3) |
| Allocation concealment (selection bias) | Low risk | "Allocations were concealed in numbered opaque sealed envelopes" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were not informed of the intervention characteristics" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Although the research assistant was not blinded to group allocation, study outcomes for effectiveness were objective and obtained from clinic data (e.g. date of surgery or wait list status)" (p 3). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol registered on ClinicalTrials.gov |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Stacey 2016.
| Study characteristics | ||
| Methods | Randomized to decision aid + usual education + preference report for surgeon vs usual education alone | |
| Participants | 174 + 169 adults aged 18 and older with moderate or severe hip or knee osteoarthritis and were determined at the orthopedic screening clinic to be appropriate for surgical consultation about joint arthroplasty in Canada | |
| Interventions | DA: video decision aid plus booklet used in preparation for consultation that included information on the clinical condition, probabilities of outcomes of options, implicit values clarification, video clips of patient experiences, guidance in communication, and preference report for the surgeon. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. The authors have a copy of the video and booklet that was evaluated in the study. Comparator: education and half page of clinical assessment findings for surgeon |
|
| Outcomes | Primary outcome: wait times Secondary outcomes: decision quality (knowledge + values + actual choice), realistic expectation of outcomes, surgical rates, perceptions of decision‐making process, costs ( Trenaman 2017 ; Trenaman 2020 ) |
|
| Notes | Source of funding: This work was supported by funding and access to the PtDA from the not‐for‐profit Informed Medical Decisions Foundation (Grant #0099‐1). Funding for graduate students was from the Faculty of Health Sciences, University of Ottawa. Conflicts of interest: The authors (DS, MT, PT, IT, AO, MPP, LB, SB, DM, GH) declare that they have no conflict of interests. GFD is a paid consultant for Stryker Corporation advising on total and partial knee replacement. At the time of the study, the Informed Medical Decisions Foundation that provided funding for the study had a licensing agreement with Health Dialog, a commercial company who markets PtDA and health coaching. The funders were not involved in the study design, data collection, analysis, interpretation of data, or writing of the report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation schedule was computer‐generated centrally by a statistician, using block randomization, with randomly varying block lengths of 4, 6, or 8." |
| Allocation concealment (selection bias) | Low risk | "To ensure concealment, call‐in telephone software was used to obtain randomized allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To minimize bias after allocation, patients reviewed the information (i.e., PtDA plus usual education or usual education only) at home, were not informed of the other intervention, and did not have contact with orthopedic screening clinic practitioners during the 2 weeks post clinic visit when measures were collected. Although the research assistant was not blinded to group allocation, the primary outcome was objective and used clinic data." Low risk because objective measures used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "343 participants were randomized to the intervention (n = 174) or usual care (n = 169) and followed for 2 years"..."At the end of the 2‐year follow‐up (October 2011), there were 165 intervention group participants and 163 controls included in the primary outcome analysis." Loss to follow‐up was 5% and 4% respectively. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT00911638) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Stamm 2017.
| Study characteristics | ||
| Methods | Randomized to decision aid alone vs decision aid + SDM vs usual care | |
| Participants | 106 + 113 + 110 men aged 50 to 75 years who were being evaluated by one of 2 primary care providers at Virginia Mason Medical Center, USA | |
| Interventions | DA: paper‐based decision aid that includes outcome probabilities and values clarification. The DA is not publicly available; a copy was provided by the author (Dr. John M. Corman; John.corman@virginiamason.org). Comparator: usual care |
|
| Outcomes | Knowledge of prostate cancer screening and the decision regarding screening | |
| Notes | Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% of participants included in analysis, similar between arms (included in usual care 85%, DA 87%, DA + SDM 83%) (P = 0.699405); provides justification for not including participants (loss to follow‐up or returning survey late) |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | There is no mention of the funding source. |
Steckelberg 2011.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 785 + 792 patients with no CRC history considering CRC screening in Germany | |
| Interventions | DA: brochure on options' outcomes, clinical problem, and outcome probabilities. The DA is no longer available (www.gesundheit.uni‐hamburg.de/upload/AltDarmkrebsinternet.pdf). The authors have a PDF version. Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: values congruent with chosen option (post‐DA) Secondary outcomes: knowledge (post‐DA), combination of actual and planned uptake (post‐DA), risk perception |
|
| Notes | Source of funding: German Federal Ministry of Education and Research Conflicts of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence (p 2, Randomization and blinding) |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed. Identity numbers were independent of allocation, and study members did not have access to the data (p 2, Randomization and blinding). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial staff who sent out questionnaires and reminders were not aware of study arm; unclear if participants were blinded (p 2, Randomization and blinding). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial staff and statistician who entered data were blinded (p 2, Randomization and blinding). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% missing one or both questionnaires in intervention group vs 9.2% in control group; judged to have low impact on study outcome (p 2) |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Unclear risk | Participants who completed the trial do not add up. |
Stephenson 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (waiting list) | |
| Participants | 464 + 463 women aged 15 to 30 years with a current or future need for contraception, attending one of the study sites in the UK | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, and summary of the 3 methods most consistent with the individual’s preferences are displayed and compared side‐by‐side, and the user can export their results by email or text message. The DA is publicly available at https://www.contraceptionchoices.org . Comparator: control (no intervention) |
|
| Outcomes | Primary outcomes: use of long‐acting reversible contraception at 6 months and satisfaction with contraceptive method at 6 months Secondary outcomes: effectiveness of contraceptive method at 6 months; change in method from baseline to 6 months; pregnancy by 6 months and diagnosed sexually transmitted infection reported at 3 or 6 months |
|
| Notes | Source of funding: National Institute for Health Research Health Technology Assessment Programme Commissioned call to increase the uptake of long‐acting contraception in young women. Study registration ISRCTN 13247829. Conflicts of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated, computerized randomization occurred immediately after baseline data collection. A randomization list was generated by a random number based algorithm in the computer software Stata25 and incorporated into the trial software program to allocate all participants to either the intervention or control group. The randomization list was stratified by setting and used varying block sizes. |
| Allocation concealment (selection bias) | Low risk | Allocation was immediate (online) and concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind (outcome analysis). Unclear how lack of blinding of participants may have influenced outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The analysis of the primary outcomes was conducted blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, "modified ITT", < 90% of participants included in analysis (loss to follow‐up similar between arms; 84% and 86% included) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (ISRCTN13247829). All outcomes of interest to the current review are reported except for "health service and out‐of‐pocket costs". |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Stubenrouch 2022.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid + consultation cards (Option grids) + decision cards vs usual care | |
| Participants | 247 + 197 patients visiting the outpatient clinics for their abdominal aortic aneurysm, varicose veins, carotid artery stenosis, or intermittent claudication and for whom more than one treatment option was possible (including the option not to treat) in the Netherlands | |
| Interventions | DA: web‐based decision aids used in preparation for consultation that include clinical information, outcome probabilities, explicit values clarification, guidance in decision‐making (step‐by‐step guide), and summary. Consultation cards (option grids) and decision cards were used during consultation to support patient involvement in decision‐making. The DAs are publicly available at: https://keuzehulp.medify.eu/KeuzehulpMedify/keuzehulp_medify.html . Comparator: usual care |
|
| Outcomes | Primary outcome: level of SDM during consultation (OPTION scale) Secondary outcomes: factors influencing SDM level, SDM as perceived by patients (SDM‐Q‐9; CollaboRATE), and by clinicians (SDM‐Q‐Doc), the degree of desired patient involvement (Control Preferences Scale), knowledge, treatment choice, consultation duration, decisional conflict, and patient’s quality of life |
|
| Notes | Source of funding: none to declare Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Patients were included consecutively and were unaware of group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the nature of this study, it is not possible to blind patients or vascular surgeon, since they actively use the intervention. However, the cluster‐randomization design does reduce potential contamination of information among the participating vascular surgeons. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded. Low risk for outcomes that were objectively measured (knowledge, decisional conflict, treatment choice, consultation duration). Unclear risk for patient‐reported subjective measures (shared decision‐making as perceived by patients, degree of desired patient involvement). High risk for observer‐reported subjective measures (level of shared decision‐making using OPTION scale). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis but missing data are balanced across groups (77% included in control group, 77% included in DA group), justifications provided |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NTR6487) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | High risk | Clustering not accounted for in the analysis of data (high risk of bias). Free of other potential biases: no evidence of selective recruitment of cluster participants. |
Subramanian 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control (no intervention) | |
| Participants | 116 + 118 adults with advanced chronic kidney disease in the USA | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, explicit values clarification, patient testimonies, guidance in decision‐making (step‐by‐step process), and guidance in communication. The version of the DA tested in the trial was provided by the author (Jarcy Zee; jarcy.zee@pennmedicine.upenn.edu). *Note: the DA was previously illustrated viahttps://choosingdialysis.org/but is no longer available. Comparator: no intervention |
|
| Outcomes | Treatment preference, decisional conflict, decision self‐efficacy, preparation for decision‐making (intervention group only), knowledge | |
| Notes | Source of funding: Research reported in this article was funded through a Patient‐Centered Outcomes Research Institute (PCORI) Award (1109) to Dr Tentori. Dr Tentori was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK087762. The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication. Conflicts of interest: Dr Tentori is an employee of DaVita HealthCare Partners, Inc. She was employed by Arbor Research Collaborative for Health, which administers the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program, which is funded by a consortium of private industry, public funders, and professional societies. Principal funders: Amgen, Kyowa Hakko Kirin, and Baxter Healthcare. Additional support for specific DOPPS projects and/or program activities in specific countries provided by: Amgen, Association of German Nephrology Centres (Verband Deutsche Nierenzentren e.V.), AstraZeneca, European Renal Association‐European Dialysis and Transplant Association, German Society of Nephrology, Hexal AG, Janssen, Japanese Society for Peritoneal Dialysis, Keryx, Proteon, Relypsa, Roche, Societa Italiana di Nefrologia, Spanish Society of Nephrology, and Vifor Fresenius Medical Care Renal Pharma. Public funding and support is provided for specific DOPPS projects, ancillary studies, or affiliated research projects by: Australia: National Health & Medical Research Council; Canada: Canadian Institutes of Health Research and Ontario Renal Network; France: Agence Nationale de la Recherche; Thailand: Thailand Research Foundation, Chulalongkorn University Matching Fund, King Chulalongkorn Memorial Hospital Matching Fund, and the National Research Council of Thailand; United Kingdom: National Institute for Health Research via the Comprehensive Clinical Research Network; and United States: National Institutes of Health and PCORI. All support is provided without restrictions on publications. The remaining authors declare that they have no relevant financial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study co‐ordinator provided the participant with a unique user login and study ID. The list of IDs provided to each recruiter was randomly generated by an independent study programmer and each ID appeared as a random sequence of letters. The list alternated between the intervention and control arms to ensure parallel assignment to the intervention or control arms of consented participants. |
| Allocation concealment (selection bias) | Low risk | Neither the study co‐ordinator nor the participant could discern the assignment based on the ID and both were therefore blinded to treatment assignment before consent and before the study started. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the study co‐ordinator nor the participant could discern the assignment based on the ID and both were therefore blinded to treatment assignment before consent and before the study started. The study co‐ordinator also remained blinded to treatment assignment throughout the study because participants engaged in the study on their own time. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the study co‐ordinator nor the participant could discern the assignment based on the ID and both were therefore blinded to treatment assignment before consent and before the study started. The study co‐ordinator also remained blinded to treatment assignment throughout the study because participants engaged in the study on their own time. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, high rate of attrition but balanced across groups. Completers: 63/118 (53%) DA group and 70/116 (60%) control group (P = 0.28284). Justification for attrition reported. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02488317) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Taylor 2006.
| Study characteristics | ||
| Methods | Randomized to print DA versus video DA versus wait list control | |
| Participants | 98 + 95 + 92 African American men with no history of prostate cancer to consider prostate cancer screening in the USA | |
| Interventions | Print DA: clinical problem; outcome probabilities; guidance (list of questions to ask at next appointment); others' opinions. The DA is not publicly available; a copy was provided by the author (taylorkl@georgetown.edu). Video DA: clinical problem; others' opinions Wait list comparator: no information provided until 1 month post‐randomization (baseline assessment for this group coincided with 1‐month assessment of print and video arms) |
|
| Outcomes | Prostate cancer screening intention (baseline and 1 month; not reported), prostate screening uptake (1 year; not included because wait list received intervention before 1 year) process variables including use and perception of the intervention materials (1 month), prostate cancer knowledge (baseline and 1 month post), decisional conflict (baseline and 1 month post), satisfaction with screening decision (baseline and 1 month post) | |
| Notes | No primary outcome reported; not found in trials registry Source of funding: Centers for Disease Control and Prevention grant TS290 and National Cancer Institute grant K07 CA72645‐01 Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information related to random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to judge blinding; however, participants were requested not to share intervention materials with others to prevent contamination between groups (p 2180). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered or published |
| Other bias | Unclear risk | "All participants were mailed $25 for their participation following completion of the 1‐month interview" (p 2181) "Men who reported that they had not yet had a chance to read/watch the materials were given an additional week to do so and called again to complete the follow‐up assessment" (p 2181) |
Tebb 2021.
| Study characteristics | ||
| Methods | Cluster randomized to decision aid vs control | |
| Participants | 693 + 667 Hispanic/Latina females aged 14 to 18 years who were sexually active, not currently pregnant, and not currently using long‐acting reversible contraception in the USA | |
| Interventions | DA: web‐based decision aid that includes clinical information, outcome probabilities, explicit values clarification, quiz, individualized recommendations based on questions answered, patient testimonies, and guidance in communication. The DA is publicly available at https://health-eyou.ucsf.edu/#eq_wellness_center . Comparator: control (no intervention) |
|
| Outcomes | Knowledge (only reports change from baseline for total sample), self‐efficacy, contraceptive use, effectiveness of clinical encounter (discussed birth control), satisfaction with DA | |
| Notes | Source of funding: This study was funded by a Patient‐Centered Outcomes Research Institute (PCORI) research award [AD‐1502‐27481]. Additional support was also provided by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS), Maternal and Child Health Bureau: Dr. Ozer (Adolescent and Young Adult Health Research Network, Cooperative Agreement: #UA6MC27378)); Dr. Brindis and Dr. Adams: (Adolescent and Young Adult Health Capacity Building Program: # U45MC27709); Ozer, Brindis and Adams also received funding from # T7IMC00003. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement of the funders. The funders mentioned above did not participate in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS, or the U.S. Government. Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinics were randomized to the control (n = 9) or intervention group (n = 9) using computer‐generated random assignment. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow diagram, high loss to follow‐up and missing data are significantly higher in DA arm (completers at 48 hours: 335/693 DA and 443/667 control (P < 0.00001)); "attrition was higher in the intervention group and dropouts tended to be younger", used multiple imputation using chained equations for attrition, no justification provided for attrition. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02847858) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | High risk | At baseline, the intervention group had significantly higher knowledge scores. Another major limitation was that, despite randomization, intervention participants, compared to controls, had significantly higher rates of sexual activity and the recruitment visit was more likely to be for a pregnancy test, EC, birth control, or birth control/pregnancy counseling. (Unclear risk). Selective recruitment of cluster participants (Low risk of bias): "All adolescent girls were offered an iPad Air upon clinic checkin (between August 2016 and May 2018). The “user” selected their preferred language and completed an online survey that obtained consent and assessed eligibility (i.e. female; 14 to 18 years; Hispanic/Latina2; sexually active; not currently pregnant; and not currently using long‐acting reversible contraception (LARC))." Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Thomson 2007.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care by clinical guidelines | |
| Participants | 69 + 67 patients with atrial fibrillation considering treatment options in the UK | |
| Interventions | DA (in consultation): computerized decision on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance/coaching by physician. The DA is not publicly available; a copy was provided by the author (computer disc sent by mail). Comparator: guidelines applied as direct advice |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: anxiety, knowledge, resource use, choice, health outcomes (stroke, transient ischemic attack, bleeding events) |
|
| Notes | Source of funding: Wellcome Trust Health Services Research Project Grants. All authors are independent of the funding bodies. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[E]lectronically‐generated random permuted blocks via a web‐based randomisation service" (p 2, Recruitment and randomization) |
| Allocation concealment (selection bias) | Low risk | "[E]lectronically‐generated random permuted blocks via a web‐based randomisation service" (p 2, Recruitment and randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Physicians were blinded. Unclear if patients are blinded and how that may affect the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram |
| Selective reporting (reporting bias) | Low risk | ISRCTN24808514 |
| Other bias | Low risk | Baseline characteristics similar, sample size similar, not stopped early |
Tilburt 2022.
| Study characteristics | ||
| Methods | Cluster randomized to 1) pre‐visit DA + within visit DA vs 2) pre‐visit DA only vs 3) within‐visit DA only vs 4) usual care | |
| Participants | 5 sites (44 participants who received during consultation DA) + 5 sites (50 participants who received usual care) aged ≥ 18 years with a positive prostate cancer biopsy within the previous 4 months in the USA | |
| Interventions | DA: web‐based decision aid used during consultation that includes explicit values clarification, individualized estimates of prostate cancer risk stratification, quality of life compared to average population, guidance in decision‐making (5‐step guide), and summary page including prostate cancer risk stratification, life expectancy, existing quality of life, and values. The within‐visit DA is available at: http://prostatecancer.takethewind.com/web/index.php . The pre‐visit DA is no longer available, therefore we only extracted data on the group that received the within‐visit DA alone vs usual care. Comparator: usual care (no details provided) |
|
| Outcomes | Primary outcome: knowledge Secondary outcomes: clinical time, decisional regret, health‐related quality of life. The latter 2 outcomes will be reported in a 1‐year follow‐up article. |
|
| Notes | Source of funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers UG1CA189823 (Alliance for Clinical Trials in Oncology NCI Community Oncology Research Program grant); UG1CA189848, UG1CA233270, UG1CA233290, UG1CA233329, UG1CA233331, UG1CA233373, UG1CA232760, and R01 MD008934 (Jon C. Tilburt, Joel E. Pacyna, Judith S. Kaur, and Simon P. Kim); and U10CA180820, UG1CA189830, and UG1CA189854 (ECOG‐ACRIN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (https://acknowledgments.alliancefound.org. Conflicts of interest: Daniel G. Petereit reports grant support from the Bristol‐Myers Squibb Foundation, the Irving A. Hansen Foundation, the Ralph Lauren Pink Pony Foundation, and the National Institutes of Health (1R01CA240080‐01); consulting fees from Boston Scientific; payments or honoraria from Boston Scientific, the University of California San Francisco, the Mayo Clinic, and the University of Pennsylvania; legal consultancy for brachytherapy cases; and a leadership role with the American Brachytherapy Society. George J. Chang reports consulting fees from Medicaroid and participation on boards for J&J and 11 Health. Ethan M. Basch reports consulting fees from AstraZeneca, Carevive Systems, Navigating Cancer, and Sivan Healthcare. Michael J. Morris is an uncompensated consultant for Bayer, Novartis, Advanced Accelerator Applications, Janssen, and Lantheus; is a compensated consultant for ORIC, Curium, Athenex, the National Comprehensive Cancer Network, and Exelixis; reports participation on boards for Curium, Athenex, Exelixis, AstraZeneca, and Amgen; and receives institutional funding for clinical trials from Bayer, Endocyte, Progenics, Corcept, Roche/ Genentech, Celgene/Bristol‐Myers Squibb, and Janssen. None of his disclosures are related to this work. Electra D. Paskett is a multiple principal investigator on a grant to her institution from the Merck Foundation and on another grant from Pfizer, and she also receives grant funding to her institution from the Breast Cancer Research Foundation. None of her disclosures are related to this work. Victor M. Montori reports that he works at the Knowledge and Evaluation Research Unit of the Mayo Clinic and conducts research into shared decision‐making; often, shared decision‐making tools are produced that are placed in the public domain and are free to use and that produce no income to the research unit or to him personally. Dominick L. Frosch reports consulting fees paid to his former employer (Sutter Health) by the Mayo Clinic/National Institutes of Health. The other authors made no disclosures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Few details: "We used a cluster randomized trial with a 2×2 factorial design. With such a design, clinical practices were identified up front and randomized with equal allocation to 1 of 4 arms receiving both previsit and within‐visit DAs, a previsit DA only, a within‐visit DA only, or no DA (usual care; Fig. 1)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% included in analysis, attrition similar between arms, no justification for attrition |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03103321) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. "The latter 2 secondary outcomes are not reported in this article because they are planned for a subsequent article devoted to 1‐year outcomes." |
| Other bias | Unclear risk | One or more of the authors are industry employees (unclear risk). Selective recruitment of cluster participants: "Because our underlying scientific question included a desire to understand the effects of DAs in minority men, particularly Black or African American men, we set aside half of all trial slots for Black or African American men to ensure a prespecified effect size analysis in this subgroup while also hoping to attract a diverse overall demographic mix of participants" (unclear risk). Few details on recruitment approaches (in article and in protocol); in protocol: "Participant recruitment will remain flexible to accommodate each site’s workflow for notifying patients about new cancer diagnoses and providing consultation about treatment choices. Some sites disclose positive cancer diagnoses by phone, with the treatment consultation occurring days later. Other sites combine notification and treatment discussion into a single consultation with the physician provider. In all cases, participating sites will need to ensure that registration and intervention (in applicable study arms) occur after diagnosis notification and prior to the specialist consultation. Each site will develop methods for identifying eligible patients ahead of visits and for recruiting patients in a way that avoids the possibility of inadvertent diagnosis disclosure by study staff" (unclear risk). Free of other potential biases: adjustment for clustering performed. |
Trevena 2008.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care by consumer guidelines | |
| Participants | 157 + 157 patients not previously screened for colorectal cancer in Australia | |
| Interventions | DA: age‐gender‐family history specific DA booklet with information on options, outcome probabilities, explicit values clarification, guidance (personal worksheet with steps in decision‐making) (Theory of planned behavior). The DA is no longer available (sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php). Comparator: consumer guidelines recommending fecal occult blood testing |
|
| Outcomes | Primary outcome: informed choice Secondary outcomes: knowledge, values, screening intention (choice); test uptake, anxiety, acceptability of the intervention, satisfaction with the decision |
|
| Notes | Source of funding: National Health and Medical Research Council (NHMRC) Program Grant Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequential ID numbers were randomly assigned by computer program to DA or Guidelines (G) in blocks of four" (p 3) |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed via the password‐protected program" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to the intervention type ‐ not sure about GPs. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded to allocation for all telephone interviews, outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics included (p 3). Fig 2 flow chart (p 5). Reasons for loss to follow‐up not mentioned. |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov ‐ NCT00148226 |
| Other bias | Low risk | Appears to be free of other potential biases. |
van Dijk 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid + usual care vs usual care | |
| Participants | 69 + 76 adults 18 years or older newly diagnosed with OA of the knee or hip in the Netherlands | |
| Interventions | DA: online decision aid provided after the first consultation when patients received the diagnosis of osteoarthritis of the knee or hip that included clinical information, outcome probabilities, explicit values clarification, knowledge test, and guidance in decision‐making (5‐step guide). The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: satisfaction, anxiety, knowledge, stage of decision‐making, preferred treatment, health outcomes, quality of life |
|
| Notes | Source of funding: none declared Conflicts of interest: There is no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomized by a computer generated randomization sequence by one of the research fellows into the control group or intervention group. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "unblinded randomized controlled trial". Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "unblinded randomized controlled trial", but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis but balanced across groups (36/69 (52%) included in usual care group, 39/76 (51%) included in DA group (P = 0.917747)); justification for attrition reported |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NL4291; old trial number: NTR4435), and 2 outcomes of interest to the review (knowledge, stage of decision‐making) were not pre‐specified; https://trialsearch.who.int/Trial2.aspx?TrialID=NTR4435 |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Van Peperstraten 2010.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 152 + 156 infertile women on wait list for in vitro fertilization in the Netherlands | |
| Interventions | DA: self‐administered booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making decision, worksheet with questions relevant to decision‐making process; 1 or more questions that asked patients to clarify their preferences; summary to be shared with practitioner), coaching (by trained in vitro fertilization nurse) + standard in vitro fertilization care. The DA is no longer available online (www.umcn.nl/ivfda‐en). The authors have a PDF version. Comparator: standard in vitro fertilization care, including a session in which the number of embryos transferred was discussed |
|
| Outcomes | Primary outcomes: actual choice (postintervention and consultation) Secondary outcomes: knowledge (pre, post‐DA and consultation), empowerment (pre, post‐DA and consultation), participation in decision‐making, decisional conflict (post‐DA and consultation), levels of anxiety (pre, post‐DA and consultation), depression (pre, post‐DA and consultation), cost evaluation of empowerment strategy (post‐DA and consultation), condition‐specific health outcomes (pregnancies) (post‐DA and consultation) |
|
| Notes | Source of funding: This study was funded by the Netherlands Organisation for Health Research and Development (grant No 945‐16‐105). All researchers are independent from this source of funding. The study sponsor had no role in the study design, collection, analysis, and interpretation of data, the writing of the article, and the decision to submit it for publication. Conflicts of interest: All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners, or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no non‐financial interests that may be relevant to the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list (p 2, Methods section) |
| Allocation concealment (selection bias) | Low risk | Central allocation (p 2, Methods section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Because of the nature of the intervention it was not possible to blind the participants or in vitro fertilisation doctors to the allocation. Participation in our trial did not change the normal in vitro routine." (p 2, Methods section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes assessed were not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are categories in each column of table 1 (p 3) where the denominators do not match the number of people in the group and no reason was given to explain why this would be or if this affects the study. |
| Selective reporting (reporting bias) | Low risk | Outcomes are the same as those registered with ClinicalTrials.gov. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
van Tol‐Geerdink 2013.
| Study characteristics | ||
| Methods | Randomized to decision aid + usual care vs usual care | |
| Participants | 163 + 77 patients with primary localized prostate cancer eligible for both radical prostatectomy and radiotherapy in the Netherlands | |
| Interventions | DA: paper‐based decision aid used after initial consultation and in preparation for decision‐making during second consultation that includes clinical information, probabilities of outcomes, implicit values clarification, and guidance in communication (space to write personal notes and questions for doctor). The DA is not publicly available; a copy was provided by the author (Julia J. van Tol‐Geerdink; Julia.vanTol‐Geerdink@radboudumc.nl). Comparator: usual care |
|
| Outcomes | Treatment preference, treatment received, decision regret, perceived participation | |
| Notes | Source of funding: Financial support for this study was provided by a grant (2007‐3809) from the Dutch Cancer Society, Amsterdam, the Netherlands. Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Enrolled patients were individually randomized to (i) the usual care group, which discussed the treatment choice with their specialist, or (ii) the decision aid group, which, in addition, had the decision aid presented by the researcher (JvTG). Randomization was imbalanced (1: 2) to have a large enough decision aid group to answer separate research questions, reported elsewhere.20 Randomization was centralized to avoid allocation bias and was blocked in groups of 3 per hospital, thus stratifying for hospital site." The investigators describe the use of stratification or permuted blocking (use of computer implied). |
| Allocation concealment (selection bias) | Low risk | Randomization was centralized to avoid allocation bias and was blocked in groups of 3 per hospital, thus stratifying for hospital site. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients and caregivers could not be blinded to the intervention." Unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, loss to follow‐up at t2 (pre‐treatment assessment) was 10/163 (6%) DA group and 7/77 control group(9%). Justification for attrition reported. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NTR1334) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Vandemheen 2009.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 70 + 79 patients with cystic fibrosis considering referral for lung transplantation in Canada | |
| Interventions | DA: self‐administered booklet with clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework). The DA is available at https://decisionaid.ohri.ca/decaids-archive.html . Comparator: blank pages |
|
| Outcomes | Primary outcomes: knowledge, accurate risk perceptions, decisional conflict Secondary outcomes: preparation for decision‐making, choice, durability of decision, undecided |
|
| Notes | Source of funding: Funded by The Ontario Thoracic Society, The Physicians’ Services Incorporated Foundation and The Australian Cystic Fibrosis Research Trust. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer‐generated random listing of two treatment allocations blocked in blocks of 2 or 4, stratified by site and infection status of Burkholderia cepacia" (p 2) |
| Allocation concealment (selection bias) | Low risk | Central allocation (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blinded RCT; patients and researchers were blinded but physicians were not because they were involved with patients before being randomized. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff, who were blinded to treatment allocation, telephoned each patient and had them complete a follow‐up questionnaire; other outcomes reported are objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline characteristics included (Flow diagram, p 2) |
| Selective reporting (reporting bias) | Low risk | Clinical trial registered with www.clinicaltrials.gov (NCT00345449) |
| Other bias | Low risk | Appears to be free of other potential biases. |
Varelas 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard consultation vs standard consultation alone | |
| Participants | 22 + 25 women aged > 18 years diagnosed with breast cancer (stage I or II only) and advised to undergo or had already undergone a mastectomy | |
| Interventions | DA: tablet‐based decision aid used in preparation for consultation that includes clinical information, and methods to clarify values. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: standard consultation |
|
| Outcomes | Primary outcomes: patient satisfaction using the Decisional Conflict Scale, knowledge Secondary outcomes: psychological status, surgeon satisfaction, time of consultation |
|
| Notes | Source of funding: none Conflicts of interest: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random number generator with a 1:1 ratio allocation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were blindly assigned to one of the two arms. The study’s surgical team were blinded to patient allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study’s surgical team were blinded to patient allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis but missing data are balanced across groups (data for 13/22 (59%) control group and 13/25 (52%) DA group (P = 0.625617)), justification for attrition provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Unclear risk | Small sample size (randomized 22 + 25); used parametric tests (student paired t‐test) to compare groups when only 13 + 13 included in the analysis. |
Vigod 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid vs control | |
| Participants | 48 + 48 women aged 18 years or older diagnosed with major depressive disorder who were planning a pregnancy or pregnant (less than 30 weeks gestation at enrolment) and for whom starting or continuing an antidepressant had been recommended as a treatment option for depression by their clinical provider in Canada | |
| Interventions | DA: web‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, guidance in decision‐making (systematic steps), guidance in communication, and printable automated summary of the information reviewed on risks and benefits, the participant’s rating of their relative importance, and the participant’s perception of external influences on their decision‐making process. The DA is not publicly available; temporary access was provided by the author (Simone N. Vigod; simone.vigod@wchospital.ca). Comparator: control (list of publicly available websites) |
|
| Outcomes | Primary outcome: feasibility, acceptability, adherence to trial protocol, DA acceptability Secondary outcomes: decisional conflict, depression, anxiety, knowledge |
|
| Notes | Source of funding: This pilot trial was funded by the Canadian Institutes for Health Research (CIHR). Conflicts of interest: DS is a Member of the Scientific Advisory Committee of the Duloxetine Pregnancy Registry. VT has done consulting work for Sunovion, Shire, NovoNordisk and Valeant. SG has received personal fees from Eli Lilly, personal fees from Psychotherapy to go, and personal fees from Compendium of pharmaceuticals over the last year, outside the submitted work. The other authors have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated by QoC using a random permuted block randomization. QoC provided a randomization identification number list with associated unique logins/passwords to sequentially assign to enrolled participants (within strata). |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was generated by QoC using a random permuted block randomization. QoC provided a randomization identification number list with associated unique logins/passwords to sequentially assign to enrolled participants (within strata). When a participant logged in to the study website, she would automatically be directed to her allocated condition. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not possible to blind participants, but they were not explicitly informed whether they were allocated to the intervention or control group. It is unclear how lack of blinding may have influenced outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were recorded by research staff who were blind to participant allocation until the final set of questions querying women's views of the PDA. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, low rate of attrition, loss to follow‐up similar between arms (10% control, 12% DA), no reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02308592) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | One or more of the authors are industry employees: DS is a Member of the Scientific Advisory Committee of the Duloxetine Pregnancy Registry. VT has done consulting work for Sunovion, Shire, NovoNordisk and Valeant. SG has received personal fees from Eli Lilly, personal fees from Psychotherapy to go, and personal fees from Compendium of pharmaceuticals over the last year, outside the submitted work. |
Vina 2016.
| Study characteristics | ||
| Methods | Randomized to video decision aid + motivational interviewing vs educational booklet | |
| Participants | 240 + 253 patients with moderate to severe knee OA, self‐identified as black, aged 50 years and older, with chronic and frequent knee pain, WOMAC score of 39 or greater, and x‐ray evidence of knee OA in the USA | |
| Interventions | DA: video decision aid that included information on the clinical condition, probabilities of outcomes of options, implicit values clarification, video clips of patient experiences, and guidance in communication, plus motivational interviewing. The DA is not publicly available; the authors have a copy of the video evaluated in previous studies (Bozic 2013; De Achaval 2012; Stacey 2014a). Comparator: educational booklet |
|
| Outcomes | Primary outcome: receipt of a referral to orthopedic surgery based on a patient’s self‐report at the 12‐month post‐intervention follow‐up Secondary outcome: change in patient preference/ willingness to undergo total knee replacement |
|
| Notes | Source of funding: Funding was received from the NIH/National Institute of Arthritis and Musculoskeletal Skin Diseases Grant# 1‐RO1‐AR‐054474‐5 (SI) and K24AR055259 (SI). Conflicts of interest: Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might post a conflict of interest in connection with the submitted article. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to one of the two study arms using a computer‐generated random assignment. The computer‐generated randomization result was sent to the study coordinator via email before the scheduled intervention session" |
| Allocation concealment (selection bias) | Low risk | "The computer‐generated randomization result was sent to the study coordinator via email before the scheduled intervention session" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Owing to the nature of the intervention, participants could not have been blinded to the study arm they were assigned to. Primary care providers were blinded from the study arm participants were assigned to." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of outcomes were blinded to which study arm the patients were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in the analysis. Attrition similar between arms (2 vs 1) and reasons for withdrawals recorded. Missing data for some outcomes, reported in limitations (there were patients with missing willingness data at different times). |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available (NCT02413411) and the secondary outcome in the trial registry is actual receipt of knee replacement, whereas the manuscript states the secondary outcome is change in patient willingness to undergo knee replacement surgery. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Vodermaier 2009.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 74 + 78 women with breast cancer considering treatment options in Germany | |
| Interventions | DA: decision board administered by research psychologists and booklet on options' outcomes, clinical problem, outcome probability. The DA is not publicly available; a copy was provided by the author (in German). Comparator: booklet on clinical problem |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: choice, length of consultation, satisfaction with decision‐making, participation in decision‐making |
|
| Notes | Source of funding: This work was supported by the German Ministry of Health as a pre‐operating study in the focus programme ‘The Patient as a Partner in the Medical Decision Making Process’ under Grant no. 217‐43794‐5/2 (Professor Dr Michael Untch, PI) and by a stipend from the Dr‐Werner‐Jackstaedt‐Stiftung in the Founder Association of the German Sciences under Grant no. S134‐10.021 (Dr Andrea Vodermaier). Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation after the patient gave written informed consent" "Random assignment was performed by means of numbered cards in envelopes", "stratified by age group" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[N]umbered cards in envelopes" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, p 5; baseline characteristics not included |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Volk 1999.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 80 + 80 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog videotape and brochure on options' outcomes, clinical problem, outcome probability, others' opinion. The DA was available from Informed Medical Decisions Foundation during the study but is no longer available. Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, preferred/uptake of option | |
| Notes | Source of funding: This project was supported by grants from the American Academy of Family Physicians Foundation and the American Academy of Family Physicians, Kansas City, Mo, and grant D32‐PE10158‐01 from the Bureau of Health Professions, Health Resources and Services Administration, Rockville, Md. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volk 1999 (primary study), p 3: "[r]andomization by permuted blocks", "Each block included the numbers 1 through 4" Volk 2003 , p 2, Methods: Randomization by permuted blocks was used to balance the number of subjects in each arm of the study. |
| Allocation concealment (selection bias) | Unclear risk | Volk 1999 (primary study): no information provided Volk 2003, p 2: "[d]etails of the study procedures, subjects, and 2‐week follow‐up results can be found elsewhere" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded to the treatment assignment, but the physicians were; therefore, outcomes were unlikely to be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were not blinded, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Volk 1999 (primary study), p 2, Procedures: baseline values included Volk 2003, p 4 Fig 1 ‐ flow diagram; baseline data not included |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Volk 1999 (primary study): appears to be free of other potential biases. Volk 2003: appears to be free of other sources of bias. |
Volk 2020.
| Study characteristics | ||
| Methods | Randomized to decision aid vs standard education | |
| Participants | 259 + 257 tobacco quit line clients (ages 55 to 77 years) who reported a 30‐plus pack‐year smoking history in the USA | |
| Interventions | DA: video decision aid used in preparation for consultation that includes clinical information, outcome probabilities, and implicit values clarification. The DA is available at https://www.youtube.com/watch?v=wir3w1eUAJk&ab_channel=MDAndersonCancerCenter . Comparator: standard education |
|
| Outcomes | Primary outcomes: preparation for decision‐making, decisional conflict Secondary outcomes: knowledge, intentions to be screened, screening behaviors, if participants had a visit with a clinician to discuss screening, underwent low‐dose computed tomography, DA acceptability |
|
| Notes | Source of funding: This study was supported by award CER‐1306‐03385 from the Patient‐Centered Outcomes Research Institute; award P30CA016672 from the National Institutes of Health, National Cancer Institute (Drs Volk, Cantor, and Lin) that used the Biostatistics Resource Group, Clinical Protocol and Data Management, and Shared Decision Making Core, and a grant from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment (Drs Volk and Cantor) that supported the Shared Decision Making Collaborative and Center for Community‐Engaged Translational Research. Conflicts of interest: Dr Volk reported receiving research support from the Patient‐Centered Outcomes Research Institute (PCORI) and receiving grants from the National Institutes of Health and The University of Texas MD Anderson Cancer Center during the conduct of the study. Dr Lowenstein reported receiving grants from PCORI, The University of Texas MD Anderson Cancer Center Duncan Family Institute, and the National Institutes of Health during the conduct of the study. Ms Leal reported receiving grants from PCORI, the National Cancer Institute, and The University of Texas MD Anderson Cancer Center Duncan Family Institute during the conduct of the study. Dr Munden reported receiving stock options from Optellum Ltd and preferred stock from TheraBionic outside the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clients within each state quit line were randomized to receive the PDA or standard educational material (EDU) using S‐plus, version 8.04 (TIBCO Software Inc) statistical software to generate a randomization schedule with various block sizes. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to intervention allocation. It is unclear how lack of blinding may have influenced outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study interviewers were blinded to participant allocation at the 3‐ and 6‐month assessments, but not the 1‐week follow‐up, because questions about the PDA were asked of participants in this group. However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram. Low attrition for outcomes of interest (knowledge, decisional conflict, preparation for DM) collected at 1‐week follow‐up (completers: 235/259 (91%) DA and 233/257 control (91%)). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02286713) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Vuorma 2003.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 184 + 179 women considering treatment for menorrhagia in Finland | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: usual care |
|
| Outcomes | Primary outcomes: uptake of option Secondary outcomes: knowledge, proportion remaining undecided, anxiety, satisfaction, health outcomes, use and cost of healthcare services |
|
| Notes | Source of funding: This study was supported by STAKES, the National Research and Development Centre for Welfare and Health, and Doctoral Programmes of Public Health of Helsinki and Tampere Universities. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Vuorma 2003 (primary study), p 2, Randomization: computer‐generated; done by a researcher who did not participate in the planning or concealment procedures "[D]one in STAKES, by researcher separately for each hospital in computer‐generated varying clusters"(p 2) Vuorma 2004: no information provided |
| Allocation concealment (selection bias) | Low risk | Vuorma 2003 (primary study), p 2 "sequentially numbered, opaque and sealed envelopes" Vuorma 2004, p 2 "sequentially numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, unclear if measurements could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff were not blinded, but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vuorma 2003 (primary study): flow chart balanced. Reasons for non‐eligibility. "One women on HRT was randomized by mistake and included in analyses." Baseline characteristics included and balanced across groups (p 4‐5) Vuorma 2004, flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | Vuorma 2003 (primary study): no mention of study protocol Vuorma 2004: no information provided |
| Other bias | Low risk | Vuorma 2003 (primary study), p 7: "increase in knowledge in both study groups, carry‐over effect; change in decision‐making process of intervention group may have altered physician's negotiation with patients" appears to be free of other potential biases. Vuorma 2004, p 5: "comparison of the baseline characteristics presented elsewhere". In the pre‐trial group compared with the control group, there was a greater increase in the dimensions of physical role functioning and emotional role functioning of the RAND‐36. |
Wallace 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 15 + 6 patients aged 18 and older eligible for consideration of a primary prevention implantable cardioverter‐defibrillator in the USA | |
| Interventions | DA: Toolkit containing 4 decision aids used in preparation for consultation comprised of (1) a one‐page Option Grid conversation aid, (2) a more in‐depth paper pamphlet, (3) a video, and (4) an interactive website that included clinical information, outcome probabilities, explicit values clarification, patient narratives, frequently asked questions, guidance in decision‐making (step‐by‐step process), and guidance in communication. The DA is publicly available at https://patientdecisionaid.org/ Comparator: usual care (pamphlets or communication normally given by the treatment facility) |
|
| Outcomes | Acceptability of the decision aid, feasibility, knowledge, decision quality (values concordance choice), choice, decision conflict, decision regret, participation | |
| Notes | Source of funding: Financial support for this study was provided entirely by the Patient Centered Outcomes Research Institute (IP2 PI000116) and the National Institutes of Health (K23AG040696). Bryan Wallace is supported National Institutes of Health: National Heart, Lung, and Blood Institute (R01HL136403). Dr. Knoepke is supported National Institutes of Health: National Heart, Lung, and Blood Institute (1K23HL153892) and the American Heart Association (18CDA34110026). Conflicts of interest: Dr. Allen receives grant funding from American Heart Association, National Institutes of Health, and the Patient Centered Outcomes Research group; and consulting fees from ACI Clinical, Amgen/ Cytokinetics, Boston Scientific, and Novartis. Glyn Elwyn has edited and published books that provide royalties: Shared Decision Making (Oxford University Press) and Groups (Radcliffe Press). Glyn Elwyn’s academic interests are focused on shared decision making and coproduction. He owns copyright in measures of shared decision making (collaboRATE) and care integration (integRATE), a measure of experience of care in serious illness (consideRATE), a measure of goal setting coopeRATE, a measure of clinician willingness to do shared decision making (incorpoRATE), an observer measures of shared decision making (Observer OPTION‐5 and Observer OPTION‐12). He is the Founder and Director of &think LLC which owns the registered trademark for Option Grids™ patient decision aids; Founder, Director of SHARPNETWORK LLC, a provider of online training for shared decision making, consultant to EBSCO Health, Bind On‐Demand Health Insurance, and Chief Clinical Research Scientist to abridge AI Inc. The authors declare no conflict of interest. The PCORI and NIH had no role in the design or development of the tools, the methods by which they were created, the analyses conducted, or the decision to publish findings. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned 2:1 to an intervention or control group with the goal of recruiting 60 patients |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram and text of the manuscript are inconsistent. Text of manuscript describes 21 participants with 15 randomized to intervention and flow diagram shows 9 randomized to intervention and 6 to control. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT02026102) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Small sample size (15 intervention + 6 control). Goal was to recruit 60 participants. |
Wang 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care (standard education) | |
| Participants | 98 + 98 pregnant women who came for routine checkups 1 month before delivery in Taiwan | |
| Interventions | DA: paper‐based decision aid used in consultation with the nurse that includes clinical information, explicit values clarification, guidance in decision making, and guidance in communication. The DA is available as a supplementary appendix in the article. Comparator: usual care (standard education) |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcome: decisional regret |
|
| Notes | Source of funding: none Conflicts of interest: The authors reported no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pregnant women were randomly divided into classic or SDM groups through computer‐generated assignment by an outpatient clinic nurse. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐masking (participant, outcomes assessor) according to trial registry |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Before the mothers were discharged after delivery, the influence of SDM was investigated by a nurse who was blinded to the participants’ group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% included in analysis but missing data are balanced across groups (23% loss to follow‐up in both arms), justification provided for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03528655) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Watson 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 475 + 522 men considering prostate cancer screening in the UK | |
| Interventions | DA: leaflet on options' outcomes, clinical problem, outcome probability. The DA is presented in Appendix A within the article. Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, screening intention, attitudes Secondary outcomes: preferred role in decision‐making |
|
| Notes | Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andom numbers generated centrally by Stata v8.2" (p 3) |
| Allocation concealment (selection bias) | Low risk | "[R]andom numbers generated centrally by Stata v8.2" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 2); reason for exclusion from analysis mentioned. Sample characteristics of risk included. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | "Adjustment for multiple testing was not accounted for and hence a degree of caution with interpretation is required, particularly in relation to findings with a P‐value close to 0.05" (p 3) |
Watts 2015.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 63 + 65 male and female veterans diagnosed with post‐traumatic stress disorder and seeking referral for treatment in the USA | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, and implicit values clarification. The DA is not publicly available; a copy was provided by the author (Bradley V. Watts; bradley.v.watts@dartmouth.edu) Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, and ability to indicate a treatment preference Secondary outcomes: satisfaction with care, symptom severity, participant functioning and quality of life |
|
| Notes | Source of funding: This work was supported by U.S. Department of Veterans Affairs (VA) Health Services Research and Development grant 07‐266‐1. The views expressed in this article do not necessarily represent the views of the VA or of the United States government. Conflicts of interest: The authors report no financial relationships with commercial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Randomization was accomplished through selection of an identical sealed envelope, which contained information about the random assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported for patients. Double‐masking (investigator, outcomes assessor) according to trial registry. Clinic providers were blinded regarding the participants’ involvement in the study. Unclear how lack of blinding of participants influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After the standard mental health evaluation, participants in both arms were seen by a research assistant who administered several assessments... The research assistant was blinded to the participants’ treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram (supplementary material), > 90% of participants included in analysis, high rate of attrition but loss to follow‐up similar across groups (DA group 16/66 (24%) and in control 13/66 (20%) (P = 0.528267)) |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT00908440) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Weymiller 2007.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 51 + 46 patients with type 2 diabetes in the USA | |
| Interventions | DA (in consultation): 1‐page decision aid options' outcomes, clinical problem, tailored outcome probability, guidance/coaching. An example DA is presented in Figure 1 of the article. Comparator: booklet on cholesterol management |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: consultation length, acceptability of the intervention, adherence, estimated personal risk, trust, patient participation (OPTION), choice |
|
| Notes | Source of funding: This study was supported by the Mayo Clinic Section of Patient Education and the American Diabetes Association. Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence (p 2) Nannenga 2009: no information provided |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation sequence, unavailable to personnel enrolling patients. "[W]ith concealed allocation" (Abstract); "maintained allocation concealment" (p 5); randomized by concealed central allocation (Nannenga 2009, p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded to the study objectives, providers and patients were naive to this study objective. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysts and statisticians blinded to allocation; intervention and outcomes; adequate blinding wherever possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3); reasons for attrition mentioned (p 4); baseline characteristics included; flow diagram Nannenga 2009, p 3: reasons for attrition mentioned and study groups balanced; baseline characteristics included |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov identifier: NCT00217061 |
| Other bias | Low risk | Enrollment of patients already receiving statin therapy and limited statin uptake decreased the precision of our results; results should best be interpreted as preliminary and requiring verification. Nannenga 2009: appears to be free of other potential biases. Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Whelan 2003.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 82 + 93 women with node negative breast cancer considering adjuvant chemotherapy in Canada | |
| Interventions | DA: decision board and booklet on options' outcomes, clinical problem, outcome probability, guidance/coaching. The DA is presented in Figure 1 of the article Comparator: booklet on clinical problem |
|
| Outcomes | Primary outcomes: knowledge, satisfaction of participant Secondary outcomes: preferred option, anxiety, accurate risk perceptions, participation in decision‐making |
|
| Notes | Source of funding: Supported by a grant from the Canadian Breast Cancer Research Initiative. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Randomization, which was performed at a central location (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Unable to blind participants in our trial for practical reasons, measures were taken to minimize bias in the design of the study and the assessment of outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram not included. "[O]ne patient excluded from analysis, determined by physician not to be candidate for chemotherapy" (p 4). Baseline data/characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if lack of blinding contributed to a potential risk of bias. |
| Other bias | Low risk | Appears to be free of other potential biases. |
Whelan 2004.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 94 + 107 women with Stage 1 or 2 breast cancer considering surgery (cluster‐RCT with 27 surgeons randomized) in Canada | |
| Interventions | DA: decision board on options' outcomes, outcome probability, guidance/coaching. The DA is presented in Figure 1 of the development article (Whelan 1999). Comparator: usual care |
|
| Outcomes | Primary outcomes: preferred option, knowledge, decisional conflict, satisfaction Secondary outcomes: accurate risk perceptions, anxiety |
|
| Notes | Source of funding: Dr Whelan is a Canada Research Chair funded by Health Canada. The Canadian Breast Cancer Research Initiative and the Ontario Ministry of Health and Long‐Term Care, Health System‐Linked Research Programme provided funding support for the study. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not specify how the sequence was generated; a paired cluster‐randomization process was used (p 2, Study design and procedures). |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned in a concealed fashion, but the method of concealment was not stated (p 2, Study design and procedures). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[C]hose cluster randomization method to avoid contamination that might have occurred if surgeons used decision board for some patients and not others" (p 6); unclear if this would introduce bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics not included; reasons given for loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Wilkens 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid plus usual care (information) vs information only | |
| Participants | 45 + 45 patients older than 18 years, seeking care for trapeziometacarpal arthritis in the USA | |
| Interventions | DA: Interactive online decision aid that includes information on the clinical problem, outcome probabilities, explicit values clarification, guidance in decision‐making (step‐by‐step process for making the decision), guidance in communication, and summary that can be taken to the consultation. The DA is publicly available at https://www.decisionaid.info/pp/thumboa/intro . Comparator: usual information provided during consultation plus informational brochure |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: disability, pain intensity, depression, treatment choice, satisfaction with the visit, consultation duration, perception of physician's empathy, decision regret, treatment satisfaction, change in treatment choice, change of surgeon |
|
| Notes | Source of funding: not reported Conflicts of interest: D.R. has received support from Wright Medical (Memphis, TN), Skeletal Dynamics (Miami, FL), Biomet (Warsaw, IN), AO North America (Paoli, PA), and AO International (Dubendorf, Switzerland). T.T. has received support from AO Trauma (Dubendorf, Switzerland), Stryker (Kalamzoo, MI), DePuy Synthes (West Chester, PA), PATIENTþ (Den Haag, The Netherlands), and VCC (Zaltbommel, The Netherlands). N.C.C. has received support from Miami Device Solutions (Miami, FL) and Depuy Synthes (Paoli, PA). The rest of the authors declare that they have no relevant conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation was based on a computer‐generated sequence of random numbers and only accessible by the independent research assistant (S.C.W.) who was present in the room when patients were going through the decision aid" |
| Allocation concealment (selection bias) | Low risk | "The allocation was based on a computer‐generated sequence of random numbers and only accessible by the independent research assistant (S.C.W.) who was present in the room when patients were going through the decision aid" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "We considered this study unblinded for all parties because blinding of the surgeon could not be guaranteed." Unclear how physicians may have influenced decisions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "We considered this study unblinded for all parties because blinding of the surgeon could not be guaranteed." However, outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: follow‐up rate of 89%. "We planned an intention‐to‐treat analysis, but everyone was managed as assigned. Multiple imputation was used to complete missing data (number of imputations set to 40) for the 7 patients (8%) with no 6‐week or 6‐month measures of pain intensity, satisfaction, and decision regret (Fig. 1). We assumed the missing data to be at random. Medical records were reviewed for change in treatment and surgeon; we assumed missing patients had not changed treatment/ surgeon when no follow‐up was noted in the medical record." |
| Selective reporting (reporting bias) | Unclear risk | No protocol/registration identified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Williams 2013.
| Study characteristics | ||
| Methods | Randomized to decision aid at home or in clinic versus usual care at home or in clinic | |
| Participants | 134 + 138 + 134 +137 men aged 40 to 70 years with no history of prostate cancer who had pre‐registered for screening in the USA | |
| Interventions | DA: content adapted from the Centers for Disease Control and Prevention's PCS educational tool. Includes clinical problem, treatment options, outcome probabilities, explicit values clarification, others' stories, summary worksheet. The DA is not publicly available; a copy was provided by the author (taylorkl@georgetown.edu). Comparator: information booklet. A 3‐page fact sheet requiring 5 minutes to read. Information presented in a Q&A format on who is recommended for testing, how to interpret results, and the limitations of testing. |
|
| Outcomes | Knowledge, decisional conflict, screening outcomes, satisfaction with decision Outcomes assessed at baseline, 2 months, 13 months, except satisfaction with decision (2 months and 13 months) |
|
| Notes | No primary outcome reported; trial registration not provided Source of funding: This work was supported by grant R01 CA98967‐01 from the National Cancer Institute, Bethesda, MD, USA. Conflicts of interest: None of the authors have any conflicts of interest or financial disclosures to report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to judge random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to judge blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any outcome data missing. |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol |
| Other bias | Low risk | Appears to be free of other potential biases. |
Wise 2019.
| Study characteristics | ||
| Methods | Randomized to decision aid + standard care vs standard care alone | |
| Participants | 148 + 149 women with one previous cesarean and singleton pregnancy < 25 weeks in New Zealand and the USA | |
| Interventions | DA: paper‐based decision aid used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, guidance in decision‐making, guidance in communication, and summary (values clarification activity) to share with doctor/midwife. The DA is not publicly available; a copy was provided by the author (Dr Michelle R. Wise; m.wise@auckland.ac.nz). Comparator: usual care (education) |
|
| Outcomes | Primary outcome: attempted vaginal birth after cesarean, also called trial of labor after cesarean, measured at the time of onset of labor Secondary outcomes: adherence to birth preference, actual mode of birth, knowledge, decisional conflict, birth mode preference, satisfaction with birth experience |
|
| Notes | Source of funding: A+ Trust, Auckland District Health Board (A+4946) Conflicts of interest: The authors report no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was a randomized trial using 1:1 allocation. Randomly assigned following simple randomization procedures (computerized random numbers). |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes containing the group allocation were sequentially used. The envelopes were prepared in advance and kept in a locked cabinet in the clinic. The allocation sequence was concealed from the researchers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were blinded to group allocation. The original intention and study protocol were to allocate either the DA or the educational pamphlet. However, PBAC clinicians argued that to provide usual care of the PBAC clinic they needed a brief encounter tool with which to guide the consultation. Moreover, they felt if women brought the DA booklet with them into the consultation rather than the pamphlet, then clinicians would not be blinded, and that it may bias the consultation. Therefore, the clinical team made the decision for all participants to receive the pamphlet. Clinicians did not report on whether women brought the DA with them to consultations. Researchers were blinded to group allocation until data analysis was complete. Not reported for participants. Unclear how lack of blinding of participants influenced the study results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded to group allocation until data analysis was complete. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flow diagram. Loss to follow‐up at Q2 when outcomes of interest were measured is significantly higher for the control group (8/148 DA and 18/149 usual care (P = 0.041838)). Justification for non‐inclusion and loss to follow‐up provided. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12611000878976) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wolf 1996.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 102 men considering PSA testing in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probability, others' opinions. The DA script is presented in Table 1 of the article. Comparator: usual care (single sentence) |
|
| Outcomes | Preferred option | |
| Notes | Source of funding: This study was supported in part by grant IRG‐72256 from the American Cancer Society, Atlanta, Ga. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Wolf 1996 (primary study): no information provided Wolf 1998, p 2: "the methodology of the randomized trial has been reported previously" |
| Allocation concealment (selection bias) | Unclear risk | Wolf 1996 (primary study): no information provided Wolf 1998, p 2: "The methodology of the randomized trial has been reported previously" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Wolf 1996 (primary study), p 2: needed a minimum sample size of 150 participants, and was achieved with a total sample size of 205. Reasons for attrition mentioned; baseline characteristics included Wolf 1998: no information provided except that the methodology of the randomized trial and the content of the informational intervention reported previously (p 2). Baseline characteristics included; flow of participants not included. |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry. |
| Other bias | Low risk | Wolf 1996 (primary study): participant population had lower SES, therefore external validity of the findings is limited, but overall it appears to be free of other potential biases. Wolf 1998: appears to be free of other potential biases. |
Wolf 2000.
| Study characteristics | ||
| Methods | Randomized to decision aid vs usual care | |
| Participants | 266 + 133 elderly (≥ 65 years) considering CRC screening in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probabilities. The DA script is presented in the Appendix within the article. Comparator: usual care (5 sentences) |
|
| Outcomes | Primary outcome: preferred option Secondary outcomes: accurate risk perceptions |
|
| Notes | Source of funding: Dr. Wolf is the recipient of an American Cancer Society Cancer Control Career Development Award for Primary Care Physicians. Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]atients were randomised" (p 2); does not indicate how |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline data not included (p 2, Results) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other potential biases. |
Wong 2006.
| Study characteristics | ||
| Methods | Randomized to decision aid vs placebo control leaflet | |
| Participants | 162 + 164 women referred for pregnancy termination in the UK | |
| Interventions | DA: decision aid leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification. The DA is not publicly available and we were unable to obtain a copy from the authors. Comparator: placebo leaflet on contraception use post pregnancy termination |
|
| Outcomes | Primary outcomes: uptake of option, knowledge, decisional conflict, anxiety | |
| Notes | Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1 ratio, balanced block of 10"; "envelope preparation by drawing slips of paper labelled either control or intervention"; "the slip determined leaflet placed into envelope" (p 2) |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, opaque trial envelopes (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics not included (p 3); reasons for attrition and incompletion mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases. |
Wyld 2021.
| Study characteristics | ||
| Methods | Cluster‐randomized to decision support intervention vs usual care | |
| Participants | 670 + 669 women aged 70 years or above at diagnosis with primary operable invasive breast cancer from 27 + 67 breast units in England and Wales | |
| Interventions | DA: online algorithm used by healthcare professional during consult to generate personalized survival outcomes followed by access to booklet decision aids (print, PDF) that include clinical information, outcome probabilities, explicit values clarification, guidance in decision‐making (step‐by‐step process), and guidance in communication. The DA is publicly available at https://agegap.shef.ac.uk/ . Comparator: usual care |
|
| Outcomes | Primary outcome: quality of life Secondary outcomes: breast cancer‐specific quality of life, treatment choices, knowledge, shared decision‐making, decision regret, anxiety, illness perception, coping strategies, breast cancer‐specific survival |
|
| Notes | Source of funding: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference number RP‐PG‐1209‐10071). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health. Conflicts of interest: The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centers (breast units) were subjected to 1: 1 block randomization, stratified by high or low current primary endocrine therapy and chemotherapy rates. Centers were randomized either to have access to the decision support interventions and training in their use, or to continue with usual care (use of computer implied) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, < 90% of participants included in analysis (excluded 311/670 (46%) DA and 288/669 (43%) usual care), however missing data are balanced across groups (P = 0.215147), justification for exclusion provided |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ISRCTN46099296) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | The low response rate to some of the questionnaires relating to decision quality metrics may also be a source of bias, with women potentially selectively agreeing to complete these if they had either a particularly positive or negative experience. Free of other potential biases: adjustment for clustering performed/no evidence of selective recruitment of cluster participants. |
Ye 2021.
| Study characteristics | ||
| Methods | Randomized to decision aid vs information | |
| Participants | 386 + 387 adults aged 50 to 80 with definite diagnosis of age‐related cataract in China | |
| Interventions | DA: paper‐based DA used in preparation for consultation that includes clinical information, outcome probabilities, explicit values clarification, and guidance in decision‐making and communication. The DA is not publicly available; a copy was provided by the authors (Yingfeng Zheng; zhyfeng@mail.sysu.edu.cn). Comparator: usual booklet developed by the National Eye Institute, available at https://www.nei.nih.gov/sites/default/files/health-pdfs/WYSK_Cataract_English_Sept2015_PRINT.pdf . |
|
| Outcomes | Primary outcome: informed choice (knowledge and attitude congruent with expressed intention) Secondary outcomes: decisional conflict, confidence in decision‐making, anxiety, worry, time perspective, anticipated regret, importance and personal chances of surgical outcomes, acceptance and usefulness of DA |
|
| Notes | Source of funding: This study was funded by the Construction Project of High‐Level Hospitals in Guangdong Province (303020107; 303010303058); National Natural Science Foundation of China (81530028; 81721003); Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201001); Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program; the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat‐sen University. Prof. Congdon is supported by the Ulverscroft Foundation (UK). Conflicts of interest: Prof. Liu reported receiving grants from the National Natural Science Foundation of China. Prof. Zheng has served on digital advisory board for Novartis. Prof. Congdon is Director of Research for Orbis International, a non‐governmental organization which carries out children’s eye health work in China. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization sequence was generated by a statistician using an online random number generator (randomization.com). |
| Allocation concealment (selection bias) | Low risk | The randomization sequence was generated by a statistician using an online random number generator (randomization.com). The statistician had no contact with participants before enrolment. An independent coworker not involved in this study randomly assigned participants to one of two study groups in a 1:1 ratio with permuted block sizes of four and eight. The coworker put each booklet into a sequentially numbered, opaque, sealed folder using an allocation sequence provided by the statistician. Interviewers’ performance was regularly monitored throughout the survey by study investigators during on‐site visits, ensuring they read questions in a structured script. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking of participants was accomplished by printing both booklets with identical cover designs and titles. Study investigators responsible for recruitment and the interviewers were unaware of study allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking of participants was accomplished by printing both booklets with identical cover designs and titles. Study investigators responsible for recruitment and the interviewers were unaware of study allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, ITT, < 90% included in ITT analysis but balanced across groups (50% in both arms), justifications for attrition provided |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (NCT03992807) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Zadro 2022.
| Study characteristics | ||
| Methods | Randomized to decision aid (side‐by‐side display) vs DA (top‐and‐bottom display) vs education material | |
| Participants | 211 + 214 people with shoulder pain considering surgery to treat their shoulder pain in Australia | |
| Interventions | DA: online printable PDF used in preparation for consultation that includes outcome probabilities, implicit values clarification, and guidance in communication. The DA is available as a supplementary appendix in the article. Comparator: education (including clinical information and options) |
|
| Outcomes | Primary outcome: treatment intention Secondary outcomes: knowledge, attitude towards surgery, informed choice, decisional conflict |
|
| Notes | Source of funding: This study was funded from JZs National Health and Medical Research Council (NHMRC) Investigator Grant (APP1194105). Conflicts of interest: KM, RT, and TH are members of the International Patient Decision Aids Standard (IPDAS) Collaboration Steering Committee. RT receives personal royalties from the sale of a book on shared decision‐making. All other authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized through the online survey platform Qualtrics (1:1 ratio; concealed to investigators). |
| Allocation concealment (selection bias) | Low risk | Participants were randomized through the online survey platform Qualtrics (1:1 ratio; concealed to investigators). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, > 90% of participants included in analysis and justification provided for attrition |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ACTRN12621000992808) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
CHD : coronary heart disease; CRC : colorectal cancer; DA : decision aid; DM : decision‐making; FAQ : frequently asked questions; GP : general practitioner; HPV : human papillomavirus; HRT : hormone replacement therapy; ICD : implantable cardioverter‐defibrillator; ITT : intention‐to‐treat; MMR : measles, mumps and rubella; NSW : New South Wales; OA : osteoarthritis; PDA : patient decision aid; PSA : prostate‐specific antigen; PTSD : post‐traumatic stress disorder; RCT : randomized controlled trial; SES : socioeconomic status; SDM : shared decision‐making; SURE : Sure of myself; Understand information; Risk‐benefit ratio; Encouragement
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadie 2009 | Study did not evaluate the decision aid (evaluated clinician use of the decision aid in one arm of a study only) |
| Abhyankar 2011 | Hypothetical choice |
| Adab 2003 | Hypothetical choice, not at a point of decision‐making |
| Adam 2018 | Two decision aids compared |
| Adekpedjou 2020 | Not a decision aid; not a treatment or screening decision |
| Akbari 2020 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Al Saffar 2008 | Study not focused on making a choice; adhering to medications only |
| Alegría 2014 | Not a patient decision aid |
| Ali 2020 | Not a patient decision aid |
| Allen 2016 | Two decision aids compared |
| Allen 2022 | Not a randomized controlled trial |
| Almario 2022 | Not a patient decision aid (no description of benefits of options) |
| AlSagheir 2020 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Altiner 2007 | Not a patient decision aid |
| Anderson 2011 | Not a randomized controlled trial |
| Arimori 2006 | Not a patient decision aid (not including benefits and harms) |
| Armstrong 2005 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Arterburn 2013 | Not evaluating a patient decision aid |
| Au 2011 | Not a randomized controlled trial |
| Bakken 2014 | Not a patient decision aid; related to lifestyle choices |
| Becker 2009 | Hypothetical choice; not at the point of decision‐making |
| Belkora 2012 | Not a patient decision aid |
| Bellmunt 2010 | Not a patient decision aid |
| Bennett 2011 | Compares 3 versions of the same patient decision aid |
| Betz 2020 | Not a treatment or screening decision |
| Betz 2021 | Not a treatment or screening decision |
| Bhattacharya 2021 | Two decision aids compared; about clinical trial entry |
| Bieber 2006 | Study did not evaluate the patient decision aid (evaluated shared decision‐making process); not a patient decision aid |
| Bombard 2020 | Hypothetical choice |
| Boulware 2013 | Not a patient decision aid (information about one choice only, no values clarification) |
| Boulware 2018 | Study does not report outcomes of interest to this review |
| Branda 2013 | Two patient decision aids with findings aggregated |
| Brenner 2014 | Not a patient decision aid |
| Breslin 2008 | Not a randomized controlled trial |
| Brown 2004 | Not focused on making a choice (no specific decision to be made) |
| Brundage 2001 | Not a randomized controlled trial |
| Brunette 2020 | Not a decision aid; not a treatment or screening decision |
| Buhse 2015 | Not a patient decision aid |
| Buhse 2018 | Not a patient decision aid |
| Burton 2007 | Not a patient decision aid (general patient education only) |
| Buzhardt 2011 | Not evaluating patient decision‐making |
| Campbell 2014 | Not evaluating a patient decision aid |
| Carling 2008 | Hypothetical choice, not at point of decision‐making |
| Carlson 2021 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Carter‐Harris 2020 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Causarano 2015 | Not a patient decision aid |
| Chadwick 1991 | Not a randomized controlled trial |
| Chan 2011 | Not a patient decision aid |
| Chewning 1999 | Not a randomized controlled trial |
| Chiew 2008 | Not a randomized controlled trial |
| Chong 2020 | Not a patient decision aid (no specific decision) |
| Christy 2022 | Not a treatment or screening decision; about clinical trial entry |
| Clark 2022 | Two decision aids compared |
| Clouston 2014 | Not a patient decision aid |
| Col 2007 | Unable to ascertain characteristics of the patient decision aid. Additional information requested from author but not provided (e.g. values clarification). |
| Colella 2004 | Not a patient decision aid (describes model of care) |
| Coronado‐Vazquez 2019 | Not a patient decision aid |
| Costanza 2011 | Not a randomized controlled trial |
| Coulter 2003 | Not a randomized controlled trial (editorial) |
| Cox 2012 | Not a randomized controlled trial |
| Crang‐Svalenius 1996 | Not a randomized controlled trial |
| Davies 2021 | Not a patient decision aid |
| Davis 2014 | Two decision aids compared |
| Davison 1999 | Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid |
| Davison 2007 | Not a patient decision aid |
| De Boer 2012 | Not a randomized controlled trial |
| De Haan 2013 | Not a randomized controlled trial of a patient decision aid |
| Deen 2012 | Not a patient decision aid |
| Dehlendorf 2019 | Not a patient decision aid (no discussion of options, benefits, harms) |
| Deinzer 2009 | Not a patient decision aid |
| Den Ouden 2017 | Study does not report outcomes of interest to this review |
| Denig 2014 | Not a patient decision aid |
| Deschamps 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Deyo 2000 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Diefenbach 2012 | Not a patient decision aid |
| Diefenbach 2018 | Two decision aids compared |
| Dobke 2008 | Not focused on making a choice |
| Dodin 2001 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Doll 2019 | Two decision aids compared |
| Donovan 2012 | Does not report results of the randomized controlled trial; descriptive article offering techniques of provision of information |
| Driscoll 2008 | Not a patient decision aid |
| Dunn 1998 | Quasi‐RCT: randomization was by days of the week |
| Eaton 2011 | Not a decision aid (no decision support) |
| Eden 2009 | Hypothetical choice, not at point of decision‐making |
| Eden 2014 | The educational brochure (control group) provided information about the options, benefits, and harms, making it a simple patient decision aid |
| Eden 2015 | Not a treatment or screening decision |
| Edwards 2012 | Hypothetical choice, not a randomized controlled trial |
| El Miedany 2019 | Pediatric population |
| El‐Jawahri 2010 | End of life decision |
| Ellison 2008 | Not a randomized controlled trial (quasi‐experimental design); unclear whether at point of decision‐making |
| Elwyn 2004 | No difference in intervention between arms; risk communication did not have values clarification |
| Elwyn 2016 | Not a randomized controlled trial |
| Emery 2007 | Not a patient decision aid |
| Emmett 2007 | Not a randomized controlled trial |
| Eneanya 2020 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Fadda 2017 | Not a patient decision aid |
| Fagerlin 2021 | Two decision aids compared |
| Fang 2021 | Two decision aids compared |
| Feldman‐Stewart 2006 | Hypothetical choice, not at point of decision‐making |
| Feldman‐Stewart 2012 | Same patient decision aid with vs without values clarification |
| Fiks 2013a | Not patient decision‐making (uptake of vaccine) |
| Fiks 2015 | Not a patient decision aid |
| Fleisher 2015 | Study does not report outcomes of interest to this review |
| Flood 1996 | Non‐randomized allocation; wait list control |
| Francis 2009 | Not a patient decision aid |
| Fraval 2015 | Not a patient decision aid; general education material to obtain informed consent for surgery |
| Frosch 2001 | Not a randomized controlled trial |
| Frosch 2003 | Same decision aid delivered on the Internet versus on DVD plus booklet |
| Frosch 2008b | Not a randomized controlled trial |
| Frosch 2011 | Not a patient decision aid |
| Frost 2009 | Qualitative study for an included RCT |
| Fujiwara 2015 | Not a patient decision aid and aims to increase screening rates |
| Garvelink 2013 | Hypothetical decision |
| Garvelink 2017 | Two decision aids compared |
| Genz 2012 | Not a patient decision aid |
| Genz 2014 | Not a patient decision aid |
| George 2021 | Not a patient decision aid |
| Giordano 2014 | Not a patient decision aid |
| Goel 2001 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Gong 2017 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Gorawara‐Bhat 2017 | Study does not report outcomes of interest to this review |
| Graham 2000 | Not a patient decision aid (general information) |
| Gray 2009 | Hypothetical choice, not at the point of decision‐making |
| Green 2001b | Not a patient decision aid (educational intervention) |
| Green 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Green 2020 | Advanced care planning |
| Greenfield 1985 | Not focused on making a choice (intervention to increase patient involvement in care) |
| Griffith 2008a | Hypothetical choice, not at the point of decision‐making |
| Griffith 2008b | Not a randomized controlled trial |
| Gruppen 1994 | Not a patient decision aid |
| Guillen 2019 | Not a patient decision aid (no description of options, benefits, or values clarification) |
| Gulliford 2014 | Not a patient decision aid |
| Gummersbach 2015 | Not a patient decision aid and a hypothetical decision |
| Hacking 2013 | Not a patient decision aid |
| Hall 2007 | Not about evaluating a patient decision aid |
| Hall 2011 | Not a patient decision aid |
| Hamann 2014 | Not a patient decision aid |
| Harmsen 2014 | Not a patient decision aid |
| Harwood 2011 | Not a randomized controlled trial |
| Hawley 2016 | Not a randomized controlled trial |
| Healton 1999 | Not a patient decision aid (education to promote compliance) |
| Heisler 2014 | Not a patient decision aid |
| Henderson 2013 | Not a treatment or screening decision |
| Henselmans 2020 | Not a patient decision aid |
| Herrera 1983 | Quasi‐RCT: assigned to 1 of 2 alternating groups |
| Hersch 2021 | Two decision aids compared |
| Hess 2015 | Conjoint analysis for values clarification without information on options, pros and cons |
| Hewison 2001 | Not a patient decision aid; no values clarification |
| Heyland 2020 | Advanced care planning |
| Heyn 2013 | Not a randomized controlled trial |
| Hickish 1995 | Not a randomized controlled trial (letter) |
| Hinsberg 2018 | Two decision aids compared |
| Hochlehnert 2006 | Not a patient decision aid (general information; no values clarification) |
| Hofbauer 2008 | Not a randomized controlled trial |
| Hoffman 2009 | Not a patient decision aid |
| Hoffmann 2022 | Study does not report outcomes of interest to this review (only clinician outcomes) |
| Holbrook 2007 | Hypothetical choice, not at the point of decision‐making |
| Hollen 2013 | Not a treatment or screening decision |
| Holloway 2003 | Not focused on making a choice (promotes complying with a recommended option) |
| Holmes‐Rovner 2011 | Not a randomized controlled trial |
| Holt 2009 | Study does not evaluate a decision aid; evaluation of spiritual versus non‐spiritual framework |
| Holt 2020 | Not a patient decision aid |
| Holzhüter 2020 | Not a patient decision aid |
| Hope 2010 | Same content |
| Hopkin 2019 | Hypothetical choice |
| Howard 2022 | Advanced care planning |
| Huang 2017 | Hypothetical choice |
| Huijbregts 2013 | Not a patient decision aid |
| Hulbaek 2021 | Study does not report outcomes of interest to this review (focused on feasibility of conducting the trial) |
| Hunt 2005 | Not focused on making a choice (promotes complying with a recommended option) |
| Hunter 1999 | Not focused on making a choice (no specific decision) |
| Hunter 2005 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Hutyra 2019 | Two decision aids compared |
| Huyghe 2009 | Hypothetical choice, not at point of decision‐making for all participants |
| Ilic 2008 | No difference in content of interventions ‐ testing mode of delivery |
| Isebaert 2007 | Not a randomized controlled trial (English paper published in 2008 Urologia Internationalis ) |
| Jackson 2011 | Not a patient decision aid |
| Jayakumar 2021 | Two decision aids compared |
| Jerant 2007 | Not focused on making a choice ‐ adherence to screening |
| Jessop 2020 | Not a patient decision aid |
| Jibaja‐Weiss 2006 | No comparison outcome data provided (only presents data for intervention group) |
| Jimbo 2019 | Two decision aids compared |
| Jimenez 2017 | Study does not report outcomes of interest to this review |
| Joosten 2009 | Not a patient decision aid |
| Joosten 2011 | Not a patient decision aid |
| Jorm 2003 | Hypothetical choice, not at point of decision‐making ‐ community sample asked to evaluate information booklet on depression |
| Juraskova 2014 | About clinical trial entry |
| Kahn 2022 | Quasi‐RCT: randomization was by odd/even days of the month |
| Kakkilaya 2011 | Hypothetical choice, not at point of decision‐making |
| Kang 2020 | Advanced care planning |
| Kaplan 2014a | Not a patient decision aid |
| Kaplan 2014b | Not randomized controlled trial results; cross‐sectional analysis of baseline data |
| Kask‐Flight 2021 | Not a treatment or screening decision |
| Kassan 2012 | Web arm only, not a randomized controlled trial |
| Kawasaki 2015 | Not a patient decision aid |
| Kayler 2020 | Two decision aids compared |
| Kellar 2008 | Hypothetical choice, not at point of decision‐making |
| Kiatpongsan 2014 | No specific decision to be made and not a true randomized controlled trial |
| Klifto 2021 | Two decision aids compared |
| Kobelka 2009 | Not a randomized controlled trial; not a patient decision aid |
| Kobewka 2021 | Advanced care planning |
| Koelewijn‐van Loon 2009 | Lifestyle only |
| Korger 2021 | Hypothetical choice; not a patient decision aid |
| Krawczyk 2012 | Uptake of a recommended option |
| Kripalani 2007 | Not a patient decision aid |
| Krones 2008 | Not a patient decision aid ‐ no benefits and harms |
| Kukafka 2018 | Not a randomized controlled trial |
| Kuppermann 2009 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Kurian 2009 | Not a randomized controlled trial; not a patient decision aid |
| Kushner 2022 | Not a patient decision aid |
| Köpke 2009 | Not a patient decision aid |
| Köpke 2014 | Not a patient decision aid |
| Labrecque 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| LaCroix 1999 | Inadequate comparison outcome data provided, secondary report of pilot study |
| Lai 2021 | Not a randomized controlled trial |
| Lairson 2011 | Not a patient decision aid (to increase uptake of screening) |
| Lalonde 2006 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Lancaster 2009 | Not a patient decision aid |
| Landrey 2013 | Not a patient decision aid |
| Langford 2020 | Two decision aids compared |
| Lazcano Ponce 2000 | Not a patient decision aid (no values clarification) |
| Legare 2003 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Leung 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Levin 2011 | Not a patient decision aid |
| Lewis 2003 | Hypothetical choice, not at the point of decision‐making |
| Lewis 2012 | Uptake of a recommended option |
| Lewis 2015 | Study is not focused on evaluation of the decision aid (focused on dissemination methods) |
| Lipnick 2020 | Advanced care planning |
| Lipstein 2021 | Not a treatment or screening decision; pediatric population |
| Logan 2022 | Not a patient decision aid |
| Lopez‐Jornet 2012 | Not a patient decision aid/not at point of decision‐making |
| Lord 2017 | Not a patient decision aid (no description of benefits and harms of options) |
| Lukens 2013 | Not a patient decision aid; results in response to clinical vignettes (hypothetical scenarios) |
| Lurie 2011 | Not a randomized controlled trial (all patients received decision aid) |
| Maisels 1983 | Not a patient decision aid (no values clarification) |
| Makimoto 2020 | Not a patient decision aid |
| Mancini 2006 | Not about evaluating a patient decision aid |
| Mangla 2019 | Two decision aids compared |
| Manne 2009 | Not focused on making a choice (about adherence not decision‐making) |
| Manne 2016 | Two decision aids compared |
| Manns 2005 | Not focused on making a choice (promotes complying with a recommended option) |
| Markham 2003 | Not a patient decision aid (review of patient information pamphlets on pre‐operative fasting) |
| Markun 2015 | Not a patient decision aid |
| Martin 2012 | Hypothetical choice, not at the point of decision‐making |
| Maslin 1998 | Insufficient outcome data provided in publication; requested from author but not provided |
| Matlock 2014 | End of life |
| Matlock 2020 | Study did not evaluate the decision aid (evaluated implementation) |
| Matloff 2006 | Not a patient decision aid ‐ genetic counseling only |
| Mazur 1994 | Hypothetical choice, not at the point of decision‐making |
| McBride 2016 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but they no longer have access |
| McCaffery 2007 | Not a patient decision aid |
| McGinley 2002 | Not a patient decision aid (no values clarification) |
| McGowan 2008 | Not a patient decision aid |
| McInerney‐Leo 2004 | Not a patient decision aid (no risk/benefit information; no values clarification) |
| Mclaren 2012 | Not a patient decision aid; hypothetical choice, not at point of decision‐making |
| Meropol 2013 | Not a patient decision aid |
| Mertz 2020 | Not a patient decision aid |
| Michael 2022 | Advanced care planning |
| Michie 1997 | Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid; additional information requested, but author was unable to provide the intervention. |
| Miller 2014a | No specific decision; related to increasing visits to healthcare provider |
| Miller 2014b | Aims to increase visits to healthcare providers; intervention targeted to partners |
| Minneci 2019 | Control group received a decision support intervention with the key elements of the patient decision aid |
| Mishel 2009 | Not a patient decision aid (information only) |
| Mohammad 2012 | Not a patient decision aid; presents only benefits, not harms |
| Molenaar 2001 | Not a randomized controlled trial |
| Mulley 2006 | Not a randomized controlled trial (editorial) |
| Myers 2005a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Myers 2005b | Not a randomized controlled trial (editorial) |
| Myers 2007 | Not a patient decision aid |
| Myers 2011 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Myers 2013 | Uptake of screening |
| Myers 2019 | Not a patient decision aid |
| Neubeck 2008 | Study protocol, does not appear to be patient decision aid |
| Newton 2001 | Not a randomized controlled trial |
| O'Cathain 2002 | Suite of 8 decision aids (not an efficacy trial) |
| O'Connor 1999a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| O'Connor 1996 | No patient decision aid ‐ framing effects |
| O'Connor 1998a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| O'Connor 2009a | Not a patient decision aid |
| O'Connor 2011 | Not a patient decision aid |
| Owens 2014A | Not an RCT; doctoral dissertation |
| Pablos 2020 | Not a randomized controlled trial |
| Paquin 2021 | Hypothetical choice |
| Parker 2017 | Pediatric population |
| Patanwala 2011 | Not a patient decision aid |
| Patel 2014 | Not an RCT |
| Pearson 2005 | Not a patient decision aid (focus on provision of information) |
| Peele 2005 | Not a patient decision aid (decision aid only supplies mortality risk information; no risk info; no values clarification) |
| Petty 2014 | Not a randomized controlled trial and not a patient decision aid |
| Philip 2010 | Not a randomized controlled trial, not a patient decision aid (promotes complying with a recommended option) |
| Phillips 1995 | Quasi‐RCT: alternating order based on patients' initial appointment sequence |
| Pignone 2013 | Not a patient decision aid; compared the effect of 3 different values clarification methods |
| Pinto 2008 | About clinical trial entry |
| Politi 2020b | Not a treatment or screening decision |
| Powers 2011 | Not a patient decision aid |
| Probst 2020 | Not a patient decision aid |
| Proctor 2006 | Not a patient decision aid (general patient education resource) |
| Prunty 2008 | About a lifestyle choice ‐ whether or not to have a child or have another child if I have multiple sclerosis |
| Qureshey 2022 | Not a patient decision aid (promotes complying with a recommended option) |
| Ramallo‐Farina 2020 | Not a patient decision aid |
| Ranta 2015 | Not a patient decision aid; intended to increase guideline adherence for transient ischemic attack/stroke |
| Rapley 2006 | Not a randomized controlled trial |
| Raynes‐Greenow 2009 | No difference in intervention content; comparison of presentation formats; audio‐guided decision aid versus booklet only |
| Raynes‐Greenow 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Reder 2017 | Two decision aids compared |
| Reder 2019 | Two decision aids compared |
| Rimer 2001 | Not focused on making a choice (promotes complying with a recommended option) |
| Rimer 2002 | Not focused on making a choice (promotes complying with a recommended option) |
| Rising 2018 | Not a patient decision aid |
| Robinson 2013 | Not a patient decision aid |
| Rogojanski 2022 | Not a randomized controlled trial |
| Ronda 2014 | Benefits or harms of self‐testing are not provided as information on the website; values clarification exercise asks users to qualify value statements as benefits or harms |
| Rosen 2022 | Hypothetical choice |
| Rostom 2002 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Roter 2012 | Not a patient decision aid |
| Rothert 1997 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Rothwell 2019 | Two decision aids compared |
| Rovner 2004 | Not a randomized controlled trial |
| Rubinstein 2011 | Not a patient decision aid |
| Ruddy 2009 | Not a patient decision aid |
| Ruehlman 2012 | Not a patient decision aid |
| Ruland 2013 | No specific decision to be made |
| Rutten 2022 | Not a patient decision aid |
| Ryser 2004 | Not focused on making a choice (promotes complying with a recommended option) |
| Sassen 2014 | Not a patient decision aid evaluation study; healthcare professionals were recruited, not patients |
| Saver 2007 | Not a patient decision aid ‐ general information; not a specific decision |
| Sawka 2011 | Not a randomized controlled trial |
| Sawka 2015a | Not a patient decision aid (information about one choice only, no values clarification reported) |
| Sawka 2015b | Not a patient decision aid (information about one choice only, no values clarification reported) |
| Scaffidi 2014 | Not an RCT |
| Schaffer 2018 | Not a patient decision aid |
| Schapira 2000 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Schapira 2007 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Scherr 2022 | Two decision aids compared |
| Schnipper 2010 | Not a patient decision aid |
| Scholl 2021 | Not a patient decision aid (no specific decision) |
| Schroy 2016 | Two decision aids compared |
| Schwartz 2009b | Hypothetical choice, not at the point of decision‐making |
| Sears 2007 | About do not resuscitate versus initiating cardiopulmonary resuscitation decision |
| Seitz 2018 | Not a patient decision aid (no description of options, benefits, risks) |
| Sepucha 2022 | Two decision aids compared |
| Sequist 2011 | Not a patient decision aid (promotes complying with a recommended option) |
| Serovich 2020 | Not a treatment or screening decision |
| Sferra 2021 | Two decision aids compared |
| Shah 2012 | Not a patient decision aid, lifestyle choices |
| Shegog 2020 | Not a patient decision aid; not a treatment or screening decision |
| Sheppard 2012 | Not a randomized controlled trial |
| Sheridan 2004 | Not a randomized controlled trial |
| Sheridan 2010 | Hypothetical choice, not at point of decision‐making |
| Sheridan 2012 | Not a patient decision aid ‐ no benefits and harms |
| Sherman 2014 | Not a randomized controlled trial |
| Sherman 2016 | Two decision aids compared |
| Sherman 2017 | Two decision aids compared |
| Shirai 2012 | Not a patient decision aid |
| Silver 2012 | Hypothetical choice, not at point of decision‐making |
| Siminoff 2006 | Not a patient decision aid (no discussion of harms) |
| Simon 2012a | Not a patient decision aid |
| Simon 2012b | Not a patient decision aid |
| Smith 2011a | No decision regarding treatment or screening to be made (decision regarding full disclosure) |
| Smith 2011b | Not a patient decision aid, not an RCT |
| Smith 2020 | Not a patient decision aid |
| Solberg 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Sorenson 2004 | Not a randomized controlled trial |
| Sparano 2006 | Not a patient decision aid |
| Stalmeier 2009 | Not a randomized controlled trial (about instrument development) |
| Stankowski‐Drengler 2019 | Two decision aids compared |
| Starosta 2015 | Not a patient decision aid ‐ benefits and harms of screening are missing. |
| Stein 2013 | End of life |
| Steiner 2003 | Not a patient decision aid (only effectiveness not cons of options; not at point of decision‐making) |
| Stephens 2008 | Not a randomized controlled trial |
| Stiggelbout 2008 | Not a patient decision aid |
| Stirling 2012 | Not a treatment or screening decision |
| Stratton 2019 | Not a treatment or screening decision |
| Street 1995 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Street 1998 | Not focused on making a choice (promotes complying with a recommended option) |
| Suen 2021 | Not a patient decision aid |
| Sundaresan 2011 | Hypothetical choice, not at the point of decision‐making, not a randomized controlled trial |
| Tabak 1995 | Not a randomized controlled trial |
| Taksler 2021 | Not a patient decision aid |
| Tanser 2021 | Not a patient decision aid |
| Tappen 2020 | Advanced care planning |
| Taylor 2013 | Not a patient decision aid ‐ benefits and harms of screening not included |
| Tebb 2019 | Not a patient decision aid |
| Ten 2008 | Not a patient decision aid; about stopping medication use |
| Ter Stege 2021 | Study does not report outcomes of interest to this review |
| Thiede 2021 | Advanced care planning |
| Thomas 2013 | Not a patient decision aid |
| Thomson 2006 | Not a randomized controlled trial; not at point of decision‐making |
| Thornton 1995 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Tiedje 2021 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Tiller 2006 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Tinsel 2013 | Not a patient decision aid |
| Tomko 2015 | Not a patient decision aid ‐ benefits and harms of screening are missing |
| Tran 2015 | Not a patient decision aid (promotes complying with a recommended option) |
| Tsai 2022 | Not a patient decision aid |
| Tucholka 2018 | Two decision aids compared |
| Ufere 2022 | Advanced care planning |
| Ukoli 2013 | Not an RCT |
| Valdez 2001 | Not a randomized controlled trial; not focused on making a choice (complying with a recommended option) |
| Van der Krieke 2013 | Not a patient decision aid, no benefits/harms |
| Van Roosmalen 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Van Steenkiste 2008 | Not a randomized controlled trial |
| Van Til 2009 | Hypothetical choice, not at the point of decision‐making |
| Van Tol‐Geerdink 2013 | Not a randomized controlled trial; insufficient information to judge random sequence generation, allocation concealment, and blinding |
| VanScoy 2017 | Advanced care planning |
| Veroff 2012 | Not a patient decision aid |
| Volandes 2009 | Advanced care planning options |
| Volandes 2011 | Hypothetical choice, end of life decision |
| Volandes 2013 | Advanced care planning |
| Volk 2008 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Von Wagner 2011 | Not a randomized controlled trial (commentary) |
| Wagner 1995 | Not a randomized controlled trial |
| Wakefield 2008a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wakefield 2008b | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wakefield 2008c | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wallston 1991 | Not a patient decision aid ‐ patient preference study |
| Wang 2004 | Not a patient decision aid ‐ intent of intervention to facilitate genetic counseling process, no focused decision |
| Wang TJ 2021 | Two decision aids compared |
| Warner 2015 | Not a treatment or screening decision |
| Waterman 2018 | Not a patient decision aid |
| Waterman 2019 | Not a patient decision aid |
| Waterman 2021 | Not a patient decision aid |
| Watts 2014 | Simple versus detailed patient decision aid |
| Wehkamp 2021 | Hypothetical choice |
| Welschen 2012 | Not a patient decision aid |
| Weng 2017 | Not a patient decision aid (information about one choice only, no values clarification) |
| Wennberg 2010 | Same decision aid in both groups |
| Werk 2019 | Not a patient decision aid |
| Westermann 2013 | Not a patient decision aid |
| Weymann 2015 | Patients not at the point of decision‐making |
| Wilhelm 2009 | Not a patient decision aid |
| Wilkes 2013 | Unable to ascertain characteristics of the patient decision aid. Additional information requested from author but not provided (e.g. values clarification). |
| Wilkie 2013 | Not treatment or screening decision |
| Wilkins 2006 | Not a randomized controlled trial |
| Willemsen 2006 | Lifestyle change |
| Williams‐Piehota 2008 | Not a randomized controlled trial |
| Williamson 2014 | Lifestyle decision ‐ not treatment or screening |
| Wilson 2019 | Study does not report outcomes of interest to this review |
| Wolff 2020 | Not a patient decision aid |
| Woltmann 2011 | Not a patient decision aid |
| Wroe 2005 | Not focused on making a choice ‐ promotes complying with a recommended option |
| Yao 2017 | Not a randomized controlled trial |
| Yee 2014 | Not a patient decision aid |
| Yu 2020 | Not a patient decision aid (no specific decision) |
| Yu 2021 | Study does not report outcomes of interest to this review (focused on feasibility of conducting the trial) |
| Yun 2011 | End of life decision |
| Zajac 2012 | Hypothetical |
| Zapka 2004 | Not focused on making a choice ‐ promotes complying with a recommendation |
| Zhong 2021 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Zikmund‐Fisher 2008 | No difference in intervention content ‐ comparison of presentation of probabilities |
| Zoffman 2012 | Not a randomized controlled trial, not a patient decision aid |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001665426.
| Study name | Navigate: Randomised controlled trial of an online treatment decision aid for men with localised prostate cancer and their partners |
| Methods | RCT |
| Participants | 304 adults diagnosed with localized prostate cancer in the last 3 months |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decisional regret, decisional satisfaction, preparedness for decision‐making, quality of life, quality of patients’ and partners’ illness communication |
| Starting date | May 2017 |
| Contact information | Ms Natalie Richards, navigate@petermac.org |
| Notes | Trial# ACTRN12616001665426 |
ACTRN12617001246370.
| Study name | Use of an internet‐based decision aid (myAID) for ulcerative colitis patients to improve quality of life, empowerment, decision making and disease control |
| Methods | RCT |
| Participants | 426 adults with a diagnosis of ulcerative colitis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Health‐related quality of life, acceptability of the decision aid, anxiety, empowerment, health literacy, adherence, quality of decision‐making |
| Starting date | October 2016 |
| Contact information | Dr Andrew Kim, andrew.h.kim@student.unsw.edu.au |
| Notes | Trial# ACTRN12617001246370 |
ACTRN12618001219279.
| Study name | The Optimise Study ‐ randomised trial of the use of a decision aid to improve informed choice regarding the benefits of low‐dose aspirin to prevent cardiovascular disease and colorectal cancer |
| Methods | RCT |
| Participants | 1780 adults aged 50 to 70 years who have not been diagnosed with a serious illness |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Measure of informed choice (MMIC) incorporating assessments of knowledge, adherence to aspirin |
| Starting date | August 2018 |
| Contact information | Lyndal Trevena, lyndal.trevena@sydney.edu.au |
| Notes | Trial# ACTRN12618001219279 |
ACTRN12620001003965.
| Study name | Should I Take Aspirin? The SITA Trial, a randomised controlled trial of a decision aid to support informed choices about taking aspirin to prevent bowel cancer for Australians aged 50 to 70 years |
| Methods | RCT |
| Participants | 258 adults aged 50 to 70 |
| Interventions | Patient decision aid vs information |
| Outcomes | Adherence, ability to make an informed choice, decisional conflict |
| Starting date | October 2020 |
| Contact information | Ms Shakira MIlton, shakira.milton@unimelb.edu.au |
| Notes | Trial# ACTRN12620001003965 |
ACTRN12620001032943.
| Study name | Comparing different information resources on the process and quality of decision making in women considering elective egg freezing |
| Methods | RCT |
| Participants | 286 females aged 18 years or over considering egg freezing |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, length of time to make a decision, knowledge, distress, informed choice, level of involvement in decision‐making, decisional regret |
| Starting date | September 2020 |
| Contact information | Dr Michelle Peate, mpeate@unimelb.edu.au |
| Notes | Trial# ACTRN12620001032943 |
ACTRN12621000515897.
| Study name | Evaluating fertility decision aids for younger women with breast cancer |
| Methods | RCT |
| Participants | 236 women aged between 18 to 40 years (inclusive) who are pre‐menopausal and have a histologically confirmed diagnosis of early‐stage breast cancer |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decision regret, informed choices, knowledge, psychological outcomes |
| Starting date | July 2021 |
| Contact information | Dr Michelle Peate, mpeate@unimelb.edu.au |
| Notes | Trial# ACTRN12621000515897 |
Al‐Itejawi 2015.
| Study name | (Cost‐)effectiveness and implementation of a decision aid for patients with prostate cancer |
| Methods | Stepped wedge cluster‐RCT |
| Participants | Newly diagnosed adult participants with localized prostate cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, quality of life, treatment preferences, participation in decision‐making, knowledge, patient‐provider communication |
| Starting date | May 2015 |
| Contact information | Hoda Al‐Itejawi, Afdeling Urologie, Amsterdam, the Netherlands |
| Notes | Trial #: NTR5177 |
Aslani 2014a.
| Study name | Computerized decision aid on mode of delivery |
| Methods | Cluster‐RCT |
| Participants | Pregnant Iranian women |
| Interventions | Computerized decision aid |
| Outcomes | Decisional conflict, knowledge |
| Starting date | Not reported |
| Contact information | Azam Aslani, Mashhad University, Iran |
| Notes | Registration number IRCT2015093010777N4 |
Aslani 2014b.
| Study name | Impact of computer‐based pregnancy‐induced hypertension and diabetes decision aids on empowering pregnant women |
| Methods | RCT |
| Participants | 420 healthy pregnant women |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Self‐efficacy, knowledge, type and frequency of doctor and/or medical center visits, anxiety |
| Starting date | November 2013 |
| Contact information | Saeid Eslami, Mashhad University of Medical Science, eslams@mums.ac.ir |
| Notes | Trial# IRCT2013103010777N2 |
Bansback 2019.
| Study name | An individualized patient‐reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial |
| Methods | RCT |
| Participants | 163 adults (age ≥ 30) patients with knee osteoarthritis (OA) who have an appointment with a surgeon for consultation about total knee arthroplasty |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision quality, quality of life, depression, knowledge, values, decisional conflict, treatment preference, preference for involvement in decision‐making, willingness to have surgery, patient‐reported shared decision‐making, decisional regret, satisfaction with knee replacement surgery, expectations, surgical consult, surgery, concordance |
| Starting date | June 2017 |
| Contact information | Nick Bansback, nick.bansback@ubc.ca |
| Notes | Trial# NCT03240913 |
Baptista 2020.
| Study name | Comparison of explicit values clarification method (VCM), implicit VCM and no VCM decision aids for men considering prostate cancer screening: protocol of a randomized trial |
| Methods | RCT |
| Participants | 276 adult men (50 to 69 years) with average risk for prostate cancer |
| Interventions | Patient decision aid with implicit values clarification vs patient decision aid with explicit values clarification vs control (information only) |
| Outcomes | Perceived clarity of personal values (3‐item subscale of the Decisional Conflict Scale), decisional conflict, screening preference, actual choice |
| Starting date | September 2019 |
| Contact information | Sofia Baptista, baptistas@med.up.pt |
| Notes | Trial# NCT03988673 |
Beach 2016.
| Study name | Protocol of a randomized controlled trial of an erythropoietin stimulating agent decision aid for anemia treatment in kidney disease |
| Methods | RCT |
| Participants | 100 adults aged 18 to 80 with a diagnosis of chronic kidney disease (CKD) or end‐stage renal disease (ESRD), and currently recieving erythropoietin‐stimulating agent (ESA) therapy |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, satisfaction with provider communications, decisional conflict, perceived efficacy in patient‐physician interactions |
| Starting date | November 2013 |
| Contact information | Kerri Cavanaugh, kerri.cavanaugh@vanderbilt.edu |
| Notes | Trial# NCT01992926 |
Benoit 2020.
| Study name | Does a web‐based decision aid improve informed choice for fertility preservation in women with breast cancer (DECISIF)? Study protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | 186 women 18 to 40 years old with breast cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Multidimensional Measure of Informed Choice (MMIC), decisional conflict, anxiety |
| Starting date | September 2018 |
| Contact information | Alexandra Benoit, alexandra.benoit@aphp.fr |
| Notes | Trial# NCT03591848 |
Carhuapoma 2021.
| Study name | Employing a mobile health decision aid to improve decision‐making for patients with advanced prostate cancer and their decision partners/proxies: the CHAMPION randomized controlled trial study design |
| Methods | RCT |
| Participants | 316 adults with stages III/IV prostate cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision conflict, decision regret, health‐related quality of life, decision‐making participation preference |
| Starting date | July 2017 |
| Contact information | Randy A Jones, raj9c@virginia.edu |
| Notes | Trial# NCT03327103 |
Chambers 2008.
| Study name | ProsCan for Men: randomized controlled trial of a decision support intervention for men with localised prostate cancer |
| Methods | RCT |
| Participants | 700 men newly diagnosed with localized prostate cancer |
| Interventions | A tele‐based nurse delivered 5‐session decision support/psychosocial intervention vs usual care |
| Outcomes | Cancer threat appraisal; decision‐related distress and bother from treatment side effects; involvement in decision‐making; satisfaction with health care; healthcare utilization; use of healthcare resources; and a return to previous activities |
| Starting date | Not yet assessed |
| Contact information | Suzanne K Chambers, Griffith University |
| Notes | Trials #: ACTRN012607000233426 |
Columbo 2019.
| Study name | Design of the PReferences for Open Versus Endovascular Repair of Abdominal Aortic Aneurysm (PROVE‐AAA) trial |
| Methods | Cluster‐RCT |
| Participants | 240 veterans with abdominal aortic aneurysm who are candidates for either endovascular or open repair |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Preferred choice, congruence between preferred and actual choice, decision regret |
| Starting date | June 2017 |
| Contact information | Philip Goodney, White River Junction VA Medical Center, White River Junction, VT |
| Notes | Trial# NCT03115346 |
CTRI/2019/06/019610.
| Study name | Effectiveness of Nursing Intervention Module on knowledge, adherence, complications and quality of life among persons receiving oral anticoagulation therapy |
| Methods | RCT |
| Participants | 320 adults aged 21 and above on oral anticoagulation therapy |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Adherence, knowledge, complication rates, quality of life |
| Starting date | June 2019 |
| Contact information | Janet Prameela Dsouza, janet.p@manipal.edu |
| Notes | Trial# CTRI/2019/06/019610 |
de Molina‐Férnandez 2019.
| Study name | The effectiveness of a digital shared decision‐making tool in hormonal contraception during clinical assessment: study protocol of a randomized controlled trial in Spain |
| Methods | Cluster‐RCT |
| Participants | 1708 women who attend clinical contraceptive counseling units |
| Interventions | Decision‐making tool vs usual care |
| Outcomes | Adherence to the chosen treatment, attitude towards compliance, actual choice, decisional conflict, satisfaction with the counselor or clinician with the use of the decision aid, knowledge |
| Starting date | January 2019 |
| Contact information | Dr Maria Inmaculada de Molina‐Fernandez, inmaculada.demolina@urv.cat |
| Notes | Trial# ISRCTN5827994 |
DRKS00014627.
| Study name | Evaluation of a patient‐oriented decision aid and the German healthcare situation in non‐metastatic prostate cancer |
| Methods | RCT |
| Participants | 1115 male patients in the age group 18 to 80 with histologically confirmed adenocarcinoma of the prostate and no clinical evidence of metastases |
| Interventions | Patient decision aid vs information |
| Outcomes | Treatment decision, knowledge, acceptability of intervention/control, decisional conflict, doctor‐patient communication, fear and depressiveness, decision regret, quality of life |
| Starting date | July 2018 |
| Contact information | Johannes Huber, johannes.huber@gmail.com |
| Notes | Trial# DRKS00014627 |
DRKS00015823.
| Study name | Development and piloting of a decision support tool to support decision making in the context of risk‐adapted prevention for patients with pathogenic BRCA1/2 mutations |
| Methods | RCT |
| Participants | 78 females aged 18 to 70 with unilateral breast cancer (first disease without metastasis) |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Decisional conflict, stage of decision‐making, knowledge, psychological stress consequences, anxiety and depression |
| Starting date | November 2018 |
| Contact information | Sibylle Kautz‐Freimuth, sibylle.kautz‐freimuth@uk‐koeln.de |
| Notes | Trial# DRKS00015823. Trial registered retrospectively. |
Geiger 2011.
| Study name | Investigating a training supporting Shared Decision Making (IT'S SDM 2011): study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | 40 physicians that contribute a sequence of 4 medical consultations including a diagnostic or treatment decision |
| Interventions | A training curriculum for doctors, intended to stimulate efforts to involve their patients in the decision‐making process |
| Outcomes | Physician‐patient communication, effect of SDM on perceived quality of the decision process and on the elaboration of the decision, decisional conflict |
| Starting date | Not yet assessed |
| Contact information | Friedemann Geiger, University Medical Center Schleswig ‐ Holstein |
| Notes | Trials #: ISRCTN78716079 |
IRCT20191229045933N1.
| Study name | Effect of a patient decision aid to select for myopia correction surgery method |
| Methods | RCT |
| Participants | 30 participants aged 15 years old to 40 years who are nearsighted and candidates for all 33 surgical procedures: Smile, Femto‐LASIK and PRK |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Knowledge, Choice |
| Starting date | April 2021 |
| Contact information | Fatemeh Zarei, f.zarei@modares.ac.ir |
| Notes | Trial# IRCT20191229045933N1 |
ISRCTN17611852.
| Study name | The impact of a decision aid on depressed patients' involvement in shared decision‐making |
| Methods | RCT |
| Participants | 44 patients aged 18 to 60 diagnosed with major depressive disorder |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Patient involvement in the decision‐making scale |
| Starting date | March 2014 |
| Contact information | Khalaf Aljumah, PO Box 33626 11458 Riyadh, Saudi Arabia |
| Notes | Trial# ISRCTN17611852 |
Isselhard 2020.
| Study name | Implementation and evaluation of a nurse‐led decision‐coaching program for healthy breast cancer susceptibility gene (BRCA1/2) mutation carriers: a study protocol for the randomized controlled EDCP‐BRCA study |
| Methods | RCT |
| Participants | 399 women aged 25 to 60 years with diagnosed, clearly pathogenic BRCA1/2 mutations who have not been diagnosed with breast or ovarian cancer |
| Interventions | Patient decision aid + decision coaching + optimized standard care vs optimized standard care only |
| Outcomes | Congruence between preferred and actual role in the decision‐making process, satisfaction with the actual role, decisional conflict, knowledge and attitude towards preventions strategies, stage of decision‐making, anxiety and depression, coping self‐efficacy |
| Starting date | November 2019 |
| Contact information | Anna Isselhard, Institute of Health Economics and Clinical Epidemiology, University Hospital of Cologne, Cologne, Germany, anna.isselhard@uk‐koeln.de |
| Notes | Trial# DRKS00015527 |
JPRN‐UMIN000024811.
| Study name | An interventional study to examine the effect of shared decision making in a family on intention of HPV vaccination in order to protect Japanese girls from HPV due to the low coverage of the vaccination and excess of mothers' responsibility to make a decision |
| Methods | RCT |
| Participants | 900 mothers with daughters aged 12 to 18 years old |
| Interventions | Patient decision aid vs information |
| Outcomes | Intention to vaccinate |
| Starting date | November 2016 |
| Contact information | Tadashi Kimura, tadashi@gyne.med.osaka‐u.ac.jp |
| Notes | Trial# JPRN‐UMIN000024811 |
JPRN‐UMIN000032623.
| Study name | A randomized controlled trial on decision aid to support the stroke with older people in decision making about location of care at recovery rehabilitation ward: efficacy of decision conflict and participation |
| Methods | RCT |
| Participants | 122 stroke survivors aged > 65 years who are admitted to the rehabilitation ward during their recovery period and facing discharge location decision‐making |
| Interventions | Patient decision aid vs information |
| Outcomes | Decision Conflict Scale, Control Preference Scale |
| Starting date | October 2018 |
| Contact information | Yoriko Aoki, yoriko18@med.u‐toyama.ac.jp |
| Notes | Trial# JPRN‐UMIN000032623 |
KCT0006945.
| Study name | Development of health information communication strategy in response to COVID‐19 crisis |
| Methods | RCT |
| Participants | Participants aged 18 to 80 years old who have been diagnosed with one or more of the following diseases: diabetes mellitus, heart failure, myocardial infarction, hypertension, renal insufficiency, pulmonary disease, chronic obstructive, asthma, liver |
| Interventions | Patient decision aid vs information |
| Outcomes | COVID‐19 vaccination intention, decisional conflict, stress, knowledge |
| Starting date | December 2021 |
| Contact information | Young‐il Jung, extra012@knou.ac.kr |
| Notes | Trial #KCT0006945 |
Kim 2020.
| Study name | A web‐based decision aid (myAID) to enhance quality of life, empowerment, decision making, and disease control for patients with ulcerative colitis: protocol for a cluster randomized controlled trial |
| Methods | Cluster‐RCT |
| Participants | 426 adults with a diagnosis of ulcerative colitis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Quality of life, empowerment, health literacy, decisional conflict, trust in physician, anxiety, intervention acceptability |
| Starting date | October 2016 |
| Contact information | Dr Andrew Kim, Ingham Institute Liverpool Hospital, andrew.h.kim@student.unsw.edu.au |
| Notes | Trial# ACTRN12617001246370 |
Lange 2021.
| Study name | An individualized decision aid for physicians and patients for total knee replacement in osteoarthritis (Value‐based TKR study): study protocol for a multi‐center, stepped wedge, cluster randomized controlled trial |
| Methods | Cluster‐RCT |
| Participants | 1080 patients with knee osteoarthritis referred for total knee replacement |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision quality, fulfillment of patient expectations |
| Starting date | June 2021 |
| Contact information | Franziska Beyer, Franziska.Beyer@uniklinikum‐dresden.de |
| Notes | Trial# NCT04837053 |
Layton 2011.
| Study name | Effects of a web‐based decision aid on African American men's prostate screening knowledge and behavior |
| Methods | — |
| Participants | 128 African American men |
| Interventions | — |
| Outcomes | — |
| Starting date | — |
| Contact information | Beverly Layton, Walden University |
| Notes | Unpublished thesis |
Lin E 2022.
| Study name | Incorporating patient‐reported outcomes into shared decision‐making in the management of patients with osteoarthritis of the knee: a hybrid effectiveness‐implementation study protocol |
| Methods | RCT |
| Participants | 200 adults aged 45 to 89 with a presumptive diagnosis of knee OA |
| Interventions | Patient decision aid + education vs education only |
| Outcomes | Patient perception of decision process and quality, concordance between patient preferences and actual outcomes, patient perception of the level of shared decision‐making, patient/provider satisfaction with discussion, total consultation time, patient‐reported overall health, choice, decisional conflict, decision regret |
| Starting date | February 2021 |
| Contact information | Lauren Uhler, lauren.uhler@austin.utexas.edu |
| Notes | Trial# NCT04805554 |
Mann 2012.
| Study name | Increasing efficacy of primary care‐based counselling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial |
| Methods | RCT |
| Participants | Primary care providers |
| Interventions | Using the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) system to enhance providers' effectiveness to counsel about lifestyle behavior changes |
| Outcomes | Outcome measurements are designed to detect changes in patient behaviors that are most likely to result from the use of ADAPT tool: difference between intervention and control patients in the change in mean steps per day at baseline and after 6 months, and 6‐month difference of differences in hemoglobin A1C and self‐reported diet between the 2 groups |
| Starting date | Not yet assessed |
| Contact information | Devin Mann, Boston University School of Medicine |
| Notes | Trial #: NCT01473654 |
NCT00813033.
| Study name | Use of a patient decision aid for gastrologic endoscopy in a paediatric setting |
| Methods | Interventional efficacy study |
| Participants | 80 parents considering gastro‐endoscopy for child |
| Interventions | Not yet assessed |
| Outcomes | Knowledge, expectations of outcomes, clarity of values, decision, decision conflict |
| Starting date | December 2008 |
| Contact information | Nancy Neilan, Children's Mercy Hospital, Kansas City |
| Notes | Trials #: NCT00813033; completed March 2011 |
NCT01152307.
| Study name | Measuring quality of decisions about treatment of depression |
| Methods | RCT |
| Participants | Patients that talked to a healthcare provider about starting or stopping a treatment (prescription medicine for depression or counseling) |
| Interventions | Decision aid (DVD/booklet) vs usual care |
| Outcomes | Knowledge, value concordance |
| Starting date | June 2010 |
| Contact information | Karen R Sepucha, Massachusetts General Hospital |
| Notes | NCT01152307; completed, study results on clinicaltrials.gov |
NCT01976325.
| Study name | Incorporation of the 'Ottawa Malaria Decision Aid' into the pre‐travel consultation process |
| Methods | RCT |
| Participants | 100 adults attending a travel clinic before traveling to an area with known chloroquine‐resistant malaria |
| Interventions | Decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict, preparation for decision‐making, medication adherence |
| Starting date | January 2014 |
| Contact information | amccarthy@toh.on.ca, Anne E McCarthy, Ottawa Hospital Research Institute |
| Notes | Trial # NCT01976325 |
NCT02027545.
| Study name | Promoting veteran‐centered colorectal cancer screening (PROM‐IS) |
| Methods | Cluster‐RCT |
| Participants | 436 older individuals (ages 70 to 75) who are "due" for colorectal screening |
| Interventions | Patient decision aid + education vs education only |
| Outcomes | Whether screening was ordered, appropriateness of screening orders, conceptual understanding of screening, elements of informed decision‐making addressed in the screening discussion, and screening utilization |
| Starting date | November 2015 |
| Contact information | Sameer D. Saini, VA Ann Arbor Healthcare System, Ann Arbor, MI |
| Notes | Trial# NCT02027545 |
NCT02084290.
| Study name | Evaluating a prediction tool and decision aid for patients with Crohn's disease |
| Methods | RCT |
| Participants | 300 adults with Crohn's disease |
| Interventions | Patient decision aid and SDM program vs usual care |
| Outcomes | Preferred choice, actual choice, adherence, cost of care, remission, patient on steroids, surgeries, Crohn's disease‐related hospitalizations |
| Starting date | March 2014 |
| Contact information | Corey A Siegel, Dartmouth‐Hitchcock Medical Center, corey.a.siegel@hitchcock.org |
| Notes | Trial #: NCT02084290 |
NCT02107794.
| Study name | Shared decision making in Graves disease ‐ Graves disease (GD) choice |
| Methods | RCT |
| Participants | 93 adults aged 18 years or older with a diagnosis of Graves disease |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional quality (knowledge, decisional conflict, satisfaction) |
| Starting date | December 2012 |
| Contact information | Victor M Montori, Mayo Clinic |
| Notes | Trial# NCT02107794 |
NCT02145481.
| Study name | Decisional quality for patients with stable coronary artery disease |
| Methods | RCT |
| Participants | 846 adults with stable coronary artery disease |
| Interventions | Patient decision aid vs standard education |
| Outcomes | Quality of the decision‐making process, knowledge, communication, involvement, treatment preferences |
| Starting date | May 2014 |
| Contact information | R. Adams Dudley, University of California, San Francisco |
| Notes | Trial # NCT02145481 |
NCT02259699.
| Study name | Ovarian cancer patient‐centered decision aid |
| Methods | RCT |
| Participants | 221 women with stage III optimally debulked advanced ovarian cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Satisfaction with decision, evidence of shared decision‐making, quality of life, satisfaction with care and satisfaction with cancer treatment |
| Starting date | December 2014 |
| Contact information | Lari Wenzel, University of California, Irvine, USA, lwenzel@uci.edu |
| Notes | Trial #: NCT02259699 |
NCT02364128.
| Study name | Improving patient decisions about bariatric surgery |
| Methods | RCT |
| Participants | 1000 adults aged 18 and older considering undergoing bariatric surgery |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Decision outcome, knowledge, preferences, weight, quality of life, comorbidity resolution, patient satisfaction |
| Starting date | January 2014 |
| Contact information | Nancy Birkmeyer, University of Michigan |
| Notes | Trial# NCT02364128 |
NCT02488603.
| Study name | Utilization of decision aids for tamoxifen treatment in breast cancer patients |
| Methods | RCT |
| Participants | 360 breast cancer patients referred for tamoxifen treatment |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict scale, satisfaction with decision, quality of life |
| Starting date | August 2015 |
| Contact information | Eun Sook Lee, National Cancer Center, Korea |
| Notes | Trial # NCT02488603 |
NCT02503553.
| Study name | Decision aids in cerebral aneurysm treatment |
| Methods | RCT |
| Participants | 60 patients undergoing treatment for cerebral aneurysm |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Participation in the shared‐decision making process; stress levels, patient satisfaction level |
| Starting date | August 2015 |
| Contact information | Kimon Bekelis, Dartmouth‐Hitchcock Medical Center; New Hampshire, USA |
| Notes | This study was withdrawn on 2 February 2016. |
NCT02540044.
| Study name | Supporting patient care with electronic resource (SuPER): efficacy of an online decision aid for patients considering biologic therapy for rheumatoid arthritis |
| Methods | RCT |
| Participants | 144 adults with rheumatoid arthritis whose rheumatologists have recommended initiating a biologic/subsequent entry biologic or switching to another biologic agent |
| Interventions | Online patient decision aid vs online standard information |
| Outcomes | Decisional conflict, knowledge, self‐efficacy, self‐management behaviors, health resource utilization, choice, evidence of shared decision‐making |
| Starting date | January 2016 |
| Contact information | Linda Li, University of British Columbia, Vancouver, Canada |
| Notes | Trial #: NCT02540044 |
NCT02611050.
| Study name | Treatment decisions for multi‐vessel CAD |
| Methods | RCT |
| Participants | 160 adults with stable multi‐vessel CAD at relative equipoise for at least 2 potential treatment options |
| Interventions | Option grid decision aid vs usual care |
| Outcomes | Decisional conflict, CollaboRATE score, knowledge, patient experience, treatment received |
| Starting date | December 2015 |
| Contact information | Elizabeth L Nichols, the Dartmouth Institute |
| Notes | This study was terminated on 25 June 2018. Enrollment was not feasible. |
NCT02759939.
| Study name | Right for me: birth control decisions made easier |
| Methods | RCT |
| Participants | 5038 females aged 15 to 49 years |
| Interventions | Patient decision aids + training vs decision aids + training + video + prompt card vs video + prompt card vs no intervention |
| Outcomes | Shared decision‐making, conversation about contraception, satisfaction with conversation, intended contraceptive method, values concordance, decision regret, contraceptive method(s) used, adherence |
| Starting date | July 2016 |
| Contact information | Rachel L. Thompson, Dartmouth‐Hitchcock Medical Center |
| Notes | Trial# NCT02759939 |
NCT02823262.
| Study name | A breast cancer treatment decision aid for women aged 70 and older |
| Methods | RCT |
| Participants | 80 women 70 years or older newly diagnosed with estrogen receptor positive, clinically lymph node negative, HER2 negative, breast cancers that are 3 centimeters or less |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Decisional conflict, knowledge, change in stage of decision‐making, self‐efficacy, values, treatment preferences, desired role in decision‐making, anxiety, quality of life, preparation for decision‐making, actual role in decision‐making, decision regret, satisfaction with treatment decision, satisfaction with the decision process, treatment received, acceptability of the intervention |
| Starting date | July 2016 |
| Contact information | Mara Schonberg, Dana‐Farber Cancer Institute |
| Notes | Trial# NCT02823262 |
NCT02914197.
| Study name | Giving information on the risks and limitations of mammography screening (GIRLS) |
| Methods | RCT |
| Participants | 608 females aged 47 to 69 due for a mammogram (have not had a mammogram ≥ 18 months) according to Canadian screening interval recommendations for routine screening |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Self‐efficacy, informed choice (knowledge), informed choice (attitude), informed choice (intention), decisional conflict, anxiety, trust in medical system, perception of health provider recommendation, information relevant to the decision‐making process, decision regret, screening participation, acceptance of a decision aid, knowledge of the benefits and risks of mammography |
| Starting date | November 2017 |
| Contact information | McMaster University |
| Notes | Trial# NCT02914197 |
NCT02963584.
| Study name | Decision aid in chronic total occlusion (CTO) patients |
| Methods | RCT |
| Participants | 160 adults aged 18 and older with coronary occlusion |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, quality of the decision‐making process, acceptability with the decision aid, rate of percutaneous coronary intervention (PCI) or medication |
| Starting date | November 2016 |
| Contact information | Rongchong Huang, The First Affiliated Hospital of Dalian Medical University |
| Notes | Trial# NCT02963584 |
NCT03088397.
| Study name | Effectiveness of a patient decision aid in immediate postpartum contraceptive counseling |
| Methods | RCT |
| Participants | 126 females aged 14 to 50 postpartum day 1 or postoperative day 1 or 2 who delivered during current admission to hospital |
| Interventions | Patient decision aid vs information vs usual care |
| Outcomes | Preparation for decision‐making, choice |
| Starting date | January 2017 |
| Contact information | Erika Levi, Montefiore Medical Center |
| Notes | Trial# NCT03088397 |
NCT03099746.
| Study name | Decision support among surrogate decision makers of the chronically critically ill (INVOLVE) |
| Methods | RCT |
| Participants | 281 surrogate decision‐makers for chronically critically ill patients in the intensive care unit requiring mechanical ventilation |
| Interventions | Patient decision aid vs information vs usual care |
| Outcomes | Preparation for decision‐making, decision‐making self‐efficacy, decisional role stress, decisional conflict, control preferences, decision regret, anxiety and depression |
| Starting date | September 2015 |
| Contact information | Ronald Hickman, Case Western Reserve University |
| Notes | Trial# NCT03099746 |
NCT03141437.
| Study name | Decision aid website in helping to make decisions about fertility in participants with cancer |
| Methods | RCT |
| Participants | 160 females aged 18 to 45 with newly diagnosed breast tumor, female genital system tumor, colorectal tumor, and/or lymphoma/myeloma and at risk for cancer‐related infertility |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decision‐making process (e.g. preparation for decision‐making, decision self‐efficacy, satisfaction) and decision quality (e.g. fertility preservation knowledge, clarity of patients' values, and congruence of preferences with the decision and/or treatment received) |
| Starting date | April 2017 |
| Contact information | Terri L Woodard MD, Anderson Cancer Center |
| Notes | Trial# NCT03141437 |
NCT03244202.
| Study name | Evaluation of a decision aid for incidental genomic findings |
| Methods | RCT |
| Participants | 133 adults with a family history of cancer who received a negative single gene test for a cancer gene mutation (e.g. BRCA1/2, MLH, MSH, PMS, etc.) or received a negative panel test |
| Interventions | Patient decision aid + counseling vs counseling only |
| Outcomes | Decisional conflict, knowledge, preparation for decision‐making, satisfaction with decision, anxiety |
| Starting date | September 2016 |
| Contact information | Yvonne Bombard, St. Michael's Hospital and University of Toronto |
| Notes | Trial# NCT03244202 |
NCT03282097.
| Study name | Decisions about cancer screening in Alzheimer's disease |
| Methods | RCT |
| Participants | 426 females aged 75 years or older who have had least one mammogram in the past 5 years and have a diagnosis of Alzheimer's disease or related dementia |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decision‐making self‐efficacy, role in decision‐making, record of mammogram |
| Starting date | November 2017 |
| Contact information | Nicole R. Fowler PhD, Indiana University |
| Notes | Trial# NCT03282097 |
NCT03374891.
| Study name | A multicenter trial of a shared decision support intervention for patients offered implantable cardioverter‐defibrillators |
| Methods | RCT |
| Participants | 790 patients aged 18 and older that have been offered a primary prevention implantable cardioverter‐defibrillator for initial implant, reimplantation, or cardiac resynchronization therapy defibrillator |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Knowledge |
| Starting date | May 2018 |
| Contact information | Daniel D Matlock, University of Colorado, Denver |
| Notes | Trial# NCT03374891 |
NCT03454022.
| Study name | Decision‐aid for renal therapy pilot trial |
| Methods | RCT |
| Participants | 31 adults aged 70 or older with chronic kidney disease stages 4 or 5, not currently on dialysis |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, congruence in patient‐caregiver goals of care, patient satisfaction, caregiver satisfaction, completion of an advance directive |
| Starting date | March 2017 |
| Contact information | Keren Ladin, Tufts University |
| Notes | Trial# NCT03454022 |
NCT03477591.
| Study name | Evaluating the impact of evidence‐based information about PSA testing on prostate cancer screening decisions |
| Methods | RCT |
| Participants | 308 English‐speaking men aged 50 and older |
| Interventions | Patient decision aid + evidence‐based information vs evidence only vs control (sham information) |
| Outcomes | Decisional conflict, decision quality, preparation for decision‐making, congruency between self‐reported screening status and stated decision |
| Starting date | October 2018 |
| Contact information | Maureen Dobbins, McMaster University |
| Notes | Trial# NCT03477591 |
NCT03500952.
| Study name | Family planning ahead |
| Methods | RCT |
| Participants | 41 pregnant women aged 15 and older that are between 28 and 38 weeks' gestation at the time of enrollment |
| Interventions | Patient decision aid vs information |
| Outcomes | Perceived support in decision‐making, feeling informed, values clarity, decisional uncertainty, decision self‐efficacy, intended contraceptive method(s), values concordance of intended contraceptive method(s), trust in health professional(s), shared decision‐making, concordance between preferred and actual decision‐making involvement, time pressure in decision‐making, pressure to use a certain contraceptive method, values concordance of contraceptive method(s) used, effective decision, contraceptive method(s) prescribed, contraceptive method(s) used, timing of decision about contraceptive method(s), perceived utility of the intervention |
| Starting date | March 2018 |
| Contact information | Rachel L. Thompson, Dartmouth‐Hitchcock Medical Center |
| Notes | Trial# NCT03500952 |
NCT03522740.
| Study name | Decision aid for renal therapy (DART) |
| Methods | RCT |
| Participants | 400 adults aged 70 and older with chronic kidney disease stages 4 or 5 (non‐dialysis) without an established dialysis start or transplant date within 3 months of expected randomization |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, Canadian Health Care Evaluation Project (CANHELP) questionnaire score, completion of an advance directive |
| Starting date | May 2018 |
| Contact information | Keren Ladin, Tufts University |
| Notes | Trial# NCT03522740 |
NCT03578211.
| Study name | Impact of decision aids on bariatric surgery choice: a randomized controlled trial |
| Methods | RCT |
| Participants | 140 adults aged 20 to 70 with a body mass index ≥ 32 kg/m 2 with obesity‐related comorbidity or body mass index ≥ 37 kg/m 2 |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decision regret |
| Starting date | May 2018 |
| Contact information | Yen‐Hao Su, Metabolic and Weight Management Center, Shuang‐Ho Hospital |
| Notes | Trial# NCT03578211 |
NCT03631758.
| Study name | Evaluating the impact of evidence‐based information about mammography on breast cancer screening decisions |
| Methods | RCT |
| Participants | 209 English speaking women, 40 to 49 years old |
| Interventions | Patient decision aid + evidence‐based information vs evidence only vs control |
| Outcomes | Decisional conflict, decision quality, preparation for decision‐making, congruency between self‐reported screening status and stated decision |
| Starting date | January 2019 |
| Contact information | Maureen Dobbins, McMaster University |
| Notes | Trial# NCT03631758 |
NCT03766009.
| Study name | Increasing patients' engagement in breast cancer surgery decision‐making |
| Methods | RCT |
| Participants | 598 females aged 18 and older newly diagnosed with stage 0‐III breast cancer planning breast surgery as a component of their definitive treatment |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Self‐efficacy, active patient participation, knowledge, concordance between personal values and surgery received |
| Starting date | March 2019 |
| Contact information | Heather B. Neuman, University of Wisconsin, Madison |
| Notes | Trial# NCT03766009 |
NCT03791138.
| Study name | The impact of a web‐based patient decision aid for women considering breast reconstruction |
| Methods | RCT |
| Participants | 250 females aged 18 and older diagnosed with breast cancer or carcinoma in situ and will be treated with mastectomy and eligible for immediate breast reconstruction |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, decision regret, knowledge, preparation for decision‐making, satisfaction with information, patient's perception of shared decision‐making, actual choice, anxiety |
| Starting date | August 2017 |
| Contact information | Eveline MA Bleiker, The Netherlands Cancer Institute |
| Notes | Trial# NCT03791138 |
NCT03834532.
| Study name | Living well after breast surgery |
| Methods | RCT |
| Participants | 17 female aged 18 and older with new diagnosis of incident or recurrent stage I‐III ductal or lobular carcinoma, or ductal carcinoma in situ |
| Interventions | Patient decision aid vs information |
| Outcomes | Knowledge, concordance of patient values and decisions, decision regret, satisfaction with decision |
| Starting date | February 2019 |
| Contact information | Michael P Pignone, University of Texas at Austin |
| Notes | Trial# NCT03834532 |
NCT03884387.
| Study name | The use of a patient decision aid in the choice of surgery for herniated disc |
| Methods | RCT |
| Participants | 142 adults aged 18 and older with lumbar disc herniation |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision quality, decisional conflict, decisional regret, quality of life |
| Starting date | May 2017 |
| Contact information | Stina B Andersen, Sygehus Lillebaelt |
| Notes | Trial# NCT03884387 |
NCT03905369.
| Study name | Focus on values to stimulate shared decisions |
| Methods | RCT |
| Participants | 128 adults aged 18 and older with thyroid cancer |
| Interventions | Patient decision aid + SDM booster + deliberation training vs training alone |
| Outcomes | Patient doctor communication, problem‐solving decision‐making scale, knowledge, decision evaluation (satisfaction, uncertainty, informed choice, and decision control), trust in oncologist, shared decision‐making process |
| Starting date | March 2020 |
| Contact information | Rosalie Koot, rosalie.koot@radboudumc.nl; Peep Stalmeier, peep.stalmeier@radboudumc.nl |
| Notes | Trial# NCT03905369 |
NCT03921437.
| Study name | Decision support for the renal replacement therapy with end‐stage renal disease |
| Methods | RCT |
| Participants | 76 adults over 20 years old with fifth stage of chronic renal failure |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Control preferences, knowledge, decision self‐efficacy, decisional conflict, satisfaction with decision, decisional regret |
| Starting date | April 2019 |
| Contact information | Tasw Jyy Wang, National Taipei University of Nursing and Health Sciences |
| Notes | Trial# NCT03921437 |
NCT03995381.
| Study name | Using decision aids to reducing decision conflict in angiography patients for choosing hemostasis: a randomized controlled trial |
| Methods | RCT |
| Participants | 150 adults aged 18 to 75 years who need an angiographic examination or treatment |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, knowledge, communication |
| Starting date | October 2019 |
| Contact information | Taipei Medical University Shuang Ho Hospital |
| Notes | Trial# NCT03995381 |
NCT04076332.
| Study name | How "shared decision making decision‐aid" help patients with obstructive sleep apnea to choose treatment plan |
| Methods | RCT |
| Participants | 90 adults aged 20 to 80 with obstructive sleep apnea |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, health literacy |
| Starting date | December 2019 |
| Contact information | Dean Wu, Taipei Medical University Shuang Ho Hospital |
| Notes | Trial# NCT04076332 |
NCT04097717.
| Study name | "My Decision" tubal sterilization decision support tool |
| Methods | RCT |
| Participants | 350 pregnant woman aged 21 to 45 considering tubal sterilization |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict, choice, satisfaction with decision making |
| Starting date | February 2020 |
| Contact information | Sonya Borrero, borrsp@UPMC.edu; Kelsey Schorr, kls234@pitt.edu |
| Notes | Trial# NCT04097717 |
NCT04101409.
| Study name | Impact of shared decision‐making with decision aids on acoustic neuroma treatment choice: a randomized controlled trial |
| Methods | RCT |
| Participants | 78 adults aged 20 and older with acoustic neuroma |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, knowledge, decision regret, depression |
| Starting date | October 2019 |
| Contact information | tsaiyichieh, Taipei Medical University Shuang Ho Hospital |
| Notes | Trial# NCT04101409 |
NCT04103931.
| Study name | Impact of a patient decision aid for treatment of aortic stenosis |
| Methods | RCT |
| Participants | 67 adults aged 18 to 85 years with severe aortic stenosis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, shared decision‐making process, treatment preference, treatment received |
| Starting date | September 2019 |
| Contact information | Karen R Sepucha, Massachusetts General Hospital |
| Notes | Trial# NCT04103931 |
NCT04122989.
| Study name | Validation of a shared decision‐making tool for multiple sclerosis |
| Methods | RCT |
| Participants | 501 adults aged 18 and older with multiple sclerosis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Proportion who start or switch therapy, patient‐provider communication, adherence, decision quality, quality of life, quality of care, decisional conflict |
| Starting date | November 2019 |
| Contact information | Nananda Col, Shared Decision Making Resources |
| Notes | Trial# NCT04122989 |
NCT04175366.
| Study name | Shared decision making in psychiatric inpatient care |
| Methods | RCT |
| Participants | 160 adults aged 18 to 100 admitted to psychiatric inpatient care |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Patient perceived participation, percentage of carried out planned outpatient visits, number of rehospitalisations, days of compulsory care, number of inpatient days, number of emergency visits, percentage of decisions on social support carried out, quality of life |
| Starting date | December 2019 |
| Contact information | Mikael Sandlund, mikael.sandlund@umu.se; Tove Janarv, tove.janarv@umu.se |
| Notes | Trial# NCT04175366 |
NCT04177628.
| Study name | Shared decision making with breast cancer patients |
| Methods | RCT |
| Participants | 664 females aged 18 and older with breast cancer or ductal carcinoma in situ breast cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Participant engagement in the decision‐making process, effectiveness in decision‐making, decision regret, quality of life |
| Starting date | March 2020 |
| Contact information | Stine R Sondergaard, stine.rauff.sondergaard@rsyd.dk |
| Notes | Trial# NCT04177628 |
NCT04240717.
| Study name | Shared decision making on immunotherapy in oncology |
| Methods | RCT |
| Participants | 90 adults aged 18 and older with a diagnosis of metastatic melanoma, stage 3 and 4 |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decision satisfaction, patient involvement in the decision‐making process, choice of treatment option, quality of physician‐patient interaction |
| Starting date | February 2020 |
| Contact information | Christiane Bieber, Heidelberg University |
| Notes | Trial# NCT04240717 |
NCT04241978.
| Study name | Development and evaluation of a web based decision aid for patients with hip osteoarthritis |
| Methods | RCT |
| Participants | 154 adults aged 18 and older with a diagnosis of hip osteoarthritis |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, knowledge, values about characteristics of treatments, treatment preference, intention to undergo the preferred treatment, concordance between values and intention to undergo treatments, decision quality, satisfaction with the decision‐making process |
| Starting date | February 2020 |
| Contact information | Pedro Serrano Aguilar, pseragu@gobiernodecanarias.org; Lilisbeth Perestelo Perez, lilisbeth.peresteloperez@sescs.es |
| Notes | Trial# NCT04241978 |
NCT04260737.
| Study name | Interactive decision aid for men diagnosed with prostate cancer |
| Methods | RCT |
| Participants | 200 adults aged 18 and older newly diagnosed with localized prostate cancer |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, decisional regret, satisfaction with decision, anxiety, depression, stress |
| Starting date | February 2020 |
| Contact information | Valgerdur K Eiriksdottir, valgerdure@ru.is |
| Notes | Trial# NCT04260737 |
NCT04270630.
| Study name | A pilot proof of concept, randomized controlled, single‐center study of a decision aid tool for older patients considering LHC as treatment for NSTEMI |
| Methods | RCT |
| Participants | 50 adults aged 75 and older with non‐ST elevation myocardial infarction eligible for non‐urgent revascularization |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, anxiety, depression, decision self‐efficacy, knowledge |
| Starting date | July 2020 |
| Contact information | John Dodson, NYU Langone Health |
| Notes | Trial# NCT04270630 |
NCT04272177.
| Study name | Influence of shared‐decision making in reducing decision conflict on the choice of awakening agent after general anesthesia |
| Methods | RCT |
| Participants | 3309 adults aged 20 and older who will receive general anesthesia |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision conflict, knowledge, percentage of choices of reversal drugs |
| Starting date | March 2020 |
| Contact information | Ka‐Wai Tam, Taipei Medical University Shuang Ho Hospital |
| Notes | Trial# NCT04272177 |
NCT04291040.
| Study name | Use of an educational multimedia tool versus routine care for the uptake of postpartum LARC in high‐risk pregnancies (SUSTAIN) |
| Methods | RCT |
| Participants | 380 females aged 13 to 50 who have high risk pregnancy due to either maternal medical conditions or obstetric/neonatal complications |
| Interventions | Decision aid vs usual care |
| Outcomes | Rate of initial LARC utilization, number of patients who keep the LARC after placement |
| Starting date | July 2020 |
| Contact information | Emma Jean Qureshey, The University of Texas Health Science Center, Houston |
| Notes | Trial #NCT04291040 |
NCT04357288.
| Study name | Randomized evaluation of decision support interventions for atrial fibrillation |
| Methods | RCT |
| Participants | 1200 adults aged 18 and older diagnosed with atrial fibrillation with additional risk of thromboembolic events |
| Interventions | Patient decision aid vs encounter decision aid vs patient and encounter decision aids vs usual care |
| Outcomes | Decisional conflict, knowledge, shared decision‐making, decision regret, preparation for decision‐making, quality of communication, control preference scale, patient satisfaction with the decision aid, concordance between the participant and the clinician, adherence, treatment choice, encounter length |
| Starting date | December 2020 |
| Contact information | Maddie McCarty, maddie.mccarty@hsc.utah.edu; Elissa Ozanne, elissa.ozanne@hsc.utah.edu |
| Notes | Trial# NCT04357288 |
NCT04364958.
| Study name | Decision aid for patients with generalized anxiety disorder: protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | 156 adults aged 18 and older with a diagnosis of generalized anxiety disorder |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, knowledge, treatment preference, actual treatment choice, concordance between preference and choice, decision quality |
| Starting date | May 2021 |
| Contact information | Lilisbeth Perestelo Pérez, lilisbeth.peresteloperez@sescs.es; Pedro Serrano Aguilar, pseragu@gobiernodecanarias.org |
| Notes | Trial# NCT04364958 |
NCT04373590.
| Study name | Decision‐making and decision support among emerging adults with first episode psychosis |
| Methods | RCT |
| Participants | 18 adults aged 18 to 25 experiencing early psychosis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decision‐making self‐efficacy, decision‐making attitudes, decisional conflict, shared decision‐making, adherence, service use, service engagement |
| Starting date | February 2019 |
| Contact information | Yaara Zisman Ilani, Temple University |
| Notes | Trial# NCT04373590 |
NCT04378816.
| Study name | A patient‐centered continuous and interdisciplinary shared decision making approach for breast cancer rehabilitation |
| Methods | RCT |
| Participants | 264 patients aged 20 and older with a diagnosis of breast cancer |
| Interventions | Patient decision aid vs no intervention |
| Outcomes | Control Preference Scale, Patient‐Physician Interactions Questionnaire, Decision Self Efficacy Scale, Patients' Perceived Involvement in Care Scale, Health Care Climate Questionnaire, SURE Test, State‐Trait Anxiety Inventory, shared decision‐making (collaboRATE) |
| Starting date | May 2020 |
| Contact information | Wen‐Hsuan Hou, Taipei Medical University |
| Notes | Trial #NCT04378816 |
NCT04397016.
| Study name | Cost talk: discussing cancer care costs |
| Methods | Stepped wedge RCT |
| Participants | 117 adults aged 18 and older with slow growing prostate cancer visiting a participating urologist/urologic surgeon to discuss treatment options |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Frequency of cost conversations, initiator (surgeon, patient, or caregiver) of cost conversations, whether or not a referral is made to address costs, decisional conflict, shared decision‐making, decision regret |
| Starting date | June 2020 |
| Contact information | Mary Politi, Washington University School of Medicine; Glyn Elwyn, Dartmouth College |
| Notes | Trial# NCT04397016 |
NCT04410029.
| Study name | Evaluation of a decision aid for early pregnancy loss |
| Methods | RCT |
| Participants | 60 adults aged 18 and older with a definitive diagnosis of early intrauterine pregnancy loss |
| Interventions | Patient decision aid vs information |
| Outcomes | Decision conflict, knowledge, decision regret, shared decision‐making |
| Starting date | July 2020 |
| Contact information | University of Pennsylvania |
| Notes | Trial# NCT04410029 |
NCT04437069.
| Study name | Improving patient and family health using family‐centered outcomes and shared decision‐making |
| Methods | RCT |
| Participants | 215 parents aged 18 and older whose fetus/neonate was diagnosed with a life‐threatening congenital heart disease |
| Interventions | Patient decision aid + values clarification exercise vs decision aid only vs usual care |
| Outcomes | Distress, decision quality ‐ values, decision quality ‐ knowledge, effectiveness of risk communication, preference for shared decision‐making, preparation for decision‐making, decision self‐efficacy, decisional conflict, decisional regret, treatment choice and treatment received, control preferences, acceptability of the decision aid, consultation quality |
| Starting date | October 2020 |
| Contact information | Angela Fagerlin, University of Utah |
| Notes | Trial# NCT04437069 |
NCT04496739.
| Study name | Making informed choices on incorporating chemoprevention into care (MiCHOICE) |
| Methods | RCT |
| Participants | 415 women aged 35 to 74 with atypical hyperplasia or lobular carcinoma in situ |
| Interventions | Patient decision aid vs information |
| Outcomes | Informed choice, perceived risk, actual risk score, accuracy of risk perception, worry, decision conflict, decision regret, chemoprevention usage, adherence, shared decision‐making |
| Starting date | September 2020 |
| Contact information | Katherine D Crew, Southwest Oncology Group |
| Notes | Trial# NCT04496739 |
NCT04504084.
| Study name | Influence of patient decision‐making aids for patients with unilateral ureteral stone: a randomized‐controlled trial |
| Methods | RCT |
| Participants | 100 adults aged 18 to 75 with ureteral stone |
| Interventions | Patient decision aid vs information |
| Outcomes | Decision conflict |
| Starting date | September 2020 |
| Contact information | Yi‐Te Chiang, Taipei Medical University Shuang Ho Hospital |
| Notes | Trial# NCT04504084 |
NCT04548531.
| Study name | Engaging patients in colon cancer screening decisions during COVID‐19 |
| Methods | RCT |
| Participants | Adults aged 45 to 75 who had screening or surveillance colonoscopy delayed or canceled from March to June 2020 |
| Interventions | Decision aid vs usual care |
| Outcomes | Shared decision‐making, decisional conflict, preferred approach to screening, number reporting "very likely" to follow through with screening, colon cancer screening rate |
| Starting date | September 2020 |
| Contact information | Karen Sepucha, Massachusetts General Hospital |
| Notes | Trial #NCT04548531 |
NCT04549571.
| Study name | Improving patient‐centered communication in breast cancer through patient and provider interventions |
| Methods | RCT |
| Participants | Women newly diagnosed with stage 0‐III breast cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, patient self‐efficacy, patient cancer worry |
| Starting date | January 2021 |
| Contact information | Sarah T. Hawley, sarahawl@umich.edu |
| Notes | Trial #NCT04549571 |
NCT04584294.
| Study name | Patient‐centered reproductive decision support tool for women veterans |
| Methods | RCT |
| Participants | 456 females aged 18 to 44 interested in receiving information or talking with their provider about pregnancy and/or birth control |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Shared decision‐making, perceived self‐efficacy in communicating with providers, knowledge, decisional conflict, confidence, choice of treatment, use of treatment, adherence, satisfaction with treatment |
| Starting date | March 2021 |
| Contact information | Lisa S Callegari, lisa.callegari@va.gov; Samantha K Benson, Samantha.Benson@va.gov |
| Notes | Trial# NCT04584294 |
NCT04621760.
| Study name | The OPENS trial: offering women PrEP (Aim 1) |
| Methods | RCT |
| Participants | 384 women aged 18 to 45 years old not known to be living with HIV |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Number of prescriptions, treatment use, perceived risk, knowledge, decisional conflict, intention for treatment, satisfaction with information received, perceived quality of information received, treatment adherence |
| Starting date | May 2021 |
| Contact information | Whitney Wilson, whitney.wilson@ucsf.edu; Dominika Seidman, dominika.seidman@ucsf.edu |
| Notes | Trial# NCT04621760 |
NCT04692987.
| Study name | Effectiveness of decision‐aid video on colorectal cancer screening |
| Methods | RCT |
| Participants | 400 Malaysian adults aged 50 to 74 with no previous history or family history of colorectal cancer who have never participated in screening |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Number who underwent screening, time from intervention to screening uptake, barriers to screening uptake |
| Starting date | March 2021 |
| Contact information | Azmawati Mohammed Nawi, azmawati@ppukm.ukm.edu.my |
| Notes | Trial# NCT04692987 |
NCT04725565.
| Study name | Genetics adviser: evaluating a digital decision support tool for genetic results |
| Methods | RCT |
| Participants | 130 cancer patients aged 18 and older who have had genomic sequencing for their cancer (but did not receive incidental findings) or adult patients who have had a negative genetic panel test |
| Interventions | Patient decision aid + counseling vs counseling only |
| Outcomes | Decisional conflict, knowledge, satisfaction with decision, preparation for decision‐making, anxiety, depression, acceptability, time with genetic counselor |
| Starting date | June 2021 |
| Contact information | Marc Clausen, Marc.Clausen@unityhealth.to |
| Notes | Trial# NCT04725565 |
NCT04741503.
| Study name | Project Insight: feasibility of a breast cancer screening decision support tool |
| Methods | RCT |
| Participants | 1277 Latina, Black, or non‐Latina White women aged 40 to 49 |
| Interventions | Patient decision aid vs information |
| Outcomes | Knowledge, decisional conflict subscales (uncertainty, informed, values clarity, and support), decision self‐efficacy, preparation for decision making |
| Starting date | April 2021 |
| Contact information | Ashley J Housten, Washington University School of Medicine |
| Notes | Trial# NCT04741503 |
NCT04748380.
| Study name | Shared decision‐making and colorectal cancer screening |
| Methods | RCT |
| Participants | 60 participants aged 75 to 85 with low health literacy |
| Interventions | Patient decision aid vs attention control |
| Outcomes | Screening intentions, knowledge, perceptions of shared decision‐making role |
| Starting date | January 2023 |
| Contact information | Tamara Cadet, cadet@upenn.edu |
| Notes | Trial #NCT04748380 |
NCT04805554.
| Study name | Incorporating patient‐reported outcomes into shared decision making with patients with osteoarthritis of the hip or knee |
| Methods | RCT |
| Participants | 200 patients aged 45 to 89 with knee OA |
| Interventions | Patient decision aid vs education |
| Outcomes | Patient perception of decision process, decision quality, concordance between patient preferences and actual outcomes, patient perception of the level of shared decision‐making, patient/provider satisfaction with discussion, total consultation time (minutes), patient‐reported overall health, treatment selected, decisional conflict, decision regret |
| Starting date | February 2021 |
| Contact information | Lauren Uhler, lauren.uhler@austin.utexas.edu |
| Notes | Trial #NCT04805554 |
NCT04837053.
| Study name | Implementation of indication criteria for total knee replacement in osteoarthritis (Value‐based TKR) |
| Methods | Cluster‐RCT |
| Participants | 1080 patients aged 18 and older with knee osteoarthritis who are candidates for knee replacement |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decision quality, fulfillment of patient expectations, Oxford Knee Score |
| Starting date | June 2021 |
| Contact information | Franziska Beyer, Franziska.Beyer@uniklinikum‐dresden.de |
| Notes | Trial #NCT04837053 |
NCT04858282.
| Study name | Application‐enabled shared decision‐making |
| Methods | RCT |
| Participants | 31 women aged 20 and older and newly diagnosed early breast cancer (stages 0‐II) |
| Interventions | Patient decision aid vs information |
| Outcomes | Knowledge, decisional conflict, decision regret |
| Starting date | August 2019 |
| Contact information | Chia‐Wen, Chuang, Chang Gung Memorial Hospital |
| Notes | Trial# NCT04858282 |
NCT04869917.
| Study name | Behavioral nudges for diabetes prevention (BEGIN) trial in primary care (BEGIN) |
| Methods | RCT |
| Participants | Adults aged 18 to 80 with prediabetes |
| Interventions | Decision aid vs usual care |
| Outcomes | Weight, participant initiation of treatment to intensive lifestyle or metformin |
| Starting date | March 2022 |
| Contact information | Matthew J O'Brien, Northwestern University |
| Notes | Trial #NCT04869917 |
NCT04879745.
| Study name | MyVoice:Rheum decision aid for women with rheumatic diseases |
| Methods | RCT |
| Participants | 50 females aged 18 to 44 with at least one of 4 rheumatic diseases diagnosed by a rheumatologist: rheumatoid arthritis, systemic sclerosis, myositis, and systemic lupus erythematosus |
| Interventions | Patient decision aid vs information vs provider experience |
| Outcomes | Acceptability/usability of intervention, knowledge, shared decision‐making, perceived efficacy in patient‐physician interactions, change in pregnancy intention, receipt of care, satisfaction with family planning conversation, decisional conflict, interpersonal quality of care |
| Starting date | July 2021 |
| Contact information | Olivia M Stransky, olivia.stransky@pitt.edu; Alison Decker, apd22@pitt.edu |
| Notes | Trial# NCT04879745 |
NCT04919941.
| Study name | A pilot study of a guide to conservative care |
| Methods | RCT |
| Participants | 92 adults aged 75 or older with advanced chronic kidney disease who do not wish to pursue maintenance dialysis |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Patient‐provider discussions of conservative care, attrition, treatment preference, treatment goals, acceptability of the intervention |
| Starting date | August 2020 |
| Contact information | Susan P Wong, University of Washington |
| Notes | Trial# NCT04919941 |
NCT04940936.
| Study name | Shared decision making on radiation dose for lung malignancies |
| Methods | RCT |
| Participants | 40 patients aged 18 and older with non‐small cell lung cancer, or metastasis from other cancer, located ≤ 1 cm from the thoracic wall |
| Interventions | Decision aid vs usual care |
| Outcomes | Shared decision‐making, decisional conflict, decision regret |
| Starting date | November 2021 |
| Contact information | Thomas L Fink, thomas.leth.fink@rsyd.dk |
| Notes | Trial #NCT04940936 |
NCT04946279.
| Study name | Decision aid for the improvement of decision‐making in patients with non‐small cell lung cancer |
| Methods | RCT |
| Participants | 100 patients with non‐small cell lung cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Feasibilty/acceptablity of the intervention, anxiety, decisional conflict, decisional regret, perceived involvement in care, shared decision‐making quality, decision‐making involvement, self‐efficacy, values‐treatment concordance |
| Starting date | August 2020 |
| Contact information | Donald Sullivan, OHSU Knight Cancer Institute |
| Notes | Trial# NCT04946279 |
NCT04948983.
| Study name | The effect of a patient decision aids for breast cancer screening |
| Methods | RCT |
| Participants | 3269 women aged 50 to 69 attending primary care centers |
| Interventions | Patient decision aid vs information |
| Outcomes | Informed choice, decisional conflict, depression, anxiety and stress, satisfaction with the decision, uptake of screening |
| Starting date | July 2021 |
| Contact information | Paulina Bravo, pbbravo@uc.cl; Alejandra Martinez, alejandra.martinez@uc.cl |
| Notes | Trial# NCT04948983 |
NCT04956978.
| Study name | Shared decision making to address racial disparities in oral anticoagulation in NVAF |
| Methods | RCT |
| Participants | 40 adults aged 18 and older with with non‐valvular atrial fibrillation |
| Interventions | Patient decision aid + counseling vs counseling only |
| Outcomes | Study feasibility outcomes, decision quality, decision to initiate treatment |
| Starting date | July 2023 |
| Contact information | Larry Jackson, larry.jackson@duke.edu |
| Notes | Trial# NCT04956978 |
NCT05033067.
| Study name | The personal patient profile decision support for patients with bladder cancer |
| Methods | RCT |
| Participants | 45 adults aged 18 and older with bladder cancer undergoing radical cystectomy (bladder removal) |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Acceptability, shared decision‐making, decisional conflict, control preferences scale, communication with providers, knowledge |
| Starting date | June 2021 |
| Contact information | Nihal Mohamed, nihal.mohamed@mountsinai.org; Holden Kata, holden.kata@mountsinai.org |
| Notes | Trial# NCT05033067 |
NCT05091944.
| Study name | An interactive web‐based birth decision aid for shared decision making |
| Methods | RCT |
| Participants | 86 pregnant women who have had one previous cesarean with at least a half year interval between current pregnancy and the previous birth |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, knowledge, preference, acceptability of the decision aid, satisfaction with the decision |
| Starting date | September 2021 |
| Contact information | Shu Wen Chen, shuwen@ntunhs.edu.tw; Chang‐Ching Yeh, ccyeh39@gmail.com |
| Notes | Trial# NCT05091944 |
NCT05130580.
| Study name | Patient‐specific decision aid system for shared decision making about breast reconstruction |
| Methods | RCT |
| Participants | 40 adults aged 21 and older planning to undergo mastectomy and considering immediate breast reconstruction |
| Interventions | Patient decision aid + enhanced consult + education vs education + standard care |
| Outcomes | Length of consultation visit, decisional conflict |
| Starting date | October 2020 |
| Contact information | Gregory Reece, greece@mdanderson.org |
| Notes | Trial# NCT05130580 |
NCT05135156.
| Study name | Lung transplant READY pilot study |
| Methods | RCT |
| Participants | 50 adults aged 18 and older with cystic fibrosis |
| Interventions | Patient decision aid vs information |
| Outcomes | Preparedness for shared decision‐making, knowledge, decisional conflict, preparedness to discuss lung transplant, anxiety |
| Starting date | December 2021 |
| Contact information | Lauren Bartlett, lrejman@uw.edu |
| Notes | Trial# NCT05135156 |
NCT05177783.
| Study name | Contraception decision aid use and patient outcomes |
| Methods | RCT |
| Participants | 500 females 18 to 34 years of age |
| Interventions | Patient decision aid vs control (no intervention) |
| Outcomes | Decisional conflict, self‐efficacy, knowledge, intention to use treatment, patient satisfaction |
| Starting date | January 2022 |
| Contact information | Sarah E Hill, s.e.hill@tcu.edu; Summer Mengelkoch, s.mengelkoch@tcu.edu |
| Notes | Trial# NCT05177783 |
NCT05182008.
| Study name | A patient decision aid for method of early abortion: a randomized control trial |
| Methods | RCT |
| Participants | 440 females of reproductive age seeking termination of pregnancy |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, satisfaction with decision, decision concordance, knowledge |
| Starting date | May 2022 |
| Contact information | Melissa Brooks, melissa.brooks@iwk.nshealth.ca |
| Notes | Trial# NCT05182008 |
NCT05219786.
| Study name | Online field test of an appendicitis decision support tool |
| Methods | RCT |
| Participants | 194 adults aged 18 and older who have not previously had appendicitis |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, acceptability, trust and accuracy of information |
| Starting date | October 2021 |
| Contact information | David R Flum, University of Washington |
| Notes | Trial# NCT05219786 |
NL7939.
| Study name | Decision aid for breast reconstruction after mastectomy: a randomized controlled trial |
| Methods | RCT |
| Participants | 120 females aged 18 and older who have undergone mastectomy |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, satisfaction (with visit, with information), shared decision‐making, treatment choice, consultation time, number of consultations, patient‐rated physician empathy, anxiety, depression, decision regret, changes in treatment choice |
| Starting date | June 2020 |
| Contact information | Claudia Bargon, c.bargon@antoniusziekenhuis.nl |
| Notes | Trial# NL7939 |
NL9666.
| Study name | RCT for evaluation of a personalized online decision aid for colorectal cancer screening participation |
| Methods | RCT |
| Participants | 324 men and women aged 45 to 55 years |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, clarity of values, deliberation, anxiety, risk perception, intention to participate, usability and acceptability |
| Starting date | July 2021 |
| Contact information | Linda Pluymen, l.p.m.pluymen@amsterdamumc.nl |
| Notes | Trial# NL9666 |
NTR4435.
| Study name | Improving patient involvement in the decision for joint replacement surgery, using decision aids |
| Methods | RCT |
| Participants | 256 adults aged > 18 and older with moderate or severe osteoarthritis in either knee or hip |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, satisfaction, physical function, anxiety, health consumption |
| Starting date | March 2014 |
| Contact information | M. Hageman, michiel.hageman@amc.uva.nl |
| Notes | Trial# NTR4435 |
NTR5467.
| Study name | Decision‐support for couples with hereditary cancer and child wish: weighing pros and cons of reproductive options regarding transmission of gene mutations |
| Methods | RCT |
| Participants | 256 woman in reproductive age (18 to 40 years) with hereditary cancer and active child wish |
| Interventions | Patient decision aid vs information |
| Outcomes | Decisional conflict, knowledge, accuracy of perceived risks, satisfaction with the decision and the decision‐making process, decision self‐efficacy, informed choice |
| Starting date | February 2017 |
| Contact information | Kelly Reumkens, kelly.reumkens@mumc.nl |
| Notes | Trial# NTR5467 |
NTR5785.
| Study name | Effect of a decision aid about postoperative epidural analgesia on patients' knowledge: a randomized controlled trial |
| Methods | RCT |
| Participants | 300 adults aged 18 and older undergoing major thoracic or abdominal surgery with indication for epidural postoperative analgesia |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, preferred and perceived participation, satisfaction/uncertainty, informed choice, decision control |
| Starting date | June 2016 |
| Contact information | Amy van den Berg, Amy.vandenBerg@Radboudumc.nl |
| Notes | Trial# NTR5785 |
NTR6070.
| Study name | Study on shared decision making in choosing a treatment for pelvic organ prolapse |
| Methods | RCT |
| Participants | 415 women with symptomatic pelvic organ prolapse for whom a (new) treatment must be chosen |
| Interventions | Patient decision aid vs information |
| Outcomes | Satisfaction with treatment decision (making), and satisfaction with information, satisfaction with care and treatment, decisional conflict, decisional regret, quality of life |
| Starting date | December 2016 |
| Contact information | M.C. Vos, c.vos@elisabeth.nl |
| Notes | Trial# NTR6070 |
NTR6379.
| Study name | Shared decision making in patients with castration‐resistant prostate cancer |
| Methods | RCT |
| Participants | 168 men that are newly diagnosed with castration‐resistant prostate cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, informed choice, correlation between G8 score and treatment decision, correlation between TUG‐test and treatment decision, quality of life, anxiety, value clarification, satisfaction with decision‐making, information and treatment, preparation for decision‐making, partner involvement in SDM, treatment outcome |
| Starting date | September 2016 |
| Contact information | Isabel de Angst, i.deangst@etz.nl |
| Notes | Trial# NTR6379 |
O'Connor 2019.
| Study name | Evaluating a patient decision aid for people with degenerative knee disease considering arthroscopic surgery: protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | 592 adults aged 45 and older with doctor‐diagnosed degenerative knee disease considering knee arthroscopy |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Referral to an orthopedic surgeon, attendance at an orthopedic surgeon consultation, attitudes towards knee arthroscopy, informed choice (composite measure of knowledge, attitudes and treatment intentions), treatment intentions, actual choice, knowledge, decisional conflict, satisfaction with preparation for making a decision |
| Starting date | February 2022 |
| Contact information | Denise O’Connor, Monash University and Cabrini Health, denise.oconnor@monash.edu |
| Notes | Trial# ACTRN12622000204741 |
Patzer 2019.
| Study name | A culturally sensitive web‐based intervention to improve living donor kidney transplant among African Americans |
| Methods | RCT |
| Participants | 850 African American or Black adults aged 18 to 65 years referred and scheduled for a transplant medical evaluation |
| Interventions | Patient decision aid + education vs education alone |
| Outcomes | Knowledge, confidence |
| Starting date | February 2019 |
| Contact information | Rachel Patzer, rpatzer@emory.edu |
| Notes | Trial# NCT03819686 |
Rahn 2021.
| Study name | Evaluation of an interactive web‐based programme on relapse management for people with multiple sclerosis (POWER@MS2): study protocol for a process evaluation accompanying a randomised controlled trial |
| Methods | RCT |
| Participants | 160 adults aged 18 to 65 years clinically isolated syndrome, suspected or diagnosed relapsing remitting multiple sclerosis |
| Interventions | Patient decision aid vs information |
| Outcomes | Change in treatment, knowledge, control preferences, patient activation measure, quality of life, depression and anxiety, health economic evaluation |
| Starting date | February 2020 |
| Contact information | Sascha Köpke, Institute of Nursing Science, University of Cologne; Anne C Rahn, Institute of Social Medicine and Epidemiology, Nursing Research Unit, University of Lübeck |
| Notes | Trial# NCT04233970 |
Rieckert 2019.
| Study name | Reduction of the long‐term use of proton pump inhibitors by a patient‐oriented electronic decision support tool (arriba‐PPI): study protocol for a randomized controlled trial |
| Methods | Cluster‐RCT |
| Participants | 3060 patients with a regular prescription of proton pump inhibitors of ≥ 6 months |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Medication use |
| Starting date | December 2018 |
| Contact information | Anja Rieckert, ed.hw‐inu@trekc |
| Notes | Trial# DRKS00016364 |
Samalin 2018.
| Study name | Efficacy of shared decision‐making on treatment adherence of patients with bipolar disorder: a cluster randomized trial (ShareD‐BD) |
| Methods | Cluster‐RCT |
| Participants | 300 adults with bipolar disorder |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Treatment adherence, decisional conflict, satisfaction with care and involvement in decision‐making, beliefs about treatment, therapeutic relationship, knowledge, clinical outcomes (depression, mania, functioning, and quality of life) and feasibility of SDM processes in clinical practice |
| Starting date | April 2018 |
| Contact information | Ludovic Samalin, lsamalin@chu‐clermontferrand.fr |
| Notes | Trial# NCT03245593 |
Schoenfeld 2021.
| Study name | Feasibility and efficacy of a decision aid for emergency department patients with suspected ureterolithiasis: protocol for an adaptive randomized controlled trial |
| Methods | RCT |
| Participants | 250 adults age 18 to 55 presenting to the emergency department with a chief complaint of flank pain who are being considered by the treating clinician for a CT scan for the diagnosis of ureterolithiasis |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, CT scan rate, patient satisfaction, patient engagement, occurrence of shared decision‐making, trust in physician, emergency department revisits, emergency department length of stay |
| Starting date | December 2019 |
| Contact information | Kye Poronsky, Kye.Poronsky@baystatehealth.org |
| Notes | Trial# NCT04234035 |
CA‐125 : cancer antigen 125; CAD : coronary artery disease; CT : computerized tomography; LARC : long‐acting reversible contraceptive; NIH : National Institutes of Health; NSW : New South Wales; OA : osteoarthritis; PSA : prostate specific antigen; RCT : randomized controlled trial; SDM : shared decision‐making.
Differences between protocol and review
There are three main differences between the original protocol and the review. We re‐structured the 2009 update, O'Connor 2009b , to organize the long list of outcomes into primary and secondary outcomes based on the new effectiveness criteria of the International Patient Decision Aid (IPDAS) Collaboration ( Elwyn 2006 ). For the 2011 update, Stacey 2011 , we changed the study quality assessment to the risk of bias tool ( Higgins 2011 ). For the 2014 update, Stacey 2014b , we used GRADE to summarize the quality of the evidence and reported the results using Table 1 . For the 2017 update, we removed 28 studies that compared detailed versus simple decision aids and limited comparisons to patient decision aids versus usual care to provide a more focused review.
For the 2023 (current) update, we stopped reporting on some outcomes, including anxiety, depression, quality of life, other condition‐specific health outcomes, total decisional conflict (SURE test and subscales of unsupported, uncertainty, ineffective choice), and litigation rates. The reduction in outcomes was based on the guidance from the Cochrane Handbook for Systematic Reviews of Interventions on staying focused on the most relevant outcomes ( Higgins 2022 ).
Contributions of authors
1999 Review ( O'Connor 1999b ): AO, AR, VF, JT, VE, HLT, MHR, VF, MB, and JJ contributed to the design of the protocol, the interpretation of results, and the revision and approved the final paper. AO led the team, and JT co‐ordinated the project. AO, MH‐R, AR, VF, and JT pilot‐tested the data extraction forms. AR, VF, and JT screened studies and extracted data. AR, JT, and AO analyzed the results.
2001 Review ( O'Connor 2001 ): AO, DS, DR, MHR, HLT, VE, MB, JT, VF, and AR contributed to the interpretation of results and the revision, and approved the final paper. AO led the team, and DS co‐ordinated the update. AO, DR, MHR, HLT, JT, DS, and JP screened studies and extracted data. DS and JP evaluated decision aids using the CREDIBLE criteria. AO and DS analyzed the results.
2002 Review ( O'Connor 2003 ): AO, DS, DR, MHR, HLT, VE, MB, JT, and VF contributed to the interpretation of results and the revision, and approved the final paper. AO led the team, and DS co‐ordinated the update. DS, JP, VT, and JT screened studies and extracted data. DS, JP, VT, and SK evaluated decision aids using the CREDIBLE criteria. AO and DS analyzed the results.
2006 Review ( O'Connor 2009b ): AO, CB, DS, MB, NC, KE, VE, VF, MHR, SK, HLT, and DR contributed to the interpretation of results, and the revision and final approval of the paper. AO led the team and CB co‐ordinated the update. CB, SK, DS, AO, and VF screened studies and extracted data. AO and CB analyzed the results.
2009 Review ( Stacey 2011 ):
DS, CB, MB, NC, KE, FL, AL, MHR, HLT, and RT contributed to the interpretation of results and the revision, and approved the final paper. DS led the team, and CB co‐ordinated the update. CB and DS screened studies; SM and AD extracted data; CB entered the data; DS verified the data entered. DS and CB analyzed the results.
2013 Review ( Stacey 2014b ):
DS, CB, MB, NC, KE, FL, AL, MHR, HLT, RT, and LT contributed to the interpretation of results and the revision, and approved the final paper. DS led the team with help co‐ordinating the update from SB and JW. CB, DS, RT, MB, MHR, NC, KE, BV, DR, and AS screened studies; SB, RW, JW, and CC extracted data; SB and JW entered the data; DS verified the data entered. DS and JW analyzed the results.
2017 Review ( Stacey 2017 ):
DS, CB, MB, KE, FL, AL, MHR, HLT, RT, LT, and KL contributed to the interpretation of results and the revision, and approved the final paper. DS led the team with help co‐ordinating the update from KL. CB, DS, RT, MB, MHR, KE, DR, and AS screened studies; KL and IS extracted data; KL entered the data; DS verified the data entered. DS analyzed the results.
2023 Review (Current):
DS, KBL, MS, MC, RJV, EED, LPB, JF, JG, MJB, CLB, PB, KS, AG, IDG, SEK, FL, HS, RT, LoT, and LyT contributed to decisions about changes in the outcomes included in this update, the interpretation of results, and revisions to the paper, and approved the final paper. DS and KBL led the team with help co‐ordinating the update from MC.
CB, MB, MC, KDS, ED, JF, AG, KBL, LPB, DS, RT, and RJV screened studies; LBP extracted data on all newly included studies; DS, KBL, RJV, EED, and PB (and graduate students in acknowledgments) extracted data on some of the newly included studies; MC entered the data; DS and KBL verified the data entered. DS, KBL, and MC analyzed the results.
Sources of support
Internal sources
-
University of Ottawa, Canada
University Research Chair in Knowledge Translation to Patients
-
Ottawa Hospital Research Institute, Canada
Scientific Director, Patient Decision Aids Research Group
External sources
-
Canadian Institutes of Health Research, Canada
Operating Grant from the Canadian Institutes of Health Research
Declarations of interest
Several of the investigators have developed patient decision aids, but none made study eligibility decisions about, extracted data from, performed risk of bias assessment for, or assessed GRADE certainty of their own studies where they were included in this review update.
DS: no relevant interests; Professor in the School of Nursing (no clinical work); Co‐chair, International Patient Decision Aid Standards Collaboration; involved in conducting a study that is eligible for inclusion in this review (to evaluate a patient decision aid produced by the Foundation in Ottawa in a RCT; funded by Foundation for Informed Decision Making); involved in conducting a study that is eligible for inclusion (Patient Decision Aid, University of Ottawa Heart Institute; funded by Canadian Institutes of Health Research; Canadian Council of Cardiovascular Nurses).
KBL: Canadian Cardiovascular Society (Grant/Contract); Canadian Institutes for Health Research (Grant/Contract); Cardiac Arrhythmia Network of Canada (Grant/Contract); Heart and Stroke Foundation of Canada (Grant/Contract); licensed Registered Nurse in Ontario, Canada; involved in conducting a study that is eligible for inclusion (Patient Decision Aid, University of Ottawa Heart Institute; funded by Canadian Institutes of Health Research; Canadian Council of Cardiovascular Nurses).
MS: none known.
MC: none known.
RJV: no relevant interests; involved in a randomized trial of a patient decision aid for lung cancer screening (JAMA Network Open. 2020;3(1):e1920362. doi:10.1001/jamanetworkopen.2019.20362. Funding source: Patient‐Centered Outcomes Research Institute); involved in a randomized trial of a patient decision aid about prostate cancer screening (Archives of Family Medicine 1999;8(4):333‐40. [CRS ID: 3133593]. Funding source: internally funded). Both studies are included in this review update.
EED: none known.
LP‐B: no relevant interests; registered physiotherapist with the Ordre Professionnel de la Physiothérapie du Québec, but has never worked as a physiotherapist; is a recipient of the Arthritis Society PhD Salary Award supporting PhD studies from September 2021 to September 2023.
JF: no relevant interests; Clinical Nurse Specialist at Aarhus University Hospital.
JG: none known.
MB: Healthwise (Employment, end date: 31 March 2017; grantee, end date: 30 June 2021); National Cancer Institute (Consultant, end date: 6 January 2017); United States Preventive Services Task Force (Chair, ongoing); Indiana University (Consultant, end date: 25 August 2017); multiple publications on shared decision‐making; Informed Medical Decisions Foundation (pre‐2017), Healthwise had statements supporting SDM (both nonprofits; USPSTF has supported and published on SDM); recipient of AHRQ grant for an RCT of a BPH decision aid, many years ago, included in the review.
CLB: none known.
PB: no relevant interests; podcasts for CDC Empowerment related to shared decision‐making; board member of the International Shared Decision Making Society.
KDS: none known.
AG: none known.
IDG: none known.
SEK: none known.
FL: no relevant interests; family medicine doctor in the public healthcare system in Canada; involved in conducting a study that is eligible for inclusion (funded by the Canadian Institute of Health Research and FRSQ).
HS: no relevant interests; volunteer at the Danish Kidney Association, a not‐for‐profit, patient‐run patient organization.
RT: no relevant interests; number of publications related to decision aids and shared decision‐making; RCT of PDA carried out at Newcastle University (Thomson R, Eccles M, Steen N, Greenaway J, Stobbart L, Murtagh M, May C. A patient decision aid to support shared decision making on antithrombotic treatment of patients with atrial fibrillation: randomised controlled trial. QSHC 2007; 16: 216‐223. Funded NHS UK R&D).
LoT: no relevant interests; published work on the development and evaluation of patient decision aids. This includes publishing work as a member of the International Patient Decision Aids Standards Collaboration (unpaid); conducted a cost‐effectiveness analysis of an RCT led by Dawn Stacey. This included a trial‐based economic evaluation and a longitudinal resource use/cost analysis. The RCT was funded by the Informed Medical Decisions Foundation (conducted analysis while being funded through a CIHR doctoral award; both analyses were published in Osteoarthritis and Cartilage while at the University of British Columbia as a PhD student).
LyT: Australian Commission on Safety and Quality in Health Care (Member of the Patients as Partners Committee); University of Sydney (Employment); Agency for Clinical Innovation (Consultant); peer reviewed publications on shared decision‐making; pro bono clinical work as a GP with refugees; involved in National Health and Medical Research Foundation (NHMRC)‐funded trial of decision aids for antibiotic use with respiratory infections (excluded from this review update) and also a NHMRC‐funded trial of decision aid for colon cancer screening (included study).
These authors should be considered joint first author
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Allen 2010 {published data only}
- Allen JD , Othus MK , Hart A Jr, Tom L , Li Y , Berry D , et al. A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the take the wheel trial . Cancer Epidemiology, Biomarkers and Prevention 2010. ; 19 ( 9 ): 2172-86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD , Othus MKD , Hart A Jr, Mohllajee AP , Bowen D . Do men make informed decisions about prostate cancer screening? Baseline results from the "Take the Wheel" Trial . Medical Decision Making 2011. ; 31 : 108-120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2018 {published data only}
- Allen LA , McIlvennan CK , Thompson JS , Dunlay SM , LaRue SJ , Lewis EF , et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial . JAMA Internal Medicine 2018. ; 178 ( 4 ): 520-9 . [DOI: 10.1001/jamainternmed.2017.8713 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvennan CK , Thompson JS , Matlock DD , Cleveland JC Jr, Dunlay SM , LaRue SJ et al. A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for advanced heart failure: DECIDE-LVAD: rationale, design, and pilot data . Journal of Cardiovascular Nursing 2016. ; 31 ( 6 ): E8-E20 . [DOI] [PubMed] [Google Scholar]
- Thompson JS , Matlock DD , McIlvennan CK , Jenkins AR , Allen LA . Development of a decision aid for patients with advanced heart failure considering a destination therapy left ventricular assist device . JACC: Heart Failure 2015. ; 3 ( 12 ): 965-76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoki 2019 {published data only}
- Aoki Y , Takaesu Y , Inoue M , Furuno T , Kobayashi Y , Chiba H , et al. Seven-day shared decision making for outpatients with first episode of mood disorders among university students: a randomized controlled trial . Psychiatry Research 2019. ; 281 : 112531 . [DOI: 10.1016/j.psychres.2019.112531 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arterburn 2011 {published data only}
- Arterburn D , Westbrook E , Bogart T , Sepucha K , Bock S , Weppner W . Randomized trial of a video-based patient decision aid for bariatric surgery . Obesity 2011. ; 19 ( 8 ): 1669-75 . [DOI] [PubMed] [Google Scholar]
Auvinen 2004 {published data only}
- Auvinen A , Hakama M , Ala-Opas M , Vornanen T , Leppilahti M , Salminen P , et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen . BJU International 2004. ; 93 ( 1 ): 52-6 . [DOI] [PubMed] [Google Scholar]
- Auvinen A , Vornanen T , Tammela TL , Ala-Opas M , Leppilahti M , Salminen P , et al. A randomized trial of the choice of treatment in prostate cancer: design and baseline characteristics . BJU International 2001. ; 88 ( 7 ): 708-15 . [DOI] [PubMed] [Google Scholar]
- Huang RC , Auvinen A , Hakama M , Tammela TLJ , Ala-Opas M , Leppilahti M , et al. Effect of intervention on decision making of treatment for disease progression, prostate-specific antigen biochemical failure and prostate cancer death . Health Expectations 2014. ; 17 ( 6 ): 776-83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2016 {published data only}
- Bailey RA , Pfeifer M , Shillington AC , Harshaw Q , Funnell MM , VanWingen J , et al. Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict . BMC Health Services Research 2016. ; 16 : 10 . [DOI: 10.1186/s12913-016-1262-4 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 1997 {published and unpublished data}
- Barry MJ , Cherkin DC , Chang Y , Fowler FJ , Skates S . A randomized trial of a multimedia shared decision-making program for men facing a treatment decision for benign prostatic hyperplasia . Disease Management and Clinical Outcomes 1997. ; 1 ( 1 ): 5-14 . [Google Scholar]
- Rovner DR , Wills CE , Bonham V , Williams G , Lillie J , Kelly-Blake K , et al. Decision aids for benign prostatic hyperplasia: applicability across race and education . Medical Decision Making 2004. ; 24 ( 4 ): 359-66 . [DOI] [PubMed] [Google Scholar]
Bekker 2004 {published data only}
- Bekker HL , Hewison J , Thornton JG . Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial . Prenatal Diagnosis 2004. ; 24 ( 4 ): 265-75 . [DOI] [PubMed] [Google Scholar]
- Bekker HL , Hewison J , Thornton JG . Understanding why decision aids work: linking process with outcome . Patient Education and Counseling 2003. ; 50 ( 3 ): 323-9 . [DOI] [PubMed] [Google Scholar]
Berger‐Hoger 2019 {published data only}
- Berger-Höger B , Liethmann K , Mühlhauser I , Haastert B , Steckelberg A . Nurse-led coaching of shared decision-making for women with ductal carcinoma in situ in breast care centers: a cluster randomized controlled trial . International Journal of Nursing Studies 2019. ; 93 : 141-52 . [DOI: 10.1016/j.ijnurstu.2019.01.013 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bergeron 2018 {published data only}
- Bergeron M , Duggins A , Chini B , Ishman SL . Clinical outcomes after shared decision-making tools with families of children with obstructive sleep apnea without tonsillar hypertrophy . Laryngoscope 2019. ; 129 ( 11 ): 2646-51 . [DOI: 10.1002/lary.27653 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bergeron M , Duggins AL , Cohen AP , Tiemeyer K , Mullen L , Crisalli J , et al. A shared decision-making tool for obstructive sleep apnea without tonsillar hypertrophy: a randomized controlled trial . Laryngoscope 2018. ; 128 ( 4 ): 1007-15 . [DOI: 10.1002/lary.26967 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernstein 1998 {published and unpublished data}
- Bernstein SJ , Skarupski KA , Grayson CE , Starling MR , Bates ER , Eagle KA . A randomized controlled trial of information-giving to patients referred for coronary angiography: effects on outcomes of care . Health Expectations 1998. ; 1 ( 1 ): 50-61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2013 {published data only}
- Berry DL , Halpenny B , Hong F , Wolpin S , Lober WB , Russell KJ , et al. The personal patient profile-prostate decision support for men with localized prostate cancer: a multi-center randomized trial . Urologic Oncology 2013. ; 31 ( 7 ): 1012-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL , Wang Q , Halpenny B , Hong F . Decision preparation, satisfaction and regret in a multi-center sample of men with newly diagnosed localized prostate cancer . Patient Education and Counseling 2012. ; 88 ( 2 ): 262-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco JLF , Halpenny B , Berry DL . Personal preferences and discordant prostate cancer treatment choice in an intervention trial of men newly diagnosed with localized prostate cancer . Health and Quality of Life Outcomes 2012. ; 10 ( 123 ): 1-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill ML , Hong F , Berry DL . When study site contributes to outcomes in a multi-center randomized trial: a secondary analysis of decisional conflict in men with localized prostate cancer . Health and Quality of Life Outcomes 2014. ; 12 : 159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2018 {published data only}
- Berry DL , Hong F , Blonquist TM , Halpenny B , Xiong N , Filson CP , et al. Decision regret, adverse outcomes, and treatment choice in men with localized prostate cancer: results from a multi-site randomized tria . Urologic Oncology 2021. ; 39 ( 8 ): 493.e9-15 . [DOI: 10.1016/j.urolonc.2020.11.038 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL , Hong FX , Blonquist TM , Halpenny B , Filson CP , Master VA , et al. Decision support with the personal patient profile-prostate: a multicenter randomized trial . Journal of Urology 2018. ; 199 ( 1 ): 89–97 . [DOI: 10.1016/j.juro.2017.07.076 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beulen 2016 {published data only}
- Beulen L , den Berg M , Faas BH , Feenstra I , Hageman M , Vugt JM , et al. The effect of a decision aid on informed decision-making in the era of non-invasive prenatal testing: a randomised controlled trial . European Journal of Human Genetics 2016. ; 24 ( 10 ): 1409-16 . [DOI: 10.1038/ejhg.2016.39 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjorklund 2012 {published data only}
- Bjorklund U , Marsk A , Levin C , Ohman SG . Audiovisual information affects informed choice and experience of information in antenatal Down syndrome screening-a randomized controlled trial . Patient Education and Counseling 2012. ; 86 ( 3 ): 390-5 . [DOI] [PubMed] [Google Scholar]
- Öhman SG , Björklund U , Marsk A . Does an informational film increase women's possibility to make an informed choice about second trimester ultrasound? Prenatal Diagnosis 2012. ; 32 ( 9 ): 833-9 . [DOI] [PubMed] [Google Scholar]
Bonner 2022 {published data only}
- Bonner C , Batcup C , Ayre J , Cvejic E , Trevena L , McCaffery K , et al. The impact of health literacy-sensitive design and heart age in a cardiovascular disease prevention decision aid: randomised controlled trial and end user testing . JMIR Cardio 2022. ; 6 ( 1 ): e34142 . [DOI: 10.2196/34142 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourmaud 2016 {published data only}
- Bourmaud A , Michel PS , Oriol M , Regnier V , Tinquaut F , Nourissat A , et al. Decision aid on breast cancer screening reduces attendance rate: results of a large-scale, randomized, controlled study by the DECIDEO group . Oncotarget 2016. ; 7 ( 11 ): 12885-92 . [DOI: 10.18632/oncotarget.7332 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bozic 2013 {published data only}
- Bozic KJ , Belkora J , Chan V , Youm J , Zhou T , Dupaix J , et al. Shared decision making in patients with osteoarthritis of the hip and knee: results of a randomized controlled trial . Journal of Bone and Joint Surgery: American Volume 2013. ; 95 ( 18 ): 1633-9 . [DOI] [PubMed] [Google Scholar]
- Bozic KJ , Chenok KE , Schindel J , Chan V , Huddleston JI , Braddock C , et al. Patient, surgeon, and healthcare purchaser views on the use of decision and communication aids in orthopaedic surgery: a mixed methods study . BMC Health Services Research 2014. ; 14 ( 366 ): 1-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm J , Chan V , Belkora J , Bozic KJ . Impact of socioeconomic factors on informed decision making and treatment choice in patients with hip and knee OA . Journal of Arthroplasty 2015. ; 30 ( 2 ): 171-5 . [DOI] [PubMed] [Google Scholar]
Brazell 2014 {published data only}
- Brazell HD , O'Sullivan DM , Forrest A , Greene JF . Effect of a decision aid on decision making for the treatment of pelvic organ prolapse . Female Pelvic Medicine & Reconstructive Surgery 2014. ; 21 ( 4 ): 231-5 . [DOI] [PubMed] [Google Scholar]
Brown 2019 {published data only}
- Brown L , Gardner G , Bonner A . A randomized controlled trial testing a decision support intervention for older patients with advanced kidney disease . Journal of Advanced Nursing 2019. ; 75 ( 11 ): 3032-44 . [DOI: 10.1111/jan.14112 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carlson 2019 {published data only}
- Carlson LM , Harris S , Hardisty EE , Hocutt G , Vargo D , Campbell E , et al. Use of a novel computerized decision aid for aneuploidy screening: a randomized controlled trial . Genetics in Medicine 2019. ; 21 ( 4 ): 923-9 . [DOI: 10.1038/s41436-018-0283-2 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2017 {published data only}
- Carroll SL , Stacey D , McGillion M , Healey JS , Foster G , Hutchings S , et al. Evaluating the feasibility of conducting a trial using a patient decision aid in implantable cardioverter defibrillator candidates: a randomized controlled feasibility trial . Pilot and Feasibility Studies 2017. ; 3 : 49 . [DOI: 10.1186/s40814-017-0189-9 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Case 2019 {published data only}
- Case BC , Qamer SZ , Gates EM , Srichai MB . Shared decision making in cardiovascular disease in the outpatient setting . JACC. Case Reports 2019. ; 1 ( 2 ): 261-70 . [DOI: 10.1016/j.jaccas.2019.06.005 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chabrera 2015 {published data only}
- Chabrera C , Zabalegui A , Bonet M , Caro M , Areal J , González JR , Font A . A decision aid to support informed choices for patients recently diagnosed with prostate cancer . Cancer Nursing 2015. ; 38 ( 3 ): E42-E50 . [DOI] [PubMed] [Google Scholar]
Chambers 2012 {published data only}
- Chambers LW , Wilson K , Hawken S , Puxty J , Crowe L , Lam PP , et al. Impact of the Ottawa influenza decision aid on healthcare personnel's influenza immunization decision: a randomized trial . Journal of Hospital Infection 2012. ; 82 ( 3 ): 194-202 . [DOI] [PubMed] [Google Scholar]
Chen C 2021 {published data only}
- Chen CH , Kang YN , Chiu PY , Huang YJ , Elwyn G , Wu MH , et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial . Patient Education and Counseling 2021. ; 104 ( 10 ): 2498-504 . [DOI: 10.1016/j.pec.2021.03.002 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen S 2021 {published data only}
- Chen SW , Yang CC , Te JC , Tsai YL , Shorten B , Shorten A . Birth choices after caesarean in Taiwan: a mixed methods pilot study of a decision aid for shared decision making . Midwifery 2021. ; 95 : 102920 . [DOI: 10.1016/j.midw.2020.102920 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clancy 1988 {published data only}
- Clancy CM , Cebul RD , Williams SV . Guiding individual decisions: a randomized, controlled trial of decision analysis . American Journal of Medicine 1988. ; 84 ( 2 ): 283-8 . [DOI] [PubMed] [Google Scholar]
Cox 2019 {published data only}
- Cox CE , White DB , Hough CL , Jones DM , Kahn JM , Olsen MK , et al. Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation: a randomized clinical trial . Annals of Internal Medicine 2019. ; 170 ( 5 ): 285-97 . [DOI: 10.7326/M18-2335 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coylewright 2016 {published data only}
- Coylewright M , Dick S , Zmolek B , Askelin J , Hawkins E , Branda M , et al. PCI choice decision aid for stable coronary artery disease: a randomized trial . Circulation: Cardiovascular Quality and Outcomes 2016. ; 9 ( 6 ): 767-76 . [DOI: 10.1161/CIRCOUTCOMES.116.002641 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Crew 2022 {published data only}
- Crew KD , Bhatkhande G , Silverman T , Amenta J , Jones T , McGuinness JE , et al. Patient and provider web-based decision support for breast cancer chemoprevention: a randomized controlled trial . Cancer Prevention Research 2022. ; 15 ( 10 ): 689-700 . [DOI: 10.1158/1940-6207.CAPR-22-0013 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuypers 2018 {published data only}
- Cuypers M , Lamers RD , Kil PM , de Poll-Franse LV , Vries M . Impact of a web-based prostate cancer treatment decision aid on patient-reported decision process parameters: results from the Prostate Cancer Patient Centered Care trial . Supportive Care in Cancer 2018. ; 26 ( 11 ): 3739-48 . [DOI: 10.1007/s00520-018-4236-8 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers M , Lamers RED , Kil PJM , de Poll-Franse LV , Vries M . Longitudinal regret and information satisfaction after deciding on treatment for localized prostate cancer with or without a decision aid. Results at one-year follow-up in the PCPCC trial . Patient Education and Counseling 2019. ; 102 ( 3 ): 424-8 . [DOI: 10.1016/j.pec.2018.10.006 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davison 1997 {published data only}
- Davison BJ , Degner LF . Empowerment of men newly diagnosed with prostate cancer . Cancer Nursing 1997. ; 20 ( 3 ): 187-96 . [DOI] [PubMed] [Google Scholar]
De Achaval 2012 {published data only}
- De Achaval S , Fraenkel L , Volk R , Cox V , Suarez-Almazor M . Impact of educational and patient decision aids on decisional conflict associated with total knee arthroplasty . Arthritis Care & Research 2012. ; 64 ( 2 ): 229-37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2002 {published data only}
- Dolan JG , Frisina S . Randomized controlled trial of a patient decision aid for colorectal cancer screening . Medical Decision Making 2002. ; 22 ( 2 ): 125-39 . [DOI] [PubMed] [Google Scholar]
Durand 2021 {published data only}
- Durand MA , Yen RW , O'Malley AJ , Schubbe D , Politi MC , Saunders CH , et al. What matters most: randomized controlled trial of breast cancer surgery conversation aids across socioeconomic strata . Cancer 2021. ; 127 ( 3 ): 422-36 . [DOI: 10.1002/cncr.33248 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi MC , Yen RW , Elwyn G , Kurien N , Czerwinski SG , Schubbe D et al. Encounter decision aids can prompt breast cancer surgery cost discussions: analysis of recorded consultations . Medical Decision Making 2020. ; 40 ( 1 ): 62-71 . [DOI: 10.1177/0272989X19893308 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Yen RW , Durand MA , Harris C , Cohen S , Ward A , O'Malley AJ , et al. Text-only and picture conversation aids both supported shared decision making for breast cancer surgery: analysis from a cluster randomized trial . Patient Education and Counseling 2020. ; 103 ( 11 ): 2235-43 . [DOI: 10.1016/j.pec.2020.07.015 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ehrbar 2019 {published data only}
- Ehrbar V , Germeyer A , Nawroth F , Dangel A , Findeklee S , Urech C , et al. Long-term effectiveness of an online decision aid for female cancer patients regarding fertility preservation: knowledge, attitude, and decisional regret . Acta Obstetricia et Gynecologica Scandinavica 2021. ; 100 ( 6 ): 1132-9 . [PMID: 10.1111/aogs.14108 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ehrbar V , Urech C , Rochlitz C , Zanetti Dällenbach R , Moffat R , Stiller R , et al. Randomized controlled trial on the effect of an online decision aid for young female cancer patients regarding fertility preservation . Human Reproduction 2019. ; 34 ( 9 ): 1726-34 . [DOI: 10.1093/humrep/dez136 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elliott 2022 {published data only}
- Elliott TE , Asche SE , O'Connor PJ , Dehmer SP , Ekstrom HL , Truitt AR , et al. Clinical decision support with or without shared decision making to improve preventive cancer care: a cluster-randomized trial . Medical Decision Making 2022. ; 42 ( 6 ): 808-21 . [DOI: 10.1177/0272989X221082083 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2010 {published data only}
- Evans R , Joseph-Williams N , Edwards A , Newcombe R , Wright P , Kinnersley P , et al. Supporting informed decision making for prostate specific antigen (PSA) testing on the web: an online randomized controlled trial . Journal of Medical Internet Research 2010. ; 12 ( 3 ): e27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerlin 2011 {published data only}
- Banegas MP , McClure JB , Barlow WE , Ubel PA , Smith DM , Zikmund-Fisher BJ , et al. Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer . Patient Education and Counseling 2013. ; 91 : 364-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A , Dillard AJ , Smith DM , Zikmund-Fisher BJ , Pitsch R , McClure JB , et al. Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid . Breast Cancer Research and Treatment 2011. ; 127 ( 3 ): 681-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A . Randomization for Guide to Decide phase II . Word document provided by the authors .
- Korfage IJ , Fuhrel-Forbis A , Ubel PA , Zikmund-Fisher BJ , Greene SM , McClure JB , et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention . Breast Cancer Research 2013. ; 15 ( R74 ): 1-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2020 {published data only}
- Fisher A , Keast R , Costa D , Sharpe L , Manicavasagar V , Anderson J , et al. Improving treatment decision-making in bipolar II disorder: a phase II randomised controlled trial of an online patient decision-aid . BMC Psychiatry 2020. ; 20 ( 1 ): 447 . [DOI: 10.1186/s12888-020-02845-0 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraenkel 2007 {published data only}
- Fraenkel L , Rabidou N , Wittink D , Fried T . Improving informed decision-making for patients with knee pain . Journal of Rheumatology 2007. ; 34 ( 9 ): 1894-8 . [PubMed] [Google Scholar]
Fraenkel 2012 {published data only}
- Fraenkel L , Street RL Jr, Towle V , O'Leary JR , Iannone L , Van Ness PH , et al. A pilot randomized controlled trial of a decision support tool to improve the quality of communication and decision-making in individuals with atrial fibrillation . Journal of the American Geriatrics Society 2012. ; 60 ( 8 ): 1434-41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraenkel 2015 {published data only}
- Fraenkel L , Matzko CK , Webb DE , Oppermann B , Charpentier B , Peters E , et al. Use of decision support for improved knowledge, values clarification, and informed choice in patients with rheumatoid arthritis . Arthritis Care & Research 2015. ; 67 ( 11 ): 1496-502 . [DOI: 10.1002/acr.22659 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2008a {published data only}
- Frosch DL , Bhatnagar V , Tally S , Hamori CJ , Kaplan RM . Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening . Archives of Internal Medicine 2008. ; 168 ( 4 ): 363-9 . [DOI] [PubMed] [Google Scholar]
Fung 2021 {published data only}
- Fung CH , Martin JL , Liang LJ , Hays RD , Col N , Patterson ES , et al. Efficacy of a patient decision aid for improving person-centered decision-making by older adults with obstructive sleep apnea . Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine 2021. ; 17 ( 2 ): 121-8 . [DOI: 10.5664/jcsm.8798 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabel 2020a {published data only}
- Gabel P , Edwards A , Kirkegaard P , Larsen MB , Andersen B . The LEAD trial—The effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: a randomised controlled trial . Patient Education and Counseling 2020. ; 103 ( 2 ): 359-68 . [DOI: 10.1016/j.pec.2019.08.029 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gabel 2020b {published data only}
- Gabel P , Larsen MB , Edwards A , Kirkegaard P , Andersen B . Effectiveness of a decision aid for colorectal cancer screening on components of informed choice according to educational attainment: a randomised controlled trial . PLOS One 2020. ; 15 ( 11 ): e0241703 . [DOI: 10.1371/journal.pone.0241703 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagne 2017 {published data only}
- Gagné ME , Légaré F , Moisan J , Boulet LP . Impact of adding a decision aid to patient education in adults with asthma: a randomized clinical trial . PLOS One 2017. ; 12 ( 1 ): e0170055 . [DOI: 10.1371/journal.pone.0170055 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattellari 2003 {published data only}
- Gattellari M , Ward JE . Does evidence-based information about screening for prostate cancer enhance consumer decision-making? A randomised controlled trial . Journal of Medical Screening 2003. ; 10 ( 1 ): 27-39 . [DOI] [PubMed] [Google Scholar]
Gattellari 2005 {published data only}
- Gattellari M , Ward JE . A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening . Patient Education and Counseling 2005. ; 57 ( 2 ): 168-82 . [DOI] [PubMed] [Google Scholar]
Gokce 2019 {published data only}
- Gökce MI , Akpınar C , Esen B , Solak V , Gülpınar O , Bedük Y. The role of a novel decision aid to support informed decision making process in patients with a symptomatic non - lower pole renal stone < 20 mm in diameter: a prospective randomized study . International Brazilian Journal of Urology 2019. ; 45 ( 5 ): 941-7 . [DOI: 10.1590/S1677-5538.IBJU.2018.0198 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2017 {published data only}
- Gordon EJ , Sohn MW , Chang CH , McNatt G , Vera K , Beauvais N , et al. Effect of a mobile web app on kidney transplant candidates' knowledge about increased risk donor kidneys: a randomized controlled trial . Transplantation 2017. ; 101 ( 6 ): 1167-76 . [DOI: 10.1097/TP.0000000000001273 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2001 {published data only}
- Green MJ , Biesecker BB , McInerney AM , Mauger D , Fost N . An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility . American Journal of Medical Genetics 2001. ; 103 ( 1 ): 16-23 . [DOI] [PubMed] [Google Scholar]
Hamann 2006 {published data only}
- Hamann J , Cohen R , Leucht S , Busch R , Kissling W . Shared decision making and long-term outcome in schizophrenia treatment . Journal of Clinical Psychiatry 2007. ; 68 ( 7 ): 992-7 . [DOI] [PubMed] [Google Scholar]
- Hamann J , Langer B , Winkler V , Busch R , Cohen R , Leucht S , et al. Shared decision making for in-patients with schizophrenia . Acta Psychiatrica Scandinavica 2006. ; 114 ( 4 ): 265-73 . [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Ersek M , Sefcik JS , Feng-Chang L , Lee TJ , Gilliam R , Hanson LC . Provider staffing effect on a decision aid intervention . Clinical Nursing Research 2014. ; 23 : 36-53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L , Carey T , Caprio A , Joon Lee T , Ersek M , Garrett J , et al. Improving decision making for feeding options in advanced dementia: a randomized, controlled trial . Journal of the American Geriatrics Society 2011. ; 59 ( 11 ): 2009-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EA , Caprio AJ , Wessell K , Lin FC , Hanson LC . Impact of a decision aid on surrogate decision-makers’ perceptions of feeding options for patients with dementia . American Medical Directors Association 2013. ; 14 ( 2 ): 114-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Heller 2008 {published data only}
- Heller L , Parker PA , Youssef A , Miller MJ . Interactive digital education aid in breast reconstruction . Plastic & Reconstructive Surgery 2008. ; 122 ( 3 ): 717-24 . [DOI] [PubMed] [Google Scholar]
Hess 2012 {published data only}
- Hess EP , Knoedler MA , Shah ND , Kline JA , Breslin M , Branda ME , et al. The chest pain choice decision aid: a randomized trial . Circulation: Cardiovascular Quality and Outcomes 2012. ; 5 ( 3 ): 251-9 . [DOI] [PubMed] [Google Scholar]
Hess 2016 {published data only}
- Hess EP , Hollander JE , Schaffer JT , Kline JA , Torres CA , Diercks DB , et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial . BMJ 2016. ; 355 : i6165 . [DOI: 10.1136/bmj.i6165 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2018 {published data only}
- Hess EP , Homme JL , Kharbanda AB , Tzimenatos L , Louie JP , Cohen DM , et al. Effect of the head computed tomography choice decision aid in parents of children with minor head trauma: a cluster randomized trial . JAMA Network Open 2018. ; 1 ( 5 ): e182430 . [DOI: 10.1001/jamanetworkopen.2018.2430 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2017 {published data only}
- Hoffman AS , Lowenstein LM , Kamath GR , Houste AJ , Leal VB , Linder SK et al. An entertainment-education colorectal cancer screening decision aid for african american patients: a randomized controlled trial . Cancer 2017. ; 123 ( 8 ): 1401-8 . [DOI: 10.1002/cncr.30489 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim SA , Hanusa BH , Hannon MJ , Kresevic D , Long J , Kent Kwoh C . Willingness and access to joint replacement among African American patients with knee osteoarthritis: a randomized, controlled intervention . Arthritis and Rheumatism 2013. ; 65 ( 5 ): 1253-61 . [DOI: 10.1002/art.37899 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2017 {published data only}
- Ibrahim SA , Blum M , Lee GC , Mooar P , Medvedeva E , Collier A , et al. Effect of a decision aid on access to total knee replacement for black patients with osteoarthritis of the knee a randomized clinical trial . JAMA Surgery 2017. ; 152 ( 1 ): e164225 . [DOI: 10.1001/jamasurg.2016.4225 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ickenroth 2016 {published data only}
- Ickenroth M , Grispen J , Vries N , Dinant G , Ronda G , Weijden T . Effects of a web-based decision aid regarding diagnostic self-testing. A single-blind randomized controlled trial . Health Education Research 2016. ; 31 ( 3 ): 395-404 . [DOI: 10.1093/her/cyw014 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jalil 2022 {published data only}
- Jalil NB , Lee PY , Nor Afiah MZ , Abdullah KL , Azizi FN , Rassip NN , et al. Effectiveness of decision aid in men with localized prostate cancer: a multicenter randomized controlled trial at tertiary referral hospitals in an Asia Pacific country . Journal of Cancer Education: the Official Journal of the American Association for Cancer Education 2022. ; 37 ( 1 ): 169-78 . [DOI: 10.1007/s13187-020-01801-6 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jibaja‐Weiss 2011 {published data only}
- Jibaja-Weiss M , Volk R , Granchi T , Neff N , Robinson E , Spann S , et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy . Patient Education and Counseling 2011. ; 84 ( 1 ): 41-8 . [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson BR , Schwartz A , Goldberg J , Koerber A . A chairside aid for shared decision making in dentistry: a randomized controlled trial . Journal of Dental Education 2006. ; 70 ( 2 ): 133-41 . [PubMed] [Google Scholar]
Karagiannis 2016 {published data only}
- Karagiannis T , Liakos A , Branda ME , Athanasiadou E , Mainou M , Boura P , et al. Use of the diabetes medication choice decision aid in patients with type 2 diabetes in Greece: a cluster randomised trial . BMJ Open 2016. ; 6 ( 11 ): e012185 . [DOI: 10.1136/bmjopen-2016-012185 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 2008 {published data only}
- Kasper J , Kopke S , Muhlhauser I , Nubling M , Heesen C . Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): a randomized controlled trial . European Journal of Neurology 2008. ; 15 ( 12 ): 1345-52 . [DOI] [PubMed] [Google Scholar]
Kennedy 2002 {published data only}
- Kennedy AD , Sculpher MJ , Coulter A , Dwyer N , Rees M , Abrams KR , et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial . JAMA 2002. ; 288 ( 21 ): 2701-8 . [DOI] [PubMed] [Google Scholar]
Khalifeh 2019 {published data only}
- Khalifeh H , Molyneaux E , Brauer R , Vigod S , Howard LM . Patient decision aids for antidepressant use in pregnancy: a pilot randomised controlled trial in the UK . BJGP Open 2019. ; 3 ( 4 ): bjgpopen19X101666 . [DOI: 10.3399/bjgpopen19X101666 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleiss 2021 {published data only}
- Kleiss II , Kortlever JT , Ring D , Vagner GA , Reichel LM . A randomized controlled trial of decision aids for upper-extremity conditions . Journal of Hand Surgery 2021. ; 46 ( 4 ): 338.e1-15 . [DOI: 10.1016/j.jhsa.2020.09.003 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Knops 2014 {published data only}
- Knops AM , Goossens A , Ubbink DT , Balm R , Koelemay MJ , Vahl AC , et al, DECAID Trial Group . A decision aid regarding treatment options for patients with an asymptomatic abdominal aortic aneurysm: a randomised clinical trial . European Journal of Vascular and Endovascular Surgery 2014. ; 48 ( 3 ): 276-83 . [DOI] [PubMed] [Google Scholar]
Korteland 2017 {published data only}
- Korteland NM , Ahmed Y , Koolbergen DR , Brouwer M , Heer F , Kluin J , et al. Does the use of a decision aid improve decision making in prosthetic heart valve selection? A multicenter randomized trial . Circulation: Cardiovascular Quality and Outcomes 2017. ; 10 ( 2 ): e003178 . [DOI: 10.1161/CIRCOUTCOMES.116.003178 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kostick 2018 {published data only}
- Kostick KM , Bruce CR , Minard CG , Volk RJ , Civitello A , Krim SR , et al. A multisite randomized controlled trial of a patient-centered ventricular assist device decision aid (VADDA Trial) . Journal of Cardiac Failure 2018. ; 24 ( 10 ): 661-71 . [DOI: 10.1016/j.cardfail.2018.08.008 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krishnamurti 2019 {published data only}
- Krishnamurti L , Ross D , Sinha C , Leong T , Bakshi N , Mittal N , et al. Comparative effectiveness of a web-based patient decision aid for therapeutic options for sickle cell disease: randomized controlled trial . Journal of Medical Internet Research 2019. ; 21 ( 12 ): e14462 . [DOI: 10.2196/14462 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krist 2007 {published data only}
- Krist AH , Woolf SH , Johnson RE , Kerns JW . Patient education on prostate cancer screening and involvement in decision making . Annals of Family Medicine 2007. ; 5 ( 2 ): 112-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukafka 2022 {published data only}
- Kukafka R , Pan S , Silverman T , Zhang T , Chung WK , Terry MB , et al. Patient and clinician decision support to increase genetic counseling for hereditary breast and ovarian cancer syndrome in primary care: a cluster randomized clinical trial . JAMA Network Open 2022. ; 5 ( 7 ): e2222092 . [DOI: 10.1001/jamanetworkopen.2022.22092 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunneman 2020 {published data only}
- Kunneman M , Branda ME , Hargraves IG , Sivly AL , Lee AT , Gorr H , et al. Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial . JAMA Internal Medicine 2020. ; 180 ( 9 ): 1215-24 . [DOI: 10.1001/jamainternmed.2020.2908 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kupke 2013 {published data only (unpublished sought but not used)}
- Kupke J , Wicht MJ , Stützer H , Derman SH , Lichtenstein NV , Noack MJ . Does the use of a visualised decision board by undergraduate students during shared decision-making enhance patients' knowledge and satisfaction? A randomised controlled trial . European Journal of Dental Education 2013. ; 17 ( 1 ): 19-25 . [DOI] [PubMed] [Google Scholar]
Kuppermann 2014 {published data only}
- Kuppermann M , Pena S , Bishop JT , Nakagawa S , Gregorich SE , Sit A , et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial . JAMA 2014. ; 312 ( 12 ): 1210-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuppermann 2020 {published data only}
- Kuppermann M , Kaimal AJ , Blat C , Gonzalez J , Thiet MP , Bermingham Y , et al. Effect of a patient-centered decision support tool on rates of trial of labor after previous cesarean delivery: the PROCEED randomized clinical trial . JAMA 2020. ; 323 ( 21 ): 2151-9 . [DOI: 10.1001/jama.2020.5952 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2013 {published data only}
- Lam WW , Chan M , Or A , Kwong A , Suen D , Fielding R . Reducing treatment decision conflict difficulties in breast cancer surgery: a randomized controlled trial . Journal of Clinical Oncology 2013. ; 31 ( 23 ): 2879-85 . [DOI] [PubMed] [Google Scholar]
Langston 2010 {published data only}
- Langston A , Rosario L , Westhoff C . Structured contraceptive counseling: a randomised controlled trial . Patient Education and Counseling 2010. ; 81 ( 3 ): 362-7 . [DOI] [PubMed] [Google Scholar]
Laupacis 2006 {published data only}
- Laupacis A , O'Connor AM , Drake ER , Rubens FD , Robblee JA , Grant FC , et al. A decision aid for autologous pre-donation in cardiac surgery - a randomized trial . Patient Education and Counseling 2006. ; 61 ( 3 ): 458-66 . [DOI] [PubMed] [Google Scholar]
LeBlanc 2015 {published data only}
- LeBlanc A , Wang AT , Wyatt K , Branda ME , Shah ND , Van Houten H , et al. Encounter decision aid vs. clinical decision support or usual care to support patient-centered treatment decisions in osteoporosis: the osteoporosis choice randomized trial II . PLOS One 2015. ; 10 ( 5 ): 1-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
LeBlanc 2015b {published data only}
- LeBlanc A , Herrin J , Williams MD , Inselman JW , Branda ME , Shah ND , et al. Shared decision making for antidepressants in primary care: a cluster randomized trial . JAMA Internal Medicine 2015. ; 175 ( 11 ): 1761-70 . [DOI: 10.1001/jamainternmed.2015.5214 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2008a {published data only}
- Legare F , Dodin S , Stacey D , Leblanc A , Tapp S . Patient decision aid on natural health products for menopausal symptoms: randomized controlled trial . Menopause International 2008. ; 14 ( 3 ): 105-10 . [DOI] [PubMed] [Google Scholar]
Legare 2011 {published data only}
- Legare F , Labrecque M , LeBlanc A , Njoya M , Laurier C , Cote L , et al. Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial . Health Expectations 2011. ; 14 : 96-110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2012 {published and unpublished data}
- Legare F , Labrecque M , Cauchon M , Castel J , Turcotte S , Grimshaw J . Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial . Canadian Medical Association Journal 2012. ; 184 ( 13 ): E726-34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Leighl 2011 {published data only}
- Leighl NB , Shepherd HL , Butow PN , Clarke SJ , McJannett M , Beale PJ , et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy . Journal of Clinical Oncology 2011. ; 29 ( 15 ): 2077-84 . [DOI] [PubMed] [Google Scholar]
Lepore 2012 {published data only}
- Lepore SJ , Wolf RL , Basch CE , Godfrey M , McGinty E , Shmukler C , et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial . Annals of Behavioral Medicine 2012. ; 44 ( 3 ): 320-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 1997 {published data only}
- Lerman C , Biesecker B , Benkendorf JL , Kerner J , Gomez-Caminero A , Hughes C , et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing . Journal of the National Cancer Institute 1997. ; 89 ( 2 ): 148-57 . [DOI] [PubMed] [Google Scholar]
Lewis 2010 {published data only}
- Lewis C , Pignone M , Schild L , Scott T , Winquist A , Rimer B , et al. Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: design and baseline findings of the CHOICE trial . Cancer 2010. ; 116 ( 7 ): 1664-73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignone M , Winquist A , Schild L , Lewis C , Scott T , Hawley J , et al. Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members . Cancer 2011. ; 117 ( 15 ): 3252-62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignone MP , Brenner AT , Hawley S , Sheridan SL , Lewis CL , Jonas DE , et al. Conjoint analysis versus rating and ranking for values elicitation and clarification in colorectal cancer screening . Journal of General Internal Medicine 2011. ; 27 ( 1 ): 45-50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2018 {published data only}
- Lewis CL , Kistler CE , Dalton AF , Morris C , Ferrari R , Barclay C , et al. A decision aid to promote appropriate colorectal cancer screening among older adults: a randomized controlled trial . Medical Decision Making 2018. ; 38 ( 5 ): 614-24 . [DOI: 10.1177/0272989X18773713 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewis 2021 {published data only}
- Lewis KB , Birnie D , Carroll SL , Brousseau-Whaley C , Clark L , Green M , et al. Decision support for implantable cardioverter-defibrillator replacement: a pilot feasibility randomized controlled trial . Journal of Cardiovascular Nursing 2021. ; 36 ( 2 ): 143-50 . [DOI: 10.1097/JCN.0000000000000694 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2020 {published data only}
- Lin SC , Tam KW , Yen JY , Lu MC , Chen EY , Kuo YT et al. The impact of shared decision making with patient decision aids on the rotavirus vaccination rate in children: a randomized controlled trial . Preventive Medicine 2020. ; 141 : 106244 . [DOI: 10.1016/j.ypmed.2020.106244 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2022 {published data only}
- Lin SQ , Su CM , Wu HC , Chou YY , Yen YC , Tam KW . Effect of patient decision aids on decisional conflict and regret associated with breast cancer surgery: a randomized controlled trial . Breast Cancer: the Journal of the Japanese Breast Cancer Society 2022. ; 29 ( 5 ): 880-8 . [DOI: 10.1007/s12282-022-01370-0 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loh 2007 {published data only}
- Loh A , Simon D , Harter M . Effects of shared decision making in primary care of depressive patients - better compliance and treatment effects . Klinikarzt 2007. ; 36 ( 1 ): 38-41 . [Google Scholar]
- Loh A , Simon D , Wills CE , Kriston L , Niebling W , Harter M . The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial . Patient Education and Counseling 2007. ; 67 ( 3 ): 324-32 . [DOI] [PubMed] [Google Scholar]
Love 2016 {published data only}
- Love EM , Manalo IF , Chen SC , Chen KH , Stoff BK . A video-based educational pilot for basal cell carcinoma (BCC) treatment: a randomized controlled trial . Journal of the American Academy of Dermatology 2016. ; 74 ( 3 ): 477-83.e7 . [DOI: 10.1016/j.jaad.2015.10.014 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luan 2016 {published data only}
- Luan A , Hui KJ , Remington AC , Liu X , Lee GK . Effects of a novel decision aid for breast reconstruction: a randomized prospective trial . Annals of Plastic Surgery 2016. ; 76 ( Suppl 3 ): S249-54 . [DOI: 10.1097/SAP.0000000000000722 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Madden 2020 {published data only}
- Madden T , Holttum J , Maddipati R , Secura GM , Nease RF , Peipert JF , et a. Evaluation of a computerized contraceptive decision aid: a randomized controlled trial . Contraception 2020. ; 102 ( 5 ): 339-45 . [DOI: 10.1016/j.contraception.2020.08.002 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann D 2010 {published data only}
- Mann DM , Ponieman D , Montori VM , Arciniega J , McGinn T . The statin choice decision aid in primary care: a randomized trial . Patient Education and Counseling 2010. ; 80 ( 1 ): 138-40 . [DOI] [PubMed] [Google Scholar]
Mann E 2010 {published data only}
- Mann E , Kellar I , Sutton S , Kinmonth AL , Hankins M , Griffin S , et al. Impact of informed-choice invitations on diabetes screening knowledge, attitude and intentions: an analogue study . BMC Public Health 2010. ; 10 : 768 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Manne 2020 {published data only}
- Manne SL , Smith BL , Frederick S , Mitarotondo A , Kashy DA , Kirstein LJ . B-Sure: a randomized pilot trial of an interactive web-based decision support aid versus usual care in average-risk breast cancer patients considering contralateral prophylactic mastectomy . Translational Behavioral Medicine 2020. ; 10 ( 2 ): 355-63 . [DOI: 10.1093/tbm/iby133 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Man‐Son‐Hing 1999 {published and unpublished data}
- Man-Son-Hing M , Laupacis A , O'Connor AM , Biggs J , Drake E , Yetisir E , et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial . JAMA 1999. ; 282 ( 8 ): 737-43 . [DOI] [PubMed] [Google Scholar]
Marteau 2010 {published data only}
- Kellar I , Mann E , Kinmonth AL , Prevost AT , Sutton S , Marteau TM . Can informed choice invitations lead to inequities in intentions to make lifestyle changes among participants in a primary care diabetes screening programme? Evidence from a randomized trial . Public Health 2011. ; 125 ( 9 ): 645-52 . [DOI] [PubMed] [Google Scholar]
- Marteau TM , Mann E , Prevost AT , Vasconcelos JC , Kellar I , Sanderson S , et al. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial . BMJ 2010. ; 340 : c2138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2012 {published data only}
- Brown I , Bradley A , Ng CJ , Colwell B , Mathers N . Investigating active ingredients in a complex intervention: a nested study within the Patient and Decision Aids (PANDAs) randomised controlled trial for people with type 2 diabetes . BMC Research Notes 2014. ; 7 : 347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers N , Ng CJ , Campbell MJ , Colwell B , Brown I , Bradley A . Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice . BMJ Open 2012. ; 2 ( 6 ): 1-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2007 {published data only}
- Mathieu E , Barratt A , Davey HM , McGeechan K , Howard K , Houssami N . Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women . Archives of Internal Medicine 2007. ; 167 ( 19 ): 2039-46 . [DOI] [PubMed] [Google Scholar]
Mathieu 2010 {published data only}
- Mathieu E , Barratt AL , McGeechan K , Davey HM , Howard K , Houssami N . Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40-year-old women . Patient Education and Counseling 2010. ; 81 ( 1 ): 63-72 . [DOI] [PubMed] [Google Scholar]
McAlister 2005 {published data only}
- McAlister FA , Man-Son-Hing M , Straus SE , Ghali WA , Anderson D , Majumdar SR , et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial . CMAJ 2005. ; 173 ( 5 ): 496-501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
McBride 2002 {published data only}
- Bastian LA , McBride CM , Fish L , Lyna P , Farrell D , Lipkus IM , et al. Evaluating participants' use of a hormone replacement therapy decision-making intervention . Patient Education and Counseling 2002. ; 48 ( 3 ): 283-91 . [DOI] [PubMed] [Google Scholar]
- McBride CM , Bastian LA , Halabi S , Fish L , Lipkus IM , Bosworth HB , et al. A tailored intervention to aid decision making about hormone replacement therapy . American Journal of Public Health 2002. ; 92 ( 7 ): 1112-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
McCaffery 2010 {published data only}
- McCaffery KJ , Irwig L , Turner R , Chan SF , Macaskill P , Lewicka M , et al. Psychosocial outcomes of three triage methods for the management of borderline abnormal cervical smears: an open randomised trial . BMJ 2010. ; 340 : b4491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrath 2017 {published data only}
- McGrath A , Sharpe L , Lah S , Parratt K . Evaluation of a decision aid for women with epilepsy who are considering pregnancy: a randomized controlled trial . Medical Decision Making 2017. ; 37 ( 5 ): 589-99 . [DOI: 10.1177/0272989X17697304 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
McIlvennan 2018 {published data only}
- McIlvennan CK , Matlock DD , Thompson JS , Dunlay SM , Blue L , LaRue SJ , et al. Caregivers of patients considering a destination therapy left ventricular assist device and a shared decision-making intervention: the DECIDE-LVAD trial . JACC. Heart Failure 2018. ; 6 ( 11 ): 904-13 . [DOI: 10.1016/j.jchf.2018.06.019 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
McLean 2020 {published data only}
- McLean D , McBride O , Samardzic T , Sisic M , Dellavalle RP , Tan J . Impact of a hidradenitis suppurativa patient decision aid on treatment decision making: a randomized controlled trial . JAAD International 2020. ; 1 ( 2 ): 190-9 . [DOI: 10.1016/j.jdin.2020.09.001 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meade 2015 {published data only}
- Meade T , Dowswell E , Manolios N , Sharpe L . The motherhood choices decision aid for women with rheumatoid arthritis increases knowledge and reduces decisional conflict: a randomized controlled trial . BMC Musculoskeletal Disorders 2015. ; 16 : 260 . [DOI: 10.1186/s12891-015-0713-0 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meier 2019 {published data only}
- Meier JD , Chorney JM , Fox SD , Hong P . Decision aid prototype for treatment of pediatric sleep disordered breathing: a randomized pilot study . Laryngoscope 2019. ; 129 ( 1 ): 229-34 . [DOI: 10.1002/lary.27204 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Metcalfe 2017 {published data only}
- Metcalfe KA , Dennis CL , Poll A , Armel S , Demsky R , Carlsson L , et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial . Genetics in Medicine: Official Journal of the American College of Medical Genetics 2017. ; 19 ( 3 ): 330-6 . [DOI: 10.1038/gim.2016.108 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller SM , Fleisher L , Roussi P , Buzaglo JS , Schnoll R , Slater E , et al. Facilitating informed decision making about breast cancer risk and genetic counseling among women calling the NCI's Cancer Information Service . Journal of Health Communication 2005. ; 10 ( Suppl 1 ): 119-36 . [DOI] [PubMed] [Google Scholar]
Miller 2011 {published data only}
- Duren-Winfield V , Onsomu EO , Case DL , Pignone M , Miller D . Health literacy and computer-assisted instruction: usability and patient preference . Journal of Health Communication 2015. ; 20 : 491-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D , Spangler J , Case D , Goff D , Singh S , Pignone M . Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population . American Journal of Preventive Medicine 2011. ; 40 ( 6 ): 608-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2018 {published data only}
- Denizard-Thompson NM , Miller DP , Snavely AC , Spangler JG , Case LD , Weaver KE . Effect of a digital health intervention on decreasing barriers and increasing facilitators for colorectal cancer screening in vulnerable patients . Cancer Epidemiology, Biomarkers and Prevention 2020. ; 29 ( 8 ): 1564-9 . [DOI: 10.1158/1055-9965.EPI-19-1199 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP Jr, Denizard-Thompson N , Weaver KE , Case LD , Troyer JL , Spangler JG , et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial . Annals of Internal Medicine 2018. ; 168 ( 8 ): 550-7 . [DOI: 10.7326/M17-2315 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moin 2019 {published data only}
- Moin T , Duru OK , Turk N , Chon JS , Frosch DL , Martin JM , et al. Effectiveness of shared decision-making for diabetes prevention: 12-month results from the prediabetes informed decision and education (PRIDE) trial . Journal of General Internal Medicine 2019. ; 34 ( 11 ): 2652-9 . [DOI: 10.1007/s11606-019-05238-6 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003 {published and unpublished data}
- Emmett CL , Montgomery AA , Peters TJ , Fahey T . Three-year follow-up of a factorial randomised controlled trial of two decision aids for newly diagnosed hypertensive patients . British Journal of General Practice 2005. ; 55 ( 516 ): 551-3 . [PMC free article] [PubMed] [Google Scholar]
- Montgomery AA , Fahey T , Peters TJ . A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients . British Journal of General Practice 2003. ; 53 ( 491 ): 446-53 . [PMC free article] [PubMed] [Google Scholar]
Montgomery 2007 {published data only}
- Frost J , Shaw. Women's views on the use of decision aids for decision making about the method of delivery following a previous caesarean section: qualitative interview study . British Journal of Obstetrics and Gynecology 2009. ; 116 ( 7 ): 896-905 . [DOI] [PubMed] [Google Scholar]
- Hollinghurst S , Emmett C , Peters TJ , Watson H , Fahey T , Murphy DJ , et al. Economic evaluation of the DIAMOND randomized trial: cost and outcomes of 2 decision aids for mode of delivery among women with previous caesarian section . BMJ 2010. ; 30 : 453-63 . [DOI] [PubMed] [Google Scholar]
- Montgomery AA , Emmett CL , Fahey T , Jones C , Ricketts I , Patel RR , et al. Two decision aids for mode of delivery among women with previous caesarean section: randomised controlled trial . BMJ 2007. ; 334 ( 7607 ): 1305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Montori 2011 {published data only}
- Montori VM , Shah ND , Pencille LJ , Branda ME , Van Houten HK , Swiglo BA . Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial . American Journal of Medicine 2011. ; 124 ( 6 ): 549-56 . [DOI] [PubMed] [Google Scholar]
- Pencille LJ , Campbell ME , Van Houten HK , Shah ND , Mullan RJ , Swiglo BA , et al. Protocol for the Osteoporosis Choice trial. A pilot randomized trial of a decision aid in primary care practice . Trials 2009. ; 10 : 113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Montoya 2019 {published data only}
- Montoya TI , Rondeau NU , Maldonado PA , Mallett VT . Decision aid video for treatment selection in latinas with symptomatic pelvic organ prolapse: a randomized pilot study . Female Pelvic Medicine & Reconstructive Surgery 2019. ; 27 ( 1 ): 39-45 . [DOI: 10.1097/SPV.0000000000000727 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2000 {published and unpublished data}
- Morgan MW , Deber RB , Llewellyn-Thomas HA , Gladstone P , Cusimano RJ , O'Rourke K , et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease . Journal of General Internal Medicine 2000. ; 15 ( 10 ): 685-93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MW . A Randomized Trial of the Ischemic Heart Disease Shared Decision Making Program: An Evaluation of a Decision Aid [Masters Thesis] . Toronto: : University of Toronto; , 1997. . [Google Scholar]
Mott 2014 {published data only}
- Mott JM , Stanley MA , Street RL Jr, Grady RH , Teng EJ . Increasing engagement in evidence-based PTSD treatment through shared decision-making: a pilot study . Military Medicine 2014. ; 179 ( 2 ): 143-9 . [DOI] [PubMed] [Google Scholar]
Mullan 2009 {published data only}
- Mullan RJ , Montori VM , Shah ND , Christianson TJ , Bryant SC , Guyatt GH , et al. The diabetes mellitus medication choice decision aid: a randomized trial . Archives of Internal Medicine 2009. ; 169 ( 17 ): 1560-8 . [DOI] [PubMed] [Google Scholar]
Murphy 2020 {published data only}
- Murphy C , Laine C , Macaulay M , Fader M . Development and randomised controlled trial of a continence product patient decision aid for men postradical prostatectomy . Journal of Clinical Nursing 2020. ; 29 ( 13-4 ): 2251-9 . [DOI: 10.1111/jocn.15223 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 2001a {published and unpublished data}
- Murray E , Davis H , Tai SS , Coulter A , Gray A , Haines A . Randomised controlled trial of an interactive multimedia decision aid on benign prostatic hypertrophy in primary care . BMJ 2001. ; 323 ( 7311 ): 493-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2001b {published and unpublished data}
- Murray E , Davis H , Tai SS , Coulter A , Gray A , Haines A . Randomized controlled trial of an interactive multimedia decision aid on hormone replacement therapy in primary care . BMJ 2001. ; 323 ( 7311 ): 490-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagle 2008 {published data only}
- Nagle C , Gunn J , Bell R , Lewis S , Meiser B , Metcalfe S , et al. Use of a decision aid for prenatal testing of fetal abnormalities to improve women's informed decision making: a cluster randomised controlled trial . BJOG: An International Journal of Obstetrics and Gynaecology 2008. ; 115 ( 3 ): 339-47 . [DOI] [PubMed] [Google Scholar]
- Nagle C , Lewis S , Meiser B , Metcalfe S , Carlin JB , Bell R , et al. Evaluation of a decision aid for prenatal testing of fetal abnormalities: a cluster randomised trial [ISRCTN22532458] . BMC Public Health 2006. ; 6 : 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Nassar 2007 {published data only}
- Nassar N , Roberts CL , Raynes-Greenow CH , Barratt A , Peat B , Decision Aid for Breech Presentation Trial Collaborators . Evaluation of a decision aid for women with breech presentation at term: a randomised controlled trial [ISRCTN14570598] . BJOG: An International Journal of Obstetrics & Gynaecology 2007. ; 114 ( 3 ): 325-33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Oakley 2006 {published data only}
- Oakley S , Walley T . A pilot study assessing the effectiveness of a decision aid on patient adherence with oral bisphosphonate medication . Pharmaceutical Journal 2006. ; 276 ( 7399 ): 536-8 . [Google Scholar]
Omaki 2021 {published data only}
- Omaki E , Castillo R , McDonald E , Eden K , Davis S , Frattaroli S , et al. A patient decision aid for prescribing pain medication: results from a pilot test in two emergency departments . Patient Education and Counseling 2021. ; 104 ( 6 ): 1304-11 . [DOI: 10.1016/j.pec.2020.11.022 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oostendorp 2017 {published data only}
- Oostendorp LM , Ottevanger PB , Donders AT , de Wouw AJ , Schoenaker IH , Smilde TJ , et al. Decision aids for second-line palliative chemotherapy: a randomised phase II multicentre trial . BMC Medical Informatics and Decision Making 2017. ; 17 ( 1 ): 130 . [DOI: 10.1186/s12911-017-0529-y ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osaka 2017 {published data only}
- Osaka W , Nakayama K . Effect of a decision aid with patient narratives in reducing decisional conflict in choice for surgery among early-stage breast cancer patients: a three-arm randomized controlled trial . Patient Education and Counseling 2017. ; 100 ( 3 ): 550-62 . [DOI: 10.1016/j.pec.2016.09.011 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ozanne 2007 {published data only}
- Ozanne EM , Annis C , Adduci K , Showstack J , Esserman L . Pilot trial of a computerized decision aid for breast cancer prevention . Breast Journal 2007. ; 13 ( 2 ): 147-54 . [DOI] [PubMed] [Google Scholar]
Partin 2004 {published and unpublished data}
- Partin MR , Nelson D , Flood AB , Friedemann-Sanchez G , Wilt TJ . Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions . Health Expectations 2006. ; 9 ( 3 ): 285-95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin MR , Nelson D , Radosevich D , Nugent S , Flood AB , Dillon N , et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors . Journal of General Internal Medicine 2004. ; 19 ( 8 ): 835-42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Patzer 2018 {published data only}
- Patzer RE , McPherson L , Basu M , Mohan S , Wolf M , Chiles M , et al. Effect of the iChoose Kidney decision aid in improving knowledge about treatment options among transplant candidates: a randomized controlled trial . American Journal of Transplantation 2018. ; 18 ( 8 ): 1954-65 . [DOI: 10.1111/ajt.14693 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perestelo‐Perez 2016 {published data only}
- Perestelo-Perez L , Rivero-Santana A , Boronat M , Sanchez-Afonso JA , Perez-Ramos J , Montori VM , et al. Effect of the statin choice encounter decision aid in Spanish patients with type 2 diabetes: a randomized trial . Patient Education and Counseling 2016. ; 99 ( 2 ): 295-9 . [DOI: 10.1016/j.pec.2015.08.032 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Perestelo‐Perez 2017 {published data only}
- Perestelo-Perez L , Rivero-Santana A , Sanchez-Afonso JA , Perez-Ramos J , Castellano-Fuentes CL , Sepucha K , et al. Effectiveness of a decision aid for patients with depression: a randomized controlled trial . Health Expectations 2017. ; 20 ( 5 ): 1096-105 . [DOI: 10.1111/hex.12553 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perestelo‐Perez 2019 {published data only}
- Perestelo-Perez L , Rivero-Santana A , Torres-Castano A , Ramos-Garcia V , Alvarez-Perez Y , Gonzalez-Hernandez N , et al. Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: a randomized trial . BMC Medical Informatics and Decision Making 2019. ; 19 ( 1 ): 8 . [DOI: 10.1186/s12911-019-0739-6 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Lacasta 2019 {published data only}
- Pérez-Lacasta MJ , Martínez-Alonso M , Garcia M , Sala M , Perestelo-Pérez L , Vidal C , et al. Effect of information about the benefits and harms of mammography on women's decision making: the InforMa randomised controlled trial . PloS One 2019. ; 14 ( 3 ): e0214057 . [DOI: 10.1371/journal.pone.0214057 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pignone 2000 {published data only}
- Pignone M , Harris R , Kinsinger L . Videotape-based decision aid for colon cancer screening. A randomized, controlled trial . Annals of Internal Medicine 2000. ; 133 ( 10 ): 761-9 . [DOI] [PubMed] [Google Scholar]
Politi 2020a {published data only}
- Politi MC , Lee CN , Philpott-Streiff SE , Foraker RE , Olsen MA , Merrill C , et al. A randomized controlled trial evaluating the BREASTChoice tool for personalized decision support about breast reconstruction after mastectomy . Annals of Surgery 2020. ; 271 ( 2 ): 230-7 . [DOI: 10.1097/SLA.0000000000003444 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Protheroe 2007 {published data only}
- Patel S , Ngunjiri A , Hee SW , Yang Y , Brown S , Friede T , et al. Primum non nocere: shared informed decision making in low back pain - a pilot cluster randomised trial . BMC Musculoskeletal Disorders 2014. ; 15 : 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protheroe J , Bower P , Chew-Graham C , Peters TJ , Fahey T . Effectiveness of a computerized decision aid in primary care on decision making and quality of life in menorrhagia: results of the MENTIP randomized controlled trial . Medical Decision Making 2007. ; 27 ( 5 ): 575-84 . [DOI] [PubMed] [Google Scholar]
- Protheroe J , Bower P , Chew-Graham C . The use of mixed methodology in evaluating complex interventions: identifying patient factors that moderate the effects of a decision aid . Family Practice 2008. ; 24 ( 6 ): 594-600 . [DOI] [PubMed] [Google Scholar]
Reuland 2017 {published data only}
- Brenner AT , Hoffman R , McWilliams A , Pignone MP , Rhyne RL , Tapp H , et al. Colorectal cancer screening in vulnerable patients: promoting informed and shared decisions . American Journal of Preventive Medicine 2016. ; 51 ( 4 ): 454-62 . [DOI: 10.1016/j.amepre.2016.03.025 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland DS , Brenner AT , Hoffman R , McWilliams A , Rhyne RL , Getrich C , et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: a randomized clinical trial . JAMA Internal Medicine 2017. ; 177 ( 7 ): 967-74 . [PMID: 10.1001/jamainternmed.2017.1294 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rivero‐Santana 2021 {published data only}
- Rivero-Santana A , Torrente-Jiménez RS , Perestelo-Pérez L , Torres-Castaño A , Ramos-García V , Bilbao A , et al. Effectiveness of a decision aid for patients with knee osteoarthritis: a randomized controlled trial . Osteoarthritis and Cartilage 2021. ; 29 ( 9 ): 1265-74 . [PMID: 10.1016/j.joca.2021.06.005 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roberto 2020 {published data only}
- Roberto A , Colombo C , Candiani G , Satolli R , Giordano L , Jaramillo L , et al. A dynamic web-based decision aid to improve informed choice in organised breast cancer screening. A pragmatic randomised trial in Italy . British Journal of Cancer 2020. ; 123 ( 5 ): 714-21 . [DOI: 10.1038/s41416-020-0935-2 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubel 2010 {published data only}
- Rubel SK , Miller JW , Stephens RL , Xu Y , Scholl LE , Holden EW , et al. Testing the effects of a decision aid for prostate cancer screening . Journal of Health Communication 2010. ; 15 ( 3 ): 307-21 . [DOI] [PubMed] [Google Scholar]
Ruffin 2007 {published data only}
- Ruffin MT , Fetters MD , Jimbo M . Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial . Preventive Medicine 2007. ; 45 ( 4 ): 267-73 . [DOI] [PubMed] [Google Scholar]
Saunier 2020 {published data only}
- Saunier F , Berthelot P , Mottet-Auselo B , Pelissier C , Fontana L , Botelho-Nevers E , et al. Impact of a decision-aid tool on influenza vaccine coverage among HCW in two French hospitals: a cluster-randomized trial . Vaccine 2020. ; 38 ( 36 ): 5759-63 . [DOI: 10.1016/j.vaccine.2020.07.011 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sawka 2012 {published and unpublished data}
- Sawka AM , Straus S , Rotstein L , Brierley JD , Tsang RW , Asa S , et al. Randomized controlled trial of a computerized decision aid on adjuvant radioactive iodine treatment for patients with early-stage papillary thyroid cancer . Journal of Clinical Oncology 2012. ; 30 ( 23 ): 2906-11 . [DOI] [PubMed] [Google Scholar]
Schapira 2019 {published data only}
- Schapira MM , Hubbard RA , Seitz HH , Conant EF , Schnall M , Cappella JN , et al. The impact of a risk-based breast cancer screening decision aid on initiation of mammography among younger women: report of a randomized trial . MDM Policy & Practice 2019. ; 4 ( 1 ): 2381468318812889 . [DOI: 10.1177/2381468318812889 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schonberg 2020 {published data only}
- Schonberg MA , Kistler CE , Pinheiro A , Jacobson AR , Aliberti GM , Karamourtopoulos M , et al. Effect of a mammography screening decision aid for women 75 years and older: a cluster randomized clinical trial . JAMA Internal Medicine 2020. ; 180 ( 6 ): 831-42 . [DOI: 10.1001/jamainternmed.2020.0440 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schott 2021 {published data only}
- Schott SL , Berkowitz J , Dodge SE , Petersen CL , Saunders CH , Sobti NK , et al. Personalized, electronic health record-integrated decision aid for stroke prevention in atrial fibrillation: a small cluster randomized trial and qualitative analysis of efficacy and acceptability . Circulation: Cardiovascular Quality and Outcomes 2021. ; 14 ( 6 ): e007329 . [DOI: 10.1161/CIRCOUTCOMES.120.007329 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schroy 2011 {published data only}
- Schroy PC 3rd, Emmons K , Peters E , Glick JT , Robinson PA , Lydotes MA , et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial . Medical Decision Making 2011. ; 31 ( 1 ): 93-107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroy PC 3rd, Emmons KM , Peters E , Glick JT , Robinson PA , Lydotes MA , et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial . American Journal of Preventive Medicine 2012. ; 43 ( 6 ): 573-83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwalm 2012 {published data only}
- Schwalm JD , Stacey D , Pericak D , Natarajan MK . Radial artery versus femoral artery access options in coronary angiogram procedures: randomized controlled trial of a patient-decision aid . Circulation: Cardiovascular Quality and Outcomes 2012. ; 5 ( 3 ): 260-6 . [DOI] [PubMed] [Google Scholar]
Schwartz 2001 {published data only}
- Schwartz MD , Benkendorf J , Lerman C , Isaacs C , Ryan-Robertson A , Johnson L . Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women . Cancer 2001. ; 92 ( 4 ): 932-40 . [DOI] [PubMed] [Google Scholar]
Schwartz 2009a {published data only}
- Hooker GW , Leventhal KG , DeMarco T , Peshkin BN , Finch C , Wahl E , et al. Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial . Medical Decision Making 2011. ; 31 ( 3 ): 412-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD , Valdimarsdottir HB , DeMarco TA , Peshkin BN , Lawrence W , Rispoli J , et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction . Health Psychology 2009. ; 28 ( 1 ): 11-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2006 {published data only}
- Sheridan SL , Shadle J , Simpson RJ Jr, Pignone MP . The impact of a decision aid about heart disease prevention on patients' discussions with their doctor and their plans for prevention: a pilot randomized trial . BMC Health Services Research 2006. ; 6 : 121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2011 {published data only}
- Sheridan SL , Draeger LB , Pignone MP , Keyserling TC , Simpson RJ Jr, Rimer B , et al. A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies . BMC Health Services Research 2011. ; 11 : 331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan SL , Draeger LB , Pignone MP , Rimer B , Bangdiwala SI , Cai J , et al. The effect of a decision aid intervention on decision making about coronary heart disease risk reduction: secondary analyses of a randomized trial . BMC Medical Informatics and Decision Making 2014. ; 14 ( 14 ): 1-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Shorten 2005 {published and unpublished data}
- Shorten A , Shorten B , Keogh J , West S , Morris J . Making choices for childbirth: a randomized controlled trial of a decision-aid for informed birth after cesarean . Birth 2005. ; 32 ( 4 ): 252-61 . [DOI] [PubMed] [Google Scholar]
Shourie 2013 {published data only}
- Shourie S , Jackson C , Cheater FM , Bekker HL , Edlin R , Tubeuf S , et al. A cluster randomised controlled trial of a web based decision aid to support parents' decisions about their child's Measles Mumps and Rubella (MMR) vaccination . Vaccine 2013. ; 31 ( 50 ): 6003-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubeuf S , Edlin R , Shourie S , Cheater FM , Bekker H , Jackson C . Cost effectiveness of a web-based decision aid for parents deciding about MMR vaccination: a three-arm cluster randomised controlled trial in primary care . British Journal of General Practice 2014. ; 64 ( 625 ): e493-9 . [DOI: 10.3399/bjgp14X680977 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2019 {published data only}
- Singh JA , Fraenkel L , Green C , Alarcón GS , Barton JL , Saag KG , et al. Individualized decision aid for diverse women with lupus nephritis (IDEA-WON): a randomized controlled trial . PLOS Medicine 2019. ; 16 ( 5 ): e1002800 . [DOI: 10.1371/journal.pmed.1002800 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smallwood 2017 {published data only}
- Smallwood AJ , Schapira MM , Fedders M , Neuner JM . A pilot randomized controlled trial of a decision aid with tailored fracture risk tool delivered via a patient portal . Osteoporosis International 2017. ; 28 ( 2 ): 567-76 . [DOI: 10.1007/s00198-016-3767-4 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2010 {published data only}
- Smith SK , Barratt A , Trevana L , Simpson JM , Jansen J , McCaffery KJ . A theoretical framework for measuring knowledge in screening decision aid trials . Patient Education and Counseling 2012. ; 89 : 330-6 . [DOI] [PubMed] [Google Scholar]
- Smith SK , Kearney P , Trevena L , Barratt A , Nutbeam D , McCaffery KJ . Informed choice in bowel cancer screening: a qualitative study to explore how adults with lower education use decision aids . Health Expectations 2012. ; 17 : 511-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SK , Simpson JM , Trevena LJ , McCaffery KJ . Factors associated with informed decisions and participation in bowel cancer screening among adults with lower education and literacy . Medical Decision Making 2014. ; 34 ( 6 ): 756-72 . [DOI] [PubMed] [Google Scholar]
- Smith SK , Trevena L , Simpson JM , Barratt A , Nutbeam D , McCaffery KJ . A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial . BMJ 2010. ; 341 : c5370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2014a {published and unpublished data}
- Stacey D , Hawker G , Dervin G , Tugwell P , Boland L , Pomey MP , et al. Decision aid for patients considering total knee arthroplasty with preference report for surgeons: a pilot randomized controlled trial . BMC Musculoskeletal Disorders 2014. ; 15 : 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2016 {published data only}
- Stacey D , Taljaard M , Dervin G , Tugwell P , O'Connor AM , Pomey MP , et al. Impact of patient decision aids on appropriate and timely access to hip or knee arthroplasty for osteoarthritis: a randomized controlled trial . Osteoarthritis and Cartilage 2016. ; 24 ( 1 ): 99-107 . [DOI: 10.1016/j.joca.2015.07.024 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Trenaman L , Stacey D , Bryan S , Payne K , Hawker G , Bansback N . Long-term effect of patient decision aids on use of joint replacement and health care costs . Osteoarthritis and Cartilage 2020. ; 28 ( 6 ): 819-23 . [DOI: 10.1016/j.joca.2020.01.019 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stamm 2017 {published data only}
- Stamm AW , Banerji JS , Wolff EM , Slee A , Akapame S , Dahl K , et al. A decision aid versus shared decision making for prostate cancer screening: results of a randomized, controlled trial . Canadian Journal of Urology 2017. ; 24 ( 4 ): 8910-7 . [PMID: ] [PubMed] [Google Scholar]
Steckelberg 2011 {published data only}
- Steckelberg A , Hulfenhaus C , Haastert B , Muhlhauser I . Effect of evidence based risk information on "informed choice" in colorectal cancer screening: randomised controlled trial . BMJ 2011. ; 342 : d3193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 2020 {published data only}
- Stephenson J , Bailey JV , Gubijev A , D'Souza P , Oliver S , Blandford A , et al. An interactive website for informed contraception choice: randomised evaluation of Contraception Choices . Digital Health 2020. ; 6 : 2055207620936435 . [DOI: 10.1177/2055207620936435 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubenrouch 2022 {published data only}
- Stubenrouch FE , Peters LJ , Mik SM , Klemm PL , Peppelenbosch AG , Schreurs SC , et al. Improving shared decision making in vascular surgery: a stepped wedge cluster randomised trial . European Journal of Vascular and Endovascular Surgery 2022. ; 64 ( 1 ): 73-81 . [DOI: 10.1016/j.ejvs.2022.04.016 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Subramanian 2019 {published data only}
- Subramanian L , Zhao J , Zee J , Knaus M , Fagerlin A , Perry E , et al. Use of a decision aid for patients considering peritoneal dialysis and in-center hemodialysis: a randomized controlled trial . American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation 2019. ; 74 ( 3 ): 351-60 . [DOI: 10.1053/j.ajkd.2019.01.030 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor KL , Davis JL 3rd, Turner RO , Johnson L , Schwartz MD , Kerner JF , et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention . Cancer Epidemiology, Biomarkers & Prevention 2006. ; 15 ( 11 ): 2179-88 . [DOI] [PubMed] [Google Scholar]
Tebb 2021 {published data only}
- Tebb KP , Rodriguez F , Pollack LM , Adams S , Rico R , Renteria R , et al. Improving contraceptive use among Latina adolescents: a cluster-randomized controlled trial evaluating an mHealth application, Health-E You/Salud iTu . Contraception 2021. ; 104 ( 3 ): 246-53 . [DOI: 10.1016/j.contraception.2021.03.004 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomson 2007 {published data only}
- Kaner E , Heaven B , Rapley T , Murtagh M , Graham R , Thomson R , et al. Medical communication and technology: a video-based process study of the use of decision aids in primary care consultations . BMC Medical Informatics and Decision Making 2007. ; 7 ( 2 ): 1-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RG , Eccles MP , Steen IN , Greenaway J , Stobbart L , Murtagh MJ , et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial . Quality & Safety in Health Care 2007. ; 16 ( 3 ): 216-23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilburt 2022 {published data only}
- Tilburt JC , Zahrieh D , Pacyna JE , Petereit DG , Kaur JS , Rapkin BD , et al. Decision aids for localized prostate cancer in diverse minority men: primary outcome results from a multicenter cancer care delivery trial (Alliance A191402CD) . Cancer 2022. ; 128 ( 6 ): 1242-51 . [DOI: 10.1002/cncr.34062 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trevena 2008 {published data only}
- Trevena LJ , Irwig L , Barratt A . Randomized trial of a self-administered decision aid for colorectal cancer screening . Journal of Medical Screening 2008. ; 15 ( 2 ): 76-82 . [DOI] [PubMed] [Google Scholar]
Vandemheen 2009 {published data only}
- Vandemheen KL , O'Connor A , Bell SC , Freitag A , Bye P , Jeanneret A , et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation . American Journal of Respiratory & Critical Care Medicine 2009. ; 180 ( 8 ): 761-8 . [DOI] [PubMed] [Google Scholar]
van Dijk 2021 {published data only}
- Dijk LA , Vervest AM , Baas DC , Poolman RW , Haverkamp D . Decision aids can decrease decisional conflict in patients with hip or knee osteoarthritis: randomized controlled trial . World Journal of Orthopedics 2021. ; 12 ( 12 ): 1026-35 . [DOI: 10.5312/wjo.v12.i12.1026 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Peperstraten 2010 {published data only}
- Kreuwel I , Peperstraten A , Hulscher M , Kremer J , Grol R , Nelen W , et al. Evaluation of an effective multifaceted implementation strategy for elective single-embryo transfer after in vitro fertilization . Human Reproduction 2013. ; 28 ( 2 ): 336-42 . [DOI] [PubMed] [Google Scholar]
- Van Peperstraten A , Nelen W , Grol R , Zielhuis G , Adang E , Stalmeier P , et al. The effect of a multifaceted empowerment strategy on decision making about the number of embryos transferred in in vitro fertilisation: randomised controlled trial . BMJ 2010. ; 341 : c2501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
van Tol‐Geerdink 2013 {published data only}
- Tol-Geerdink JJ , Leer JW , Wijburg CJ , Oort IM , Vergunst H , Lin EJ , et al. Does a decision aid for prostate cancer affect different aspects of decisional regret, assessed with new regret scales? A randomized, controlled trial . Health Expectations 2016. ; 19 ( 2 ): 459-70 . [DOI: 10.1111/hex.12369 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol-Geerdink JJ , Willem Leer J , Weijerman PC , Oort IM , Vergunst H , Lin EN , et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid . BJU International 2013. ; 111 ( 4 ): 564-73 . [DOI: 10.1111/j.1464-410X.2012.11402.x ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Varelas 2020 {published data only}
- Varelas L , Egro FM , Evankovich N , Nguyen V . A randomized controlled trial to assess the use of a virtual decisional aid to improve knowledge and patient satisfaction in women considering breast reconstruction following mastectomy . Curēus 2020. ; 12 ( 12 ): e12018 . [DOI: 10.7759/cureus.12018 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vigod 2019 {published data only}
- Vigod SN , Hussain-Shamsy N , Stewart DE , Grigoriadis S , Metcalfe K , Oberlander TF , et al. A patient decision aid for antidepressant use in pregnancy: pilot randomized controlled trial . Journal of Affective Disorders 2019. ; 251 : 91-9 . [DOI: 10.1016/j.jad.2019.01.051 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vina 2016 {published data only}
- Vina ER , Richardson D , Medvedeva E , Kent Kwoh C , Collier A , Ibrahim SA . Does a patient-centered educational intervention affect African American access to knee replacement? A randomized trial . Clinical Orthopaedics and Related Research 2016. ; 474 ( 8 ): 1755-64 . [DOI: 10.1007/s11999-016-4834-z ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vodermaier 2009 {published data only}
- Vodermaier A , Caspari C , Koehm J , Kahlert S , Ditsch N , Untch M . Contextual factors in shared decision making: a randomised controlled trial in women with a strong suspicion of breast cancer . British Journal of Cancer 2009. ; 100 ( 4 ): 590-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Volk 1999 {published and unpublished data}
- Volk RJ , Cass AR , Spann SJ . A randomized controlled trial of shared decision making for prostate cancer screening . Archives of Family Medicine 1999. ; 8 ( 4 ): 333-40 . [DOI] [PubMed] [Google Scholar]
- Volk RJ , Spann SJ , Cass AR , Hawley ST . Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up . Annals of Family Medicine 2003. ; 1 ( 1 ): 22-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Volk 2020 {published data only}
- Volk RJ , Lowenstein LM , Leal VB , Escoto KH , Cantor SB , Munden RF , et al. Effect of a patient decision aid on lung cancer screening decision-making by persons who smoke: a randomized clinical trial . JAMA Network Open 2020. ; 3 ( 1 ): e1920362 . [DOI: 10.1001/jamanetworkopen.2019.20362 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuorma 2003 {published data only}
- Vuorma S , Rissanen P , Aalto AM , Hurskainen R , Kujansuu E , Teperi J . Impact of patient information booklet on treatment decision - a randomized trial among women with heavy menstruation . Health Expectations 2003. ; 6 ( 4 ): 290-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorma S , Teperi J , Aalto AM , Hurskainen R , Kujansuu E , Rissanen P . A randomized trial among women with heavy menstruation - impact of a decision aid on treatment outcomes and costs . Health Expectations 2004. ; 7 ( 4 ): 327-37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2021 {published data only}
- Wallace BC , Jones J , Masoudi FA , Nowels CT , Varosy P , Thomson R , et al. Development and piloting of four decision aids for implantable cardioverter-defibrillators in different media formats . Pacing and Clinical Electrophysiology 2021. ; 44 ( 11 ): 1842-52 . [DOI: 10.1111/pace.14365 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2021 {published data only}
- Wang MM , Loh EW , Chou JF , Sung PM , Chou YY , Lin YK , et al. Influence of shared decision making on decisional conflict and regret in postpartum mother-infant care: a randomized controlled trial . Value in Health: the Journal of the International Society for Pharmacoeconomics and Outcomes Research 2021. ; 24 ( 9 ): 1335-42 . [DOI: 10.1016/j.jval.2021.03.011 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Watson 2006 {published data only}
- Watson E , Hewitson P , Brett J , Bukach C , Evans R , Edwards A , et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men's knowledge, attitudes and intention to be tested . Patient Education and Counseling 2006. ; 63 ( 3 ): 367-79 . [DOI] [PubMed] [Google Scholar]
Watts 2015 {published data only}
- Watts BV , Schnurr PP , Zayed M , Young-Xu Y , Stender P , Llewellyn-Thomas H . A randomized controlled clinical trial of a patient decision aid for posttraumatic stress disorder . Psychiatric Services: a Journal of the American Psychiatric Association 2015. ; 66 ( 2 ): 149-54 . [DOI: 10.1176/appi.ps.201400062 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weymiller 2007 {published data only}
- Jones LA , Weymiller AJ , Shah N , Bryant SC , Christianson TJH , Guyatt GH , et al. Should clinicians deliver decision aids? Further exploration of the statin choice randomized trial results . Medical Decision Making 2009. ; 29 ( 4 ): 468-74 . [DOI] [PubMed] [Google Scholar]
- Nannenga MR , Montori VM , Weymiller AJ , Smith SA , Christianson TJ , Bryant SC , et al. A treatment decision aid may increase patient trust in the diabetes specialist. The Statin Choice randomized trial . Health Expectations 2009. ; 12 ( 1 ): 38-44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymiller AJ , Montori VM , Jones LA , Gafni A , Guyatt GH , Bryant SC , et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial . Archives of Internal Medicine 2007. ; 167 ( 10 ): 1076-82 . [DOI] [PubMed] [Google Scholar]
Whelan 2003 {published and unpublished data}
- Whelan T , Sawka C , Levine M , Gafni A , Reyno L , Willan A , et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer . Journal of the National Cancer Institute 2003. ; 95 ( 8 ): 581-7 . [DOI] [PubMed] [Google Scholar]
Whelan 2004 {published and unpublished data}
- Whelan T , Levine M , Willan A , Gafni A , Sanders K , Mirsky D , et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial . JAMA 2004. ; 292 ( 4 ): 435-41 . [DOI] [PubMed] [Google Scholar]
Wilkens 2019 {published data only}
- Wilkens SC , Ring D , Teunis T , Lee SP , Chen NC . Decision aid for trapeziometacarpal arthritis: a randomized controlled trial . The Journal of Hand Surgery 2019. ; 44 ( 3 ): 247.e1-9 . [DOI: 10.1016/j.jhsa.2018.06.004 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2013 {published and unpublished data}
- Williams RM , Davis KM , Luta G , Edmond SN , Dorfman CS , Schwartz MD , et al. Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer . Patient Education and Counseling 2013. ; 91 : 329-36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wise 2019 {published data only}
- Wise MR , Sadler L , Shorten B , Westhuizen K , Shorten A . Birth choices for women in a 'Positive Birth after Caesarean' clinic: randomised trial of alternative shared decision support strategies . The Australian & New Zealand Journal of Obstetrics & Gynaecology 2019. ; 59 ( 5 ): 684-92 . [DOI: 10.1111/ajo.12955 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wolf 1996 {published data only}
- Wolf AM , Nasser JF , Wolf AM , Schorling JB . The impact of informed consent on patient interest in prostate-specific antigen screening . Archives of Internal Medicine 1996. ; 156 ( 12 ): 1333-6 . [PubMed] [Google Scholar]
- Wolf AM , Schorling JB . Preferences of elderly men for prostate-specific antigen screening and the impact of informed consent . Journals of Gerontology Series A-Biological Sciences & Medical Sciences 1998. ; 53 ( 3 ): M195-200 . [DOI] [PubMed] [Google Scholar]
Wolf 2000 {published and unpublished data}
- Wolf AM , Schorling JB . Does informed consent alter elderly patients' preferences for colorectal cancer screening? Results of a randomized trial . Journal of General Internal Medicine 2000. ; 15 ( 1 ): 24-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong SS , Thornton JG , Gbolade B , Bekker HL . A randomised controlled trial of a decision-aid leaflet to facilitate women's choice between pregnancy termination methods . BJOG: An International Journal of Obstetrics & Gynaecology 2006. ; 113 ( 6 ): 688-94 . [DOI] [PubMed] [Google Scholar]
Wyld 2021 {published data only}
- Wyld L , Reed MW , Collins K , Burton M , Lifford K , Edwards A , et al. Bridging the age gap in breast cancer: cluster randomized trial of the effects of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices . British Journal of Surgery 2021. ; 108 ( 5 ): 499-510 . [DOI: 10.1093/bjs/znab005 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2021 {published data only}
- Ye G , Qu B , Tham YC , Zhong Y , Jin L , Lamoureux E , et al. A decision aid to facilitate informed choices among cataract patients: a randomized controlled trial . Patient Education and Counseling 2021. ; 104 ( 6 ): 1295-303 . [DOI: 10.1016/j.pec.2020.10.036 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zadro 2022 {published data only}
- Zadro J , Jones C , Harris I , Buchbinder R , O'Connor DA , McCaffery K , et al. Can a patient decision aid reduce people's intentions to have shoulder surgery . BMJ Open 2022. ; 11 ( 8 ): e054032 . [DOI: 10.1136/bmjopen-2021-054032 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abadie 2009 {published data only}
- Abadie R , Weymiller AJ , Tilburt J , Shah ND , Charles C , Gafni A , et al. Clinician's use of the Statin Choice decision aid in patients with diabetes: a videographic study nested in a randomized trial . Journal of Evaluation in Clinical Practice 2009. ; 15 ( 3 ): 492-7 . [DOI] [PubMed] [Google Scholar]
Abhyankar 2011 {published data only}
- Abhyankar P , Bekker HL , Summers BA , Velikova G . Why values elicitation techniques enable people to make informed decisions about cancer trial participation . Health Expectations 2011. ; 14 ( Suppl 1 ): 20-32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Adab 2003 {published data only}
- Adab P , Marshall T , Rouse A , Randhawa B , Sangha H , Bhangoo N . Randomised controlled trial of the effect of evidence based information on women's willingness to participate in cervical cancer screening . Journal of Epidemiology & Community Health 2003. ; 57 ( 8 ): 589-93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Adam 2018 {published data only}
- Adam S , Birch PH , Coe RR , Bansback N , Jones AL , Connolly MB , et al. Assessing an interactive online tool to support parents' genomic testing decisions . Journal of Genetic Counseling 2018. ; 28 ( 1 ): 10-7 . [DOI] [PubMed] [Google Scholar]
Adekpedjou 2020 {published data only}
- Adekpedjou R , Stacey D , Brière N , Freitas A , Garvelink MM , Dogba MJ , et al. Engaging caregivers in health-related housing decisions for older adults with cognitive impairment: a cluster randomized trial . Gerontologist 2020. ; 60 ( 5 ): 947-57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Akbari 2020 {published data only}
- Akbari Z , Mehrabi E , Mirghafourvand M , Nourizadeh R . The effect of decision aid on breast cancer screening behaviors based on theory of stage of change: an interventional study . Crescent Journal of Medical and Biological Sciences 2020. ; 7 ( 3 ): 404-8 . [Google Scholar]
Alegría 2014 {published data only}
- Alegría M , Carson N , Flores M , Li X , Shi P , Lessios AS , et al. Activation, self-management, engagement, and retention in behavioral health care: a randomized clinical trial of the DECIDE intervention . JAMA Psychiatry 2014. ; 71 ( 5 ): 557-65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2020 {published data only}
- Ali MK , Chwastiak L , Poongothai S , Emmert-Fees KMF , Patel SA , Anjana RM , et al. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT randomized clinical trial . JAMA 2020. ; 324 ( 7 ): 651-62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2016 {published data only}
- Allen KD , Sanders LL , Olsen MK , Bowlby L , Katz JN , Mather RC 3rd , et al. Internet versus DVD decision aids for hip and knee osteoarthritis . Musculoskeletal Care 2016. ; 14 ( 2 ): 87-97 . [DOI] [PubMed] [Google Scholar]
Allen 2022 {published data only}
- Allen JD , Porteny T , Kaplan A , Ladin K , Monahan K , Berry DL . Does shared decision-making for prostate cancer screening among African American men happen? It depends on who you ask . Journal of Racial and Ethnic Health Disparities 2022. ; 9 ( 4 ): 1225-33 . [DOI] [PubMed] [Google Scholar]
Almario 2022 {published data only}
- Almario CV , Deen WK , Chen M , Gale R , Sidorkiewicz S , Choi SY , et al. Interactive inflammatory bowel disease biologics decision aid does not improve patient outcomes over static education: results from a randomized trial . American Journal of Gastroenterology 2022. ; 117 ( 9 ): 1508-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Saffar 2008 {published data only}
- Al Saffar N , Abdulkareem A , Abdulhakeem A , Salah AQ , Heba M . Depressed patients' preferences for education about medications by pharmacists in Kuwait . Patient Education and Counseling 2008. ; 72 ( 1 ): 94-101 . [DOI] [PubMed] [Google Scholar]
AlSagheir 2020 {published data only}
- AlSagheir AI , Alrowais NA , Alkhudhair BK , AlYousefi NA , Al Sagheir AI , Ali AM , et al. Comparing the use of Arabic decision aid to usual care. A multicenter randomized controlled trial for Arabic speaking metastatic colorectal cancer patients in Saudi Arabia . Saudi Medical Journal 2020. ; 41 ( 5 ): 499-507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Altiner 2007 {published data only}
- Altiner A , Brockmann S , Sielk M , Wilm S , Wegscheider K , Abholz HH . Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: a cluster-randomized intervention study . Journal of Antimicrobial Chemotherapy 2007. ; 60 ( 3 ): 638-44 . [DOI] [PubMed] [Google Scholar]
Anderson 2011 {published data only}
- Anderson C , Carter J , Nattress K , Beale P , Philp S , Harrison J , et al. "The booklet helped me not to panic": a pilot of a decision aid for asymptomatic women with ovarian cancer and with rising CA-125 levels . International Journal of Gynecological Cancer 2011. ; 21 ( 4 ): 737-43 . [DOI] [PubMed] [Google Scholar]
Arimori 2006 {published data only}
- Arimori N . Randomized controlled trial of decision aids for women considering prenatal testing: the effect of the Ottawa Personal Decision Guide on decisional conflict . Japan Journal of Nursing Science 2006. ; 3 ( 2 ): 119-30 . [Google Scholar]
Armstrong 2005 {published data only}
- Armstrong K , Weber B , Ubel PA , Peters N , Holmes J , Schwartz JS . Individualized survival curves improve satisfaction with cancer risk management decisions in women with BRCA1/2 mutations . Journal of Clinical Oncology 2005. ; 23 ( 36 ): 9319-28 . [DOI] [PubMed] [Google Scholar]
Arterburn 2013 {published data only}
- Arterburn D , Flum DR , Westbrook EO , Fuller S , Shea M , Bock SN , et al, CROSSROADS Study Team . A population-based, shared decision-making approach to recruit for a randomized trial of bariatric surgery versus lifestyle for type 2 diabetes . Surgery for Obesity and Related Diseases 2013. ; 9 ( 6 ): 837-44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Au 2011 {published data only}
- Au AH , Lam WW , Chan MC , Or AY , Kwong A , Suen D , et al. Development and pilot-testing of a decision aid for use among Chinese women facing breast cancer surgery . Health Expectations 2011. ; 14 ( 4 ): 405-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakken 2014 {published data only}
- Bakken S , Jia H , Chen ES , Choi J , John RM , Lee NJ , et al. The effect of a mobile health decision support system on diagnosis and management of obesity, tobacco use, and depression in adults and children . The Journal for Nurse Practitioners 2014. ; 10 ( 10 ): 774-80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2009 {published data only}
- Becker H , Stuifbergen AK , Dormire SL . The effects of hormone therapy decision support for women with mobility impairments . Health Care for Women International 2009. ; 30 ( 9 ): 845-54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Belkora 2012 {published data only}
- Belkora J , Stupar L , O'Donnell S , Loucks A , Moore D , Jupiter C , et al. Decision support by telephone: randomized controlled trial in a rural community setting . Patient Education and Counseling 2012. ; 89 ( 1 ): 134-42 . [DOI] [PubMed] [Google Scholar]
Bellmunt 2010 {published data only}
- Bellmunt J , Eisen T , Szczylik C , Mulders P , Porta C . A new patient-focused approach to the treatment of metastatic renal cell carcinoma: establishing customized treatment options . BJU International 2010. ; 107 ( 8 ): 1190-9 . [DOI] [PubMed] [Google Scholar]
Bennett 2011 {published data only}
- Bennett PA . Making the Choice: Cesarean Delivery by Maternal Request versus Planned Vaginal Birth [PhD thesis] . Ann Arbor: : University of Colorado at Denver; , 2011. . [Google Scholar]
Betz 2020 {published data only}
- Betz ME , Knoepke CE , Simpson S , Siry BJ , Clement A , Saunders T , et al. An interactive web-based lethal means safety decision aid for suicidal adults (lock to live): pilot randomized controlled trial . Journal of Medical Internet Research 2020. ; 22 ( 1 ): e16253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Betz 2021 {published data only}
- Betz ME , Polzer E , Nearing K , Knoepke CE , Johnson RL , Meador L , et al. Feasibility and acceptability of a web-based caregiver decision aid (safety in dementia) for firearm access: pilot randomized controlled trial . JMIR Formative Research 2021. ; 5 ( 9 ): e30990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharya 2021 {published data only}
- Bhattacharya IS , Haviland JS , Turner L , Stobart H , Balasopoulou A , Stones L , et al. Can patient decision aids reduce decisional conflict in a de-escalation of breast radiotherapy clinical trial? The PRIMETIME study within a trial implemented using a cluster stepped-wedge trial design . Trials 2021. ; 22 ( 1 ): 397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieber 2006 {published data only}
- Bieber C , Muller KG , Blumenstiel K , Eich W . Participative decision-making as a measure to improve the doctor-patient interaction with fibromyalgia patients [Partizipative Entscheidungsfindung als Maßnahme zur Verbesserung der Arzt-Patient-Interaktion mit Fibromyalgie-Patientinnen]. Zeitschrift fur Medizinische Psychologie 2006. ; 15 ( 2 ): 53-60 . [Google Scholar]
- Bieber C , Muller KG , Blumenstiel K , Hochlehnert A , Wilke S , Hartmann M , et al. A shared decision-making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial . Journal of Psychosomatic Research 2008. ; 64 ( 1 ): 13-20 . [DOI] [PubMed] [Google Scholar]
Bombard 2020 {published data only}
- Bombard Y , Clausen M , Shickh S , Mighton C , Casalino S , Kim TH , et al. Effectiveness of the Genomics ADvISER decision aid for the selection of secondary findings from genomic sequencing: a randomized clinical trial . Genetics in Medicine 2020. ; 22 ( 4 ): 727-35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulware 2013 {published data only}
- Boulware LE , Hill-Briggs F , Kraus ES , Melancon JK , Falcone B , Ephraim PL , . Effectiveness of educational and social worker interventions to activate patients' discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial . American Journal of Kidney Diseases 2013. ; 61 ( 3 ): 476-86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulware 2018 {published data only}
- Boulware LE , Ephraim PL , Ameling J , Lewis-Boyer L , Rabb H , Greer RC , et al. Effectiveness of informational decision aids and a live donor financial assistance program on pursuit of live kidney transplants in African American hemodialysis patients . BMC Nephrology 2018. ; 19 ( 1 ): 107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Branda 2013 {published data only}
- Branda ME , LeBlanc A , Shah ND , Tiedje K , Ruud K , Van Houten H , et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care . BMC Health Services Research 2013. ; 13 ( 301 ): 1-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenner 2014 {published data only}
- Brenner A , Howard K , Lewis C , Sheridan S , Crutchfield T , Hawley S , et al. Comparing 3 values clarification methods for colorectal cancer screening decision-making: a randomized trial in the US and Australia . Journal of General Internal Medicine 2014. ; 29 ( 3 ): 507-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Breslin 2008 {published data only}
- Breslin M , Mullan RJ , Montori VM . The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes . Patient Education and Counseling 2008. ; 73 ( 3 ): 465-72 . [DOI] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown RF , Butow PN , Sharrock MA , Henman M , Boyle F , Goldstein D , et al. Education and role modelling for clinical decisions with female cancer patients . Health Expectations 2004. ; 7 ( 4 ): 303-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Brundage 2001 {published data only}
- Brundage MD , Feldman-Stewart D , Cosby R , Gregg R , Dixon P , Youssef Y , et al. Phase I study of a decision aid for patients with locally advanced non-small-cell lung cancer . Journal of Clinical Oncology 2001. ; 19 ( 5 ): 1326-35 . [DOI] [PubMed] [Google Scholar]
Brunette 2020 {published data only}
- Brunette MF , Ferron JC , McGurk SR , Williams JM , Harrington A , Devitt T , et al. Brief, web-based interventions to motivate smokers with schizophrenia: randomized controlled trial . JMIR Mental Health 2020. ; 7 ( 2 ): e16524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhse 2015 {published data only}
- Buhse S , Mühlhauser I , Heller T , Kuniss N , Müller UA , Kasper J , . Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial . BMJ Open 2015. ; 5 ( 11 ): e009116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhse 2018 {published data only}
- Buhse S , Kuniss N , Liethmann K , Müller UA , Lehmann T , Mühlhauser I . Informed shared decision-making programme for patients with type 2 diabetes in primary care: cluster randomised controlled trial . BMJ Open 2018. ; 8 ( 12 ): e024004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2007 {published data only}
- Burton MJ . Booklet-based education in vestibular rehabilitation or symptom control improved subjective health in Meniere disease . Evidence-Based Medicine 2007. ; 12 ( 4 ): 111 . [DOI] [PubMed] [Google Scholar]
Buzhardt 2011 {published data only}
- Buzhardt J , Greenwood CR , Walker D , Anderson R , Howard W , Carta JJ . Effects of web-based support on early head start home visitors' use of evidence-based intervention decision making and growth in children's expressive communication . NHSA Dialog 2011. ; 14 ( 3 ): 121-46 . [Google Scholar]
Campbell 2014 {published data only}
- Campbell SR , Holter MC , Manthey TJ , Rapp CA . The effect of CommonGround Software and Decision Support Center . American Journal of Psychiatric Rehabilitation 2014. ; 17 ( 2 ): 166-80 . [Google Scholar]
Carling 2008 {published data only}
- Carling C , Kristoffersen DT , Herrin J , Treweek S , Oxman AD , Schunemann H , et al. How should the impact of different presentations of treatment effects on patient choice be evaluated? A pilot randomized trial . PLOS One 2008. ; 3 ( 11 ): e3693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 2021 {published data only}
- Carlson DS , Grivas P , Wei W , Dhillon PK , Abraksia S . The effectiveness of shared compared to informed decision making for prostate cancer screening in a high-risk African American population: a randomized control trial . Cancer Investigation 2021. ; 39 ( 2 ): 124-32 . [DOI] [PubMed] [Google Scholar]
Carter‐Harris 2020 {published data only}
- Carter-Harris L , Comer RS , Slaven Ii JE , Monahan PO , Vode E , Hanna NH , et al. Computer-tailored decision support tool for lung cancer screening: community-based pilot randomized controlled trial . Journal of Medical Internet Research 2020. ; 22 ( 11 ): e17050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Causarano 2015 {published data only}
- Causarano N , Platt J , Baxter NN , Bagher S , Jones JM , Metcalfe KA , et al. Pre-consultation educational group intervention to improve shared decision-making for postmastectomy breast reconstruction: a pilot randomized controlled trial . Support Cancer Care 2015. ; 23 : 1365-75 . [DOI] [PubMed] [Google Scholar]
Chadwick 1991 {published data only}
- Chadwick DJ , Gillatt DA , Gingell JC . Medical or surgical orchidectomy: the patients' choice . BMJ 1991. ; 302 ( 6776 ): 572 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan EC , McFall SL , Byrd TL , Mullen PD , Volk RJ , Ureda J , et al. A community-based intervention to promote informed decision making for prostate cancer screening among Hispanic American men changed knowledge and role preferences: a cluster RCT . Patient Education and Counseling 2011. ; 84 ( 2 ): e44-51 . [DOI] [PubMed] [Google Scholar]
Chewning 1999 {published data only}
- Chewning B , Mosena P , Wilson D , Erdman H , Potthoff S , Murphy A , et al. Evaluation of a computerized contraceptive decision aid for adolescent patients . Patient Education and Counseling 1999. ; 38 ( 3 ): 227-39 . [DOI] [PubMed] [Google Scholar]
Chiew 2008 {published data only}
- Chiew KS , Shepherd H , Vardy J , Tattersall MHN , Butow PN , Leighl NB . Development and evaluation of a decision aid for patients considering first-line chemotherapy for metastatic breast cancer . Health Expectations 2008. ; 11 ( 1 ): 35-45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2020 {published data only}
- Chong WQ , Mogro MJ , Arsad A , Tai BC , Lee SC . Use of decision aid to improve informed decision-making and communication with physicians on the use of oral complementary and alternative medicine (CAM) among cancer patients on chemotherapy treatment: a randomised controlled trial . Supportive Care in Cancer 2020. ; 29 ( 7 ): 3689-96 . [DOI] [PubMed] [Google Scholar]
Christy 2022 {published data only}
- Christy SM , Livingstone AS , Byrne MM . Feasibility, acceptability, and effectiveness of a decision aid versus an informational website to promote clinical trial decision-making among cancer patients: a pilot randomized controlled trial . Patient Education and Counseling 2022. ; 105 ( 5 ): 1082-8 . [DOI] [PubMed] [Google Scholar]
Clark 2022 {published data only}
- Clark SD , Reuland DS , Brenner AT , Jonas DE . Effect of incidental findings information on lung cancer screening intent: a randomized controlled trial . Journal of General Internal Medicine 2022. ; 37 ( 14 ): 3676-83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Clouston 2014 {published data only}
- Clouston K , Katz A , Martens PJ , Sisler J , Turner D , Lobchuk M , et al, CIHR/CCMB Team in Primary Care Oncology (PCO-NET) . Does access to a colorectal cancer screening website and/or a nurse-managed telephone help line provided to patients by their family physician increase fecal occult blood test uptake? Results from a pragmatic cluster randomized controlled trial . BMC Cancer 2014. ; 14 : 263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Col 2007 {published data only}
- Col NF , Ngo L , Fortin JM , Goldberg RJ , O'Connor AM . Can computerized decision support help patients make complex treatment decisions? A randomized controlled trial of an individualized menopause decision aid . Medical Decision Making 2007. ; 27 ( 5 ): 585-98 . [DOI] [PubMed] [Google Scholar]
Colella 2004 {published data only}
- Colella KM , DeLuca G . Shared decision making in patients with newly diagnosed prostate cancer: a model for treatment education and support . Urologic Nursing 2004. ; 24 ( 3 ): 187-91, 195-6 . [PubMed] [Google Scholar]
Coronado‐Vazquez 2019 {published data only}
- Coronado-Vázquez V , Gómez-Salgado J , Cerezo-Espinosa de Los Monteros J , Ayuso-Murillo D , Ruiz-Frutos C . Shared decision-making in chronic patients with polypharmacy: an interventional study for assessing medication appropriateness . Journal of Clinical Medicine 2019. ; 8 ( 6 ): 904 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Costanza 2011 {published data only}
- Costanza ME , Luckmann RS , Rosal M , White MJ , LaPelle N , Partin M , et al. Helping men make an informed decision about prostate cancer screening: a pilot study of telephone counseling . Patient Education and Counseling 2011. ; 82 ( 2 ): 193-200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulter 2003 {published data only}
- Coulter A . Patient information and shared decision-making in cancer care . British Journal of Cancer 2003. ; 89 ( Suppl 1 ): S15-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2012 {published data only}
- Cox CE , Lewis CL , Hanson LC , Hough CL , Kahn JM , White DB , et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation . Critical Care Medicine 2012. ; 40 ( 8 ): 2327-34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Crang‐Svalenius 1996 {published data only}
- Crang-Svalenius E , Dykes AK , Jorgensen C . Women's informed choice of prenatal diagnosis: early ultrasound examination-routine ultrasound examination-age-independent amniocentesis . Fetal Diagnosis & Therapy 1996. ; 11 ( 1 ): 20-5 . [DOI] [PubMed] [Google Scholar]
Davies 2021 {published data only}
- Davies C , Marshall HS , Zimet G , McCaffery K , Brotherton JML , Kang M , et al. Effect of a school-based educational intervention about the human papillomavirus vaccine on psychosocial outcomes among adolescents: analysis of secondary outcomes of a cluster randomized trial . JAMA Network Open 2021. ; 4 ( 11 ): e2129057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2014 {published data only}
- Davis SN , Sutton SK , Vadaparampil ST , Meade CD , Rivers BM , Patel MV , et al. Informed decision making among first-degree relatives of prostate cancer survivors: a pilot randomized trial . Contemporary Clinical Trials 2014. ; 39 ( 2 ): 327-34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Davison 1999 {published data only}
- Davison BJ , Kirk P , Degner LF , Hassard TH . Information and patient participation in screening for prostate cancer . Patient Education and Counseling 1999. ; 37 ( 3 ): 255-63 . [DOI] [PubMed] [Google Scholar]
Davison 2007 {published data only}
- Davison BJ , Goldenberg SL , Wiens KP , Gleave ME . Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer . Cancer Nursing 2007. ; 30 ( 5 ): E7-15 . [DOI] [PubMed] [Google Scholar]
De Boer 2012 {published data only}
- De Boer JC , Blijderveen G , Dijk G , Duivenvoorden HJ , Williams M . Implementing structured, multiprofessional medical ethical decision-making in a neonatal intensive care unit . Journal of Medical Ethics 2012. ; 38 ( 10 ): 596-601 . [DOI] [PubMed] [Google Scholar]
Deen 2012 {published data only}
- Deen D , Lu WH , Weintraub MR , Maranda MJ , Elshafey S , Gold MR . The impact of different modalities for activating patients in a community health center setting . Patient Education and Counseling 2012. ; 89 ( 1 ): 178-83 . [DOI] [PubMed] [Google Scholar]
De Haan 2013 {published data only}
- De Haan MC , Wijkerslooth TR , Stoop E , Bossuyt P , Fockens P , Thomeer M , et al. Informed decision-making in colorectal cancer screening using colonoscopy or CT-colonography . Patient Education and Counseling 2013. ; 91 ( 3 ): 318-25 . [DOI] [PubMed] [Google Scholar]
Dehlendorf 2019 {published data only}
- Dehlendorf C , Fitzpatrick J , Fox E , Holt K , Vittinghoff E , Reed R , et al. Cluster randomized trial of a patient-centered contraceptive decision support tool, My Birth Control . American Journal of Obstetrics and Gynecology 2019. ; 220 ( 6 ): 565.e1-12 . [DOI] [PubMed] [Google Scholar]
Deinzer 2009 {published data only}
- Deinzer A , Veelken R , Kohnen R , Schmieder RE . Is a shared decision-making approach effective in improving hypertension management? Journal of Clinical Hypertension 2009. ; 11 ( 5 ): 266-70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Denig 2014 {published data only}
- Denig P , Schuling J , Haaijer-Ruskamp F , Voorham J . Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial . BMJ 2014. ; 349 : g5651 . [DOI] [PubMed] [Google Scholar]
Den Ouden 2017 {published data only}
- Den Ouden H , Vos RC , Rutten GE . Effectiveness of shared goal setting and decision making to achieve treatment targets in type 2 diabetes patients: a cluster-randomized trial (OPTIMAL) . Health Expectations 2017. ; 20 ( 5 ): 1172-80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Deschamps 2004 {published and unpublished data}
- Deschamps MA , Taylor JG , Neubauer SL , Whiting S , Green K . Impact of pharmacist consultation versus a decision aid on decision making regarding hormone replacement therapy . International Journal of Pharmacy Practice 2004. ; 12 ( 1 ): 21-8 . [Google Scholar]
Deyo 2000 {published and unpublished data}
- Deyo RA , Cherkin DC , Weinstein J , Howe J , Ciol M , Mulley AG . Involving patients in clinical decisions: impact of an interactive video program on use of back surgery . Medical Care 2000. ; 38 ( 9 ): 959-69 . [DOI] [PubMed] [Google Scholar]
- Phelan EA , Deyo RA , Cherkin DC , Weinstein JN , Ciol MA , Kreuter W , et al. Helping patients decide about back surgery: a randomized trial of an interactive video program . Spine 2001. ; 26 ( 2 ): 206-12 . [DOI] [PubMed] [Google Scholar]
Diefenbach 2012 {published data only}
- Diefenbach MA , Mohamed NE , Butz BP , Bar-Chama N , Stock R , Cesaretti J , et al. Acceptability and preliminary feasibility of an internet/CD-ROM-based education and decision program for early-stage prostate cancer patients: randomized pilot study . Journal of Medical Internet Research 2012. ; 14 ( 1 ): e6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Diefenbach 2018 {published data only}
- Diefenbach MA , Benedict C , Miller SM , Stanton AL , Ropka ME , Wen KY , et al. Examining the impact of a multimedia intervention on treatment decision-making among newly diagnosed prostate cancer patients: results from a nationwide RCT . Translational Behavioral Medicine 2018. ; 8 ( 6 ): 876-86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobke 2008 {published data only}
- Dobke MK , Bhavsar D . Pilot trial of telemedicine as a decision aid for patients with chronic wounds . Telemedicine Journal and e-health 2008. ; 14 ( 3 ): 245-9 . [DOI] [PubMed] [Google Scholar]
Dodin 2001 {published and unpublished data}
- Dodin S , Legare F , Daudelin G , Tetroe J , O'Connor A . Making a decision about hormone replacement therapy. A randomized controlled trial [Prise de decision en matière d'hormonothérapie de remplacement]. Canadian Family Physician 2001. ; 47 : 1586-93 . [PMC free article] [PubMed] [Google Scholar]
Doll 2019 {published data only}
- Doll JA , Jones WS , Lokhnygina Y , Culpepper S , Parks RL , Calhoun C , et al. PREPARED Study: a study of shared decision-making for coronary artery disease . Circulation. Cardiovascular Quality and Outcomes 2019. ; 12 ( 2 ): e005244 . [DOI] [PubMed] [Google Scholar]
Donovan 2012 {published data only}
- Donovan JL . Presenting treatment options to men with clinically localized prostate cancer: the acceptability of active surveillance/monitoring . Journal of the National Cancer Institute. Monographs 2012. ; 45 : 191-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 2008 {published data only}
- Driscoll DL , Rupert DJ , Golin CE , McCormack LA , Sheridan SL , Welch BM . Promoting prostate-specific antigen informed decision-making. Evaluating two community-level interventions . American Journal of Preventive Medicine 2008. ; 35 ( 2 ): 87-94 . [DOI] [PubMed] [Google Scholar]
Dunn 1998 {published and unpublished data}
- Dunn RA , Shenouda PE , Martin DR , Schultz AJ . Videotape increases parent knowledge about poliovirus vaccines and choices of polio vaccination schedules . Pediatrics 1998. ; 102 ( 2 ): e26 . [DOI] [PubMed] [Google Scholar]
Eaton 2011 {published data only}
- Eaton L , Cherry C , Cain D , Pope H . A novel approach to prevention for at-risk HIV negative men who have sex with men: creating a teachable moment to promote informed sexual decision making . American Journal of Public Health 2011. ; 101 ( 3 ): 539-45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Eden 2009 {published data only}
- Eden KB , Dolan JG , Perrin NA , Kocaoglu D , Anderson N , Case J , et al. Patients were more consistent in randomized trial at prioritizing childbirth preferences using graphic-numeric than verbal formats . Journal of Clinical Epidemiology 2009. ; 62 ( 4 ): 415-24 . [DOI] [PubMed] [Google Scholar]
Eden 2014 {published data only}
- Eden KB , Perrin NA , Vesco KK , Guise JM . A randomized comparative trial of two decision tools for pregnant women with prior cesareans . Journal of Obstetric, Gynecologic, & Neonatal Nursing 2014. ; 43 : 568-79 . [DOI] [PubMed] [Google Scholar]
Eden 2015 {published data only}
- Eden KB , Perrin NA , Hanson GC , Messing JT , Bloom TL , Campbell JC , et al. Use of online safety decision aid by abused women . American Journal of Preventive Medicine 2015. ; 48 ( 4 ): 372-83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2012 {published data only}
- Edwards JA , Snyder FJ , Allen PM , Makinson KA , Hamby DM . Decision making for risk management: a comparison of graphical methods for presenting quantitative uncertainty . Risk Analysis 2012. ; 32 ( 12 ): 2055-70 . [DOI] [PubMed] [Google Scholar]
El‐Jawahri 2010 {published data only}
- El-Jawahri A , Podgurski LM , Eichler AF , Plotkin SR , Temel JS , Mitchell SL . Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial . Journal of Clinical Oncology 2010. ; 28 ( 2 ): 305-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellison 2008 {published data only}
- Ellison GL , Weinrich SP . A randomized trial comparing web-based decision aids on prostate cancer knowledge for African-American men . Journal of the National Medical Association 2008. ; 100 ( 10 ): 1139-45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
El Miedany 2019 {published data only}
- El Miedany Y , El Gaafary M , Lotfy H , El Aroussy N , Mekkawy D , Nasef SI , et al. Shared decision-making aid for juvenile idiopathic arthritis: moving from informative patient education to interactive critical thinking . Clinical Rheumatology 2019. ; 38 ( 11 ): 3217-25 . [DOI] [PubMed] [Google Scholar]
Elwyn 2004 {published data only}
- Elwyn G , Edwards A , Hood K , Robling M , Atwell C , Russell I , et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice . Family Practice 2004. ; 21 ( 4 ): 337-46 . [DOI] [PubMed] [Google Scholar]
Elwyn 2016 {published data only}
- Elwyn G , Pickles T , Edwards A , Kinsey K , Brain K , Newcombe RG , . Supporting shared decision making using an option grid for osteoarthritis of the knee in an interface musculoskeletal clinic: a stepped wedge trial . Patient Education and Counseling 2016. ; 99 ( 4 ): 571-7 . [DOI] [PubMed] [Google Scholar]
Emery 2007 {published data only}
- Emery J , Morris H , Goodchild R , Fanshawe T , Prevost AT , Bobrow M , et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care . British Journal of Cancer 2007. ; 97 ( 4 ): 486-93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmett 2007 {published data only}
- Emmett CL , Murphy DJ , Patel RR , Fahey T , Jones C , Ricketts IW , et al. Decision-making about mode of delivery after previous caesarean section: development and piloting of two computer-based decision aids . Health Expectations 2007. ; 10 ( 2 ): 161-72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Eneanya 2020 {published data only}
- Eneanya ND , Percy SG , Stallings TL , Wang W , Steele DJR , Germain MJ , et al. Use of a supportive kidney care video decision aid in older patients: a randomized controlled trial . American Journal of Nephrology 2020. ; 51 ( 9 ): 736-44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fadda 2017 {published data only}
- Fadda M , Galimberti E , Fiordelli M , Romanò L , Zanetti A , Schulz PJ . Effectiveness of a smartphone app to increase parents' knowledge and empowerment in the MMR vaccination decision: a randomized controlled trial . Human Vaccines and Immunotherapeutics 2017. ; 13 ( 11 ): 2512-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerlin 2021 {published data only}
- Fagerlin A , Holmes-Rovner M , Hofer TP , Rovner D , Alexander SC , Knight SJ , et al. Head to head randomized trial of two decision aids for prostate cancer . BMC Medical Informatics and Decision Making 2021. ; 21 ( 1 ): 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2021 {published data only}
- Fang SY , Lin PJ , Kuo YL . Long-term effectiveness of a decision support app (Pink Journey) for women considering breast reconstruction surgery: pilot randomized controlled trial . JMIR mHealth and uHealth 2021. ; 9 ( 12 ): e31092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman‐Stewart 2006 {published data only}
- Feldman-Stewart D , Brennenstuhl S , Brundage MD , Roques T . An explicit values clarification task: development and validation . Patient Education and Counseling 2006. ; 63 ( 3 ): 350-6 . [DOI] [PubMed] [Google Scholar]
Feldman‐Stewart 2012 {published data only}
- Feldman-Stewart D , Tong C , Siemens R , Alibhai S , Pickles T , Robinson J , et al. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial . Medical Decision Making 2012. ; 32 ( 4 ): 616-26 . [DOI] [PubMed] [Google Scholar]
Fiks 2013a {published data only}
- Fiks AG , Grundmeier RW , Mayne S , Song L , Feemster K , Karavite D , et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt . Pediatrics 2013. ; 131 ( 6 ): 1114-24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiks 2015 {published data only}
- Fiks AG , Mayne SL , Karavite DJ , Suh A , O'Hara R , Localio AR , et al. Parent-reported outcomes of a shared decision-making portal in asthma: a practice-based RCT . Pediatrics 2015. ; 135 ( 4 ): e965-73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleisher 2015 {published data only}
- Fleisher L , Wen KY , Miller SM , Diefenbach M , Stanton AL , Ropka M , et al. Development and utilization of complementary communication channels for treatment decision making and survivorship issues among cancer patients: the CIS Research Consortium Experience . Internet Interventions 2015. ; 2 ( 4 ): 392-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Flood 1996 {published data only}
- Flood AB , Wennberg JE , Nease RF Jr , Fowler FJ Jr, Ding J , Hynes LM . The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team . Journal of General Internal Medicine 1996. ; 11 ( 6 ): 342-9 . [DOI] [PubMed] [Google Scholar]
Francis 2009 {published data only}
- Francis NA , Butler CC , Hood K , Simpson S , Wood F , Nuttall J . Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial . BMJ 2009. ; 339 : b2885 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraval 2015 {published data only}
- Fraval A , Chandrananth J , Chong YM , Tran P , Coventry LS . Internet based patient education improves informed consent for elective orthopaedic surgery: a randomized controlled trial . BMC Musculoskeletal Disorders 2015. ; 16 : 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2001 {published data only}
- Frosch DL , Kaplan RM , Felitti V . Evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test . Journal of General Internal Medicine 2001. ; 16 ( 6 ): 391-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2003 {published data only}
- Frosch DL , Kaplan RM , Felitti VJ . A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test . Journal of General Internal Medicine 2003. ; 18 ( 10 ): 781-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2008b {published data only}
- Frosch DL , Legare F , Mangione CM . Using decision aids in community-based primary care: a theory-driven evaluation with ethnically diverse patients . Patient Education and Counseling 2008. ; 73 ( 3 ): 490-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2011 {published data only}
- Frosch DL , Uy V , Ochoa S , Mangione CM . Evaluation of a behavior support intervention for patients with poorly controlled diabetes . Archives of Internal Medicine 2011. ; 171 ( 22 ): 2011-7 . [DOI] [PubMed] [Google Scholar]
Frost 2009 {published data only}
- Frost J , Shaw A , Montgomery A , Murphy DJ . Women's views on the use of decision aids for decision making about the method of delivery following a previous caesarean section: qualitative interview study . BJOG: An International Journal of Obstetrics & Gynaecology 2009. ; 116 ( 7 ): 896-905 . [DOI] [PubMed] [Google Scholar]
Fujiwara 2015 {published data only}
- Fujiwara H , Shimoda A , Ishikawa Y , Taneichi A , Ohashi M , Takahashi Y , et al. Effect of providing risk information on undergoing cervical cancer screening: a randomized controlled trial . Archives of Public Health 2015. ; 73 : 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvelink 2013 {published data only}
- Garvelink MM , ter Kuile MM , Fischer MJ , Louwé LA , Hilders CG , Kroep JR , et al. Development of a decision aid about fertility preservation for women with breast cancer in the Netherlands . Journal of Psychosomatic Obstetrics and Gynecology 2013. ; 34 ( 4 ): 170-8 . [DOI] [PubMed] [Google Scholar]
Garvelink 2017 {published data only}
- Garvelink MM , Ter Kuile MM , Louwé LA , Hilders CG , Stiggelbout AM . Feasibility and effects of a decision aid about fertility preservation . Human Fertility 2017. ; 20 ( 2 ): 104-12 . [DOI] [PubMed] [Google Scholar]
Genz 2012 {published data only}
- Genz J , Haastert B , Müller H , Verheyen F , Cole D , Rathmann W , et al. Blood glucose testing and primary prevention of type 2 diabetes - evaluation of the effect of evidence-based patient information: a randomized controlled trial . Diabetic Medicine 2012. ; 29 ( 8 ): 1011-20 . [DOI] [PubMed] [Google Scholar]
Genz 2014 {published data only}
- Genz J , Haastert B , Müller H , Verheyen F , Cole D , Rathmann W , et al. Socioeconomic factors and effect of evidence-based patient information about primary prevention of type 2 diabetes mellitus--are there interactions? BMC Research Notes 2014. ; 7 : 541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2021 {published data only}
- George M , Bruzzese JM , Lynn S , Sommers M , Pantalon MV , Jia H , et al. Group-randomized trial of tailored brief shared decision-making to improve asthma control in urban black adults . Journal of Advanced Nursing 2021. ; 77 ( 3 ): 1501-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Giordano 2014 {published data only}
- Giordano A , Lugaresi A , Confalonieri P , Granella F , Radice D , Trojano M , et al. Implementation of the "Sapere Migliora" information aid for newly diagnosed people with multiple sclerosis in routine clinical practice: a late-phase controlled trial . Multiple Sclerosis Journal 2014. ; 20 ( 9 ): 1234-43 . [DOI] [PubMed] [Google Scholar]
Goel 2001 {published and unpublished data}
- Goel V , Sawka CA , Thiel EC , Gort EH , O'Connor AM . Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer . Medical Decision Making 2001. ; 21 ( 1 ): 1-6 . [DOI] [PubMed] [Google Scholar]
Gong 2017 {published data only}
- Gong HS , Park JW , Shin YH , Kim K , Cho KJ , Baek GH . Use of a decision aid did not decrease decisional conflict in patients with carpal tunnel syndrome . BMC Musculoskeletal Disorders 2017. ; 18 ( 1 ): 118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorawara‐Bhat 2017 {published data only}
- Gorawara-Bhat R , O'Muircheartaigh S , Mohile S , Dale W . Patients' perceptions and attitudes on recurrent prostate cancer and hormone therapy: qualitative comparison between decision-aid and control groups . Journal of Geriatric Oncology 2017. ; 8 ( 5 ): 368-73 . [DOI] [PubMed] [Google Scholar]
Graham 2000 {published data only}
- Graham W , Smith P , Kamal A , Fitzmaurice A , Smith N , Hamilton N . Randomised controlled trial comparing effectiveness of touch screen system with leaflet for providing women with information on prenatal tests . BMJ 2000. ; 320 ( 7228 ): 155-60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2009 {published data only}
- Gray SW , O'Grady C , Karp L , Smith D , Schwartz JS , Hornik RC , et al. Risk information exposure and direct-to-consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer . Cancer Epidemiology, Biomarkers & Prevention 2009. ; 18 ( 4 ): 1303-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2001b {published data only}
- Green MJ , McInerney AM , Biesecker BB , Fost N . Education about genetic testing for breast cancer susceptibility: patient preferences for a computer program or genetic counselor . American Journal of Medical Genetics 2001. ; 103 ( 1 ): 24-31 . [DOI] [PubMed] [Google Scholar]
Green 2004 {published data only}
- Green MJ , Peterson SK , Baker MW , Friedman LC , Harper GR , Rubinstein WS , et al. Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions . Genetics in Medicine 2005. ; 7 ( 4 ): 221-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ , Peterson SK , Baker MW , Harper GR , Friedman LC , Rubinstein WS , et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial . JAMA 2004. ; 292 ( 4 ): 442-52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2020 {published data only}
- Green MJ , Van Scoy LJ , Foy AJ , Dimmock AEF , Lehman E , Levi BH . Patients with advanced cancer choose less aggressive medical treatment on vignettes after using a computer-based decision aid . American Journal of Hospice & Palliative Medicine 2020. ; 37 ( 7 ): 537-41 . [DOI] [PubMed] [Google Scholar]
Greenfield 1985 {published data only}
- Greenfield S , Kaplan S , Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes . Annals of Internal Medicine 1985. ; 102 ( 4 ): 520-8 . [DOI] [PubMed] [Google Scholar]
Griffith 2008a {published data only}
- Griffith JM , Lewis CL , Brenner AR , Pignone MP . The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial . BMC Medical Informatics and Decision Making 2008. ; 8 : 4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffith 2008b {published data only}
- Griffith JM , Fichter M , Fowler FJ , Lewis C , Pignone MP . Should a colon cancer screening decision aid include the option of no testing? A comparative trial of two decision aids . BMC Medical Informatics and Decision Making 2008. ; 8 : 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruppen 1994 {published data only}
- Gruppen LD , Margolin J , Wisdom K , Grum CM . Outcome bias and cognitive dissonance in evaluating treatment decisions . Academic Medicine 1994. ; 69 ( 10 Suppl ): S57-9 . [DOI] [PubMed] [Google Scholar]
Guillen 2019 {published data only}
- Guillén Ú, Mackley A , Laventhal N , Kukora S , Christ L , Derrick M , et al. Evaluating the use of a decision aid for parents facing extremely premature delivery: a randomized trial . Journal of Pediatrics 2019. ; 209 : 52-60.e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 2014 {published data only}
- Gulliford MC , Staa T , Dregan A , McDermott L , McCann G , Ashworth M , et al. Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study) . Annals of Family Medicine 2014. ; 12 ( 4 ): 344-51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Gummersbach 2015 {published data only}
- Gummersbach E , in der Schmitten J , Mortsiefer A , Abholz HH , Wegscheider K , Pentzek M . Willingness to participate in mammography screening - a randomized controlled questionnaire study of responses to two patient information leaflets with different factual content . Deutsches Ärzteblatt International 2015. ; 112 ( 5 ): 61-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacking 2013 {published data only}
- Hacking B , Wallace L , Scott S , Kosmala-Anderson J , Belkora J , McNeill A . Testing the feasibility, acceptability and effectiveness of a 'decision navigation' intervention for early stage prostate cancer patients in Scotland - a randomised controlled trial . Psycho-Oncology 2013. ; 22 ( 5 ): 1017-24 . [DOI] [PubMed] [Google Scholar]
Hall 2007 {published data only}
- Hall S , Chitty L , Dormandy E , Hollywood A , Wildschut HIJ , Fortuny A , et al. Undergoing prenatal screening for Down's syndrome: presentation of choice and information in Europe and Asia . European Journal of Human Genetics 2007. ; 15 ( 5 ): 563-9 . [DOI] [PubMed] [Google Scholar]
Hall 2011 {published data only}
- Hall MJ , Manne SL , Winkel G , Chung DS , Weinberg DS , Meropol NJ . Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing . Cancer Epidemiology, Biomarkers and Prevention 2011. ; 20 ( 2 ): 249-54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamann 2014 {published data only}
- Hamann J , Maris N , Iosifidou P , Mendel R , Cohen R , Wolf P , Kissling W . Effects of a question prompt sheet on active patient behaviour: a randomized controlled trial with depressed outpatients . International Journal of Social Psychiatry 2014. ; 60 ( 3 ): 227-35 . [DOI] [PubMed] [Google Scholar]
Harmsen 2014 {published data only}
- Harmsen CG , Kristiansen IS , Larsen PV , Nexøe J , Støvring H , Gyrd-Hansen D , et al. Communicating risk using absolute risk reduction or prolongation of life formats: cluster-randomised trial in general practice . British Journal of General Practice 2014. ; 64 ( 621 ): e199-207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Harwood 2011 {published data only}
- Harwood R , Douglas C , Clark D . Decision aids for breast and nodal surgery in patients with early breast cancer: development and a pilot study . Asia-Pacific Journal of Clinical Oncology 2011. ; 7 : 114-22 . [DOI] [PubMed] [Google Scholar]
Hawley 2016 {published data only}
- Hawley ST , Newman L , Griggs JJ , Kosir MA , Katz SJ . Evaluating a decision aid for improving decision making in patients with early-stage breast cancer . The Patient: Patient-Centered Outcomes Research 2016. ; 9 ( 2 ): 161-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Healton 1999 {published data only}
- Healton C , Taylor S , Messeri P , Weinberg G , Bamji M . Effects of ZDV-based patient education on intentions toward ZDV use, HIV testing and reproduction among a US cohort of women . AIDS Care 1999. ; 11 ( 6 ): 675-86 . [DOI] [PubMed] [Google Scholar]
Heisler 2014 {published data only}
- Heisler M , Choi H , Palmisano G , Mase R , Richardson C , Fagerlin A , et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial . Annals of Internal Medicine 2014. ; 161 ( 10 Suppl ): S13-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2013 {published data only}
- Henderson C , Brohan E , Clement S , Williams P , Lassman F , Schauman O , et al. Decision aid on disclosure of mental health status to an employer: feasibility and outcomes of a randomised controlled trial . British Journal of Psychiatry 2013. ; 203 ( 5 ): 350-7 . [DOI] [PubMed] [Google Scholar]
Henselmans 2020 {published data only}
- Henselmans I , Laarhoven HW , Maarschalkerweerd P , Haes HC , Dijkgraaf MG , Sommeijer DW , et al. Effect of a skills training for oncologists and a patient communication aid on shared decision making about palliative systemic treatment: a randomized clinical trial . Oncologist 2020. ; 25 ( 3 ): e578-88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrera 1983 {published data only}
- Herrera AJ , Cochran B , Herrera A , Wallace B . Parental information and circumcision in highly motivated couples with higher education . Pediatrics 1983. ; 71 ( 2 ): 233-4 . [PubMed] [Google Scholar]
Hersch 2021 {published data only}
- Hersch J , Barratt A , McGeechan K , Jansen J , Houssami N , Dhillon H , et al. Informing women about overdetection in breast cancer screening: two-year outcomes from a randomized trial . Journal of the National Cancer Institute 2021. ; 113 ( 11 ): 1523-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2015 {published data only}
- Hess LM , Litwiller A , Byron J , Stutsman J , Kasper K , Learman LA . Preference elicitation tool for abnormal uterine bleeding treatment: a randomized controlled trial . The Patient: Patient Centered Outcomes Research 2015. ; 8 ( 2 ): 217-27 . [DOI] [PubMed] [Google Scholar]
Hewison 2001 {published data only}
- Hewison J , Cuckle H , Baillie C , Sehmi I , Lindow S , Jackson F , et al. Use of videotapes for viewing at home to inform choice in Down syndrome screening: a randomised controlled trial . Prenatal Diagnosis 2001. ; 21 ( 2 ): 146-9 . [DOI] [PubMed] [Google Scholar]
Heyland 2020 {published data only}
- Heyland DK , Heyland R , Bailey A , Howard M . A novel decision aid to help plan for serious illness: a multisite randomized trial . CMAJ Open 2020. ; 8 ( 2 ): E289-96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Heyn 2013 {published data only}
- Heyn L , Finset A , Eide H , Ruland CM . Effects of an interactive tailored patient assessment on patient-clinician communication in cancer care . Psycho-Oncology 2013. ; 22 ( 1 ): 89-96 . [DOI] [PubMed] [Google Scholar]
Hickish 1995 {published data only}
- Hickish TF , Smith IE , Middleton G , Nicolson M . Patient preference for extended palliative chemotherapy for non-small cell lung cancer . Lancet 1995. ; 345 ( 8953 ): 857-8 . [DOI] [PubMed] [Google Scholar]
Hinsberg 2018 {published data only}
- Hinsberg L , Marques F , Leavitt L , Skubisz C , Sepucha K , Wasfy JH . Comparing the effectiveness of two different decision AIDS for stable chest discomfort . Coronary Artery Disease 2018. ; 29 ( 3 ): 230-6 . [DOI] [PubMed] [Google Scholar]
Hochlehnert 2006 {published data only}
- Hochlehnert A , Richter A , Bludau HB , Bieber C , Blumenstiel K , Mueller K , et al. A computer-based information-tool for chronic pain patients: computerized information to support the process of shared decision-making . Patient Education and Counseling 2006. ; 61 ( 1 ): 92-8 . [DOI] [PubMed] [Google Scholar]
Hofbauer 2008 {published data only}
- Hofbauer GFL , Buhler RPN , French LE , Brockes M , Scheuer E . Patient-centered care in dermatology: an online system that provides accessible and appropriate information to guide patients' decision making . Archives of Dermatology 2008. ; 144 ( 9 ): 1225-7 . [DOI] [PubMed] [Google Scholar]
Hoffman 2009 {published data only}
- Hoffman RM , Walter LC . Colorectal cancer screening in the elderly: the need for informed decision making . Journal of General Internal Medicine 2009. ; 24 ( 12 ): 1336-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2022 {published data only}
- Hoffmann TC , Jones M , Glasziou P , Beller E , Trevena L , Mar CD . A brief shared decision-making intervention for acute respiratory infections on antibiotic dispensing rates in primary care: a cluster randomized trial . Annals of Family Medicine 2022. ; 20 ( 1 ): 35-41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Holbrook 2007 {published data only}
- Holbrook A , Labiris R , Goldsmith CH , Ota K , Harb S , Sebaldt RJ . Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial . CMAJ 2007. ; 176 ( 11 ): 1583-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollen 2013 {published data only}
- Hollen PJ , Tyc VL , Donnangelo SF , Shannon SV , O'Laughlen MC , Hinton I , et al. A substance use decision aid for medically at-risk adolescents: results of a randomized controlled trial for cancer-surviving adolescents . Cancer Nursing 2013. ; 36 ( 5 ): 355-67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Holloway 2003 {published data only}
- Holloway RM , Wilkinson C , Peters TJ , Russell I , Cohen D , Hale J , et al. Cluster-randomised trial of risk communication to enhance informed uptake of cervical screening . British Journal of General Practice 2003. ; 53 ( 493 ): 620-5 . [PMC free article] [PubMed] [Google Scholar]
Holmes‐Rovner 2011 {published data only}
- Holmes-Rovner M , Kelly-Blake K , Dwamena F , Dontje K , Henry R , Olomu A , et al. Shared decision making guidance reminders in practice (SDM-GRIP) . Patient Education and Counseling 2011. ; 85 ( 2 ): 219-24 . [DOI] [PubMed] [Google Scholar]
Holt 2009 {published data only}
- Holt CL , Wynn TA , Litaker MS , Southward P , Jeames S , Schulz E . A comparison of a spiritually based and non-spiritually based educational intervention for informed decision making for prostate cancer screening among church-attending African-American men . Urologic Nursing 2009. ; 29 ( 4 ): 249-58 . [PMC free article] [PubMed] [Google Scholar]
Holt 2020 {published data only}
- Holt K , Kimport K , Kuppermann M , Fitzpatrick J , Steinauer J , Dehlendorf C . Patient-provider communication before and after implementation of the contraceptive decision support tool My Birth Control . Patient Education and Counseling 2020. ; 103 ( 2 ): 315-20 . [DOI] [PubMed] [Google Scholar]
Holzhüter 2020 {published data only}
- Holzhüter F , Hamann J . Nocebo effects by providing informed consent in shared decision making? Not necessarily: a randomized pilot-trial using an open-label placebo approach . BMC Medical Ethics 2020. ; 21 ( 1 ): 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Hope 2010 {published data only}
- Hope N , Rombauts L . Can an educational DVD improve the acceptability of elective single embryo transfer? A randomized controlled study . Fertility and Sterility 2010. ; 94 ( 2 ): 489-95 . [DOI] [PubMed] [Google Scholar]
Hopkin 2019 {published data only}
- Hopkin G , Au A , Collier VJ , Yudkin JS , Basu S , Naci H . Combining multiple treatment comparisons with personalized patient preferences: a randomized trial of an interactive platform for statin treatment selection . Medical Decision Making 2019. ; 39 ( 3 ): 264-77 . [DOI] [PubMed] [Google Scholar]
Howard 2022 {published data only}
- Howard M , Elston D , Borhan S , Hafid A , Arora N , Forbes R , et al. Randomised trial of a serious illness decision aid (Plan Well Guide) for patients and their substitute decision-makers to improve engagement in advance care planning . BMJ Supportive & Palliative Care 2022. ; 12 ( 1 ): 99-106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang ES , Nathan AG , Cooper JM , Lee SM , Shin N , John PM , . Impact and feasibility of personalized decision support for older patients with diabetes: a pilot randomized trial . Medical Decision Making 2017. ; 37 ( 5 ): 611-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Huijbregts 2013 {published data only}
- Huijbregts KML , Jong FJ , Marwijk HWJ , Beekman ATF , Adèr HJ , Hakkaart-van Roijen L , et al. A target-driven collaborative care model for major depressive disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative . Journal of Affective Disorders 2013. ; 146 : 328-37 . [DOI] [PubMed] [Google Scholar]
Hulbaek 2021 {published data only}
- Hulbaek M , Primdahl J , Birkelund R , Al-Kozai SAH , Barawi S , Ebbesen NT , et al. A preference-sensitive online instrument to support shared decision making for patients with pelvic organ prolapse: a pilot multicenter randomized controlled trial . CIN: Computers, Informatics, Nursing 2021. ; 39 ( 11 ): 714-24 . [DOI] [PubMed] [Google Scholar]
Hunt 2005 {published data only}
- Hunt LM , Voogd KB , Castaneda H . The routine and the traumatic in prenatal genetic diagnosis: does clinical information inform patient decision-making? Patient Education and Counseling 2005. ; 56 ( 3 ): 302-12 . [DOI] [PubMed] [Google Scholar]
Hunter 1999 {published data only}
- Hunter M , O'Dea I . An evaluation of a health education intervention for mid-aged women: five year follow-up of effects upon knowledge, impact of menopause and health . Patient Education and Counseling 1999. ; 38 ( 3 ): 249-55 . [DOI] [PubMed] [Google Scholar]
Hunter 2005 {published data only}
- Hunter AG , Cappelli M , Humphreys L , Allanson JE , Chiu TT , Peeters C , et al. A randomized trial comparing alternative approaches to prenatal diagnosis counseling in advanced maternal age patients . Clinical Genetics 2005. ; 67 ( 4 ): 303-13 . [DOI] [PubMed] [Google Scholar]
Hutyra 2019 {published data only}
- Hutyra CA , Smiley S , Taylor DC , Orlando LA , Mather RC 3rd. Efficacy of a preference-based decision tool on treatment decisions for a first-time anterior shoulder dislocation: a randomized controlled trial of at-risk patients . Medical Decision Making 2019. ; 39 ( 3 ): 253-63 . [DOI] [PubMed] [Google Scholar]
Huyghe 2009 {published data only}
- Huyghe E , Martinetti P , Sui D , Schover LR . Banking on Fatherhood: pilot studies of a computerized educational tool on sperm banking before cancer treatment . Psycho-Oncology 2009. ; 18 ( 9 ): 1011-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilic 2008 {published data only}
- Ilic D , Egberts K , McKenzie JE , Risgridger G , Green S . Informing men about prostate cancer screening: a randomized controlled trial of patient education materials . Journal of General Internal Medicine 2008. ; 23 ( 4 ): 466-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Isebaert 2007 {published data only}
- Isebaert S , Van Audenhove C , Haustermans K , DeRidder K , Junius S , Joniau S , et al. A decision aid for patients with localized prostate cancer: first results [Een beslissingshulp voor patienten met gelokaliseerde prostaatkanker: eerste resultaten]. Tijdschrift voor Geneeskunde 2007. ; 63 ( 1 ): 15-21 . [Google Scholar]
Jackson 2011 {published data only}
- Jackson C , Cheater FM , Harrison W , Peacock R , Bekker H , West R , et al. Randomised cluster trial to support informed parental decision-making for the MMR vaccine . BMC Public Health 2011. ; 11 : 475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Jayakumar 2021 {published data only}
- Jayakumar P , Moore MG , Furlough KA , Uhler LM , Andrawis JP , Koenig KM , et al. Comparison of an artificial intelligence-enabled patient decision aid vs educational material on decision quality, shared decision-making, patient experience, and functional outcomes in adults with knee osteoarthritis: a randomized clinical trial . JAMA Network Open 2021. ; 4 ( 2 ): e2037107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2007 {published data only}
- Jerant A , Kravitz RL , Rooney M , Amerson S , Kreuter M , Franks P . Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices . Patient Education and Counseling 2007. ; 66 ( 1 ): 67-74 . [DOI] [PubMed] [Google Scholar]
Jessop 2020 {published data only}
- Jessop AB , Bass SB , Brajuha J , Alhajji M , Burke M , Gashat MT , et al. "Take Charge, Get Cured": pilot testing a targeted mHealth treatment decision support tool for methadone patients with hepatitis C virus for acceptability and promise of efficacy . Journal of Substance Abuse Treatment 2020. ; 109 : 23-33 . [DOI] [PubMed] [Google Scholar]
Jibaja‐Weiss 2006 {published data only}
- Jibaja-Weiss ML , Volk RJ , Granchi TS , Neff NE , Spann SJ , Aoki N , et al. Entertainment education for informed breast cancer treatment decisions in low-literate women: development and initial evaluation of a patient decision aid . Journal of Cancer Education 2006. ; 21 ( 3 ): 133-9 . [DOI] [PubMed] [Google Scholar]
Jimbo 2019 {published data only}
- Jimbo M , Sen A , Plegue MA , Hawley S , Kelly-Blake K , Rapai M , et al. Interactivity in a decision aid: findings from a decision aid to technologically enhance shared decision making RCT . American Journal of Preventive Medicine 2019. ; 57 ( 1 ): 77-86 . [DOI] [PubMed] [Google Scholar]
Jimenez 2017 {published data only}
- Jimenez ME , DuRivage NE , Bezpalko O , Suh A , Wade R , Blum NJ , et al. A pilot randomized trial of a video patient decision aid to facilitate early intervention referrals from primary care . Clinical Pediatrics 2017. ; 56 ( 3 ): 268-77 . [DOI] [PubMed] [Google Scholar]
Joosten 2009 {published data only}
- Joosten EA , Jong CA , Weert-van Oene GH , Sensky T , Staak CP . Shared decision-making reduces drug use and psychiatric severity in substance-dependent patients . Psychotherapy and Psychosomatics 2009. ; 78 : 245-53 . [DOI] [PubMed] [Google Scholar]
Joosten 2011 {published data only}
- Joosten EA , De Jong CA , Weert-van Oene GH , Sensky T , Staak CP . Shared decision-making: increases autonomy in substance-dependent patients . Substance Use and Misuse 2011. ; 48 : 1037-48 . [DOI] [PubMed] [Google Scholar]
Jorm 2003 {published data only}
- Jorm AF , Griffiths KM , Christensen H , Korten AE , Parslow RA , Rodgers B . Providing information about the effectiveness of treatment options to depressed people in the community: a randomized controlled trial of effects on mental health literacy, help-seeking and symptoms . Psychological Medicine 2003. ; 33 ( 6 ): 1071-9 . [DOI] [PubMed] [Google Scholar]
Juraskova 2014 {published data only}
- Juraskova I , Butow P , Bonner C , Bell ML , Smith AB , Seccombe M , et al. Improving decision making about clinical trial participation - a randomised controlled trial of a decision aid for women considering participation in the IBIS-II breast cancer prevention trial . British Journal of Cancer 2014. ; 111 ( 1 ): 1-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 2022 {published data only}
- Kahn BJ , Morales-Pico BM , Blalock TW , Zhang C , Stoff BK . Educational video promotes durable knowledge about actinic keratoses in patients with field cancerization: a pseudorandomized, single-blind, controlled pilot study . Journal of Dermatological Treatment 2022. ; 33 ( 1 ): 240-6 . [DOI] [PubMed] [Google Scholar]
Kakkilaya 2011 {published data only}
- Kakkilaya V , Groome L , Platt D , Kurepa D , Pramanik A , Caldito G , et al. Use of a visual aid to improve counseling at the threshold of viability . Pediatrics 2011. ; 128 ( 6 ): e1511-9 . [DOI] [PubMed] [Google Scholar]
Kang 2020 {published data only}
- Kang E , Lee J , Choo J , Min J , Yun YH . Randomized controlled trial of advance care planning video decision aid for the general population . Journal of Pain and Symptom Management 2020. ; 59 ( 6 ): 1239-47 . [DOI] [PubMed] [Google Scholar]
Kaplan 2014a {published data only}
- Kaplan CP , Livaudais-Toman J , Tice JA , Kerlikowske K , Gregorich SE , Pérez-Stable EJ , et al. A randomized, controlled trial to increase discussion of breast cancer in primary care . Cancer Epidemiology, Biomarkers & Prevention 2014. ; 23 ( 7 ): 1245-53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2014b {published data only}
- Kaplan AL , Crespi CM , Saucedo JD , Connor SE , Litwin MS , Saigal CS . Decisional conflict in economically disadvantaged men with newly diagnosed prostate cancer . Cancer 2014. ; 120 ( 17 ): 2721-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kask‐Flight 2021 {published data only}
- Kask-Flight L , Durak K , Suija K , Rätsep A , Kalda R . Reduction of cardiovascular risk factors among young men with hypertension using an interactive decision aid: cluster-randomized control trial . BMC Cardiovascular Disorders 2021. ; 21 ( 1 ): 543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassan 2012 {published data only}
- Kassan EC , Williams RM , Kelly SP , Barry SA , Penek S , Fishman MB , et al. Men's use of an internet-based decision aid for prostate cancer screening . Journal of Health Communication 2012. ; 17 ( 6 ): 677-97 . [DOI] [PubMed] [Google Scholar]
Kawasaki 2015 {published data only}
- Kawasaki Y . Development of nursing shared-structured decision-making model to support cancer patients . Journal of Japan Academy of Nursing Science 2015. ; 35 ( 1 ): 277-85 . [Google Scholar]
Kayler 2020 {published data only}
- Kayler LK , Dolph BA , Cleveland CN , Keller MM , Feeley TH . Educational animations to inform transplant candidates about deceased donor kidney options: an efficacy randomized trial . Transplant Direct 2020. ; 6 ( 7 ): e575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellar 2008 {published data only}
- Kellar I , Sutton S , Griffin S , Prevost AT , Kinmonth AL , Marteau TM . Evaluation of an informed choice invitation for type 2 diabetes screening . Patient Education and Counseling 2008. ; 72 ( 2 ): 232-8 . [DOI] [PubMed] [Google Scholar]
Kiatpongsan 2014 {published data only}
- Kiatpongsan S , Carlson K , Feibelmann S , Sepucha K . Decision aid reduces misperceptions about hormone therapy: a randomized controlled trial . Menopause: The Journal of The North American Menopause Society 2014. ; 21 ( 1 ): 33-8 . [DOI] [PubMed] [Google Scholar]
Klifto 2021 {published data only}
- Klifto KM , Khan H , Manahan MA , Sacks JM , Broderick KP , Aliu O , et al. Decision aid for women with newly diagnosed breast cancer seeking breast reconstruction surgery: a prospective, randomized, controlled, single-blinded, pilot study . Journal of Plastic, Reconstructive and Aesthetic Surgery 2021. ; 74 ( 10 ): 2519-26 . [DOI] [PubMed] [Google Scholar]
Kobelka 2009 {published data only}
- Kobelka C , Mattman A , Langlois S . An evaluation of the decision-making process regarding amniocentesis following a screen-positive maternal serum screen result . Prenatal Diagnosis 2009. ; 29 ( 5 ): 514-9 . [DOI] [PubMed] [Google Scholar]
Kobewka 2021 {published data only}
- Kobewka D , Heyland DK , Dodek P , Nijjar A , Bansback N , Howard M , et al. Randomized controlled trial of a decision support intervention about cardiopulmonary resuscitation for hospitalized patients who have a high risk of death . Journal of General Internal Medicine 2021. ; 36 ( 9 ): 2593-600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Koelewijn‐van Loon 2009 {published data only}
- Koelewijn-van Loon MS , Weijden T , Steenkiste B , Ronda G , Winkens B , Severens JL , et al. Involving patients in cardiovascular risk management with nurse-led clinics: a cluster randomized controlled trial . CMAJ 2009. ; 181 ( 12 ): E267-74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Köpke 2009 {published data only}
- Köpke S , Kasper J , Mühlhauser I , Nübling M , Heesen C . Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized-controlled trial . Multiple Sclerosis 2009. ; 15 ( 1 ): 96-104 . [DOI] [PubMed] [Google Scholar]
Köpke 2014 {published data only}
- Köpke S , Kern S , Ziemssen T , Berghoff M , Kleiter I , Marziniak M , et al. Evidence-based patient information programme in early multiple sclerosis: a randomised controlled trial . Journal of Neurology, Neurosurgery, and Psychiatry 2014. ; 85 ( 4 ): 411-18 . [DOI] [PubMed] [Google Scholar]
Korger 2021 {published data only}
- Korger S , Eggeling M , Cress U , Kimmerle J , Bientzle M . Decision aids to prepare patients for shared decision making: two randomized controlled experiments on the impact of awareness of preference-sensitivity and personal motives . Health Expectations 2021. ; 24 ( 2 ): 257-68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Krawczyk 2012 {published data only}
- Krawczyk A . Cancer Prevention and the Human Papillomavirus Vaccine: Psychosocial and Behavioural Factors Involved in Vaccination Decision-making [PhD thesis] . Montreal: : McGill Library; , 2012. . [Google Scholar]
Kripalani 2007 {published data only}
- Kripalani S , Sharma J , Justice E , Justice J , Spiker C , Laufman LE , et al. Low-literacy interventions to promote discussion of prostate cancer: a randomized controlled trial . American Journal of Preventive Medicine 2007. ; 33 ( 2 ): 83-90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Krones 2008 {published data only}
- Krones T , Keller H , Becker A , Sonnichsen A , Baum E , Donner-Banzhoff N . The theory of planned behaviour in a randomized trial of a decision aid on cardiovascular risk prevention . Patient Education and Counseling 2009. ; 78 ( 2 ): 169-76 . [DOI] [PubMed] [Google Scholar]
- Krones T , Keller H , Sönnichsen A , Sadowski EM , Baum E , Wegscheider K , et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial . Annals of Family Medicine 2008. ; 6 ( 3 ): 218-27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukafka 2018 {published data only}
- Kukafka R , Fang J , Vanegas A , Silverman T , Crew KD . Pilot study of decision support tools on breast cancer chemoprevention for high-risk women and healthcare providers in the primary care setting . BMC Medical Informatics and Decision Making 2018. ; 18 ( 1 ): 134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuppermann 2009 {published data only}
- Kuppermann M , Norton ME , Gates E , Gregorich SE , Learman LA , Nakagawa S , et al. Computerized prenatal genetic testing decision-assisting tool: a randomized controlled trial . Obstetrics & Gynecology 2009. ; 113 ( 1 ): 53-63 . [DOI] [PubMed] [Google Scholar]
Kurian 2009 {published data only}
- Kurian B , Trivedi M , Grannemann B , Claassen C , Daly E , Sunderajan P . A computerized decision support system for depression in primary care . Primary Care Companion to the Journal of Clinical Psychiatry 2009. ; 11 ( 4 ): 140-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Kushner 2022 {published data only}
- Kushner BS , Holden T , Han B , Sehnert M , Majumder A , Blatnik JA , et al. Randomized control trial evaluating the use of a shared decision-making aid for older ventral hernia patients in the Geriatric Assessment and Medical Preoperative Screening (GrAMPS) Program . Hernia 2022. ; 26 ( 3 ): 901-9 . [DOI] [PubMed] [Google Scholar]
Labrecque 2010 {published data only}
- Labrecque M , Paunescu C , Plesu I , Stacey D , Legare F . Evaluation of the effect of a patient decision aid about vasectomy on the decision-making process: a randomized trial . Contraception 2010. ; 82 ( 6 ): 556-62 . [DOI] [PubMed] [Google Scholar]
LaCroix 1999 {published data only}
- LaCroix AZ , Newton KM , Buist DSM , Curry SJ , Scholes D , Anderson LA , et al. Population-based strategy for improving informed decision making about hormone replacement therapy in managed care settings . Women's Health Issues 1999. ; 9 ( 6 ): 306-18 . [Google Scholar]
Lai 2021 {published data only}
- Lai CH , DeBaun MR , Van Rysselberghe N , Abrams GD , Kamal RN , Bishop JA , et al. Can upstream patient education improve fracture care in a digital world? Use of a decision aid for the treatment of displaced diaphyseal clavicle fractures . Journal of Orthopaedic Trauma 2021. ; 35 ( 3 ): 160-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lairson 2011 {published data only}
- Lairson DR , Chan W , Chang YC , Junco DJ , Vernon SW . Cost-effectiveness of targeted versus tailored interventions to promote mammography screening among women military veterans in the United States . Evaluation and Program Planning 2011. ; 34 ( 2 ): 97-104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalonde 2006 {published data only}
- Lalonde L , O'Connor AM , Duguay P , Brassard J , Drake E , Grover SA . Evaluation of a decision aid and a personal risk profile in community pharmacy for patients considering options to improve cardiovascular health: the OPTIONS pilot study . International Journal of Pharmacy Practice 2006. ; 14 ( 1 ): 51-62 . [Google Scholar]
Lancaster 2009 {published data only}
- Lancaster T . Physician training in the use of a decision aid increased patient participation in decision making for CVD prevention . Evidence-Based Medicine 2009. ; 14 ( 1 ): 24 . [DOI] [PubMed] [Google Scholar]
Landrey 2013 {published data only}
- Landrey AR , Matlock DD , Andrews L , Bronsert M , Denberg T . Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam . Journal of Primary Care & Community Health 2013. ; 4 ( 1 ): 67-74 . [DOI] [PubMed] [Google Scholar]
Langford 2020 {published data only}
- Langford AT , Scherer LD , Ubel PA , Holmes-Rovner M , Scherr KA , Fagerlin A . Racial differences in veterans' response to a standard vs. patient-centered decision aid for prostate cancer: implications for decision making in African American and White men . Patient Education and Counseling 2020. ; S0738-3991 ( 20 ): 30322-0 . [DOI] [PubMed] [Google Scholar]
Lazcano Ponce 2000 {published data only}
- Lazcano Ponce EC , Sloan NL , Winikoff B , Langer A , Coggins C , Heimburger A , et al. The power of information and contraceptive choice in a family planning setting in Mexico . Sexually Transmitted Infections 2000. ; 76 ( 4 ): 277-81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2003 {published data only}
- Legare F , O'Connor AM , Graham ID , Wells GA , Jacobsen MJ , Elmslie T , et al. The effect of decision aids on the agreement between women's and physicians' decisional conflict about hormone replacement therapy . Patient Education and Counseling 2003. ; 50 ( 2 ): 211-21 . [DOI] [PubMed] [Google Scholar]
Leung 2004 {published data only}
- Leung KY , Lee CP , Chan HY , Tang MH , Lam YH , Lee A . Randomised trial comparing an interactive multimedia decision aid with a leaflet and a video to give information about prenatal screening for Down syndrome . Prenatal Diagnosis 2004. ; 24 ( 8 ): 613-8 . [DOI] [PubMed] [Google Scholar]
Levin 2011 {published data only}
- Levin W , Campbell D , McGovern K , Gau J , Kosty D , Seeley J , Lewinsohn P . A computer-assisted depression intervention in primary care . Psychological Medicine 2011. ; 41 ( 7 ): 1373-83 . [DOI] [PubMed] [Google Scholar]
Lewis 2003 {published data only}
- Lewis CL , Pignone MP , Sheridan SL , Downs SM , Kinsinger LS . A randomized trial of three videos that differ in the framing of information about mammography in women 40 to 49 years old . Journal of General Internal Medicine 2003. ; 18 ( 11 ): 875-83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
- Lewis CL , Brenner AT , Griffith JM , Moore CG , Pignone MP . Two controlled trials to determine the effectiveness of a mailed intervention to increase colon cancer screening . North Carolina Medical Journal 2012. ; 73 ( 2 ): 93-8 . [PMC free article] [PubMed] [Google Scholar]
Lewis 2015 {published data only}
- Lewis CL , Adams J , Tai-Seale M , Huang Q , Knowles SB , Nielsen ME , et al. A randomized controlled effectiveness trial for PSA screening decision support interventions in two primary care settings . Journal of General Internal Medicine 2015. ; 30 ( 6 ): 810-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipnick 2020 {published data only}
- Lipnick D , Green M , Thiede E , Smith TJ , Lehman EB , Johnson R , et al. Surrogate decision maker stress in advance care planning conversations: a mixed-methods analysis from a randomized controlled trial . Journal of Pain and Symptom Management 2020. ; 60 ( 6 ): 1117-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipstein 2021 {published data only}
- Lipstein EA , Breslin M , Dodds CM , Kappelman MD , Ollberding NJ , Margolis P , et al. Integrating shared decision making into trial consent: a nested, cluster-randomized trial . Patient Education and Counseling 2021. ; 104 ( 7 ): 1575-82 . [DOI] [PubMed] [Google Scholar]
Logan 2022 {published data only}
- Logan PA , Horne JC , Allen F , Armstrong SJ , Clark AB , Conroy S , et al. A multidomain decision support tool to prevent falls in older people: the FinCH cluster RCT . Health Technology Assessment (Winchester, England) 2022. ; 26 ( 9 ): 1-136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Jornet 2012 {published data only}
- López-Jornet P , Camacho-Alonso F , Sanchez-Siles M . Patient information preferences and behaviour in relation to oral biopsies . British Journal of Oral & Maxillofacial Surgery 2012. ; 50 ( 8 ): e115-8 . [DOI] [PubMed] [Google Scholar]
Lord 2017 {published data only}
- Lord K , Livingston G , Cooper C . A feasibility randomised controlled trial of the DECIDE intervention: dementia carers making informed decisions . British Journal of Psychiatry Open 2017. ; 3 ( 1 ): 12-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lukens 2013 {published data only}
- Lukens JM , Solomon P , Sorenson SB . Shared decision-making for clients with mental illness: a randomized factorial survey . Research on Social Work Practice 2013. ; 23 ( 6 ): 694-705 . [Google Scholar]
Lurie 2011 {published data only}
- Lurie J , Spratt K , Blood E , Tosteson T , Tosteson A , Weinstein J . Effects of viewing an evidence based video decision aid on patients' treatment preferences for spine surgery . Spine 2011. ; 36 ( 18 ): 1501-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Maisels 1983 {published data only}
- Maisels MJ , Hayes B , Conrad S , Chez RA . Circumcision: the effect of information on parental decision making . Pediatrics 1983. ; 71 ( 3 ): 453-5 . [PubMed] [Google Scholar]
Makimoto 2020 {published data only}
- Makimoto G , Hotta K , Oze I , Ninomiya K , Nakanishi M , Hara N , et al. Patients' preferences and perceptions of lung cancer treatment decision making: results from Okayama lung cancer study group trial 1406 . Acta Oncologica 2020. ; 59 ( 3 ): 324-8 . [DOI] [PubMed] [Google Scholar]
Mancini 2006 {published data only}
- Mancini J , Santin G , Chabal F , Julian-Reynier C . Cross-cultural validation of the Decisional Conflict Scale in a sample of French patients . Quality of Life Research 2006. ; 15 ( 6 ): 1063-8 . [DOI] [PubMed] [Google Scholar]
Mangla 2019 {published data only}
- Mangla M , Bedair H , Dwyer M , Freiberg A , Sepucha K . Pilot study examining feasibility and comparing the effectiveness of decision aids for hip and knee osteoarthritis: a randomized trial . MDM Policy & Practice 2019. ; 4 ( 1 ): 2381468319827270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Manne 2009 {published data only}
- Manne SL , Coups EJ , Markowitz A , Meropol NJ , Haller D , Jacobsen PB , et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings . Annals of Behavioral Medicine 2009. ; 37 ( 2 ): 207-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Manne 2016 {published data only}
- Manne SL , Topham N , D'Agostino TA , Myers Virtue S , Kirstein L , Brill K , et al. Acceptability and pilot efficacy trial of a web-based breast reconstruction decision support aid for women considering mastectomy . Psycho-oncology 2016. ; 25 ( 12 ): 1424-33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Manns 2005 {published data only}
- Manns B J , Taub K , Vanderstraeten C , Jones H , Mills C , Visser M , et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial . Kidney International 2005. ; 68 ( 4 ): 1777-83 . [DOI] [PubMed] [Google Scholar]
Markham 2003 {published data only}
- Markham R , Smith A . Limits to patient choice: example from anaesthesia . BMJ 2003. ; 326 ( 7394 ): 863-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Markun 2015 {published data only}
- Markun S , Dishy A , Neuner-Jehle S , Rosemann T , Frei A . The chronic care for wet age related macular degeneration (CHARMED) study: a randomized controlled trial RTY - Journal article . PLOS One 2015. ; 10 ( 11 ): e0143085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2012 {published data only}
- Martin R , Brower M , Geralds A , Gallagher P , Tellinghuisen D . An experimental evaluation of patient decision aid design to communicate the effects of medications on the rate of progression of structural joint damage in rheumatoid arthritis . Patient Education and Counseling 2012. ; 86 ( 3 ): 329-34 . [DOI] [PubMed] [Google Scholar]
Maslin 1998 {published data only}
- Maslin AM , Baum M , Walker JS , A'Hern R , Prouse A . Shared decision-making using an interactive video disk system for women with early breast cancer . NT Research 1998. ; 3 ( 6 ): 444-55 . [Google Scholar]
- Maslin AM , Baum M , Walker JS , A'Hern R , Prouse A . Using an interactive video disk in breast cancer patient support . Nursing Times 1998. ; 94 ( 44 ): 4-10 . [PubMed] [Google Scholar]
Matlock 2014 {published data only}
- Matlock DD , Keech TA , McKenzie MB , Bronsert MR , Nowels CT , Kutner JS . Feasibility and acceptability of a decision aid designed for people facing advanced or terminal illness: a pilot randomized trial . Health Expectations 2014. ; 17 ( 1 ): 49-59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Matlock 2020 {published data only}
- Matlock DD , McIlvennan CK , Thompson JS , Morris MA , Venechuk G , Dunlay SM , et al. Decision aid implementation among left ventricular assist device programs participating in the DECIDE-LVAD stepped-wedge trial . Medical Decision Making 2020. ; 40 ( 3 ): 289-301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Matloff 2006 {published data only}
- Matloff ET , Moyer A , Shannon KM , Niendorf KB , Col NF . Healthy women with a family history of breast cancer: impact of a tailored genetic counseling intervention on risk perception, knowledge, and menopausal therapy decision making . Journal of Women's Health 2006. ; 15 ( 7 ): 843-56 . [DOI] [PubMed] [Google Scholar]
Mazur 1994 {published data only}
- Mazur DJ , Hickam DH . The effect of physician's explanations on patients' treatment preferences: five-year survival data . Medical Decision Making 1994. ; 14 ( 3 ): 255-8 . [DOI] [PubMed] [Google Scholar]
McBride 2016 {published data only}
- McBride E , Hacking B , O'Carroll R , Young M , Jahr J , Borthwick C , et al. Increasing patient involvement in the diabetic foot pathway: a pilot randomized controlled trial . Diabetic Medicine 2016. ; 33 ( 11 ): 1483-92 . [DOI] [PubMed] [Google Scholar]
McCaffery 2007 {published data only}
- McCaffery K , Irwig L , Bossuyt P . Patient decision aids to support clinical decision making: evaluating the decision or the outcomes of the decision . Medical Decision Making 2007. ; 27 ( 5 ): 619-25 . [DOI] [PubMed] [Google Scholar]
McGinley 2002 {published data only}
- McGinley AM . Effect of Web-based Computer-tailoring on Women's Intention to Continue or Begin to Use Hormone Replacement Therapy to Lower their Risk for Osteoporosis [PhD thesis] . Philadelphia: : University of Pennsylvania; , 2002. . [Google Scholar]
McGowan 2008 {published data only}
- McGowan J , Hogg W , Campbell C , Rowan M . Just-in-time information improved decision-making in primary care: a randomized controlled trial . PLOS One 2008. ; 3 ( 11 ): e3785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
McInerney‐Leo 2004 {published data only}
- McInerney-Leo A , Biesecker BB , Hadley DW , Kase RG , Giambarresi TR , Johnson E , et al. BRCA1/2 testing in hereditary breast and ovarian cancer families: effectiveness of problem-solving training as a counseling intervention . American Journal of Medical Genetics. Part A 2004. ; 130 ( 3 ): 221-7 . [DOI] [PubMed] [Google Scholar]
Mclaren 2012 {published data only}
- Mclaren PJ , Hyde MK , White KM . Exploring the role of gender and risk perceptions in people's decisions to register as a bone marrow donor . Health Education Research 2011. ; 27 ( 3 ): 513-22 . [DOI] [PubMed] [Google Scholar]
Meropol 2013 {published data only}
- Meropol NJ , Egleston BL , Buzaglo JS , Balshem A , Benson AB 3rd, Cegala DJ , et al. A web-based communication aid for patients with cancer: the CONNECT study . Cancer 2013. ; 119 ( 7 ): 1437-45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertz 2020 {published data only}
- Mertz K , Shah RF , Eppler SL , Yao J , Safran M , Palanca A , et al. A simple goal elicitation tool improves shared decision making in outpatient orthopedic surgery: a randomized controlled trial . Medical Decision Making 2020. ; 40 ( 6 ): 766-73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Michael 2022 {published data only}
Michie 1997 {published data only}
- Michie S , Smith D , McClennan A , Marteau TM . Patient decision making: an evaluation of two different methods of presenting information about a screening test . British Journal of Health Psychology 1997. ; 2 ( 4 ): 317-26 . [Google Scholar]
Miller 2014a {published data only}
- Miller MJ , Allison JJ , Cobaugh DJ , Ray MN , Saag KG . A group-randomized trial of shared decision making for non-steroidal anti-inflammatory drug risk awareness: primary results and lessons learned . Journal of Evaluation in Clinical Practice 2014. ; 20 : 638-48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2014b {published data only}
- Miller SM , Roussi P , Scarpato J , Wen KY , Zhu F , Roy G . Randomized trial of print messaging: the role of the partner and monitoring style in promoting provider discussions about prostate cancer screening among African American men . Psycho-Oncology 2014. ; 23 : 404-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Minneci 2019 {published data only}
- Minneci PC , Cooper JN , Leonhart K , Nacion K , Sulkowski J , Porter K , et al. Effects of a patient activation tool on decision making between surgery and nonoperative management for pediatric appendicitis: a randomized clinical trial . JAMA Network Open 2019. ; 2 ( 6 ): e195009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishel 2009 {published data only}
- Mishel MH , Germino BB , Lin L , Pruthi RS , Wallen EM , Crandell J , et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial . Patient Education and Counseling 2009. ; 77 ( 3 ): 349-59 . [DOI] [PubMed] [Google Scholar]
Mohammad 2012 {published data only}
- Mohammad-Alizadeh-Charandabi S , Shahnazi M , Jahanbakhsh. Communicating contraceptive effectiveness: a randomized controlled trial . Journal of Caring Sciences 2012. ; 1 ( 1 ): 1-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Molenaar 2001 {published data only}
- Molenaar S , Sprangers MA , Rutgers EJ , Luiten EJ , Mulder J , Bossuyt PM , et al. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life . Journal of Clinical Oncology 2001. ; 19 ( 6 ): 1676-87 . [DOI] [PubMed] [Google Scholar]
Mulley 2006 {published data only}
- Mulley AG Jr. Developing skills for evidence-based surgery: ensuring that patients make informed decisions . Surgical Clinics of North America 2006. ; 86 ( 1 ): 181-92 . [DOI] [PubMed] [Google Scholar]
Myers 2005a {published data only}
- Myers RE , Daskalakis C , Cocroft J , Kunkel EJ , Delmoor E , Liberatore M , et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening . Journal of the National Medical Association 2005. ; 97 ( 8 ): 1143-54 . [PMC free article] [PubMed] [Google Scholar]
Myers 2005b {published data only}
- Myers RE . Decision counseling in cancer prevention and control . Health Psychology 2005. ; 24 ( 4 Suppl ): S71-7 . [DOI] [PubMed] [Google Scholar]
Myers 2007 {published data only}
- Myers RE , Sifri R , Hyslop T , Rosenthal M , Vernon SW , Cocroft J , et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening . Cancer 2007. ; 110 ( 9 ): 2083-91 . [DOI] [PubMed] [Google Scholar]
Myers 2011 {published data only}
- Myers RE , Daskalakis C , Kunkel EJ , Cocroft JR , Riggio JM , Capkin M , et al. Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counseling . Patient Education and Counseling 2011. ; 83 ( 2 ): 240-6 . [DOI] [PubMed] [Google Scholar]
Myers 2013 {published data only}
- Myers RE , Bittner-Fagan H , Daskalakis C , Sifri R , Vernon SW , Cocroft J , et al. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening . Cancer Epidemiology, Biomarkers & Prevention 2013. ; 22 ( 1 ): 109-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2019 {published data only}
- Myers RE , Stello B , Daskalakis C , Sifri R , González ET , DiCarlo M , et al. Decision support and navigation to increase colorectal cancer screening among Hispanic patients . Cancer Epidemiology, Biomarkers & Prevention 2019. ; 28 ( 2 ): 384-91 . [DOI] [PubMed] [Google Scholar]
Neubeck 2008 {published data only}
- Neubeck L , Redfern J , Briffa T , Bauman A , Hare D , Freedman SB . The CHOICE (Choice of Health Options In prevention of Cardiovascular Events) replication trial: study protocol . BMC Cardiovascular Disorders 2008. ; 8 : 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2001 {published data only}
- Newton KM , LaCroix AZ , Buist DS , Delaney KM , Anderson LA . Women's responses to a mailed hormone replacement therapy workbook . Menopause 2001. ; 8 ( 5 ): 361-7 . [DOI] [PubMed] [Google Scholar]
O'Cathain 2002 {published data only}
- O'Cathain A , Walters SJ , Nicholl JP , Thomas KJ , Kirkham M . Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice . BMJ 2002. ; 324 ( 7338 ): 643-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1996 {published data only}
- O'Connor AM , Pennie RA , Dales RE . Framing effects on expectations, decisions, and side effects experienced: the case of influenza immunization . Journal of Clinical Epidemiology 1996. ; 49 ( 11 ): 1721-6 . [DOI] [PubMed] [Google Scholar]
O'Connor 1998a {published and unpublished data}
- O'Connor AM , Tugwell P , Wells GA , Elmslie T , Jolly E , Hollingworth G , et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy . Medical Decision Making 1998. ; 18 : 295-303 . [DOI] [PubMed] [Google Scholar]
O'Connor 1999a {published data only}
- O'Connor AM , Wells GA , Tugwell P , Laupacis A , Elmslie T , Drake E . The effects of an 'explicit' values clarification exercise in a women's decision aid regarding postmenopausal hormone therapy . Health Expectations 1999. ; 2 : 21-32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2009a {published data only}
- O'Connor PJ , Sperl-Hillen J , Johnson PE , Rush WA , Crain AL . Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial . Diabetes Care 2009. ; 32 ( 7 ): 1158-63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2011 {published data only}
- Connor PJ , Sperl-Hillen JM , Rush WA , Johnson PE , Amundson GH , Asche SE , et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial . Annals of Family Medicine 2011. ; 9 ( 1 ): 12-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Owens 2014A {published data only}
- Owens OL . A Community-driven Approach to the Development of a Digital Decision Aid to Facilitate Informed Decision Making for Prostate Cancer Screening among African-American men in Communities of Faith [PhD thesis] . Columbia: : University of South Carolina; , 2014. . [Google Scholar]
Pablos 2020 {published data only}
- Pablos JL , Jover JA , Roman-Ivorra JA , Inciarte-Mundo J , Dilla T , Sacristan JA , et al. Patient decision aid (PDA) for patients with rheumatoid arthritis reduces decisional conflict and improves readiness for treatment decision making . The Patient: Patient-Centered Outcomes Research 2020. ; 13 ( 1 ): 57-69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Paquin 2021 {published data only}
- Paquin RS , Peinado S , Lewis MA , Biesecker BB , Rini C , Roche M , et al. A behavior-theoretic evaluation of values clarification on parental beliefs and intentions toward genomic sequencing for newborns . Social Science & Medicine 2021. ; 271 : 112037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2017 {published data only}
- Parker K , Cunningham SJ , Petrie A , Ryan FS . Randomized controlled trial of a patient decision-making aid for orthodontics . American Journal of Orthodontics and Dentofacial Orthopedics 2017. ; 152 ( 2 ): 154-60 . [DOI] [PubMed] [Google Scholar]
Patanwala 2011 {published data only}
- Patanwala IM , Brocklebank V , Inglis J , Trewby PN . A randomized questionnaire-based study on the impact of providing numerical information on colorectal cancer screening . Journal of the Royal Society of Medicine Short Reports 2011. ; 2 ( 6 ): 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2014 {published data only}
- Patel S , Ngunjiri A , Wan Hee S , Yang Y , Brown S , Friede T , et al. Primum non nocere: shared informed decision making in low back pain - a pilot cluster randomised trial . BMC Musculoskeletal Disorders 2014. ; 15 : 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearson 2005 {published data only}
- Pearson S , Maddern GJ , Hewett P . Interacting effects of preoperative information and patient choice in adaptation to colonoscopy . Diseases of the Colon & Rectum 2005. ; 48 ( 11 ): 2047-54 . [DOI] [PubMed] [Google Scholar]
Peele 2005 {published data only}
- Peele PB , Siminoff LA , Xu Y , Ravdin PM . Decreased use of adjuvant breast cancer therapy in a randomized controlled trial of a decision aid with individualized risk information . Medical Decision Making 2005. ; 25 ( 3 ): 301-7 . [DOI] [PubMed] [Google Scholar]
Petty 2014 {published data only}
- Petty J . Exploring the effectiveness of an interactive, technology-enabled learning tool to enhance knowledge for neonatal nurses . Neonatal, Paediatric and Child Health Nursing 2014. ; 17 ( 1 ): 2-10 . [Google Scholar]
Philip 2010 {published data only}
- Philip E , DuHamel K , Jandorf L . Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: a preliminary study . Cancer Causes Control 2010. ; 21 ( 10 ): 1685-91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 1995 {published data only}
- Phillips C , Hill BJ , Cannac C . The influence of video imaging on patients' perceptions and expectations . Angle Orthodontist 1995. ; 65 ( 4 ): 263-70 . [DOI] [PubMed] [Google Scholar]
Pignone 2013 {published data only}
- Pignone MP , Howard K , Brenner AT , Crutchfield TM , Hawley ST , Lewis CL , et al. Comparing 3 techniques for eliciting patient values for decision making about prostate-specific antigen screening: a randomized controlled trial . JAMA Internal Medicine 2013. ; 173 ( 5 ): 362-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2008 {published data only}
- Pinto H , Rumball D , Maskrey V , Holland R . A pilot study for a randomized controlled and patient preference trial of buprenorphine versus methadone maintenance treatment in the management of opiate dependent patients . Journal of Substance Use 2008. ; 13 ( 2 ): 73-82 . [Google Scholar]
Politi 2020b {published data only}
- Politi MC , Grant RL , George NP , Barker AR , James AS , Kuroki LM , et al. Improving cancer patients' insurance choices (I Can PIC): a randomized trial of a personalized health insurance decision aid . Oncologist 2020. ; 25 ( 7 ): 609-19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Powers 2011 {published data only}
- Powers B , Danus S , Grubber J , Olsen M , Oddone E , Bosworth H . The effectiveness of personalized coronary heart disease and stroke risk communication . American Heart Journal 2011. ; 161 ( 4 ): 673-80 . [DOI] [PubMed] [Google Scholar]
Probst 2020 {published data only}
- Probst MA , Lin MP , Sze JJ , Hess EP , Breslin M , Frosch DL , et al. Shared decision making for syncope in the emergency department: a randomized controlled feasibility trial . Academic Emergency Medicine 2020. ; 27 ( 9 ): 853-65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2006 {published data only}
- Proctor A , Jenkins TR , Loeb T , Elliot M , Ryan A . Patient satisfaction with 3 methods of postpartum contraceptive counseling: a randomized, prospective trial . Journal of Reproductive Medicine 2006. ; 51 ( 5 ): 377-82 . [PubMed] [Google Scholar]
Prunty 2008 {published data only}
- Prunty MC , Sharpe L , Butow P , Fulcher G . The motherhood choice: a decision aid for women with multiple sclerosis . Patient Education and Counseling 2008. ; 71 ( 1 ): 108-15 . [DOI] [PubMed] [Google Scholar]
Qureshey 2022 {published data only}
- Qureshey EJ , Chauhan SP , Wagner SM , Batiste O , Chen HY , Ashimi S , et al. Educational multimedia tool compared with routine care for the uptake of postpartum long-acting reversible contraception in individuals with high-risk pregnancies: a randomized controlled trial . Obstetrics and Gynecology 2022. ; 139 ( 4 ): 571-8 . [DOI] [PubMed] [Google Scholar]
Ramallo‐Farina 2020 {published data only}
- Ramallo-Fariña Y , García-Bello MA , García-Pérez L , Boronat M , Wägner AM , Rodríguez-Rodríguez L , et al. Effectiveness of internet-based multicomponent interventions for patients and health care professionals to improve clinical outcomes in type 2 diabetes evaluated through the indica study: multiarm cluster randomized controlled trial . JMIR mHealth and uHealth 2020. ; 8 ( 11 ): e18922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranta 2015 {published data only}
- Ranta A , Dovey S , Weatherall M , O'Dea D , Gommans J , Tilyard M . Cluster randomized controlled trial of TIA electronic decision support in primary care . American Academy of Neurology 2015. ; 84 ( 15 ): 1545-51 . [DOI] [PubMed] [Google Scholar]
Rapley 2006 {published data only}
- Rapley T , May C , Heaven B , Murtagh M , Graham R , Kaner EF , et al. Doctor-patient interaction in a randomised controlled trial of decision-support tools . Social Science & Medicine 2006. ; 62 ( 9 ): 2267-78 . [DOI] [PubMed] [Google Scholar]
Raynes‐Greenow 2009 {published data only}
- Raynes-Greenow CH , Roberts CL , Nassar N , Trevena L . Do audio-guided decision aids improve outcomes? A randomized controlled trial of an audio-guided decision aid compared with a booklet decision aid for Australian women considering labour analgesia . Health Expectations 2009. ; 12 ( 4 ): 407-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Raynes‐Greenow 2010 {published data only}
- Raynes-Greenow CH , Nassar N , Torvaldsen S , Trevena L , Roberts CL . Assisting informed decision making for labour analgesia: a randomised controlled trial of a decision aid for labour analgesia versus a pamphlet . BMC Pregnancy and Childbirth 2010. ; 10 : 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Reder 2017 {published data only}
- Reder M , Kolip P . Does a decision aid improve informed choice in mammography screening? Results from a randomised controlled trial . PLoS One 2017. ; 12 ( 12 ): e0189148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Reder 2019 {published data only}
- Reder M , Soellner R , Kolip P . Do women with high ehealth literacy profit more from a decision aid on mammography screening? Testing the moderation effect of the eHEALS in a randomized controlled trial . Frontiers in Public Health 2019. ; 7 : 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rimer 2001 {published data only}
- Rimer BK , Halabi S , Sugg Skinner C , Kaplan EB , Crawford Y , Samsa GP , et al. The short-term impact of tailored mammography decision-making interventions . Patient Education and Counseling 2001. ; 43 ( 3 ): 269-85 . [DOI] [PubMed] [Google Scholar]
Rimer 2002 {published data only}
- Rimer BK , Halabi S , Sugg Skinner C , Lipkus IM , Strigo TS , Kaplan EB , et al. Effects of a mammography decision-making intervention at 12 and 24 months . American Journal of Preventive Medicine 2002. ; 22 ( 4 ): 247-57 . [DOI] [PubMed] [Google Scholar]
Rising 2018 {published data only}
- Rising KL , Hollander JE , Schaffer JT , Kline JA , Torres CA , Diercks DB , et al. Effectiveness of a decision aid in potentially vulnerable patients: a secondary analysis of the Chest Pain Choice multicenter randomized trial . Medical Decision Making 2018. ; 38 ( 1 ): 69-78 . [DOI] [PubMed] [Google Scholar]
Robinson 2013 {published data only}
- Robinson JK , Gaber R , Hultgren B , Eilers S , Blatt H , Stapleton J , et al. Skin self-examination education for early detection of melanoma: a randomized controlled trial of internet, workbook and in-person interventions . Journal of Medical Internet Research 2013. ; 16 ( 1 ): 1-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogojanski 2022 {published data only}
- Rogojanski J , Zeifman RJ , Antony MM , Walker JR , Monson CM , Mobilizing Minds Research Group . Evaluation of a decision aid for the treatment of depression among college students . Journal of American College Health 2022. ; 70 ( 6 ): 1634-43 . [DOI] [PubMed] [Google Scholar]
Ronda 2014 {published and unpublished data}
- Ronda G , Grispen JEJ , Ickenroth M , Dinant GJ , Vries NK , Weijden T . The effects of a web-based decision aid on the intention to diagnostic self-testing for cholesterol and diabetes: a randomized controlled trial . BMC Public Health 2014. ; 14 : 921 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 2022 {published data only}
- Rosen JE , Flum DR , Davidson GH , Liao JM . Randomized pilot test of a decision support tool for acute appendicitis: decisional conflict and acceptability in a healthy population . Annals of Surgery Open 2022. ; 3 ( 4 ): e213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostom 2002 {published data only}
- Rostom A , O'Connor A , Tugwell P , Wells G . A randomized trial of a computerized versus an audio-booklet decision aid for women considering post-menopausal hormone replacement therapy . Patient Education and Counseling 2002. ; 46 ( 1 ): 67-74 . [DOI] [PubMed] [Google Scholar]
Roter 2012 {published data only}
- Roter DL , Wexler R , Naragon P , Forrest B , Dees J , Almodovar A , Wood J . The impact of patient and physician computer mediated communication skill training on reported communication and patient satisfaction . Patient Education and Counseling 2012. ; 88 ( 3 ): 406-13 . [DOI] [PubMed] [Google Scholar]
Rothert 1997 {published and unpublished data}
- Holmes-Rovner M , Kroll J , Rovner DR , Schmitt N , Rothert M , Padonu G , et al. Patient decision support intervention: increased consistency with decision analytic models . Medical Care 1999. ; 37 ( 3 ): 270-84 . [DOI] [PubMed] [Google Scholar]
- Rothert ML , Holmes-Rovner M , Rovner D , Kroll J , Breer L , Talarczyk G , et al. An educational intervention as decision support for menopausal women . Research in Nursing & Health 1997. ; 20 ( 5 ): 377-87 . [DOI] [PubMed] [Google Scholar]
Rothwell 2019 {published data only}
- Rothwell E , Johnson E , Wong B , Rose NC , Latendresse G , Altizer R , et al. The use of a game-based decision aid to educate pregnant women about prenatal screening: a randomized controlled study . American Journal of Perinatology 2019. ; 36 ( 3 ): 322-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovner 2004 {published data only}
- Rovner DR , Wills CE , Bonham V , Williams G , Lillie J , Kelly-Blake K , et al. Decision aids for benign prostatic hyperplasia: applicability across race and education . Medical Decision Making 2004. ; 24 ( 4 ): 359-66 . [DOI] [PubMed] [Google Scholar]
Rubinstein 2011 {published data only}
- Rubinstein W , Acheson L , O'Neill S , Ruffin M , Wang C , Beaumont J , et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial . Genetics in Medicine 2011. ; 13 ( 11 ): 956-65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruddy 2009 {published data only}
- Ruddy KJ , Partridge AH . Breast cancer in young women: clinical decision-making in the face of uncertainty . Oncology 2009. ; 23 ( 6 ): 474-7 . [PubMed] [Google Scholar]
Ruehlman 2012 {published data only}
- Ruehlman LS , Karoly P , Enders C . A randomized controlled evaluation of an online chronic pain self management program . Pain 2012. ; 153 ( 2 ): 319-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruland 2013 {published data only}
- Ruland CM , Andersen T , Jeneson A , Moore S , Grimsbø GH , Børøsund E , et al. Effects of an internet support system to assist cancer patients in reducing symptom distress: a randomized controlled trial . Cancer Nursing 2013. ; 36 ( 1 ): 6-17 . [DOI] [PubMed] [Google Scholar]
Rutten 2022 {published data only}
- Rutten JJ , Buul LW , Smalbrugge M , Geerlings SE , Gerritsen DL , Natsch S , et al. An electronic health record integrated decision tool and supportive interventions to improve antibiotic prescribing for urinary tract infections in nursing homes: a cluster randomized controlled trial . Journal of the American Medical Directors Association 2022. ; 23 ( 3 ): 387-93 . [DOI] [PubMed] [Google Scholar]
Ryser 2004 {published data only}
- Ryser FG . Breastfeeding attitudes, intention, and initiation in low-income women: the effect of the best start program . Journal of Human Lactation 2004. ; 20 ( 3 ): 300-5 . [DOI] [PubMed] [Google Scholar]
Sassen 2014 {published data only}
- Sassen B , Kok G , Schepers J , Vanhees L . Supporting health care professionals to improve the processes of shared decision making and self-management in a web-based intervention: randomized controlled trial . Journal of Medical Internet Research 2014. ; 16 ( 10 ): e211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Saver 2007 {published data only}
- Saver BG , Gustafson D , Taylor TR , Hawkins RP , Woods NF , Dinauer S , et al. A tale of two studies: the importance of setting, subjects and context in two randomized, controlled trials of a web-based decision support for perimenopausal and postmenopausal health decisions . Patient Education and Counseling 2007. ; 66 ( 2 ): 211-22 . [DOI] [PubMed] [Google Scholar]
Sawka 2011 {published data only}
- Sawka AM , Straus S , Gafni A , Brierley JD , Tsang RW , Rotstein L , et al. How can we meet the information needs of patients with early stage papillary thyroid cancer considering radioactive iodine remnant ablation? Clinical Endocrinology 2011. ; 74 : 419-23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawka 2015a {published data only}
- Sawka AM , Straus S , Rodin G , Heus L , Brierley JD , Tsang RW , et al. Thyroid cancer patient perceptions of radioactive iodine treatment choice: follow-up from a decision-aid randomized trial . Cancer 2015. ; 121 ( 20 ): 3717-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawka 2015b {published data only}
- Sawka AM , Straus S , Rodin G , Tsang RW , Brierley JD , Rotstein L , et al. Exploring the relationship between patients' information preference style and knowledge acquisition process in a computerized patient decision aid randomized controlled trial . BMC Medical Informatics and Decision Making 2015. ; 15 : 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Scaffidi 2014 {published data only}
- Scaffidi RM , Posmontier B , Bloch JR , Wittmann-Price R . The relationship between personal knowledge and decision self-efficacy in choosing trial of labor after cesarean . Journal of Midwifery & Women's Health 2014. ; 59 ( 3 ): 246-53 . [DOI] [PubMed] [Google Scholar]
Schaffer 2018 {published data only}
- Schaffer JT , Hess EP , Hollander JE , Kline JA , Torres CA , Diercks DB , et al. Impact of a shared decision making intervention on health care utilization: a secondary analysis of the chest pain choice multicenter randomized trial . Academic Emergency Medicine 2018. ; 25 ( 3 ): 293-300 . [DOI] [PubMed] [Google Scholar]
Schapira 2000 {published data only}
- Schapira MM , VanRuiswyk J . The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests . Journal of Family Practice 2000. ; 49 ( 5 ): 418-24 . [PubMed] [Google Scholar]
Schapira 2007 {published data only}
- Schapira MM , Gilligan MA , McAuliffe T , Garmon G , Carnes M , Nattinger AB . Decision-making at menopause: a randomized controlled trial of a computer-based hormone therapy decision-aid . Patient Education and Counseling 2007. ; 67 ( 1-2 ): 100-7 . [DOI] [PubMed] [Google Scholar]
Scherr 2022 {published data only}
- Scherr K , Delaney RK , Ubel P , Kahn VC , Hamstra D , Wei JT , et al. Preparing patients with early stage prostate cancer to participate in clinical appointments using a shared decision making training video . Medical Decision Making 2022. ; 42 ( 3 ): 364-74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnipper 2010 {published data only}
- Schnipper JL , Linder JA , Palchuk MB , Yu DT , McColgan KE , Volk LA , et al. Effects of documentation-based decision support on chronic disease management . American Journal of Managed Care 2010. ; 16 ( 12 Suppl HIT ): SP72-81 . [PubMed] [Google Scholar]
Scholl 2021 {published data only}
- Scholl I , Hahlweg P , Lindig A , Frerichs W , Zill J , Cords H , et al. Evaluation of a program for routine implementation of shared decision-making in cancer care: results of a stepped wedge cluster randomized trial . Implementation Science 2021. ; 16 ( 1 ): 106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroy 2016 {published data only}
- Schroy PC 3rd, Duhovic E , Chen CA , Heeren TC , Lopez W , Apodaca DL . Risk stratification and shared decision making for colorectal cancer screening: a randomized controlled trial . Medical Decision Making 2016. ; 36 ( 4 ): 526-35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2009b {published data only}
- Schwartz LM , Woloshin S , Welch HG . Using a drug facts box to communicate drug benefits and harms: two randomized trials . Annals of Internal Medicine 2009. ; 150 ( 8 ): 516-27 . [DOI] [PubMed] [Google Scholar]
Sears 2007 {published data only}
- Sears SR , Woodward JT , Twillman RK . What do I have to lose? effects of a psycho-educational intervention on cancer patient preference for resuscitation . Journal of Behavioral Medicine 2007. ; 30 ( 6 ): 533-44 . [DOI] [PubMed] [Google Scholar]
Seitz 2018 {published data only}
- Seitz HH , Schapira MM , Gibson LA , Skubisz C , Mello S , Armstrong K , et al. Explaining the effects of a decision intervention on mammography intentions: the roles of worry, fear and perceived susceptibility to breast cancer . Psychology & Health 2018. ; 33 ( 5 ): 682-700 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sepucha 2022 {published data only}
- Sepucha KR , Vo H , Chang Y , Dorrwachter JM , Dwyer M , Freiberg AA , et al. Shared decision-making is associated with better outcomes in patients with knee but not hip osteoarthritis: the DECIDE-OA randomized study . Journal of Bone and Joint Surgery. American Volume 2022. ; 104 ( 1 ): 62-9 . [DOI] [PubMed] [Google Scholar]
Sequist 2011 {published data only}
- Sequist T , Zaslavsky A , Colditz G , Ayanian J . Electronic patient message to promote colorectal cancer screening . Archives of Internal Medicine 2011. ; 171 ( 7 ): 636-41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Serovich 2020 {published data only}
- Serovich JM , Laschober TC , Brown MJ , Kimberly JA , Lescano CM . Effects of a decision-making intervention to help decide whether to disclose HIV-positive status to family members on well-being and sexual behavior . Archives of Sexual Behavior 2020. ; 49 ( 6 ): 2091-101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sferra 2021 {published data only}
- Sferra SR , Cheng JS , Boynton Z , DiSesa V , Kaiser LR , Ma GX , et al. Aiding shared decision making in lung cancer screening: two decision tools . Journal of Public Health (Oxford, England) 2021. ; 43 ( 3 ): 673-80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah S , Singh K , Ali MK , Mohan V , Kadir MM , Unnikrishnan AG , et al, CARRS Trial Writing Group . Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia - the CARRS multi-center translation trial . Diabetes Research and Clinical Practice 2012. ; 98 ( 2 ): 285-94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Shegog 2020 {published data only}
- Shegog R , Begley C , Chong J , Sepulveda R , Addy R , Martin K , et al. MINDSET: clinic-based decision support demonstrates longitudinal efficacy for increased epilepsy self-management adherence among Spanish speaking patients . Epilepsy & Behavior 2020. ; 113 : 107552 . [DOI] [PubMed] [Google Scholar]
Sheppard 2012 {published data only}
- Sheppard VB , Wallington SF , Williams KP , Lucas W . A decision-support intervention for black women eligible for adjuvant systematic therapy: Sisters informing sisters about breast cancer treatment - An intervention to reduce treatment disparities . In: Elk R , Landrine H , editors(s). Cancer Disparities: Causes and Evidence-Based Solutions . American Cancer Society; , 2012. . [Google Scholar]
Sheridan 2004 {published data only}
- Sheridan SL , Felix K , Pignone MP , Lewis CL . Information needs of men regarding prostate cancer screening and the effect of a brief decision aid . Patient Education and Counseling 2004. ; 54 ( 3 ): 345-51 . [DOI] [PubMed] [Google Scholar]
Sheridan 2010 {published data only}
- Sheridan SL , Griffith JM , Behrend L , Gizlice Z , Jianwen C , Pignone MP . Effect of adding a values clarification exercise to a decision aid on heart disease prevention: a randomized trial . Medical Decision Making 2010. ; 30 ( 4 ): E28-39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2012 {published data only}
- Sheridan SL , Golin C , Bunton A , Lykes JB , Schwartz B , McCormack L , et al. Shared decision making for prostate cancer screening: the results of a combined analysis of two practice-based randomized controlled trials . BMC Medical Informatics & Decision Making 2012. ; 12 : 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2014 {published data only}
- Sherman KA , Harcourt DM , Lam TC , Shaw LK , Boyages J . BRECONDA: Development and acceptability of an interactive decisional support tool for women considering breast reconstruction . Psycho-Oncology 2014. ; 23 : 835-8 . [DOI] [PubMed] [Google Scholar]
Sherman 2016 {published data only}
- Sherman KA , Shaw LE , Winch CJ , Harcourt D , Boyages J , Cameron LD , et al. Reducing decisional conflict and enhancing satisfaction with information among women considering breast reconstruction following mastectomy: results from the BRECONDA randomized controlled trial . Plastic and Reconstructive Surgery 2016. ; 138 ( 4 ): 592e-602e . [DOI] [PubMed] [Google Scholar]
Sherman 2017 {published data only}
- Sherman KA , Kilby CJ , Shaw LK , Winch C , Kirk J , Tucker K , et al. Facilitating decision-making in women undergoing genetic testing for hereditary breast cancer: BRECONDA randomized controlled trial results . Breast (Edinburgh, Scotland) 2017. ; 36 : 79-85 . [DOI] [PubMed] [Google Scholar]
Shirai 2012 {published data only}
- Shirai Y , Fujimori M , Ogawa A , Yamada Y , Nishiwaki Y , Ohtsu A , Uchitomi Y . Patients' perception of the usefulness of a question prompt sheet for advanced cancer patients when deciding the initial treatment: a randomized, controlled trial . Psycho-Oncology 2012. ; 21 ( 7 ): 706-13 . [DOI] [PubMed] [Google Scholar]
Silver 2012 {published data only}
- Silver B , Zaman IF , Ashraf K , Majed Y , Norwood EM , Schuh LA , et al. A randomized trial of decision-making in asymptomatic carotid stenosis . Neurology 2012. ; 78 ( 5 ): 315-21 . [DOI] [PubMed] [Google Scholar]
Siminoff 2006 {published data only}
- Siminoff LA , Gordon NH , Silverman P , Budd T , Ravdin PM . A decision aid to assist in adjuvant therapy choices for breast cancer . Psycho-Oncology 2006. ; 15 ( 11 ): 1001-13 . [DOI] [PubMed] [Google Scholar]
- Vickers AJ , Elkin EB , Peele PB , Dickler M , Siminoff LA . Long-term health outcomes of a decision aid: data from a randomized trial of adjuvant! in women with localized breast cancer . Medical Decision Making 2009. ; 29 ( 4 ): 461-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2012a {published data only}
- Simon D , Kriston L , Wolff A , Buchholz A , Vietor C , Hecke T , et al. Effectiveness of a web-based, individually tailored decision aid for depression or acute low back pain: a randomized controlled trial . Patient Education and Counseling 2012. ; 87 ( 3 ): 360-8 . [DOI] [PubMed] [Google Scholar]
Simon 2012b {published data only}
- Simon W , Lambert MJ , Harris MW , Busath G , Vazquez A . Providing patient progress information and clinical support tools to therapists: effects on patients at risk of treatment failure . Psychotherapy Research 2012. ; 22 ( 6 ): 638-47 . [DOI] [PubMed] [Google Scholar]
Smith 2011a {published data only}
- Smith T , Dow L , Virago E , Khatcheressian J , Matsuyama R , Lyckholm L . A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer . Journal of Supportive Oncology 2011. ; 9 ( 2 ): 79-86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011b {published data only}
- Smith SW , Nazione S , LaPlante C , Clark-Hitt R , Park HS , Sung R , Leichtman A . Living kidney donor decision making and communication . Journal of Health Communication: International Perspectives 2011. ; 16 ( 8 ): 870-88 . [DOI] [PubMed] [Google Scholar]
Smith 2020 {published data only}
- Smith SK , Westbrook K , MacDermott K , Amarasekara S , LeBlanc M , Pan W . Four conversations: a randomized controlled trial of an online, personalized coping and decision aid for metastatic breast cancer patient . Journal of Palliative Medicine 2020. ; 23 ( 3 ): 353-8 . [DOI] [PubMed] [Google Scholar]
Solberg 2010 {published data only}
- Solberg LI , Asche SE , Sepucha K , Thygeson NM , Madden JE , Morrissey L , et al. Informed choice assistance for women making uterine fibroid treatment decisions: a practical clinical trial . Medical Decision Making 2010. ; 30 ( 4 ): 444-52 . [DOI] [PubMed] [Google Scholar]
Sorenson 2004 {published data only}
- Sorenson JR , Lakon C , Spinney T , Jennings-Grant T . Assessment of a decision aid to assist genetic testing research participants in the informed consent process . Genetic Testing 2004. ; 8 ( 3 ): 336-46 . [DOI] [PubMed] [Google Scholar]
Sparano 2006 {published data only}
- Sparano JA . TAILORx: trial assigning individualized options for treatment (Rx) . Clinical Breast Cancer 2006. ; 7 ( 4 ): 347-50 . [DOI] [PubMed] [Google Scholar]
Stalmeier 2009 {published data only}
- Stalmeier PF , Roosmalen MS . Concise evaluation of decision aids . Patient Education and Counseling 2009. ; 74 ( 1 ): 104-9 . [DOI] [PubMed] [Google Scholar]
Stankowski‐Drengler 2019 {published data only}
- Stankowski-Drengler TJ , Tucholka JL , Bruce JG , Steffens NM , Schumacher JR , Greenberg CC , et al. A randomized controlled trial evaluating the impact of pre-consultation information on patients' perception of information conveyed and satisfaction with the decision-making process . Annals of Surgical Oncology 2019. ; 26 ( 10 ): 3275-81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Starosta 2015 {published data only}
- Starosta AJ , Luta G , Tomko CA , Schwartz MD , Taylor KL . Baseline attitudes about prostate cancer screening moderate the impact of decision aids on screening rates . Annals of Behavioral Medicine 2015. ; 49 : 762-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2013 {published data only}
- Stein RA , Sharpe L , Bell ML , Boyle FM , Dunn SM , Clarke SJ . Randomized controlled trial of a structured intervention to facilitate end-of-life decision making in patients with advanced cancer . Journal of Clinical Oncology 2013. ; 31 ( 27 ): 3403-10 . [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner MJ , Dalebout S , Condon S , Dominik R , Trussell J . Understanding risk: a randomized controlled trial of communicating contraceptive effectiveness . Obstetrics & Gynecology 2003. ; 102 ( 4 ): 709-17 . [DOI] [PubMed] [Google Scholar]
Stephens 2008 {published data only}
- Stephens RL , Xu Y , Volk RJ , Scholl LE , Kamin SL , Holden EW . Influence of a patient decision aid on decisional conflict related to PSA testing: a structural equation model . Health Psychology 2008. ; 27 ( 6 ): 711-21 . [DOI] [PubMed] [Google Scholar]
Stiggelbout 2008 {published data only}
- Stiggelbout AM , Molewijk AC , Otten W , Bockel JH , Bruijninckx CM , Salm I , et al. The impact of individualized evidence-based decision support on aneurysm patients' decision making, ideals of autonomy, and quality of life . Medical Decision Making 2008. ; 28 ( 5 ): 751-62 . [DOI] [PubMed] [Google Scholar]
Stirling 2012 {published data only}
- Stirling C , Leggett S , Lloyd B , Scott J , Blizzard L , Quinn S , Robinson A . Decision aids for respite service choices by carers of people with dementia: development and pilot RCT . BMC Medical Informatics and Decision Making 2012. ; 12 : 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratton 2019 {published data only}
- Stratton E , Choi I , Calvo R , Hickie I , Henderson C , Harvey SB , et al. Web-based decision aid tool for disclosure of a mental health condition in the workplace: a randomised controlled trial . Occupational and Environmental Medicine 2019. ; 76 ( 9 ): 595-602 . [DOI] [PubMed] [Google Scholar]
Street 1995 {published data only}
- Street RLJ , Voigt B , Geyer CJ , Manning T , Swanson GP . Increasing patient involvement in choosing treatment for early breast cancer . Cancer 1995. ; 76 ( 11 ): 2275-85 . [DOI] [PubMed] [Google Scholar]
Street 1998 {published data only}
- Street RL Jr, Van Order A , Bramson R , Manning T . Preconsultation education promoting breast cancer screening: does the choice of media make a difference? Journal of Cancer Education 1998. ; 13 ( 3 ): 152-61 . [DOI] [PubMed] [Google Scholar]
Suen 2021 {published data only}
- Suen AO , Butler RA , Arnold RM , Myers B , Witteman HO , Cox CE , et al. A pilot randomized trial of an interactive web-based tool to support surrogate decision makers in the intensive care unit . Annals of the American Thoracic Society 2021. ; 18 ( 7 ): 1191-201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundaresan 2011 {published data only}
- Sundaresan P , Turner S , Kneebone A , Pearse M , Butow P . Evaluating the utility of a patient decision aid for potential participants of a prostate cancer trial (RAVES-TROG 08.03) . Radiotherapy and Oncology 2011. ; 101 ( 3 ): 521-4 . [DOI] [PubMed] [Google Scholar]
Tabak 1995 {published data only}
- Tabak N . Decision making in consenting to experimental cancer therapy . Cancer Nursing 1995. ; 18 ( 2 ): 89-96 . [PubMed] [Google Scholar]
Taksler 2021 {published data only}
- Taksler GB , Hu B , DeGrandis F Jr, Montori VM , Fagerlin A , Nagykaldi Z , et al. Effect of individualized preventive care recommendations vs usual care on patient interest and use of recommendations: a pilot randomized clinical trial . JAMA Network Open 2021. ; 4 ( 11 ): e2131455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanser 2021 {published data only}
- Tanser FC , Kim HY , Mathenjwa T , Shahmanesh M , Seeley J , Matthews P , et al. Home-based Intervention to Test and Start (HITS): a community-randomized controlled trial to increase HIV testing uptake among men in rural South Africa . Journal of the International AIDS Society 2021. ; 24 ( 2 ): e25665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappen 2020 {published data only}
- Tappen RM , Worch SM , Newman DO , Hain D . Evaluation of a novel decision guide "Go to the Hospital or Stay Here?" for nursing home residents and families: a randomized trial . Research in Gerontological Nursing 2020. ; 13 ( 6 ): 309-19 . [DOI] [PubMed] [Google Scholar]
Taylor 2013 {published data only}
- Taylor KL , Williams RM , Davis K , Luta G , Penek S , Barry S , et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial . JAMA Internal Medicine 2013. ; 173 ( 18 ): 1704-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tebb 2019 {published data only}
- Tebb KP , Leng Trieu S , Rico R , Renteria R , Rodriguez F , Puffer M . A mobile health contraception decision support intervention for Latina adolescents: implementation evaluation for use in school-based health centers . JMIR mHealth and uHealth 2019. ; 7 ( 3 ): e11163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ten 2008 {published data only}
- Ten Wolde GB , Dijkstra A , Empelen P , den Hout W , Neven AK , Zitman F . Long-term effectiveness of computer-generated tailored patient education on benzodiazepines: a randomized controlled trial . Addiction 2008. ; 103 ( 4 ): 662-70 . [DOI] [PubMed] [Google Scholar]
Ter Stege 2021 {published data only}
- Ter Stege JA , Oldenburg HS , Woerdeman LA , Witkamp AJ , Kieffer JM , Huizum MA , et al. Decisional conflict in breast cancer patients considering immediate breast reconstruction . The Breast : Official Journal of the European Society of Mastology 2021. ; 55 : 91-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiede 2021 {published data only}
- Thiede E , Levi BH , Lipnick D , Johnson R , Seo La I , Lehman EB , et al. Effect of advance care planning on surrogate decision makers' preparedness for decision making: results of a mixed-methods randomized controlled trial . Journal of Palliative Medicine 2021. ; 24 ( 7 ): 982-93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013 {published data only}
- Thomas KL , Zimmer LO , Dai D , Al-Khatib SM , Allen LaPointe NM , Peterson ED . Educational videos to reduce racial disparities in ICD therapy via innovative designs (VIVID): a randomized clinical trial . American Heart Journal 2013. ; 166 ( 1 ): 157-63 . [DOI] [PubMed] [Google Scholar]
Thomson 2006 {published data only}
- Thomson P , Dowding D , Swanson V , Bland R , Mair C , Morrison A , et al. A computerised guidance tree (decision aid) for hypertension, based on decision analysis: development and preliminary evaluation . European Journal of Cardiovascular Nursing 2006. ; 5 ( 2 ): 146-9 . [DOI] [PubMed] [Google Scholar]
Thornton 1995 {published data only}
- Thornton JG , Hewison J , Lilford RJ , Vail A . A randomised trial of three methods of giving information about prenatal testing . BMJ 1995. ; 311 ( 7013 ): 1127-30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiedje 2021 {published data only}
- Tiedje D , Borowski M , Simbrich A , Schlößler K , Kruse K , Bothe C , et al. Decision aid and cost compensation influence uptake of PSA-based early detection without affecting decisional conflict: a cluster randomised trial . Scientific Reports 2021. ; 11 ( 1 ): 23503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiller 2006 {published data only}
- Tiller K , Meiser B , Gaff C , Kirk J , Dudding T , Phillips KA , et al. A randomized controlled trial of a decision aid for women at increased risk of ovarian cancer . Medical Decision Making 2006. ; 26 ( 4 ): 360-72 . [DOI] [PubMed] [Google Scholar]
Tinsel 2013 {published data only}
- Tinsel I , Buchholz A , Vach W , Siegel A , Dürk T , Buchholz A , et al. Shared decision-making in antihypertensive therapy: a cluster randomised controlled trial . BMC Family Practice 2013. ; 14 : 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomko 2015 {published data only}
- Tomko C , Davis K , Ludin S , Kelly S , Stern A , Luta G , et al. Decisional outcomes following use of an interactive web-based decision aid for prostate cancer screening . Translational Behavioral Medicine: Practice, Policy, Research 2015. ; 5 ( 2 ): 189-97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2015 {published data only}
- Tran VT , Kisseleva-Romanova E , Rigal L , Falcoff H . Impact of a printed decision aid on patients' intention to undergo prostate cancer screening: a multicentre, pragmatic randomised controlled trial in primary care . British Journal of General Practice 2015. ; 65 ( 634 ): e295-304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2022 {published data only}
- Tsai PS , Wang CC , Lan YH , Tsai HW , Hsiao CY , Wu JC , et al. Effectiveness of question prompt lists in patients with breast cancer: a randomized controlled trial . Patient Education and Counseling 2022. ; 105 ( 9 ): 2984-94 . [DOI] [PubMed] [Google Scholar]
Tucholka 2018 {published data only}
- Tucholka JL , Yang DY , Bruce JG , Steffens NM , Schumacher JR , Greenberg CC , et al. A randomized controlled trial evaluating the impact of web-based information on breast cancer patients' knowledge of surgical treatment options . Journal of the American College of Surgeons 2018. ; 226 ( 2 ): 126-33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Ufere 2022 {published data only}
- Ufere NN , Robinson B , Donlan J , Indriolo T , Bloom J , Scherrer A , et al. Pilot randomized controlled trial of an advance care planning video decision tool for patients with advanced liver disease . Clinical Gastroenterology and Hepatology 2022. ; 20 ( 10 ): 2287-95.e3 . [DOI] [PubMed] [Google Scholar]
Ukoli 2013 {published data only}
- Ukoli FA , Patel K , Hargreaves M , Beard K , Moton PJ , Bragg R , et al. A tailored prostate cancer education intervention for low-income African Americans: impact on knowledge and screening . Journal of Health Care for the Poor and Underserved 2013. ; 24 ( 1 ): 311-31 . [DOI] [PubMed] [Google Scholar]
Valdez 2001 {published data only}
- Valdez A , Banerjee K , Fernandez M , Ackerson L . Impact of a multimedia breast cancer education intervention on use of mammography by low-income Latinas . Journal of Cancer Education 2001. ; 16 ( 4 ): 221-4 . [DOI] [PubMed] [Google Scholar]
Van der Krieke 2013 {published data only}
- Van der Krieke L , Emerencia AC , Boonstra N , Wunderink L , Jonge P , Sytema S . A web-based tool to support shared decision making for people with a psychotic disorder: randomized controlled trial and process evaluation . Journal of Medical Internet Research 2013. ; 15 ( 10 ): e216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Roosmalen 2004 {published and unpublished data}
- Van Roosmalen MS , Stalmeier PF , Verhoef LC , Hoekstra-Weebers JE , Oosterwijk JC , Hoogerbrugge N , et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation . British Journal of Cancer 2004. ; 90 ( 2 ): 333-42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roosmalen MS , Stalmeier PF , Verhoef LC , Hoekstra-Weebers JE , Oosterwijk JC , Hoogerbrugge N , et al. Randomized trial of a shared decision-making intervention consisting of trade-offs and individualized treatment information for BRCA1/2 mutation carriers . Journal of Clinical Oncology 2004. ; 22 ( 16 ): 3293-301 . [DOI] [PubMed] [Google Scholar]
VanScoy 2017 {published data only}
- Van Scoy LJ , Chiarolanzio PJ , Kim C , Heyland DK . Development and initial evaluation of an online decision support tool for families of patients with critical illness: a multicenter pilot study . Journal of Critical Care 2017. ; 39 : 18-24 . [DOI] [PubMed] [Google Scholar]
Van Steenkiste 2008 {published data only}
- Van Steenkiste B , Weijden TM , Stoffers JHEH , Grol RPTM . Patients' responsiveness to a decision support tool for primary prevention of cardiovascular diseases in primary care . Patient Education and Counseling 2008. ; 72 ( 1 ): 63-70 . [DOI] [PubMed] [Google Scholar]
Van Til 2009 {published data only}
- Van Til JA , Stiggelbout AM , IJzerman MJ . The effect of information on preferences stated in a choice-based conjoint analysis . Patient Education and Counseling 2009. ; 74 ( 2 ): 264-71 . [DOI] [PubMed] [Google Scholar]
Van Tol‐Geerdink 2013 {published data only}
- Van Tol-Geerdink JJ , Leer JW , Weijerman PC , Oort IM , Vergunst H , Lin EN , et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid . BJU International 2013. ; 111 ( 4 ): 564-73 . [DOI] [PubMed] [Google Scholar]
Veroff 2012 {published data only}
- Veroff D , Sullivan L , Shoptaw EJ , Venator B , Ochoa-Arvelo T , Baxter J , et al. Improving self-care for heart failure for seniors: Impact of video and written education and decision aids . Population Health Management 2012. ; 15 ( 1 ): 37-45 . [DOI] [PubMed] [Google Scholar]
Volandes 2009 {published data only}
- Volandes AE , Paasche-Orlow MK , Barry MJ , Gillick MR , Minaker KL , Chang Y , et al. Video decision support tool for advance care planning in dementia: randomised controlled trial . BMJ 2009. ; 338 : b2159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Volandes 2011 {published data only}
- Volandes A , Ferguson L , Davis A , Hull N , Green M , Chang Y , et al. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomised controlled trial . Journal of Palliative Medicine 2011. ; 14 ( 2 ): 169-77 . [DOI] [PubMed] [Google Scholar]
Volandes 2013 {published data only}
- Volandes AE , Paasche-Orlow MK , Mitchell SL , El-Jawahri A , Davis AD , Barry MJ , et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced care . Journal of Clinical Oncology 2013. ; 31 ( 3 ): 380-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Volk 2008 {published data only}
- Volk RJ , Jibaja-Weiss ML , Hawley ST , Kneuper S , Spann SJ , Miles BJ , et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy . Patient Education and Counseling 2008. ; 73 ( 3 ): 482-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Wagner 2011 {published data only}
- Von Wagner C . A decision aid to support informed choice about bowel cancer screening in people with low educational level improves knowledge but reduces screening uptake . Evidence-Based Nursing 2011. ; 14 ( 2 ): 36-7 . [DOI] [PubMed] [Google Scholar]
Wagner 1995 {published data only}
- Wagner EH , Barrett P , Barry MJ , Barlow W , Fowler FJ Jr. The effect of a shared decision making program on rates of surgery for benign prostatic hyperplasia. Pilot results . Medical Care 1995. ; 33 ( 8 ): 765-70 . [DOI] [PubMed] [Google Scholar]
Wakefield 2008a {published data only}
- Wakefield CE , Meiser B , Homewood J , Ward R , O'Donnell S , Kirk J , et al. Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk . Cancer 2008. ; 113 ( 5 ): 956-65 . [DOI] [PubMed] [Google Scholar]
Wakefield 2008b {published data only}
- Wakefield CE , Meiser B , Homewood J , Peate M , Taylor A , Lobb E , et al. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk . Breast Cancer Research and Treatment 2008. ; 107 ( 2 ): 289-301 . [DOI] [PubMed] [Google Scholar]
Wakefield 2008c {published data only}
- Wakefield CE , Meiser B , Homewood J , Taylor A , Gleeson M , Williams R . A randomized trial of a breast/ovarian cancer genetic testing decision aid used as a communication aid during genetic counseling . Psycho-Oncology 2008. ; 17 ( 8 ): 844-54 . [DOI] [PubMed] [Google Scholar]
Wallston 1991 {published data only}
- Wallston KA , Smith RA , King JE , Smith MS , Rye P , Burish TG . Desire for control and choice of antiemetic treatment for cancer chemotherapy . Western Journal of Nursing Research 1991. ; 13 ( 1 ): 12-23 . [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang C , Gonzalez R , Milliron KJ , Strecher VJ , Merajver SD . Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process . American Journal of Medical Genetics. Part A 2005. ; 134 ( 1 ): 66-73 . [DOI] [PubMed] [Google Scholar]
Wang TJ 2021 {published data only}
- Wang TJ , Chiu PP , Chen KK , Hung LP . Efficacy of a decision support intervention for reducing decisional conflict in patients with elevated serum prostate-specific antigen: a randomized controlled trial . European Journal of Oncology Nursing 2021. ; 50 : 101865 . [DOI] [PubMed] [Google Scholar]
Warner 2015 {published data only}
- Warner DO , LeBlanc A , Kadimpati S , Vickers KS , Shi Y , Montori V . Decision aid for cigarette smokers scheduled for elective surgery . Anesthesiology 2015. ; 123 ( 1 ): 18-28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterman 2018 {published data only}
- Waterman AD , Peipert JD . An explore transplant group randomized controlled education trial to increase dialysis patients' decision-making and pursuit of transplantation . Progress in Transplantation 2018. ; 28 ( 2 ): 174-83 . [DOI] [PubMed] [Google Scholar]
Waterman 2019 {published data only}
- Waterman AD , Peipert JD , McSorley AM , Goalby CJ , Beaumont JL , Peace L . Direct delivery of kidney transplant education to black and low-income patients receiving dialysis: a randomized controlled trial . American Journal of Kidney Diseases 2019. ; 74 ( 5 ): 640-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterman 2021 {published data only}
- Waterman AD , Peipert JD , Cui Y , Beaumont JL , Paiva A , Lipsey AF , et al. Your Path to Transplant: a randomized controlled trial of a tailored expert system intervention to increase knowledge, attitudes, and pursuit of kidney transplant . American Journal of Transplantation 2021. ; 21 ( 3 ): 1186-96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 2014 {published data only}
- Watts KJ , Meiser B , Wakefield CE , Barratt AL , Howard K , Cheah BC , et al. Online prostate cancer screening decision aid for at-risk men: a randomized trial . Health Psychology 2014. ; 33 ( 9 ): 986-97 . [DOI] [PubMed] [Google Scholar]
Wehkamp 2021 {published data only}
- Wehkamp K , Kiefer FB , Geiger F , Scheibler F , Rueffer JU , Donner-Banzhoff N , et al. Enhancing specific health literacy with a digital evidence-based patient decision aid for hypertension: a randomized controlled trial . Patient Preference and Adherence 2021. ; 15 : 1269-79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Welschen 2012 {published data only}
- Welschen LM , Bot SD , Kostense PJ , Dekker JM , Timmermans DR , Weijden T , et al. Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study . Diabetes Care 2012. ; 35 : 2485-92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Weng 2017 {published data only}
- Weng FL , Peipert JD , Holland BK , Brown DR , Waterman AD . A clustered randomized trial of an educational intervention during transplant evaluation to increase knowledge of living donor kidney transplant . Progress in Transplantation 2017. ; 27 ( 4 ): 377-85 . [DOI] [PubMed] [Google Scholar]
Wennberg 2010 {published data only}
- Wennberg DE , Marr A , Lang L , O'Malley S , Bennett G . A randomized trial of a telephone care-management strategy . New England Journal of Medicine 2010. ; 363 ( 13 ): 1245-55 . [DOI] [PubMed] [Google Scholar]
Werk 2019 {published data only}
- Werk LN , Diaz MC , Cadilla A , Franciosi JP , Hossain MJ . Promoting adherence to influenza vaccination recommendations in pediatric practice . Journal of Primary Care & Community Health 2019. ; 10 : 2150132719853060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Westermann 2013 {published data only}
- Westermann GM , Verheij F , Winkens B , Verhulst FC , Van Oort FV . Structured shared decision-making using dialogue and visualization: a randomized controlled trial . Patient Education and Counseling 2013. ; 90 ( 1 ): 74-81 . [DOI] [PubMed] [Google Scholar]
Weymann 2015 {published data only}
- Weymann N , Dirmaier J , Wolff A , Kriston L , Härter M . Effectiveness of a web-based tailored interactive health communication application for patients with type 2 diabetes or chronic low back pain: randomized controlled trial . Journal of Medical Internet Research 2015. ; 17 ( 3 ): e53 1-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 2009 {published data only}
- Wilhelm D , Gillen S , Wirnhier H , Kranzfelder M , Schneider A , Scmidt A , et al. Extended preoperative patient education using a multimedia DVD: impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial . Langenbeck's Archives of Surgery 2009. ; 394 ( 2 ): 227-33 . [DOI] [PubMed] [Google Scholar]
Wilkes 2013 {published data only}
- Wilkes MS , Day FC , Srinivasan M , Griffin E , Tancredi DJ , Rainwater JA , et al. Pairing physician education with patient activation to improve shared decisions in prostate cancer screening: a cluster randomized controlled trial . Annals of Family Medicine 2013. ; 11 ( 4 ): 324-34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkie 2013 {published data only}
- Wilkie DJ , Gallo AM , Yao Y , Molokie RE , Stahl C , Hershberger PE , et al. Reproductive health choices for young adults with sickle cell disease or trait: randomized controlled trial immediate posttest effects . Nursing Research 2013. ; 62 ( 5 ): 352-61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 2006 {published data only}
- Wilkins EG , Lowery JC , Copeland LA , Goldfarb SL , Wren PA , Janz NK . Impact of an educational video on patient decision making in early breast cancer treatment . Medical Decision Making 2006. ; 26 ( 6 ): 589-98 . [DOI] [PubMed] [Google Scholar]
Willemsen 2006 {published data only}
- Willemsen MC , Wiebing M , Emst A , Zeeman G . Helping smokers to decide on the use of efficacious smoking cessation methods: a randomized controlled trial of a decision aid . Addiction 2006. ; 101 ( 3 ): 441-9 . [DOI] [PubMed] [Google Scholar]
Williamson 2014 {published data only}
- Williamson LEA , Lawson KL , Downe PJ , Pierson RA . Informed reproductive decision-making: the impact of providing fertility information on fertility knowledge and intentions to delay childbearing . Journals of Obstetrics and Gynaecology Canada 2014. ; 36 ( 5 ): 400-5 . [DOI] [PubMed] [Google Scholar]
Williams‐Piehota 2008 {published data only}
- Williams-Piehota PA , McCormack LA , Treiman K , Bann CM . Health information styles among participants in a prostate cancer screening informed decision-making intervention . Health Education Research 2008. ; 23 ( 3 ): 440-53 . [DOI] [PubMed] [Google Scholar]
Wilson 2019 {published data only}
- Wilson LS , Blonquist TM , Hong F , Halpenny B , Wolpin S , Chang P , et al. Assigning value to preparation for prostate cancer decision making: a willingness to pay analysis . BMC Medical Informatics and Decision Making 2019. ; 19 ( 1 ): 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2020 {published data only}
- Wolff JC , Garcia A , Kelly LM , Frazier EA , Jones RN , Spirito A . Feasibility of decision rule-based treatment of comorbid youth: a pilot randomized control trial . Behaviour Research and Therapy 2020. ; 131 : 103625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Woltmann 2011 {published data only}
- Woltmann EM , Wilkniss SM , Teachout A , McHugo GJ , Drake RE . Trial of an electronic decision support system to facilitate shared decision making in community mental health . Psychiatric Services 2011. ; 62 ( 1 ): 54-60 . [DOI] [PubMed] [Google Scholar]
Wroe 2005 {published data only}
- Wroe AL , Turner N , Owens RG . Evaluation of a decision-making aid for parents regarding childhood immunizations . Health Psychology 2005. ; 24 ( 6 ): 539-47 . [DOI] [PubMed] [Google Scholar]
Yao 2017 {published data only}
- Yao K , Belkora J , Bedrosian I , Rosenberg S , Sisco M , Barrera E , et al. Impact of an in-visit decision aid on patient knowledge about contralateral prophylactic mastectomy: a pilot study . Annals of Surgical Oncology 2017. ; 24 ( 1 ): 91-9 . [DOI] [PubMed] [Google Scholar]
Yee 2014 {published data only}
- Yee LM , Wolf M , Mullen R , Bergeron AR , Cooper Bailey S , Levine R , Grobman WA . A randomized trial of a prenatal genetic testing interactive computerized information aid . Prenatal Diagnosis 2014. ; 34 ( 6 ): 552-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2020 {published data only}
- Yu C , Choi D , Bruno BA , Thorpe KE , Straus SE , Cantarutti P , et al. Impact of MyDiabetesPlan, a web-based patient decision aid on decisional conflict, diabetes distress, quality of life, and chronic illness care in patients with diabetes: cluster randomized controlled trial . Journal of Medical Internet Research 2020. ; 22 ( 9 ): e16984 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2021 {published data only}
- Yu CH , Medleg F , Choi D , Spagnuolo CM , Pinnaduwage L , Straus SE , . Integrating shared decision-making into primary care: lessons learned from a multi-centre feasibility randomized controlled trial . BMC Medical Informatics and Decision Making 2021. ; 21 ( 1 ): 323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2011 {published data only}
- Yun YH , Lee MK , Park S , Lee JL , Park J , Choi YS , et al. Use of a decision aid to help caregivers discuss terminal disease status with a family member with cancer: a randomized controlled trial . Journal of Clinical Oncology 2011. ; 29 ( 36 ): 4811-9 . [DOI] [PubMed] [Google Scholar]
Zajac 2012 {published data only}
- Zajac LE . Making Difficult Health Decisions: A Motivated Decision Processing Model [PhD thesis] . Pittsburgh: : University of Pittsburgh; , 2012. . [Google Scholar]
Zapka 2004 {published data only}
- Zapka JG , Lemon SC , Puleo E , Estabrook B , Luckmann R , Erban S . Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination . Annals of Internal Medicine 2004. ; 141 ( 9 ): 683-92 . [DOI] [PubMed] [Google Scholar]
Zhong 2021 {published data only}
- Zhong T , Quong WL , Cheng T , Kerrebijn I , Butler K , Hofer SO , et al. Preconsultation educational group intervention can address the knowledge gap in postmastectomy breast reconstruction . Annals of Plastic Surgery 2021. ; 86 ( 6 ): 695-700 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Zikmund‐Fisher 2008 {published data only}
- Zikmund-Fisher BJ , Ubel PA , Smith DM , Derry HA , McClure JB , Stark A , et al. Communicating side effect risks in a tamoxifen prophylaxis decision aid: the debiasing influence of pictographs . Patient Education and Counseling 2008. ; 73 ( 2 ): 209-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoffman 2012 {published data only}
- Zoffman V , Kirkevold M . Realizing empowerment in difficult diabetes care: a guided self-determination intervention . Qualitative Health Research 2012. ; 22 ( 1 ): 103-18 . [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616001665426 {published data only}
- ACTRN12616001665426. Navigate: Randomised controlled trial of an online treatment decision aid for men with localised prostate cancer and their partners . https://anzctr.org.au/ACTRN12616001665426.aspx (first received 11 October 2016) .
ACTRN12617001246370 {published data only}
- ACTRN12617001246370. Use of an internet-based decision aid (myAID) for ulcerative colitis patients to improve quality of life, empowerment, decision making and disease control [A cluster randomised controlled trial of a decision aid (myAID) for ulcerative colitis patients to enhance patients quality of life, empowerment, quality of decision making and disease control ]. https://anzctr.org.au/ACTRN12617001246370.aspx (first received 19 August 2017) . [DOI] [PMC free article] [PubMed]
ACTRN12618001219279 {published data only}
- ACTRN12618001219279. The Optimise Study - randomised trial of the use of a decision aid to improve informed choice regarding the benefits of low-dose aspirin to prevent cardiovascular disease and colorectal cancer . https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375268 (first received 19 June 2018) .
ACTRN12620001003965 {published data only}
- ACTRN12620001003965. Should I Take Aspirin? The SITA Trial, a randomised controlled trial of a decision aid to support informed choices about taking aspirin to prevent bowel cancer for Australians aged 50 to 70 years [Should I Take Aspirin? The SITA Trial: an RCT of a decision aid to support informed choices about taking aspirin to prevent bowel cancer and other chronic diseases]. https://anzctr.org.au/ACTRN12620001003965.aspx (first received 10 August 2020) .
ACTRN12620001032943 {published data only}
- ACTRN12620001032943. Comparing different information resources on the process and quality of decision making in women considering elective egg freezing [In women considering egg freezing, what is the effect of a Decision Aid plus information, compared to information alone on the process and quality of decision making]. https://anzctr.org.au/ACTRN12620001032943.aspx (first received 11 August 2020) .
ACTRN12621000515897 {published data only}
- ACTRN12621000515897. Evaluating fertility decision aids for younger women with breast cancer [Evaluating the impact of a fertility decision aid developed using health literacy principles, compared to a gold-standard decision aid, on decision-related outcomes in younger women with breast cancer]. https://anzctr.org.au/ACTRN12621000515897.aspx (first received 12 March 2021) .
Al‐Itejawi 2015 {published data only}
- Al-Itejawi HH , Uden-Kraan CF , Vis AN , Nieuwenhuijzen JA , Hofstee MJ , Moorselaar RJ , Verdonck-de Leeuw IM . Development of a patient decision aid for the treatment of localised prostate cancer: a participatory design approach . Journal of Clinical Nursing 2016. ; 25 ( 7-8 ): 1131-44 . [DOI] [PubMed] [Google Scholar]
Aslani 2014a {published data only}
- Aslani A , Tara F , Ghalichi L , Eslami S . The impact of computerized decision aid on mode of delivery - a study protocol . Studies in Health Technology and Informatics 2014. ; 200 : 170-2 . [PubMed] [Google Scholar]
Aslani 2014b {published data only}
- Aslani A , Tara F , Ghalighi L , Pournik O , Ensing S , Abu-Hanna A , et al. Impact of computer-based pregnancy-induced hypertension and diabetes decision aids on empowering pregnant women . Healthcare Informatics Research 2014. ; 20 ( 4 ): 266-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansback 2019 {published data only}
- Bansback N , Trenaman L , MacDonald KV , Hawker G , Johnson JA , Stacey D , et al. An individualized patient-reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial . BMC Musculoskeletal Disorders 2019. ; 20 ( 1 ): 89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Baptista 2020 {published data only}
- Baptista S , Heleno B , Teixeira A , Taylor KL , Martins C . Comparison of explicit values clarification method (VCM), implicit VCM and no VCM decision aids for men considering prostate cancer screening: protocol of a randomized trial . BMC Medical Informatics and Decision Making 2020. ; 20 ( 1 ): 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Beach 2016 {published data only}
- Beach LB , Wild M , Ramachandran G , Ikizler HO , Cavanaugh KL . Protocol of a randomized controlled trial of an erythropoietin stimulating agent decision aid for anemia treatment in kidney disease . BMC Nephrology 2016. ; 17 ( 1 ): 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Benoit 2020 {published data only}
- Benoit A , Grynberg M , Morello R , Sermondade N , Grandazzi G , Moutel G . Does a web-based decision aid improve informed choice for fertility preservation in women with breast cancer (DECISIF)? Study protocol for a randomised controlled trial . BMJ Open 2020. ; 10 ( 2 ): e031739 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Carhuapoma 2021 {published data only}
- Carhuapoma LR , Thayer WM , Elmore CE , Gildersleeve J , Singh T , Shaukat F , et al. Employing a mobile health decision aid to improve decision-making for patients with advanced prostate cancer and their decision partners/proxies: the CHAMPION randomized controlled trial study design . Trials 2021. ; 22 ( 1 ): 631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambers 2008 {published data only}
- Chambers SK , Ferguson M , Gardiner RA , Nicol D , Gordon L , Occhipinti S , et al. ProsCan for men: randomised controlled trial of a decision support intervention for men with localised prostate cancer . BMC Cancer 2008. ; 8 : 207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Columbo 2019 {published data only}
- Columbo JA , Kang R , Spangler EL , Newhall K , Brooke BS , Dosluoglu H , et al. Design of the PReferences for Open Versus Endovascular Repair of Abdominal Aortic Aneurysm (PROVE-AAA) trial . Annals of Vascular Surgery 2019. ; 8 : 247-53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2019/06/019610 {published data only}
- CTRI/2019/06/019610. Effectiveness of Nursing Intervention Module on knowledge, adherence, complications and quality of life among persons receiving oral anticoagulation therapy . http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=34370 (first received 11 June 2019) .
de Molina‐Férnandez 2019 {published data only}
- Molina-Férnandez MI , Raigal-Aran L , la Flor-Lopez M , Prata P , Font-Jimenez I , Valls-Fonayet F , et al. The effectiveness of a digital shared decision-making tool in hormonal contraception during clinical assessment: study protocol of a randomized controlled trial in Spain . BMC Public Health 2019. ; 19 ( 1 ): 1224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00014627 {published data only}
- DRKS00014627. Evaluation of a patient-oriented decision aid and the German healthcare situation in non-metastatic prostate cancer . http://www.drks.de/DRKS00014627 (first received 12 June 2018) .
DRKS00015823 {published data only}
- DRKS00015823. Development and piloting of a decision support tool to support decision making in the context of risk-adapted prevention for patients with pathogenic BRCA1/2 mutations . http://www.drks.de/DRKS00015823 (first received 14 June 2019) .
Geiger 2011 {published data only}
- Geiger F , Liethmann K , Hoffmann F , Paschedag J , Kasper J . Investigating a training supporting Shared Decision Making (IT'S SDM 2011): study protocol for a randomized controlled trial . Trials 2011. ; 12 : 232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20191229045933N1 {published data only}
- IRCT20191229045933N1. Effect of a patient decision aid to select for myopia correction surgery method [Design, implementation, and evaluation of educational intervention through the patient decision making aid (PDA) to select correction method for patients with myopia]. http://en.irct.ir/trial/44617 (first received 19 March 2021) .
ISRCTN17611852 {published data only}
- ISRCTN17611852. The impact of a decision aid on depressed patients' involvement in shared decision-making [The impact of a decision aid on depressed patients' involvement in shared decision-making: a pilot randomized controlled double blind study ]. http://isrctn.com/ISRCTN17611852 (first received 25 November 2014) .
Isselhard 2020 {published data only}
- Isselhard A , Topper M , Berger-Hoger B , Steckelberg A , Fischer H , Vitinius F , et al. Implementation and evaluation of a nurse-led decision-coaching program for healthy breast cancer susceptibility gene (BRCA1/2) mutation carriers: a study protocol for the randomized controlled EDCP-BRCA study . Trials 2020. ; 21 ( 1 ): 501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
JPRN‐UMIN000024811 {published data only}
- JPRN-UMIN000024811. An interventional study to examine the effect of shared decision making in a family on intention of HPV vaccination in order to protect Japanese girls from HPV due to the low coverage of the vaccination and excess of mothers' responsibility to make a decision . https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000028553 (first received 12 November 2016) .
JPRN‐UMIN000032623 {published data only}
- JPRN-UMIN000032623. A randomized controlled trial on decision aid to support the stroke with older people in decision making about location of care at recovery rehabilitation ward: efficacy of decision conflict and participation . https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000036967 (first received 1 October 2019) .
KCT0006945 {published data only}
- KCT0006945. Development of health information communication strategy in response to COVID-19 crisis . https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0006945 (first received 20 January 2022) .
Kim 2020 {published data only}
- Kim AH , Girgis A , Karimi N , Sechi AJ , Descallar J , Andrews JM , et al. A web-based decision aid (myAID) to enhance quality of life, empowerment, decision making, and disease control for patients with ulcerative colitis: protocol for a cluster randomized controlled trial . JMIR Research Protocols 2020. ; 9 ( 7 ): e15994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Lange 2021 {published data only}
- Lange T , Deckert S , Beyer F , Hahn W , Einhart N , Roessler M , et al. An individualized decision aid for physicians and patients for total knee replacement in osteoarthritis (Value-based TKR study): study protocol for a multi-center, stepped wedge, cluster randomized controlled trial . BMC Musculoskeletal Disorders 2021. ; 22 ( 1 ): 783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Layton 2011 {published data only}
- Layton B . Effects of a Web-Based Decision Aid on African American Men's Prostate Screening Knowledge and Behavior [Doctoral Thesis] . Minneapolis (USA) : Walden Dissertations and Doctoral Studies; , 2011. . [Google Scholar]
Lin E 2022 {published data only}
- Lin E , Uhler LM , Finley EP , Jayakumar P , Rathouz PJ , Bozic KJ , et al. Incorporating patient-reported outcomes into shared decision-making in the management of patients with osteoarthritis of the knee: a hybrid effectiveness-implementation study protocol . BMJ Open 2022. ; 12 ( 2 ): e055933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 2012 {published data only}
- Mann DM , Lin JJ . Increasing efficacy of primary care-based counseling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial . Implementation Science 2012. ; 7 : 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00813033 {published data only}
- NCT00813033. Use of a Patient Decision Aid for Gastrologic Endoscopy in a Pediatric Setting [Creation and Pilot Evaluation of a Patient Decision Aid as an Adjunct to the Consenting Process for Gastrointestinal Endoscopy in a Pediatric Setting]. https://clinicaltrials.gov/show/NCT00813033 (first received 19 December 2008) .
NCT01152307 {published data only}
- NCT01152307. Measuring quality of decisions about treatment of depression [Measuring quality of decisions about treatment of depression]. clinicaltrials.gov/show/NCT01152307 (first received 22 June 2010) .
NCT01976325 {published data only}
- NCT01976325. Evaluating the Ottawa Malaria Decision Aid (OMDA) [Incorporation of the 'Ottawa Malaria Decision Aid' into the pre-travel consultation process: assessment of travelers' knowledge, decisional conflict, preparation for decision-making and medication adherence compared to standard care]. clinicaltrials.gov/show/NCT01976325 (first received 29 October 2013) .
NCT02027545 {published data only}
- NCT02027545. Promoting veteran-centered colorectal cancer screening (PROM-IS) . https://clinicaltrials.gov/ct2/show/record/NCT02027545 (first received 18 December 2013) .
NCT02084290 {published data only}
- NCT02084290. Evaluating a shared decision making program for Crohn's disease [Evaluating a prediction tool and decision aid for patients with Crohn's disease]. clinicaltrials.gov/show/NCT02084290 (first received 29 January 2014) .
NCT02107794 {published data only}
- NCT02107794. Shared decision making in graves disease - graves disease (GD) choice . https://clinicaltrials.gov/ct2/show/record/NCT02107794 (first received 24 March 2014) .
NCT02145481 {published data only}
- NCT02145481. Decisional quality for patients with coronary artery disease (DeQCAD) . clinicaltrials.gov/show/NCT02145481 (first received 15 May 2014) .
NCT02259699 {published data only}
- NCT02259699. Ovarian cancer patient-centered decision aid (PCOA) [Ovarian cancer patient-centered decision aid]. clinicaltrials.gov/show/NCT02259699 (first received 15 May 2014) .
NCT02364128 {published data only}
- NCT02364128. Improving patient decisions about bariatric surgery . https://clinicaltrials.gov/ct2/show/record/NCT02364128 (first received 22 January 2015) .
NCT02488603 {published data only}
- NCT02488603. Decision aids for tamoxifen treatment in breast cancer patients [Utilization of Decision aids for tamoxifen treatment in breast cancer patients: a randomized controlled trial]. clinicaltrials.gov/show/NCT02488603 (first received 25 June 2015) .
NCT02503553 {published data only}
- NCT02503553. Decision aids in cerebral aneurysm treatment . clinicaltrials.gov/show/NCT02503553 (first received 17 July 2015) .
NCT02540044 {published data only}
- NCT02540044. Supporting Patient care with Electronic Resource (SuPER) (SuPER) [Supporting Patient care with Electronic Resource (SuPER): efficacy of an online decision aid for patients considering biologic therapy for rheumatoid arthritis]. clinicaltrials.gov/show/NCT02540044 (first received 1 September 2015) .
NCT02611050 {published data only}
- NCT02611050. Treatment decisions for multi-vessel CAD [Treatment decisions for multi-vessel coronary artery disease patients]. clinicaltrials.gov/show/NCT02611050 (first received 9 November 2015) .
NCT02759939 {published data only}
- NCT02759939. Right for me: birth control decisions made easier . https://clinicaltrials.gov/ct2/show/record/NCT02759939 (first received 3 February 2016) .
NCT02823262 {published data only}
- NCT02823262. A breast cancer treatment decision aid for women aged 70 and older . https://clinicaltrials.gov/ct2/show/record/NCT02823262 (first received 29 June 2016) .
NCT02914197 {published data only}
- NCT02914197. Giving information on the risks and limitations of mammography screening (GIRLS) . https://clinicaltrials.gov/ct2/show/record/NCT02914197 (first received 22 September 2016) .
NCT02963584 {published data only}
- NCT02963584. Decision aid in chronic total occlusion (CTO) patients [A pilot randomized trial of a decision aid in CTO patients]. https://clinicaltrials.gov/ct2/show/record/NCT02963584 (first received 27 October 2016) .
NCT03088397 {published data only}
- NCT03088397. Effectiveness of a patient decision aid in immediate postpartum contraceptive counseling . https://clinicaltrials.gov/ct2/show/record/NCT03088397 (first received 7 September 2016) .
NCT03099746 {published data only}
- NCT03099746. Decision support among surrogate decision makers of the chronically critically ill (INVOLVE) [A clinical trial of decision support for end of life care among surrogate decision makers of the chronically critically ill ]. https://clinicaltrials.gov/ct2/show/record/NCT03099746 (first received 22 February 2017) .
NCT03141437 {published data only}
- NCT03141437. Decision aid website in helping to make decisions about fertility in participants with cancer [Patient-centered decision counseling for women at risk of cancer-related infertility: efficacy study and comparative-effectiveness randomized trial]. https://clinicaltrials.gov/ct2/show/record/NCT03141437 (first received 3 May 2017) .
NCT03244202 {published data only}
- NCT03244202. Evaluation of a decision aid for incidental genomic findings [Randomized controlled trial of a decision aid for incidental genomic findings]. https://clinicaltrials.gov/ct2/show/record/NCT03244202 (first received 6 August 2017) .
NCT03282097 {published data only}
- NCT03282097. Decisions about cancer screening in Alzheimer's disease . https://clinicaltrials.gov/ct2/show/record/NCT03282097 (first received 11 September 2017) .
NCT03374891 {published data only}
- NCT03374891. A multicenter trial of a shared decision support intervention for patients offered implantable cardioverter-defibrillators [DECIDE - ICD: a multicenter trial of a shared decision support intervention for patients offered implantable cardioverter-defibrillators]. https://clinicaltrials.gov/ct2/show/record/NCT03374891 (first received 29 November 2017) . [DOI] [PMC free article] [PubMed]
NCT03454022 {published data only}
- NCT03454022. Decision-aid for renal therapy pilot trial [Decision-aid for renal therapy pilot trial (DART pilot trial) ]. https://clinicaltrials.gov/ct2/show/record/NCT03454022 (first received 16 January 2018) .
NCT03477591 {published data only}
- NCT03477591. Evaluating the impact of evidence-based information about PSA testing on prostate cancer screening decisions [McMaster choices study: evaluating the impact of evidence-based information about PSA testing on prostate cancer screening decisions]. https://clinicaltrials.gov/ct2/show/record/NCT03477591 (first received 12 March 2018) .
NCT03500952 {published data only}
- NCT03500952. Family planning ahead . https://clinicaltrials.gov/ct2/show/record/NCT03500952 (first received 10 April 2018) .
NCT03522740 {published data only}
- NCT03522740. Decision aid for renal therapy (DART) [Decision aid for renal therapy: promoting knowledge and autonomy in chronic kidney disease patients and their care-partners]. https://clinicaltrials.gov/ct2/show/record/NCT03522740 (first received 1 May 2018) .
NCT03578211 {published data only}
- NCT03578211. Impact of decision aids on bariatric surgery choice: a randomized controlled trial . https://clinicaltrials.gov/ct2/show/record/NCT03578211 (first received 25 June 2018) .
NCT03631758 {published data only}
- NCT03631758. Evaluating the impact of evidence-based information about mammography on breast cancer screening decisions [McMaster choices study: evaluating the impact of cancer screening patient decision aids on breast cancer screening decisions]. https://clinicaltrials.gov/ct2/show/record/NCT03631758 (first received 13 August 2018) .
NCT03766009 {published data only}
- NCT03766009. Increasing patients' engagement in breast cancer surgery decision-making [Increasing socioeconomically disadvantaged patients' engagement in breast cancer surgery decision making through a shared decision making intervention]. https://clinicaltrials.gov/ct2/show/record/NCT03766009 (first received 30 November 2018) . [DOI] [PMC free article] [PubMed]
NCT03791138 {published data only}
- NCT03791138. The impact of a web-based patient decision aid for women considering breast reconstruction [The impact of a web-based decision aid for women considering breast reconstruction: a randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT03791138 (first received 17 December 2018) .
NCT03834532 {published data only}
- NCT03834532. Living well after breast surgery . https://clinicaltrials.gov/ct2/show/record/NCT03834532 (first received 28 January 2019) .
NCT03884387 {published data only}
- NCT03884387. The use of a patient decision aid in the choice of surgery for herniated disc [The use of a decision aid in the choice of surgery for herniated disc]. https://clinicaltrials.gov/ct2/show/record/NCT03884387 (first received 3 January 2019) .
NCT03905369 {published data only}
- NCT03905369. Focus on values to stimulate shared decisions [Focus on values to stimulate shared decisions in patients with thyroid cancer: a multifaceted communication booster (COMBO)]. https://clinicaltrials.gov/ct2/show/record/NCT03905369 (first received 21 March 2019) .
NCT03921437 {published data only}
- NCT03921437. Decision support for the renal replacement therapy with end-stage renal disease [The efficacy of a decision support intervention on reducing conflict and improving satisfaction in making the renal replacement therapy decision among patients with end-stage renal disease]. https://clinicaltrials.gov/ct2/show/record/NCT03921437 (first received 16 April 2019) .
NCT03995381 {published data only}
- NCT03995381. Using decision aids to reducing decision conflict in angiography patients for choosing hemostasis: a randomized controlled trial . https://clinicaltrials.gov/ct2/show/record/NCT03995381 (first received 21 June 2019) .
NCT04076332 {published data only}
- NCT04076332. How "shared decision making decision-aid" help patients with obstructive sleep apnea to choose treatment plan . https://clinicaltrials.gov/ct2/show/record/NCT04076332 (first received 28 June 2019) .
NCT04097717 {published data only}
- NCT04097717. "My Decision" tubal sterilization decision support tool [Developing and testing a decision support tool for women making tubal sterilization decisions]. https://clinicaltrials.gov/ct2/show/record/NCT04097717 (first received 19 September 2019) .
NCT04101409 {published data only}
- NCT04101409. Impact of shared decision-making with decision aids on acoustic neuroma treatment choice: a randomized controlled trial . https://clinicaltrials.gov/ct2/show/record/NCT04101409 (first received 23 January 2019) .
NCT04103931 {published data only}
- NCT04103931. Impact of a patient decision aid for treatment of aortic stenosis [Pilot randomized trial of decision aid for treatment of aortic stenosis]. https://clinicaltrials.gov/ct2/show/record/NCT04103931 (first received 24 September 2019) .
NCT04122989 {published data only}
- NCT04122989. Validation of a shared decision-making tool for multiple sclerosis [Validation of a shared decision-making tool, ms-support, to improve decisions about disease modifying therapies (DMT) for multiple sclerosis]. https://clinicaltrials.gov/ct2/show/record/NCT04122989 (first received 8 October 2019) .
NCT04175366 {published data only}
- NCT04175366. Shared decision making in psychiatric inpatient care [Shared decision making in psychiatric inpatient care to enhance patient participation]. https://clinicaltrials.gov/ct2/show/record/NCT04175366 (first received 14 November 2019) .
NCT04177628 {published data only}
- NCT04177628. Shared decision making with breast cancer patients [Shared decision making with breast cancer patients offered adjuvant radiotherapy]. https://clinicaltrials.gov/ct2/show/record/NCT04177628 (first received 20 November 2019) .
NCT04240717 {published data only}
- NCT04240717. Shared decision making on immunotherapy in oncology [Shared decision making on immunotherapy in oncology - prospective, randomized, controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT04240717 (first received 23 December 2019) .
NCT04241978 {published data only}
- NCT04241978. Development and evaluation of a web based decision aid for patients with hip osteoarthritis [The effectiveness of a web-based decision aid for patients with hip osteoarthritis: study protocol for a randomized controlled trial ]. https://clinicaltrials.gov/ct2/show/record/NCT04241978 (first received 22 January 2020) .
NCT04260737 {published data only}
- NCT04260737. Interactive decision aid for men diagnosed with prostate cancer [Developing and testing an interactive decision aid for newly diagnosed prostate cancer patients]. https://clinicaltrials.gov/ct2/show/record/NCT04260737 (first received 8 January 2020) .
NCT04270630 {published data only}
- NCT04270630. A pilot proof of concept, randomized controlled, single-center study of a decision aid tool for older patients considering LHC as treatment for NSTEMI [A pilot proof of concept, randomized controlled, single-center study of a decision aid tool for older patients (age ≥75) considering left heart catheterization as treatment for non-st elevation myocardial infarction]. https://clinicaltrials.gov/ct2/show/record/NCT04270630 (first received 13 February 2020) .
NCT04272177 {published data only}
- NCT04272177. Influence of shared-decision making in reducing decision conflict on the choice of awakening agent after general anesthesia [Influence of shared-decision making in reducing decision conflict on the choice of awakening agent after general anesthesia: a multicenter randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT04272177 (first received 13 February 2020) .
NCT04291040 {published data only}
- NCT04291040. Use of an educational multimedia tool versus routine care for the uptake of postpartum LARC in high-risk pregnancies (SUSTAIN) . https://clinicaltrials.gov/study/NCT04291040 (first received 27 February 2020) .
NCT04357288 {published data only}
- NCT04357288. Randomized evaluation of decision support interventions for atrial fibrillation . https://clinicaltrials.gov/ct2/show/record/NCT04357288 (first received 13 April 2020) .
NCT04364958 {published data only}
- NCT04364958. Decision aid for patients with generalized anxiety disorder: protocol for a randomized controlled trial [The effectiveness of a web-based decision aid for patients with generalized anxiety disorder: protocol for a randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT04364958 (first received 24 April 2020) .
NCT04373590 {published data only}
- NCT04373590. Decision-making and decision support among emerging adults with first episode psychosis [Mental healthcare decision-making and decision support among emerging adults enrolled in coordinated specialty care for early psychosis]. https://clinicaltrials.gov/ct2/show/record/NCT04373590 (first received 29 April 2020) .
NCT04378816 {published data only}
- NCT04378816. A Patient-centered Continuous and Interdisciplinary Shared Decision Making Approach for Breast Cancer Rehabilitation . https://clinicaltrials.gov/study/NCT04378816 (first received 5 May 2020) .
NCT04397016 {published data only}
- NCT04397016. Cost talk: discussing cancer care costs [Cost talk: a randomized stepped wedge trial of interventions helping patients discuss cancer care costs with clinicians during shared decision making]. https://clinicaltrials.gov/ct2/show/record/NCT04397016 (first received 11 May 2020) .
NCT04410029 {published data only}
- NCT04410029. Evaluation of a decision aid for early pregnancy loss [Evaluation of a decision aid for early pregnancy loss: a pilot RCT study]. https://clinicaltrials.gov/ct2/show/record/NCT04410029 (first received 18 May 2020) .
NCT04437069 {published data only}
- NCT04437069. Improving patient and family health using family-centered outcomes and shared decision-making . https://clinicaltrials.gov/ct2/show/record/NCT04437069 (first received 29 May 2020) .
NCT04496739 {published data only}
- NCT04496739. Making informed choices on incorporating chemoprevention into care (MiCHOICE) [Cluster randomized controlled trial of patient and provider decision support to increase chemoprevention informed choice among women with atypical hyperplasia or lobular carcinoma in situ - making informed choices on incorporating chemoprevention into care (MiChoice) ]. https://clinicaltrials.gov/ct2/show/record/NCT04496739 (first received 29 July 2020) .
NCT04504084 {published data only}
- NCT04504084. Influence of patient decision-making aids for patients with unilateral ureteral stone: a randomized-controlled trial . https://clinicaltrials.gov/ct2/show/record/NCT04504084 (first received 5 August 2020) .
NCT04548531 {published data only}
- NCT04548531. Engaging patients in colon cancer screening decisions during COVID-19 . https://clinicaltrials.gov/study/NCT04548531 (first received 9 September 2020) .
NCT04549571 {published data only}
- NCT04549571. Improving patient-centered communication in breast cancer through patient and provider interventions . https://www.clinicaltrials.gov/study/NCT04549571 (first received 9 September 2020) .
NCT04584294 {published data only}
- NCT04584294. Patient-centered reproductive decision support tool for women veterans [MyPath: a patient-centered web-based intervention to improve reproductive planning for women veterans]. https://clinicaltrials.gov/ct2/show/record/NCT04584294 (first received 5 October 2020) .
NCT04621760 {published data only}
- NCT04621760. The OPENS trial: offering women PrEP (Aim 1) [Offering women PrEP with education and shared decision-making ]. https://clinicaltrials.gov/ct2/show/record/NCT04621760 (first received 9 October 2020) .
NCT04692987 {published data only}
- NCT04692987. Effectiveness of decision-aid video on colorectal cancer screening [Effectiveness of decision-aid video on colorectal cancer screening in primary healthcare setting: a randomized controlled trial ]. https://clinicaltrials.gov/ct2/show/record/NCT04692987 (first received 22 December 2020) .
NCT04725565 {published data only}
- NCT04725565. Genetics adviser: evaluating a digital decision support tool for genetic results . https://clinicaltrials.gov/ct2/show/record/NCT04725565 (first received 13 January 2021) .
NCT04741503 {published data only}
- NCT04741503. Project Insight: feasibility of a breast cancer screening decision support tool . https://clinicaltrials.gov/ct2/show/record/NCT04741503 (first received 25 January 2021) .
NCT04748380 {published data only}
- NCT04748380. Shared decision-making and colorectal cancer screening . https://www.clinicaltrials.gov/study/NCT04748380 (first received 10 February 2021) .
NCT04805554 {published data only}
- NCT04805554. Incorporating patient-reported outcomes into shared decision making with patients with osteoarthritis of the hip or knee . https://www.clinicaltrials.gov/study/NCT04805554 (first received 15 March 2021) .
NCT04837053 {published data only}
- NCT04837053. Implementation of indication criteria for total knee replacement in osteoarthritis (Value-based TKR) . https://www.clinicaltrials.gov/study/NCT04837053 (first received 22 February 2021) .
NCT04858282 {published data only}
- NCT04858282. Application-enabled shared decision-making . https://clinicaltrials.gov/ct2/show/record/NCT04858282 (first received 16 April 2021) .
NCT04869917 {published data only}
- NCT04869917. Behavioral nudges for diabetes prevention (BEGIN) trial in primary care . https://www.clinicaltrials.gov/study/NCT04869917 (first received 28 April 2021) .
NCT04879745 {published data only}
- NCT04879745. MyVoice:Rheum decision aid for women with rheumatic diseases [A pilot study of the MyVoice:Rheum decision aid to address the reproductive health needs of women with rheumatic diseases]. https://clinicaltrials.gov/ct2/show/record/NCT04879745 (first received 4 May 2021) .
NCT04919941 {published data only}
- NCT04919941. A pilot study of a guide to conservative care . https://clinicaltrials.gov/ct2/show/record/NCT04919941 (first received 28 May 2021) .
NCT04940936 {published data only}
- NCT04940936. Shared decision making on radiation dose for lung malignancies . https://www.clinicaltrials.gov/study/NCT04940936 (first received 11 June 2021) .
NCT04946279 {published data only}
- NCT04946279. Decision aid for the improvement of decision-making in patients with non-small cell lung cancer [Improving decision-making encounters in lung cancer (I DECide): a low-literacy conversation tool]. https://clinicaltrials.gov/ct2/show/record/NCT04946279 (first received 23 June 2021) .
NCT04948983 {published data only}
- NCT04948983. The effect of a patient decision aids for breast cancer screening [Patient decision aids for women facing the decision of breast cancer screening in the public health sector]. https://clinicaltrials.gov/ct2/show/record/NCT04948983 (first received 7 June 2021) .
NCT04956978 {published data only}
- NCT04956978. Shared decision making to address racial disparities in oral anticoagulation in NVAF [Shared decision making to address racial disparities in oral anticoagulation use in patients with non-valvular atrial fibrillation ]. https://clinicaltrials.gov/ct2/show/record/NCT04956978 (first received 25 June 2021) .
NCT05033067 {published data only}
- NCT05033067. The personal patient profile decision support for patients with bladder cancer . https://clinicaltrials.gov/ct2/show/record/NCT05033067 (first received 27 August 2021) .
NCT05091944 {published data only}
- NCT05091944. An interactive web-based birth decision aid for shared decision making [An interactive web-based decision aid for shared decision making: birth choice after cesarean in Taiwan]. https://clinicaltrials.gov/ct2/show/record/NCT05091944 (first received 14 September 2021) .
NCT05130580 {published data only}
- NCT05130580. Patient-specific decision aid system for shared decision making about breast reconstruction [Evaluation of a patient-specific decision aid system for shared decision-making about breast reconstruction ]. https://clinicaltrials.gov/ct2/show/record/NCT05130580 (first received 9 September 2021) .
NCT05135156 {published data only}
- NCT05135156. Lung transplant READY pilot study [Lung transplant resources for education and decision-making for your cystic fibrosis (READY): a pilot randomized controlled trial ]. https://clinicaltrials.gov/ct2/show/record/NCT05135156 (first received 22 November 2021) .
NCT05177783 {published data only}
- NCT05177783. Contraception decision aid use and patient outcomes . https://clinicaltrials.gov/ct2/show/record/NCT05177783 (first received 10 December 2021) .
NCT05182008 {published data only}
- NCT05182008. A patient decision aid for method of early abortion: a randomized control trial . https://clinicaltrials.gov/ct2/show/record/NCT05182008 (first received 12 December 2021) .
NCT05219786 {published data only}
- NCT05219786. Online field test of an appendicitis decision support tool . https://clinicaltrials.gov/ct2/show/record/NCT05219786 (first received 8 November 2021) .
NL7939 {published data only}
- NL7939. Decision aid for breast reconstruction after mastectomy: a randomized controlled trial . https://trialsearch.who.int/Trial2.aspx?TrialID=NL7939 (first received 6 August 2019) .
NL9666 {published data only}
- NL9666. RCT for evaluation of a personalized online decision aid for colorectal cancer screening participation [Development and evaluation of a personalized online decision aid for colorectal cancer screening participation: a randomized controlled trial ]. https://trialsearch.who.int/Trial2.aspx?TrialID=NL9666 (first received 9 August 2021) .
NTR4435 {published data only}
- NTR4435. Improving patient involvement in the decision for joint replacement surgery, using decision aids . https://trialsearch.who.int/Trial2.aspx?TrialID=NTR4435 (first received 2 February 2014) .
NTR5467 {published data only}
- NTR5467. Decision-support for couples with hereditary cancer and child wish: weighing pros and cons of reproductive options regarding transmission of gene mutations . https://trialsearch.who.int/Trial2.aspx?TrialID=NTR5467 (first received 21 October 2015) .
NTR5785 {published data only}
- NTR5785. Effect of a decision aid about postoperative epidural analgesia on patients' knowledge: a randomized controlled trial . https://trialsearch.who.int/Trial2.aspx?TrialID=NTR5785 (first received 17 February 2016) .
NTR6070 {published data only}
- NTR6070. Study on shared decision making in choosing a treatment for pelvic organ prolapse [Shared decision making in pelvic organ prolapse - a cluster randomized controlled trial on use of the decision aid]. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR6070 (first received 30 October 2016) .
NTR6379 {published data only}
- NTR6379. Shared decision making in patients with castration-resistant prostate cancer . https://trialsearch.who.int/Trial2.aspx?TrialID=NTR6379 (first received 20 April 2017) .
O'Connor 2019 {published data only}
- O'Connor D , Hoffmann T , McCaffery K , Maher C , Harris I , Glasziou P , et al. Evaluating a patient decision aid for people with degenerative knee disease considering arthroscopic surgery: protocol for a randomised controlled trial . BMJ Evidence-Based Medicine 2019. ; 24 : A48 . [Google Scholar]
Patzer 2019 {published data only}
- Patzer RE , McPherson L , Redmond N , DuBay D , Zayas C , Hartmann E , et al. A culturally sensitive web-based intervention to improve living donor kidney transplant among African Americans . Kidney International Reports 2019. ; 4 ( 9 ): 1285-95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahn 2021 {published data only}
- Rahn AC , Wenzel L , Icks A , Stahmann A , Scheiderbauer J , Grentzenberg K , et al. Evaluation of an interactive web-based programme on relapse management for people with multiple sclerosis (POWER@MS2): study protocol for a process evaluation accompanying a randomised controlled trial . Trials 2021. ; 22 ( 1 ): 139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Rieckert 2019 {published data only}
- Rieckert A , Becker A , Donner-Banzhof N , Viniol A , Bucker B , Wilm S , et al. Reduction of the long-term use of proton pump inhibitors by a patient-oriented electronic decision support tool (arriba-PPI): study protocol for a randomized controlled trial . Trials 2019. ; 20 ( 1 ): 636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Samalin 2018 {published data only}
- Samalin L , Honciuc M , Boyer L , Chazeron I , Blanc O , Abbar M , et al. Efficacy of shared decision-making on treatment adherence of patients with bipolar disorder: a cluster randomized trial (ShareD-BD) . BMC Psychiatry 2018. ; 18 ( 1 ): 103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoenfeld 2021 {published data only}
- Schoenfeld EM , Poronsky KE , Westafer LM , DiFronzo BM , Visintainer P , Scales CD , et al. Feasibility and efficacy of a decision aid for emergency department patients with suspected ureterolithiasis: protocol for an adaptive randomized controlled trial . Trials 2021. ; 22 ( 1 ): 201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alonso Coello 2022
- Alonso Coello P , Álvarez Pérez Y , Bono Vega M , Gavín Benavent P , Perestelo Pérez L , Prieto Remón L , et al. Aplicación de las recomendaciones de las guías de práctica clínica a la toma de decisiones compartida . https://portal.guiasalud.es/aplicacion-de-las-recomendaciones-de-las-guias-de-practica-clinica-a-la-toma-de-decisiones-compartida-manual-metodologico/ 2022.
Bachtiger 2021
- Bachtiger P , Adamson A , Chow JJ , Sisodia R , Quint JK , Peters NS . The impact of the COVID-19 pandemic on the uptake of influenza vaccine: UK-wide observational study . JMIR Public Health and Surveillance 2021. ; 7 ( 4 ): e26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bekker 2003
- Bekker HL , Legare F , Stacey D , O'Connor A , Lemyre L . Is anxiety a suitable measure of decision aid effectiveness: a systematic review . Patient Education and Counselling 2003. ; 50 ( 3 ): 255-62. [DOI] [PubMed] [Google Scholar]
Bennett 2010
- Bennett C , Graham ID , Kristjansson E , Kearing SA , Clay KF , O'Connor AM . Validation of a preparation for decision making scale . Patient Education and Counseling 2010. ; 78 ( 1 ): 130-3. [DOI] [PubMed] [Google Scholar]
Bowen 2013
- Bowen SJ , Graham ID . From knowledge translation to engaged scholarship: promoting research relevance and utilization . Archives of Physical Medicine and Rehabilitation 2013. ; 94 ( 1 Suppl ): S3-8. [DOI] [PubMed] [Google Scholar]
Bravo 2022
- Bravo P , Härter M , McCaffery K , Giguère A , Hahlweg P , Elwyn G . Editorial: 20 years after the start of international Shared Decision-Making activities: Is it time to celebrate? Probably… . Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2022. ; 171 : 1-4. [DOI] [PubMed] [Google Scholar]
Brehaut 2003
- Brehaut JC , O'Connor AM , Wood TJ , Hack TF , Siminoff L , Gordon E , et al. Validation of a decision regret scale . Medical Decision Making 2003. ; 23 ( 4 ): 281-92. [DOI] [PubMed] [Google Scholar]
Brouwers 2010
- Brouwers M , Stacey D , O’Connor A . Knowledge creation: synthesis, tools and products . CMAJ 2010. ; 182 ( 2 ): E68-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015
- Brown JG , Joyce KE , Stacey D , Thomson MD . Patients or volunteers? The impact of motivation for trial participation on the efficacy of patient decision aids: a secondary analysis of a Cochrane Systematic Review . Medical Decision Making 2015. ; 35 ( 4 ): 419-35. [DOI] [PubMed] [Google Scholar]
CDSR 2022
- Cochrane Database of Systematic Reviews . 2021 Impact Report (corrected November 2022) . https://www.cochranelibrary.com/documents/20182/141048232/CDSR+-+2021+Impact+Report_Nov22.pdf/8a5495b9-6551-4557-42a7-876b677869c6 2022.
CIHR 2015
- Canadian Institutes of Health Research . Guide to Knowledge Translation Planning at CIHR: Integrated and End-of-Grant Approaches . https://cihr-irsc.gc.ca/e/45321.html 2015.
Covidence 2022 [Computer program]
- Covidence systematic review software . Melbourne, Australia: Veritas Health Innovation , accessed 21 March 2017. Available at www.covidence.org.
Coyne 2013
- Coyne I , O'Mathuna DP , Gibson F , Shields L , Sheaf G . Interventions for promoting participation in shared decision-making for children with cancer . Cochrane Database of Systematic Reviews 2013. , Issue 6 . Art. No: CD008970. [DOI: 10.1002/14651858.CD008970.pub2 ] [DOI] [PubMed] [Google Scholar]
Dahl Steffensen 2022
- Dahl Steffensen K , Mølri Knudsen B , Finderup J , Willemann Würgler M , Olling K . Implementation of patient-centred care in Denmark: the way forward with shared decision-making . Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2022. ; 171 : 36-41. [DOI] [PubMed] [Google Scholar]
Davidson 2022
- US Preventive Services Task Force . Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations . JAMA 2022. ; 327 ( 12 ): 1171-6. [DOI] [PubMed] [Google Scholar]
Degner 1992
- Degner LF , Sloan JA . Decision making during serious illness: what role do patients really want to play . Journal of Clinical Epidemiology 1992. ; 45 ( 9 ): 941-50. [DOI] [PubMed] [Google Scholar]
Degner 1997
- Degner LF , Sloan JA , Venkatesh P . The Control Preferences Scale . Canadian Journal of Nursing Research 1997. ; 29 ( 3 ): 21-43. [PubMed] [Google Scholar]
Duncan 2010
- Duncan E , Best C , Hagen S . Shared decision making interventions for people with mental health conditions . Cochrane Database of Systematic Reviews 2010. , Issue 1 . Art. No: CD007297. [DOI: 10.1002/14651858.CD007297.pub2 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durand 2008
- Durand MA , Stiel M , Boivin J , Elwyn G . Where is the theory? Evaluating the theoretical frameworks described in decision support technologies . Patient Education and Counseling 2008. ; 71 ( 1 ): 125-35. [DOI] [PubMed] [Google Scholar]
Durand 2014
- Durand MA , Carpenter L , Dolan H , Bravo P , Mann M , Bunn F , et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis . PLOS One 2014. ; 9 ( 4 ): 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2003
- Edwards A , Elwyn G , Hood K , Robling M , Atwell C , Holmes-Rovner M , et al. The development of COMRADE--a patient-based outcome measure to evaluate the effectiveness of risk communication and treatment decision making in consultations . Patient Education and Counseling 2003. ; 50 ( 3 ): 311-22. [DOI] [PubMed] [Google Scholar]
Elwyn 2005
- Elwyn G , Hutchings H , Edwards A , Rapport F , Wensing M , Cheung WY , et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks . Health Expectations 2005. ; 8 ( 1 ): 34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2006
- Elwyn G , O’Connor A , Stacey D , Volk R , Edwards A , Coulter A , et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process . BMJ 2006. ; 333 ( 7565 ): 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2013
- Elwyn G , I Scholl, Tietbohl C , Mann M , Edwards AGK , Clay C et al. "Many miles to go...": A systematic review of the implementation of patient decision support interventions into routine clinical practice . BMC: Medical Informatics and Decision Making 2013. ; 13 ( Suppl 2 ): S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2013b
- Elwyn G , Barr PJ , Grande SW , Thompson R , Walsh T , Ozanne EM . Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters . Patient Education and Counseling 2013. ; 93 ( 1 ): 102-7. [DOI] [PubMed] [Google Scholar]
Fergusson 2018
- Fergusson D , Monfaredi Z , Pussegoda K , Garritty C , Lyddiatt A , Shea B , et al. The prevalence of patient engagement in published trials: a systematic review . Res Involv Engagem 2018. ; 4 : 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagnon 2009
- Gagnon M . Knowledge dissemination and exchange of knowledge . In: Straus S , Tetroe J , Graham ID , editors(s). Knowledge Translation in Health Care: Moving from Evidence to Practice . Oxford: : Blackwell Publishing Ltd; , 2009. : 235-45. [Google Scholar]
Geiger 2022
- Geiger F . Hospital-wide implementation of SDM increased SDM level, cost effectiveness and patient safety . In: International Shared Decision Making Society Conference . 2022.
Gentles 2013
- Gentles SJ , Stacey D , Bennett C , Alshurafa M , Walter SD . Factors explaining the heterogeneity of effects of patient decision aids on knowledge of outcome probabilities: a systematic review sub-analysis . Systematic Reviews 2013. ; 2 : 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gogovor 2022
- Gogovor A , Fakhfakh M , Asmaou Bouba D , Acakpo O , Ayivi-Vinz G , Musabyimana A , et al. Shared decision-making and person-centred care approaches in three African regions . Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2022. ; 171 : 6-10. [DOI] [PubMed] [Google Scholar]
Grabinski 2018
- Grabinski VF , Myckatyn TM , Lee CN , Philpott-Streiff SE , Politi MC . Importance of shared decision-making for vulnerable populations: examples from postmastectomy breast reconstruction . Health Equity 2018. ; 2 ( 1 ): 234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT . Version accessed 21 March 2017. Hamilton (ON) : McMaster University (developed by Evidence Prime) . Available at gradepro.org.
Hartling 2012
- Hartling L , Hamm M , Milne A , Vandermeer B , Santaguida PL , Ansari M , et al. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments [Internet]. Appendix B, Guidelines for Risk of Bias Assessments. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92290/ . Rockville (MD) : Agency for Healthcare Research and Quality (US) , 2012. [PubMed] [Google Scholar]
Helsedirektoratet Norway 2017
- Helsedirektoratet Norway. Nasjonale kvalitetskrav tilsamvalgsverktøy som skal publiserespå helsenorge.no . https://www.helsedirektoratet.no/tema/pasient-og-brukerrettighetsloven/nasjonale-kvalitetskrav-til-samvalgsverktoy-som-skal-publiseres-pa-helsenorge.no?tidligere-versjoner#257923 2017.
Hibbard 1997
- HIbbard JH , Slovic P , Jewett JJ . Informing consumer decisions in health care: Implications from decision-making research . Milbank Quarterly 1997. ; 75 ( 3 ): 395-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbard 2013
- Hibbard JH , Greene J . What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs . Health Affairs 2013. ; 32 ( 2 ): 207-14. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT , Altman DG , Sterne JAC (editors) . Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 . Available from www.handbook.cochrane.org.
Higgins 2022
- Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , Welch VA (editors) . Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022 . Available from www.training.cochrane.org/handbook.
Hollinghurst 2010
- Hollinghurst S , Emmett C , Peters TJ , Watson H , Fahey T , Murphy DJ , et al. Economic evaluation of the DIAMOND randomized trial: cost and outcomes of 2 decision aids for mode of delivery among women with previous caesarian section . BMJ 2010. ; 30 : 453-63. [DOI] [PubMed] [Google Scholar]
Horowitz 1979
- Horowitz M , Wilner N , Alvarez W . Impact of Event Scale: a measure of subjective stress . Psychosomatic Medicine 1979. ; 41 : 209-18. [DOI] [PubMed] [Google Scholar]
Housten 2019
- Housten AJ , Lowenstein LM , Hoffman A , Jacobs LE , Zirari Z , Hoover DS , et al. A review of the presentation of overdiagnosis in cancer screening patient decision aids . MDM Policy & Practice 2019. ; 4 ( 2 ): 2381468319881447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2018
- Hughes TM , Merath K , Chen Q , Sun S , Palmer E , Idrees JJ , et al. Association of shared decision-making on patient-reported health outcomes and healthcare utilization . American Journal of Surgery 2018. ; 216 ( 1 ): 7-12. [DOI] [PubMed] [Google Scholar]
IPDAS 2005a
- International Patient Decision Aid Standards Collaboration . Background Document. 2005 . ipdas.ohri.ca/IPDAS_Background.pdf (accessed 29 Oct 2013).
IPDAS 2005b
- International Patient Decision Aid Standards Collaboration . IPDAS Voting Document - 2nd Round. 2005 . ipdas.ohri.ca/IPDAS_Second_Round.pdf (accessed 29 Oct 2013).
Irish 2023
- Irish GL , Weightman A , Hersch J , Coates PT , Clayton PA . Do patient decision aids help people who are facing decisions about solid organ transplantation? A systematic review . Clinical Transplantation 2023. ; 37 ( 4 ): e14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph‐Williams 2013
- Joseph-Williams N , Newcombe R , Politi M , Durand MA , Sivell S , Stacey D , et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process . Medical Decision Making 2014. ; 34 ( 6 ): 699-710 . [DOI: 10.1177/0272989X13501721 ] [DOI] [PubMed] [Google Scholar]
Kasper 2012
- Kasper J , Hoffmann F , Heesen C , Köpke S , Geiger F . MAPPIN'SDM--the multifocal approach to sharing in shared decision making . PLOS One 2012. ; 7 ( 4 ): e34849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kriston 2010
- Kriston L , Scholl I , Hölzel L , Simon D , Loh A , Härter M . The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample . Patient Education and Counseling 2010. ; 80 ( 1 ): 94-9. [DOI] [PubMed] [Google Scholar]
Lewis 2017
- Lewis KB , Wood B , Sepucha KR , Thomson RG , Stacey D . Quality of reporting of patient decision aids in recent randomized controlled trials: a descriptive synthesis and comparative analysis . Patient Education and Counseling 2017. ; 100 ( 7 ): 1387-93. [DOI] [PubMed] [Google Scholar]
Lewis 2023
- Lewis KB , Smith M , Stacey D , Carley M , Graham ID , Cochrane Review of Patient Decision Aids Research Team . Evaluation of an integrated knowledge translation approach used for updating the Cochrane Review of Patient Decision Aids [preprint] . https://doi.org/10.21203/rs.3.rs-3314629/v1 2023. [DOI] [PMC free article] [PubMed]
Lin 2009
- Lin GA , Aaronson DS , Knight SJ , Carroll PR , Dudley RA . Patient decision aids for prostate cancer treatment: a systematic review of the literature . CA: A Cancer Journal for Clinicians 2009. ; 59 ( 6 ): 379-90. [DOI] [PubMed] [Google Scholar]
Légaré 2018
- Légaré F , Adekpedjou R , Stacey D , Turcotte S , Kryworuchko J , Graham ID , et al. Interventions for increasing the use of shared decision making by healthcare professionals . Cochrane Database of Systematic Reviews 2018. , Issue 7 . Art. No: CD006732. [DOI: 10.1002/14651858.CD006732.pub4 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makoul 2006
- Makoul G , Clayman ML . An integrative model of shared decision making in medical encounters . Patient Education and Counseling 2006. ; 60 ( 3 ): 301-12. [DOI] [PubMed] [Google Scholar]
McAlpine 2018
- McAlpine K , Lewis KB , Trevena LJ , Stacey D . What is the effectiveness of patient decision aids for cancer-related decisions? A systematic review subanalysis . JCO Clinical Cancer Informatics 2018. ; 2 : 1-13. [DOI] [PubMed] [Google Scholar]
Michie 2002
- Michie S , Dormandy E , Marteau TM . The multi-dimensional measure of informed choice: a validation study . Patient Education and Counseling 2002. ; 48 ( 1 ): 87-91. [DOI] [PubMed] [Google Scholar]
Mitropoulou 2022
- Mitropoulou P , Grüner-Hegge N , Reinhold J , Papadopoulou C . Shared decision making in cardiology: a systematic review and meta-analysis . Heart 2022. ; 109 ( 1 ): 34-9. [DOI] [PubMed] [Google Scholar]
Mulley 1995
- Mulley A . Outcomes research: implications for policy and practice . In: Smith R , Delamother T , editors(s). Outcomes in Clinical Practice . London: : BMJ Publishing Group; , 1995. [Google Scholar]
Munro 2016
- Munro S , Stacey D , Lewis KB , Bansback N . Choosing treatment and screening options congruent with values: do decision aids help? Sub-analysis of a systematic review . Patient Education & Counseling 2016. ; 99 ( 4 ): 491-500. [DOI] [PubMed] [Google Scholar]
NCGC/NICE 2012
- National Clinical Guideline Centre . Patient experience in adult NHS services: improving the experience of care for people using adult NHS services. 2012 . www.nice.org.uk/nicemedia/live/13668/58283/58283.pdf (accessed prior to 27 March 2017).
NCGC/NICE 2021
- National Clinical Guideline Centre . Patient experience in adult NHS services: improving the experience of care for people using adult NHS services . https://www.nice.org.uk/guidance/cg138 2021. [PubMed]
NICE 2021
- National Institute for Health and Care Excellence . Standards framework for shared-decision-making support tools, including patient decision aids . https://www.nice.org.uk/corporate/ecd8/resources/standards-framework-for-shareddecisionmaking-support-tools-including-patient-decision-aids-pdf-1124019137221 17 June 2021.
Nundy 2022
- Nundy S , Cooper LA , Mate KS . The quintuple aim for health care improvement: a new imperative to advance health equity . JAMA 2022. ; 327 ( 6 ): 521-2. [DOI] [PubMed] [Google Scholar]
O'Connor 1995
- O'Connor AM . Validation of a decisional conflict scale . Medical Decision Making 1995. ; 15 ( 1 ): 25-30. [DOI] [PubMed] [Google Scholar]
O'Connor 1998b
- O'Connor AM , Tugwell P , Wells GA , Elmslie T , Jolly E , Hollingworth G , et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation . Patient Education and Counselling 1998. ; 33 ( 3 ): 267-79. [DOI] [PubMed] [Google Scholar]
O'Connor 2000
- O'Connor AM . User Manual – Stage of Decision Making [document on the Internet] . Ottawa Hospital Research Institute; © 2000. Available from http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Stage_Decision_Making.pdf.
O'Connor 2002
- O'Connor AM . User Manual – Decision Self-Efficacy Scale [document on the Internet] . Ottawa Hospital Research Institute; © 1995. [modified 2002] Available from http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf.
O'Neill 2017
- O'Neill ES , Grande SW , Sherman A , Elwyn G , Coylewright M . Availability of patient decision aids for stroke prevention in atrial fibrillation: a systematic review . American Heart Journal 2017. ; 191 : 1-11. [DOI] [PubMed] [Google Scholar]
Ottawa Hospital Research Institute 2023
- Patient Decision Aids Research Group OHRI . A to Z inventory of decision aids . https://decisionaid.ohri.ca/AZinvent.php 2023.
RevMan Web 2023 [Computer program]
- Review Manager Web (RevMan Web) . Version 4.27.0. The Cochrane Collaboration , 2023. . Available at revman.cochrane.org.
Rothert 1987
- Rothert M , Talarcyzk GJ . Patient compliance and the decision making process of clinicians and patients . Journal of Compliance in Health Care 1987. ; 2 ( 1 ): 55-71. [Google Scholar]
Rutherford 2019
- Rutherford C , King MT , Butow P , Legare F , Lyddiatt A , Souli I , et al. Is quality of life a suitable measure of patient decision aid effectiveness? Sub-analysis of a Cochrane systematic review . Quality of Life Research 2019. ; 28 ( 3 ): 593-607. [DOI] [PubMed] [Google Scholar]
Scalia 2019
- Scalia P , Durand MA , Berkowitz JL , Ramesh NP , Faber MJ , Kremer JA , et al. The impact and utility of encounter patient decision aids: systematic review, meta-analysis and narrative synthesis . Patient Education and Counseling 2019. ; 102 ( 5 ): 817-41. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ , Higgins JPT , Vist GE , Glasziou P , Akl EA , Skoetz N , Guyatt GH . Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022 . Available from www.training.cochrane.org/handbook.
Sepucha 2013
- Sepucha KR , Borkhoff CM , Lally J , Levin CA , Matlock DD , Ng CJ , et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments . BMC: Medical Informatics and Decision Making 2013. ; 13 ( Suppl 2 ): S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shay 2015
- Shay LA , Lafata JE . Where is the evidence? A systematic review of shared decision making and patient outcomes . Medical Decision Making 2015. ; 35 ( 1 ): 114-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skivington 2021
- Skivington K , Matthews L , Simpson SA , Craig P , Baird J , Blazeby JM , et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance . BMJ 2021. ; 374 : n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spatz 2016
- Spatz ES , Krumholz HM , Moulton BW . The new era of informed consent: getting to a reasonable-patient standard through shared decision making . JAMA 2016. ; 315 ( 19 ): 2063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2019
- Stacey D , Suwalska V , Boland L , Lewis KB , Presseau J , Thomson R . Are patient decision aids used in clinical practice after rigorous evaluation? A survey of trial authors . Medical Decision Making 2019. ; 39 ( 7 ): 805-15. [DOI] [PubMed] [Google Scholar]
Stacey 2021
- Stacey D , Volk RJ , IPDAS Evidence Update Leads. The International Patient Decision Aid Standards (IPDAS) Collaboration: Evidence Update 2.0 . Medical Decision Making 2021. ; 41 ( 7 ): 729-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2023
- Stacey D , Ludwig C , Archambault P , Smith M , Taljaard M , Carley M , et al. Decisions and decisional needs of Canadians from all provinces and territories during the COVID-19 pandemic: population-based cross-sectional surveys . JMIR Public Health and Surveillance 2023. ; 9 : e43652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staniszewska 2017
- Staniszewska S , Brett J , Simera I , Seers K , Mockford C , Goodlad S , et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research . BMJ 2017. ; 358 : j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trenaman 2014
- Trenaman L , Stirling B , Bansback N . The cost-effectiveness of patient decision aids: a systematic review . Healthcare 2014. ; 2 ( 4 ): 2510257. [DOI] [PubMed] [Google Scholar]
Trenaman 2016
- Trenaman L , Selva A , Desroches S , Singh K , Bissonnette J , Bansback N , et al. A measurement framework for adherence in patient decision aid trials applied in a systematic review subanalysis . Journal of Clinical Epidemiology 2016. ; 77 : 15-23. [DOI] [PubMed] [Google Scholar]
Trenaman 2017
- Trenaman L , Stacey D , Bryan S , Taljaard M , Hawker G , Dervin G , et al. Decision aids for patients considering total joint replacement: a cost-effectiveness analysis alongside a randomised controlled trial . Osteoarthritis and Cartilage 2017. ; 25 ( 10 ): 1615-22. [DOI] [PubMed] [Google Scholar]
Trenaman 2020
- Trenaman L , Stacey D , Bryan S , Payne K , Hawker G , Bansback N . Long-term effect of patient decision aids on use of joint replacement and health care costs . Osteoarthritis and Cartilage 2020. ; 28 ( 6 ): 819-23. [DOI] [PubMed] [Google Scholar]
Trikalinos 2014
- Trikalinos TA , Wieland LS , Adam GP , Zgodic A , Ntzani EE . Decision Aids for Cancer Screening and Treatment . Rockville, MD: : Agency for Healthcare Research and Quality; , 2014. [PubMed] [Google Scholar]
Turkson‐Ocran 2021
- Turkson-Ocran RN , Ogunwole SM , Hines AL , Peterson PN . Shared decision making in cardiovascular patient care to address cardiovascular disease disparities . Journal of the American Heart Association 2021. ; 10 ( 20 ): e018183. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Weijden 2019
- Weijden T , Dreesens D , Faber MJ , Bos N , Drenthen T , Maas I , et al. Developing quality criteria for patient-directed knowledge tools related to clinical practice guidelines. A development and consensus study . Health Expectations 2019. ; 22 ( 2 ): 201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Veenendaal 2022
- Veenendaal H , Peters LJ , Weele E , Hendriks MP , Schuurman M , Visserman E , et al. Effects and working mechanisms of a multilevel implementation program for applying shared decision-making while discussing systemic treatment in breast cancer . Current Oncology 2022. ; 30 ( 1 ): 236-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Washington State Health Care Authority 2016
- Washington State Health Care Authority . Patient decision aid certification criteria . Available from: http://www.hca.wa.gov/hw/Documents/sdm_cert_criteria.pdf 2016.
References to other published versions of this review
O'Connor 1999b
- O'Connor AM , Rostom A , Fiset V , Tetroe J , Entwistle V , Llewellyn-Thomas H , et al. Decision aids for patients facing health treatment or screening decisions: systematic review . BMJ 1999. ; 319 ( 7212 ): 731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2001
- O'Connor AM , Stacey D , Rovner D , Holmes-Rovner M , Tetroe J , Llewellyn-Thomas H , et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2001. , Issue 3 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431 ] [DOI] [PubMed] [Google Scholar]
O'Connor 2003
- O'Connor AM , Stacey D , Rovner D , Holmes-Rovner M , Tetroe J , Llewellyn-Thomas H , et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2003. , Issue 1 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431 ] [DOI] [PubMed] [Google Scholar]
O'Connor 2009b
- O'Connor AM , Bennett C , Stacey D , Barry M , Col NF , Eden KB , et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2009. , Issue 3 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431.pub2 ] [DOI] [PubMed] [Google Scholar]
Stacey 2011
- Stacey D , Bennett CL , Barry MJ , Col NF , Eden KB , Holmes-Rovner M , et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2011. , Issue 10 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431.pub3 ] [DOI] [PubMed] [Google Scholar]
Stacey 2014b
- Stacey D , Legare F , Col NF , Bennett CL , Barry MJ , Eden KB , et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2014. , Issue 1 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431.pub4 ] [DOI] [PubMed] [Google Scholar]
Stacey 2017
- Stacey D , Légaré F , Lewis K , Barry MJ , Bennett CL , Eden KB et al. Decision aids for people facing health treatment or screening decisions . Cochrane Database of Systematic Reviews 2017. , Issue 4 . Art. No: CD001431. [DOI: 10.1002/14651858.CD001431.pub5 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


